Genome-scale identification of plant defensin (PDF) family genes and molecular characterization of their responses to diverse nutrient stresses in allotetraploid rapeseed
- Published
- Accepted
- Received
- Academic Editor
- Yongping Cai
- Subject Areas
- Agricultural Science, Plant Science
- Keywords
- Brassica napus, Gene family, Nutrient stress, Plant defensin, Transcriptional profiling
- Copyright
- © 2021 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Genome-scale identification of plant defensin (PDF) family genes and molecular characterization of their responses to diverse nutrient stresses in allotetraploid rapeseed. PeerJ 9:e12007 https://doi.org/10.7717/peerj.12007
Abstract
Plant defensins (PDFs), short peptides with strong antibacterial activity, play important roles in plant growth, development, and stress resistance. However, there are few systematic analyses on PDFs in Brassica napus. Here, bioinformatics methods were used to identify genome-wide PDFs in Brassica napus, and systematically analyze physicochemical properties, expansion pattern, phylogeny, and expression profiling of BnaPDFs under diverse nutrient stresses. A total of 37 full-length PDF homologs, divided into two subgroups (PDF1s and PDF2s), were identified in the rapeseed genome. A total of two distinct clades were identified in the BnaPDF phylogeny. Clade specific conserved motifs were identified within each clade respectively. Most BnaPDFs were proved to undergo powerful purified selection. The PDF members had enriched cis-elements related to growth and development, hormone response, environmental stress response in their promoter regions. GO annotations indicate that the functional pathways of BnaPDFs are mainly involved in cells killing and plant defense responses. In addition, bna-miRNA164 and bna-miRNA172 respectively regulate the expression of their targets BnaA2.PDF2.5 and BnaC7.PDF2.6. The expression patterns of BnaPDFs were analyzed in different tissues. BnaPDF1.2bs was mainly expressed in the roots, whereas BnaPDF2.2s and BnaPDF2.3s were both expressed in stamen, pericarp, silique, and stem. However, the other BnaPDF members showed low expression levels in various tissues. Differential expression of BnaPDFs under nitrate limitation, ammonium excess, phosphorus starvation, potassium deficiency, cadmium toxicity, and salt stress indicated that they might participate in different nutrient stress resistance. The genome-wide identification and characterization of BnaPDFs will enrich understanding of their molecular characteristics and provide elite gene resources for genetic improvement of rapeseed resistance to nutrient stresses.
Introduction
Plant antimicrobial peptides (AMPs), short peptides produced by plants, are the first line of defense against bacterial and fungal invasion (Ebbensgaard et al., 2015). There are many types of AMPs, including plant defensins (PDFs), hevein-like peptides, knottin-type peptides, lipid transfer proteins, α-hairpinin, and snakins(Nawrot et al., 2014).
As an important part of the innate immune system of plants, PDFs are ubiquitously expressed in seeds, roots, stems, leaves, flowers, and fruits (Silverstein et al., 2007; Parisi et al., 2019). PDFs, first found in wheat endosperm, were originally classified as γ-thionine, which were later changed to the PDFs after being found similar in sequences and structures to insect defensins (Mendez et al., 1990; Broekaert et al., 1995). PDFs, containing 45–54 amino acids, are rich in cysteine (Cys) and basic amino acids (lysine and arginine). Eight cysteines form four pairs of disulfide bonds to stabilize α helix and three β folding sheets, forming CSαβ conformation (Lay & Anderson, 2005; Sathoff et al., 2019; Odintsova, Slezina & Istomina, 2020). Cys pairing patterns in PDFs are Cys1-Cys8, Cys2-Cys5, Cys3-Cys6, and Cys4-Cys7 (García-Olmedo et al., 1998). The characteristic sequences of α-helix and β-strand are Cys-Xaa-Xaa-Xaa-Cys and Cys-Xaa-Cys, respectively, both of which are common to most PDFs. Although the CSαβ motif of plant defensins is conserved, the amino acid sequences of their primary structure differ greatly (Tam et al., 2015).
Current studies have shown that PDFs play important roles in plant growth and development, and have strong antibacterial activity (Van Loon, Rep & Pieterse, 2006). Most PDFs display antibacterial activity mainly by changing membrane potential and permeability, destroying plasma membrane integrity, or inhibiting bacterial growth (Tam et al., 2015). The expression of Raphanus sativus defensin RsAFP2 in wheat enhanced the resistance to Fusarium graminearum and Rhizoctonia cerealis (Li et al., 2011). Nicotiana alata defensin NaD1 enters the cytoplasm of Fusarium oxysporum hyphae, resulting in granulation of the cytoplasm and cell death (van der Weerden, Lay & Anderson, 2008)
PDFs are also inhibitors of trypsin and amylase, and they can block ion channels and enhance metal tolerance. VrD1 and TvD1 exhibit insecticidal activity against alpha-amylase (Lin et al., 2007; Vijayan et al., 2012). It has been proved that Arg at position 38 of MsDef1 is key to antifungal activity. In addition, MsDef1 also effectively blocks Ca2+ channels (Spelbrink et al., 2004). The Arabidopsis thaliana defensin AtPDF2.3 contains a toxin signature sequence (K-C5-R-G), which effectively blocks potassium channels (Vriens et al., 2016). PDFs are also involved in heavy metal detoxification. Overexpressed AhPDF1.1 increases the tolerance of Arabidopsis halleri to zinc excess (Mirouze et al., 2006). Overexpression of AtPDF2.6 increases the resistance of A. thaliana to cadmium toxicity by reducing the content of cadmium ion in cytoplasm through chelation (Luo et al., 2019a). OsCAL1, a rice defensin-like proteins, is mainly expressed in parenchyma cells of root and xylem, and is involved in the cadmium transport and distribution in the aboveground, promoting the leaf cadmium accumulation (Luo et al., 2018). CAL2 is the closest homolog of CAL1. CAL2 is also cadmium chelating activity. Overexpression of CAL2 increases the accumulation of cadmium in Arabidopsis and rice seedlings (Luo et al., 2020). Moreover, heterologous expression of CAL2 enhanced cadmium sensitivity in Arabidopsis. The heterologous overexpression of BnaPDFL plays a positive role in the cadmium tolerance of Arabidopsis (Luo & Zhang, 2019).
Brassica napus is a main oil crop species in the world. It can be used as human edible oil and animal protein feed, showing important economic value (Raza, 2020). B. napus (An An Cn Cn, 2n = 4x = 38) is a allotetraploid plant species formed by natural hybridization of two diploids, B. rapa (Ar Ar, 2n = 2x = 20) and B. oleracea (Co Co, 2n = 2x = 18 (Bayer et al., 2017)). Compared with A. thaliana, allopolyploid events in B. napus produce many repetitive fragments and homologous regions in the genome (Pelé et al., 2017).
At present, hundreds of PDFs have been isolated from A. thaliana (Thomma, Cammue & Thevissen, 2002), Nicotiana alata (van der Weerden, Hancock & Anderson, 2010), Dahlia merkii (Thevissen et al., 2000), and other plant species. However, genome-wide identification and comprehensive analysis of PDF family members has not been reported in B. napus yet. Therefore, bioinformatics methods were used to comprehensively analyze gene structure, phylogeny, and chromosomal distribution of genome-scale PDFs. In recent years, previous studies have shown that the PDFs participate in the adaptive response of plants to cadmium stress and salt stress (Luo et al., 2018; Luo et al., 2019a; Khadka et al., 2020; Wu, Lin & Chuang, 2016), but there are few studies on the response to nitrate limitation, ammonium excess, phosphorus starvation, and potassium deficiency. In addition, we also discussed gene expression and transcriptional responsive characteristics of BnaPDFs in different organs and diverse nutrient stresses, respectively. The genome-wide identification and characterization of BnaPDFs will enrich the understanding of their molecular characteristics, and will also provide elite gene resources for the genetic improvement of rapeseed resistance to nutrient stresses.
Materials and Methods
Retrieval of gene sequences
In the Arabidopsis information resource (https://www.arabidopsis.org/), we obtained AtPDF amino acid sequences. Through a BLASTp analysis, the PDF protein sequences in B. napus, B. rapa, and B. oleracea were retrieved from the following database: The Arabidopsis Information Resource (TAIR10, https://www.arabidopsis.org/) for A. thaliana, the Brassica Database (BRAD) v. 1.1 (http://brassicadb.org/brad/) for B. rapa (Wang et al., 2015), Genoscope (http://www.genoscope.cns.fr/brassicanapus/) and BnPIR (http://cbi.hzau.edu.cn/bnapus/index.php) for B. napus (Chalhoub et al., 2014), B. oleracea v2.1 (http://plants.ensembl.org/ Brassica oleracea) for B. oleracea (Yu et al., 2013), National Center for Biotechnology Information (NCBI, https://www.ncbi.nlm.nih.gov/), Phytozome v.10 (http://phytozome.jgi.doe.gov/pz/portal.html) (Goodstein et al., 2012), and EnsemblPlants (http://plants.ensembl.org/index.html). All sequences with an E-value < 1e−10 were selected as candidate genes.
Gene nomenclature
In this study, according to the nomenclature previously proposed, BnaPDFs were named according to the following criterion: genus name (one uppercase letter) + plant species (two lowercase letters) + chromosome (followed by a period) + PDF homolog in A. thaliana (Ostergaard & King, 2008; Li et al., 2015). In this study, multiple homologs of BnaPDFs on the same chromosomes were named by serial numbering. For example, BnaC2.PDF1.1a and BnaC2.PDF1.1b indicated two AtPDF1.1 homologs on the chromosome C2 of B. napus.
Physical mapping and family expansion analysis
Genomic annotations of BnaPDFs were used to determine their location and length on chromosomes (Camacho et al., 2009). We submitted AtPDFs and BnaPDFs at the start sites of chromosomes to MapGene2Chrom Web v2 (http://mg2c.iask.in/mg2c_v2.0/) online drawing tool to locate the physical location of AtPDFs and BnaPDFs. In this study, tandem repeats genes were defined as arrays of two or more PDFs within a genomic 100-kb region (Smith & Waterman, 1987).
Sequence alignment and phylogeny analysis
To further analyze evolutionary relationships between PDFs of B. napus and A. thaliana, the PDF protein sequences of B. napus and A. thaliana were compared through performing multiple alignment of homologous protein sequences using ClustalW (Thompson, Higgins & Gibson, 1994). MEGA X (Molecular Evolutionary Genetics Analysis, http://www.megasoftware.net/) (Kumar et al., 2018) was used to construct a phylogenetic tree using the neighbor-joining method (Saitou & Nei, 1987). The parameters were set as follows: Bootstrap replications value of 1,000, Poisson correction, and complete deletion. Further, the online tool ITOL (https://itol.embl.de/) was used to edit and beautify the evolutionary tree.
Analysis of evolutionary selection pressure and divergence of BnaPDFs
To understand selection pressure on BnaPDFs during the evolution process, synonymous (Ks) and non-synonymous (Ka) nucleotide substitution values and Ka/Ks of the duplicated gene pairs of PDFs were calculated (Yang & Nielsen, 2000). First, Clustal Omega (http://www.clustal.org/omega/) (Sievers et al., 2011) was used to pairwise alignment the amino acid sequences of AtPDFs and BnaPDFs. Subsequently, the Ka/Ks Calculator (Wang et al., 2010) (https://sourceforge.net/projects/kakscalculator2/) software was used to calculate the values of Ka, Ks, and Ka/Ks. According to Darwinian evolution, Ka/Ks > 1.0 is generally considered as positive selection, while Ka/Ks < 1.0 indicates purification selection occurs, and Ka/Ks = 1.0 means neutral selection. Ks was used to estimate the divergence time (T) according to the following formula: T = Ks/2λ. In this formula, λ refers to the molecular replacement rate (λ = 1.5 × 10−8 for Brassicaceae species) (Blanc & Wolfe, 2004).
Molecular characterization of BnaPDFs
ExPASy ProtoParam (https://web.expasy.org/protparam/) (Wilkins et al., 1999) was used to analyze physicochemical parameters of BnaPDFs, including amino acid number, molecular weight (MW, kD), theoretical isoelectric point (pI), grand average of hydropathy (GRAVY), and instability index (II). A positive value of GRAVY indicates that the protein is hydrophobic, and a negative value is hydrophilic (Kyte & Doolittle, 1982). Instability index > 40.0 means the protein is unstable (Guruprasad, Reddy & Pandit, 1990).
The online website WoLF PSORT (http://www.genscript.com/wolf-psort.html) (Horton et al., 2007) was used to analyze subcellular localization of AtPDFs and BnaPDFs. The amino acid sequences of AtPDFs and BnaPDFs were submitted to the TMHMM v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) program for transmembrane domain prediction. The online tool NetPhos 3.1 Server (http://www.cbs.dtu.dk/services/NetPhos/) was used to predict potential phosphorylation sites of AtPDFs and BnaPDFs (Blom et al., 2004).
The online SignalP v. 4.1 (http://www.cbs.dtu.dk/services/SignalP/) was used to analyze signal peptide sites of their amino acid positions in BnaPDFs (Petersen et al., 2011). To determine recombinant protein solubility of BnaPDFs, the recombinant protein solubility prediction (RPSP) (http://biotech.ou.edu) program was used to assume that recombinant proteins that were over-expressed in Escherichia coli (Harrison & Bagajewicz, 2015).
The STRING (Search Tool for Recurring Instances of Neighboring Genes) v 11.0 (https://string-db.org) program was used to retrieve PDF association networks (Szklarczyk et al., 2019). The online phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/webscripts/jobmonitor) program was used to predict three-dimensional structures of BnaPDFs (Kelley et al., 2016).
Conserved motif identification of BnaPDFs
To further study conserved motifs of PDFs of Arabidopsis and B. napus, their protein sequences were submitted to the MEME (Multiple Expectation maximization for Motif Elicitation) v. 4.12.0 (http://meme-suite.org/tools/meme) program (Bailey et al., 2009). Motif length was set from 10–50 amino acid residues, and the maximum number of motifs was set at ten, with other parameters as default values. Finally, the online Weblogo (https://weblogo.berkeley.edu/logo.cgi) was used to display the conserved amino acid sequences in BnaPDFs (Crooks et al., 2004).
Elucidation of gene structure and promoter regulatory cis-elements
The full-length genomic and CDS sequences of PDF family genes were obtained from A. thaliana and B. napus databases, and exon-intron structures of PDFs were displayed with Gene Structure Display Server (GSDS) (http://gsds.cbi.pku.edu.cn/). The 2.0 kb genomic sequence upstream of the start codon (ATG) of BnaPDFs and AtPDFs were downloaded in the B. napus Genome Browser (http://www.genoscope.cns.fr/brassicanapus/) and TAIR (https://www.arabidopsis.org/). These sequence files were submitted to PLACE v. 30.0 (http://www.dna.affrc.go.jp/PLACE/) program to predict promoter cis-acting regulatory DNA elements (Higo et al., 1999). The results were statistically classified, and the cis-acting elements of PDFs were visualized. In order to reveal the potential biological functions of PDFs, the R package GOseq was used for Gene Ontology (GO) analysis. In order to analyze miRNAs targeting BnaPDF genes, the psRNATarget database (http://plantgrn.noble.org/psRNATarget/) was used to predict different miRNAs.
Growth conditions
To further investigate transcriptional responses of BnaPDFs to various nutrient stresses, rapeseed seeds were selected for germination for 7 days, and the uniformly growing seedlings (cv. Zhongshuang 11) were transferred to a black plastic container with ten L of Hoagland nutrient solution. The basic nutrient solution contained 1.0 mM KH2PO4, 5.0 mM KNO3, 5.0 mM Ca(NO3)2·4H2O, 2.0 mM MgSO4·7H2O, 0.050 mM EDTA-Fe, 9.0 µM MnCl2·4H2O, 0.80 µM ZnSO4·7H2O, 0.30 µM CuSO4·5H2O, 0.10 µM Na2MoO4·2H2O, and 46 µM H3BO3. To maintain ion concentrations in the nutrient solution, the solution was refreshed every 4 days. B. napus seedlings were cultured in the light chamber under the following growth conditions: light intensity of 200 μmol m−2 s−1, daytime temperature of 25 °C/night temperature of 22 °C, photoperiod 16 h light/8 h dark, and relative humidity of 70%.
Under treatment of nitrate (NO3−) deficiency, 7-day-old B. napus seedlings after seed germination were grown under high nitrate (6.0 mM NO3−) for 10 days, and then the plants were transferred to low nitrate (0.30 mM NO3-) for 3 d until sampling. Under treatment of ammonium (NH4+) toxicity, 7-day-old B. napus seedlings after seed germination were grown under high nitrate (6.0 mM NO3-) for 10 days, then were transferred to a nitrogen-free nutrient solution to grow for 3 d, and finally were grown for 6 h under excess ammonium (6.0 mM NH4+) until sampling. Under phosphate starvation treatment, 7-day-old uniformly growing B. napus seedlings after seed germination, which were grown under 250 µM phosphate (KH2PO4) for 10 days, were grown under five µM phosphate for 3 d until sampling. Under potassium deficiency treatment, 7-day-old uniformly growing B. napus seedlings after seed germination, which were first grown under high potassium (6.0 mM K+) for 10 days, were then transferred to low potassium (0.03 mM K+) for 3 d until sampling. Under salt stress treatment, 7-day-old uniformly growing B. napus seedlings after seed germination were cultured in NaCl-free nutrient solution for 10 days, and then were transferred to 200 mM NaCl for 1 d until sampling. Under cadmium (Cd) toxicity treatment, 7-day-old B. napus seedlings after seed germination are cultured in a Cd-free nutrient solution for 10 days, and then transferred to ten µM CdCl2 for 12 h until sampling.
The shoots and roots of fresh rapeseed seedlings above-mentioned were sampled separately and were immediately stored at −80 °C. Each sample contained three independent biological replicates for transcriptional analysis of BnaPDFs under different nutrient stresses.
Reverse transcription quantitative PCR assays
Total RNA of each sample was extracted by using pre-chilled TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations. Purified total RNA was used as templates for cDNA synthesis using PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Shiga, Japan), and then quantitative PCR assays were used to analyze the relative expression of BnaPDFs. The PCR program was set as follows: 95 °C for 3 min, 95°C for 10 s, 40 cycles, and 60 °C for 30 s (Hua et al., 2018). BnaEF1-α and BnaGDI1 were used as internal references, and expression levels of BnaPDFs were calculated using the 2−ΔΔCT method (Livak & Schmittgen, 2001). Each sample contained three independent biological replicates, and each cDNA template had three technical replicates.
Statistical data analysis
All data were presented as mean ± SD. Comparisons among different treatments were performed using Student’s t-test or one-way ANOVA. A value of p < 0.05 was considered statistically significant. Pearson correlation and statistical analysis were carried out using GraphPad Prism 8.0 software.
Results
Genome-wide identification of PDFs in Brassica species
In A. thaliana, the PDF family included three subfamilies consisting of 13 members, including AtPDF1s (AtPDF1.1, AtPDF1.2a, AtPDF1.2b, AtPDF1.2c, AtPDF1.3, AtPDF1.4, and AtPDF1.5), and AtPDF2s (AtPDF2.1, AtPDF2.2, AtPDF2.3, AtPDF2.4, AtPDF2.5, and AtPDF2.6). Subsequently, we identified a total of 17, 16, and 44 PDF homologs in B. rapa, B. oleracea, and B. napus, respectively (Table 1). In detail, the number of BnaPDF family members ranged from one (BnaPDF1.2c and BnaPDF1.3) to nine (BnaPDF1.2b). However, no homologous sequences of AtPDF1.5, AtPDF2.1, and AtPDF2.4 were identified in B. napus. It suggested that they might have been lost in the evolution of Brassica species due to functional redundancy. Also, they could loss due to genetic drift or loss during duplication process.
| Item |
Brassica napus (1,130 Mb) |
Brassica rapa (465 Mb) |
Brassica oleracea (485 Mb) |
Arabidopsis thaliana (125 Mb) |
|---|---|---|---|---|
| PDF1 | 25 | 10 | 6 | 7 |
| PDF2 | 12 | 4 | 5 | 6 |
| Total | 37 | 14 | 11 | 13 |
Genomic distribution and expansion patterns of BnaPDFs
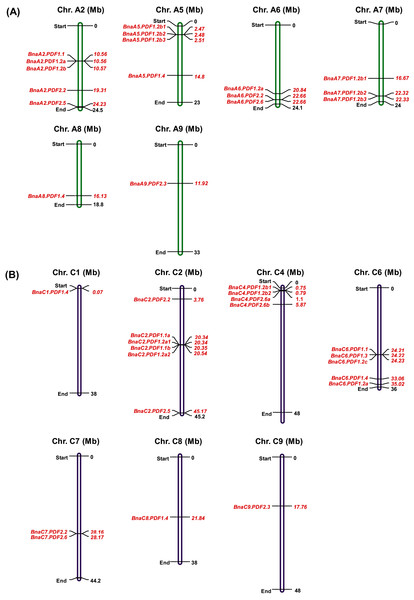
Chromosomal locations of PDFs on their respective chromosomes were listed in Fig. 1. Chromosomal location analysis of BnaPDFs revealed that the PDF family were distributed in the B. napus genome. A total of 37 BnaPDFs were distributed on 13 chromosomes, and 17 BnaPDFs were identified to be located on six chromosomes (A2, A5, A6, A7, A8, and A9) of the A subgenome. Likewise, 20 BnaPDFs are identified on seven chromosomes (C1, C2, C4, C6, C7, C8, and C9) of the C subgenome (Table 2, Fig. 1B).
Figure 1: Physical mapping of the plant defensin (PDF) family members in Brassica napus.
The BnaPDFs were physically mapped onto 13 chromosomes of B. napus, (A) A subgenome: A2, A5, A6, A7, A8 and A9; (B) C subgenome: C1, C2, C4, C6, C7, C8, and C9.| Gene ID | Gene name | Block | CDS (bp) | Exon/ intron |
Amino acid (aa) |
Ka | Ks | Ka/Ks | Divergent time (Mya) |
|---|---|---|---|---|---|---|---|---|---|
| BnaA02g17500D | BnaA2.PDF1.1 | E | 243 | 2/1 | 80 | 0.1772 | 0.4305 | 0.4116 | 14.35 |
| BnaC02g23400D | BnaC2.PDF1.1a | E | 243 | 2/1 | 80 | 0.0586 | 0.3035 | 0.1930 | 10.11 |
| BnaC02g23420D | BnaC2.PDF1.1b | E | 243 | 2/1 | 80 | 0.1775 | 0.4289 | 0.4137 | 14.29 |
| BnaC06g22110D | BnaC6.PDF1.1 | E | 243 | 2/1 | 80 | 0.0993 | 0.3175 | 0.3128 | 10.58 |
| BnaA02g17510D | BnaA2.PDF1.2a | V | 249 | 2/1 | 82 | 0.2324 | 0.5643 | 0.4118 | 18.81 |
| BnaA06g30850D | BnaA6.PDF1.2a | V | 243 | 2/1 | 80 | 0.2157 | 0.4763 | 0.4529 | 15.87 |
| BnaC02g23410D | BnaC2.PDF1.2a1 | V | 249 | 2/1 | 82 | 0.2313 | 0.4939 | 0.4684 | 16.46 |
| BnaC02g23620D | BnaC2.PDF1.2a2 | V | 258 | 2/1 | 85 | 0.4893 | 0.7062 | 0.6929 | 23.54 |
| BnaC06g36530D | BnaC6.PDF1.2a | V | 243 | 2/1 | 80 | 0.0947 | 0.4695 | 0.2018 | 15.65 |
| BnaA02g17520D | BnaA2.PDF1.2b | I | 243 | 2/1 | 80 | 0.0749 | 0.3831 | 0.1956 | 12.77 |
| BnaA05g04680D | BnaA5.PDF1.2b1 | I | 243 | 2/1 | 80 | 0.5090 | 0.5004 | 1.0172 | 16.68 |
| BnaA05g04690D | BnaA5.PDF1.2b2 | I | 246 | 2/1 | 81 | 0.4842 | 0.4739 | 1.0218 | 15.79 |
| BnaA05g04760D | BnaA5.PDF1.2b3 | I | 243 | 2/1 | 80 | 0.4710 | 0.5987 | 0.7866 | 19.95 |
| BnaA07g21570D | BnaA7.PDF1.2b1 | I | 243 | 2/1 | 80 | 0.1011 | 0.3673 | 0.2753 | 12.24 |
| BnaA07g32130D | BnaA7.PDF1.2b2 | I | 243 | 2/1 | 80 | 0.0853 | 0.3282 | 0.2600 | 10.94 |
| BnaA07g32150D | BnaA7.PDF1.2b3 | I | 240 | 2/1 | 79 | 0.0705 | 0.3482 | 0.2023 | 11.60 |
| BnaC04g53180D | BnaC4.PDF1.2b1 | I | 243 | 2/1 | 80 | 0.5064 | 0.5078 | 0.9972 | 16.92 |
| BnaC04g53190D | BnaC4.PDF1.2b2 | I | 243 | 2/1 | 80 | 0.4662 | 0.6050 | 0.7706 | 20.16 |
| BnaC06g22140D | BnaC6.PDF1.2c | V | 240 | 2/1 | 79 | 0.0952 | 0.2719 | 0.3502 | 9.06 |
| BnaC06g22120D | BnaC6.PDF1.3 | I | 240 | 2/1 | 79 | 0.0868 | 0.3933 | 0.2206 | 13.11 |
| BnaA05g19430D | BnaA5.PDF1.4 | B | 288 | 3/2 | 95 | 0.1206 | 0.5015 | 0.2405 | 16.72 |
| BnaA08g22000D | BnaA8.PDF1.4 | B | 237 | 2/1 | 78 | 0.4702 | 3.1360 | 0.1499 | 104.53 |
| BnaC01g00250D | BnaC1.PDF1.4 | B | 231 | 2/1 | 76 | 0.4451 | 3.0059 | 0.1481 | 100.19 |
| BnaC06g33060D | BnaC6.PDF1.4 | B | 267 | 2/1 | 88 | 0.3904 | 5.6153 | 0.0695 | 187.17 |
| BnaC08g18880D | BnaC8.PDF1.4 | B | 237 | 2/1 | 78 | 0.0465 | 0.2858 | 0.1626 | 9.52 |
| BnaA02g26210D | BnaA2.PDF2.2 | K | 234 | 2/1 | 77 | 0.0572 | 0.5222 | 0.1096 | 17.40 |
| BnaA06g34320D | BnaA6.PDF2.2 | K | 234 | 2/1 | 77 | 0.0505 | 0.2948 | 0.1713 | 9.82 |
| BnaC02g47680D | BnaC2.PDF2.2 | K | 234 | 2/1 | 77 | 0.0572 | 0.5903 | 0.0969 | 19.67 |
| BnaC07g21510D | BnaC7.PDF2.2 | K | 231 | 2/1 | 76 | 0.0525 | 0.3780 | 0.1390 | 12.60 |
| BnaA09g18990D | BnaA9.PDF2.3 | K | 234 | 2/1 | 77 | 0.0743 | 0.3135 | 0.2371 | 10.45 |
| BnaC09g20670D | BnaC9.PDF2.3 | K | 234 | 2/1 | 77 | 0.0826 | 0.3291 | 0.2511 | 10.97 |
| BnaA02g33850D | BnaA2.PDF2.5 | X | 222 | 2/1 | 73 | 0.1430 | 0.8075 | 0.1771 | 26.91 |
| BnaC02g42620D | BnaC2.PDF2.5 | X | 222 | 2/1 | 73 | 0.1452 | 0.7109 | 0.2043 | 23.69 |
| BnaA06g34310D | BnaA6.PDF2.6 | K | 222 | 2/1 | 73 | 0.1761 | 0.3469 | 0.5076 | 11.56 |
| BnaC04g53620D | BnaC4.PDF2.6a | K | 222 | 2/1 | 73 | 0.4993 | 1.5605 | 0.3200 | 52.01 |
| BnaC04g07810D | BnaC4.PDF2.6b | K | 222 | 2/1 | 73 | 0.4988 | 1.7036 | 0.2928 | 56.78 |
| BnaC07g21520D | BnaC7.PDF2.6 | K | 222 | 2/1 | 73 | 0.1744 | 0.3814 | 0.4573 | 12.71 |
| AT1G75830 | AtPDF1.1 | E | 243 | 2/1 | 80 | ||||
| AT1G75830 | AtPDF1.2a | V | 243 | 2/1 | 80 | ||||
| AT2G26020 | AtPDF1.2b | I | 243 | 2/1 | 80 | ||||
| AT5G44430 | AtPDF1.2c | V | 243 | 2/1 | 80 | ||||
| AT2G26010 | AtPDF1.3 | I | 243 | 2/1 | 80 | ||||
| AT1G19610 | AtPDF1.4 | B | 237 | 2/1 | 78 | ||||
| AT1G55010 | AtPDF1.5 | C | 243 | 2/1 | 80 | ||||
| AT2G02120 | AtPDF2.1 | K | 234 | 2/1 | 77 | ||||
| AT2G02100 | AtPDF2.2 | K | 234 | 2/1 | 77 | ||||
| AT2G02130 | AtPDF2.3 | K | 234 | 2/1 | 77 | ||||
| AT1G61070 | AtPDF2.4 | D | 231 | 2/1 | 76 | ||||
| AT5G63660 | AtPDF2.5 | X | 222 | 2/1 | 73 | ||||
| AT2G02140 | AtPDF2.6 | K | 222 | 2/1 | 73 |
Note:
CDS, coding sequence; Ka, non-synonymous nucleotide substitution rate; Ks, synonymous nucleotide substitution rate.
Gene amplification is used as a main driving force for adaptive evolution of species (Tang et al., 2010; Jiao et al., 2011). Gene amplification mainly includes tandem duplication, segmental duplication, whole-genome duplication/polyploidization, and replicative transposition (Freeling, 2009). A total of twenty-four of BnaPDFs existed in the form of tandem duplication gene clusters, which indicated that tandem duplication might be a primary way of the BnaPDF family amplification. The Arabidopsis genome can be divided into 24 ancestral cruciferous blocks, labeled A–X (Schranz, Lysak & Mitchell-Olds, 2006). Table 2 showed that AtPDFs and their corresponding BnaPDF homologs were located on the same chromosomal blocks. In detail, the PDFs were located on ten chromosomal blocks, including B, C, D, E, I, K, S, U, V, X.
Phylogeny analysis of BnaPDFs
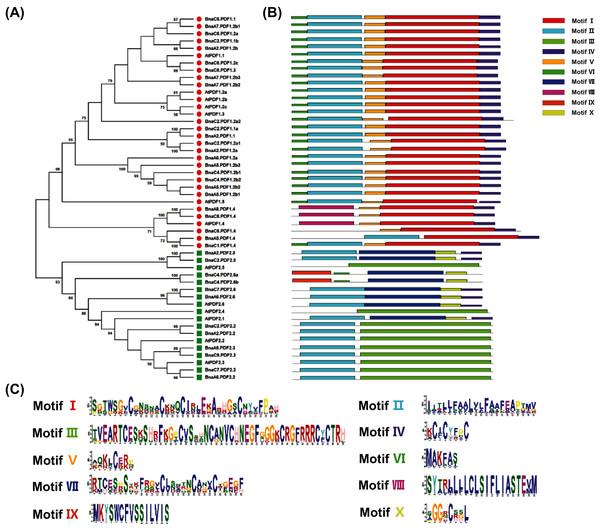
To reveal molecular and phylogenetic relationships between the PDFs of B. napus and A. thaliana, protein sequences from BnaPDFs and AtPDFs were used to construct unrooted phylogenetic trees (Fig. 2). In Arabidopsis, PDF family members are mainly divided into two evolutionary branches: PDF1s and PDF2s. Besides, a phylogenetic analysis of 13 PDFs of A. thaliana and 37 PDFs of B. napus was performed. As shown in Fig. 2, the phylogenetic tree was clearly clustered into two well-supported classes, namely PDF1 and PDF2. In each evolutionary clade, BnaPDF members were clustered closely together with the corresponding homolog in Arabidopsis. The results showed that PDFs had diverged before the formation of Brassica species. Most of the PDFs in each subfamily had short branch lengths (Fig. 2), suggesting that genetic differentiation has occurred recently.
Figure 2: Phylogeny analysis and conserved motifs of the plant defensins (PDFs) in Arabidopsis thaliana and Brassica napus.
(A) Phylogeny analysis of AtPDFs and BnaPDFs. The PDF protein sequence was multiple-aligned using ClustalW, and then the neighboring method in the MEGA X was used to construct a phylogenetic tree. In the phylogenetic tree, related taxa are clustered together (1,000 repeats), The evolution distance is calculated using the poisson correction method, and the unit is the number of amino acid substitutions at each position. Molecular identification (B) and sequence characterization (C) of the conserved motifs in the PDFs. In (B) the 10 different colored boxes represent ten conserved motifs (patterns I–X), and grey lines represent without conserved regions. In (C) the larger the fonts, the more conserved the motifs.Molecular characterization of BnaPDFs
Physicochemical properties of PDF family proteins were analyzed by the ExPASy online software. The results showed that CDS lengths of BnaPDFs stretched from 222 bp (BnaPDF2.5s and BnaPDF2.6s) to 288 bp (BnaA5.PDF1.4), and protein lengths of PDFs were also identified to be varied between 73 and 95 amino acids. In compliance with protein lengths, molecular weights of PDFs also differed ranging from 7.6 to 10.5 kD (Table 2). Isoelectric points (pI) of PDFs ranged from 5.01 (BnaC1.PDF1.4) and 9.82 (BnaA9.PDF2.3 and BnaC9.PDF2.3). The pIs of most PDF proteins were above seven, except for BnaC1.PDF1.4, BnaC4.PDF2.6a, BnaC4.PDF2.6b (Table 3). In terms of instability index (IIs), 51.35% (19/37) of BnaPDFs with IIs greater than 40 was presumed to be stable proteins, and the other part is weakly stable proteins. Grand average of hydropathy index (GRAVY) of BnaA6.PDF1.2a, BnaA5.PDF1.2b2, BnaPDF1.4, BnaC7.PDF2.2, and BnaPDF2.5 was negative (Table 3), the results showed that the PDF family proteins are hydrophilic proteins.
| Gene ID | Gene name | pI | MW (kDa) |
II | Aliphatic inedx | GRAVY | TM domains | Subcellular localization |
|---|---|---|---|---|---|---|---|---|
| BnaA02g17500D | BnaA2.PDF1.1 | 9.00 | 8918.54 | 32.13 | 71.00 | 0.065 | 1 | Extr |
| BnaC06g22110D | BnaC6.PDF1.1 | 8.70 | 8764.31 | 30.55 | 74.50 | 0.259 | 1 | Extr |
| BnaC02g23400D | BnaC2.PDF1.1a | 8.47 | 8700.26 | 30.55 | 83.12 | 0.348 | 1 | Extr |
| BnaC02g23420D | BnaC2.PDF1.1b | 9.01 | 8946.55 | 33.19 | 71.00 | 0.057 | 1 | Extr |
| BnaA02g17510D | BnaA2.PDF1.2a | 9.24 | 9240.08 | 43.41 | 73.90 | 0.237 | 1 | Extr |
| BnaA06g30850D | BnaA6.PDF1.2a | 9.41 | 9232.09 | 45.29 | 65.88 | −0.005 | 1 | Extr |
| BnaC02g23410D | BnaC2.PDF1.2a1 | 9.24 | 9240.08 | 43.41 | 73.90 | 0.237 | 1 | Extr |
| BnaC02g23620D | BnaC2.PDF1.2a2 | 8.47 | 9145.64 | 38.52 | 73.65 | 0.148 | 1 | Extr |
| BnaC06g36530D | BnaC6.PDF1.2a | 8.70 | 8778.33 | 29.49 | 75.75 | 0.295 | 1 | Extr |
| BnaA02g17520D | BnaA2.PDF1.2b | 8.47 | 8718.23 | 32.96 | 73.38 | 267 | 1 | Extr |
| BnaA05g04680D | BnaA5.PDF1.2b1 | 9.18 | 9223.98 | 52.62 | 70.75 | 0.075 | 1 | Extr |
| BnaA05g04690D | BnaA5.PDF1.2b2 | 9.20 | 9382.06 | 72.77 | 61.48 | −0.101 | 1 | Extr |
| BnaA05g04760D | BnaA5.PDF1.2b3 | 9.06 | 9185.82 | 73.26 | 62.25 | 0.025 | 1 | Extr |
| BnaA07g21570D | BnaA7.PDF1.2b1 | 8.70 | 8820.41 | 29.49 | 78.12 | 0.294 | 1 | Extr |
| BnaA07g32130D | BnaA7.PDF1.2b2 | 8.70 | 8830.37 | 29.45 | 78.12 | 0.336 | 1 | Extr |
| BnaA07g32150D | BnaA7.PDF1.2b3 | 8.15 | 8555.01 | 30.42 | 76.71 | 0.411 | 1 | Extr |
| BnaC04g53180D | BnaC4.PDF1.2b1 | 9.03 | 9164.87 | 67.38 | 70.75 | 0.033 | 1 | Extr |
| BnaC04g53190D | BnaC4.PDF1.2b2 | 9.18 | 9251.99 | 63.15 | 68.25 | 0.04 | 1 | Extr |
| BnaC06g22140D | BnaC6.PDF1.2c | 8.49 | 8599.07 | 28.57 | 66.84 | 0.363 | 1 | Extr |
| BnaC06g22120D | BnaC6.PDF1.3 | 8.47 | 8659.12 | 33.36 | 63.16 | 0.209 | 1 | Extr |
| BnaA05g19430D | BnaA5.PDF1.4 | 8.67 | 10567.17 | 52.94 | 67.79 | −0.028 | 1 | Extr |
| BnaA08g22000D | BnaA8.PDF1.4 | 8.85 | 8814.28 | 57.46 | 60.13 | 0.103 | 1 | Extr |
| BnaC01g00250D | BnaC1.PDF1.4 | 5.01 | 8335.5 | 30.87 | 79.61 | 0.312 | 0 | Extr |
| BnaC06g33060D | BnaC6.PDF1.4 | 8.82 | 10136.95 | 45.86 | 73.30 | −0.13 | 1 | Extr |
| BnaC08g18880D | BnaC8.PDF1.4 | 8.85 | 8844.3 | 53.33 | 58.85 | 0.071 | 1 | Extr |
| BnaA02g26210D | BnaA2.PDF2.2 | 9.33 | 8511.05 | 49.43 | 68.31 | 0.048 | 1 | Extr |
| BnaA06g34320D | BnaA6.PDF2.2 | 9.63 | 8510.06 | 34.44 | 61.95 | 0.032 | 1 | Extr |
| BnaC02g47680D | BnaC2.PDF2.2 | 9.33 | 8497.02 | 46.93 | 68.31 | 0.048 | 1 | Extr |
| BnaC07g21510D | BnaC7.PDF2.2 | 9.49 | 8382.83 | 35.88 | 57.63 | −0.012 | 1 | Extr |
| BnaA09g18990D | BnaA9.PDF2.3 | 9.82 | 8691.31 | 49.86 | 63.25 | −0.069 | 1 | Extr |
| BnaC09g20670D | BnaC9.PDF2.3 | 9.82 | 8707.35 | 50.84 | 68.31 | 0.001 | 1 | Extr |
| BnaA02g33850D | BnaA2.PDF2.5 | 9.14 | 8414.81 | 61.47 | 69.45 | −0.036 | 1 | Extr |
| BnaC02g42620D | BnaC2.PDF2.5 | 9.14 | 8414.81 | 61.47 | 69.45 | −0.036 | 1 | Extr |
| BnaA06g34310D | BnaA6.PDF2.6 | 8.71 | 7687.19 | 37.47 | 86.85 | 0.495 | 1 | Extr |
| BnaC04g53620D | BnaC4.PDF2.6a | 6.49 | 8605.98 | 37.43 | 64.11 | 0.034 | 1 | Extr |
| BnaC04g07810D | BnaC4.PDF2.6b | 6.49 | 8605.98 | 37.43 | 64.11 | 0.034 | 1 | Extr |
| BnaC07g21520D | BnaC7.PDF2.6 | 8.71 | 7687.19 | 37.47 | 86.85 | 0.495 | 1 | Extr |
| AT1G75830 | AtPDF1.1 | 8.47 | 8709.22 | 27.49 | 74.5 | 0.339 | 1 | Extr |
| AT1G75830 | AtPDF1.2a | 8.14 | 8518.03 | 27.68 | 81.88 | 0.454 | 1 | Extr |
| AT2G26020 | AtPDF1.2b | 8.14 | 8640.16 | 28.23 | 78.12 | 0.389 | 1 | Extr |
| AT5G44430 | AtPDF1.2c | 8.14 | 8550.03 | 25.27 | 75.75 | 0.358 | 1 | Extr |
| AT2G26010 | AtPDF1.3 | 8.14 | 8580.1 | 25.27 | 78.25 | 0.436 | 1 | Extr |
| AT1G19610 | AtPDF1.4 | 8.44 | 8840.28 | 55.88 | 52.69 | 0.113 | 1 | Extr |
| AT1G55010 | AtPDF1.5 | 5.65 | 9139.47 | 31.07 | 65.88 | 0.006 | 0 | Extr |
| AT2G02120 | AtPDF2.1 | 9.14 | 8578.05 | 41.13 | 56.88 | 0.017 | 1 | Extr |
| AT2G02100 | AtPDF2.2 | 9.37 | 8524.07 | 40.13 | 63.25 | 0.201 | 1 | Extr |
| AT2G02130 | AtPDF2.3 | 9.63 | 8544.07 | 39.18 | 59.48 | 0.004 | 1 | Extr |
| AT1G61070 | AtPDF2.4 | 8.52 | 8289.68 | 51.47 | 80.79 | 0.182 | 1 | Extr |
| AT5G63660 | AtPDF2.5 | 8.92 | 8387.69 | 56.73 | 54.79 | −0.13 | 0 | Extr |
| AT2G02140 | AtPDF2.6 | 8.92 | 7718.14 | 76.05 | 73.56 | 0.281 | 1 | Extr |
Note:
GRAVY, grand average of hydropathy; II, instability index; MW, molecular weight; pI, isoelectric point; Extr, extracellular region.
The Wolf Psort software was used to predict subcellular locations of BnaPDFs and AtPDFs (Table 3). The prediction results indicated that PDF1s and PDF2s of Arabidopsis were potentially located in the extracellular matrix. The subcellular localization of BnaPDFs was predicted to have the same position.
Phosphorylation, a ubiquitous protein activity regulation mechanism in organisms, plays an important role in cell signal transduction and usually occurs at the sites of serine, threonine, and tyrosine (Mijakovic, Grangeasse & Turgay, 2016). The NetPhos 3.1 software was used to analyze the phosphorylation sites of AtPDF and BnaPDF proteins (Fig. S1). The PDF proteins had differential preference for phosphorylation on serine, threonine, and tyrosine sites. The phosphorylation sites of most PDFs were mainly serine and threonine residues.
Transmembrane domains are main sites where proteins in the membrane combine with lipid (Kim et al., 2020). AtPDF1.5, AtPDF2.5, and BnaC1.PDF1.4 were predicted to have no transmembrane domains, and the remaining BnaPDF and AtPDF proteins contained a transmembrane domain. Therefore, most PDF family members functioned on the cell membrane (Fig. S2).
The members of BnaPDFs and AtPDFs contained a signal peptides, which guided the newly synthesized protein to secret the organelles and performed functions.
Recombinant protein solubility prediction showed that the recombinant BnaC2.PDF1.1b, BnaC2.PDF1.2a1, BnaA2.PDF1.2a, BnaA2.PDF1.2b, and BnaA5.PDF1.4 expressed in plant might be unstable, whereas the rest of BnaPDF recombinant proteins expressed in plant showed strong stability (Table 3).
Identification of evolutionary selection pressure on BnaPDFs
Selection pressure analysis also reflect differentiation of gene families in the process of evolution. Ratios of substitution (Ka) and synonymous substitution (Ks) were used to estimate selection pressure on BnaPDFs during evolution (Table 2). The Ka values of BnaPDFs ranged from 0.0465 (BnaC8.PDF1.4) to 0.5090 (BnaA5.PDF1.2b1) with an average value of 0.2312. The Ks values of BnaPDFs ranged from 0.1648 (BnaA8.PDF1.4) to 5.6153 (BnaC6.PDF1. 4) with average value of 0.8204. The Ka/Ks ratios of 34 BnaPDFs were between 0.0695 and 0.7866, which indicated that BnaPDFs had undergone strong negative evolution to preserve their functionality. The ratio of Ka/Ks of BnaC4.PDF1.2b1 was 0.9972, which indicated BnaC4.PDF1.2b1 underwent neutral selection. The ratios of Ka/Ks of BnaA5.PDF1.2b1 and BnaA5.PDF1.2b2 were greater than one, indicating that these two genes were subject to positive selection during evolutionary selection.
The separation of Arabidopsis and Brassica species occurred 12 to 20 million years ago (Mya). The Ks value results showed that duplication events of most PDFs occurred 10–100 Mya, which indicated that the species formation of Brassica plants might be accompanied by the functional divergence of PDF genes (Fig. 2A).
Conserved domain, gene structure, protein interaction and transcriptional regulatory analysis
The MEME program was used to analyze the conservation of all PDF proteins, and ten conservative motifs were found (Fig. 2B). This study showed that each PDF contained one to five motifs. Motif II were highly conserved in all PDF sequences, except for several PDF family members (BnaA8.PDF1.4, BnaC8.PDF1.4, BnaC6.PDF1.4, BnaC4.PDF2.6a, BnaC4.PDF2.6b, AtPDF1.4, AtPDF2.4 and AtPDF2.5) (Fig. 2B). The composition patterns of protein conserved motifs in each subgroup were similar, indicating that the same subgroup might had similar biological functions. Each subfamily had its specific conserved motif. All PDF1 subfamily members include motif I, motif IV and motif V. Motif I, Motif V, and Motif VI were unique to the PDF1 subfamily, and Motif III and Motif VII were unique to the PDF2 subfamily (Fig. 2B).
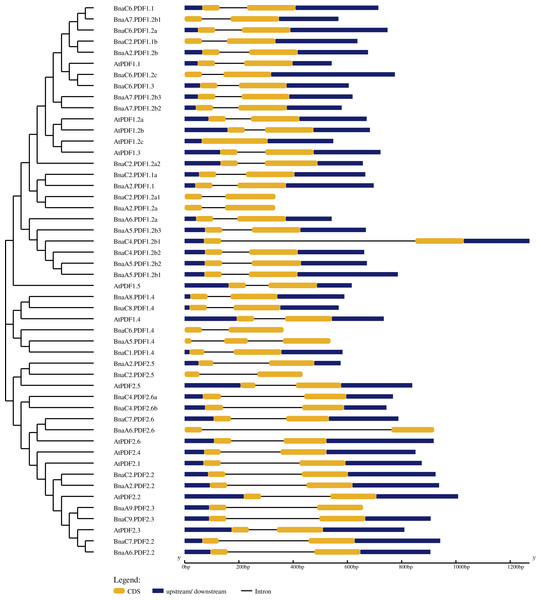
The exon-intron structure of BnaPDFs was determined by comparing the genomic sequences and its corresponding coding sequences (Fig. 3). The statistical results showed that except AtPDF1.3 had only one exon, BnaA5.PDF1.4 contained three exons and two introns, and all other genes contained only two exons and one intron. In addition, several BnaPDF genes contained longer intron structures, especially for, BnaC4.PDF1.2b1, BnaA6.PDF2.6.
Figure 3: Gene structures of the PDF family genes in Arabidopsis thaliana and Brassica napus.
In the GSDS server, the exon-intron structures of the PDFs were determined by comparing the coding sequence with the corresponding genome sequence. The yellow boxes represent exons, blue boxes indicate upstream or downstream, and the lines represent introns. The diagram was obtained using the GSDS web server.Phyre2 was used to predict secondary and three-dimensional structures of BnaPDFs (Fig. S4). Secondary structures of most BnaPDFs are mainly composed of alpha helix, beta strand, disordered and transmembrane helix. Alpha helix was the main component of BnaPDF secondary structures, accounting for 30% (BnaC4.PDF2.6) to 60% (BnaC6.PDF1.4), with an average value of 52%. The proportion of disordered structures ranged from 25% (BnaC4.PDF1.2b1) to 46% (BnaA8.PDF1.4), with an average of 35%, whereas the proportion of β-turns and transmembrane helix was relatively small. The three-dimensional structures of BnaPDFs, formed by further winding and folding on the basis of secondary structure, were mainly composed of alpha helix and disordered structures.
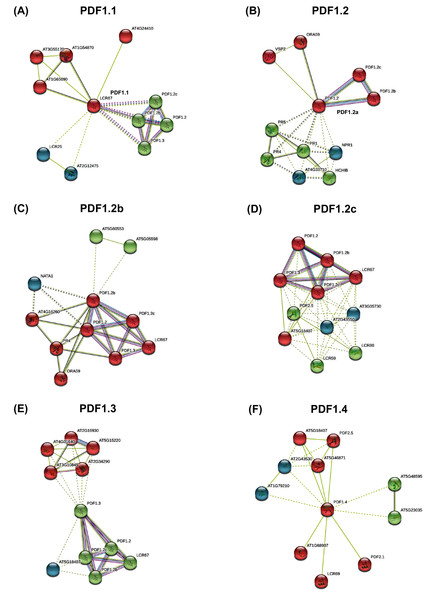
PDF proteins interaction analysis results showed that PDFs showed closely interaction with each other (Fig. 4). Almost all PDF proteins were associated with innate immune proteins (such as disease-related proteins) and the DEFL-encoding proteins. From PDF interaction networks involving PDF1.3, PDF2.1, PDF2.3, and PDF2.5, the genes regulating plant stress response, phosphorylation-mediated growth and development, ubiquitination-responsive gene expression, and hormone-mediated signals were highly enriched (Fig. 4, Fig. S5).
Figure 4: The protein-protein interaction network of plant defensins.
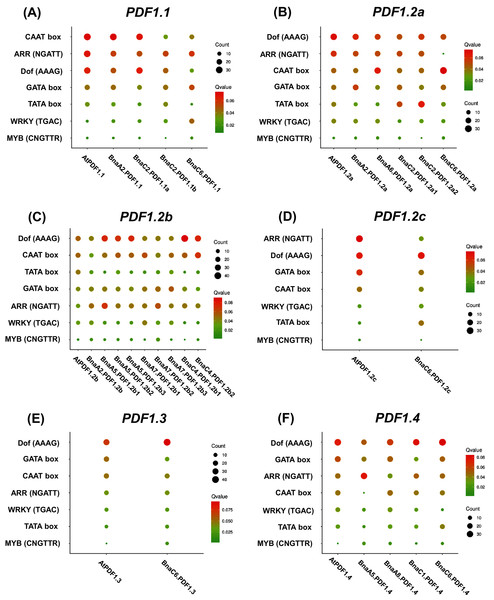
Constructed PDF1.1 (A), PDF1.2a (B), PDF1.2b (C), PDF1.2c (D), PDF1.3 (E), PDF1.4 (F) and other protein interaction networks provided by the STRING web server. The protein interaction network of other members of the PDF family is shown in the Fig. S5. The network nodes represent proteins. The network is divided into three clusters, represented by red, green and blue nodes. The edges represent the binding of proteins to proteins. The edges of different colors have different interactions. The purple edges represent the interactions between proteins, which has been experimentally proven. The black edges represent co-expression between proteins, and the yellow edges represent that they are still being explored.Analysis of 2.0 kb genome sequences upstream of the PDFs start codon (ATG) showed that PDFs had rich cis-elements related to growth and development, hormone response, environmental stress, and light response. The identified environmental stress responsive cis-elements included ARE (anaerobic induced response element), DRE (drought response element), and LTR (low temperature response element). We also investigated the presence of ARR, CAAT-box, Dof, GATA box, TATA Box, WRKY, and MYB in the PDF promoter regions of both B. napus and A. thaliana (Fig. 5). As shown in Fig. 5, the results showed that DNA binding with one finger (Dof, AAAG) and Age-Related Resistance (ARR, GATT) were highly enriched, while WRKY and MYB binding sites were fewer in the PDF promoter regions.
Figure 5: Enrichment analysis of cis-acting regulatory elements (CRE) in the promoter region of Brassica napus plant defensins (PDF) genes.
Enrichment analysis of cis-acting regulatory elements of PDF1.1 (A), PDF1.2a (B), PDF1.2b (C), PDF1.2c (D), PDF1.3 (E), PDF1.4 (F). the cis-acting elements of other members of the PDF family are shown in the Fig. S6. In the scatter chart, the larger the circle, the more CREs.The GO annotation results of BnaPDFs showed that they were mainly enriched in biological processes, such as killing of cells of other organism (GO:0031640), defense response (GO:0006952), and defense response to fungus (GO:0050832) (Fig. S7).
We predicted miRNAs targeting BnaPDF genes, and the results showed that the miRNAs only targeted BnaA2.PDF2.5 and BnaC7.PDF2.6. The miRNAs including bna-miR164a, bna-miR164b, bna-miR164c, and bna-miR164d targeted BnaA2.PDF2.5, and these miRNAs exerted an inhibitory effect by directly cleaving mRNA. The miRNAs including bna-miR172a, bna-miR172d, and bna-miR6031 targeted BnaC7.PDF2.6. bna-miR172a, bna-miR172d inhibited the translation process, whereas bna-miR6031 directly cleaved mRNA (Table S1).
Transcriptional analysis of BnaPDFs under diverse nutrient stresses
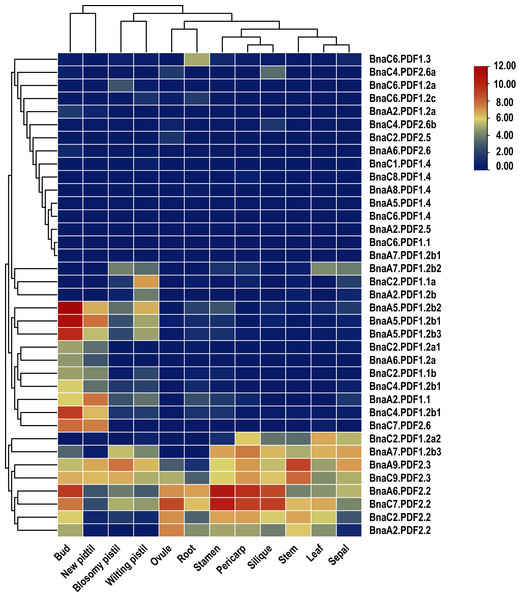
In order to explore potential biological functions of BnaPDFs in the growth and development of B. napus, the expression patterns of BnaPDFs were analyzed in different tissues, including blossomy pistil, bud ovule, leaf, new pistil, pericarp, root, sepal, silique, stamen, and stem (Fig. 6). It was observed that BnaA6.PDF2.2 and BnaC7.PDF2.2 were predominantly expressed in bud, silique, pericarp, and stamen, and BnaA9.PDF2.3 was expressed at a high level in leaves, whereas BnaA5.PDF1.2b1, BnaA5.PDF1.2b2, and BnaA5.PDF1.2b3 had the highest expression abundances in roots. However, the other BnaPDF members showed very low expression levels in various tissues.
Figure 6: Summarization of tissue/organ specificity expression of PDFs in Brassica napus.
Summarization of bud, new pistil, blosomy pistil, wilting pistil, ovule, root, silique, stamen, leaf, sepal, pericarp, stem specificity expression of PDFs in Brassica napus.In order to gain a deeper understanding of biological functions of BnaPDFs under abiotic stress, we analyzed the expression patterns of nitrate deficiency, ammonium toxicity, phosphorus deficiency, potassium deficiency, salt stress, and cadmium toxicity.
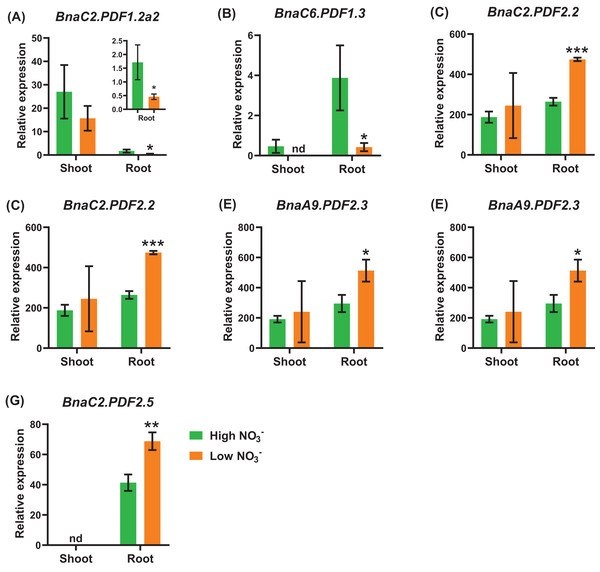
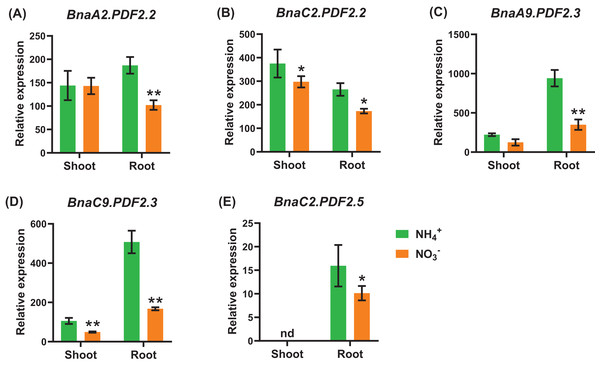
Under nitrate deficiency, all the 37 BnaPDFs were not differentially expressed in the shoots, whereas seven DEGs were identified in the roots (Fig. 7). Most of the DEGs, particularly BnaPDF2.2s, BnaPDF2.3s, and BnaPDF2.5s, were obviously upregulated under nitrate deficiency (Figs. 7C–7G). However, the expression of BnaC2.PDF1.2a2 and BnaC6.PDF1.3 were suppressed in (Figs. 7A, 7B). Five members of BnaPDFs were differentially expressed under ammonium toxicity. It was noteworthy that the expression of BnaPDF2.2s, BnaPDF2.3s, and BnaPDF2.5s were induced in the shoots and roots under ammonium toxicity (Fig. 8).
Figure 7: The qRT-PCR-assisted transcriptional characterization of the plant defensins (PDF) genes in Brassica napus under different nitrate (NO3−) supply levels.
Differential expression of BnaC2.PDF1.2a2 (A), BnaC6.PDF1.3 (B), BnaC2.PDF2.2 (C), BnaC7.PDF2.2 (D), BnaA9.PDF2.3 (E), BnaC9.PDF2.3 (F), and BnaC2.PDF2.5 (G) under high and low nitrate conditions. For high-throughput transcript profiling, the 7-day-old uniform B. napus seedlings after seed germination were hydroponic cultured in 6.0 mM nitrate for 10 d, and then were transferred to 0.30 mM nitrate for 3 d until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). nd stands for “not detected”. An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01, ***P < 0.001.Figure 8: The qRT-PCR-assisted transcriptional characterization of the plant defensins (PDF) in Brassica napus under different nitrogen (N) form conditions.
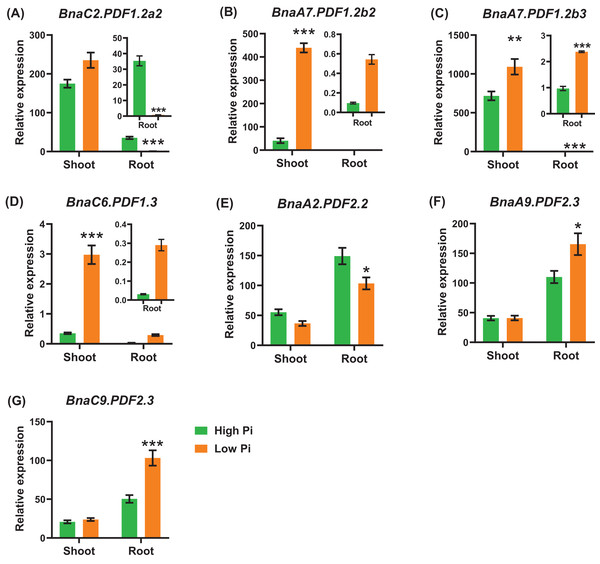
Differential expression of BnaA2.PDF2.2 (A), BnaC2.PDF2.2 (B), BnaA9.PDF2.3 (C), BnaC9.PDF2.3 (D), and BnaC2.PDF2.5 (E) under nitrate (NO3−) and ammonium (NH4+) conditions. For high-throughput transcript profiling, the 7-day-old uniform B. napus seedlings after seed germination were hydroponic cultured in 6.0 mM nitrate for 10d, and then were transferred to an N-free solution for 3 d. Subsequently, seedlings were treated with 6.0 mM ammonium (NH4+) for 3 d until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). nd stands for “not detected”. An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01.In general, the expression level of BnaPDFs was lower in the roots than in the shoots. Under low phosphate, we identified seven BnaPDFs that exhibited differential expression in shoots or roots, and a larger proportion of the DEGs were upregulated (Fig. 9).
Figure 9: The qRT-PCR-assisted transcriptional characterization of the plant defensins (PDFs) in Brassica napus under different phosphate (Pi) levels.
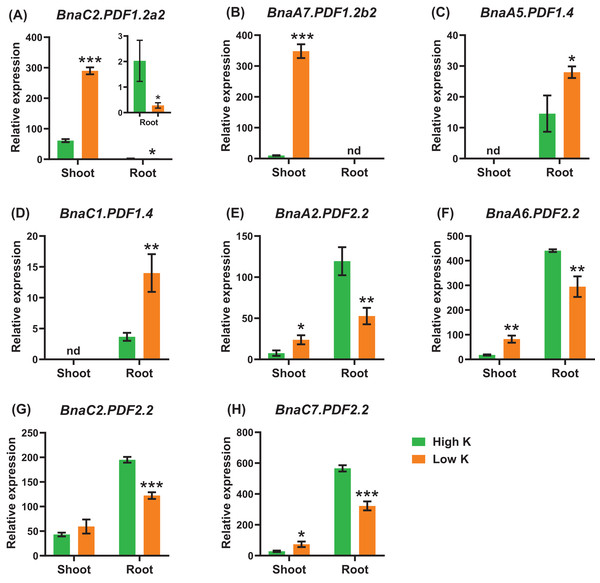
Differential expression of BnaC2.PDF1.2a2 (A), BnaA7.PDF1.2b2 (B), BnaA7.PDF1.2b3 (C), BnaC6.PDF1.3 (D), BnaA2.PDF2.2 (E), BnaA9.PDF2.3 (F), and BnaC9.PDF2.3 (G) under high Pi and low Pi levels. For high-throughput transcript profiling, the 7-day-old uniform B. napus seedlings after seed germination were hydroponic cultured in 250 μM Pi for 10 d, and then were transferred to 5 μM Pi for 3 d until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01, ***P < 0.001.Potassium deficiency leads to a decrease in the stomatal conductance of leaves and inhibition of photosynthesis. Potassium deficiency also reduces leaf expansion rate and leaf area (Wang, Garvin & Kochian, 2002; Battie-Laclau et al., 2014). Under potassium deficiency, a total of eight DEGs were identified in the shoots or roots (Fig. 10). In detail, BnaC2.PDF1.2a2 and BnaA7.PDF1.2b2 exhibited the highest levels in the shoots (Figs. 10A, 10B). In the shoots, five DEGs of BnaPDF1.2b and BnaPDF1.2c were upregulated under potassium deficiency (Figs. 10B–10D). In the roots, five downregulated (including BnaPDF1.2s and BnaPDF2.2s) and two up-regulated BnaPDFs (BnaPDF1.4s) were identified, respectively.
Figure 10: The qRT-PCR-assisted transcriptional characterization of the plant defensins (PDFs) in Brassica napus under different potassium (K) levels.
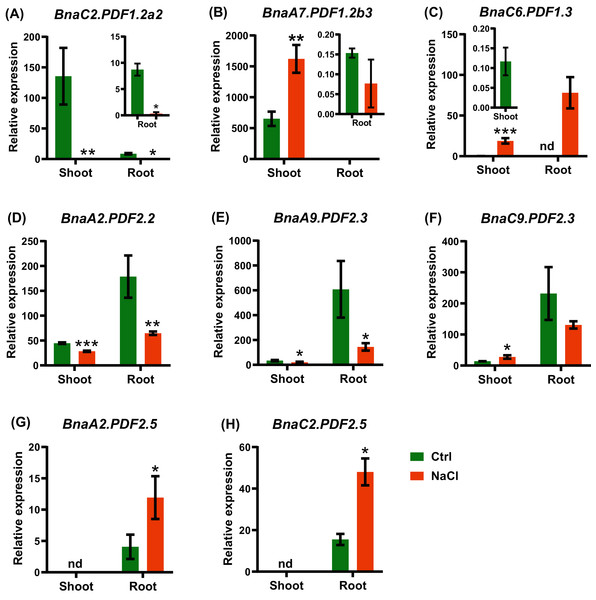
Differential expression of BnaC2.PDF1.2a2 (A), BnaA7.PDF1.2b2 (B), BnaA7.PDF1.2b3 (C), BnaC6.PDF1.3 (D), BnaA2.PDF2.2 (E), BnaA9.PDF2.3 (F), and BnaC9.PDF2.3 (G) under high K and low K levels. For the transcriptional analysis, the 7 d-old uniform B. napus seedlings after seed germination were first hydroponically grown under 6 mM K+ 10 d, and then were transferred to 0.03 mM K+ for 1 d until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). nd stands for “not detected”. An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01, ***P < 0.001.B. napus was a moderate-salt-tolerant crop and can be used as a phytoremediation resource for saline-alkali soils. In the shoots and roots, a total of eight BnaPDF DEGs were identified under salt stress. Under salt stress, BnaA7.PDF1.2b3 had the highest expression level in the shoots, and BnaA9.PDF2.3 had the highest expression level in the roots (Figs. 11B, 11E). The shoots upregulated genes included BnaA7.PDF1.2b3, BnaC6.PDF1.3, and BnaC9.PDF2.3, The roots upregulated genes included BnaA7.PDF1.2b3, BnaA2.PDF2.5, and BnaC2.PDF2.5 (Fig. 11).
Figure 11: The qRT-PCR assisted transcriptional characterization of the plant defensins (PDFs) in Brassica napus under salt stress.
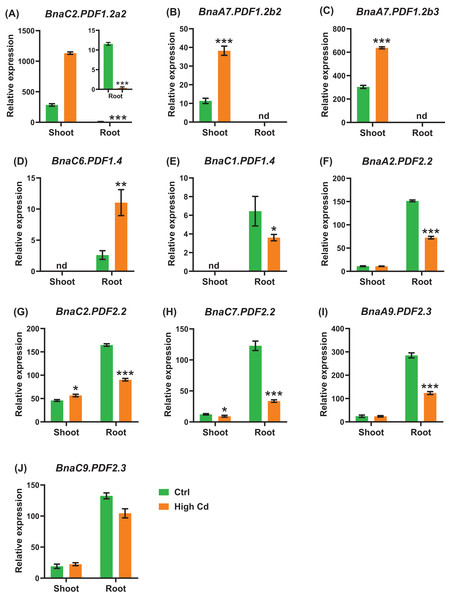
(A–H) Differential expression profiling of: BnaC2.PDF1.2a2 (A), BnaA7.PDF1.2b3 (B), BnaC6.PDF1.3 (C), BnaA2.PDF2.2 (D), BnaA9.PDF2.3 (E), BnaC9.PDF2.3 (F), BnaA2.PDF2.5 (G), and BnaC2.PDF2.5 (H) under salt stress. For high-throughput transcript profiling, the 7-day-old uniform B. napus seedlings after seed germination were hydroponic cultured in NaCl-free solution for 10 d, and then transferred to 200 mM NaCl for 12 h until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). nd stands for “not detected”. An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01, ***P < 0.001.Cadmium, a heavy metal with strong biotoxicity, which can cause plant tissue cells to produce reactive oxygen species, cause membrane lipid peroxidation, and inhibit plant growth (Pereira de Araújo et al., 2017; Ben Ghnaya et al., 2009). We identified a total of ten BnaPDF DEGs in the shoots and roots under cadmium toxicity (Fig. 12). All the expression of BnaC2.PDF1.2a2, BnaC2.PDF2.2, and BnaC7.PDF2.2 was induced by cadmium in the shoots and roots (Figs. 12A, 12G, 12H). In the shoots, we found that the cadmium toxicity significantly induced the expression of the BnaPDF1.2a, BnaPDF1.2b, and BnaPDF2.2 DEGs, whereas repressed the expression of the BnaC7.PDF2.2. In the roots,the expression of most BnaPDFs was significantly downregulated by cadmium toxicity except that the BnaC6.PDF1.4 expression was obviously upregulated by cadmium toxicity.
Figure 12: The qRT-PCR-assisted transcriptional characterization of the plant defensins (PDF) genes in Brassica napus under cadmium (Cd) toxicity.
Differential expression of BnaC2.PDF1.2a2 (A), BnaA7.PDF1.2b2 (B), BnaA7.PDF1.2b3 (C), BnaC1.PDF1.4 (D), BnaC6.PDF1.4 (E), BnaA2.PDF2.2 (F), BnaC2.PDF2.2 (G), BnaC7.PDF2.2 (H), BnaA9.PDF2.3 (I), and BnaC9.PDF2.3 (J) under Cd-free (−Cd) and Cd (10 μM CdCl2) toxicity. For the transcriptional analysis, the 7-d-old uniform B. napus seedlings after seed germination were hydroponically cultivated in a cadmium-free solution for 10 d, and then were transferred to 10 μM CdCl2 for 12 h until sampling. The shoots and roots are three independent biological replicates. Error bars indicate SD (n = 3). nd stands for “not detected”. An asterisk indicates that the BnaPDFs differentially expressed are significant at *P < 0.05, **P < 0.01, ***P < 0.001.According to the transcriptional responses of BnaPDFs to multiple nutrient stresses, we conducted a summary analysis. These results showed that all the BnaPDFs were not identified to responsive to the six nutrient stresses above-mentioned, whereas BnaC2.PDF1.2a2 was simultaneously responsive to five nutrient stresses, including low nitrate, limited phosphate, potassium deficiency, toxic cadmium and salt stress. In addition, BnaA9.PDF2.3, BnaC9.PDF2.3, BnaA2.PDF2.2, BnaA7.PDF1.2b3, BnaC7.PDF2.2, BnaC2.PDF2.2, and BnaC6.PDF1.3 were simultaneously responsive to three or four nutritional stresses (Table 4).
| Term | Nitrate limitation | Ammonium excess | Phosphorus starvation | Potassium deficiency | Cadmium toxicity | Salt stress |
|---|---|---|---|---|---|---|
| PDF1s | 2 | 3 | 3 | 4 | 5 | 3 |
| PDF2s | 5 | 6 | 4 | 4 | 5 | 5 |
Discussion
In the process of gene evolution, conserved regions are retained, but several mutations have also occurred, along with changes in gene function, leading to the differentiation of genes into subfamily (Ohno, 1999). B. napus is an allotetraploid plant species formed by natural hybridization of two diploids, B. rapa and B. oleracea, and undergoes several rounds of whole-genome triplication and duplication compared with Arabidopsis (Bayer et al., 2017). Therefore, these processes usually result in the formation of multicopy gene families in B. napus (Parkin et al., 2005). Previous studies have shown that the existence of defensins is universal, small in size (45–54 amino acid residues), relative molecular mass (5–7 kDa). The defensins usually contain eight cysteines forming four disulfide bonds, forming three antiparallel β-chains and one α-helix in a conservative conformation, which is considered necessary for structure and function (Cools et al., 2017a; Campos et al., 2018; Bhattacharya et al., 2017). However, there have been few systematic studies on PDFs in B. napus so far. The genome-wide identification of BnaPDFs will provide a comprehensive insight into their family evolution and provide elite gene resources for the genetic improvement of rapeseed resistance to nutrient stresses.
Comprehensive analysis of molecular characteristics of BnaPDFs
The secondary structures of BnaPDFs were predicted to contain α-helix, extended chain, β-turn, and random coils, of which α-helix and random coils were the main components (Fig. S4). Most members contained 1–3 exons, and the gene structure was relatively simple.
Plant defensins can be divided into two classes according to their precursors (Lay & Anderson, 2005). Class I defensins composed of signal peptides and mature defensins and secreted into the extracellular space (Parisi et al., 2019). Class II defensins contained a C-terminal propeptide, and is mainly expressed constitutively in flowers and fruits of Solanaceae plants (Balandín et al., 2005). We used WoLF PSORT to subcellular localization of the PDF gene, and the result showed that it is extracellular region or secreted. Our predicted results are consistent with the characteristics of class I of defensins. In addition, all BnaPDF members contain signal peptides. Previous studies have confirmed that the signal peptide guides the newly synthesized protein to be secreted outside the organelles to perform functions (Hiller et al., 2004). Therefore, BnaPDF is synthesized in the cell and functions in the extracellular region.
Based on the protein-protein interaction network, we found that PDFs may interact with ethylene-responsive transcription factors, disease-related proteins, 1,3-glucanase and chitinase. Plants produced ethylene response transcription factors to reduce the attraction of herbivorous pests, and also activated the downstream transcriptional response of jasmonic acid, conferring resistance to several necrotrophic fungi (Berrocal-Lobo, Molina & Solano, 2002; Miyamoto et al., 2019). PDFs were also related to 1,3-glucanase and chitinase. Glucan and chitin were the main components of fungal cells, therefore, PDFs had strong antibacterial activity (Tian et al., 2007). According to the evolutionary relationship, most gene pairs had short branch lengths, indicating that differentiation has occurred recently.
Transcription factors initiated the transcription process of specific genes by interacting with cis-acting elements, thereby regulating gene expression (Wittkopp & Kalay, 2011). In this study, a variety of plant hormones and stress-related cis-elements were identified in the promoter regions of the BnaPDF family members, indicating that BnaPDFs might play an essential role in the adaption of rapeseed plants to stresses. miRNA is an important participant in mediating plant immunity to biological stress. It enhances the plant immune system by regulating the expression of plant hormones and target genes (Fei et al., 2016). At present, there are few researches on the interaction of PDFs and miRNAs, but this field should be studied more deeply. Several bioinformatic tools were employed to predict miRNAs upstream of PDFs in the study, and this approach may provide a new perspective for understanding plant defense mechanisms. It has been reported that miR-124 and miR-924 are involved in the pathogenesis of inflammatory bowel disease by negatively regulating α-defensin 5 mRNA and protein expression (Miles et al., 2016).
Potential involvement of BnaPDFs in rapeseed responses to diverse nutrient stresses
Plant defense response regulates the growth and development of plant roots, leaves, stems, bud, new pistil, blosomy pistil, ovule, silique, stamen, and sepal (Hegedüs & Marx, 2013). Tomato SlDef2 regulates flower development. Overexpression SlDef2 reduces pollen viability and seed production (Stotz, Spence & Wang, 2009). In this study, BnaA6.PDF2.2 and BnaC7.PDF2.2 were identified to be predominantly expressed in bud, silique, and stamen. However, BnaA5.PDF1.2b1, BnaA5.PDF1.2b2, and BnaA5.PDF1.2b3 had the highest expression levels in the bud, showed that different PDF members might play differential roles in different parts of rapeseed plants. It also implied that PDFs might be involved in the growth and development of the leaf, root, stamen, stem, and other organs of B. napus.
Nitrogen is an essential nutrient for plants, nitrate and ammonium are the two primary inorganic nitrogen sources absorbed and utilized by plants (Konishi & Yanagisawa, 2014). After plants absorb nitrate, part of it is directly transported to shoots or stored in vacuoles. The other part is transported to the shoot and converted to nitrite under nitrate reductase in the leaves. Nitrite reductase produces ammonium, which is assimilated into amino acids by the GS/GOGAT pathway (Tischner, 2000; Clément et al., 2018). Nitrogen absorption is a key step in nitrogen metabolism, and is one of the most important limiting factors for plant growth. Nitrogen deficiency seriously affects plants’ growth and development. Therefore, improving nitrogen use efficiency (NUE) is an important aspect of improving plant resistance to nitrogen deficiency (Hammad et al., 2017). This study analyzed the expression of BnaPDF family members under nitrate deficiency and ammonium toxicity treatments. Under nitrogen deficiency, chloroplast proteins in senescent leaves are degraded into amide-nitrogen compounds and redistributed to newly developed leaves (Hammad et al., 2017).We found that the general expression of PDF1.2s, PDF2.2s, PDF2.3s, and PDF2.5 was induced by nitrate limitation (Fig. 7). Several previous studies have shown that small peptides can be used as signal molecules in the protein kinase pathway to indirectly regulate the expression of other genes. AtPDF2.1 mediates ammonia metabolism by regulating the activity of glutamine synthase in Arabidopsis (Yao et al., 2019). When plants are exposed to ammonium stresses, PDF might regulate the activity of glutamine synthetase activity, thereby affecting the concentration of glutamine and the movement of NADH-GOGAT (Yao et al., 2019).
Phosphorus is one of indispensable macronutrient elements in the process of plant growth and development. It is an important part of many metabolites and macromolecules such as ATP, phospholipids, and nucleic acids in plants (Wang, Garvin & Kochian, 2002; Hu et al., 2019). In this study, most BnaPDFs are upregulated under phosphate starvation (Fig. 9), which indicated that BnaPDFs might play important roles in the resistance of rapeseed plants to phosphate deficiency. OsAFP1 interacts with phosphate ion, this ion is fixed by OsAFP1 dimer interface, the side chains of His37 in OsAFP1 coordinate with phosphate ion (Ochiai et al., 2020). HsAFP1 interacts with the phosphate group in phosphatidylinositol phosphate to induce changes in membrane permeability and exert antifungal activity (Cools et al., 2017b).
Potassium plays an important role in various physiological and metabolic processes, such as cell osmotic regulation, enzyme activation, chlorophyll synthesis, stomatal movement, and signal transduction (Wang, Garvin & Kochian, 2002). Under potassium deficiency, it was noted that the expression of most BnaPDF DEGs was significantly induced in the shoots but was inhibited in the roots (Fig. 10). Plant defensins are used as potassium channel blockers (Parisi et al., 2019; Vriens et al., 2016). For example, the sequence of AtPDF2.3 contains a toxin characteristic sequence (K-C5-R-G) that can block potassium channels (Vriens et al., 2016). The defensin-like ZmES4 is exclusively expressed in female gametes and interacts with the potassium channel KZM1, further leading to potassium influx (Amien et al., 2010). We also found the same toxin feature (K-C5-RG) in BnaPDF2.3s, so we speculate that BnaPDF2.3s and potassium channels exist interaction. We speculated that BnaPDFs might be involved in regulating the ion balance in cells and maintaining plant cell homeostasis (Fisher et al., 2012).
Soil salinity is one of the important environmental factors that restrict plant growth and development, and salt stress significantly reduces rapeseed yield (Munns & Tester, 2008; Ashraf & McNeilly, 2004). The expression of most BnaPDF DEGs was induced by salt stress (Fig. 11), and they might improve the salt tolerance of rapeseed plants. Indeed, PDFs were reported to be upregulated in salt stress, which activate the ROS scavenging system in plant cells, thereby protecting the photosystem and enhancing plant tolerance to abiotic stress (Khadka et al., 2020; Wu, Lin & Chuang, 2016).
Plant defensins chelate with cadmium through sulfhydryl groups, promote the secretion of cadmium outside the cell, reduce the intracellular cadmium content, and participate in cadmium transport and distribution (Luo et al., 2019b; Ben Ghnaya et al., 2009). AtPDF2.5 is significantly induced under cadmium stress. AtPDF2.5 reduces the free cadmium in the cell and enhance the resistance of Arabidopsis to cadmium by chelating the intracellular cadmium and mediating its efflux (Luo et al., 2019b). AtPDF2.6 also improves plant tolerance to cadmium by chelating with cadmium (Luo et al., 2019a). In this study, we found that the expression of some BnaPDFs was altered in response to cadmium toxicity, which indicated their potential participation in alleviating the toxicity of cadmium to rapeseed plants, and further promoted the combination of cadmium-chelators. In summary, analysis of tissue-specificity expression patterns and the transcriptional responses of BnaPDFs to various nutrient stresses indicated that BnaPDFs might play an essential role in different growth stages and stress responses of B. napus.
Plant diseases, especially fungal diseases, are one of the main reasons for crop yield reduction. At present, the application of plant defensins with broad-spectrum antibacterial activity in crop disease-resistant breeding is called a hot spot (Fisher et al., 2012). After the alfalfa defensin, MsDef1 was expressed in potatoes, and potatoes showed strong resistance to Verticillium Dahliae (Gao et al., 2000). The expression of alfalfa defensin MsDef4.2 in wheat enhances wheat resistance to leaf rust but does not affect the colonization of beneficial arbuscular mycorrhizal fungi on the roots (Kaur et al., 2017). The co-expression of Rs-AFP1 and chimeric chitinase in rapeseed enhances the resistance of Brassica napus to Sclerotinia sclerotiorum.
Conclusion
In this study, based on the identification and molecular characterization of the PDFs, the BnaPDF DEGs that were identified under various nutrient stresses might be used as elite gene resources for the genetic improvement of rapeseed plants to stresses. In addition, the research results could also provide references for a deeper understanding of the molecular evolution mechanism and potential functions of the PDF family members in B. napus.