Investigation of the protein profile of silkworm (Bombyx mori) pupae reared on a well-calibrated artificial diet compared to mulberry leaf diet
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Agricultural Science, Biochemistry, Entomology
- Keywords
- Insect, Farming, Artificial diet, Mass spectrometry
- Copyright
- © 2019 Lamberti et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Investigation of the protein profile of silkworm (Bombyx mori) pupae reared on a well-calibrated artificial diet compared to mulberry leaf diet. PeerJ 7:e6723 https://doi.org/10.7717/peerj.6723
Abstract
Background
Silkworm pupae is the main by-product of the sericulture industry with an interesting nutritional profile, especially in terms of proteins. In consideration of its possible use as a food or food ingredient in Western countries, a comparative proteomic experiment has been performed to investigate the differences of the protein profile of male and female silkworm pupae reared on mulberry leaves or on an artificial diet.
Methods
The nutritional profile of lyophilized silkworm pupae in terms of dry matter and ash was evaluated according to the AOAC procedures, the total nitrogen content was determined by a nitrogen analyzer and the silkworm pupae gross energy value was measured using an adiabatic calorimetric bomb. The comparative proteomic analysis was performed on male and female silkworm pupae reared on mulberry leaves or on the artificial diet. Proteins were separated by two-dimensional electrophoresis and, after a multivariate statistical analysis, the differentially expressed proteins were identified by LC-MS/MS.
Results
The comparative proteomic approach highlighted 47 silkworm pupae proteins differentially expressed comparing diet and gender. PCA analysis showed that seven proteins were more effective in discriminating the sex and five were more effective in discriminating the diet type. In spite of the above-mentioned differences in the silkworm pupae protein profile, no strong alteration of the pupa physiological traits have been demonstrated, suggesting a general silkworm pupae flexibility to adapt to a well-balanced artificial diet. Differences in lipid transport and metabolism were found among the experimental groups, that might have a relevant effect on the timing and on hormone secretion. This aspect may also affect silk production, as univoltine strains are the most productive. The proteomic data provided in this work, may offer a contribution in understanding also the influence of gender and farming strategy on the allergen profile of Bombyx mori, when used as food or as a food ingredient. Female silkworm pupae reared on mulberry leaves seemed to contain lower levels of known allergens than those reared in the other experimental conditions; these findings will have to be taken into account when farming B. mori for food production purposes. However, our results need to be supported by further characterization of the allergenic potential of B. mori.
Introduction
Silkworms (Bombyx mori) are insects that are able to convert plant proteins to produce silk, and, while silkworm pupae is considered the main by-product of the sericulture industry. Silkworm pupae has been used as a food, a medicine and as an animal feed in many Asian countries for a long time (Dong et al., 2017), due to its interesting nutritional profile, in terms of protein, fat, and chitin contents.
Traditionally, silkworm larvae are fed with fresh mulberry leaves, which are also their natural diet. However, in order to obviate the serious drawbacks of mulberry leaves, such as the seasonal limitation concerning the supply of fresh leaves, the possible harm from parasites or pesticides and the high labor costs, different artificial diets containing essential nutrients have been studied (Cappellozza et al., 2005; Dong et al., 2017; Zhou et al., 2008). These artificial diets may affect the larval mortality and/or the length of the larval cycle to various extents, and the resulting silk production is often slightly reduced (Cappellozza et al., 2005). Another important aspect of the silkworm farming is related to gender. Male and female silkworm have shown different silk production abilities, in particular as far as the quality and quantity of silk are concerned. Gender has also been found to affect the growth rate at various larval stages, possibly due to a difference in nutrient utilization by the midgut, as reported by Qin et al. (2014).
Owing to the increasing interest in insects as a new food and feed protein source over the last few years, EFSA issued a scientific opinion on the topic in October 2015. They highlighted that a specific risk assessment should be performed, taking into account the whole production chain from farming to consumption, including the species to raise and the substrate to use as well as the methods for farming and processing (EFSA Scientific Committee, 2015). Among the edible insect candidates, with the greatest potential for use as food on the EU market, the silkworm seems a promising candidate from a nutritional point of view (EFSA Scientific Committee, 2015).
In light of these considerations, we designed a comparative proteomic study to characterize the protein profile of male and female silkworm pupae reared on two diets, in order to identify any possible differences in protein expression, for obtaining basic understanding of how to optimize the rearing strategies for the use of this edible insect in the food and feed sector.
Materials and Methods
Experimental animals
The larvae of hybrid silkworm strain (four-way polyhybrid (57 × 76)–(76 × 57) belonging to the germplasm collection of the CREA Research Centre for Agriculture and Environment (CREA-AA)) were reared under the same environmental conditions (temperature (25 ± 1 °C) and relative humidity), but on two different diets: mulberry leaves (L) or an artificial diet (A) according to Cappellozza et al. (2005) (Table S1). Seven days after reaching the cocoon stage, the pupae were harvested and sexed according to their morphological features. A vertical line across the center of the ventral side of the eighth segment and a genital aperture in the ninth sternum were considered to identify females (F), whereas only the presence of an aperture situated at the ninth sternum was used to identify males (M).
All the analyses were carried out on three batches for each treatment: males reared on A (MA) and on L (ML), and females reared on A (FA) and on L (FL).
Nutritional profile analysis
In order to evaluate the nutritional profile of lyophilized silkworm pupae, the dry matter (DM) (#930.15) and the ash (#924.05) were assessed according to the AOAC procedures (AOAC International, 2003). The total nitrogen (N) content was determined using a nitrogen analyzer (Rapid N III; Elementar Analysen system GmbH, Hanau, Germany) according to the Dumas method and the gross energy was measured using an adiabatic calorimetric bomb (C7000; IKA, Staufen, Germany).
Proteomic analysis
The comparative proteomic analysis was performed on three extraction replicates for each biological replicate and for each experimental condition (for a total of 36 two-dimensional electrophoresis (2DE) gels).
Preparation of the soluble protein extracts
A pool of 10 frozen pupae (−80 °C), corresponding to 0.5 g, pulverized by means of a mincer, was solubilized in 1.5 mL of PBS (0.1M, pH 7.4) and a Complete™ (Sigma-Aldrich S.r.l., St. Louis, MO, USA) protease inhibitor was added (one tablet per 50 mL extraction solution). Each sample was sonicated on ice, under agitation, for a total of 30 s, for seven cycles, with 30 min of break after each cycle. After centrifugation (13,201×g, 4 °C, 10 min), the upper phase and the pellet were discarded and the supernatant protein content was determined by means of the 2D-Quant-kit (GE Healthcare, Chicago, IL, USA).
Two-dimensional electrophoresis
Each protein extract (50 μg) was diluted in an appropriate volume of IPG rehydration buffer (7M urea, 2M thiourea, 66 mM DTT, 4% CHAPS, 0.5% ampholytes) and loaded on immobilized pH gradient strips (seven cm, linear pI gradient from 3 to 10) (Bio-Rad Italia, Hercules, CA, USA). The IPG strips were actively rehydrated for 6 h at 50 V and 20 °C, and isoelectrofocusing was carried out on a Protean IEF Cell (Bio-Rad, Hercules, CA, USA), starting with a voltage of 200 V for 1 h, then 1,000 V for 1 h and finally up to 4,000 V for a total of 25,000 Vh. The focused strips were incubated at RT in a reduction buffer (6M urea, 30% v/v glycerol, 2% w/v SDS, 50 mM Tris–HCl, pH 8.6, 2% w/v DTT) for 15 min and then in an alkylation buffer (6M urea, 30% v/v glycerol, 2% w/v SDS, 50 mM Tris–HCl, pH 8.6, 4.5% w/v iodoacetamide) for 15 min in the dark. The equilibrated strips were then embedded at the top of LDS precast homogeneous gels (NuPAGE 10% Bis–Tris, Invitrogen Corporation, Carlsbad, CA, USA) and electrophoretic separation was performed in an XCell SureLock Mini-Cell System (Invitrogen, Carlsbad, CA, USA) at RT, 200 V constant, 125 mA, 100 W for 45 min. The gels were stained with Colloidal Coomassie Blue (Candiano et al., 2004) and scanned with a ChemiDoc MP System densitometer (Bio-Rad, Hercules, CA, USA) at the resolution of 600 dpi.
Image analysis
The image analysis was performed with PDQuest Advanced 2D Gel Analysis Software (Bio-Rad, Hercules, CA, USA). Spot detection was automatically performed using the software algorithm and the spots were verified manually. After the insertion of an appropriate number of user seeds, the matching was performed automatically and then checked manually. To ensure normalization of the spot quantities, the protein spot densities were normalized (%V) on total volumes of all the spots in each gel image.
Mass spectrometry protein identification
The protein spots selected as being differentially expressed, excised from fresh 2DE gels, were destained overnight with 40% ethanol/50 mM NH4HCO3, washed three times with 25 mM NH4CO3 and three times with acetonitrile (ANC) and then dried in Eppendorf Concentrator 5301 (Eppendorf, Hamburg, Germany). The proteins were in-gel digested with 75 ng/μL of sequencing-grade, modified porcine trypsin (Promega, Madison, WI, USA). The peptide digests were desalted on a Discovery® DSC-18 solid phase extraction 96-well plate (25 mg/well) (Sigma-Aldrich Inc., St. Louis, MO, USA), prior to mass spectrometry analysis. The LC-MS/MS analyses were performed by means of a micro-LC Eksigent Technologies (Dublin, OH, USA) system, which included a micro LC200 Eksigent pump with a 5–50 μL flow module and a programmable autosampler CTC PAL with a Peltier unit (1.0–45.0 °C). The stationary phase was a Halo Fused C18 column (0.5 × 100 mm, 2.7 μm; Eksigent Technologies, Dublin, OH, USA). The mobile phase was a mixture of 0.1% (v/v) formic acid in water (A) and 0.1% (v/v) formic acid in ANC (B), and it was eluting at a flow-rate of 15.0 μL/min and at an increasing concentration of solvent B, that is, from 2% to 40% in 30 min. The injection volume was 4.0 μL. The oven temperature was set at 40 °C. The LC system was interfaced with a 5600+ Triple TOFTM system (AB Sciex, Concord, Canada), equipped with DuoSprayTM Ion Source and a calibrant delivery system. The mass spectrometer worked in data dependent acquisition mode (DDA). Peptide profiling was performed using a 100–1,300 Da mass range (TOF scan with an accumulation time of 100.0 ms), followed by an MS/MS product ion scan from 200 to 1,250 Da (accumulation time of 5.0 ms) with the abundance threshold set at 30 cps (35 candidate ions can be monitored per cycle). The ion source parameters were set in electrospray positive mode as follows: curtain gas (N2) at 25 psig, nebulizer gas GAS1 at 25 psig and GAS2 at 20 psig, ion spray floating voltage at 5,000 V, source temperature at 450 °C and declustering potential at 25 V (Cvijetic et al., 2017; Martinotti et al., 2016).
Protein database search
The DDA files were searched using Mascot v. 2.4 (Matrix Science Inc., Boston, MA, USA). Trypsin was specified as a digestion enzyme with two missed cleavages. The instrument was set at ESI-QUAD-TOF, and the following modifications were allowed for the search: carbamidomethylcysteins as fixed modification and oxidized methionine as variable modification. A search tolerance of 50 ppm was specified for the peptide mass tolerance, and 0.1 Da for the MS/MS tolerance. The peptide charges searched for were set at 2+, 3+, and 4+, and the search was performed on monoisotopic mass. The unreviewed UniProt Swiss-Prot B. mori database (version 2017.06.21, containing 18320 sequence entries) was used. Only proteins with at least four peptides with a peptide score > peptide identity were considered for identification purposes. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD012869.
Statistical analysis
Data from the nutrient profile analysis were compared by means of ANOVA and successively with the Tukey multiple comparison test, with the significance threshold set at P < 0.05.
The normalized spot intensity data were exported and analyzed using R statistic software (R version 3.3.2 - 2016-10-31). Data quality was assessed using distribution plots (frequency histograms), a box plot and the Shapiro Wilks normality test for all the considered proteins (Pedreschi et al., 2008). A control of the quality data was carried out, according to these preliminary data analyses, and the missing values were substituted with intra-spot medians when there were 3 or fewer missing values, or the intra-spots were erased and an experimental treatment was conducted in the case of 4 or 5 missing values; when the number of missing values was greater, the spot was eliminated from the statistical analysis. Data were analyzed by means of ANOVA, and the Tukey multiple comparison test was then used as a post hoc test for comparison of the means between treatments. Protein spots with a fold change ≥±1.5 and P < 0.05 were selected and excised from the gel for identification.
A multivariate analysis of the normalized spot quantities was performed using PAST software, version 2.17 (Hammer, Harper & Ryan, 2001). In order to further normalize the spot intensities, the quantitative data were standardized by subtraction of the mean spot values, and then dividing them by their standard deviations (N = 36). The standardized intensities were then ordered by means of a principal component analysis, in which each sample was labeled with differently shaped points. The same analysis was performed another time, but only on the significantly different spots, as selected by means of ANOVA and fold variation. Finally, the Manhattan algorithm was used to cluster the different samples according to the standardized intensities of those spots that showed a PCA correlation value >± 65%.
Results
Insect growth and nutrient composition
The mean weight, proximate composition, and energy value of the silkworm pupae are reported in Table 1. The insect growth was comparable for the two diets, in terms of the length of the cycle, albeit with a slight delay, ranging from 1 to 2 days, over the whole larval life span. The larvae reared on the A diet showed a slightly (but not significant) lower weight at the end of the fifth instar. The nutrient composition of the silkworm pupae grown on either the A or L diets was significantly different as far as its crude protein content and energy value are concerned. The highest protein content (16.8%) was recorded in female reared on artificial diet (FA), while the lowest (13.8%) was found in male reared on mulberry leaves diet (ML), as a result of the different protein contents (on a DM basis) of the diets (Table 1). The energy silkworm pupae results showed an opposite trend, with respect to the protein content, with the highest values recorded in both the ML and FL, that is, 6.82 ± 0.79 and 6.02 ± 0.39 Mj/kg, respectively.
| Experimental groups | ||||
|---|---|---|---|---|
| MA | ML | FA | FL | |
| Mean weight (g) | 0.80 ± 0.05b | 0.83 ± 0.16b | 1.00 ± 0.13a | 1.01 ± 0.20a |
| Dry matter (% FM) | 23.40 ± 0.18 | 25.10 ± 1.47 | 23.26 ± 0.65 | 25.90 ± 1.39 |
| Crude protein (% FM) | 14.38 ± 1.63ab | 13.81 ± 2.41b | 16.83 ± 1.43a | 14.58 ± 1.94ab |
| Ash (% FM) | 1.33 ± 0.07 | 1.16 ± 0.17 | 1.27 ± 0.10 | 1.30 ± 0.29 |
| Gross energy (Mj/kg FM) | 5.38 ± 0.19bc | 6.82 ± 0.79a | 5.09 ± 0.21c | 6.02 ± 0.39b |
Note:
Values are means ± standard deviations of triplicate analyses; FM, fresh matter means with the different letters in the same row are significantly different (P < 0.05).
Proteomic analysis: identification of differentially expressed proteins
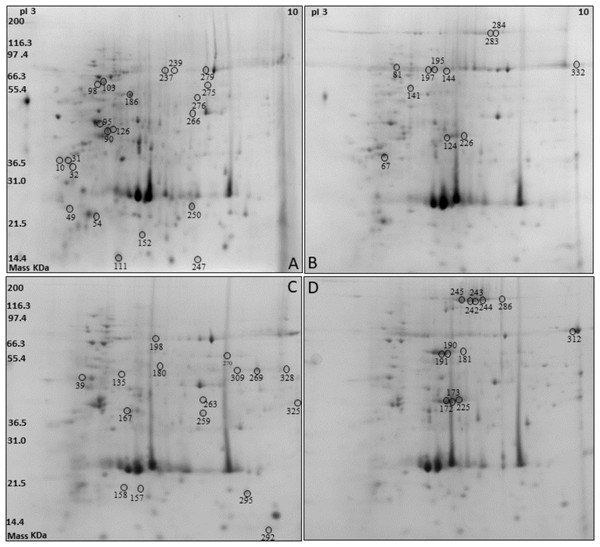
Two-dimensional electrophoresis was performed on protein extracts from silkworm pupae reared on two different diets (A and L diets) and considering males (M) separately from females (F) (Fig. 1). Overall, 153 ± 9, 158 ± 15 spots were detected in the M and F reared on the A diet, while 157 ± 21, 163 ± 26 spots were detected in the M and F reared on the L diet. The protein spots that resulted to be differentially expressed (P < 0.05) with a fold change ≥± 1.5, under different sex and diet conditions, were selected and excised from the gel for their identification by means of mass spectrometry analysis.
Figure 1: Two-dimensional electrophoresis (2DE) silkworm reared on two different diets considering male separated from female: Artificial diet (A, male; B, female) and fresh mulberry leaves diet (C, male; D, female).
Following the mass spectrometry analyses, the proteins listed in Table 2 were chosen as the best candidates obtained by bioinformatics search, with at least four valid peptides (Table S2). Some proteins and/or isoforms were identified in more than one spot on the same gel: Vitellogenin (spots 124, 172, 173, 226 as Vitellogenin light chain; spots 242, 243, 244, 245, 283, 284, 286 as Vitellogenin heavy chain, and spot 225), Catalase (spots 269, 276, and 309), Chitinase (spots 98 and 103), Transferrin (spots 239 and 279) and Egg specific protein (spots 190 and 191). In some cases, two or more proteins were identified in one spot: for instance, sex specific storage protein 1, sex specific storage protein 2, and arylphorin were identified in spots 144, 195, and 197. However, it was not possible to discriminate which of these proteins was responsible for the spot volume variation.
| Spot | Entry UniProt | Name | Masst/Masse | pIt/pIe | Peptides | Protein score | Coverage (%) |
|---|---|---|---|---|---|---|---|
| 10 | C0H6F9 | Putative cuticle protein | 28,335/36,000 | 4.63/4.30 | 8 | 545 | 49.6 |
| 31 | C0H6F9 | Putative cuticle protein | 28,335/36,000 | 4.63/4.50 | 11 | 1,733 | 59.6 |
| 32 | B9VTR5 | 32 kDa apolipoprotein | 32,299/34,000 | 4.79/4.60 | 11 | 1,625 | 51.2 |
| 39 | Q8T8B2 | Tubulin beta chain | 50,638/52,000 | 4.75/4.80 | 15 | 1,414 | 33.8 |
| Q8I9N4 | Masquerade-like serine proteinase homolog | 46,764/52,000 | 4,96/4,80 | 15 | 633 | 40 | |
| 49 | B9VTR5 | 32 kDa apolipoprotein | 32,299/22,000 | 4.79/4.90 | 8 | 1,981 | 27.4 |
| 54 | Q8T113 | 27 kDa glycoprotein | 25,571/23,000 | 5.12/5.10 | 9 | 1,387 | 56.4 |
| 67 | Q03383 | Antichymotrypsin-1 | 44,715/40,000 | 5.21/5.00 | 12 | 458 | 29.5 |
| 81 | H9JP12 | Sex-specific storage-protein 1 | 88,007/82,000 | 5.28/5.00 | 20 | 689 | 22.5 |
| 90 | H9IXK0 | Antichymotrypsin-1 | 41,893/45,000 | 5.14/5.20 | 27 | 2,417 | 60.2 |
| 95 | C4PAW6 | Hemolin | 45,335/50,000 | 5.12/5.20 | 32 | 4,911 | 80.2 |
| 98 | Q9GQC4 | Chitinase | 61,886/65,000 | 5.01/5.20 | 20 | 1,270 | 41 |
| 100 | I6XKQ0 | Heat shock protein 70-5 | 75,536/80,000 | 5.84/5.70 | 13 | 623 | 22.5 |
| H9IXK0 | Heat shock cognate protein | 71,359/80,000 | 5.33/5.70 | 9 | 354 | 17.4 | |
| 103 | Q8WR52 | Chitinase | 64,280/65,000 | 5.14/5.20 | 15 | 576 | 31.4 |
| 111 | Q2QEH2 | Cellular retinoic acid binding protein | 14,963/65,000 | 5.66/5.20 | 16 | 1,760 | 76.5 |
| 124 | Q27309 | Vitellogenin | 203,725/40,000 | 6.85/6.00 | 16 | 1,087 | 10.5 |
| 126 | H9IXK0 | Antichymotrypsin-1 | 41,893/45,000 | 5.14/5.10 | 29 | 2,062 | 57.6 |
| 135 | Q2F5Y9 | Mitochondrial aldehyde dehydrogenase | 53,127/54,000 | 5.57/5.70 | 14 | 696 | 31.1 |
| 141 | P49010 | Chitooligosaccharidolytic beta-N-acetylglucosaminidase | 68,968/60,000 | 5.17/5.70 | 24 | 1,871 | 39.3 |
| H9J8Q7 | Beta-hexosaminidase | 61,914/60,000 | 5.33/5.30 | 24 | 1,836 | 44.5 | |
| 144 | Q1HPP4 | Arylphorin | 83,569/80,000 | 5.7/6.00 | 37 | 1,925 | 53.9 |
| H9JP12 | Sex-specific storage-protein 1 | 88,007/80,000 | 6.78/6.00 | 35 | 2,164 | 43.9 | |
| P20613 | Sex-specific storage-protein 2 | 83,698/80,000 | 6.04/6.00 | 34 | 1,785 | 48.4 | |
| 152 | Q1HPP5 | Actin-depolymerizing factor 1 | 17,227/18,000 | 6.17/6.00 | 17 | 1,219 | 81.8 |
| 157 | Q5CCJ4 | Glutathione S-transferase sigma | 23,382/23,000 | 5.85/6.20 | 17 | 1,237 | 71.1 |
| 158 | Q5CCJ4 | Glutathione S-transferase sigma | 23,382/23,000 | 5.85/5.80 | 16 | 1,177 | 67.2 |
| 167 | Q2F5T5 | Arginine kinase | 40,308/40,000 | 5.87/5.90 | 24 | 1,841 | 60.6 |
| 172 | Q27309 | Vitellogenin (light chain) | 40,203/40,000 | 6.85/6.30 | 22 | 3,021 | 65.6 |
| 173 | Q27309 | Vitellogenin (light chain) | 40,203/40,000 | 6.85/6.30 | 23 | 2,861 | 71.3 |
| 180 | H9J859 | Fascin | 57,239/55,000 | 6.25/6.50 | 16 | 967 | 39.3 |
| 181 | H9JLS3 | Dynein heavy chain 2, axonemal-like | 386,433/60,000 | 6.42/6.50 | 20 | 759 | 6 |
| 186 | H9JGR2 | Chitinase precursor | 61,037/58,000 | 5.58/5.90 | 36 | 4,098 | 66.4 |
| 190 | Q17219 | Egg-specific protein | 63,545/60,000 | 6.14/6.30 | 30 | 3,745 | 71.2 |
| 191 | Q17219 | Egg-specific protein | 63,545/60,000 | 6.14/6.20 | 38 | 4,668 | 78.5 |
| 195 | Q1HPP4 | Arylphorin | 83,569/80,000 | 5.70/6.50 | 55 | 3,186 | 74.1 |
| P09179 | Sex-specific storage-protein 1 | 87,890/80,000 | 6.78/6.50 | 57 | 5,231 | 67.3 | |
| P20613 | Sex-specific storage-protein 2 | 83,698/80,000 | 6.04/6.50 | 43 | 2,619 | 58.1 | |
| 197 | Q1HPP4 | Arylphorin | 83,569/80,000 | 5.70/6.50 | 55 | 3,186 | 74.1 |
| P09179 | Sex-specific storage-protein 1 | 87,890/80,000 | 6.78/6.50 | 57 | 5,231 | 67.3 | |
| P20613 | Sex-specific storage-protein 2 | 83,698/80,000 | 6.04/6.50 | 43 | 2,619 | 58.1 | |
| 198 | H9JTA2 | Uncharacterized protein | 74,049/73,000 | 6.31/6.40 | 16 | 498 | 30.5 |
| Q27451 | Phenoloxidase subunit 1 | 79,305/73,000 | 6.25/6.40 | 16 | 402 | 26.0 | |
| 225 | Q27309 | Vitellogenin | 203,725/40,000 | 6.85/6.50 | 21 | 2,198 | 17.6 |
| 226 | Q27309 | Vitellogenin (light chain) | 40,203/40,000 | 6.85/6.80 | 18 | 1,779 | 62.3 |
| 237 | H9JP12 | Sex-specific storage-protein 1 | 88,007/80,000 | 6.78/6.80 | 31 | 1,728 | 44.1 |
| 239 | O97158 | Transferrin | 77,156/80,000 | 6.89/7.00 | 21 | 1,032 | 39.4 |
| 242 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/6.80 | 36 | 2,062 | 26.8 |
| 243 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/7.00 | 35 | 1,921 | 24.1 |
| 244 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/7.10 | 37 | 1,835 | 27.1 |
| 245 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/6.80 | 40 | 2,323 | 30.7 |
| 247 | Q1HPS1 | ML-domain containing secreted protein | 17,360/17,000 | 6.28/7.20 | 5 | 324 | 27.9 |
| 250 | Q1HQ02 | Ferritin | 26,245/25,000 | 6.75/7.00 | 12 | 843 | 49.8 |
| 259 | Q1HPN7 | Fructose-bisphosphate aldolase | 39,971/40,000 | 8.38/7.70 | 15 | 1,376 | 46.4 |
| 263 | H9ITY5 | Probable medium-chain specific acyl-CoA dehydrogenase. mitochondrial isoform X2 | 46,461/45,000 | 5.91/7.50 | 16 | 856 | 44.1 |
| 266 | A7BEX9 | Imaginal disk growth factor | 48,362/50,000 | 7.64/7.20 | 19 | 1,734 | 52.8 |
| 269 | Q68AP5 | Catalase | 57,092/55,000 | 8.11/8.20 | 34 | 2,149 | 67.5 |
| 270 | H9IYX7 | Bifunctional purine biosynthesis protein | 64,577/60,000 | 7.19/7.80 | 31 | 1,977 | 49.6 |
| 275 | H9IYX7 | Bifunctional purine biosynthesis protein | 64,577/62,000 | 7.19/7.50 | 25 | 1,193 | 49.2 |
| 276 | H9IZ23 | Pyruvate kinase | 68,697/55,000 | 9.00/7.20 | 16 | 941 | 27.8 |
| Q68AP5 | Catalase | 57,092/55,000 | 8.11/7.20 | 10 | 362 | 22 | |
| H9J8X4 | Glucose-6-phosphate 1-dehydrogenase | 56,942/55,000 | 6.86/7.20 | 11 | 288 | 28 | |
| 279 | O97158 | Transferrin | 77,156/80,000 | 6.89/7.50 | 50 | 4,089 | 71.8 |
| 283 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/7.30 | 57 | 3,470 | 47.7 |
| 284 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/7.40 | 65 | 4,638 | 51.4 |
| 286 | Q27309 | Vitellogenin (heavy chain) | 161,327/160,000 | 6.85/7.50 | 50 | 3,175 | 40.6 |
| 292 | Q69FX2 | Promoting protein | 17,625/16,000 | 8.37/8.80 | 10 | 863 | 68.8 |
| 295 | Q60GK5 | Glutathione S-transferase delta | 24,269/23,000 | 7.61/8.30 | 20 | 2,143 | 91.2 |
| 309 | Q68AP5 | Catalase | 57,092/55,000 | 8.11/8.00 | 25 | 1,430 | 58.6 |
| 312 | G1UIS8 | Apolipophorin protein | 371,420/73,000 | 7.94/9.00 | 24 | 1,511 | 7.8 |
| 325 | C6L8Q2 | Putative acetyl transferase | 41,580/40,000 | 8.91/9.30 | 16 | 1,082 | 59.3 |
| A0A0A0QY84 | Elongation factor 1-alpha | 50,626/40,000 | 9.24/9.30 | 17 | 1,013 | 41.7 | |
| 328 | Q2F5T3 | ATP synthase subunit alpha | 59,792/55,000 | 9.21/9.00 | 26 | 1,447 | 51.2 |
| 332 | H9JP12 | Sex-specific storage-protein 1 | 88,007/80,000 | 6.78/9.30 | 17 | 679 | 20.02 |
| Q1HPP4 | Arylphorin | 83,569/80,000 | 5.70/9.30 | 18 | 476 | 26.3 |
Among the diet-modulated spots, confirmed for both genders, seven were only detected in the silkworms reared on the A diet (spots 10, 32, 54, 67, 98, 103, and 141), nine were only detected in the silkworms reared on the L diet (spots 157, 180, 181, 198, 269, 270, 292, 325, and 328), 10 spots were up-regulated in the silkworms reared on the A diet (spots 49, 124, 144, 186, 237, 244, 266, 279, 283, and 284) and only one spot was up-regulated in the silkworms reared on the L diet (spot 167) (Table 3).
| Protein | Spot N | Statistically significant RATIO | Fold-change | Loading value |
|---|---|---|---|---|
| Spots detected only in the silkworm reared on A diet comparing the same gender | ||||
| Putative cuticle protein | 10 | MA/ML | Only in MA | 0.359 |
| FA/FL | Only in FA | |||
| 31 | FA/FL | Only in FA | 0.391 | |
| 32 kDa apolipoprotein | 32 | MA/ML | Only in MA | 0.677 |
| FA/FL | Only in FA | |||
| 27 kDa glycoprotein | 54 | MA/ML | Only in MA | 0.580 |
| FA/FL | Only in FA | |||
| Antichymotrypsin-1 | 67 | MA/ML | Only in MA | 0.086 |
| FA/FL | Only in FA | |||
| 90 | MA/ML | Only in MA | −0.224 | |
| Hemolin | 95 | FA/FL | Only in FA | 0.292 |
| Chitinase | 98 | MA/ML | Only in MA | 0.556 |
| FA/FL | Only in FA | |||
| 103 | MA/ML | Only in MA | 0.614 | |
| FA/FL | Only in FA | |||
| Heat shock protein 70-5 | 100 | MA/ML | Only in MA | −0.055 |
| Heat shock cognate protein | ||||
| Antichymotrypsin | 126 | MA/ML | Only in MA | 0.320 |
| Beta-hexosaminidase | 141 | MA/ML | Only in MA | 0.515 |
| Acetylglucosamidase | FA/FL | Only in FA | ||
| Actin-depolymerizing1 | 152 | MA/ML | Only in MA | 0.214 |
| Glutathione S-transferase sigma | 158 | FA/FL | Only in FA | 0.379 |
| Transferrin | 239 | MA/ML | Only in MA | 0.312 |
| ML-domain containing secreted protein | 247 | MA/ML | Only in MA | 0.167 |
| Imaginal disk growth factor | 266 | FA/FL | Only in FA | 0.495 |
| SP1 | 332 | FA/FL | Only in FA | 0.227 |
| Arylphorin | ||||
| Spots detected only in the silkworm reared on L diet comparing the same gender | ||||
| Tubulin beta chain | 39 | ML/FL | Only in ML | −0.493 |
| Masquerade-like serine proteinase homolog | ||||
| SP1 | 81 | FA/FL | Only in FL | 0.223 |
| Mitochondrial aldehyde dehydrogenase | 135 | FA/FL | Only in FL | −0.448 |
| Glutathione S-transferase sigma | 157 | MA/ML | Only in ML | −0.428 |
| FA/FL | Only in FL | |||
| Fascin | 180 | MA/ML | Only in ML | −0.717 |
| FA/FL | Only in FL | |||
| Dynein heavy chain 2 | 181 | MA/ML | Only in ML | −0.508 |
| FA/FL | Only in FL | |||
| Egg-specific protein | 190 | FA/FL | Only in FL | −0.039 |
| 191 | FA/FL | Only in FL | 0.103 | |
| SP1 | 195 | MA/ML | Only in ML | 0.101 |
| SP2 | 197 | MA/ML | Only in ML | 0.209 |
| Arylphorin | ||||
| Phenoloxidase subunit 1 | 198 | MA/ML | Only in ML | −0.214 |
| FA/FL | Only in FL | |||
| Spots detected only in the silkworm reared on L diet comparing the same gender | ||||
| SP1 | 237 | FA/FL | Only in FL | 0.222 |
| acyl-CoA dehydrogenase isoform X2 | 263 | FA/FL | Only in FL | −0.734 |
| Catalase | 269 | FA/FL | Only in FL | −0.783 |
| MA/ML | Only in ML | |||
| 309 | MA/ML | Only in ML | −0.745 | |
| Bifunctional purine biosynthesis protein | 270 | MA/ML | Only in ML | −0.779 |
| FA/FL | Only in FL | |||
| Promoting protein | 292 | MA/ML | Only in ML | −0.422 |
| FA/FL | Only in FL | |||
| Spots detected only in the silkworm reared on L diet comparing the same gender | ||||
| Apolipophorin protein | 312 | FA/FL | Only in FL | −0.208 |
| Putative acetyl transferase | 325 | MA/ML | Only in ML | −0.619 |
| Elongation factor 1-alpha | FA/FL | Only in FL | ||
| ATP synthase α subunit | 328 | MA/ML | Only in ML | −0.636 |
| FA/FL | Only in FL | |||
| Spots up-regulated in the silkworm reared on A diet | ||||
| 32 kDa-apolipoprotein | 49 | MA/ML | 2.35* | 0.460 |
| Vitellogenin LC | 124 | FA/FL | 2.25* | 0.209 |
| SP1 | 144 | FA/FL | 2.94* | 0.499 |
| SP2 | ||||
| Arylphorin | ||||
| Chitinase precursor | 186 | MA/ML | 2.29* | 0.574 |
| SP1 | 237 | MA/ML | 2.16* | 0.222 |
| Imaginal disk growth factor | 266 | MA/ML | 2.17** | 0.495 |
| Transferrin | 279 | MA/ML | 2.64** | 0.606 |
| Vitellogenin HC | 244 | FA/FL | 1.78** | 0.460 |
| 283 | 1.71* | 0.419 | ||
| 284 | 3.55* | 0.437 | ||
| Spots up-regulated in the silkworm reared on L diet | ||||
| Arginine kinase | 167 | ML/MA | 1.88* | 0.046 |
Considering the sex-modulated spots confirmed for both diets, three were only detected in M (spots 167, 275, and 276), eight were only detected in F (spots 124, 172, 173, 225, 242, 244, 283, and 284), 11 were up-regulated in M (spots 10,32, 95, 11, 135, 157, 198, 250, 259, 295, and 328) and two were up-regulated in F (spots 67 and 181) (Table 4). In addition, 15 spots were only present in one condition: spots 100, 126, 152, 239, and 247 were only detected in MA, spots 39 and 309 were only detected in ML, spots 81 and 332 were only detected in FA and spots 190, 191, 243, 245, 286, and 312 were only detected in FL.
| Protein | Spot N | Statistically significant RATIO | Fold-change | Loading value |
|---|---|---|---|---|
| Spots detected only in Male comparing silkworm reared on the same diet | ||||
| Putative cuticle protein | 31 | ML/FL | Only in ML | 0.218 |
| Tubulin beta chain | 39 | ML/FL | Only in ML | 0.064 |
| Masquerade-like serine proteinase homolog | ||||
| Hemolin | 95 | ML/FL | Only in ML | 0.544 |
| Heat shock protein 70-5 | 100 | MA/FA | Only in MA | 0.571 |
| Heat shock cognate protein | ||||
| Antichymotrypsin | 126 | MA/FA | Only in MA | 0.553 |
| Mitochondrial aldehyde dehydrogenase | 135 | MA/FA | Only in MA | 0.497 |
| Actin-depolymerizing factor 1 | 152 | MA/FA | Only in MA | 0.474 |
| Arginine kinase | 167 | MA/FA | Only in MA | 0.219 |
| ML/FL | Only in ML | |||
| SP1 | 237 | MA/FA | Only in MA | −0.159 |
| Transferrin | 239 | MA/FA | Only in MA | −0.138 |
| ML-domain containing secreted protein | 247 | MA/FA | Only in MA | 0.336 |
| acyl-CoA dh isoform X2 | 263 | MA/FA | Only in MA | 0.257 |
| Imaginal disk growth factor | 266 | ML/FL | Only in ML | 0.674 |
| Bifunctional purine biosynthesis protein | 275 | MA/FA | Only in MA | 0.545 |
| ML/FL | Only in ML | |||
| Pyruvate kinase | 276 | MA/FA | Only in MA | 0.716 |
| Catalase | ||||
| Glucose-6P-1 dehydrogenase | ML/FL | Only in ML | ||
| Transferrin | 279 | MA/FA | Only in MA | 0.534 |
| Spots detected only in Female comparing silkworm reared on the same diet | ||||
| SP1 | 81 | MA/FA | Only in FA | −0.422 |
| Antichymotrypsin-1 | 90 | ML/FL | Only in FL | 0.492 |
| Egg-specific protein | 190 | ML/FL | Only in FL | −0.531 |
| 191 | ML/FL | Only in FL | −0.681 | |
| Vitellogenin LC | 124 | MA/FA | Only in FA | −0.536 |
| ML/FL | Only in FL | |||
| 172 | MA/FA | Only in FA | −0.575 | |
| ML/FL | Only in FL | |||
| 173 | MA/FA | Only in FA | −0.674 | |
| ML/FL | Only in FL | |||
| 225 | MA/FA | Only in FA | −0.663 | |
| ML/FL | Only in FL | |||
| 226 | MA/FA | Only in FA | −0.437 | |
| Vitellogenin HC | 242 | MA/FA | Only in FA | −0.631 |
| ML/FL | Only in FL | |||
| 243 | ML/FL | Only in FL | −0.619 | |
| 244 | MA/FA | Only in FA | −0.571 | |
| ML/FL | Only in FL | |||
| 245 | ML/FL | Only in FL | −0.591 | |
| 283 | MA/FA | Only in FA | −0.494 | |
| ML/FL | Only in FL | |||
| 284 | MA/FA | Only in FA | −0.437 | |
| ML/FL | Only in FL | |||
| 286 | ML/FL | Only in FL | −0.529 | |
| Apolipophorin protein | 312 | ML/FL | Only in FL | −0.255 |
| SP1 | 332 | MA/FA | only in FA | −0.544 |
| Arylphorin | ||||
| Spots up-regulated in Male | ||||
| Putative cuticle protein | 10 | MA/FA | 2.96** | 0.633 |
| 32 kDa apolipoprotein | 32 | MA/FA | 1.70** | 0.677 |
| Hemolin | 95 | MA/FA | 2.34* | 0.544 |
| Cellular retinoic acid binding protein | 111 | MA/FA | 3.23** | 0.729 |
| Mitochondrial aldehyde dehydrogenase | 135 | ML/FL | 1.87* | 0.497 |
| Glutathione S-transferase sigma | 157 | ML/FL | 2.63** | 0.100 |
| Phenoloxidase subunit 1 | 198 | ML/FL | 1.72 | −0.081 |
| Ferritin | 250 | MA/FA | 2.31** | 0.706 |
| Fructose-bisphosphate aldolase | 259 | MA/FA | 1.71* | 0.767 |
| ML/FL | 2.04* | |||
| Glutathione S-transferase delta | 295 | ML/FL | 2.23* | 0.474 |
| ATP synthase α subunit | 328 | ML/FL | 1.75** | 0.051 |
| Spots up-regulated in Female | ||||
| Antichymotrypsin-1 | 67 | FA/MA | 1.73** | 0.011 |
| Dynein heavy chain 2 | 181 | FL/ML | 1.84** | −0.593 |
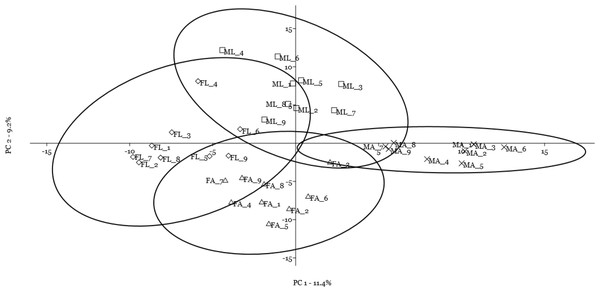
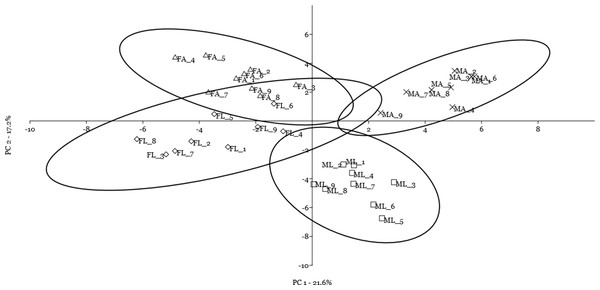
A multivariate statistical approach (PCA) was used, in two steps, to investigate the clustering tendencies and to outline the contribution of single spots to the differences between samples. Figure 2 reports the grouping tendency of the four samples when all standardized quantitative data from 2DE gel image analysis were included. The different samples seemed to cluster quite separately according to their spot intensity, with the sex disclosed along the second principal component, and the diet separated, although less sharply, along the first principal component. The PCA was then repeated by including only quantitative data of the spots that were significantly different according to univariate statistics and up/downregulated more than 1.5-fold (Fig. 3). Again in this case, the four samples showed a tendency to group, according to sex, along the first principal component, and to diet along the second component. By analyzing the contribution of the single spots to these components (loading values in Tables 2 and 3), we identified the spots that were the most relevant for the variability between groups.
Figure 2: Principal component analysis plot of all quantitative data from two-dimensional electrophoresis gel image analysis.
Female silkworm reared on artificial diet (FA empty triangle) or on mulberry leaves (FL empty diamond); Male silkworm reared on artificial diet (MA black cross) or on mulberry leaves (ML empty square).Figure 3: Principal component analysis plot of the spots that were significantly different according to univariate statistics and up/downregulated more than 1.5-fold.
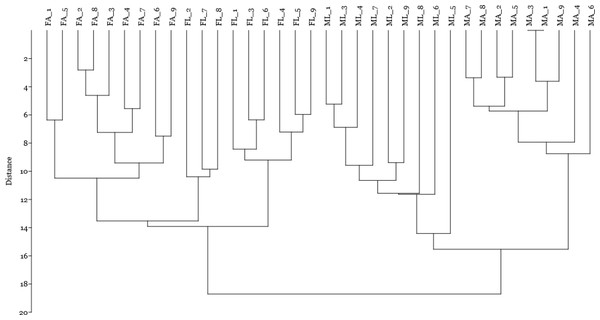
Female silkworm reared on artificial diet (FA empty triangle) or on mulberry leaves (FL empty diamond); Male silkworm reared on artificial diet (MA black cross) or on mulberry leaves (ML empty square).Finally, by including the quantitative data on spot volume for the 14 selected spots in a cluster analysis using the Manhattan algorithm (Fig. 4), we observed that these proteins were suitable for discriminating sex and diet effects as separate clusters, with a higher sex- than diet-related effect.
Figure 4: Cluster analysis by Manhattan algorithm of quantitative data on spot volume for those 14 spots with loading value greater than 0.65.
Female silkworm reared on artificial diet (FA) or on mulberry leaves (FL); Male silkworm reared on artificial diet (MA) or on mulberry leaves (ML).Discussion
Insect growth and nutrient composition
It is worth noting that the insect growth results of the silkworm pupae reared on mulberry leaves are referred to good quality leaves produced in springtime; however, if the quality of the leaves had not been optimal (e.g., late summer leaves), the differences might not have been significant, or the reverse situation might have been obtained, where a lighter cocoon weight would have been obtained for the leaves rather than for the diet (Kumar et al., 2013).
Regardless of which rearing substrate was utilized, the protein content of the silkworm pupae was found to be higher than that of other data reported for silkworm pupae that are produced as by-products of reeling industry (Rao, 1994; Pereira et al., 2003). The lower caloric value of the silkworm pupae reared on the A diet could be related to its lower cholesterol content, as previously reported by Dong et al. (2017) in a metabolomics study in which a significantly lower cholesterol content was found in both F (62%) and M (71.4%) reared on A diet compared to L diet.
Protein discriminating gender effect and protein discriminating diet effect
By using the multivariate statistical approach, seven proteins were found to better discriminate the sex effect, whereas five proteins were better at discriminating the diet effect. Egg-specific protein (Q17219) and vitellogenin (Q27309) were only present in females. The imaginal disk growth factor (IDGF, A7BEX9), cellular retinoic acid (RA) binding protein (CRABP, Q2QEH2), ferritin (Q1HQ02), fructose-bisphosphate aldolase (Q1HPN7), and 32 kDa apolipoprotein (B9VTR5) were up-regulated in males. The bifunctional purine biosynthesis protein (H9IYX7), acyl-CoA dehydrogenase (H9ITY5), Fascin (H9J859), and Catalase (Q68AP5) were up-regulated in the L diet; while 32 kDa apolipoprotein (B9VTR5) were up-regulated in the A diet.
Vitellogenin (Vg) is the major precursor of the egg-yolk protein Vitellin, together with the egg-specific protein are the major proteins in yolk. In our experiments, both Vg and the egg-specific proteins were only detected in the female silkworms, as expected.
The Vg protein in B. mori (BmVg), is a tetramer with a molecular mass of 440 kDa, composed of two heavy chains and two light chains (Izumi, Tomino & Chino, 1980). In our 2DE experiments, we found Vg in 12 spots, separated at different pI (from 6.5 to 7.5) and molecular weight (40 kDa for the light chain and 160 kDa for the heavy chain). Vg was found in the female pupae without any differences between the L and A diets, thus making Vg the best discriminating gender-protein.
The egg-specific protein showed a correlation with gender, but, unlike Vg, it was only found in females reared on the L diet. Since the synthesis of egg-specific protein is stimulated by ecdysone (Ono, Nagayama & Shimura, 1975), its absence from the silkworm pupae reared on the A diet could be correlated with this hormone-dependent regulation, thus suggesting a hormonal balance alteration between the L and A diets. In previous data obtained for the same rearing conditions (Cappellozza et al., 2005), the silkworm pupae fed on an A diet often enclosed into moths that laid non-diapausing eggs, while they were usually monovoltine when reared on an L diet. This silkworm pupae behavior, which is mainly linked to hormone secretion, has already been demonstrated by Yamamura et al. (2011).
Moving on to the proteins that are more abundant in males, IDGF, showed up regulation in both of the diets, with more abundance in the A diet. IDGF is the first polypeptide growth factor to be reported for invertebrates, and it cooperates with insulin to stimulate the proliferation, polarization, and motility of imaginal disc cells (Hipfner & Cohen, 1999). IDGF has been suggested to be a systemic regulator in response to environmental inputs, such as nutritional status: the amount of BmIDGF dropped significantly after starvation and increased again upon re-feeding (Wang et al., 2009). A proteomic analysis on B. mori performed by Zhou et al. (2008) gave analogous results to ours, showing that the concentration of BmIDGF in the hemolymph was double in the larvae reared on A diet compared to those reared on L diet. CRABP is an exclusively sex-regulated protein belonging to the RA signal transduction pathway. RA is a vitamin A metabolite, that is, involved in the proliferation, cellular differentiation, remodeling of adult tissues, and in apoptosis, through the modulation of target gene expression. CRABP protects the B. mori cells from RA excesses, by sequestering RA and inducing its degradation (Wang et al., 2007). In our experiments, CRABP was over expressed in the males, for both of the diets, thus supporting the idea that RA, involved in several biological processes in females, have to be more suitable for females than for males.
Two other proteins were more up-regulated in the males than in the females: Fructose-bisphosphate aldolase (glycolytic pathway), and bifunctional purine biosynthesis protein. The bifunctional purine biosynthesis protein was found to be regulated in a similar way by Qin et al. (2014), who compared midgut proteins from B. mori male and female larvae. The authors demonstrated an enhancement in pyrimidine and purine biosynthesis in silkworm males. This up regulation may result in improved DNA/RNA synthesis and metabolism, which subsequently allow the male larvae to grow faster than the female ones in the fifth instar. In our experiment, a faster growth of the male larvae than the female ones was observed, as the cocoon emergence distributed over 3 days was recorded earlier for the male moths. This general behavior of anticipated emergence of male silk moths is well-known and it has been explored carefully to synchronize males and females for mating in the egg production process of silkworms for commercial purposes (Wang, 1989).
Moreover, the bifunctional purine biosynthesis protein, together with acetyl-CoA dehydrogenase were also upregulated (or found to be exclusively present) in the B. mori reared on the L diet. These results are in agreement with those of Dong, Pan & Zhang (2018), who demonstrated, by means of a metabolite analysis of B. mori, that both the carbohydrate and purine metabolisms were slowed down in silkworms reared on an A diet. Another diet-related protein was Fascin only biosynthesized in the silkworms reared on mulberry leaves, at the same extent between males and females. This is a globular actin cross-linking protein that bundles actin filaments into organized structures (Cant et al., 1994). A functional study on sea urchin demonstrated the importance of Fascin in the organization of F-actin in the egg microvillus core, which forms shortly after fertilization (Otto, Kane & Bryan, 1982). Zhou et al. (2008) showed a decreased expression of Tropomyosin 1 in B. mori reared on an A diet compared to an L diet: they speculated that the down-regulation of tropomiosin might inhibit the formation of actin filaments, therefore, weakening the contraction ability of the smooth muscle in the midgut of silkworms. In our experiments, Fascin, which is involved in the same biological process as Tropomyosin, showed the same expression profile, and it might, therefore, also be responsible for the reduction of actin structure organization in B. mori reared on A diets. Catalase, just like Fascin, was only present in the silkworms reared on the L diet. This is the protein mainly considered to be responsible for the scavenging of the reactive oxygen species (ROS) (Sohal, Arnold & Orr, 1990). ROS are produced as a consequence of aerobic respiration and substrate oxidation and are responsible for the damage of DNA, proteins, and lipid membranes. The cells biosynthesize antioxidative enzymes, such as Catalase, to protect themselves from ROS. Yamamoto et al. (2005) were the first to sequence and characterize B. mori Catalase (BmCAT), and some years later Nabizadeh & Kumar (2010) demonstrated a significant decrease in BmCAT activity in silkworms reared under thermal stress (at 40 ± 1 °C). In our experiments, BmCAT was absent in the silkworms reared on the A diet. Considering that, BmCAT in silkworm pupae is also linked to voltinism of the eggs, Zhao & Shi (2009) observed that the CAT activity in univoltine strains of B. mori was higher from the fifth to the seventh day of pupal development than that of polyvoltine strains. Therefore, this behavior might be linked to a variation in the hormonal balance rather than to a physiological disorder.
Potentially allergenic proteins
The proteomic approach setup adopted in this study allowed us to verify whether the expression of the already known allergenic proteins in B. mori were differentially affected by sex and rearing substrates. Among the differentially regulated proteins identified in this study, we found three proteins that have already been demonstrated to be allergens in B. mori: arginine kinase (AK; Liu et al., 2009), 27 kDa glycoprotein (Jeong et al., 2016), and chitinase (Zhao et al., 2015).
Arginine kinases are enzymes involved in energy catabolism and are found exclusively in invertebrates. Several AKs have recently been characterized as allergens and they have subsequently been proposed to be panallergens (García-Orozco et al., 2007; Sookrung et al., 2006). Using sequence alignment analysis, Liu et al. (2009) determined that BmAK shows significant similarity (ranging from 81% to 92%) with other AKs that have been associated with allergenicity. Moreover, they demonstrated that BmAK reacts with sera from patients who have shown a reaction to the crude extract of silkworms during a skin prick test, and that cross-reacts with the AK from the Periplaneta americana, rPaAK cockroach.
The 27 kDa glycoprotein is synthesized in the fat body of silkwarm and it is present at all stages of development in both sexes. However, its function is still unknown. A 27-kDa hemolymph protein from the wax moth, Galleria mellonella, has been reported to be an inhalant allergen in a patient suffering from rhinoconjunctivitis (Madero et al., 2007), and it shares a 54.9% amino acid sequence identity with the 27-kDa glycoprotein of silkworms. This report suggests the possibility of a different sensitization route for the 27 kDa hemolymph allergen in insects. In the study of Jeong et al. (2016), a 27-kDa glycoprotein was identified from a silkworm pupa as a heat stable IgE binding component. Specific IgE to recombinant 27-kDa glycoprotein was detected for one third of the tested silkworm allergic subjects, and IgE reactivity was shown to be increased after the protein extract was heated, so Jeong et al. (2016) suggested that food processing might increase allergenicity of the 27-kDa glycoprotein as a result of chemical modifications and/or structural changes.
The main function of insect Chitinases pertains to the turnover of such chitin-containing extracellular matrices as the insect cuticle and the peritrophic matrix during molting. In addition, chitinases may have a digestive function in insects, if their diet contains chitin. Zhao et al. (2015) found that silkworm chitinase resembles the Der f 18 of Dermatophagoides farinae (Q86R84) (24.8% of identical amino acid and 57.4% similar). They investigated IgE reactivity to BmChitinase using sera of patients allergic to silkworm pupa protein, and speculated that silkworm chitinase might be a cross-reactive allergen of house dust mites (Der f 18). Further studies are needed to identify the specific epitopes of these potentially allergenic proteins.
In our experiment, AK (spot 167) resulted to be upregulated in the males for both of the diets. 27-kDa glycoprotein (spot 54) was upregulated in the A diet while Chitinase (spots 98 and 103) was only present in the silkworms reared on the A diet and was upregulated in the males. From an allergenic point of view, our data indicate that female silkworms reared on mulberry leaves contain lower levels of known allergens, compared to the other experimental conditions that were considered. Further studies to assess the safety of B. mori, from the allergenic point of view, if used as food or a food ingredient, including the use of the sera of patients allergic to insect/crustaceous/dust mite are necessary.
Conclusions
A comparative proteomic experiment has been conducted to investigate the difference in the B. mori pupa protein profile, as affected by diet and gender. A PCA analysis allowed to outline the contribution of single proteins to differences in the experimental conditions: seven and five pupa proteins were found to be more effective in discriminating the sex and the diet type, respectively. Overall, we found that the pupae derived from silkworms grown on artificial diets and mulberry leaves show differences in their protein composition, although these differences did not lead to any different physiological traits. On the other hand, the differential protein expression between the two diets has highlighted a general flexibility of the insect to adapt to the artificial diet. Larvae developed on the two alternative feeding substrates show important differences in proteins related to lipid transport and metabolism; this phenomenon might be responsible for the recorded variation in silk production and, through the egg composition, might have an influence on the progeny physiological behavior.
Although this is a preliminary study, it has been possible to claim that female silkworm pupae reared on mulberry leaves contain lower levels of known allergens than those reared in the other experimental conditions. However, these results need to be supported by further immunoblotting experiments with the sera of potentially allergic patients.
The present work can provide some basic understanding of B. mori growth and physiology in relation to gender and farming. In addition, the data presented here offer a contribution to the evaluation of the influence of these two factors on the allergen profile of B. mori for its use as food or as a food ingredient.