Current situation and future perspectives for the use of fungi in the biomaterial industry and proposal for a new classification of fungal-derived materials
- Published
- Accepted
- Received
- Academic Editor
- Andre Fajardo
- Subject Areas
- Bioinspired Materials, Biomaterials, Composites
- Keywords
- Biological materials, Fungi, Mycelium, Mycomaterials, Bioindustry
- Copyright
- © 2023 Ballen et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Materials Science) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Current situation and future perspectives for the use of fungi in the biomaterial industry and proposal for a new classification of fungal-derived materials. PeerJ Materials Science 5:e31 https://doi.org/10.7717/peerj-matsci.31
Abstract
The potential applications of fungi in the development of new biomaterials derived from fungal mycelium have captured the attention from both the scientific community and the society. The notable ability of mycelium networks to self-construct and aggregate can be used to produce diverse biomaterials. These biomaterials can be created in a pure state, or both in conjunction with other organic/inorganic compounds. Recent advancements in mycomaterials have gained attention due to their sustainability and mechanical, thermogravimetric, and compression properties. Such properties contribute to reducing the reliance on environmentally problematic substrates within the industry. After a standardized and comprehensive review of publications on mycomaterials across different fields, such as biology, health, agriculture, engineering, and material sciences, we detected that publications on this theme are utterly scattered. This critical review enabled us to also propose a novel classification system for these fungal-derived materials to help to structure and standardize this emerging transdisciplinary field of knowledge.
Introduction
Fungi are a highly diverse group of organisms, occurring in all kinds of habitats on the planet and with high biotechnological importance in the industry. They have caught the attention of the scientific community and society for their applications in the development of new biomaterials (Hyde et al., 2019; Appels et al., 2020). Biomaterials derived from fungal mycelium are a recent type of material that takes advantage of the mycelial interlocking self-aggregating networks and can be explored to offer more competitive and environmentally friendly, and self-regenerative products to the market (Camere & Karana, 2017; Kavanagh, 2017; Karana et al., 2018; Gandia et al., 2021). Moreover, materials derived from mycelial fungi represent an alternative dissociation of products of petrochemical origin, which is a very significant aspect of sustainable development and innovation (Faruk et al., 2012; Koronis, Silva & Fontul, 2013; Lelivelt et al., 2015; Appels et al., 2020). The search for better solutions and opportunities for these alternative biomaterials has led to a growing increase in scientific production (deeply analyzed in the current study), resulting in options for improving the initially proposed materials as well as the emergence of new types of materials with various applications.
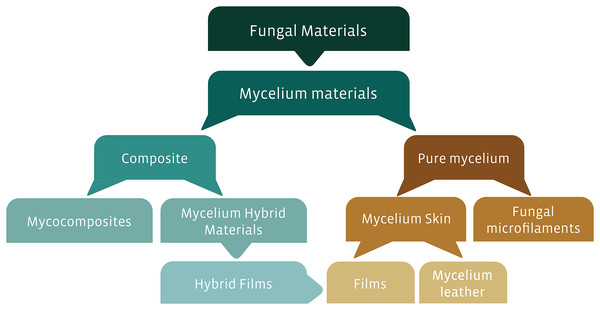
The rationale of our study is to offer a standardized classification proposal for these fungal-derived materials and, thus, offer help to the audience of all researchers who are interested in the subject, understanding its importance as a modern example of transdisciplinarity, in the way it will be addressed: (i) composite materials, comprising mycocomposites and hybrid materials derived from mycelium; and (ii) pure materials, which encompass materials such as films and foams of pure mycelium and microfilament materials of chitin derived from mycelia. More recently, an intersection between the hybrids and the pure ones has arisen, which contemplates the inclusion of additives on the pure mycelia to improve the mechanical characteristics, the latter will also be addressed in the current study.
Initially, the basic components to produce materials of fungal origin or mycomaterials will be addressed, namely fungi, substrates, additives, and the enzymes and fermentation methods commonly used for their processing. Next, the proposed classification will be directed to the corresponding definition and most relevant parameters for the production of bio-based materials.
The wood-colonizing fungi
Wood-decaying fungi perform a key role in the decomposition of organic matter, carbon cycle, and nutrients allocation (Mosquera, 2007; Blackwell, 2011; Badotti et al., 2017; Tedersoo et al., 2018). Despite the different evolutionary origins, two groups of fungi show similar ecological roles in decaying plant biomass: Basidiomycota and Ascomycota. These phyla contain macroscopic (mushroom) and microscopic unicellular (yeast) species. They are also present in different symbiotic relationships and as pathogens of other fungi, plants, and animals (Blackwell, 2011; Tomé et al., 2019). A range of fungal species from these phyla can naturally produce enzymes degrading cellulose, hemicellulose, and lignin into smaller molecules (Gautam et al., 2019). According to the type of wood decomposition and their growth preferences, Basidiomycota fungi have been classified as white-rot fungi (decomposing the three components of wood: cellulose, hemicellulose, and lignin) and brown-rot fungi (rapidly decomposing cellulose and hemicellulose, but also modifying the lignin) (Gupta et al., 2019). Most of the soft-rot fungi are Ascomycota, which penetrate the wood by forming characteristic cavities (Daniel, 2014). Their role in the carbon biogeochemical cycle as powerful wood decomposers is related to the highly efficient enzymatic repertoire in these organisms (Peralta et al., 2017; Ferreira et al., 2018).
Regarding the morphology of their cells, fungi can exhibit a somatic phase in the unicellular and pluricellular (or hyphal) forms. The yeast-like phase comprises single, delimited, and small cells (Loguercio-Leite, 2004). The hyphal phase, which mycomaterials (i.e., fungal-based materials) are made of, has elongated and cylindrical cells that extend continuously from their ends (apical growth). Depending on the interaction of the physical-chemical environment and the set of hyphae, called mycelium, which corresponds to the fungal body, it can be long or diffuse, short or branched, or both (Loguercio-Leite, 2004; Girometta et al., 2019). These traits can be related to features such as the strength and cohesion of the final product based on the mycomaterial. Delimiting the intracellular content and the external environment is the role of the cell wall of the fungal hyphae, which consists of glucans, chitin, and proteins (mainly mannoproteins), with ergosterol as the main constituent of the plasma membrane (Free, 2013). The cell wall performs functions such as giving rigidity and shape, allowing metabolism and ion exchange and is responsible for interactions with other organisms or materials (Free, 2013; Money, 2016).
In order to grow, the great majority of fungi require water, an anaerobic environment, an organic source of carbon and energy as well as nitrogen, phosphorus, sulfur, magnesium, potassium, and calcium, among other elements in different proportions (Money, 2016). The processes for obtaining these elements are diverse and take place mainly due to enzymatic mechanisms, such as those that occur in the degradation of plant biomass, which is carried out mainly by Basidiomycota fungi (Peralta et al., 2017; Ferreira et al., 2018; Tomé et al., 2019).
Usually, mycomaterials are produced from substrates derived from organic supports of plants or agro-industrial residues. The composition of plant tissues varies depending on the species, but, in general, they are made up of cellulose, hemicelluloses, and lignin (Motta et al., 2018). More commonly, several agro-industrial residues have been studied for the growth of fungi for the production of bioactive compounds, such as phenolic compounds, antioxidants (Torres-León et al., 2019), and enzymes (de Souza et al., 2006; Anto, Trivedi & Patel, 2006; Singh, Kapoor & Kumar, 2012; Diaz et al., 2016), but also mycelium-based composites (Lima et al., 2020). The fungal decomposition process results in a higher degradation pattern of lignocellulosic substances of agro-industrial residues, as is evident from the breakdown of the plant cell wall constituents as well as the increase in fungal enzyme activities.
The development of fungi on these residues is influenced by the production of enzymes that enable them to grow on the surface and is probably related to its composition (Geethanjali, Gowtham & Jayashankar, 2020). Studies carried out with Pleurotus pulmonarius, a Basidiomycota (Agaricomycetes) species, showed that the type of residue used for fermentation (wheat straw, corn straw, and soybean straw) directly influences the development of the fungus as mycelial growth rate, stipe length, pileus length, pileus width, and time to harvest (Wu et al., 2019). The areca husk, coffee husk, and paddy straw also are effectively used to grow ligninolytic fungi that showed high degradation of these wastes due to enzymatic action, such as laccase, manganese peroxidase (MnP), lignin peroxidase (LiP), and carboxymethyl cellulase (CMCase) (Geethanjali, Gowtham & Jayashankar, 2020). Therefore, the complete degradation process of the agro-industrial waste by the filamentous fungi hyphae occurs due to its hydrolytic enzymes that enable them to grow on the substrate’s surface and penetrate the intraparticle spaces to colonize all the substrate.
Basidiomycota is the most common fungal group for working with mycomaterials. This phylum has enzymatic and non-enzymatic mechanisms to degrade lignocellulosic biomass (Pelletier et al., 2013; Girometta et al., 2019). The enzymatic ones are two types, the first is an important hydrolytic system in the degradation of polysaccharides, which contains carbohydrate-active enzymes such as xylanases and cellulases, and the second is an oxidative ligninolytic system, which encompasses peroxidases (Peralta et al., 2017; Ferreira et al., 2018). It is worth noting that both mechanisms are extracellular, occurring due to the development of mycelial fungal hyphae on dense substrates (Pelletier et al., 2013; Peralta et al., 2017; Girometta et al., 2019), which can facilitate the handling of the fungal metabolites for different purposes.
The exceptional ligninolytic system of basidiomicotan fungi has led these organisms and their metabolites to be used in different types of industries, such as food, pharmaceutical, textile, and biotechnological industries (Peralta et al., 2017; Badotti et al., 2017; Tomé et al., 2019). In this last application, this edge has generated great interest due to the use of fungal structures (e.g., mycelia) or subproducts (e.g., enzymes) in the development of different types of materials. These fungal-based materials, or more strictly, mycelial materials, can be classified as pure or composite materials. Among the composite mycelium materials, there are the mycocomposites—a blend formed by substrate and mycelia—and mycelium hybrid materials, which comprise, for instance, hybrid films. Alternatively, pure mycelial materials can comprise the mycelial skins, such as films or amadou; and fungal microfilaments, such as nanopapers, and nanofibrils (Fig. 1). Usually, these fungal-based materials have great potential to replace materials of petrochemical origin, a source extensively recognized for their polluting profile (Jones et al., 2017; Girometta et al., 2019). In the development of new bio-based materials, there are several variables inherent to each species, type of substrates, and production system that enhance the generation of products with different characteristics and uses. We can highlight, for instance, characteristics that improve the use of these materials as structural, acoustic, insulating material, shock absorber, fire protection, biodegradable, flexible, rigid, absorbent, or water-resistant (Holt et al., 2012; Arifin & Yusuf, 2013; Pelletier et al., 2013, 2017; Lelivelt et al., 2015; Haneef et al., 2017).
Figure 1: Proposed classification of fungal-based materials, derived from the literature investigated in this review.
Survey methodology
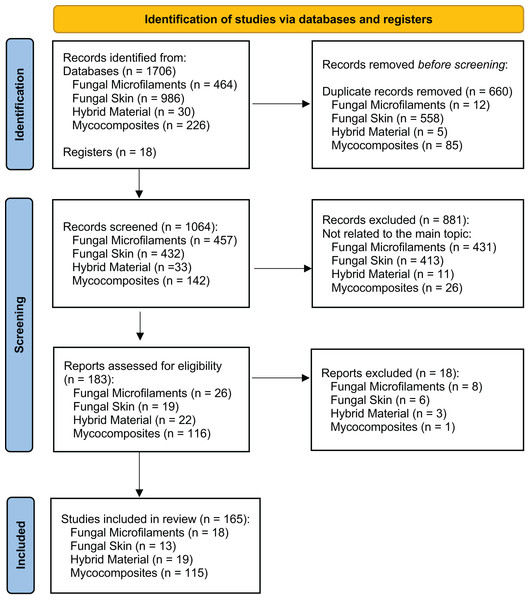
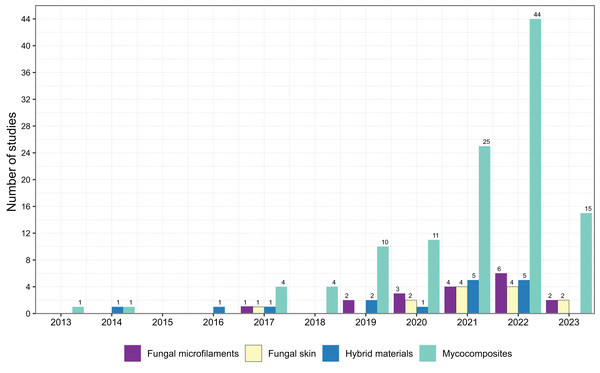
A comprehensive review of the scientific literature was performed to investigate when bio-based materials have become a new and important field of biotechnology and to indicate the relevant terms for describing the bio-based materials. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 protocol as a standardized method for reporting a systematic review (Fig. 2) (Page et al., 2021). We conducted the literature searches in May 2023 in three databases: Web of Science—Core Collection, Scopus (Elsevier), and PubMed. We performed four searches using the three databases, considering a group of keywords for each search separately, grouped in four main topics: ‘Fungal Microfilaments’, ‘Fungal Skin’, ‘Hybrid Material’, and ‘Mycocomposites’ (Fig. 3). All the selected keywords and search strategies are shown in Table S1. These keywords were also used to determine which terms are used as synonyms in the literature.
Figure 2: Global view of the records through database searching.
PRISMA diagram shows the main results of the searches for articles in three databases: Web of Science—Core Collection, Scopus (Elsevier), and PubMed. We performed four searches in May 2023 using the three databases, considering a group of keywords for each search separately, grouped in four main topics: ‘Fungal Microfilaments’, ‘Fungal Skin’, ‘Hybrid Material’, and ‘Mycocomposites’, according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) suggested flow-chart for literature selection process (Page et al., 2021).Figure 3: Scientific literature available in the field of biotechnology that discusses bio-based materials with the four most prevalent terms in literature and the number of studies published by year.
For each record identified through database searching (n = 1,706), we evaluated the title, the abstract, and their keywords. Results were exported and analyzed using two scripts developed in Python 3. Firstly, results were submitted in a unique format because they came from different sources. For this purpose, the format_input.py script (Jasper, 2022a) was developed. Then, the script filtered the records based on the DOI or the document title, creating an output file divided into unique records (with DOI), without DOI, and duplicated items. For removing duplicates, we used the script remove_duplicates.py (Jasper, 2022b). We added 18 studies that were not retrieved in the database searches that we identified in the literature, totaling 1,724 records. After removing duplicates, we screened 1064 studies considered appropriate for further investigation, according to our eligibility criteria. The eligibility criteria for inclusion of studies consisted of considering articles that present any term related to bio-based materials, such as those presented in Fig. 1, without restrictions of date, file format, or language. We considered the ‘material application’, which is the field in which this bio-based material is used; and the ‘origin’ of the material, which includes only materials strictly derived from filamentous fungi (Table S2). We excluded 881 records that were not related to the four main topics, evaluated the remained 183 records, and included 165 studies in the systematic review, considering the topics fungal microfilaments (n = 18), fungal skin (n = 13), hybrid material (n = 19), and mycocomposites (n = 115) (Fig. 2).
We identified that the first term that arose in the literature was ‘mycocomposites’, after 2013 (Fig. 3), and is the most used term to describe composite materials based on mycelium. The other terms were published only in 2017. It is worth noting that the literature cited by these articles is intimately connected and overlapped, which indicates that the same study frequently describes more than one type of bio-based materials.
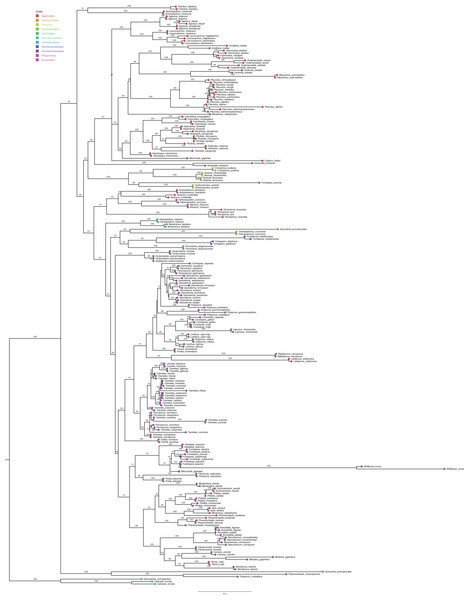
After retrieving the studies, we identified the wood-decaying fungal groups that can produce laccase enzymes, which can be important for mycomaterials. We selected the fungal species and searched for the internal transcribed spacer (ITS), the region used as a barcode for most of the fungal species, at GenBank database (https://www.ncbi.nlm.nih.gov/nucleotide/) to perform a phylogenetic analysis. We then checked the ability of these species to produce laccase at the articles that were retrieved and at the CAZy database (Carbohydrate Active Enzymes database) (http://www.cazy.org/) (Table S3) (Drula et al., 2022). We aligned the sequences from GenBank using Geneious Prime 2023.1.2 with the MUSCLE algorithm, which resulted in 2,760 bp from 237 sequences. The GTR+F+I+G4 evolutionary model was selected using ModelFinder (Chernomor, von Haeseler & Minh, 2016; Kalyaanamoorthy et al., 2017), based on Bayesian information criterion (BIC) scores. Phylogeny was performed using a maximum likelihood with IQ-TREE multicore v.1.6.12 (Hoang et al., 2018; Nguyen et al., 2020), generating 1,000 bootstraps replicates (Fig. 4) and edited in Figtree v.1.4.3 (Rambaut, 2017).
Figure 4: Maximum likelihood phylogenetic of laccase-enzymes producers from white-rot Basidiomycota fungi.
Tree was generated with 1,000 bootstraps replicates using the internal transcribed spacer and comprise 237 taxa. Orders are indicated at the tips of nodes.Enzymes and mycomaterials
Since the carbohydrate-active enzymes (CAZymes) produced by the wood-decaying fungi can promote the break of recalcitrant plant-based materials into subproducts of industrial interest, such as lignin, cellulose, and pectin, the production of these enzymes can be a focal point to be industrially exploited during the production of mycomaterials. A typical lignocellulolytic enzyme produced by basidiomycetes is laccase. It is commonly secreted by several genera, such as Phanerochaete, Trametes, Pycnoporus, Nematoloma, Sporotrichum, and Stropharia, with more than 125 different laccase-producing basidiomycetes genes described (Mikolasch & Schauer, 2009).
Fungal laccases are multi-copper oxidases with high versatility and low catalytic requirements, requiring only atmospheric O2 for activation, and reducing it to water. This high redox potential, especially of certain basidiomycotan laccases, significantly increases their oxidation capacity compared to bacterial laccases, which provides these enzymes with a great potential for applications as biocatalysts in various biological activities related to the formation of pigments, degradation of lignin, detoxification, and pathogenesis. Moreover, the high industrial potential of these fungal-borne laccases is mainly due to their high capacity for relative non-specific oxidation, their independence from cofactors, and also by the use of available oxygen as an electron acceptor (de Salas & Camarero, 2021).
White-rot fungi are the most common laccase producers, and the major structural changes that occur by their action include demethylation, oxidation of carbon atoms, modification of the chains between carbon-carbon of the phenylpropane units, direct modifications of a chain arylglycerol-β-aryl ether, and hydroxylation and modification of aromatic rings. Laccases catalyze oxidation by extracting an electron from a phenolic substrate, generating a phenoxy radical (Kawai, Nakagawa & Ohashi, 2002). The radicals formed act in non-catalytic reactions, such as radical coupling, deprotonation, and nucleophilic attack by water. Therefore, these multiple reactions produce polymerization, alkyl-aryl breaks, carbon oxidation, and demethylation (Kawai, Nakagawa & Ohashi, 2002).
In addition to laccase, enzymatic treatments of lignocellulosic materials with oxidases have also been reported, based on enzymatic oxidation and subsequent cross-linking of lignin and/or other plant-derived phenolics, such as tannins, promoting the modification of the chemical structure of lignin with exposure to these enzymes. The performance of peroxidase is similar to laccase; however, these require hydrogen peroxide as an electron acceptor. The most commonly used peroxidases for modifying lignocellulosic materials are lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP), and horseradish peroxidase (HRP) (Widsten & Kandelbauer, 2008).
The mechanism of action of MnP includes the oxidation of Mn2+ to Mn3+, which oxidizes a wide range of phenolic lignin substrates. It also can oxidize non-phenolic structures, oxidize, and depolarize natural and synthetic lignin and recalcitrant compounds. On the other hand, LiP action involves the formation of cationic radicals through the oxidation of an electron, leading to side-chain cleavage, demethylation, intramolecular addition, and rearrangements. It also performs other oxidative processes such as hydroxylation of the methylene benzyl group, oxidation of benzyl alcohols, aldehydes, or ketones corresponding to the oxidation of phenol. Additionally, LiP also has an additional ability to oxidize a variety of phenolic compounds, with veratyl alcohol suggested as a redox mediator to improve lignin degradation activity. The enzyme catalytic cycle involves three main steps: (i) oxidation of the iron enzyme (Fe(III)) by hydrogen peroxide as an electron acceptor; (ii) the oxo-ferryl intermediate is reduced by a substrate molecule, which donates an electron to form the second intermediate, and (iii) subsequent donation of a second electron to compound II by the reduced substrate, returning the LiP to the oxidized ferric (Rekik et al., 2019).
Substrates for fungal growth and development of biomaterials
The agro-industrial activity is responsible for a large percentage of lignocellulosic residues that are still underutilized, such as palm oil and sisal residues, resulting from the defibration and processing of sisal, among other residues (Semedo, 2006; Moraes et al., 2017; Santos & da Silva, 2018). For instance, only in Brazil, significant amounts of biomass are generated, and it is estimated that the volume may reach 1 Gt in 2030 (Moraes et al., 2017). The residues generated in the agro-industrial activity are useful for obtaining products for energy, food, and other biotechnological applications, as they are abundant, renewable, and cheap (Siqueira et al., 2010; FitzPatrick et al., 2010; Jones et al., 2017).
Many approaches have been proposed to add value to agro-industrial waste, such as the development of materials derived from fungi since they can break plant-based substrates (Siqueira et al., 2010; FitzPatrick et al., 2010; Jones, Huynh & John, 2018; Jones et al., 2020). In the production of these mycocomposites, the substrate can serve as the basic structure of the matrix for the material and as a source of nutrients for the growth of the fungus. Factors related to the size and structure of the substrate are relevant in the development of this type of material (Lelivelt et al., 2015; Pelletier et al., 2017; Girometta et al., 2019).
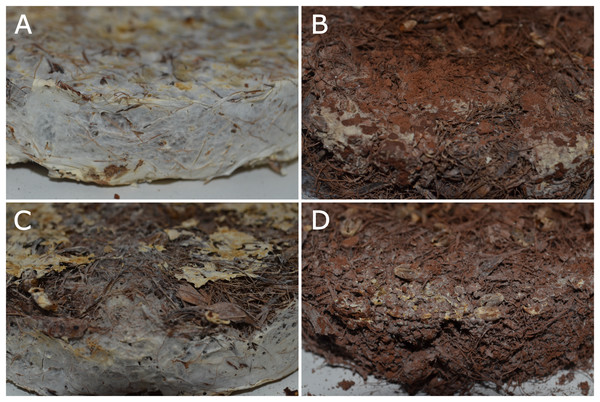
The type of substrate used for the growth of fungi can induce specific physiological and biochemical changes, influencing the density of cell biomass, which is reflected in the mechanical characteristics when it comes to the development of fungal-based materials (Elisashvili & Kvesitadze, 2005; Pelletier et al., 2013; Lelivelt et al., 2015; Girometta et al., 2019). Several substrates have been used for the growth of fungi belonging to Basidiomycota, aiming at products with different mechanical characteristics (Elisashvili & Kvesitadze, 2005; Pelletier et al., 2013; Jones et al., 2017; Girometta et al., 2019). For instance, when the same isolate of Ganoderma lucidum (strain CCMB601) was inoculated to substrates based on different concentrations of mining waste and palm oil residues (Fig. 5) and incubated at the same conditions of temperature and humidity, the fungal mycelia grew and formed a different matrix depending upon the concentration of substrate particles (Fig. 5; LAB Sierra, 2022, personal observation). Fungal mycelium freely grew in control (without waste, Fig. 5A) and in the presence of 50% of mining waste proportional to the substrate (substrate:mining waste = 2:1, Fig. 5C) while in presence of 100% (substrate:mining waste = 1:1, Fig. 5B) and 200% of mining waste (substrate:mining waste = 1:2, Fig. 5D), mycelium growth was reduced.
Figure 5: The same isolate of the fungus Ganoderma lucidum CCMB601 (Basidiomycota) growing on different substrates, with different compositions and sizes of particles.
(A) Palm oil residues; (B) palm oil residues associated with 100% of mining waste (1:1); (C) palm oil residues associated to 50% of mining waste (2:1); and (D) palm oil residues associated to 200% of mining waste (1:2). All substrates were incubated at the same temperature, time, and relative humidity, using the same size of mold.Additives to enhance fungal growth on substrates
The use of additives for fungal growth is mostly addressed to supply macronutrients and/or micronutrients, often absent in the substrate, and their use, in many cases, represents a considerable increase in substrate colonization by fungal mycelia (Donini et al., 2006; Sánchez, 2010). In the development of materials derived from fungi, the use of additives has been explored in two ways, as a nutritional supplement or as growth support for fungi (Lelivelt et al., 2015; Jiang et al., 2016). For fungal growth, the use of additives is mainly to produce edible fungi, with preference being given to supplementation with wheat bran, sorghum, or corn, due to their rich content in carbon, nitrogen, and other elements (Donini et al., 2006; Sánchez, 2010). As the additives directly influence fungal nutrition and growth, they induce mycelial production, contributing to improve the mechanical features of the final product (Bayer, McIntyre & Swersey, 2008; Arifin & Yusuf, 2013; Lelivelt et al., 2015) (Table 1).
| Fungal species | Additive | Usage of additive | Effect of additive | Reference |
|---|---|---|---|---|
| Ganoderma lucidum | Chitin and β-glucan based oligosaccharides | Nutritional supplement and growth support | Increase the internal bond strength, the rupture and elasticity point | Liu et al. (2019) |
| Corn flour, KH2PO4, K2HPO4, MgSO4, and glucose | Nutritional supplement | Improve growth and correct pH | ||
| T. flavus | Lizardite | Growth support | Increase the internal bond strength | Li et al. (2022) |
| G. lucidum | D-glucose, alkali lignin | Growth support | Increase the internal bond strength | Antinori et al. (2021) |
| Pleurotus ostreatus, P. citrinopileatus, P. eryngii, and G. lucidum | Flour, whole husk psyllium, and whole chicken feather | Nutritional supplement and growth support | Improve growth, increase the internal bond strength | Silverman, Cao & Cobb (2020) |
| Trichoderma asperellum, Agaricus bisphorus, G. lucidum, P. ostreatus | Oat husk, rapeseed cake, pine sawdust milled, oat straw, birch sawdust | Nutritional supplement and growth support | Improve growth, increase the internal bond strength | Tacer-Caba et al. (2020) |
| Bacillus amyloliquefaciens | Polypropylene | Growth support | Increase the tensile strength | Răut et al. (2021) |
| P. ostreatus, Oudemansiella radicata, Acremonium sp. | CaCO3 | Growth support | Increase the tensile strength | Gou et al. (2021) |
| P. ostreatus | Latex | Slowing the growth rate, growth support | Late fungal growth and produced strength | He et al. (2014) |
| G. lucidum, P. ostreatus | Clay (65.50% SiO2, 1.10% TiO2, 21.50% Al2O3, 8.9% Fe2O3, 0.30% CaO, 0.80% MgO, 1.80% K2O, 0.10% Na2O, 0.4% Mn) | Growth support | Increase the tensile strength | Jauk et al. (2021) |
| Not specified | Bacterial cellulose | Growth support | No conclusions | Hoenerloh, Ozkan & Scott (2022) |
| T. versicolor | Cellulose nanofibrils | Growth support | Enhance strength | Sun et al. (2019) |
| T. versicolor | Bacterial cellulose | Growth support | Strengthening of internal bonding | Elsacker et al. (2021) |
| G. lucidum | Cotton plant materials (starch, gypsum, cotton cores and cottonseed hulls) | Growth support | Strengthening of internal bonding | Holt et al. (2012) |
| Pleurotus sp | Carrageenan, chitosan, and xanthan gum |
Growth support | Increase mechanical properties | López Nava et al. (2016) |
| Not specified | Carbohydrates, minerals (not specified) | Nutritional supplement | Improve growth | Tudryn et al. (2018) |
| Mortierella alpina, Mucor circinelloides | Polyunsaturated fatty acids, glucan, proteins, chitin, and other natural polymers | Nutritional supplement and growth support | Improve growth, strength the internal bonding |
Meyer et al. (2020) |
| Lentinula edodes | Wheat bran | Nutritional supplement | Nutritional supplement | Matos et al. (2019) |
Fungal production or growth systems for fungal-based material
Industrial-type systems for fungal growth have been developed, mainly to obtain the metabolites produced by them during growth. Such systems are called the fermentation process (or better-named bioprocess), which can be of the submerged (liquid) type (LSF) or in a solid state (SSF). Fermentation (or bioprocess) is the process by which a microorganism develops from a source of carbon and nutrients, aiming to obtain a product (Chahal, 1985; Mantovani et al., 2012; Chilakamarry et al., 2022).
The LSF occurs in the presence of water and usually with soluble substrates, and SSF occurs in the absence, or almost absence, of free water (Chahal, 1985; Mantovani et al., 2012; Chilakamarry et al., 2022). In SSF systems, growth takes place on the surface of the substrate, functioning as a source of growth and as a support, one of the advantages of these systems is the feasibility of using agro-industrial waste, especially for countries with an abundance of these kinds of wastes, such as Brazil, focusing on environmental sustainability (Chilakamarry et al., 2022). The use of these LSF and SSF systems is recently gaining new uses and is no longer limited only to the production of its metabolites but also to the direct improvement of the structure of the organism after growth, such as mushroom cultivation, mainly for the development of bio-derivative materials.
Fungal-based material
Materials derived from fungi, which are bio-based, have been gradually positioning themselves within the area of materials science in an important way. The increasing interest in fungal-based materials is mainly due to the use of renewable raw materials, biodegradability, low energy consumption for their production, profitability potential, competitiveness with materials of petrochemical origin (cost-benefit ratio for the environment), and little generation of polluting waste; these being definitive factors in the circular economy and within sustainability policies (Macarthur, Waughray & Stuchtey, 2006; Jiang et al., 2016; Girometta et al., 2019; Appels et al., 2020; Appels & Wösten, 2021).
The development of materials derived from fungi is shown as a solution to the challenges posed by sustainability, mainly as a successful example of transdisciplinarity, partially overcoming some obstacles related to the standardization of the production process and the use of technical standards for characterization of the final product (Tejedor, Segalàs & Rosas-Casals, 2018; Girometta et al., 2019). Transdisciplinarity has allowed the integration of knowledge and collaboration between areas such as microbiology, design, and materials engineering. Moreover, it has led in a short time to the production of innovative and competitive materials of fungal origin in different markets, such as plastics, where EPS expanded polystyrene had a world market of 6.62 million tons for 2016 and is responsible for the large amount of plastic waste, which has already been found in coastal regions, and in the digestive system of eight species of fish (Macarthur, Waughray & Stuchtey, 2006; Tejedor, Segalàs & Rosas-Casals, 2018; Girometta et al., 2019; Appels et al., 2020; Song et al., 2020; Appels & Wösten, 2021).
Standards for the mechanical characterization of materials derived from fungi
The use of technical standards for the characterization of materials allows to determine the physical, chemical, and mechanical characteristics of the product, aiming to know the material and establish concomitantly the different applications. Its use seeks to standardize processes to reach reliable and reproducible results, thus achieving safe materials of quality and good performance (da Luz & de Almeida, 2012; Ratner et al., 2013). Different countries use specific standards according to their criteria and needs; however, it is common to use international standards that serve as a reference. An example is the standards established by the American Society for Testing and Materials (ASTM), which has around 12,800 voluntary agreements on globally applicable standards (da Luz & de Almeida, 2012; Ratner et al., 2013).
To date, no specific standards have been established for the characterization of materials derived from fungi; however, in some cases, the existing standards for synthetic materials are used, such as ASTM and ISO (Table 2), which together with discretion in the publication of data (due to the preference in the production of patents), have led to few reliable comparisons due to the variation of methods and standard settings (Holt et al., 2012; Girometta et al., 2019). It should be noted that, despite this type of materials being a recent and pioneering field, the standardization of production systems and know-how is being mostly in the hands of the companies that carry out the economic exploitation of these materials, which is one of the great challenges for their industrial production (Girometta et al., 2019).
| Mechanical characteristic | Standards used | Reference |
|---|---|---|
| Bending by the three-point bending method to determine the stiffness of the material and the geometry of the material | -ASTM D7250/D7250M-16 Standard practice for determining sandwich beam flexural and shear stiffness -ASTM C393/C393M-11e1 Standard test method for core shear properties of sandwich constructions by beam flexure |
Jiang et al. (2016) |
| Acoustic material properties, impedance, and absorption of acoustic materials | -ASTM E1050, 2010 Standard test method for impedance and absorption of acoustical materials using a tube, two microphone, and a digital frequency analysis system. ASTM International -ISO 10534-1, 1996. Acoustics—determination of sound absorption coefficient and impedance in impedance tubes part 1: method using standing wave ratio. International Organization for Standardization, Geneva. |
Pelletier et al. (2013) |
| Density determination | -ASTM D792-00 Standard test methods for density and specific gravity (relative density) of plastics by displacement |
Arifin & Yusuf (2013) |
| Acoustic properties of the material were characterized to determine acoustic absorption, reflection, and continuous transmission | -ASTM E1050, 2010 Standard test method for impedance and absorption of acoustical materials using a tube, two microphone, and a digital frequency analysis system. ASTM International -ISO 10534-1, 1996. Acoustics—determination of sound absorption coefficient and impedance in impedance tubes—part 1: method using standing wave ratio. International Organization for Standardization, Geneva. -ISO 10534-2, 1998. Acoustics—determination of sound absorption coefficient and impedance in impedance tubes—part 2: Transfer function method. International Organization for Standardization, Geneva. |
Pelletier et al. (2017) |
| The tests were carried out to evaluate the physical and mechanical properties of the material, such as compressive strength, bending strength, modulus of elasticity, density, dimensional stability, accelerated aging, water absorption, calorimetry, and thermal conductivity | -ASTM C165-07 Standard test method for measuring compressive properties for thermal insulations, ASTM International (2007). -ASTM C203-05a Standard test methods for breaking load and flexural properties of block-type thermal insulation, ASTM International (2005). -ASTM C303-10 Standard test method for dimensions and density of performed block and board-type thermal insulation, ASTM International (2010). -ASTM C481-99 Standard test method for laboratory aging of sandwich constructions, ASTM International (2005). -ASTM C1134-90 Standard test method for water retention of rigid thermal insulations following partial immersion, ASTM International (2007). -ASTM E1354-11 Standard test method for heat and visible smoke release rates for materials and products using oxygen consumption calorimeter, ASTM International (2011). |
Holt et al. (2012) |
| Use of standards for comparative purposes of density, compression, and flexion of mycocomposites with EPS | -ASTM C578-04 Standard specification for rigid, cellular polystyrene thermal insulation, West Conshohocken, PA (2004). -ASTM C62-13a Standard specification for building brick (solid masonry units made from clay or shale), West Conshohocken, PA (2013). |
Jones et al. (2017) |
| Density was evaluated without following any standard and compressive strength was also evaluated following the standard | -ASTM C165-2007 Standard test method for measuring compressive properties of thermal insulations. |
He et al. (2014) |
| Thermal conductivity and compressive strength were evaluated | -ASTM D5334-14 Standard test method for determination of thermal conductivity of soil and soft rock by thermal needle probe procedure -ASTM D2166/D2166M-13 Standard test method for unconfined compressive strength of cohesive soil |
Yang et al. (2017) |
Some study approaches have tested fungal components as biomaterials, such as films, mycocomposites, and fungal microfilaments. To facilitate the understanding of the material derived from fungi according to its composition and final product, we proposed a diagram (Fig. 1) based on classifications described in the literature (Karana et al., 2018; Girometta et al., 2019; Appels et al., 2020; Gandia et al., 2021). We emphasize that in this review only materials derived from mycelium will be addressed exclusively. Materials derived from fungi of leveduriform origin (unicellular fungi) used in symbiosis with bacteria for the production of biomaterials (e.g., Kombucha-derived biofilm), as well as products derived from fungi such as hydrophobins, used in materials as fire retardants, are outside the scope of our systematic review (Alongi et al., 2014; Gilbert et al., 2021).
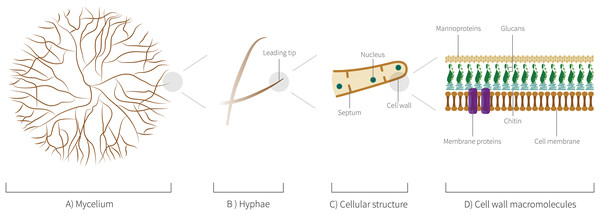
Among the criteria for choosing the species of fungi to be used in the field of these biomaterials, it is important to reinforce that the fungi cannot be pathogenic. Thus, preferably edible fungi or those that are harmless to man and other animals are considered excellent candidates for the study and development. Therefore, it is not surprising that among the genera, most studied as biomaterials are wood-decomposing Basidiomycota, especially species of the genera Pleurotus, Ganoderma, Schizophyllum, Trametes, and Agaricus. The 24 species of 18 distinct genera mentioned here also have a morphological and biochemical ultrastructure that give naturally interesting characteristics and that will be discussed ahead. In Fig. 6, we highlight the level of organization of mycelium structure from hyphae to cellular structure.
Figure 6: Mycelium cellular structure and macromolecules.
(A) Schematic representation of mycelium showing a branched network of hyphae. (B) Schematic representation showing the leading tip, the last cell at the end of each hyphae, a central point of the metabolic activities. (C) Schematic representation of the hyphal cellular structure. (D) Schematic representation of cell wall macromolecules, such as proteins of membrane.Mycelium materials
Materials derived from mycelium are a recent type of material that takes advantage of forming interlocking networks capable of self-aggregating or adding other components that exhibit no inhibitions for fungal growth (Camere & Karana, 2017; Kavanagh, 2017; Karana et al., 2018; Gandia et al., 2021). This aggregation capacity has been explored to optimize and offer the market more competitive products. In general, the macroscopic and mechanical characteristics of mycelium-based materials are determined by variables such as the fungal strains, substrates, additives, growth conditions, and production system, which means that a change in any of these parameters will reflect in the characteristics of the final product (Lelivelt et al., 2015; Jiang et al., 2016; Camere & Karana, 2017; Jones et al., 2017; Karana et al., 2018; Appels et al., 2018, 2019, 2020; Girometta et al., 2019; Appels & Wösten, 2021).
Materials derived from mycelium can be classified into two types: (i) compounds (or composites), which refer to materials made up of two or more materials, one of which must necessarily be the fungal structures; (ii) pure materials, consisting exclusively of the presence of fungal hyphae (Karana et al., 2018; Appels et al., 2020; Gandia et al., 2021). For both types of materials, the pre-and post-treatment variables are diverse, aiming at optimizing their mechanical behavior. Each type of material, its generalities, and alternative names will be better subsequently described.
Composites of mycelium materials
In the area of materials science, composites are materials composed of two or more different phases, which differ in shape and constitution, in which one phase functions as a matrix and another as a reinforcement or aggregative. In the case of materials of fungal origin, hyphae function as binders (Meyers et al., 2008; Ramakrishna & Huang, 2016; Girometta et al., 2019). Remarkably, the mechanical properties are significantly different from those that occur in their constituents individually (Meyers et al., 2008; Ramakrishna & Huang, 2016). Biocomposites are a type of compounds in which one or more phases are of biological origin, that is, they are bio-derived and, in many cases, they can be bio-inspired materials (Faruk et al., 2012; Koronis, Silva & Fontul, 2013; Lelivelt et al., 2015; Jiang et al., 2016; Girometta et al., 2019). For instance, one product that uses agro-industrial waste as a surface for fungal growth can be considered as one type of biocomposite.
Mycocomposites
The term mycocomposite was coined by Jiang et al. (2016) and will be adopted in this review to refer to composite materials (Jiang et al., 2016). In mycocomposites (Table S4), the two main phases are: (i) the matrix phase, consisting mainly of agro-industrial residues, which function as a carbon source for the growth of fungal mycelium; (ii) the aggregating phase, constituted by the mycelium (Fig. 1), which, in turn, functions as a reinforcing material due to its chemical constitution, mainly chitin and glucans (Meyers et al., 2008; Arifin & Yusuf, 2013; Pelletier et al., 2013; Lelivelt et al., 2015; Jiang et al., 2016; Yang et al., 2017; Girometta et al., 2019; Hyde et al., 2019). It is important to highlight that the mycelium functions as a highly branched network, acting as an interfacial binder, which adds not only the substrate but other components of both organic and inorganic constitution (Arifin & Yusuf, 2013; Lelivelt et al., 2015; Jiang et al., 2016).
The development of mycocomposites has gained prominence as a disruptive, bioinspired, and bio-derived technology that was first introduced to the market as an innovative substitute for polystyrene, recognized as an inherently sustainable material due to the initial origin of its constituents (Bayer, McIntyre & Swersey, 2008; Holt et al., 2012; Arifin & Yusuf, 2013; Jiang et al., 2016; Teixeira et al., 2018; Girometta et al., 2019). The emergence of mycocomposites especially represents a dissociation of materials of petrochemical origin, presented as a great contribution to the actions necessary for sustainable development and the fulfillment of the objectives of the 2030 agenda for sustainable development (Macarthur, Waughray & Stuchtey, 2006; Jones et al., 2017; Girometta et al., 2019; Appels et al., 2020; Appels & Wösten, 2021).
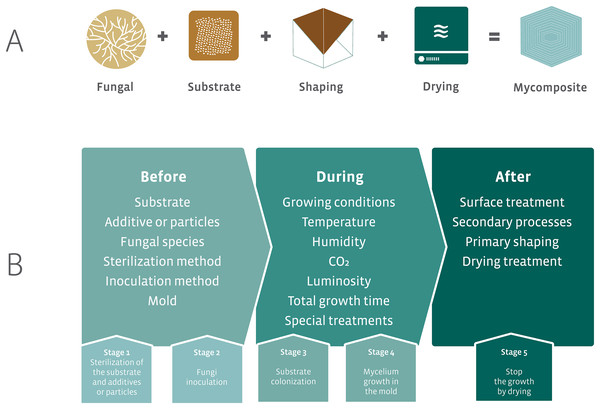
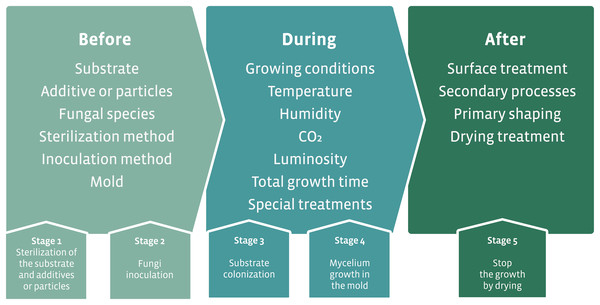
The initial proposal for the development of mycocomposites was inspired by the natural growth of fungi on substrates, such as wood. The industrial proposal differs from the natural process by optimizing the fungal growth in different substrates that result in products with different mechanical characteristics using additives for fungal growth and nutrition, and manipulation of physicochemical parameters. The accelerated fungal growth is followed by a final drying phase made in the industrial process (Fig. 7A). This last phase in the industrial process aims to stop the growth of the fungus and avoid the formation of basidiomata (in the case of the use of Basidiomycota species). The fungal strain is associated with a substrate, submitted to a process of shaping followed by a drying step to produce the mycocomposite (Fig. 7A) (Bayer, McIntyre & Swersey, 2008; Lelivelt et al., 2015; Jiang et al., 2016).
Figure 7: Elements required for the development of mycocomposites.
(A) Basic elements for the development of industrial mycocomposites. (B) Variables that influence the mechanical and physical characteristics of the mycocomposites, according to the stages of the production process. Modified from Karana et al. (2018) and Girometta et al. (2019).In the production process of mycocomposites, several variables can be considered, all of which are subject to research and closely related to both substrate and fungi, the raw materials used, and the processes used to obtain a product with differentiated and competitive mechanical characteristics for distinct purposes (Fig. 7B) (Jones et al., 2017; Girometta et al., 2019).
Hybrid mycelium materials
As with mycocomposites, hybrid mycelium materials are compounds based on fungal mycelium (Table S4), with the difference that the latter can be added with organic and inorganic compounds (Attias, 2020; Trabelsi et al., 2021; Elsacker et al., 2021; Elsacker, De Laet & Peeters, 2022). The role of additives in the composition of these hybrids arises as a response to the need to improve the mechanical behavior of mycocomposites in natura (only mycelium and substrate). It is expected that these additives allow an improvement in the production systems of these materials, work as binders supplying the limitations caused by the weak union between the substrate and the mycelium, fill the interfacial spaces, function as a carbon source, and concomitantly as a skeleton or support for growth (Sun et al., 2019; Attias, 2020; Elsacker et al., 2021).
Among the additives described are cellulose derivatives, whether microfibrils or cellulosic nanomaterials, the latter include cellulose nanocrystals (CNC), cellulose nanofibrils (CNF), and bacterial nanocellulose (NCB). Other additives are carbon nanotubes (CNT), polyacrylonitrile nanofibers (PAN), montmorillonite nanoclay, ground glass, and latex, among others (Nawawi et al., 2020a; Trabelsi et al., 2021; Sayfutdinova et al., 2022; Elsacker, De Laet & Peeters, 2022). Of special attention is the need for separation between compositions of coarse materials, a term used for compounds of agricultural residues or biowaste linked to the mycelium, and compositions with refined or sensitive materials, a term used when nanomaterials are part of the composition. The exploitation of the latter for the production of materials derived from mycelium is more recent and has a smaller number of publications, and this nanobiotechnology is considered promising for the future (El Naschie, 2006; Trabelsi et al., 2021).
The use of nanostructures as additional binders to mycelial structures, produced from renewable, biodegradable raw materials of low density and economic value, such as lignocellulosic fibers, which are very abundant in the world, has ecological and environmental benefits, in addition to providing excellent properties for mechanical features such as rigidity and tensile strength, flexibility, greater air permeability, filtration efficiency and economic advantages (Huang et al., 2003; Abdul Khalil et al., 2014; Irbe et al., 2021; Elsacker et al., 2021). This hybrid association with the use of nanostructures seems to influence the morphology of the hyphae, making them more regular, rigid, and flat, besides having a large surface area and uniting the natural fibers by hydrogen bonds and mechanical interlocking (Attias et al., 2021; Elsacker et al., 2021). From an ecologically correct point of view, Ahn et al. (2020) and Li et al. (2016) showed how it is possible to adopt hybrid fungal mycelia associated with environmentally benign clay nanotubes and blackberry tannin in their work, respectively, for the treatment of wastewater (Li et al., 2016; Ahn et al., 2020).
The improvement of the physical and mechanical properties of two-hybrid composites was also verified by Elsacker et al. (2021) that combined mycelium and bacterial cellulose as an organic additive, and by Sun et al. (2019) who investigated the use of hybrid systems of wood, fungal mycelium, and CNF as a binder, proving to be a good product to replace formaldehyde-based composites, such as MDF, MDP, agglomerates, and plywood, which can be a toxic and carcinogenic substance (Sun et al., 2019).
The growth of nanofiber mats associated with biological structures may be suitable to increase the mechanical stability of the product compared to the pure nanofiber mat. In this case, the mycelium confers good mechanical properties (Sabantina et al., 2019; Trabelsi et al., 2021). In the aforementioned studies, the polyacrylonitrile nanofiber mat was adopted, a synthetic acrylic polymer widely used in the manufacture of rugs, carpets, furniture upholstery, and clothes, since PAN is petroleum-derived and non-biodegradable.
In order to assure structural stability and surface area properties to mycocomposites, sphere fungal hyphae (FH) and CNTs were designed from a biological assembly method that fixed CNTs onto fungal hyphae (Zhu et al., 2018). The developed biomaterial has water pollution removal capability by surface adsorption and was tested under various conditions to evaluate the removal of uranium (U(VI)), and anionic (Congo red (CR)) and cationic (methyl violet (MV)) dyes. The authors used well-fitted Langmuir and Freunlich models to calculate the adsorption isotherms. In a similar work, although the degradation process is taking place, the authors proposed an assembly of fungal mycelium-carbon nanotube composites. This novel biomaterial was designed by adding carbon nanotubes into a fungal mycelium (Penicillium oxalicum SYJ-1) to evaluate PAH-degrading rates. The authors conclude the efficiency of inorganic nanomaterials and microorganisms as chemical adsorption and biodegradation composites (Zhou et al., 2022). On the other hand, a functionalized fungal mycelium was prepared as a bioceramic hybrid for enhanced water treatment (Ahn et al., 2020). The authors developed a scalable and simple methodology that structurally modified the fungal hyphae to maximize the rate of pollutant sorption, controlling the fungal dispersion growth and development. Pellets of Aspergillus fumigatus were dipped in halloysite nanotubes, a mesoporous clay mineral presenting a large surface area and sorption capacities, with room enough to the mycelium growth. In conclusion, heavy metals sorption was observed in the mycomaterial, when compared to the hybrid nanocomposite–mycelium without the fungal growth. Thus, hybrid nanocomposite–mycelium materials are promising for the fields of biotechnology in medicine, agriculture, environment, architecture, food packaging, and textile industry, among others (Sun et al., 2019; Sabantina et al., 2019; Irbe et al., 2021; Trabelsi et al., 2021).
The production process differs greatly in each case depending on the type and application of the hybrid mycelium material but can be summarized as shown in Fig. 8. Table 3 lists all the fungal taxa used and their associated characteristics for the development of hybrid materials.
Figure 8: Schematic of the production process of hybrid mycelium materials.
Modified from Karana et al. (2018).| Hybrid materials | ||
|---|---|---|
| Fungi | Characteristics | Reference |
| Trametes ochracea | Good compatibility with nanocellulose observed in previous work | Attias et al. (2021) |
| Pleurotus ostreatus | Used for medical, nutritional, filter or packaging purposes, and promising candidate for the mechanical stabilization of nanofiber mats | Sabantina et al. (2019) |
| Ganoderma applanatum | Good phytochemical properties and great potential for application in clinical nanotechnology engineering | Irbe et al. (2021) |
| Fomes fomentarius | Used in medicine for centuries as a source of medicinal and nutraceutical products | Irbe et al. (2021) |
| Agaricus bisporus | Leader among edible mushrooms | Irbe et al. (2021) |
| Trametes versicolor | Used in traditional medicine for great health effects | Irbe et al. (2021) |
| Pleurotus ostreatus | Is a well-known edible mushroom, which grows in a short period and is the second mushroom cultivated in the world | Trabelsi et al. (2021) |
| White-rot basidiomycetes by ecovative design | Unmentioned | Sun et al. (2019) |
| Aspergillus fumigatus | Already adopted for removal of pollutants in aquatic environments | Ahn et al. (2020) |
| Ganoderma lucidum | Powerful oxidants | |
| Xylaria | Unmentioned | Li et al. (2019) |
| Trametes versicolor | Commonly used | Elsacker et al. (2021) |
Pure mycelium
Pure fungal materials are either the result of complete degradation of the substrate or are obtained by removing the fungal “skin” from the substrate. The properties of pure mycelium materials depend on the substrate, the type of fungus, and its growing conditions, as well as post-processing (Appels et al., 2019). Pure mycelium biomasses can be generated by growing the organism separately from its substrate, resulting in sustainable materials with adaptable properties ranging from foam, paper, and leather to materials with polymer-like characteristics. Furthermore, novel fermentation processes for pure mycelium materials can also increase the functionalities of this unique material when combined with appropriate post-growth treatments (Vandelook et al., 2021).
Pure mycelium foams are formed by aerial hyphae that grow on the surface of substrates when exposed to air. The production cycle extends over about 14 days, and the resulting biomass, of low density and high surface area due to the lack of lignocellulosic material in its structure foams, is then heat-treated at temperatures above 60 °C to neutralize all the biological activity, being converted into products. The low density of this fungal biomass results in a highly competitive material in the sector of synthetic foams such as polystyrene and polyurethane ones, as well as in lightweight cargo packaging and thermal insulation materials sectors. An increasing diversity of sectors current commercialize applications of mycelium foams (Gandia et al., 2021). These innovative products range from skin care, such as sponges, synthetic leather and supports for paints and dyes, to shoe insoles, upholstery and textiles. Other relevant sectors that apply mycelium foam as biocompatible cellular support are the biomedical area and the food sector (Gandia et al., 2021).
Pure mycelium products have a major advantage over their synthetic counterparts. When not combined with non-biodegradable elements, they are a great solution for low carbon and sustainable modes of production. Moreover, mycelium materials have been shown to have flame retardant properties and are less prone to combustion compared to petrochemical derived plastics (Vandelook et al., 2021).
Fungal leather
In the context of shifting social standards and increasing emphasis on environmental sustainability, the fungal derived-leather is now emerging as a promising alternative to replace, not only bovine leather but also the synthetic ones, that require hazardous chemicals use derived from fossil fuels. This results in a lack of biodegradability presenting the same limited end-of-life options as most plastics (Jones et al., 2021).
Mycelium leathers, also known as mycelium-based leather, is mainly originated from Polyporales species (Basidiomycota) that use lignocellulosic substrates as source. The biomass generated by the development of hyphae might have light or rigid consistency, depending on their chemical structure, which is formed by flexible structures composed of chitin, glucans and glycoproteins (Latgé, 2010; Raman et al., 2022). Agricultural and forest wastes are converted into substrates for most species, which allow the bioconversion of these resources without causing any unbalance or degradation of the natural ecosystem (Latgé, 2010). Mostly, fungal-derived leather can be developed by two ways, growing hyphal filaments in a liquid medium (liquid fermentation), pressed into a pure fiber pulp, and a solid state fermentation process, with physical and chemical treatments, where the mycelium layers are formed on a surface composed of solid lignocellulosic nutrients (Raimbault, 1998; Viniegra-González et al., 2003; Islam et al., 2017; Kaplan-Bei, 2018; Ross et al., 2020; Jones et al., 2021). Different fermentation processes are commonly mentioned in literature, and the liquid state is based on standard media or low-cost agricultural waste, such as blackstrap molasses as raw material to fungal growth. Moreover, they can be separated into fibers, and are processed by traditional papermaking techniques such as fiber suspension, filtration, pressing and drying (Jones, Huynh & John, 2018; Weiland et al., 2019; Nawawi et al., 2020a; Jones et al., 2021).
The industrial and construction materials sectors have risen interest in mycelium-based technologies (Raman et al., 2022). However, experimental raw data is hardly accessible, whether from individual knowledge or intellectual property, avoiding the development of innovative technologies in different sectors, varying from mycology to engineering. The commercial Startups and established companies include Ecovative, MycoWorks, Bolt Threads, Mycotech Lab, and Mycel (Table 4).
| Company | Products | Reference |
|---|---|---|
| Ecovative LLC. | Food, leather, foams, packaging | www.ecovative.com (accessed on June 11th, 2023) |
| Mogu | Acoustic panel, wall panel, flooring | www.mogu.bio (accessed on June 11th, 2023) |
| Mycoworks | Leather | www.mycoworks.com (accessed on June 11th, 2023) |
| Magical mushroom | Packaging | www.magicalmushroom.com (accessed on June 11th, 2023) |
| Okom wrks labs | Construction material | www.okomwrks.co (accessed on June 11th, 2023) |
| Norwegian mycelium AS | Food, new materials | www.nomy.no (accessed on June 11th, 2023) |
| Biomyc | Styrofoam | www.biomyc.com (accessed on June 11th, 2023) |
| MyCell Co., Ltd | Leather | www.mycelproject.com (accessed on June 11th, 2023) |
| Mushroom material | Packaging | www.mushroommaterial.com (accessed on June 11th, 2023) |
| Mycotech lab | Leather | www.mycl.bio (accessed on June 11th, 2023) |
| Mycelium materials Europe | Foam, packaging | www.myceliummaterials.nl (accessed on June 11th, 2023) |
| Mycellium Co. | Planter pot, acoustic panel, insulation panel | www.mycellium.co (accessed on June 11th, 2023) |
| Bolt threads | Leather, silk | www.boltthreads.com (accessed on June 30th, 2023) |
Fungal chitin nanofibers or microfilaments
Chitin microfibrils and nanofibrils are materials from chitin that are covalently linked to glucan, galactomannans, and proteins (such as mannoproteins and hydrophobins) and are part of a fungal cell wall, thus, forming a native compound (Nawawi et al., 2020a; Zin, Jimat & Nawawi, 2022). The main biological function of chitin is to provide structural support, being found with the same function in other organisms such as crustaceans, insect exoskeletons, nematode eggs, and protozoan cysts (Góes-Neto et al., 2010; Khan et al., 2017; Nawawi et al., 2020a).
Structurally, chitin is a long unbranched polysaccharide, formed by N-acetylglucosamine in a similar way to a fiber and it is this fibrillar structure that makes it interesting to be explored as a material (Khan et al., 2017). Chitin is the second most abundant renewable polymer and commercially it has been explored in a common way from the crustacean industry, thus, giving them added value; however, their exploration on an industrial scale is inefficient due to factors associated with the availability and quality of the material, as well as the dependence on seasonal and regional fluctuation (Jones, Huynh & John, 2018; Nawawi et al., 2020a; Salehinik et al., 2021; Zin, Jimat & Nawawi, 2022). Other limitations of the use of crustacean chitin are those associated with the presence of the allergenic protein known as tropomyosin, limiting its use in food applications and as medical devices (Jones, Huynh & John, 2018; Zin, Jimat & Nawawi, 2022).
The extraction and defibrillation process to obtain chitin from crustaceans requires the demineralization of the shells, thus, adding an acid step, which together with the need to use specialized equipment, reduces advantages in this type of commercial exploration. This is due to the generation of chemical residues that are environmental pollutants and because of the additional energy expenditure (Jones, Huynh & John, 2018; Zin, Jimat & Nawawi, 2022). On the other hand, in the separation of microfibers from the matrix of origin, the presence of β-glucans bound to fungal-derived chitin, contrary to that of crustaceans, leads to superior tensile properties, diversifying applications in areas such as engineering (Nawawi et al., 2020a). Considering the aforementioned disadvantages, the exploration of chitin of fungal origin arises as a renewable alternative, easily isolated and abundant by the production or fermentation systems that can be used to obtain the mycelium (raw material for obtaining chitin) (Yousefi et al., 2021; Zin, Jimat & Nawawi, 2022).
The study of fungal chitin nanofibrils emerges as a recent mechanism for optimizing the mechanical performance of materials derived from fungal mycelium, by eliminating both the non-structural elements of the mycelium and the substrates, the latter being used only as a source of carbon of the fungus and not as a structuring part of the material (Jones et al., 2018; Janesch et al., 2019). This allows exploration to improve resistance in materials derived from mycelium, since it seems to be associated with low mycelium-substrate affinity and with the presence of non-structural elements of the mycelium itself, such as some proteins and lipids (Jones, Huynh & John, 2018; Salehinik et al., 2021). It is worth noting that, although agricultural waste in the production of chitin micro and nanofibrils is not used as a structuring part but as a carbon source for fungal growth, its use continues to gain relevance due to the conversion of agricultural biomass into fungal biomass, transforming the biomass from low-value materials into high-value chitinous materials, and, thus, resulting in the production of low-cost, sustainable materials (Jones et al., 2018; Janesch et al., 2019; Nawawi et al., 2020a; Yousefi et al., 2021). Regarding yield, it was estimated that twice as many fungal chitin fibers are obtained as crab fibers, starting from the same initial mass of the raw matrix for each material (Nawawi et al., 2020a).
Chitin microfibrils and nanofibrils in their pure form or as hybrids with components such as cellulose can be used for the development of high-performance membranes and filters, both for organic solvents and for water treatment, as well as for the production of plastic films, packaging, composites, cosmetics, and pharmaceuticals. All of them mainly due to the physicochemical and surface properties and composition, where chitin provides strength and rigidity, while glucan increases flexibility and hardness (Jones et al., 2018; Nawawi et al., 2020a; Yousefi et al., 2021). It should be noted that the diversification of specific applications can be expanded by regulating the physicochemical parameters of fungal growth (Table 6), which includes the variation in nutritional factors, which has led some researchers to develop methodologies to evaluate the suitability of the substrate to guarantee an optimal rate of fungal growth (Jones et al., 2018; Nawawi et al., 2020b). The extraction process of chitin microfibrils is relatively simple; however, it has some variations to improve the mechanical behavior of the material. Alternative names can be checked in Table S4 and fungi used for the development of fungal chitin nanofibers in Table 5.
| Type of material | Mechanical characteristic | Standards used | Reference |
|---|---|---|---|
| Hybrid materials | Tensile strength | ISO 1924-1:1992 | Irbe et al. (2021) |
| Tensile | MTS ISA 3125 | Attias (2020) | |
| Without mechanical tests | Sabantina et al. (2019) | ||
| Without mechanical tests | Trabelsi et al. (2021) | ||
| Three-point bending test to determine the modulus of rupture, modulus of elasticity, and internal bond strength | ASTM D1037:2012 | Sun et al. (2019) | |
| Without mechanical tests | Ahn et al. (2020) | ||
| Without mechanical tests | Li et al. (2016) | ||
| Three-point bending test to bending behavior analysis | ISO 16978 | Elsacker et al. (2021) | |
| ISO 12344 | |||
| Tensile strength parallel | ASTM 1037 | Elsacker et al. (2021) | |
| Tensile behavior perpendicular | EN 319:1993 | Elsacker et al. (2021) | |
| Fungal chitin nanofibers | Evaluate the tensile properties of paper and board using a constant rate of elongation apparatus | ASTM D828-97 | Zin, Jimat & Nawawi (2022) |
| Ammonium molybdate spectrometric method for determining fungal cell wall phosphate content (AIM) | Norma Europea ISO6878 | Salehinik et al. (2021) | |
| Method for evaluating the tensile strength and Young’s modulus of fibers | ASTM C1557-14 | Nawawi et al. (2020a) | |
| Standard test method for tensile properties of plastics adapted by authors | ASTM D638-14 | Nawawi et al. (2020a) | |
| Plastics—determination of tensile properties—part 2: test conditions for molding and extrusion plastics | ISO 527-2:2012 | Jones, Huynh & John (2018) | |
| Fungal chitin nanofibers | ||
|---|---|---|
| Fungi | Characteristics | Reference |
| Agaricus bisporus | The large-scale production of A. bisporus also makes it abundant and relatively stable in composition and properties, and subsequently an ideal model system for the investigation of nanofibrils and fungal chitin-glucan products. | Yousefi et al. (2021) |
| Pleurotus ostreatus | Probably because the chitin fiber is relatively shorter, the results related to hardness were better, on the other hand, a denser fibrous network could indicate that it can support a greater external load. This tensile strength is maintained when using only the fibers or when used as an additive to biopolymers such as PLA. | Zin, Jimat & Nawawi (2022) |
| Flammulina velutipes | Higher tensile strength, which may be due to the long fiber length, seems to be related to greater chitin preservation. This tensile strength is maintained when using only the fibers or when used as an additive to biopolymers such as PLA. | Zin, Jimat & Nawawi (2022) |
| Lentinula edodes | The papers obtained have a darker color, probably because the intensity of the melanin is greater. This tensile strength is maintained when using only the fibers or when used as an additive to biopolymers such as PLA. | Zin, Jimat & Nawawi (2022) |
| Mucor indicus | According to some studies, the composition of the cell wall of this fungus, which includes the content of chitin and chitosan, can be manipulated by controlling the culture conditions. | Salehinik et al. (2021) |
| Agaricus bisporus | The resulting nanopapers presented differentiated physical-chemical surface properties, being more hydrophobic than chitin from crustaceans. | Nawawi et al. (2020a) |
| Trametes versicolor | Good growth performance evidenced the viability of mycelial biomass production to be faster than in other fungi, it is considered a reliable source of chitin for applications in materials science due to its significant conversion performance (biomass into chitin-glucan), being industrially scalable using bioreactors and continuous culture techniques | Jones et al. (2018) |
| Agaricus bisporus | Good yield but the growth rate is temporarily very slow and competes with food supply. | Jones et al. (2018) |
Molecular biology as a tool to produce sustainable biomaterials
An extensive number of fungal species of Basidiomycota and Ascomycota were shown here as potential sources of an enzymatic arsenal involved in different steps of the production of mycomaterials. Herein, we cited 18 genera of wood-decaying fungi (and 24 species) used in biomaterials. Although most of these fungi are well studied in metabolic and enzymatic assays, genomic studies are still a prominent field. At present, at least 20 species described herein present the whole genome available at the National Center for Biotechnology Information (NCBI) database (National Library of Medicine, 2023). A combination of molecular tools with the results of enzymatic and mechanical assays for choosing the best fungal species and their strains and substrates is one of the strategies that can accelerate the discovery of novel genes of interest for increasing the quality of mycocomposites.
The improvement of mechanical properties is one of the most desirable goals during the optimization of fungal-based biomaterials (Girometta et al., 2019; Womer, Huynh & John, 2023). Moreover, most of the basidiomycotan fungi whose mycelium is used in generating mycomaterials are white-rot decayers with di- or trimitic hyphal systems (Porter & Naleway, 2022; Hotz et al., 2023). Thus, using genomic data mining, the identification and characterization of the protein-coding genes involved with the morphogenesis of skeletal and binding hyphae could provide important clues to investigate novel ways to make reinforced mycomaterials. Furthermore, a phylogenetic-oriented search for prospecting possible distinct species to be used as new fungi in mycomaterials would be paramount for widening the horizon of likely potential species.
Depending on the carbon source or fungal developmental state, differential gene expressions of CAZymes can be induced. Several putative wood-degrading genes were detected from the transcriptome of the white-rot fungus G. lucidum, such as 13 potential lignin oxidases (LO families) and 9 potential lignin-degrading auxiliary enzymes (LDA families) differentially expressed by this fungal species in different development states: as mycelium or as basidioma (Yu et al., 2012). Additionally, more than four families of polysaccharide-degrading enzymes in 11 species of Polyporales deserve attention due to the enzymatic activity detected in lignocellulose breakdown when exposed to different carbon sources (Hori et al., 2013). As Polyporales is a group that has been studied as a potential source for enzymes that act in lignocellulose breakdown for many years, the discovery and better understanding of such differential expression and its control by the transcriptomics and proteomics would help to better understand the enzyme production by these wood-decaying fungi.
Therefore, using genomic, transcriptomic, and proteomic approaches, different points of control can be exploited to maximize the efficient conversion of the agro-industrial substrates on molecules or subproducts of interest. As, for instance, the identification of family genes encoding proteins involved in fungal colonization and adherence to surface (e.g., hydrophobins), in cellular wall constitution (e.g., ergosterol) (Free, 2013); and their impacts on the mechanical characteristics of bio-based materials, in the regulation of substrate degradation (e.g., peroxidases) (Peralta et al., 2017; Ferreira et al., 2018), and the effect of genetic mutations that improve fungal biomass production (e.g., chitin) (Jones et al., 2018). Hydrophobins are proteins responsible for fungal adherence to hydrophobic surfaces reported in Ascomycota and Basidiomycota (Lo, Lai & Sunde, 2019), and the hydrophobin sc3 gene impacts cell wall composition in the fungus S. commune (Appels et al., 2018; Wösten, 2019). The mechanical properties of the strain which has the sc3 mutation are analogous to thermoplastics, which differs from the wild type (Appels et al., 2018). This suggests that the mechanical properties and water retention of the mycelium associated with the substrate can be influenced by the expression of proteins related to the fungal cell wall, giving greater resistance to compression, among others (Karana et al., 2018; Appels et al., 2018; Attias et al., 2021). Hence, the investigation of genes of a fungal phenotype of interest in different stages of substrate colonization can play a central role to improve fungal development in several relevant steps to produce mycocomposites.
Conclusions
The comprehensive review of the literature has permitted: (i) a global understanding of fungal-derived biomaterials; (ii) to compile and explain all the terms used according to each type of material, which is extremely necessary to unify the nomenclature in relation to mycomaterials; (iii) to expand the scope of the information; (iv) to detect the applications of those mycomaterials and the possible improvements that can be proposed in this field of knowledge to the biotechnological processes associated with their production. The use of mineral additives confers variability in the application of mycocomposites since the additive interferes with their mechanical behavior. This variation in composition can result from flexible materials without additives to materials that are less flexible but more resistant to higher weight loads. One of the perspectives for this transdisciplinary field is the expansion of the analyses and characterization of the mycomaterials, overcoming the challenges brought by the production of limited mechanical characterization assays or with variable standardization.
The success and mechanical efficiency of mycocomposites is established by mycelial growth as an aggregator of the components of the composite matrix. Fungal mycelium growth can be manipulated by optimizing the physicochemical conditions, carbon sources and intrinsic characteristics of the fungal isolate, which can bring great contributions to the improvement of the mycocomposites.