Light dependent courtship behavior in Drosophila simulans and D. melanogaster
- Published
- Accepted
- Received
- Academic Editor
- Claudio Ramirez
- Subject Areas
- Animal Behavior, Ecology, Evolutionary Studies
- Keywords
- Drosophila, Courtship, Signals, Perception, Vision, Variation
- Copyright
- © 2020 Shahandeh et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Light dependent courtship behavior in Drosophila simulans and D. melanogaster. PeerJ 8:e9499 https://doi.org/10.7717/peerj.9499
Abstract
Differences in courtship signals and perception are well-known among Drosophila species. One such described difference is the dependency on light, and thus presumably vision, for copulation success. Many studies have described a difference in light-dependent copulation success between D. melanogaster and D. simulans, identifying D. simulans as a light-dependent species, and D. melanogaster as a light-independent one. However, many of these studies use assays of varying design and few strains to represent the entire species. Here, we attempt to better characterize this purported difference using 11 strains of each species, paired by collection location, in behavioral assays conducted at two different exposure times. We show that, while there is a species-wide difference in magnitude of light-dependent copulation success, D. melanogaster copulation success is, on average, still impaired in the dark at both exposure times we measured. Additionally, there is significant variation in strain-specific ability to copulate in the dark in both species across two different exposure times. We find that this variation correlates strongly with longitude in D. melanogaster, but not in D. simulans. We hypothesize that differences in species history and demography may explain behavioral variation. Finally, we use courtship assays to show that light-dependent copulation success in one D. simulans strain is driven in part by both males and females. We discuss potential differences in courtship signals and/or signal importance between these species and potential for further comparative studies for functional characterization.
Introduction
Courtship in Drosophila is a multimodal form of communication, involving chemosensory, auditory, tactile, and visual signals (Greenspan & Ferveur, 2000). Often, male signals are more conspicuous and easily observed, and thus are more widely studied; for example, males of many species produce a courtship song by extending and vibrating thier wings (Spieth, 1952). The resulting song can be recorded and separated into discrete parts, such as pulse and sine song (Von Schilcher, 1976), and quantified using metrics like inter-pulse interval and pulse duration (Kyriacou & Hall, 1982). These metrics show clear signs of species specificity that are important to conspecific reproductive success (Kyriacou & Hall, 1982; Spieth, 1974). Another signal that can be easily quantified, and has been extensively studied, is variation in chemotactic pheromones (Cobb & Jallon, 1990; Jallon & David, 1987; Pardy et al., 2019; Pischedda et al., 2014). These pheromones, present on the fly cuticle, sometimes act as sex- and species-specific identifiers that stimulate courtship among conspecific pairings, but suppress courtship between heterospecific pairs in some species (Billeter et al., 2009; Manning, 1959b; Savarit et al., 1999; Shahandeh, Pischedda & Turner, 2018; Shahandeh & Thomas, 2020).
In the case of visual signals, for Drosophila melanogaster, visual perception of a moving courtship target is necessary for males to initiate and maintain courtship (Agrawal, Safarik & Dickinson, 2014; Cook, 1980), with males preferring to initiate courtship towards moving targets over stationary ones (Tompkins et al., 1982). D. melanogaster males prefer larger females (Byrne & Rice, 2006; Edward & Chapman, 2012), though it remains to be determined if this choice is driven by visual perception or some other cryptic correlate of female quality. Given that these prior results highlight the potential importance of visual signaling in courtship, it is noteworthy that D. melanogaster is said to copulate successfully independent of light (Manning, 1959a; Spieth & Hsu, 1950). Indeed, for many other Drosophila species, copulation success is relatively light dependent (Ewing, 1983; Grossfield, 1971; Spieth, 1974). However, the specific visual signals that make courtship light dependent for other species remain relatively unclear, with a few notable exceptions where males or females have evolved an additional postural display whereby they presumably send visual signals using specific, repeated, positions or movements (Brown, 1965; Ewing, 1983).
For the commonly studied cosmopolitan species, D. simulans, no such postural behavior has been described. Nonetheless, this species is said to differ largely from D. melanogaster in their light-dependent copulation success (Grossfield, 1971; Manning, 1959a). Specifically, D. melanogaster copulates successfully independent of light, while D. simulans copulates significantly less in the dark (Manning, 1959a; Spieth & Hsu, 1950). The ubiquity of this purported difference is debatable, however, as genetically blinded D. melanogaster do not copulate as successfully as wild-type males when kept in bright light (Tompkins et al., 1982). Reported differences in Drosophila light-dependent copulation behavior may be a result of strain-specific behavior or may reflect experimentally induced variation. Indeed, studies often use just one or two strains as a representative of a species and conduct assays of variable lengths, ranging from minutes to a week, and designs, ranging from individual pairs of flies to large groups that are blinded or compared under varying light regimes (Cobb & Ferveur, 1995; Giglio & Dyer, 2013; Gleason et al., 2012; Grossfield, 1971; Manning, 1959a; Spieth & Hsu, 1950; Tompkins et al., 1982).
In the present study, we seek to more accurately quantify the level of light dependency for these two sister species of Drosophila: D. melanogaster and D. simulans. To do so, we measure light dependent copulation success at two exposure times for 11 strains of D. melanogaster and 11 strains of D. simulans collected from paired locations around the globe. By doing so, we are able to quantify species differences in light dependent copulation behavior as well as assess intraspecific variation and time-dependency. Further, there is some evidence that light-dependent copulation success is inversely correlated with ecological generality (Grossfield, 1971). Using our D. melanogaster and D. simulans strains, we also test for correlations among behavior and geographic variables to gain insight into potential factors underlying global behavioral variation. Finally, we use the most extreme lines from either side of the behavioral spectrum for each species in courtship observation to begin to understand the mechanistic causes of light-dependent behavior.
Material and Methods
Fly strains and maintenance
We selected 11 wild-type D. melanogaster and 11 wild-type D. simulans strains (Table 1) from the National Drosophila Species Stock center that were collected across 6 continents. These 22 strains constitute 11 pairs of D. simulans and D. melanogaster strains that were collected at approximately equal latitudes and longitudes. Whenever possible, we chose strains that were collected from the same location at the same time.
| Stock # | Species | Collection location (date) | Longitude | Latitude | Strain label |
|---|---|---|---|---|---|
| 14021-0251.005 | D. simulans | Lima, Peru (1956) | −77.0428 | −12.0464 | PER005 |
| 14021-0231.01 | D. melanogaster | Ica, Peru (1956) | −75.7342 | −14.0755 | M-PER01 |
| 14021-0251.009 | D. simulans | Gorak, New Guinea (1961) | 145.3863 | −6.0835 | NG009 |
| 14021-0231.120 | D. melanogaster | Port Moresby, Papua New Guinea (1982) | 147.1803 | −9.4438 | M-NG120 |
| 14021-0251.169 | D. simulans | South Africa | 22.9375 | −30.5595 | SA169 |
| 14021-0231.51 14021-0231.62 | D. melanogaster | Cape Town, South Africa (2007) | 18.4241 | −33.9249 | M-SA51 |
| 14021-0251.181 | D. simulans | Crete Island, Greece (2002) | 24.8093 | 35.2401 | GRE181 |
| 14021-0231.69 | D. melanogaster | Athens, Greece (1965) | 23.7275 | 37.9838 | M-GRE69 |
| 14021-0251.261 | D. simulans | Lujeri, Malawi (2009) | 35.6484 | −16.0400 | MAL261 |
| 14021-0231.76 | D. melanogaster | Lujeri, Malawi (2009) | 35.6484 | −16.04 | M-MAL76 |
| 14021-0251.288 | D. simulans | Athens, Georgia (2009) | 83.3576 | 33.9519 | GEO288 |
| 14021-0231.183 | D. melanogaster | Athens, Georgia (2009) | 83.3576 | 33.9519 | M-GEO181 |
| 14021-0251.004 | D. simulans | Australia (1955) | 133.7751 | −25.2744 | AUS004 |
| 14021-0231.03 | D. melanogaster | Queensland, Australia | 142.7028 | −20.9176 | M-AUS05 |
| 14021-0251.166 | D. simulans | IslaMorada, Florida | −80.6278 | 24.9243 | FLO166 |
| 14021-0231.14 | D. melanogaster | Orlando, Florida | −81.3792 | 28.5383 | M-FLO14 |
| 14021-0251.001 | D. simulans | Georgetown, Guyana (1956) | −58.1551 | 6.8013 | GUY001 |
| 14021-0231.15 | D. melanogaster | Bahia, Brazil | −41.7007 | −12.5797 | M-BRAZ15 |
| 14021-0251.196 | D. simulans | Ansirabe, Madagascar (1998) | 47.0291 | −19.873 | MAD196 |
| 14021-0231.125 | D. melanogaster | Tananarive, Madagascar (1982) | 47.5079 | −18.8792 | M-MAD125 |
| 14021-0251.006 | D. simulans | Nueva, California (1961) | −117.1459 | 33.8014 | NUE006 |
| 14021-0231.131 | D. melanogaster | La Jolla, California (2009) | −117.2713 | 32.8328 | M-SD131 |
We maintained each strain on non-overlapping, alternating 2-week life cycles. We reared all strains on a standard cornmeal-yeast-molasses medium in 25 mm vials at 25 °C and ∼50% humidity under a 12:12 h light/dark cycle. At the beginning of each cycle, we transferred roughly 20-30 adult flies to a culture vial with fresh media. We allowed the flies to oviposit for 48 h before transferring them to a second collection vial with fresh media, where flies were allowed to oviposit for an additional 24 h before being discarded. We repeated this process every fourteen days using offspring from the culture vials to maintain each strain. For use in experiments, we collected male and female offspring as virgins from the collection vials 4–5 h following “lights-on” under light CO2 anesthesia 11 days after oviposition. For all experiments described below, we aged males and females separately in holding vials with fresh food media at a density of 10 flies for 3–4 days before each assay. We aged flies in groups prior to assay, because flies held in isolation display increased aggressive behaviors we were concerned would affect copulation success, skewing our data (Hoffmann, 1990).
Light dependent copulation success assay
To measure each strain’s ability to successfully copulate independent of light, we measured copulation success in a normal light (control) treatment, and in an entirely dark (experimental) treatment, side-by-side on the same day. On the morning of each assay, immediately following “lights-on”, we aspirated a single virgin male and female into a 20 mm vial with fresh food media sealed with a foam plug. We chose to use single pairs, as there is some evidence from other Drosophila that males approach females sequentially in the wild, and females that are approached singly are more likely to copulate (Noor & Ortiz-Barrientos, 2006). We assayed flies in vials with food media because adult Drosophila are most likely to encounter mates on or near a food substrate (Soto-Yéber et al., 2018). We held these vials at 25 °C and ∼50% humidity for either 2 or 6 h in an incubator illuminated (control), with a Phillips “Cool white” 32-watt fluorescent light bulb or in the same incubator sealed in a light-proof box (experimental). We chose these time-points, rather than a day or week-long assay, because they represent a short, more realistic exposure time for flies in the wild, and a longer exposure time with which we could assess time dependency. At the end of the assay, we used an aspirator to remove the male from the vial so no post-assay copulation could occur. We then held females in vials at 25 °C and ∼50% humidity in a 12 h:12 h light/dark cycle to oviposit for 7 days. On day seven, we checked each vial for the presence of larvae or early stage pupae, indicating whether insemination successfully occurred during the assay time. We collected all data on a weekly basis over the course of 6 weeks.
For each of the 11 D. melanogaster and D. simulans strains, we observed 25–31 pairings in the control and experimental treatments for each exposure time (2 or 6 h). For each strain, we calculated copulation success using the proportion of vials that produced offspring as a proxy for the proportion of vials where successful copulation occurred. We used Fisher’s exact tests to compare copulation success between control and experimental treatments for each strain at each exposure time, followed by post-hoc correction for multiple comparisons (Holm, 1979). We tested for a species-wide difference between light/dark treatments at each exposure time using paired t-tests or paired Wilcoxon rank-sum tests when the data did not fit a normal distribution.
We next calculated relative dark copulation success for each exposure time as the percent of successful copulations in the dark treatment divided by the proportion of successful copulations for the same strain in the light treatment (% dark/% light). We compared relative dark copulation success between the 2-hour and 6-hour exposure times within species, and for each exposure time between species, using paired t-tests. We also tested for a correlation between relative dark copulation success at each exposure time and three collection variables: longitude, the absolute value of latitude (i.e., distance from the equator), and collection date (when available). We used Pearson’s correlation test when the data fit a normal distribution, and Spearman’s rank correlation when the data was not normally distributed.
Courtship assays
To test if males from D. melanogaster and D. simulans strains with relatively light-dependent and light-independent copulation success actively court females in the dark, we conducted courtship assays under two treatments. For each treatment, we gently aspirated single virgin males into vials containing a thin layer of food media 24 h prior to the assay. The morning of the assay, 1–2 h following “lights on,” we aspirated a single female into the vial and pushed a foam plug down into the vial until it was just 1–2 cm from the food surface. Because these flies were held in a much smaller space, they were forced to interact even when held in the dark, so we observed courtship for just 30 min to avoid observer fatigue. We scored each minute for one of three easily distinguished courtship behaviors: (1) singing (single-wing extension and vibration), (2) attempted copulation, and (3) successful copulation. For males exhibiting multiple behaviors within a minute, we scored each pairing once per minute per behavior. As a control, we observed male and female pairings under bright light. As an experimental treatment, we observed male and female pairings in a dark room illuminated solely with red light because the Drosophila compound eye is insensitive to red wavelengths of light (Salcedo et al., 1999).
We selected the single most light-independent and light-dependent D. simulans strains (MAL261 and SA169, respectively) and D. melanogaster strains (M-BAZ15 and M-NG120, respectively) from the two-hour exposure period for courtship observation. First, for all four strains, we observed males with females of their own strain. For the D. simulans strains, we also observed males with females collected from the opposing strain because SA169 displayed significantly less frequent courtship towards SA169 females when observed in the dark (see results). In either treatment, we considered any male that spent more than 10% of the assay time exhibiting any courtship behavior as successfully courting. We compared the proportion of males that courted females in the light and in the dark using Fisher’s exact tests followed with post-hoc correction for multiple comparisons (Holm, 1979). For males that displayed courtship, we also calculated courtship latency (the time from the start of assay until the male initiates courtship) and courtship effort (the total proportion of time a male spends courting during the 30-minute assay). If a pair successfully copulated, we calculated courtship effort as the percent of time a male spent courting from start of assay until copulation. We did not apply the same 10% cut-off for courtship latency and effort as we did for the proportion of males that courted, due to exceedingly low sample sizes. We compared courtship latencies and courtship efforts between pairings using Wilcoxon rank-sum tests followed by post-hoc correction for multiple comparisons (Holm, 1979).
Results
Light dependent copulation success
D. simulans
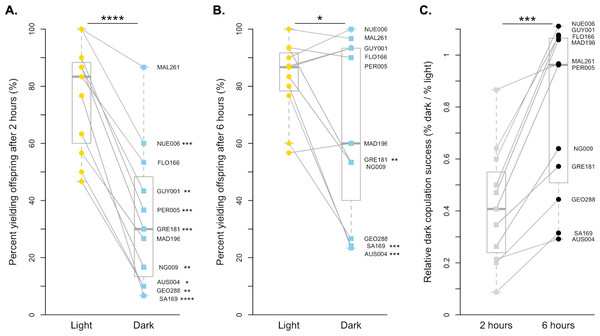
For D. simulans, copulation is much more successful in the light than in the dark when males and females were held together for 2 h (paired t-test, p < 0.0001, Fig. 1A). On average, D. simulans strains were 41.77% as successful at copulating in the dark compared to in the light when given 2 h. Each of the 11 strains had decreased copulation success in the dark, and 8 of 11 strains were statistically significant after correcting for multiple comparisons (Fig. 1A, Table S1). The species-level pattern is still detectable when males and females are held together for 6 h, but far less significant (paired Wilcoxon, p < 0.05, Fig. 1B). Overall, D. simulans strains were 77.36% as successful at copulating in the dark as they were when held in the light for 6 h. This marked improvement appears to be driven by six lines (NUE006, MAL261, GUY001, FLO166, PER005, and MAD196), which copulate approximately equally as successfully in the light as they do in the dark when given increased time (all p = 1, Fig. 1B, Table S1). Still, the remaining lines displayed reduced copulation successes in the dark compared to the light treatment, with 3 remaining significantly lower following correction for multiple comparisons (Fig. 1B, Table S1). When we compared the relative dark copulation success for our D. simulans strains across the two exposure times, we found a significant difference between the 2 and 6-hour treatments (paired Wilcoxon p < 0.001), with strains showing decreased light dependency for copulation at the 6-hour exposure time (Fig. 1C). Overall, the proportion of flies that successfully copulated increased by 0.36, on average, when given extra time.
Figure 1: Copulation success of 11 D. simulans strains by treatment and time point.
(A) The copulation success of pairs held for 2 h in the light (yellow circles) compared to in the dark (blue squares). (B) The copulation success of pairs held for 6 h in the light (yellow circles) compared to in the dark (blue squares). (C). The relative light independence of copulation success in the dark compared to in the light is shown for each strain for 2 h (light grey squares) and 6 h (black circles). For all, paired data points between treatments are connected with a line. Corresponding points are labelled with their strain label (Table 1). Individual strains and significance values after correction for multiple comparisons are indicated to the right of each point. Species wide differences are indicated with asterisks above plots ( ∗ = p < 0.05, ∗∗∗ = p < 0.001, and ∗∗∗∗ = p < 0.0001).D. melanogaster
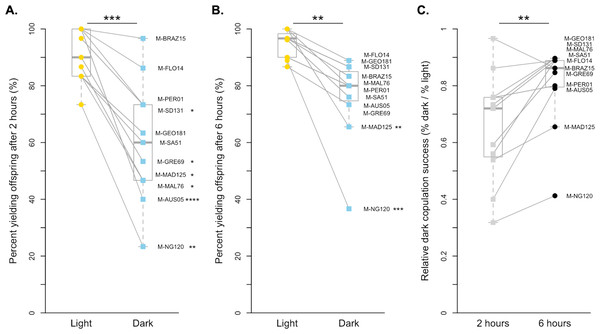
We also found that copulation is more successful in the light for D. melanogaster when pairs were given 2 h to mate (paired t-test p < 0.001, Fig. 2A). On average, D. melanogaster was 65.54% as effective at mating in the dark as they were in the light when given 2 h, with 6 of 11 strains significantly worse in the dark after correcting for multiple comparisons (Fig. 2A, Table S1). Like with D. simulans, we were still able to detect an overall effect of light vs. dark treatments at 6 h, albeit less significantly (paired Wilcoxon p < 0.01, Fig. 2B, Table S1). While just two lines individually copulated significantly less at this exposure time (M-MAD125 and M-NG120), all lines showed a reduced proportion of copulating pairs in the dark relative to the light. Overall, the difference between treatments at 6 h was smaller, with strains copulating 80.24% as successfully in the dark as they did in the light. Again, we find that D. melanogaster males show reduced light-dependent copulation behavior at 6 h relative to 2 h (paired Wilcoxon p < 0.01, Fig. 2C). Overall, the proportion of flies that successfully copulated increased by 0.15, on average, when given extra time.
Figure 2: Copulation success of 11 D. melanogaster strains by treatment and time point.
(A) The copulation success of pairs held for 2 h in the light (yellow circles) compared to in the dark (blue squares). (B) The copulation success of pairs held for 6 h in the light (yellow circles) compared to in the dark (blue squares). (C). The relative light independence of copulation success in the dark compared to in the light is shown for each strain for 2 h (light grey squares) and 6 h (dark grey circles). For all, paired data points between treatments are connected with a line. Corresponding points are labelled with their strain label (Table 1). Individual strains and significance values after correction for multiple comparisons are indicated to the right of each point. Species wide differences are indicated with asterisks above plots ( ∗∗ = p < 0.01 and ∗∗∗ = p < 0.001).Comparing D. simulans and D. melanogaster
When we compare relative copulation success in the dark between D. simulans and D. melanogaster, we see a significant difference at the 2-hour exposure time (paired t-test p < 0.05). Specifically, the relative copulation success in the dark for D. simulans (41.77%) is significantly lower than that of D. melanogaster (65.54%). Contrastingly, we do not find a significant difference at the 6-hour exposure time (paired Wilcoxon p = 0.5988). The loss of the effect is driven by D. simulans strains improving their relative copulation success in the dark significantly when given more time (77.36%), compared to a more minor improvement by D. melanogaster (80.24%). We also found no correlation between the light-dependent copulation behavior of D. simulans strains and the D. melanogaster strains collected from the same (or similar) geographic location at 2 h (Pearson’s r = 0.1679, p = 0.6217) or 6 h (Spearman’s rho = 0.2115, p = 0.5324).
Correlations of light-dependent copulation success
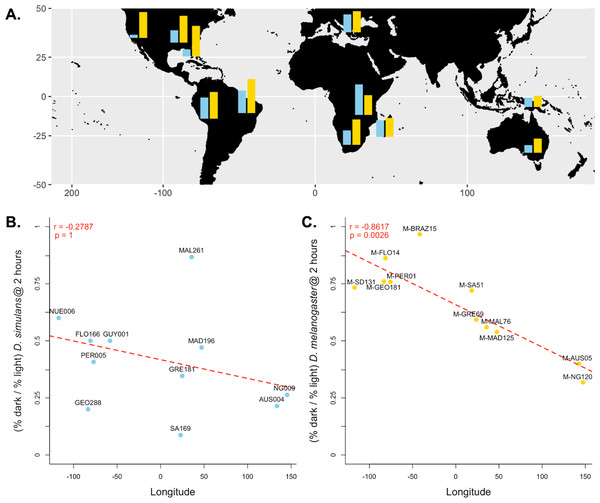
Because we found the largest effect of light-dependent copulation success at the 2-hour exposure time, we used the relative copulation success in the dark of our 11 D. melanogaster and D. simulans strains at 2 h to test for a correlation with other variables: aspects of collection location and date. For our D. simulans strains, we found no correlation between behavior and longitude (Pearson’s r = − 0.2787, p = 1, Fig. 3B), distance from the equator (Pearson’s r = − 0.2579, p = 1, Fig. S1A ), or collection date (Spearman’s rho = 0.2152, p = 1, Fig. S2 A). For our D. melanogaster strains, we found no correlation between behavior and distance from the equator (Pearson’s r = 0.2490, p = 1, Fig. S1B) or collection date (Pearson’s r = 0.0700, p = 1, Fig. S2B ). We did, however, detect a significant correlation between light-dependent copulation success at 2 h and longitude (Pearson’s r = − 0.8617, p < 0.01, Fig. 3C).
Figure 3: Differences in dark courtship ability correlate with longitude in D. melanogaster but not D. simulans.
(A) The relative dark copulation success at 2 h is plotted by geographic sample for D. simulans strains (blue) and D. melanogaster strain (yellow). (B) There is no correlation between relative light dependence at 2 h (y-axis) and longitude (x-axis) for D. simulans strains. (C) There is a significant correlation between relative light dependence at 2 h (y-axis) and longitude (x-axis) for D. melanogaster strains. For B and C, individual points are labelled with their strain label (Table 1). The red dashed line represents the best fit line from a linear model Pearson’s correlation coefficient and significance values, corrected for multiple comparisons, are displayed in the upper left corner of the plot.Light-dependent courtship behavior
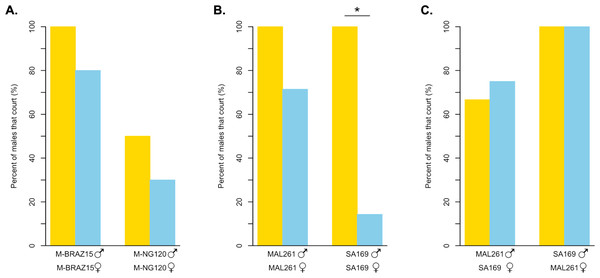
We wanted to know if light-dependent copulation behavior was mediated by male or female behavior. To test for differences in courtship behavior, we observed the courtship of two D. melanogaster strains under bright light and in darkness (Fig. 4A, Table S2A). For the strain we identified as relatively light-independent using our copulation assay, M-BRAZ15, we found that males court females at high frequencies in both treatments. Specifically, 100% of M-BRAZ15 males courted their own females under bright light, while 80% displayed courtship towards females when observed in the dark. Strain M-NG120, which displayed the most light-dependent copulation in our assay, courted females 50% of the time under bright light, and 30% of the time in the dark. While each strain showed a 20% decrease in overall courtship, the difference was not significant in either case (p = 0.9474 for both). Additionally, we detected no significant differences between courtship latency or effort for pairings observed in the light compared to those in the dark, however sample sizes are quite small, as not all males displayed courtship (Table S3).
Figure 4: Courtship behavior of light dependent and light independent D. melanogaster and D. simulans under bright light and in darkness.
(A) The percent of D. melanogaster strain males that court their own females in the light (yellow bars) compared to in darkness (blue bars). M-BRAZ15 was the most light-independent strain from our copulation assay, while M-NG120 was the most light-dependent (N = 10 for both). (B) The percent of D. simulans strain males that court their own females in the light (yellow bars) compared to in darkness (blue bars). N = 4 in the light, and N = 7 in the dark for both strains. (C) The percent of D. simulans strain males that court the opposing strain’s females in the light (yellow bars) compared to in darkness (blue bars). N = 3 for all except for MAL261 males with SA169 females in the dark, where N = 4. For B and C, MAL261 was the most light-independent strain from our copulation assay, while SA169 was the most light-dependent. ∗ = p < 0.05.We also observed the courtship behavior of two D. simulans strains under the same conditions (Fig. 4B, Table S2A). MAL261, which copulated successfully independent of light, displayed high courtship towards their own females in both scenarios; 100% of males courted in the light, while 71.4% displayed courtship in the dark (p = 0.4909). For SA169 males, which showed the strongest signal of light-dependent copulation success in our mating trials, we observed a significant difference in the proportion of males that courted under bright light, 100%, compared to in the dark, 14.3% (p < 0.05). For both MAL261 and SA169 we did not detect significant differences in courtship latency or effort when courting in the dark relative to the light (Table S3). To identify whether the difference in the proportion of courtship we observed is driven by male or female behavior, we observed MAL261 males with SA169 females and SA169 males with MAL261 females under the same conditions (Fig. 4C, Table S2B). We found that 75% of MAL261 males still court SA169 females in the dark, compared to 66.7% in the light (p = 1). Interestingly, we also found that SA169 males court MAL261 equally as well in the dark as they do in the light (100% for both, p = 1). Again, there were no detectable differences in courtship latency or effort at these small sample sizes (Table S3).
Discussion
A species difference in light-dependent copulation behavior
In some respects, our findings confirm a previously described species difference in light-dependent copulation behavior (Grossfield, 1971; Spieth, 1974). Specifically, D. melanogaster strains had, on average, greater copulation success in the dark than D. simulans strains when assayed for 2 h. However, this difference disappears when strains of each species are given 4 more hours. While both species show improvement in copulation success in the dark when given increased time, the loss of a species difference at 6 h is driven by a significant improvement in ability to copulate in the dark among D. simulans strains relative to D. melanogaster strains. Interestingly, the overall improvement is largely driven by 6 D. simulans strains (Fig. 1), that copulate equally as well in the dark as in the light when given 6 h.
In other measures, our results refute some of the species-wide conclusions made by previous studies of light-dependent copulation success, and address some of the inconsistencies among previously published results. First, we show that D. melanogaster strains, as a whole, do not copulate successfully independent of light. At both the 2 and 6-hour exposure times, we detect a significant difference in copulation success between our light and dark treatments. Individually, in both treatments, each strain has a higher copulation success in the light compared to the dark (6 strains and 2 strains significantly so at 2 and 6 h respectively). This is in contrast to D. simulans, where a handful of strains show relatively unchanged, if not slightly increased, copulation success in the dark relative to the light at the 6-hour exposure time. Overall, while these data do demonstrate a difference between species in light-dependent copulation behavior, they also highlight an important role of both genetic variation and assay time in detecting this species difference. Our data show that a high level of intraspecific variation for both species creates largely overlapping distributions of behavior that are greatly affected by exposure time. Had we chosen fewer strains or sampled at a single exposure time, our conclusions may have been very different.
Environmental correlates of light-dependent copulation behavior
We observed substantial variation in light-dependent copulation behavior among our D. simulans and D. melanogaster strains. Previous results have suggested a role of ecological generality in dark copulation success (Grossfield, 1971); species that occupy greater geographic range tend to have increased ability to copulate successfully in the dark. However, both D. simulans and D. melanogaster are near cosmopolitan human commensal species that overlap nearly entirely in their geographic ranges (Kliman et al., 2000). To attempt to identify other potential causes of the species difference we detected at the 2-hour exposure time, we tested for correlations between light-dependent copulation success and other aspects of strain collection (latitude, longitude, and collection date). We found no correlation between collection date and behavior for either D. melanogaster or D. simulans, which reduces (but does not conclusively eliminate) the likelihood that variation in light-dependent copulation behavior is a product of laboratory adaptation. For D. simulans, light-dependent copulation behavior did not correlate with either longitude or distance from the equator. Further studies including more strains would have increased power to detect less significant correlations, however. For D. melanogaster, behavioral variation correlated strongly with longitude at the strain’s collection site (Fig. 3C). This correlation is unlikely to be a result of differences in habitat, as longitude does not correlate strongly with measures of environment. Instead, this correlation might reflect the demographic history of D. melanogaster. If so, the lack of correlation between variation in D. simulans behavior and longitude potentially reflects differences in species demography.
D. melanogaster originated in sub-Saharan Africa, eventually expanding out of Africa and colonizing the rest of the world, first colonizing Eurasia (Li & Stephan, 2006). Much later, an admixed American population was established, likely during the modern colonization of the Americas (<500 years ago) (Duchen et al., 2013). Australia’s population is similarly admixed and likely very recently colonized with modern sea travel (Arguello et al., 2019). Importantly, there is still significant gene flow between populations (Arguello et al., 2019). Our data potentially mirror these two recent independent trans-oceanic colonization events. American populations show the highest level of light-independent copulation success, while Australian/south east Asian populations display the lowest. The African and European populations fall in the middle (for both behavior and longitude). If we consider the African phenotypes ancestral, then there appears to be little change with the colonization of Europe, but dramatic shifts in behavior with opposing valence for the colonization of the Americas and Oceania. Whether these differences are due to founder effects or divergent selection remains an open question. In contrast, D. simulans, which originated in East Africa or Madagascar, spread across the globe much more recently (Dean & Ballard, 2004), and globally distributed samples show significantly less population structure (Irvin et al., 1998) and clinal variation (Machado et al., 2016) than D. melanogaster. Similarly, there is no discernable structure to light-dependent copulation success in our data. The effects of species demography on light-dependent copulation success are hypotheses that still require explicit testing, however. We cannot make strong conclusions regarding these effects with the data we have presented here.
Courtship behavior differs between relatively light-dependent and light-independent strains
To begin to identify the mechanistic drivers of differences in light-dependent copulation behavior, we selected the most light-dependent and light-independent strains from each species to compare male courtship rates in both the light and the dark. For D. melanogaster, we found that both the light-independent (M-BRAZ15) and light-dependent (MNG120) strains courted at statistically indistinguishable rates in both treatments. Although, M-NG120 showed reduced courtship overall. This relative reduction in courtship is congruent with the result of our mating assay, where M-NG120 showed the lowest copulation success in both the light and the dark, indicating this pattern is more likely a result of differences in male courtship vigor. These results imply that for these strains of D. melanogaster, reduced copulation success may depend partially on a male’s ability to locate females in the dark. Partly supporting this hypothesis, recent work has shown that other Drosophila species display increased courtship latency in the absence of visual cues (Roy & Gleason, 2019). We did not detect significant differences in courtship latency, but further observation at larger sample sizes may find a similar trend. Differences in ability to locate females in the dark may be driven by a unique male scanning behavior described among dark-courting D. melanogaster strains, presumably used to locate females without visual input (Krstic, Boll & Noll, 2009). D. melanogaster males also depend on olfactory cues and female movement (and the resulting sound/vibration) to initiate courtship with females in the dark (Ejima & Griffith, 2008; Stockinger et al., 2005). Thus, in the absence of visual detection of movement, D. melanogaster males can rely on another sensory modalities to identify the presence of courtship targets and adopt a different strategy to locate them in the dark. The variation we observed among strains also potentially reflects variations in male’s ability to locate females, or variation in female signals that males use to locate females in the dark (Trajković et al., 2017).
For D. simulans, we found that the light-independent strain (MAL261) courted females with high frequency in the light, and somewhat reduced, although statistically insignificantly, frequency in the dark. For the light-dependent D. simulans strain (SA169), we found that males courted females at high frequencies in the light, but at significantly lower frequencies in the dark (Fig. 4B). Thus, SA169 males are less willing or able to court SA169 females in the dark than MAL261 males are able to court MAL261 females, despite having equal courtship in the light. From these results, it is unclear if the difference in the amount of courtship in the dark is driven by males or females. It is possible that SA169 males cannot identify potential courtship targets because of the lack of a perceivable moving courtship target. It could be equally likely that SA169 female signals are missing or become indiscernible in the dark, leading to a reduction in male courtship.
To test if the loss of SA169 male courtship is driven by female signals, we swapped female types for our light-dependent and light-independent strains. We observed SA169 males with MAL261 females, and vice versa. MAL261 males were equally able to court SA169 females in the light and in the dark. Surprisingly, we found the same pattern for SA169 males: they court MAL261 females 100% of the time in both the light and in the dark. So, SA169 males can locate and court females in the dark, but do not do so when those females are also SA169. In contrast, MAL261 males will court either female in the dark. These results indicate that there is both a difference in male strains’ ability/willingness to court and in female strains’ attractiveness in the dark. It is possible that these differences are the result of differences in female activity or male olfactory or vibratory/acoustic perception ability in the dark, but we do not know if D. simulans males rely on olfactory, auditory, or vibratory cues to identify females in the dark in the same way that D. melanogaster males do (Ejima & Griffith, 2008). Ultimately, these results highlight the complex coordination of signals and receivers that underlies Drosophila courtship. They also suggest that the variation we observe among lines, potentially in both species, can reflect variation in male behavior, female behavior, or both. More careful observation of a greater number of flies and strains (and strain combinations) is necessary to understand the contributions of males and females.
The role of sensory perception in D. simulans light-independent courtship behavior
The above results highlight an important, yet unknown aspect of male mate choice in D. simulans. While we know quite a bit about the signals that males send to females during courtship (Greenspan & Ferveur, 2000), it remains unclear what signals D. simulans males use to discriminate between males and females. Unlike its sister species, D. melanogaster, D. simulans males and females express the same primary chemotactic pheromone, 7-tricosene (7T) (Cobb & Jallon, 1990). It is possible that males discriminate between sexes using a difference in 7T abundance, a difference in one of the many low-abundance cuticular hydrocarbons (CHCs) (Pardy et al., 2019), or the male-specific expression of cis-vaccenyl acetate (Jallon, 1984). While D. simulans males and females can differ quantitatively in CHCs, this only seems to be the case in some strains (Sharma et al., 2012). Additionally, the perception of these chemotactic signals is light-independent, and are unlikely to explain the species-wide reduction in dark copulation success anyway. Our results suggest that male courtship initiation in D. simulans may partially depend on a female visual signal. They also suggest that strains can vary in the importance of visual perception to initiate male courtship, which might reflect variation in ability to rely on other sensory modalities to identify female courtship targets.
Conclusions
The above conclusions highlight two potential areas of further investigation. First, for males that show higher light-dependent copulation success, what are the visual signals necessary for courtship initiation? Second, for males that show relatively light-independent copulation success, what other signals allow them to successfully initiate courtship in the dark? In either case, the role of vision needs to be further examined in combination with the other senses in a larger comparative framework to understand how sensory modalities interact to determine variation male courtship behavior both within and between species. Should visual signals prove important to D. simulans male mate discrimination, there is ample opportunity for a more precise characterization of the neural substrate of signal hierarchies in D. simulans courtship behavior. In D. melanogaster, sensory receptors (Ahmed et al., 2019; Ejima & Griffith, 2008; Göpfert & Robert, 2003; Montell, 2009) and specific neurons necessary to detect a variety of courtship signals (Lu et al., 2014; Pan, Meissner & Baker, 2012; Seeholzer et al., 2018; Starostina et al., 2012; Thistle et al., 2012; Toda, Zhao & Dickson, 2012) and produce male courtship behavior (Kohatsu, Koganezawa & Yamamoto, 2011; Von Philipsborn et al., 2011) have been identified. Our results highlight that D. simulans may rely on different (or differentially weighted) female signals. A comparative analysis of the molecular underpinnings of signal perception in these species will help to identify differences in signal perception or signal hierarchies.
Supplemental Information
The proportion of pairs that copulated
Data is split by species, time point, and strain. The number of mated pairs of the total vials set up is provided in both the dark and the light treatments. The light dependent copulation success (Pdark/Plight) is calculated by dividing the proportion of successful copulations in the dark by the proportion of successful copulations in the light. Also provided are the p-value’s for within strain comparisons after correcting for multiple copmparisons.
The proportion of males displaying courtship towards their own females in the light and in the dark
The number of males that courted their own female types for two light-dependent and two light-independent strains (one D. melanogaster and one D. simulans for each). The corrected p-value for differences between treatments is shown.
Courtship latency and courtship effort of D. melanogaster and D. simulans males when observed in the dark and when observed in the light
The species, male and female strain identity, and treatment are provided in columns 1–4, respectively. Mean courtship latency is presented with standard deviation in parentheses. The corrected p-value for a Wilcoxon rank-sum test is also provided. Mean courtship effort and corrected p-values are presented similarly. The number of pairs with courtship latency/effort values recorded (N) is provided.
Differences in dark courtship ability do not correlate with distance from the equator in D. melanogaster or D. simulans
There is no correlation between relative light dependence at 2 hours (y-axis) and distance from the equator (absolute value of longitude, x-axis) for D. melanogaster strains (A.) or D. simulans strains (B.). For both, individual points are labelled with their strain label (Table 1). The red dashed line represents the best fit line from a linear model Pearson’s correlation coefficient and significance values, corrected for multiple comparisons, are displayed in the upper left corner of the plot.
Differences in dark courtship ability do not correlate with collection year in D. melanogaster or D. simulans
There is no correlation between relative light dependence at 2 h (y-axis) and collection year (x-axis) for D. melanogaster strains (A.) or D. simulans strains (B.). For both, individual points are labelled with their strain label (Table 1). The red dashed line represents the best fit line from a linear model Pearson’s correlation coefficient and significance values, corrected for multiple comparisons, are displayed in the upper left corner of the plot.