Evaluation of salt tolerance in Eruca sativa accessions based on morpho-physiological traits
- Published
- Accepted
- Received
- Academic Editor
- Renato Benesperi
- Subject Areas
- Agricultural Science, Biodiversity, Plant Science, Biosphere Interactions, Ecotoxicology
- Keywords
- Environment, Abiotic stress, Salinity, Diversity, Chlorophyl, Photosynthesis, Morpho-physiological traits
- Copyright
- © 2020 Afsar et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Evaluation of salt tolerance in Eruca sativa accessions based on morpho-physiological traits. PeerJ 8:e9749 https://doi.org/10.7717/peerj.9749
Abstract
Background
Salinity is one of the most lethal abiotic stresses which affect multiple aspects of plant physiology. Natural variations in plant germplasm are a great resource that could be exploited for improvement in salt tolerance. Eruca sativa (E. sativa) exhibits tolerance to abiotic stresses. However, thorough evaluation of its salt stress tolerance and screening for traits that could be reliably applied for salt tolerance needs to be studied. The current study was designed to characterize 25 E. sativa accessions, originating from diverse geographical regions of Pakistan, for the salt stress tolerance.
Methods
Salt stress (150 mM NaCl) was applied for 2 weeks to the plants at four leaf stage in hydroponics. Data of the following morpho-physiological traits were collected from control and treated plants of all the accessions: root length (RL), shoot length (SL), plant height (PH), leaf number (LN), leaf area (LA), fresh weight (FW), dry weight (DW), chlorophyl content (SPAD), electrolyte leakage (EL), relative water content (RWC), gas exchange parameters and mineral ion content. Salt tolerance was determined based on membership function value (MFV) of the tested traits.
Results
Compared with control, the salt-stressed group had significantly reduced mean SL, RL, PH, LN, LA, FW, DW and SPAD. NaCl treatment triggered a slight increase in EL in few accessions. Mean RWC of control and treated groups were not significantly different although few accessions exhibited variation in this trait. Salt stress caused a significant reduction in photosynthesis rate (PR), transpiration rate (TR) and stomatal conductance (SC) but intercellular CO2 (Ci) was not significantly different between control and treated groups. Compared with control, the salt-stressed plants accumulated significantly higher Na+, K+ and Ca2+ while significantly lower Mg2+. K+/Na+ ratio was significantly decreased in salt-stressed plants compared with control. Importantly, significant inter-accession variations were found for all the tested traits. The principal component analysis identified SL, RL, PH, LN, LA, FW, DW and PR as the most significant traits for resolving inter-accession variability. Based on MFV of the tested traits, accessions were categorized into five standard groups. Among 25 accessions, one accession was ranked as highly tolerant, four as tolerant while 15 accessions were ranked as moderately tolerant. Of the remaining five accessions, four were ranked as sensitive while one accession as highly sensitive.
Conclusion
E. sativa accessions were found to exhibit significant genetic diversity in all the tested traits. A few most significant traits for dissecting the genetic variability were identified that could be used for future large-scale germplasm screening in E. sativa. Salt tolerant accessions could be a good resource for future breeding programs aiming to improve salt stress tolerance.
Introduction
Salt stress is one of the major abiotic stresses affecting the productivity of cultivated soils. The salt-affected areas are increasing, and the irrigated soils are more prone to damage by salinity. Presently about 45 million hectares of irrigated land is affected by salinity. Furthermore, it is estimated that about half of the world’s arable land could become salinized by 2050 (Li et al., 2014; Machado & Serralheiro, 2017). For addressing challenges to the global food security, genetic engineering to create salt tolerant species is considered as a promising strategy (Bhardwaj et al., 2010; Shokri-Gharelo & Noparvar, 2018).
Salt stress is one of the most common abiotic stresses which affect multiple aspects of plant physiology (Munns & Tester, 2008; Fageria, Stone & Santos, 2012). It disrupts nutrient ion balance, decreases stomatal conductance (SC), and negatively affects the photosynthetic activity. Excessive salt accumulation severely impairs the membrane integrity, water relations and pigment content of plants (Acosta-Motos et al., 2017; Munns & Tester, 2008). The plants undergo morphological alterations which include a reduction in root length (RL) and shoot length (SL), plant height (PH) and leaves size and number (Munns & Tester, 2008). As most of the important crops are glycophytes hence their growth is seriously hindered by salt stress (Yang & Guo, 2018).
Natural variations in plant germplasm are a great resource that could be exploited for improvement in salt tolerance without affecting valuable agronomic traits. Genetic variations could be exploited by plant biologists to identify the physiological mechanisms, sets of genes and proteins involved in stress tolerance. Subsequently, these genes can be incorporated in suitable plant species to yield salt stress tolerant varieties (Gupta & Huang, 2014; Ismail & Horie, 2017; Singh, Singh & Sharma, 2018). Moreover, a reliable and extensive phenotypic evaluation of germplasm is important for the identification of tolerance-associated traits (Ismail & Horie, 2017).
Several factors are responsible for the lack of success in developing salt tolerant genotypes. These include low efficiency of morphological, biochemical and physiological parameters being used as screening criteria, limited availability of genetic diversity for breeding programs, and the lack of efficient evaluation methods for identifying salt tolerant genotypes through multivariable screening criteria (Zeng, Shannon & Grieve, 2002; Oyiga et al., 2016).
Eruca sativa (also known as rocket or arugula, and locally as taramira) is a diploid herbaceous plant from Brassicaceae family. It is an important industrial crop that can grow in poor fertility lands and diverse climatic conditions. E. sativa exhibits tolerance to abiotic stresses like salt, drought, and temperature stress (Ashraf, 1994; Shannon & Grieve, 1999; Garg & Sharma, 2014). The seed oil of E. sativa possesses antimicrobial, anti-cancerous and antioxidant properties (Khoobchandani et al., 2010; Azarenko, Jordan & Wilson, 2014). Furthermore, due to high erucic acid content, E. sativa is considered as a potential source for industrial oil (Pignone & Gomez-Campo, 2011). E. sativa exhibits significant genetic diversity and a broader market range. There is huge potential for germplasm improvement; however, limited work on this crop has been carried out so far (Slater, 2013). The genetic variability in E. sativa is a valuable resource that could be exploited in order to screen for high salt tolerance. However, a thorough evaluation of its salt stress tolerance or screening for traits that could be reliably applied for salt tolerance needs to be carried out.
Keeping in mind the above mentioned research gaps, major objectives of the current research were as follows: (1) to understand the effect of salt stress on growth and development of E. sativa accessions, (2) to determine the extent of variability in salinity tolerance among E. sativa accessions, (3) to identify morpho-physiological traits with a maximum contribution towards variability and (4) to identify the salt tolerant and salt sensitive accessions for further studies and future breeding programs.
Materials and Methods
Plant material, growth conditions and salt stress treatment
Twenty five E. sativa accessions were used in this study. The seeds of these accessions were kindly provided by Bio-Resources Conservation Institute, National Agricultural Research Centre, Islamabad, Pakistan. The list of accessions along with other details is given in Table S1. The seeds were surface sterilized and sown in a thick moist sheet of foam which was then placed in 72-cell seed starter trays in a growth chamber under controlled conditions of 16 h/8h day night (350 µmol/ m2/s2) at 23 °C and 60% relative humidity. After one week of growth, uniform sized seedlings were transferred to the Hoagland-type solution (Hoagland & Arnon, 1938) in 4-liter plastic containers. The set up was placed in a growth chamber under controlled conditions (as mentioned above). The solution was changed every 2–3 days. The experiment was carried out in a completely randomized design (CRD). Plants were divided into two groups; one as control (0 mM NaCl) and other as treated (150 mM NaCl). At the four-leaf stage, the salt stress was applied for 2 weeks. Before the determination of gas exchange parameters plants were kept in full sunlight for 2 h (9.00 a.m.–11.00 a.m.).
Determination of plant growth and development traits
Data from control and treated plants was collected after 14 days of growth. Morphological traits like, SL, RL, PH and leaf attributes i.e., leaf number (LN) and leaf area (LA) were recorded. The experiment was performed in six replicates for each parameter. SL, RL and PH were measured from digital images by using image analysis software Digimizer. The LA was manually measured using a grid paper. Fresh weight (FW) of control and treated plants was determined immediately after harvesting by using a digital lab scale. For determination of dry weight (DW), whole plants (root + shoot) were dried in an incubator at 75 °C for 72 h, and then weight was determined by using a digital lab scale. Plant biomass assay was carried out in three replicates.
Electrolyte leakage
Electrolyte leakage (EL) was determined following the previously described method (Yildirim, Karlidag & Turan, 2009). Briefly, ten equal size leaf discs (10 mm diameter) from fully expanded leaves of control and treated plants were prepared and washed with deionized water to remove electrolytes adhered to the surface. These were then incubated at 10 °C for 24 h in glass tubes filled with 10 ml of deionized water and the first electrical conductivity reading (EC1) was recorded. The tubes were heated at 95 °C in a water bath for 20 min to release all the electrolytes. After cooling at room temperature, final electrical conductivity reading (EC2) was recorded. The EC readings were determined by using a portable conductometer (HI-98129 Pocket EC/TDS and pH Tester). The experiment was performed in three replicates. Following equation was used for the calculation of EL:
Determination of chlorophyl content
Relative chlorophyl content was measured by using Chlorophyll Meter (CCM-200plus; Opti-Sciences, Hudson, NH, USA). Fully expanded fourth leaf from all the plants (control and treated) was selected for the reading. The data are presented in the form of chlorophyl content Index (CCI). All the measurements were recorded in six replicates.
Relative water content
Relative Water Content (RWC) was determined from the data of fresh, dry and turgid weight of control and treated plants, as described previously (Loutfy et al., 2012). The experiment was performed in three replicates. RWC was calculated by using the formula: where FW, stands for fresh weight TW for turgid weight and DW for dry weight.
Mineral ion content
For the determination of mineral ions, 100 mg of dried whole plant samples were placed in a furnace for 5 h at 520 °C for ash formation. It was then mixed with the nitric acid-perchloric acid mixture (5:1) and the final volume was raised to 15 ml with distilled water. The filtrate was used to determine the concentrations of Na+, K+, Ca2+ and Mg2+ by atomic absorption spectrometry. For standard curves, different concentrations of Na+, K+, Ca2+ and Mg2+ were prepared by diluting stock solutions of CaSO4, KCl, NaCl and MgSO4. The standard curve was used to determine the content of each element and the values were expressed in mg/g DW. The experiment was performed in three replicates.
Leaf gas exchange parameters
The photosynthesis rate (PR), intercellular CO2 concentration (Ci), transpiration rate (TR), and stomatal conductance (SC) were measured with a portable gas exchange system iFL (ADC BioScientific Ltd., Hoddesdon, UK). All the measurements were conducted on a sunny day with full light intensity (11.00 a.m.–4.00 p.m.). Young fully expanded leaves (third and fourth) were used in situ for recording the above mentioned gas exchange parameters. All the measurements were recorded in four replicates. The following conditions were applied for the assay: Leaf surface diameter 6 cm, ambient atmospheric CO2 concentration (Cref) 352 μmol mol−1, PAR (Qleaf): 1,200 μmol/m2s and wide-range of chamber water vapor pressure 4.4–6.6 mbar. Three plants from each accession and treatment were analyzed for leaf gas exchange parameters.
Salt tolerance evaluation
The data of all morpho-physiological parameters (control and treatment) for each accession was converted to the salt-tolerance index (STI) which is the ratio of the value for the NaCl-treated plant/value for the control. For the categorization of E. sativa accessions according to their salt tolerance, membership function value (MFV) was applied as previously described (Chen et al., 2012). The MFV was calculated according to the following formula:
For the traits inversely related to salt tolerance (e.g., EL), following formula for MFV calculation was used: where Xp is the MFV value of the salt stress parameter “P” in a specific accession, X is the actual value of salt tolerance parameter while Xmin and Xmax represent the minimum and maximum MFV values, respectively, for that parameter in all accessions. A single MFV value (Xc) was obtained for each accession by taking the mean of MFV values of all tested morpho-physiological traits. E. sativa accessions were divided into five standard groups according to the average MFV value (Xa) and S.D. The accession was considered as highly tolerant if Xc ≥ Xa + 1.64 S.D., tolerant if the Xa + 1 SD ≤ Xc < Xa + 1.64 S.D., moderately tolerant if Xa − 1 S.D. ≤ Xc < Xa + 1 S.D., sensitive if Xa − 1.64 S.D. ≤ Xc < Xa − 1 S.D., and highly sensitive if Xc < Xa − 1.64 S.D.
Statistical analysis
Differences among accessions and treatments were considered statistically significant at p < 0.05 by Duncan’s multiple-range test performed using IBM SPSS Statistics for Windows, V.20 (IBM Corporation, Armonk, NY, USA). Principal component analysis (PCA), cluster analysis, and correlation matrix analyses were performed on MFV values of studied salt tolerance traits using STATISTICA Statsoft (version 10).
Results
Morphological traits and leaf attributes
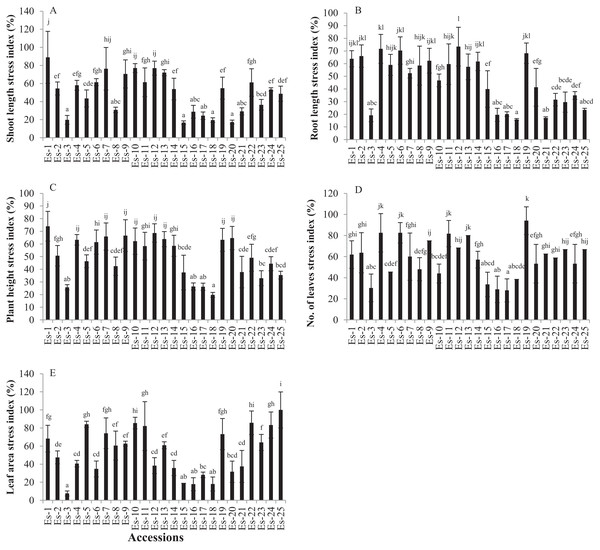
Shoot length was significantly reduced in the salt-stressed plants compared with the control (p < 0.05). The mean SL values for the control and treatments groups were 2.30 ± 0.56 and 1.10 ± 0.48 inches, respectively (Table 1). To determine the salt stress response of accessions based on shoot growth, SL of each accession was expressed as stress index, as described in the material and methods section. Accessions showing the higher stress index were considered more salt tolerant and vise versa. SL stress index of accessions varied from 16.74 to 88.96. Es-1 and Es-15 exhibited the highest and the lowest SL stress index, respectively (Fig. 1A).
| Accessions | SL (inch) | RL (inch) | PH (inch) | LN | LA (inches2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | |
| Es-1 | 1.89Aijk | 1.69Aghij | 2.40Aopq | 1.54Bghij | 4.15Amnop | 3.08Bij | 4.2Aklmn | 2.6Bcdef | 0.05A,fghij | 0.03A,bcdef |
| Es-2 | 2.83Aq | 1.55Bfghi | 1.93Aklm | 1.27Bdefgh | 4.99Arst | 2.53Befgh | 4.4Almno | 2.8Bdefg | 0.08A,op | 0.04B,efgh |
| Es-3 | 3.51Ar | 0.70Bab | 2.35Aop | 0.45Ba | 4.62Aopqr | 1.18Ba | 4.4Almno | 1.33Ba | 0.16A,r | 0.01B,a |
| Es-4 | 3.56Ar | 2.07Bjklmn | 2.75Aqr | 1.98Blmn | 6.05Au | 3.83Bklm | 4.6Amnop | 3.8Bijkl | 0.09A,q | 0.04B,efghi |
| Es-5 | 2.48Aop | 1.08Bcde | 1.65Aijkl | 0.97Bbcd | 4.50Anopqr | 2.08Bbcde | 4.4Almno | 2Babc | 0.06A,lmno | 0.06B,ijkl |
| Es-6 | 2.01Ajkl | 1.23Bdef | 1.48Afghij | 1.04Bcd | 3.55Ajkl | 2.18Bcdef | 4.6Amnop | 3.8Bijkl | 0.08A,op | 0.03B,bcde |
| Es-7 | 1.95Ajkl | 1.49Bfgh | 2.19Amno | 1.15Bdef | 4.08Almn | 2.69Bfghi | 4Ajklm | 2.4Bcde | 0.07A,op | 0.06B,ijkl |
| Es-8 | 2.97Aq | 0.92Bbcd | 1.80Ajkl | 1.05Bcde | 4.66Apqrs | 1.97Bbcd | 5Aopq | 2.4Bcde | 0.07A,mnop | 0.04B,efghi |
| Es-9 | 2.42Anop | 1.71Bghij | 2.23Amno | 1.39Befghi | 4.64Apqrs | 3.10Bij | 4Ajklm | 3Befgh | 0.07A,mnop | 0.04B,efghi |
| Es-10 | 1.38Aefg | 1.06Acde | 1.61Ahijk | 0.75Babc | 3.04Ahij | 1.89Bbcd | 5Aopq | 2.2Bbcd | 0.08A,p | 0.07A,mnop |
| Es-11 | 2.07Ajklmn | 1.27Bdef | 1.89Aklm | 1.13Bdef | 3.98Almn | 2.32Bdefg | 4.4A,lmno | 3.6Bhijk | 0.06A,ijkl | 0.04A,fghij |
| Es-12 | 1.78Ahij | 1.37Befg | 1.66Aijkl | 1.22Bdefg | 3.44Ajk | 2.36Bdefg | 4.4Almno | 3Befgh | 0.06A,jklm | 0.02B,abcd |
| Es-13 | 2.73Apq | 1.97Bjkl | 2.50Aopq | 1.44Bfghi | 5.34At | 3.40Bjk | 5Aopq | 4Bjklm | 0.07A,nop | 0.04B,efghi |
| Es-14 | 2.29Almno | 1.23Bdef | 2.41Aopq | 1.49Bfghij | 4.78Aqrs | 2.80Bghi | 5.6Aq | 3.2Bfghi | 0.08A,pq | 0.03B,bcde |
| Es-15 | 2.07Ajklmn | 0.35Ba | 2.42Aopq | 0.97Bbcd | 4.38Anopq | 1.64Bab | 4.75Anop | 1.6Bab | 0.05A,hijk | 0.01B,a |
| Es-16 | 2.42Anop | 0.69Bab | 2.31Ano | 0.45Ba | 4.64Apqrs | 1.22Ba | 4.6Amnop | 1.33Ba | 0.07A,mnop | 0.01B,a |
| Es-17 | 2.44Anop | 0.60Bab | 2.23Amno | 0.45Ba | 4.69Apqrs | 1.22Ba | 5Aopq | 1.4Ba | 0.07A,op | 0.02B,abc |
| Es-18 | 3.05Aq | 0.60Bab | 2.90Ar | 0.46Ba | 5.87Au | 1.16Ba | 5.2Apq | 2Babc | 0.07A,nop | 0.01B,a |
| Es-19 | 2.25Aklmno | 1.23Bdef | 2.18Amno | 1.49Bfghij | 4.42Anopq | 2.80Bghi | 3.4Aghij | 3.2Afghi | 0.04A,efghi | 0.03B,bcde |
| Es-20 | 1.99Ajkl | 0.35Ba | 2.34Aop | 0.97Bbcd | 4.33Amnopq | 2.80Bghi | 3Aefgh | 1.6Bab | 0.03A,defg | 0.01B,a |
| Es-21 | 1.99Ajkl | 0.60Bab | 2.68Apqr | 0.46Ba | 4.71Apqrs | 1.78Bbc | 3.2Afghi | 2Babc | 0.04A,efgh | 0.01B,ab |
| Es-22 | 1.73Aghij | 1.22Bdef | 2.47Aopq | 0.78Babc | 4.33Amnopq | 2.12Bbcde | 3.4Aghij | 2Babc | 0.042A,efghi | 0.03A,cdefg |
| Es-23 | 1.40Aefg | 0.63Bab | 1.8Ajkl | 0.53Ba | 3.53Ajkl | 1.16Ba | 3Aefgh | 2Babc | 0.05A,ghij | 0.03B,bcdef |
| Es-24 | 2.39Amnop | 0.75Bbc | 1.41Afghi | 0.49Ba | 2.84Aghi | 1.26Ba | 3Aefgh | 1.6Bab | 0.04A,efgh | 0.03A,bcdef |
| Es-25 | 1.9Aijk | 1.17Bdef | 2.74Aqr | 0.64Bab | 5.18Ast | 1.83Bbcd | 3Aefgh | 2Babc | 0.04A,efghi | 0.04A,efghi |
| Mean | 2.30A | 1.10B | 2.17A | 0.98B | 4.43A | 2.18B | 4.22A | 2.43B | 0.07A | 0.03B |
| S.D | 0.56 | 0.48 | 0.42 | 0.43 | 0.77 | 0.76 | 0.78 | 0.82 | 0.03 | 0.02 |
Notes:
SL, shoot length; RL, root length; PH, plant height; LN, leaf number; LA, leaf area.
Different lowercase letters show significant differences (p < 0.05) among accessions while the different upper case letters show significant differences (p < 0.05) among treatments (Control vs. NaCl).
Figure 1: Effect of salt stress on growth parameters in E. sativa (A) Shoot length stress index (B) Root length stress index (C) Plant height stress index (D) leaf number stress index (E) leaf area stress index.
Values are mean (±SD) of six replicates. Means with same letters are not significantly different tested by Duncan’s multiple range test at 5% level.Salt stress significantly inhibited RL as compared to control (p < 0.05). Mean RL values in control and treated groups were 2.17 ± 0.42 and 0.98 ± 0.43 inches, respectively (Table 1). RL stress index ranged from 15.88 to 73.47. The highest RL stress index was exhibited by Es-12 while the lowest by Es-18 (Fig. 1B).
Plant height was also significantly reduced in salt-stressed plants compared with control (p < 0.05). Mean PH values for control and treated groups were 4.43 ± 0.77 and 2.18 ± 0.76 inches, respectively (Table 1). PH stress index varied from 19.80 to 73.98; accessions Es-1 and Es-18 showed the highest and the lowest stress index, respectively (Fig. 1C).
Leaf number and LA were also significantly reduced in salt-stressed plants compared to control (p < 0.05). Mean LN for control and treatment groups were 4.22 ± 0.78 and 2.43 ± 0.82, respectively (Table 1). LN stress index varied from 28 to 94.12; Es-19 exhibited the highest stress index while Es-17 the lowest (Fig. 1D). Mean LA was also significantly less in the treated group compared with control (p < 0.05) (Table 1). Mean LA for control and treatment groups was 0.07 ± 0.025 and 0.03 ± 0.016 inch2, respectively (Table 1). LA stress index varied from 7.6 to100; Es-25 and Es-3 showed the highest and the lowest stress index, respectively (Fig. 1E). To summarize, these data show that salt stress significantly inhibited morphological traits in all the accessions. Moreover, the extent of inhibition of these traits, by salt stress, was variable in E. sativa accessions.
Plant biomass
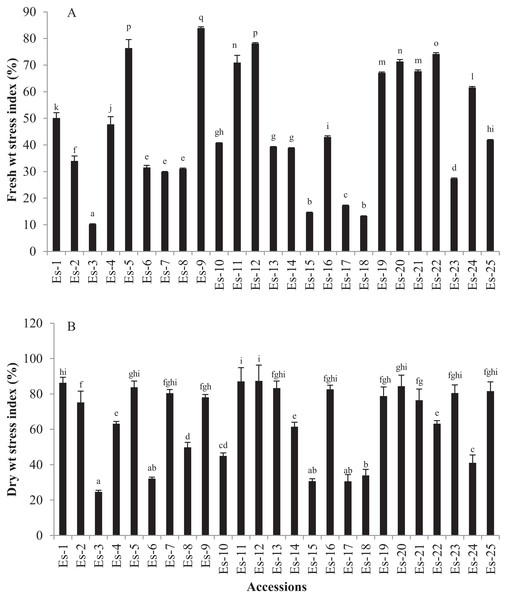
Salt stress significantly reduced the FW and DW of E. sativa plants (p < 0.05). Mean FW values in control and treatment groups were 241 ± 164 mg and 96 ± 66 mg, respectively (Table 2). FW stress index ranged from 10.2 to 83.92 (Fig. 2A). Cultivar Es-9 showed the highest FW stress index while Es-3 the lowest (Fig. 2). Mean DW values for control and treated groups were 10.6 ± 6.9 and 6.29 ± 3.9 mg, respectively (Table 2). DW stress index varied from 24.65 to 87.42. Es-12 and Es-3 exhibited the highest and the lowest DW stress index, respectively (Fig. 2B).
| Accessions | FW (mg) | DW (mg) | RWC (%) | SPAD (CCI) | ||||
|---|---|---|---|---|---|---|---|---|
| Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | |
| Es-1 | 343.1Av | 171.97Bmn | 20.93At | 18.05Br | 75.84Ab | 89.81Bcd | 16.88Ahijklm | 13.65Bdefg |
| Es-2 | 173.13Amn | 58.83Bfg | 11.96Amn | 9.00Bk | 71.59Aa | 91.73Bcdefghijkl | 21.16Anopq | 15.2Bdefghi |
| Es-3 | 321.26Au | 32.76Bab | 15.93Aq | 3.93Befg | 76.89Ab | 89.22Bcd | 18.66Ajklmno | 13.2Bdef |
| Es-4 | 347.55Av | 165.95Bm | 19.72As | 12.45Bno | 96.03Amno | 96.34Amno | 16.9Ahijklm | 15.88Afghijk |
| Es-5 | 222.8Aq | 170.2Bmn | 12.93Ao | 10.83Bl | 91.39Acdefghij | 94.63Ahijklmn | 23.71Ars | 16.82Bhijkl |
| Es-6 | 754.8Az | 238.13Br | 32.53Au | 10.46Bl | 98.8Ao | 96.00Amno | 17.08Ahijklm | 12.58Bcd |
| Es-7 | 323.13Au | 96.47Bj | 10.6Al | 8.52Bk | 97.48Ano | 96.00Amno | 26.3Ast | 31.31Bu |
| Es-8 | 245.13Ars | 76.17Bi | 11.2Alm | 5.58Bh | 94.58Aghijklmn | 92.02Acdefghijkl | 9.8Aab | 6.93Aa |
| Es-9 | 60.59Ag | 50.85Bef | 3.48Acdefg | 2.72Abcd | 91.55Acdefghijk | 90.26Acde | 27.4At | 31.31Bu |
| Es-10 | 435.06Ax | 177.52Bn | 16Aq | 7.18Bij | 96.41Amno | 94.72Ahijklmn | 18.51Ajklmn | 13Bdef |
| Es-11 | 203.71Ap | 144.60Bl | 7.38Aj | 6.43Bi | 94.69Ahijklmn | 95.89Amno | 14.31Adefgh | 9.35Bab |
| Es-12 | 247.26As | 193.26Bo | 10.6Al | 9.26Bk | 94.84Aijklmn | 95.2Ajklmno | 15.66Aefghij | 14.36Adefgh |
| Es-13 | 449.33Ay | 176.71Bn | 11Al | 9.17Bk | 96.06Amno | 94.59Aghijklmn | 15Adefghi | 9.08Bab |
| Es-14 | 426Aw | 165.55Bm | 14Ap | 8.61Bk | 97.35Ano | 94.23Afghijklmn | 24.88Ast | 17.51Bijklm |
| Es-15 | 251.2As | 36.76Babc | 11.93Amn | 3.66Bdefg | 95.45Aklmno | 91.86Acdefghijkl | 16.55Aghijkl | 9.63Bab |
| Es-16 | 77.00Ai | 33.09Babc | 3.67Adefg | 3.03Acde | 90.81Acdefg | 89.22Acd | 21.21Anopq | 12.66Bcde |
| Es-17 | 210.2Ap | 36.22Babc | 10.4Al | 3.18Bcde | 94.24Afghijklmn | 91.31Acdefghi | 15.18Adefghi | 9.21Bab |
| Es-18 | 307.93At | 40.94Bbcd | 8.66Ak | 2.94Bbcd | 96.56Amno | 88.6Bc | 9.61Aab | 12.5Bcd |
| Es-19 | 104.92Ak | 70.43Bhi | 5.33Ah | 4.20Bfg | 92.96Adefghijklm | 94.27Afghijklmn | 18.75Aklmno | 16.51Aghijkl |
| Es-20 | 42.43Acde | 30.3Ba | 3.2Acde | 2.7Abc | 88.29Ac | 91.90Acdefghijkl | 22.21Aqr | 14.66Bdefghi |
| Es-21 | 90.55Aj | 61.33Bg | 4.18Afg | 3.2Bcde | 94.99Aijklmn | 95.25Ajklmno | 9.95Abc | 16.9Bhijklm |
| Es-22 | 48.40Ade | 35.92Babc | 2.09Aab | 1.32Aa | 96.49Amno | 95.93Amno | 18.98Almno | 8.01Bab |
| Es-23 | 138.11Al | 37.79Babc | 4.16Afg | 3.35Acdef | 94.44Aghijklmn | 91.63Acdefghijkl | 21.5Apqr | 8.83Bab |
| Es-24 | 107.8Ak | 66.40Bgh | 7.66Aj | 3.14Bcde | 90.97Acdefgh | 89.62Acd | 14.21Adefgh | 9.41Bab |
| Es-25 | 95.33Aj | 39.93Bbcd | 5.28Ah | 4.31Bg | 93.99Aefghijklmn | 90.57Acdef | 19.83Amnop | 12.95Bdef |
| Mean | 241.07A | 96.33B | 10.60A | 6.29B | 92.11A | 92.83A | 18.17A | 14.06B |
| S.D. | 164.93 | 66.23 | 6.88 | 3.99 | 7.01 | 2.58 | 4.79 | 6.02 |
Notes:
FW, fresh weight; DW, dry weight; RWC, relative water content; SPAD, chlorophyll content.
Different lowercase letters show significant differences (p < 0.05) among accessions while the different upper case letters show significant differences (p < 0.05) among treatments (Control vs. NaCl).
Figure 2: Effect of salt stress on biomass of E. sativa accessions (A) Fresh weight stress index (B) dry weight stress index.
Values are mean (±SD) of three replicates. Means with same letters are not significantly different tested by Duncan’s multiple range test at 5% level.RWC, EL and chlorophyl content
Mean RWC values of control and treatment groups were not significantly different, being 92.11 ± 7% and 92.83 ± 2.6%, respectively (p > 0.05) (Table 2). However, moderate but significant variations in RWC were observed between control and its treated counterpart for few accessions (p < 0.05) (Table 2). RWC, in the control group, varied from 71.6% to 98.8% while in the treated group from 88.6% to 96.3%. Among the control group, Es-2 and Es-6 showed the lowest and the highest RWC, respectively. In the treated group Es-18 showed the lowest while Es-4 showed the highest RWC (Table 2).
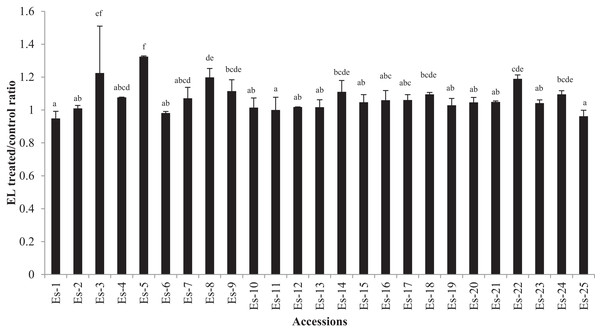
The EL data is presented in Fig. 3 as the ratio of treated to control for each accession. A higher ratio showed an increased EL due to salt stress. Moderate but statistically significant differences in EL were found in few accessions (p < 0.05) (Fig. 3). EL ratio of the accessions ranged from 0.949 to1.324 (mean EL ratio 1.07). The lowest ratio was recorded for accessions Es-1 while the highest for Es-5 (Fig. 3).
Figure 3: Electrolyte leakage in E. sativa accessions presented as ratio of treated/ control plants.
Data are mean (±SD) of three replicates. Means with same letters are not significantly different tested by Duncan’s multiple range test at 5% level.Compared with control, the salt-treated group had significantly less chlorophyl (p < 0.05). The mean chlorophyl content values for control and treated groups were 18.17 ± 4.79 and 14.06 ± 6.02 CCI, respectively (Table 2). Under salt stress, chlorophyl content of the accessions was highly variable. Compared to their respective control, four accessions showed a significant SPAD increase, 17 accessions showed a significant decrease while four accessions did not show any significant SPAD change under salt stress (p < 0.05) (Table 2).
Gas exchange attributes
Salt stress caused a marked and significant reduction in net PR in all accessions (p < 0.05). PR in control was 7.5 ± 4.69 µmol m−2s−1 while in treated group it was only 1.91 ± 0.97 µmol m−2s−1 (Table 3). A significant variation was shown by the accessions in this attribute (p < 0.05). At 0 mM NaCl, Es-3 and Es-17 had maximum and minimum PR, respectively while at 150 mM NaCl, Es-1 and Es-25 showed the maximum and minimum PR, respectively (Table 3).
| Accessions | Photosynthesis rate (µmolm−2s−1) |
Intercellular CO2 μmol mol–1 |
Transpiration rate (mmol m−2 s−1) |
Stomatal conductance (mmolm−2s−1) |
||||
|---|---|---|---|---|---|---|---|---|
| Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | |
| Es-1 | 12.5Agh | 4.24Bbcde | 286Abcdefghij | 245.5Aabcd | 3.16Agh | 1.64Bbcde | 0.22Ade | 0.08Bab |
| Es-2 | 2.74Aabcde | 1.78Aabc | 271.5Aabcdefgh | 288.5Abcdefghij | 0.91Aabcd | 1.12Aabcd | 0.04Aab | 0.04Aab |
| Es-3 | 17Ai | 1.34Ba | 290.5Abcdefghij | 258Aabcde | 4.18Ah | 0.56Bab | 0.31Af | 0.02Ba |
| Es-4 | 13Agh | 3.4Babcde | 279Aabcdefgh | 273Aabcdefgh | 3.55Agh | 1.38Babcde | 0.21Ade | 0.06Bab |
| Es-5 | 9.10Af | 2.62Babcde | 280Aabcdefgh | 312.5Adefghijk | 3.45Agh | 1.94Bdef | 0.2Ade | 0.05Bab |
| Es-6 | 4.96Ade | 1.22Ba | 281.5Aabcdefghi | 315Aefghijk | 3.88Ah | 1.03Babcd | 0.2Ade | 0.03Bab |
| Es-8 | 2.85Aabcde | 1.53Aab | 300.5Acdefghijk | 333Aghijk | 1.57Abcde | 1.3Aabcd | 0.07Aab | 0.05Aab |
| Es-9 | 14.01Agh | 2.46Babcde | 255Aabcde | 251Aabcde | 3.46Agh | 0.68Babcd | 0.23Ade | 0.03Bab |
| Es-10 | 8.8Af | 2.51Babcde | 281.5Aabcdefghi | 258.5Aabcde | 3.48Agh | 1.24Babcd | 0.17Acde | 0.06Bab |
| Es-11 | 3.28Aabcde | 1.16Aa | 290Abcdefghij | 329Afghijk | 1.65Abcde | 0.63Aabc | 0.07Aab | 0.03Aa |
| Es-12 | 9.06Af | 3.31Babcde | 255.5Aabcde | 281.5Aabcdefghi | 3.14Agh | 1.85Bcdef | 0.15Acd | 0.07Bab |
| Es-13 | 14.8Ahi | 2.5Babcde | 294Acdefghijk | 223.5Bab | 3.64Agh | 0.68Babcd | 0.2Ade | 0.03Ba |
| Es-14 | 8.83Af | 3.18Babcde | 294.5Acdefghijk | 274.5Aabcdefgh | 2.59Aefg | 1.25Babcd | 0.2Ade | 0.05Bab |
| Es-15 | 5.16Ae | 1.37Ba | 315.5Aefghijk | 215.5Ba | 1.93Adef | 0.26Ba | 0.11Abc | 0.01Ba |
| Es-17 | 1.14Aa | 0.76Aa | 340Ahijk | 315Aefghijk | 1.07Aabcd | 0.37Aab | 0.05Aab | 0.03Aa |
| Es-18 | 2.84Aabcde | 1.42Aa | 306Acdefghijk | 349.5Aijk | 1.40Aabcde | 1Aabcd | 0.07Aab | 0.03Aa |
| Es-19 | 2.16Aabc | 1.62Aab | 287Abcdefghij | 293Acdefghij | 3.64Agh | 0.57Babc | 0.2Ade | 0.04Bab |
| Es-20 | 12.7Agh | 1.75Babc | 238Aabc | 215.5Aa | 3Afgh | 0.46Bab | 0.16Acd | 0.02Ba |
| Es-21 | 12.18Ag | 2.57Babcde | 274Aabcdefgh | 265.5Aabcdefg | 4.14Ah | 1.23Babcd | 0.25Ae | 0.06Bab |
| Es-22 | 4.26Abcde | 1.24Ba | 259Aabcde | 275Aabcdefgh | 1.33Aabcd | 0.83Aabcd | 0.06Aab | 0.03Aab |
| Es-23 | 5.04Ae | 0.77Ba | 246Aabcd | 264Aabcdef | 1.56Abcde | 0.66Aabcd | 0.06Aab | 0.03Aa |
| Es-24 | 4.47Acde | 0.9Ba | 276.5Aabcdefgh | 361Bk | 1.52Aabcde | 0.61Aabc | 0.06Aab | 0.03Aa |
| Es-25 | 2.27Aabcd | 0.71Aa | 266Aabcdefg | 350.5Bjk | 0.99Aabcd | 0.38Aab | 0.06Aab | 0.03Aab |
| Mean | 7.5A | 1.91B | 281.20A | 284.70A | 2.57A | 0.94B | 0.14A | 0.04B |
| S.D | 4.69 | 0.97 | 22.90 | 42.57 | 1.13 | 0.48 | 0.08 | 0.02 |
Note:
Different lowercase letters show significant differences (p < 0.05) among accessions while the different upper case letters show significant differences (p < 0.05) among treatments (Control vs. NaCl).
Over all, no significant differences were found in mean intercellular CO2 concentration (Ci) between control and treatment groups (p > 0.05). Mean Ci values in control and treatment groups were 281.2 ± 22.9 μmol mol−1 and 284.7 ± 42.6 μmol mol−1, respectively (Table 3). However, significant inter-accession variations in Ci were detected in both control and treatment groups (p < 0.05). The Ci ranged from 238 to 340 μmol mol−1 in control group while from 215.5 to 361 μmol mol−1 in treatment group (Table 3). Es-20 and Es-17 showed the highest and the lowest Ci respectively among control group while Es-24 and Es-15 showed the highest and the lowest Ci respectively among treatment group (Table 3).
The TR in the salt-stressed group was significantly lower compared with the control group (p < 0.05). Mean TR values for control and treated groups were 2.57 ± 1.13 mmol m−2s−1 and 0.94 ± 0.48 mmol m−2s−1, respectively (Table 3). In control plants TR ranged from 0.91 (Es-2) to 4.18 mmol m−2s−1 (Es-3) while in salt-stressed plants it ranged from 0.26 (Es-15) to 1.94 mmol m−2s−1 (Es-5) (Table 3).
The salt-stressed group exhibited significantly reduced SC compared with the control group (p < 0.05). Mean SC values for control and treated groups were 0.14 ± 0.08 mmol m−2s−1 and 0.04 ± 0.02 mmol m−2s−1, respectively (Table 3). Significant inter-accession variations were also observed in SC within control as well as treatment groups (p < 0.05). SC, in control group, ranged from 0.04 mmol m−2s−1 to 0.31 mmol m−2s−1. Es-3 and Es-2 exhibited the highest and the lowest SC, respectively (Table 3). On the other hand, SC in treatment group ranged from 0.01 mmol m−2s−1 to 0.075 mmol m−2s−1; Es-12 and Es-15 exhibiting the highest and the lowest SC, respectively. The accession Es-2 exhibited an interesting phenotype; SC under control and salt stress was not significantly different (Table 3). Gas exchange parameters could not be assayed in two accessions (Es-7 and Es-16) due to the unavailability of enough seeds.
Mineral ion content
Significantly higher Na+ accumulated in salt-stressed plants compared with control (p < 0.05). Mean Na+ content values for control and treated groups were 13.52 ± 5.01 and 29.55 ± 8.8 mg/g DW, respectively (Table 4). Significant inter-accession variations were found in Na+ content in both control and treatment groups (p < 0.05). In control group, Na+ content varied from 4.6 to 24.90 mg/g DW; Es-1 and Es-21 showing the lowest and the highest Na+ content, respectively (Table 4). Na+ content, in treatment group, ranged from 16.99 to 48.11 mg/g DW; accessions Es-3 and Es-20 accumulated the lowest and the highest Na+, respectively (Table 4).
| Accessions | Na+ (mg g−1 DW) | K+ (mg g−1 DW) | Ca2+ (mg g−1 DW) | Mg2+ (mg g−1 DW) | K+/Na+ ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | Control | NaCl | |
| Es-1 | 4.6Aa | 21.5Bklmnopq | 8.72Aab | 11.55Abcdef | 4.02Aabcde | 3.51Aabcd | 2.74Aabc | 2.53Aab | 1.89Au | 0.53Babc |
| Es-2 | 6.035Aab | 23.86Bopqrs | 8.81Aabc | 12.98Bdefghi | 4.28Aabcde | 3.73Aabcd | 3.49Aabcdefg | 2.82Aabcd | 1.46At | 0.54Babc |
| Es-3 | 7.43Aabcd | 16.99Bhijklm | 9.59Aabcd | 14.46Befghijk | 5.69Adefghi | 5.73Adefghi | 3.78Aabcdefghi | 3.55Aabcdefgh | 1.29Aqrst | 0.86Bdefghijklm |
| Es-4 | 7.07Aabc | 22.16Bmnopqr | 6.95Aa | 9.74Aabcd | 2.68Aa | 2.92Aab | 5.10Acdefghijk | 3.88Aabcdefghi | 0.98Aijklmnop | 0.43Ba |
| Es-5 | 9.97Aabcdef | 27.53Brst | 12.06Abcdefg | 13.02Adefghi | 5.29Abcdefgh | 4.01Aabcde | 7.24Aklm | 4.89Abcdefghijk | 1.20Aopqrst | 0.47Bab |
| Es-6 | 10.67Abcdefg | 25.01Bpqrs | 12.20Abcdefg | 14.71Aefghijkl | 3.095Aabc | 2.87Aab | 5.18Acdefghijk | 3.51Aabcdefg | 1.14Amnopqrs | 0.58Babcde |
| Es-7 | 13.15Adefghi | 30.72Btu | 16.65Aijklmn | 16.97Ajklmno | 3.98Aabcde | 3.04Aabc | 3.69Aabcdefgh | 2.71Aabc | 1.27Apqrst | 0.55Babcd |
| Es-8 | 15.59Aefghij | 31.9Btu | 17.59Aklmno | 21.96Bpqrs | 7.78Ahijklm | 6.38Aefghij | 9.49Anop | 8.85Amno | 1.12Almnopqr | 0.69Babcdefghi |
| Es-9 | 16.21Aghijkl | 31.85Btu | 33.4Av | 41.30Bw | 8.99Aklmn | 13.46Bq | 8.11Almn | 5.33Bdefghijk | 2.06Au | 1.29Bqrst |
| Es-10 | 9.81Aabcde | 35.98Buv | 12.51Acdefgh | 16.71Bijklmn | 4.68Aabcdef | 8.51Bjklm | 3.44Aabcdef | 2.90Aabcd | 1.29Aqrst | 0.46Bab |
| Es-11 | 15.51Aefghij | 18.54Aijklmno | 18.38Almnop | 20.71Aopqr | 9.86Amnop | 11Anop | 4.42Aabcdefghij | 4.25Aabcdefghi | 1.18Anopqrst | 1.11Almnopqr |
| Es-12 | 16.83Ahijklm | 20.31Ajklmnop | 14.54Aefghijkl | 11.98Abcdefg | 3.98Aabcde | 7.14Bfghijk | 6.07Ahijkl | 5.50Aefghijk | 0.86Aefghijklm | 0.59Aabcde |
| Es-13 | 10.55Abcdefg | 27.91Bst | 15.09Afghijklm | 15.77Aghijklmn | 5.47Acdefgh | 7.99Bijklm | 4.50Aabcdefghij | 3.15Aabcde | 1.43Ast | 0.56Babcde |
| Es-14 | 12.84Adefghi | 35.57Buv | 13.03Adefghi | 18.91Bmnopq | 4.43Aabcde | 10.12Bmnop | 4.19Aabcdefghi | 3.73Aabcdefghi | 1.01Aklmnopqr | 0.53Babc |
| Es-15 | 15.76Afghijk | 21.62Blmnopq | 12.65Adefgh | 17.02Bjklmno | 4.13Aabcde | 5.79Adefghi | 4.34Aabcdefghij | 3.22Aabcde | 0.80Acdefghijk | 0.79Acdefghijk |
| Es-16 | 10.68Abcdefg | 22.97Bnopqrs | 13.63Aefghij | 19.24Bnopqr | 5.48Acdefgh | 12.09Bpq | 3.87Aabcdefghi | 2.78Aabc | 1.30Arst | 0.83Bcdefghijkl |
| Es-17 | 17.87Ahijklmn | 47.04Bxy | 17.13Ajklmno | 26.58Bu | 7.26Aghijkl | 23.16Br | 6.25Aijkl | 4.61Aabcdefghij | 0.96Ahijklmno | 0.58Babcde |
| Es-18 | 24.72Apqrs | 38.14Bvw | 22.81Arst | 25.36Astu | 4.88Aabcdefg | 13.56Bq | 5.05Abcdefghijk | 3.76Aabcdefghi | 0.92Aghijklmno | 0.67Aabcdefgh |
| Es-19 | 12.51Acdefgh | 32.4Btu | 18.36Almnop | 22.16Bqrs | 2.60Aa | 6.94Bfghijk | 5.99Aghijkl | 3.78Aabcdefghi | 1.47At | 0.69Babcdefghi |
| Es-20 | 13.82Aefghi | 48.11By | 26.50Au | 33.35Bv | 13.46Aq | 21.62Br | 6.83Ajklm | 4.77Aabcdefghijk | 1.91Au | 0.71Babcedfghij |
| Es-21 | 24.9Apqrs | 44.65Bxy | 24.81Astu | 30.41Bv | 8.28Ajklm | 12.02Bpq | 10.53Aop | 5.82Bfghijkl | 1.00Ajklmnopq | 0.68Babcdefghi |
| Es-22 | 16.87Ahijklm | 28.21Bst | 12.70Adefgh | 15.63Aghijklmn | 9.59Almno | 13.33Bq | 5.55Aefghijk | 4.65Aabcdefghij | 0.75Abcdefghijk | 0.56Aabcde |
| Es-23 | 17.41Ahijklmn | 26.70Bqrst | 10.94Abcde | 16.50Bhijklmn | 8.63Ajklmn | 9.90Amnop | 3.17Aabcde | 2.27Aa | 0.63Aabcdefg | 0.62Aabcdef |
| Es-24 | 13.83Aefghi | 28.32Bst | 13.90Aefghijk | 19.10Bnopq | 6.39Aefghij | 7.4Ahijkl | 14.25Aq | 5.6Befghijk | 1.00Ajklmnopq | 0.67Babcdefgh |
| Es-25 | 15.45Aefghij | 41.97Bwx | 13.94Aefghijk | 26.22Btu | 5.81Adefghi | 11.76Bopq | 11.35Ap | 9.95Anop | 0.90Afghijklmn | 0.62Aabcdefg |
| Mean | 13.52A | 29.55B | 15.61A | 19.44B | 6.03A | 9.08B | 5.35A | 4.06B | 1.22A | 0.67B |
| S.D | 5.01 | 8.80 | 6.09 | 7.46 | 2.63 | 5.34 | 2.86 | 1.84 | 0.36 | 0.20 |
Note:
Different lowercase letters show significant differences (p < 0.05) among accessions while the different upper case letters show significant differences (p < 0.05) among treatments (Control vs. NaCl).
Salt-stressed group, compared with control, exhibited a moderate but statistically significant increase in K+ content (p < 0.05). The mean K+ content values for control and treatment groups were 15.61 ± 6.08 mg/g DW and 19.44 ± 7.46 mg/g DW, respectively (Table 4). Furthermore, in both control and treated groups, accessions showed significant variability in K+ content. The K+ content in control group varied from 6.95 to 33.40 mg/g DW while in treatment group from 9.74 to 41.30 mg/g DW (Table 4). In control as well as stressed group, Es-4 and Es-9 exhibited the lowest and the highest K+ content, respectively (Table 4).
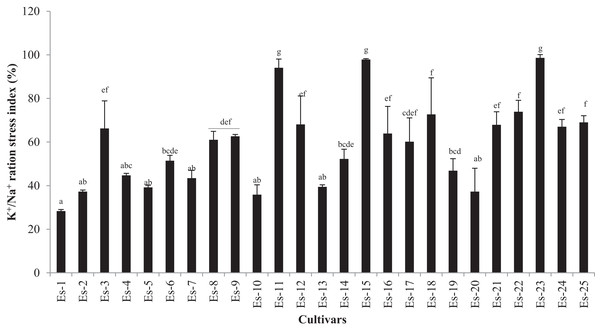
K+/Na+ ratio was significantly decreased in the salt-treated group as compared with control (p < 0.05). The mean K+/Na+ ratio in control and treatment groups was 1.22 ± 0.36 and 0.67 ± 0.2, respectively (Table 4). Accessions exhibited diverse trends for K+/Na+ ratio which ranged from 0.63 to 2.07 and 0.44 to 1.3 in control and treated plants, respectively (Table 4). To further distinguish the accessions in which K+/Na+ ratio was least affected by the salt stress, K+/Na+ ratio was expressed as the stress index. As shown in Fig. 4, accessions Es-23, Es-15 and Es-11 showed the highest while Es-1, Es-10 and Es-20 the lowest K+/Na+ ratio stress index (Fig. 4).
Figure 4: K+/Na+ ratio stress index of Eruca sativa accessions.
Stress index was calculated as ratio of stressed to control and expressed as percentage. Data are mean (±SD) of three replicates. Means with same letters are not significantly different tested by Duncan’s multiple range test at 5% level.We also determined two other important cations Ca2+ and Mg2+ in E. sativa accessions. Salt-stressed group accumulated significantly more Ca2+ than the control group (p < 0.05) (Control mean 6.03 ± 2.63 mg/g DW vs treated mean 9.08 ± 5.34 mg/g DW) (Table 4). Accessions, growing under controlled conditions as well as under NaCl treatment, showed significant variations in Ca2+ content (p < 0.05) (Table 4). Ca2+ content, in the control group varied from 2.61 to 13.47 mg/g DW while, for the treatment group, it varied from 2.87 to 23.17 mg/g DW (Table 4).
Salt-stressed group showed significantly low Mg2+ content compared with control group (p < 0.05). Mean Mg2+ content for the control group was 5.35 ± 2.86 mg/g DW while for the salt treatment group it was 4.06 ± 1.84 mg/g DW (Table 4). Significant variations in Mg2+ content were observed among accessions growing under controlled conditions as well as under NaCl treatment (Table 4). Mg2+ content, of the control group, ranged from 2.74 to14.25 mg/g DW while in the treatment group from 2.28 to 9.95 mg/g DW (Table 4).
Principal component analysis
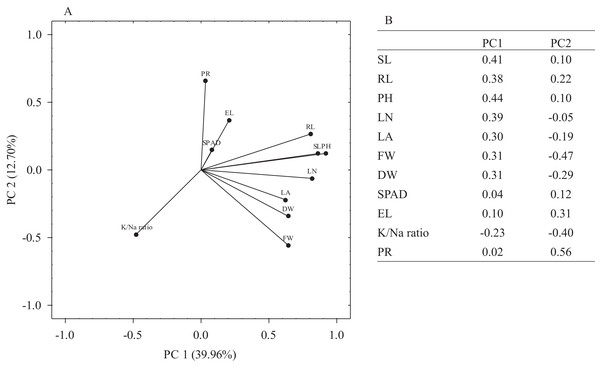
The principal component analysis (PCA) was performed on mean MFV values of morpho-physiological traits to understand the weightage of each trait towards observed variation. Overall, PCA1 and PCA2 represented 52.66% of the observed variation whereas individually PCA1 and PCA2 accounted for 39.96% and 12.7% of the variation, respectively (Fig. 5A). The most significant traits for PC1 were PH, SL, RL, LN, LA, FW and DW while for PC2, PR and EL were the most significant traits as these accounted for higher contributions towards PC2 (loadings ≥0.30). K+/Na+ ratio was the least significant trait for both PC1 and PC2 (Fig. 5B).
Figure 5: Principal component analysis (PCA).
(A) The loading plot of morpho-physiological traits used for the determination of salt stress tolerance in E. sativa accessions. (B) Eigenvalues of salt tolerance traits in PC1 and PC2.Ranking and grouping of E. sativa accessions for evaluation of salt tolerance
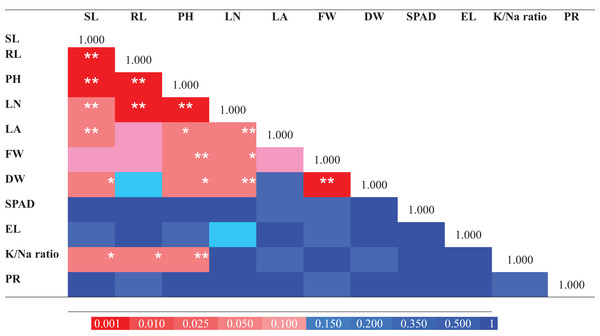
For determination of the extent of variation in salinity tolerance among E. sativa accessions, the data of morpho-physiological traits (for control and treatment) were standardized by calculating the STI which is the ratio of the value for the NaCl treated plant/value for the control. STI was then used for computing the MFV of E. sativa accessions. The following traits were used for MFV calculation: SL, RL, PH, LN, LA, FW, DW, SPAD, EL, K+/Na+ ratio and PR. Moreover, Pearson’s correlation analysis was done for the determination of the relationship among traits. As shown in Fig. 6A strong and highly significant correlations were found among morphological traits like RL, SL and PH (p < 0.001). Leaf attributes also showed strong correlations. LN showed significant correlation with PH, RL (p < 0.001), SL, LA, DW (p < 0.01) and FW (p < 0.05). LA exhibited a strong correlation with SL (p < 0.01) and PH (p < 0.05). FW and DW were also strongly correlated (p < 0.001). K+/Na+ ratio also exhibited significant correlations with PH, RL (p < 0.01) and SL (p < 0.05). Three of the tested traits EL, SPAD and PR did not show any significant correlation (Fig. 6).
Figure 6: Pearson correlation matrix of the salt tolerance traits from 25 Eruca sativa accessions grown under 0 mM and 150 mM NaCl.
The color scale represents the nature of correlation. The asterisks ***, ** and * show that correlation is significant at 0.001, 0.01 and 0.05 level, respectively.The ranking of the degree of salt tolerance among E. sativa accessions was done based on MFV. The higher mean MFV represents higher salt tolerance and vise versa. MFV data of the accessions are presented in Table 5. Mean and standard deviation (S.D) of overall MFV data were 0.43 and 0.11, respectively. The highest MFV score was observed for Es-11 (0.62, highly tolerant) while the lowest score for Es-3 (0.18, highly sensitive). Furthermore, E. sativa accessions were categorized into five standard groups according to the previously described criteria (Chen et al., 2012). Among 25 accessions, one accession was ranked as highly tolerant (MFV ≥ 0.62), four as tolerant (0.55 ≤ MFV < 0.62), fifteen as moderately tolerant (0.33 ≤ MFV < 0.55), four as sensitive (0.26 ≤ MFV < 0.33) while one accession as highly sensitive (MFV < 0.26). Accession Es-11 was ranked as highly tolerant while accessions Es-1, Es-9, Es-12 and Es-19 as tolerant. Es-3 was ranked as highly sensitive while Es-15, Es-16, Es-17 and Es-18 as sensitive. The remaining 15 accessions were placed in moderately tolerant group (Table 5).
| Accessions | SL | RL | PH | LN | LA | FW | DW | SPAD | EL | KNR | PR | Mean MFV | Category | Age (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Es-11 | 0.45 | 0.59 | 0.60 | 0.63 | 0.62 | 0.82 | 0.77 | 0.21 | 0.81 | 0.92 | 0.38 | 0.62 | HT | 4 |
| Es-19 | 0.38 | 0.69 | 0.67 | 0.70 | 0.55 | 0.77 | 0.69 | 0.35 | 0.75 | 0.26 | 0.87 | 0.61 | T | 16 |
| Es-12 | 0.59 | 0.76 | 0.75 | 0.49 | 0.26 | 0.92 | 0.77 | 0.37 | 0.77 | 0.56 | 0.39 | 0.60 | T | |
| Es-9 | 0.53 | 0.62 | 0.72 | 0.56 | 0.46 | 0.99 | 0.73 | 0.51 | 0.55 | 0.48 | 0.15 | 0.58 | T | |
| Es-1 | 0.70 | 0.64 | 0.82 | 0.43 | 0.51 | 0.54 | 0.76 | 0.31 | 0.93 | 0.01 | 0.36 | 0.55 | T | |
| Es-7 | 0.59 | 0.49 | 0.71 | 0.41 | 0.56 | 0.27 | 0.70 | 0.54 | 0.65 | 0.22 | NA | 0.51 | MT | 60 |
| Es-6 | 0.45 | 0.72 | 0.64 | 0.64 | 0.77 | 0.29 | 0.24 | 0.24 | 0.85 | 0.33 | 0.24 | 0.49 | MT | |
| Es-4 | 0.42 | 0.74 | 0.67 | 0.64 | 0.28 | 0.51 | 0.54 | 0.39 | 0.64 | 0.24 | 0.26 | 0.48 | MT | |
| Es-13 | 0.55 | 0.56 | 0.68 | 0.61 | 0.45 | 0.40 | 0.73 | 0.18 | 0.77 | 0.16 | 0.15 | 0.48 | MT | |
| Es-2 | 0.38 | 0.66 | 0.49 | 0.45 | 0.34 | 0.33 | 0.66 | 0.25 | 0.79 | 0.13 | 0.74 | 0.47 | MT | |
| Es-25 | 0.33 | 0.13 | 0.27 | 0.48 | 0.77 | 0.43 | 0.72 | 0.21 | 0.90 | 0.57 | 0.33 | 0.47 | MT | |
| Es-22 | 0.44 | 0.23 | 0.47 | 0.40 | 0.71 | 0.86 | 0.54 | 0.06 | 0.38 | 0.64 | 0.30 | 0.46 | MT | |
| Es-21 | 0.15 | 0.05 | 0.30 | 0.44 | 0.26 | 0.78 | 0.67 | 0.86 | 0.70 | 0.56 | 0.20 | 0.45 | MT | |
| Es-10 | 0.59 | 0.42 | 0.65 | 0.25 | 0.65 | 0.42 | 0.37 | 0.24 | 0.78 | 0.11 | 0.29 | 0.43 | MT | |
| Es-14 | 0.38 | 0.61 | 0.60 | 0.38 | 0.24 | 0.39 | 0.52 | 0.24 | 0.56 | 0.34 | 0.38 | 0.42 | MT | |
| Es-24 | 0.37 | 0.27 | 0.40 | 0.34 | 0.63 | 0.69 | 0.33 | 0.21 | 0.59 | 0.55 | 0.19 | 0.42 | MT | |
| Es-5 | 0.28 | 0.58 | 0.43 | 0.26 | 0.64 | 0.89 | 0.74 | 0.23 | 0.07 | 0.16 | 0.30 | 0.42 | MT | |
| Es-23 | 0.21 | 0.21 | 0.23 | 0.48 | 0.47 | 0.24 | 0.71 | 0.06 | 0.72 | 0.98 | 0.13 | 0.40 | MT | |
| Es-20 | 0.03 | 0.36 | 0.69 | 0.34 | 0.21 | 0.83 | 0.74 | 0.21 | 0.71 | 0.13 | 0.11 | 0.40 | MT | |
| Es-8 | 0.16 | 0.57 | 0.37 | 0.29 | 0.44 | 0.29 | 0.41 | 0.24 | 0.36 | 0.46 | 0.61 | 0.38 | MT | |
| Es-16 | 0.14 | 0.08 | 0.14 | 0.09 | 0.09 | 0.45 | 0.73 | 0.17 | 0.68 | 0.50 | NA | 0.31 | S | 16 |
| Es-15 | 0.03 | 0.34 | 0.30 | 0.14 | 0.10 | 0.07 | 0.23 | 0.16 | 0.70 | 0.97 | 0.27 | 0.30 | S | |
| Es-18 | 0.05 | 0.03 | 0.04 | 0.19 | 0.06 | 0.05 | 0.26 | 0.61 | 0.59 | 0.62 | 0.56 | 0.28 | S | |
| Es-17 | 0.10 | 0.09 | 0.14 | 0.08 | 0.18 | 0.10 | 0.23 | 0.18 | 0.67 | 0.45 | 0.77 | 0.27 | S | |
| Es-3 | 0.06 | 0.08 | 0.13 | 0.11 | 0.01 | 0.01 | 0.17 | 0.24 | 0.61 | 0.53 | 0.03 | 0.18 | HS | 4 |
Note:
HT, highly tolerant; T, tolerant; MT, moderately tolerant; S, sensitive; HS, highly sensitive.
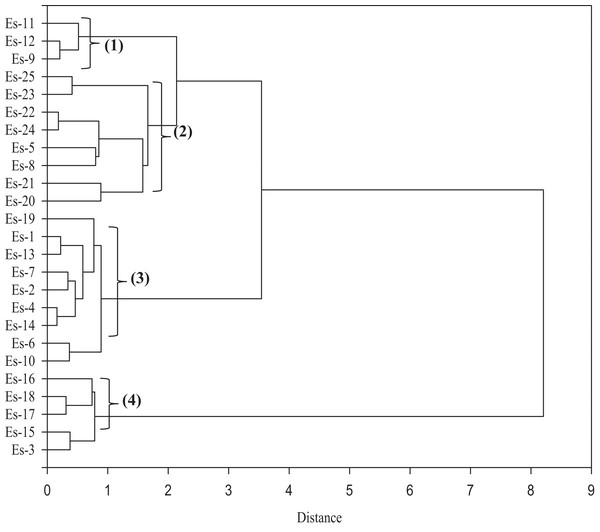
Furthermore, the MFV data was used to construct a similarity matrix based on Euclidean distance (Fig. 7). The accessions were separated into four distinct clusters; two smaller clusters (clusters 1 and 4) and two larger clusters (clusters 2 and 3). Clusters 1 and 4 consisted of three and five accessions, respectively, while clusters 2 and 3 consisted of eight and nine accessions, respectively. Es-9, Es-11 and Es-12 were placed in cluster 1. The members of cluster 2 included Es-5, Es-8 and Es-20 to Es-25. The largest cluster that is, cluster 3 comprised of the following members: Es-1, Es-2, Es-4, Es-6, Es-7, Es-10, Es-13, Es-14 and Es-19. Five accessions namely Es-3, Es-15, Es-16, Es-17 and Es-18 were included in cluster 4 (Fig. 7).
Figure 7: A dendrogram depicting the clustering of 25 Eruca sativa accessions based on Euclidean distance of salt stress tolerance-related traits.
Discussion
It is well known that salt stress negatively affects plant growth and development attributes like PH and biomass in many plant species resulting in significant yield losses (Munns & Tester, 2008; Arzani & Ashraf, 2016). E. sativa can grow in poor fertility lands and exhibit tolerance to various abiotic stresses including salinity (Ashraf, 1994; Shannon & Grieve, 1999; Garg & Sharma, 2014). In the current research, we thoroughly assessed the 25 E. sativa accessions to determine the extent of genetic variation in salt tolerance based on 11 morpho-physiological traits. The growth and development traits like RL, SL, PH, LN, LA, FW and DW were significantly reduced in stressed plants compared with control (Figs. 1 and 2; Table 1). The growth inhibition could be the result of ion toxicity, nutrients imbalance, osmotic stress, the decline in PR, and changes in SC (Munns & Tester, 2008; Fageria, Stone & Santos, 2012). The reduction in LN and LA under salt stress could be a physiological response of the plant to prevent excessive water loss (Parida & Das, 2005). Salt stress significantly reduced PH, shoot biomass and LA in six rocket genotypes (Petretto et al., 2019). Similar data regarding the effect of salt stress on growth parameters in E. sativa was also reported by Bakhshandeh et al. (2019). The data presented in the current research are in good agreement with the above-cited studies.
Relative water content is a key marker and could be applied as a simple indicator for screening plant salt tolerance. By maintaining RWC under stress conditions, plant resumes metabolic activities and continues growth and development in saline conditions (Slabbert & Krüger, 2014). In our study, mean RWC values for control and treated groups were not significantly different however moderate variations in RWC between control and its treated counterpart were observed for few accessions (Table 2). According to Ashraf (1994), RWC was not significantly different between the tolerant and sensitive E. sativa cultivars under salt stress. In another study, E. sativa cv Astro did not exhibit any significant change in RWC up to 100 mMol/L NaCl however it was significantly reduced at 200 and 300 mmol/L NaCl (Hniličková et al., 2017). On the other hand, in a study by Al Gehani & Ismail (2016), significant changes in RWC in E. sativa were evident at 40 mMol/L NaCl. In the light of our data and above-cited studies, it seems that although E. sativa has a general tendency to maintain RWC under moderate salt stress however significant variation in this trait also seems to exist.
The EL can be triggered by different stresses including salinity. These stresses lead to increased ROS production which damages cell membrane causing more EL (Demidchik et al., 2003, 2010). It is regarded as one of the main selection criteria for identifying salt tolerant plants (Ashraf & Ali, 2008). ROS can be detoxified in plants by up-regulating the antioxidant enzymes and by non- enzymatic antioxidants. According to our data, only a minute increase in EL was observed for few accessions in salt stress (which is evident from treated/ control EL ratio close to 1 for most of the accessions) (Fig. 3). The mean EL ratio for all the accessions was 1.07 which shows that plants might not be undergoing any extensive membrane damage. It seems that E. sativa accessions exhibit a good level of tissue tolerance and protect the membrane from any significant damage.
It is generally believed that salt stress causes a decrease in chlorophyl content (Khoshbakht, Ramin & Baninasab, 2015; Qiu et al., 2017). Some reports have, however, shown an increase in chlorophyl content for a few plant species under salt stress (Stefanov et al., 2016; Shah, Houborg & McCabe, 2017). In current study we observed an overall reduction in chlorophyl content in treatment group compared with control. As presented in Table 2, the mean chlorophyl content of the stressed group was significantly lower than control (p < 0.05). However, at accession level response was variable; in most of the accessions (68%) salt stress triggered a decline in chlorophyl content while in 16% accessions chlorophyl did not significantly change (Table 2). The remaining 16% accessions exhibited an increase in chlorophyl content under salt stress (Table 2). According to these data, although a decline in chlorophyl content was a general trend however, at accession level, the response was variable.
It is known that TR, SC, intercellular CO2 concentration and PR are negatively affected by salt stress (Sudhir & Murthy, 2004; Chaves, Flexas & Pinheiro, 2009; Ullah et al., 2019). Salt stress induced inhibition of photosynthesis could be the result of stomatal as well as nonstomatal factors (Xu & Zhou, 2008; Saibo, Lourenço & Oliveira, 2009). The latter include inhibition of Rubisco, reduced regeneration of Ribulose-1,5- bisphosphate, and hindrances in internal CO2 conductance (Von Caemmerer & Farquhar, 1981). In some cases, non-stomatal factors are more responsible for the decrease in photosynthesis (Jacob & Lawlor, 1991). We have shown that PR, SC, and TR were markedly reduced in salt-stressed plants while Ci was the least affected gas exchange parameter (Table 3). A comparatively less pronounced effect of salinity on Ci shows that possibly non-stomatal factors could be the major contributors to PR reduction in E. sativa accessions. If this was not the case, we might have observed a higher decline in Ci along with other gas exchange parameters. Similar findings have been reported in rice wherein significantly higher Na+ accumulation in leaves reduced SC and photosynthesis without significantly affecting the Ci (Yeo et al., 1991). Hniličková et al. (2017) reported a significant reduction in all gas exchange parameters by salt stress in E. sativa. Interestingly Ci remained unaffected in salt-stressed plants till 100 mM/L NaCl but was significantly reduced at higher concentrations (above 200 mM/L NaCl) (Hniličková et al., 2017). Since we used 150 mM NaCl in our study, it could be possible that Ci reduction takes place at higher NaCl concentrations. Overall, our findings are in agreement with those reported by Hniličková et al. (2017).
It has been reported that exposure to NaCl disturbs the homeostasis of cations like Na+, Mg+2, Ca+2 and K+ in plants (Rabie, Aboul-Nasr & Al-Humiany, 2005). Na+ and K+ homeostasis is crucial for plants salt tolerance (Chen et al., 2007). Most of the plant species are susceptible to elevated concentrations of Na+ because it results in ion toxicity and osmotic stress (Horie, Hauser & Schroeder, 2009). It is also well known that K+ is the most crucial monovalent cation for many plant processes (Benito et al., 2014). Salt tolerant plants have efficient mechanisms in place for the sequestration of Na+ or the elevation of cytoplasmic K+ levels relative to Na+ (Gierth & Mäser, 2007; Horie, Hauser & Schroeder, 2009). Our data shows that, compared with the control group’s mean, about two-fold higher Na+ accumulated in salt treated group (Table 4). These data show that E. sativa could be an includer type and it might possess an active Na+ tolerance mechanism. We have reported a moderate but statistically significant increase in K+ concentration in the salt-treated group as compared with control (Table 4) which shows that possibly E. sativa possesses mechanisms for retaining/ accumulating K+ under salt stress. It has been reported that barley accessions actively retain K+ under salt stress (Genc, McDonald & Tester, 2007). Salt stress-induced K+ elevation has also been previously reported for a tolerant wheat genotype, barley, watermelon and quinoa (Shabala et al., 2010; Orsini et al., 2011; Hariadi et al., 2011; Adolf, Jacobsen & Shabala, 2013; Ekbic et al., 2017; Dugasa et al., 2018). Tissue tolerance to Na+ accumulation and K+ retention under salt stress could be important factors for salt tolerance in E. sativa.
Plants are known to exhibit diversity in salt stress tolerance (Sabir, Ashraf & Akram, 2011; Al-Ashkar et al., 2019; Wu et al., 2019; Mohamed et al., 2020; Benabderrahim, Guiza & Haddad, 2020). E. sativa has been reported to exhibit significant genetic diversity (Slater, 2013). The evaluation of different E. sativa genotypes holds great promise for future breeding programs (Petretto et al., 2019). In the current research, we thoroughly characterized 25 E. sativa accessions to determine the extent of genetic variation in salt tolerance based on 11 morpho-physiological traits. Significant inter-accession variability was observed in all the tested traits (Table 5). Different traits showed different rankings of accessions for salt tolerance, indicating genetic diversity among E. sativa accessions. The contribution of the individual traits in observed variability could not be necessarily the same; few traits could be more contributing than others toward overall variability. PCA is an effective tool for dissecting the traits according to their contribution in variability and has been applied to screen the traits in previous studies on salt tolerance (Barhoumi, 2019; Al-Ashkar et al., 2019; Benabderrahim, Guiza & Haddad, 2020; Efisue & Dike, 2020). In the current research, PCA identified PH, SL, RL, LN, LA, FW, DW, PR and EL as the most significant traits (loadings ≥ 0.30) since these accounted for maximum inter-accession variation (Fig. 5). These traits could, therefore, be used for any larger scale E. sativa germplasm screening in the future.
For screening of salt tolerance, different methods based on multiple trait results have been used (Bandeh et al., 2018; Hussain et al., 2013; Long et al., 2013). Among these, MFV is an effective screening method (Mohamed et al., 2020), which has been previously applied for ranking germplasm for drought tolerance (Chen et al., 2012) and salt tolerance (Wu et al., 2019). In the current study, 25 E. sativa accessions were ranked according to the degree of salt tolerance (as reflected by their MFV score). As shown in Table 5, 4% of the accessions were ranked as highly tolerant, 16% as tolerant, 60% as moderately tolerant, 16% as sensitive and 4% as highly sensitive. Moreover, the results of cluster analysis were largely in agreement with the MFV-based categorization (Fig. 7; Table 5). Interestingly, all the sensitive/ highly sensitive accessions were placed together in cluster 4. Similarly, one highly tolerant and two tolerant accessions were placed in cluster 1. All the tolerant/ moderately tolerant accessions were placed in clusters 2 and 3 (Fig. 7; Table 5).
Conclusions
Salt stress significantly affected most of the morpho-physiological traits. We identified a few traits which could be most effective in resolving the inter-accession variability. Using the MFV of tested traits, E. sativa accessions were ranked according of their salt tolerance. The accessions exhibited significant variations in the extent of salt stress tolerance. The tolerant accessions could be good material for future breeding programs aiming to improve salt tolerance. Further insights into the salt tolerance mechanism could be gained through carrying out the comparative transcriptomics in highly tolerant and sensitive accessions under salt stress conditions.
Supplemental Information
Details of Eruca sativa accessions used in current study.
*D.I. Khan; Dera Ismail Khan, KPK; Khyber Pakhtunkhwa