Adult body size measurement redundancies in Osmia lignaria and Megachile rotundata (Hymenoptera: Megachilidae)
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Agricultural Science, Entomology, Zoology
- Keywords
- Body size, Intertegular distance, Head capsule width, Fresh weight, Dry weight, Solitary bee
- Licence
- This is an open access article distributed under the terms of the Open Government License.
- Cite this article
- 2021. Adult body size measurement redundancies in Osmia lignaria and Megachile rotundata (Hymenoptera: Megachilidae) PeerJ 9:e12344 https://doi.org/10.7717/peerj.12344
Abstract
Metrics to assess relative adult bee body size have included both mass and morphometrics, but these metrics may not equally or reliably estimate body size for all bee species and in all situations, due to bee age, diet, and/or environment. Understanding the relationships between different metrics and possible redundancies in the information they afford is important but not always known. Body size measurements provide valuable data for interpreting research outcomes for managed solitary bees, including Osmia lignaria Say and Megachile rotundata F. (Hymenoptera: Megachilidae). Applied studies of these important and readily available U.S. crop pollinators focus on refining commercial management practices, and basic empirical studies in various scientific disciplines (from genomics to ecology) employ them as model systems to study solitary bees. To examine common metrics of body size, we measured head capsule width (HCW), intertegular distance (ITD), and fresh and dry weights of newly emerged adults of both species. Using linear and exponential models, we determined relationships between these body size metrics. For M. rotundata, linear models best described relationships between ITD and all other metrics, and between HCW and fresh and dry weights. For O. lignaria, linear models best fit relationships between all metrics except for fresh weight with both ITD and HCW, which were fitted better with exponential models. For both species, model fits were strongest when males and females were pooled. Depending on the study question, knowing that only one metric may reliably measure body size can simplify evaluations of O. lignaria and M. rotundata responses to artificial or environmental variables.
Introduction
Managed O. lignaria (the blue orchard bee) and M. rotundata (the alfalfa leafcutting bee) are solitary bees that are commercially available for pollinating U.S. crops in western states. Broad knowledge of their natural history, biology, and physiology has resulted in healthy, managed bee populations to serve competitive U.S. industries for which large quantities of bees are reared, bought, and sold (Bosch & Kemp, 2002; Pitts-Singer & Cane, 2011). For many studies, appropriate bee body size estimates are necessary to compare populations or individuals subjected to different manipulations or environmental conditions. As well, some studies include body size because it may be a covariate that influences other variables of interest. Bee body size is a response variable that is frequently correlated with behavioral and physiological outcomes, such as when assessing the effects of larval feeding on manipulated ratios or quantities of pollen, sugars, yeast, bacteria, viruses, and chemical contaminants on individual growth, performance, and survival (Bukovinszky et al., 2017; Fischman, Pitts-Singer & Robinson, 2017; Frohlich & Tepedino, 1986; Guedot, Bosch & Kemp, 2009; Helm et al., 2017; Kim & Thorp, 2001; Mokkapati, Bednarska & Laskowski, 2021; Radmacher & Strohm, 2010). Additionally, size estimates can inform ecological investigations of bee response to climate change (McCabe, Cobb & Butterfield, 2019). Understanding and interpreting solitary bee responses to different nutritional and environmental conditions are important for both managed and wild pollinator populations, especially considering recent and alarming declines in global diversity and abundance of bees (Potts et al. 2010).
Presently, the most common metrics used by researchers interested in quantifying bee body size include body mass (fresh and dry weight), head capsule width (HCW), and intertegular distance (ITD). Less commonly used is the length of the marginal cell of the wing (Foster & Cartar, 2010). While mass is a weight measure, HCW and ITD are morphometric measurements. HCW is the distance between lateral margins of the compound eye (Rust, 1991), and ITD is the distance between the medial edges of the tegulae (i.e., the two wing attachment points) (Cane, 1987). The use of multiple options for estimating bee body size can complicate efforts to obtain and compare experimental responses across studies and species.
No one measure of bee body size is always appropriate or feasible. Although fresh weight and dry weight, which are measures of mass, are used to report relative bee size (Bosch & Vicens, 2002; Frohlich & Tepedino, 1986; Giovanetti & Lasso, 2005), they may not be comparative measures within or between studies due to the inclusion/exclusion of the meconium in reported weights or due to variation in individual food consumption rates. Wing marginal cell length may be a reliable discrete body size estimator, but this measure can be difficult to obtain without access to microscopy and requires lethal sampling of individuals. HCW may not offer a predictable allometric measure for body size across bee species, because HCW may reflect other morphological variances, such as the size of mandibles and glands needed for nest construction, gathering nest construction materials, or chewing out of nests at adult emergence (Renauld et al., 2016). Nonetheless, both HCW and ITD can be obtained by immobilizing live specimens for taking direct measurements with calipers or using photographs for digital measurements (Fischman, Pitts-Singer & Robinson, 2017), which can take place in the laboratory or field. Body volume also can measure body size for individual bees, and although this metric can yield results similar to ITD, it is very labor-intensive (McCabe, Cobb & Butterfield, 2019).
Inconsistent methodologies for estimating bee body size, specifically in O. lignaria and M. rotundata, can inhibit accurate and comparable measurements for studies that are critical for assessing and managing species across and within populations. In addition, because these solitary bees can be acquired in large numbers from established O. lignaria and M. rotundata retail and consulting industries, these species can serve as model bees that could inform non-Apis bee lethal and sublethal responses to variable diets, climatic conditions, or agrochemical exposure. For these two species, determining a robust and singular estimate of bee body size would streamline future research efforts.
We examined individual O. lignaria and M. rotundata adults to determine species-specific relationships between measurement types and weights that are used as bee body estimates. Therefore, the main goals of this study were to examine the relationships of two body size estimations (ITD and HCW) and how they related to each other and to other body size metrics (i.e., fresh weight & dry weight) and to explore whether these relationships vary by sex.
Materials and Methods
Osmia lignaria were sourced from 2018 progeny recovered from a tart cherry orchard in Santaquin, Utah. Adult bees had been released during crop bloom and allowed to nest in artificial cavities. Progeny were wintered (4–5 °C) and in mid-February 2019, cocooned adults (n = 96 males and 96 females) were selected at random, removed from cold storage, and placed into individual wells of 48-well polystyrene culture plates with lids (Corning Glass Works, Corning, NY, USA) kept at ambient laboratory temperature (approx. 20 °C). On days that O. lignaria adults emerged, they were promptly removed from their wells prior to the expulsion of their meconium and stored at −80 °C. A total of 62 female and 61 males emerged.
Approximately 150 M. rotundata cells containing overwintering cocooned prepupae were obtained from a Utah bee manager in March 2019. In late March, prepupae were incubated in Petri dishes (29 °C) so that adults would emerge in 17–21 days. Bees were checked daily for emergence beginning on 10 April 2019 where 64 females and 49 males emerged. The first M. rotundata male emerged 12 April 2019. Adults were collected on the day of emergence before meconium was expelled and were stored at −80 °C.
In July 2019, all frozen bees were retrieved and thawed for data collection. Each bee was weighed to the nearest 10−5g to collect their fresh weight. Next, the bee body was placed into a furrow of soft foam to assure a uniform and level positioning, and the dorsal side of each bee was digitally photographed (Fischman, Pitts-Singer & Robinson, 2017). From each photograph, ITD and HCW were measured in mm via ImageJ. To ensure consistent and even placement of the bee on the foam, only a single, trained user conducted bee placement and image capture of specimens. Nonetheless, not all images were used for data collection due to poor bee positioning or image quality. Each image was independently calibrated by the presence of a fine metric ruler included within the frame of each image. Measurements in ImageJ are generally more accurate or just as accurate as measurements taken by calipers or a standard ruler (Igathinathane et al., 2008). For both species, ITD was measured from the anterior, medial edge of one tegula to the same location on the other tegula. Head capsule width was measured on the posterior edge of the compound eyes, from one lateral margin of the head to the other (Fig. S1). After all specimens were photographed and labeled, they were placed into a drying oven (Model 05015-58; Cole Parmer Instrument Co., Niles, IL, USA) held at 45 °C for four days when the specimens had ceased to lose weight. Finally, each dried specimen was again weighed to the nearest 10−5g as a measure of dry weight.
For each species, we used linear and exponential regression models to examine the relationship between ITD and each of the following: HCW, fresh weight, and dry in three separate models. Additionally, we tested the relationship between HCW and fresh weight and dry weight in two additional models. Exponential model variables were transformed for the response and predictor variables (Table 1). All variables and residuals were tested for normality using Shapiro-Wilk tests (Fig. S2). Linear and exponential regression models were generated separately for each species with both sexes combined and with the sexes separated (i.e., O. lignaria ensemble, O. lignaria female, O. lignaria male, etc.). Model selection was determined using Akaike’s Information Criterion (AIC), which ranks competing models according to calculated AIC values. This AIC approach was used to test the hypothesis that ITD has a non-linear relationship with other body size measurements (i.e., HCW, fresh weight, and dry weight) as found in Cane (1987). An AIC approach was also used to test the model for HCW in relationship to fresh and dry weight. Model statistical differences were determined by chi-square analysis and R2 values. All statistical analyses were performed using R.3.1.2 and R packages lme4 (Bates et al., 2015) and arm (Gelman & Su, 2018).
| Osmia lignaria | Megachile rotundata | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | R2 | p-value | AIC | DF | R2 | p-value | AIC | |||
| ITD ~ Dry WT | 121 | 0.825 | 2.00E−16 | −29.476 | * | 109 | 0.725 | 2.00E−16 | −201.46 | * |
| LOG(ITD) ~ log(Dry WT) | 121 | 0.811 | 2.00E−16 | −19.044 | 109 | 0.671 | 2.00E−16 | −180.95 | ||
| ΔAIC | 10.432 | ΔAIC | 20.51 | |||||||
| ITD ~ Fresh WT | 121 | 0.814 | 2.00E−16 | −24.33 | 109 | 0.706 | 2.00E−16 | −197.97 | * | |
| LOG(ITD) ~ log(Fresh WT) | 121 | 0.826 | 2.00E−16 | −29.58 | * | 109 | 0.601 | 2.00E−16 | −159.81 | |
| ΔAIC | 5.25 | ΔAIC | −38.16 | |||||||
| ITD ~ HCW | 121 | 0.874 | 2.00E−16 | −69.34 | * | 109 | 0.701 | 2.00E−16 | −189.9 | |
| LOG(ITD) ~ log(HCW) | 121 | 0.868 | 2.00E−16 | −63.93 | 109 | 0.703 | 2.00E−16 | −190.04 | ||
| ΔAIC | 5.41 | ΔAIC | −0.14 | |||||||
| HCW ~ Fresh WT | 121 | 0.844 | 2.00E−16 | −57.02 | 109 | 0.539 | 2.00E−16 | −162.63 | * | |
| LOG(HCW) ~ log(Fresh WT) | 121 | 0.849 | 2.00E−16 | −60.8 | * | 109 | 0.437 | 3.75E−15 | −140.66 | |
| ΔAIC | 3.78 | ΔAIC | −21.97 | |||||||
| HCW ~ Dry WT | 121 | 0.842 | 2.00E−16 | −55.17 | * | 109 | 0.551 | 2.00E−16 | −165.54 | * |
| LOG(HCW) ~ log(Dry WT) | 121 | 0.822 | 2.00E−16 | −40.76 | 109 | 0.472 | 2.00E−16 | −147.59 | ||
| ΔAIC | 14.41 | ΔAIC | 17.95 | |||||||
Note:
ΔAIC represents the difference between AIC values of each model. Significantly smaller AIC value indicating best fit model (indicated by*) based on chi-square analysis. ITD, intertegular distance; HCW, head capsule width; WT, weight.
Results
On average (±standard deviation) for all individuals examined, O. lignaria ITD was 3.18 ± 0.51 mm, HCW was 3.62 ± 0.47 mm, fresh weight was 0.066 ± 0.024 g, and dry weight was 0.027 ± 0.011 g. For M. rotundata, average ITD was 2.59 ± 0.18 mm, HCW was 2.92 ± 0.23 mm, fresh weight was 0.029 ± 0.007 g, and dry weight was 0.011 ± 0.006 g.
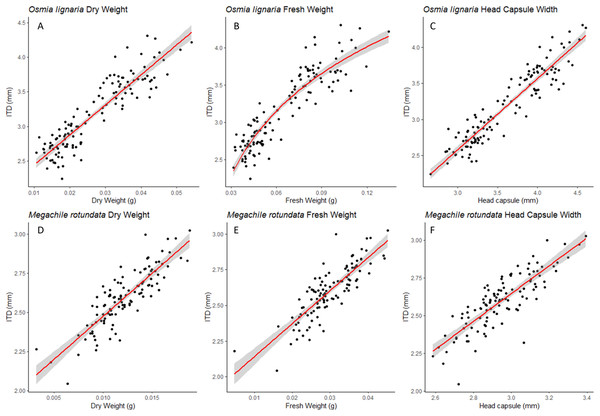
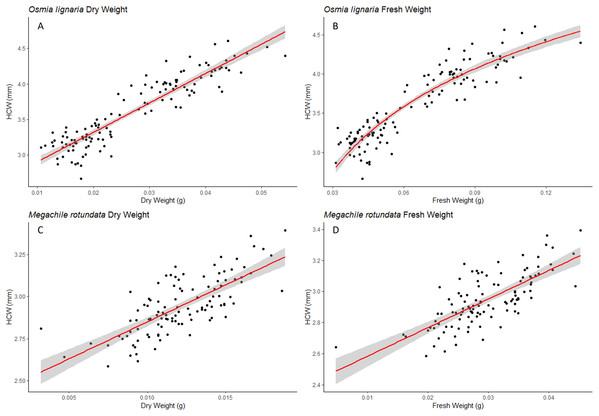
For both ITD and HCW in O. lignaria, linear regression models confirmed significant relationships for every combination of body size metrics evaluated. AIC and R2 values showed that the linear models were a better fit for all interactions except for those with O. lignaria fresh weight, for which the exponential linear models were better fits (p < 0.001; Table 1, Figs. 1 & 2). The relationship between ITD and HCW when plotted by a linear regression revealed a positive relationship (R2 = 0.873, p < 0.001, Fig. 1A). Positive relationships were also found between ITD and fresh weight (R2 = 0.814, p < 0.001, Fig. 1B) and between ITD and dry weight (R2 = 0.801, p < 0.001, Fig. 1C). The relationship between HCW and fresh weight (R2 = 0.845, p < 0.001, Fig. 2A) and between HCW and dry weight were positive relationships (R2 = 0.842, p < 0.001, Fig. 2B).
Figure 1: ITD relationships.
For Osmia lignaria (A–C) and Megachile rotundata (D–F) adults (females and males), the intertegular distance relationships with other body metrics are shown. The linear regression model is used for dry weight and head capsule width, and the exponential regression model is used for fresh weight in O. lignaria, with 95% confidence intervals shown in shaded areas.Figure 2: HCW Relationships.
For Osmia lignaria (A–B) and Megachile rotundata (C–D) adult females and males combined, the head capsule width relationships with other body metrics, the linear regression model is used for dry weight and the exponential regression model is used for fresh weight. 95% confidence intervals shown in shaded areas.For M. rotundata, the linear regression model was the best fit for all relationships between the predictor variables (ITD and HCW) and between these variables and bee weights (Table 1, Figs. 1 & 2). The relationships between ITD and HCW (R2 = 0.696, p < 0.001, Fig. 1D), ITD and fresh weight (R2 = 0.648, p < 0.001, Fig. 1E), and ITD and dry weight (R2 = 0.675, p < 0.001, Fig. 1F) were all positive. Positive relationships were also shown between HCW and fresh weight (R2 = 0.539, p < 0.001, Fig. 2C) and between HCW and dry weight (R2 = 0.552, p < 0.001, Fig. 2D).
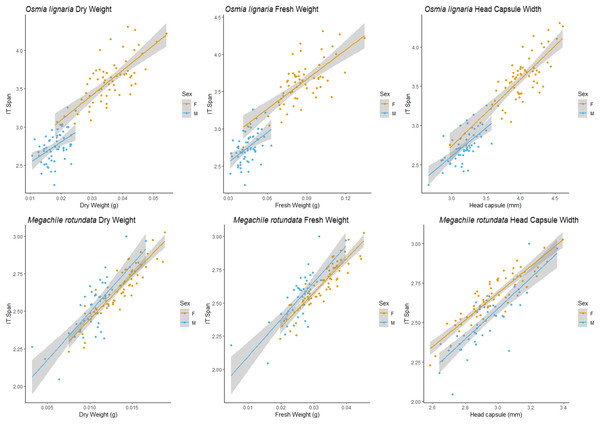
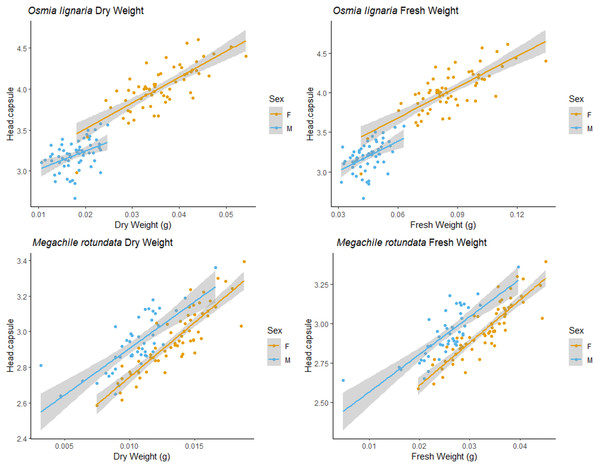
We examined each sex independently as well. For O. lignaria, ITD slopes differed between the sexes (ITD ~ Fresh.Wt: p = 0.023, ITD ~ Dry.Wt: p < 0.001, ITD ~ HCW: p < 0.001) (Figs. 3 & 4). Less model variation was explained when sexes were treated separately (female R2 = 0.532, male R2 = 0.197, pooled R2 = 0.825) versus when they were pooled. These patterns held true for all analyses of O. lignaria variable combinations. For both ITD and HCW by sex, linear regression models were the best fit for all relationship pairings (Table 2, Figs. 3 & 4). Female and male sizes were largely separated across all comparisons for O. lignaria. Females were larger (fresh weight = 0.086 ± 0.016 g; dry weight = 0.036 ± 0.007 g) than males (fresh weight = 0.045 ± 0.007 g; dry weight = 0.018 ± 0.006 g). Correspondingly, females had a larger ITD and HCW than male O. lignaria (ITD: males = 2.75 ± 0.20 mm; females = 3.18 ± 0.51 mm; HCW: males = 3.19 ± 0.18 mm; females = 3.61 ± 0.47 mm).
Figure 3: ITD male/female relationships.
For Osmia lignaria (top row) and Megachile rotundata (bottom row) adult females and males separately, linear regression model analysis for intertegular distance relationships between fresh weight, dry weight, and head capsule width. 95% confidence intervals shown in shaded areas.Figure 4: HCW male/female relationships.
For Osmia lignaria (top row) and Megachile rotundata (bottom row) adult females and males separately, linear regression model analysis for head capsule width relationships between fresh weight and dry weight. 95% confidence intervals shown in shaded areas.| Dry weight | Fresh weight | Head capsule width | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | p-value | R2 | p-value | R2 | p-value | ||||
| O. lignaria Male | 0.1977 | 3.00E−04 | * | 0.2145 | 1.71E−04 | * | 0.4317 | 8.88E−09 | * |
| O. lignaria Female | 0.5327 | 1.70E−11 | * | 0.5117 | 6.47E−11 | * | 0.6118 | 3.73E−14 | * |
| M. rotundata Male | 0.6118 | 8.48E−11 | * | 0.6038 | 1.35E−10 | * | 0.6783 | 1.18E−12 | * |
| M. rotundata Female | 0.7926 | 2.00E−16 | * | 0.7824 | 2.00E−16 | * | 0.8201 | 2.00E−16 | * |
Note:
Linear models relating intertegular distance to other body size metrics, examining Osmia lignaria and Megachile rotundata males and females separately. Asterisks denotes significance.
For M. rotundata, both ITD and HCW models explained more or the same variation when sexes were examined individually (female R2 = 0.793, male R2 = 0.612, pooled R2 = 0.725, Table 2, Figs. 3 & 4) vs when they were pooled. Slopes did not differ between the sexes (ITD ~ Fresh.Wt: p = 0.918, ITD ~ Dry.Wt: p = 0.494, ITD ~ HCW: p = 0.386). On average, females were slightly larger (fresh weight = 0.032 +/− 0.006; dry weight = 0.009 +/- 0.007) than males (fresh weight = 0.025 +/− 0.005 g; dry weight = 0.013 +/− 0.002 g), and weights of M. rotundata females and males were more overlapping than for O. lignaria. Females had a marginally larger ITD and HCW compared to males (ITD: females = 2.65 +/− 0.16 mm, males = 2.52 +/− 0.18 mm; HCW: females = 2.96 +/− 0.17, males = 2.89 +/− 0.29).
Discussion
Having examined a robust sample of O. lignaria and M. rotundata adults, we found strong relationships between measurements of simple morphological traits and bee fresh and dry weights. For O. lignaria, the positive relationships were best fit to a linear model, except for models that included fresh weight of pooled males and females, which were best fitted to an exponential model. For M. rotundata, ITD had a positive linear relationship with HCW, fresh weight and dry weight; HCW also correlated well with these same measurements. Cane’s (1987) data of one female each of 20 different solitary bee species were best fitted using an exponential relationship between ITD and weight, primarily due to the fit of smaller bee species. This might be expected if variables for each bee species follow a series of linear relationships with gradually shifting slopes that create a curve. Although the use of ITD was proposed by Cane (1987) to be a reliable and more feasible means to assess bee size compared to obtaining dry weights, he cautioned that intraspecific comparisons should be based on examination of such relationships for each species of interest, as we have done in this study. Here we show that relationships between two body metric variables across individuals within a species may not follow the same patterns as this relationship when measured across species within a taxon (e.g., all wild bees) as presented in Cane (1987). For social bees, such metrics as ITD and HCW are unreliable estimators (e.g., Burdine et al. 2018) due to inherent size variations between individuals and over time due to caste differentiations and phases of colony cycles.
A further important finding for body size estimators of O. lignaria and M. rotundata is that when the sexes of each species are combined, the measured parameters for the pooled populations are better explained by the models of relationships (i.e., the R2 values were higher) than when the sexes are treated separately. The fits for O. lignaria metrics with both sexes included are stronger than those for M. rotundata, which may be a consequence of the smaller size of this species, the narrow range of individual values, and the presence of several extreme M. rotundata outliers compared to values for O. lignaria. The sexual dimorphism of M. rotundata is less pronounced (with metric values for sexes broadly overlapping) than for O. lignaria. On the other hand, for O. lignaria when the sexes are examined separately, the fits are weaker because few female metric values overlap with male values, and the value ranges for each sex are more constricted than when sexes are pooled.
Our results also show that relationships between ITD and other metrics for females of both O. lignaria and M. rotundata are less variable than for males. Offspring size and sex are strongly linked to provision size, which represents maternal investment (Frohlich & Tepedino, 1986; Helm et al., 2021). Such investments by nesting females may be due to resource availability (amount and distance to resources), seasonality (time of year and duration of nesting), or even as a precursor to nutritional manipulation that can lead to caste determination for social bees (Grula et al., 2021; Peterson & Roitberg, 2006; Pitts-Singer & Bosch, 2010). Thus, offspring number, size, and sex ratio likely represent strategic bet-hedging or reproductive maximization under the influence of current conditions. Therefore, variability in body size may be directly dependent on environmental conditions. For example, if floral resources are limited or distant, a mother bee may make fewer but more equally-sized mass provisions for daughters that are large enough to assure their survival and future reproduction. Meanwhile, the same mother bee may vary either or both the amount of food in male provisions and the number of male cells per nest assuming male size does not limit sperm production or access to females. Indeed, estimated mean mass of M. rotundata prepupae from one summer was higher than prepupae raised on less floral resources in the next summer, and the prepupal masses were more variable (higher standard error of the mean) in males than females when the floral resources were limited (Pitts-Singer & Bosch, 2010).
Many studies report multiple body size measurements due to the concern that one method will not give an accurate representation for body size (Greenleaf et al., 2007; O’Neill et al., 2010; O’Neill & O’Neill, 2011; Torné-Noguera et al., 2014). We contend that, especially for managed bees such as O. lignaria and M. rotundata, using more than one body measurement is not necessary. ITD and HCW measurements are simple, quickly attainable in the laboratory and field, and more strongly related to each other than they are to either fresh or dry weight. Because of the positive correlation between these metrics, they are inherently redundant measurements for O. lignaria and M. rotundata. ITD measurements remain constant and are independent from changes in adult diet (Hagen & Dupont, 2013), reproductive status, or age. Additionally, ITD is not misconstrued due to solitary bee allometric growth, as may be the case for HCW (Cane, 1987). One more benefit of ITD measurements is that they can be harmlessly collected from museum specimens (Gilbert, 2011) or used in the field for observation-mark-recapture and behavioral studies without sacrificing a subset of the population. By using one accurate body measurement as a proxy for body size within a species, this metric can be applied reliably as a dependent or independent variable in studies that require large sample sizes.
Our study concludes that for O. lignaria and M. rotundata, ITD is strongly correlated with HCW, fresh weight at emergence, and dry weight. Use of ITD to estimate adult body size can be especially important in behavioral, nutritional, toxicological, and ecological studies, because it can provide a baseline assessment of adult size that is constant despite experimental or environmental conditions. Determining a single, appropriate metric to assess how or whether toxicological responses to larval chemical exposure scale to bee body size may fill important research gaps in the consideration of non-Apis risk assessment protocols (Boyle et al., 2019). Care should be taken that the measurement obtained, size or weight, is appropriate for the question being asked. Bee size may be indicative of the nutritional quality/quantity of the provision mass. However, bee weight may give inferences about the current condition of an adult (variations in stored lipids, muscle mass, water content, maturity of reproductive organs, etc.), while ITD and HCW can be used as standard metrics to assess size effects on developmental outcomes on newly emerged progeny and their later performance.
Supplemental Information
M. rotundata raw measurements.
This data was used for statistical analysis to compare between body metrics.
O. lignaria raw measurements.
This data was used for statistical analysis to compare between body metrics.
Images documenting how the measurements were taken for intertegular distance (A) and head capsule width (B), as shown for Osmia lignaria.
ITD was measured from the anterior, medial edge of one tegula to the same location on the other tegula. Head capsule width was measured on the posterior edge of the compound eyes, from one lateral margin of the head to the other.