Estimating intraspecific genetic diversity from community DNA metabarcoding data
- Published
- Accepted
- Received
- Academic Editor
- Donald Baird
- Subject Areas
- Biogeography, Bioinformatics, Molecular Biology, Freshwater Biology
- Keywords
- Metabarcoding, High-throughput sequencing, Population genetics, Haplotyping, Ecosystem assessment, Exact sequence variant, CO1
- Copyright
- © 2018 Elbrecht et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Estimating intraspecific genetic diversity from community DNA metabarcoding data. PeerJ 6:e4644 https://doi.org/10.7717/peerj.4644
Abstract
Background
DNA metabarcoding is used to generate species composition data for entire communities. However, sequencing errors in high-throughput sequencing instruments are fairly common, usually requiring reads to be clustered into operational taxonomic units (OTUs), losing information on intraspecific diversity in the process. While Cytochrome c oxidase subunit I (COI) haplotype information is limited in resolving intraspecific diversity it is nevertheless often useful e.g. in a phylogeographic context, helping to formulate hypotheses on taxon distribution and dispersal.
Methods
This study combines sequence denoising strategies, normally applied in microbial research, with additional abundance-based filtering to extract haplotype information from freshwater macroinvertebrate metabarcoding datasets. This novel approach was added to the R package “JAMP” and can be applied to COI amplicon datasets. We tested our haplotyping method by sequencing (i) a single-species mock community composed of 31 individuals with 15 different haplotypes spanning three orders of magnitude in biomass and (ii) 18 monitoring samples each amplified with four different primer sets and two PCR replicates.
Results
We detected all 15 haplotypes of the single specimens in the mock community with relaxed filtering and denoising settings. However, up to 480 additional unexpected haplotypes remained in both replicates. Rigorous filtering removes most unexpected haplotypes, but also can discard expected haplotypes mainly from the small specimens. In the monitoring samples, the different primer sets detected 177–200 OTUs, each containing an average of 2.40–3.30 haplotypes per OTU. The derived intraspecific diversity data showed population structures that were consistent between replicates and similar between primer pairs but resolution depended on the primer length. A closer look at abundant taxa in the dataset revealed various population genetic patterns, e.g. the stonefly Taeniopteryx nebulosa and the caddisfly Hydropsyche pellucidula showed a distinct north–south cline with respect to haplotype distribution, while the beetle Oulimnius tuberculatus and the isopod Asellus aquaticus displayed no clear population pattern but differed in genetic diversity.
Discussion
We developed a strategy to infer intraspecific genetic diversity from bulk invertebrate metabarcoding data. It needs to be stressed that at this point this metabarcoding-informed haplotyping is not capable of capturing the full diversity present in such samples, due to variation in specimen size, primer bias and loss of sequence variants with low abundance. Nevertheless, for a high number of species intraspecific diversity was recovered, identifying potentially isolated populations and taxa for further more detailed phylogeographic investigation. While we are currently lacking large-scale metabarcoding datasets to fully take advantage of our new approach, metabarcoding-informed haplotyping holds great promise for biomonitoring efforts that not only seek information about species diversity but also underlying genetic diversity.
Introduction
High-throughput analysis of DNA barcodes retrieved from environmental samples, i.e. DNA metabarcoding, allows for the rapid and standardized assessment of community composition without the need for morpho-taxonomy (Taberlet et al., 2012a; Creer et al., 2016). This new surge of data enables biodiversity surveys at speeds and scales that were previously inconceivable in ecological and evolutionary studies. While the approach has major strengths and is generally regarded as a game changer for ecological research (Creer et al., 2016), it still has limitations such as the fact that sequences are typically clustered into operational taxonomic units (OTUs, Fig. S1) thereby ignoring any intraspecific sequence variation (Callahan, McMurdie & Holmes, 2017). However, clustering is often used to reduce the influence of PCR and sequencing errors that can otherwise generate false OTUs (Edgar, 2013). The inability to detect sequence variation within OTUs hampers our ability to detect impacts at population level. Simultaneous assessment of inter- and intraspecific diversity, however, represents a leap forward in ecological research and management because haplotype data are direct proxies for spatio-temporal dynamics of populations and both parameters can differ substantially (Taberlet et al., 2012b). In particular the assessment of fragmentation (Weiss & Leese, 2016) or changes in population size in response to environmental impacts are key areas of basic and applied ecological research (Sutherland et al., 2012). For management, this parameter is also important because genetic variation is typically lost long before species or OTUs disappear (Bálint et al., 2011). Unfortunately, methods to extract haplotype information from metabarcoding datasets are generally not widely available and thus most studies are based on single-specimen analyses. Some of those are based on denoising algorithms capable of distinguishing between true haplotypes and sequencing noise (Tikhonov, Leach & Wingreen, 2015; Eren et al., 2015; Edgar, 2016; Callahan et al., 2016; Amir et al., 2017) and have been tested for microbial samples (Eren et al., 2015; Callahan et al., 2016; Needham, Sachdeva & Fuhrman, 2017). Wares & Pappalardo (2016) suggested that haplotype information in metazoan datasets can be used to, for instance, improve taxa abundance estimates, which was successfully demonstrated with freshwater fish fecal samples (Corse et al., 2017). Recent studies were also able to infer haplotypes with metabarcoding for single specimens (Shokralla et al., 2014), arthropod bulk samples (Elbrecht & Leese, 2015; Pedro et al., 2017) and environmental water samples (Sigsgaard et al., 2016), all highlighting the possibility to extract sequence variant information within OTUs when targeting metazoan taxa.
We here further explored bioinformatics strategies in order to unlock the potential of metabarcoding-based haplotyping of entire and complex metazoan communities. We combined stringent quality filtering of reads with the recently developed unoise3 denoising strategy (Edgar, 2016) and calibrated this approach using a previously characterized single-species mock sample composed of specimens with known haplotypes (Elbrecht & Leese, 2015; Vamos, Elbrecht & Leese, 2017). Subsequently, we used multi-species metabarcoding data from 18 sample sites that were part of a large-scale governmental freshwater macroinvertebrate biomonitoring program (Elbrecht et al., 2017). These were denoised with the developed strategy and we tested the potential to detect intraspecific variation over a broad geographic gradient across multiple taxa.
Materials and Methods
We tested our haplotyping strategy on two available DNA metabarcoding datasets, (1) a single-species mock sample containing 31 specimens with known haplotypes from an earlier population genetics project (Elbrecht et al., 2014; Vamos, Elbrecht & Leese, 2017) and (2) a multi-species macroinvertebrate community dataset from the Finnish governmental stream monitoring program (Elbrecht et al., 2017). Haplotypes were determined by bidirectional Sanger sequencing for the single-species mock samples (Elbrecht et al., 2014), while the multi-species sample was metabarcoded on Illumina systems using several primer sets (Elbrecht & Leese, 2015, 2017; Vamos, Elbrecht & Leese, 2017). Resulting OTU centroids were assembled into haplotypes as described in Elbrecht & Leese (2017). The samples were sequenced for a region nested within the classical Folmer Cytochrome c oxidase subunit I (COI) region (Folmer et al., 1994) with two replicates each. The single-species sample was sequenced using a short primer set amplifying 178 bp (Vamos, Elbrecht & Leese, 2017), while the multi-species monitoring samples were amplified using four different primer sets targeting a region of up to 421 bp (Elbrecht & Leese, 2017). Paired-end sequencing (250 bp) was performed on Illumina MiSeq and HiSeq systems with great sequencing depth (on average 1.53 million reads per sample, SD = 0.29).
To extract individual haplotypes from the metabarcoding datasets, we used strict quality filtering followed by denoising (unoise3 Edgar, 2016, with additional threshold-based filtering steps, see Fig. 1B). The full metabarcoding and haplotyping pipelines are available as part of the “Just Another Metabarcoding Pipeline” (JAMP) R package (https://github.com/VascoElbrecht/JAMP), which uses Usearch v10.0.240 (Edgar, 2013), Vsearch v2.4.3 (Rognes et al., 2016) and Cutadapt 1.9 (Martin, 2011) for most of the data processing. The advantage of the JAMP wrapper is its modularity and the automated generation of additional summary statistics and extended quality filtering options. All pipeline commands used are also available as Supporting Information (Fig. S2, Scripts S1, JAMP v0.28). In short, pre-processing of reads involved sample demultiplexing, paired-end merging, primer trimming, generation of reverse complements where needed (to align all reads in the forward direction), maximum expected error (ee) filtering = 0.5 (Edgar & Flyvbjerg, 2015), only keeping reads of exact length targeted by the respective primer set and subsampling to 1 and 0.4 million reads, respectively, to generate the same sequencing depth for the single-species and multi-species samples. To further reduce the amount of sequences affected by sequencing errors, we discarded sequences below 10 reads or 0.001% abundance in each sample and applied read denoising with unoise3 after pooling all samples as implemented in Usearch (Edgar, 2016) using only reads with ≥10 abundance in each sample after dereplication. Different ee cutoffs and alpha values were tested, with ee = 0.5 and alpha = 5 being used for the final analysis of the 18 monitoring samples. With lower ee values, more low quality sequences were discarded (Edgar & Flyvbjerg, 2015). Similarly, lower alpha values led to more strict denoising with unoise3 (Edgar, 2016).
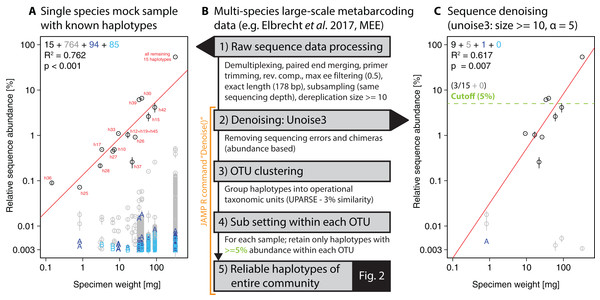
Figure 1: Overview of DNA metabarcoding data of a single-species mock sample containing specimens with 15 distinct haplotypes amplified with the fwh1 primer set (black circles), with red numbers above each circle showing the original 31 haplotypes using the full 658 bp barcoding region (Elbrecht & Leese, 2015; Vamos, Elbrecht & Leese, 2017).
Detected haplotypes (unexpected ones shown in grey and blue) are plotted against specimen biomass for the processed data (A) and followed by read denoising using unoise3 (C). Denoising was applied to both replicates individually, with a circle if the read was detected in both samples (error bar = SD) and “A” or “B” if the read was found in only one replicate. For processing of the multi-species samples (B, Fig. 2), all samples were pooled and jointly denoised, followed by OTU clustering and read mapping, then followed by discarding of haplotypes below a 5% threshold within each sample.For the single-species mock sample, the denoised and quality filtered reads (prior to denoising) were mapped against the expected 15 haplotype sequences using Vsearch (Rognes et al., 2016). The unoise3 implementation in the JAMP package adds additional threshold-based filtering after the denoising step, which we used for the Finnish multi-species monitoring samples in order to discard haplotypes with less than 0.01% abundance in at least one sample and OTUs with less than 0.1% abundance in at least one sample (“Denoise(…, minhaplosize = 0.01, OTUmin = 0.1)”). All read mapping steps of denoised data were done with Vsearch. Additionally, within each OTU and sample site, only haplotypes with at least 5% abundance per sample were considered for generating haplotype maps and networks, in order to exclude low abundance OTUs, which can be difficult to separate from PCR artifacts and sequencing errors (withinOTU = 5). The Denoise function also includes presence-based filtering for larger datasets, requiring a specific haplotype or OTU being present in a minimum number of samples (minHaploPresence = 1 or minOTUPresence = 1). However, as we had only 18 sample sites available, this filtering was not applied to the dataset.
Results
Our approach starts with denoising of quality filtered reads using unoise3 (Edgar, 2016) followed by an additional threshold-based filtering step which includes OTU clustering of denoised reads (Edgar, 2013) and the removal of low abundant OTUs/haplotypes (see Fig. 1B). We validated this approach by using a single-species mock community of known haplotype composition (Elbrecht & Leese, 2015), in which we found 943 unexpected haplotypes above 0.003% abundance with no ee filtering applied (Fig. 1A). Filtering the raw sequence data with different quality thresholds (max ee, Edgar & Flyvbjerg, 2015) reduced the number of unexpected haplotypes by only up to 10.22% (Fig. S3). The consistency between the two independent sequencing replicates indicates that a major fraction of the detected haplotypes represent in fact real biological signal (e.g. somatic mutations, numts or heteroplasmy (Bensasson et al., 2001; Shokralla et al., 2014)), which is difficult to differentiate from PCR and sequencing errors. Even after using different alpha values for the unoise3 algorithm some unexpected sequence variants remained (Fig. S4). An error filtering of max ee = 0.5 in combination with an alpha of 5 was chosen for subsequent analysis (Fig. 1C), as it offers the best trade-off between expected and unexpected haplotypes (nine of 15 expected, six unexpected with low abundance), while retaining 67.08% (SD = 17.69%) of the original sequence data after quality filtering and before denoising.
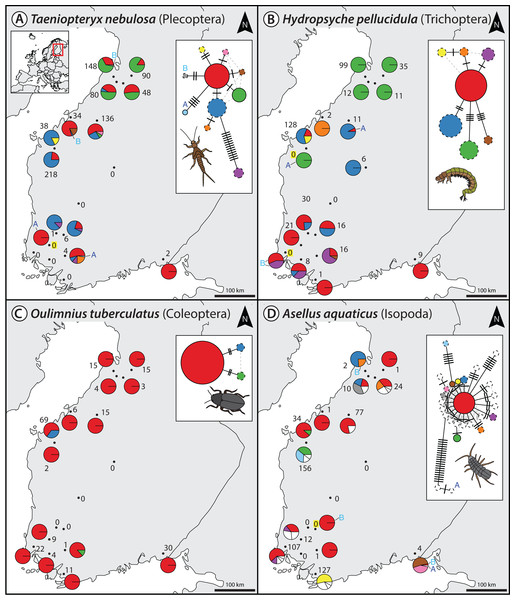
For the denoising of our multi-species monitoring samples, additional and more conservative filtering steps were introduced to ensure only true sequence variants are included in the analysis (discarding low abundant OTUs and haplotypes below 0.1% and 0.01%, as well as haplotypes below 5% read abundance within each OTU of the respective sample, Fig. 1C green line). Denoising of metabarcoding data from 18 macroinvertebrate samples of the Finnish stream monitoring programme, recovered 177–200 OTUs containing 534–646 haplotypes (on average 2.40–3.30 haplotypes per OTU, SD = 2.13–3.26) for the different primer pairs (Table S1). Most OTUs were only present in a few sample locations, allowing for only limited analyses of intraspecific genetic variation patterns (Fig. S5, see also Fig. S7 in Elbrecht et al., 2017). Figure 2 shows some examples of haplotype diversity and geographic distribution for more common and widely distributed taxa in this study. For Taeniopteryx nebulosa (Plecoptera) and Hydropsyche pellucidula (Trichoptera) we found distinct patterns of latitudinal variation in haplotype composition (Figs. 2A and 2B), while Oulimnius tuberculatus (Coleoptera) showed low genetic variation across all primer combinations (Fig. 2C; Fig. S6). Asellus aquaticus (Isopoda) on the other hand showed very high genetic diversity and several probably regionally endemic haplotypes (Fig. 2D).
Figure 2: Haplotype maps and networks extracted from multi-species monitoring metabarcoding datasets amplified with the BF2+BR2 primer set for four abundant macroinvertebrate taxa (A = Taeniopteryx nebulosa, B = Hydropsyche pellucidula, C = Oulimnius tuberculatus, D = Asellus aquaticus).
Numbers next to each sampling site indicate sample size of the respective taxa based on morphological identification in a sample (Elbrecht et al., 2017). Conflicts between DNA and morphology-based detections are highlighted in yellow. Haplotype frequency composition per site is indicated by pie charts. For A. aquaticus only the 10 most common haplotypes are visualised with different colors (remaining ones in white). Each crossline in a network represents one base pair difference between the respective haplotypes. Dashed lines around a circle indicate novel haplotypes that were not available in the BOLD reference database. An “A” or “B” next to a haplotype in the map or network indicates the presence of this haplotype only in one replicate. Shapefile-data© OpenStreetMap contributors, licensed under Creative Commons 2.0 (CC BY-SA).Extracted haplotype patterns between replicates were highly reproducible (R2 = 0.751, SD = 0.242), while at the same time recovering more sequence variants with longer amplicons (Fig. S6). Taxon occurrence for the four taxa analyzed in detail matched morphology based identifications (Elbrecht et al., 2017) in most cases (only four false positive detections, Fig. 2). The few inconsistencies between replicates in haplotypes and taxa occurrence are mostly affecting low abundance reads. In the sequence alignments, all four primer sets shared most of the variable positions (Fig. S6).
Discussion
In this case study, we first developed and demonstrated a bioinformatic strategy to process metabarcoding data using a controlled single-species approach, in order to then extract intraspecific genetic diversity information from complex multi-species metazoan environmental samples. While our multi-species dataset was limited to only 18 sampling sites, and many taxa were not widely distributed (Elbrecht et al., 2017), we could still infer COI-based population genetic patterns for some of the abundant and more widespread taxa. Where available, observed population genetic patterns were also consistent with previous studies, e.g. earlier work reported high genetic diversity for A. aquaticus (Sworobowicz et al., 2015). Other published work, e.g. on H. pellucidula (Múrria et al., 2010) and O. tuberculatus (Čiampor & Kodada, 2010), was too limited in sampling size and region for a proper comparison.
Deriving haplotypes from metabarcoding data does not require specialized field or laboratory protocols, as existing data is analyzed. And while our dataset is very limited with just 18 sample sites, there are efforts underway to implement DNA metabarcoding-based monitoring of stream water quality in Europe, potentially generating HTS data for thousands of sample sites every year (Leese et al., 2016). Such haplotype data, even though limited in resolution and based only on a single gene marker, could be used to test or derive hypotheses about taxa dispersal and distribution at an unprecedented scale (Hughes, Schmidt & Finn, 2009), which would be highly beneficial for basic research but also ecological restoration and management of aquatic ecosystems.
While the detection of haplotypes from bulk samples was demonstrated in this and other studies (Sigsgaard et al., 2016; Corse et al., 2017; Pedro et al., 2017), the limitations of metabarcoding-based haplotyping remain relatively unexplored. Metabarcoding datasets can be affected by primer bias (Elbrecht & Leese, 2015), tag switching (Esling, Lejzerowicz & Pawlowski, 2015; Schnell, Bohmann & Gilbert, 2015), as well as PCR and sequencing errors (Nakamura et al., 2011; Tremblay et al., 2015). Such issues can lead to artificial haplotypes, which are usually sufficiently different to distinguish them from actual haplotypes in the samples, especially if they are less abundant and thus likely influenced by stochastic effects (Leray & Knowlton, 2017). We applied very strict quality filtering in our pipeline, and cautiously discarded all haplotypes below 5% abundance within an OTU. This is necessary, as low abundant haplotypes cannot be separated from sequencing errors (Nakamura et al., 2011; Tremblay et al., 2015), somatic mutations (Shokralla et al., 2014) and other noise in the data, as we have shown for the single-species mock samples. Strict filtering will remove rare and low abundant haplotypes, but it is necessary to reduce the amount of false positive artificial sequences that result from the currently rather high error rates of HTS instruments. Even with such strict filtering settings, we cannot be fully confident that all false haplotypes were excluded, e.g. as the result of undetected chimeric sequences (Edgar et al., 2011) or systematic sequencing errors (Nakamura et al., 2011; Schirmer et al., 2015; Schirmer, 2016) that likely persist across replicates. Approaches relying on the comparison of replicate samples could be an appropriate strategy in particular when working with unicellular organisms (Lange et al., 2015). However, for our metazoan communities many variants were found in both replicates (Fig. 1). Macroinvertebrate communities can vary considerably in biomass, which means rare and small specimens will be underrepresented when extracting DNA from bulk samples (Elbrecht, Peinert & Leese, 2017). Thus, taxa in the sample are sequenced at different sequencing depth, which likely has an influence on the amount of false haplotypes detected within each OTU. Additionally, differences in specimen biomass can skew the detection of haplotypes, as only those of large specimens will be retained in bioinformatics analysis (haplotypes of small specimens are likely below 5% abundance). Such uncertainties need to be considered when performing population genetic analyses, which are usually done at specimen level, with the exact number of specimens and haplotypes known for each sampling site. It has to be emphasized that at this point metabarcoding-based haplotyping only provides very limited information of genetic diversity and phylogeography of a given taxon. However, interesting patterns emerging from such studies can be subsequently explored by re-collecting taxa of interest and using standard population genetic markers with a higher resolution (e.g. microsatellites or ddRAD Peterson et al., 2012). Our study demonstrates the feasibility and potential of metabarcoding data for the investigation of population genetic patterns of entire complex environmental communities. The shortcomings and the level of resolution of this novel approach need to be carefully tested (e.g. by constructing mock samples using synthesized DNA). Additionally, more bioinformatics approaches suited for the analysis of metazoan bulk samples need to be developed, especially with respect to variation in specimen biomass (Elbrecht, Peinert & Leese, 2017). Furthermore, most software currently used in this field was developed for microbial samples and should therefore be further tested and benchmarked for its feasibility in studies involving eukaryotes. Despite the clear limitations of this haplotyping approach, we are confident that it will be useful in future large-scale studies of genetic diversity. While metabarcoding studies will remain affected by sequencing errors (potentially leading to false haplotypes), we expect that most of these issues can be mitigated by increasing the number of sampling sites to several hundred or even thousands. For large-scale efforts such as routine monitoring using metabarcoding (Baird & Hajibabaei, 2012; Gibson et al., 2015; Elbrecht et al., 2017), this might soon become a feasible option if not standard. Additionally, references databases should be further completed and extended to cover a large geographic range in order to assign species names and ground truth the detected haplotypes (Carew et al., 2017; Curry et al., 2018).
Conclusion
Our study demonstrates that haplotypes can be extracted from complex metazoan metabarcoding datasets. This proof of concept work already shows emerging population genetic patterns for a few species, but more large-scale validation studies are needed to explore the limitations and the potential of metabarcoding-based haplotyping. While some shortcomings such as occasional false positive detections and loss of rare and small taxa for such complex communities are difficult to overcome on a per sample bases, these might be partly offset by studying comparative patterns of intraspecific variation across many taxa and sites. As metabarcoding becomes more accessible and larger DNA-based biodiversity assessment and monitoring initiatives emerge, sampling and extracting haplotypes from hundreds of sites might become a feasible path of future research.
Supplemental Information
Figure S1: Schematic overview of errors affecting metabarcoding data and clustering/denoising strategies to reduce them.
Overview of the metabarcoding process, with key biases potentially affecting sequence accuracy (shown in red). In the bulk sample (A) several species with different biomass (indicated by circle size) and distinct haplotypes (indicated by colour) are present. After tissue homogenization and DNA extraction the COI marker is amplified using PCR (B), which can not only skew sequence abundance but also fail to amplify taxa due to primer bias (Elbrecht & Leese, 2015) or insufficient sequencing depth in the case of underrepresented/rare taxa (Elbrecht, Peinert & Leese, 2017). In the process of HTS (C) many new false sequence variants are generated due to sequencing errors (Schirmer et al., 2015), chimera formation (Edgar et al., 2011) and mixing of multiplexed samples (Esling, Lejzerowicz & Pawlowski, 2015; Schnell, Bohmann & Gilbert, 2015). The impact of these errors is usually reduced by strict quality filtering and clustering of similar sequences into operational taxonomic units (OTUs). Normally, only the most abundant sequence in an OTU is considered and used to identify the respective species, which in turn means that information on genetic diversity is lost (Callahan, McMurdie & Holmes, 2017) (D). Recently alternative denoising strategies have been developed to remove sequences affected by errors from a dataset and retain the actual haplotype sequences present in a sample (Eren et al., 2015; Edgar & Flyvbjerg, 2015; Callahan et al., 2016; Amir et al., 2017). Figure based on Figure S1 in Callahan et al., 2016.
Figure S2: Overview of the haplotyping strategy used here and their implementation in the JAMP R package.
Detailed bioinformatic processing of metabarcoding to extract haplotype sequences using the JAMP R package. A) Metabarcoding raw data is processed and quality filtered. These steps are integrated in JAMP, but most other standard metabarcoding pipelines could be used as well. B) The processed and quality filtered samples from step A would be usually clustered into operational taxonomic units, but are here additionally filtered (retaining reads of only the expected amplicon length and discarding reads of low abundance) and then denoised. C) In denoising with usearch unoise3 the strictness of denoising is controlled by the alpha value (low alpha = less noise, however more true haplotypes get discarded). D) The denoised reads (=haplotypes) are clustered into OTUs grouped by similarity and the abundance of each haplotype for each sample is exported in a table. E) The haplotype table is additionally filtered using different thresholds, to reduce the presence of low abundant OTUs and haplotypes and increase data reliability. F) The final filtered haplotype table can be used for phylogeographic and population genetic analysis.
Figure S3: Effect of different quality filtering (max ee) on reads of the single species mock sample.
Effect of different expected error filtering thresholds on haplotype recovery (no denoising applied). All filtered reads are mapped against the expected haplotypes (black circles). Not all reads are shared between both replicates (indicated by A or B instead of a circle). The 15 expected haplotypes are shown in black, while unexpected ones are highlighted in gray or blue. Error bars show the standard deviation of relative read abundance between both replicates, for the respective haplotype.
Figure S4: Effect of different alpha values in read denoising of the single-species mock sample.
Effect of different haplotype recovery of in the single species mock sample, when using different alpha values with Unoise3 (as integrated in the JAMP package). Not all reads are shared between both replicates (indicated by A or B instead of a circle). The 15 expected haplotypes are shown in black, while unexpected ones are highlighted in gray or blue. Error bars show the standard deviation of relative read abundance between both replicates, for the respective haplotype.
Figure S5: Bar plots of haplotype distribution within each OTU.
Bar plots showing the haplotype composition of all 199 OTUs obtained with the BF2+BR2 primer combination. The OTU number is indicated above each bar, with the four taxa shown in Figure 2 being highlighted. Haplotypes are shown in different colours, with white bars indicating the proportion of sites where the respective OTU was not detected. Most OTUs were only present at a few sample sites.
Figure S6: Detailed plots of four taxa from the denoised multi-species monitoring samples, showing haplotype maps & networks, similarity between replicates and sequence alignments.
Detailed haplotype maps, networks and sequence alignment for all 4 primer combinations and replicates of selected taxa. a) Haplotype maps for both replicates for each of the four primer combinations. For A. aquaticus only the 10 most common haplotypes are shown in different colours (remaining ones in white). For each primer combination, the haplotypes in the map and network have the same corresponding colours. b) Haplotype networks for each primer pair. Each cross line represents one base pair difference between the respective haplotypes. Haplotypes present in just one replicate are indicated by A or B next to the network node. Dashed lines around a circle indicate novel haplotypes that were not available in the BOLD reference database. c) Quantification of similarity between both replicates, by plotting abundance of individual haplotypes of each sampling point against each other. The red line indicates the best fit (with significance and adjusted R2 value given in each plot). d) Sequence alignment of all haplotypes, with mismatching nucleotides between sequences highlighted (green = T, red = A, yellow = G and blue = C). See the following pages for example plots of: Page 2: Taeniopteryx nebulosa Page 3: Hydropsyche pellucidula Page 4: Oulimnius tuberculatus Page 5: Asellus aquaticus.