The evolution of feeding within Euchelicerata: data from the fossil groups Eurypterida and Trigonotarbida illustrate possible evolutionary pathways
- Published
- Accepted
- Received
- Academic Editor
- Bruce Lieberman
- Subject Areas
- Evolutionary Studies, Paleontology, Zoology
- Keywords
- Eurypterida, Trigonotarbida, Arachnida, Character evolution, Mouthparts, Endites, Rhynie chert
- Copyright
- © 2020 Haug
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. The evolution of feeding within Euchelicerata: data from the fossil groups Eurypterida and Trigonotarbida illustrate possible evolutionary pathways. PeerJ 8:e9696 https://doi.org/10.7717/peerj.9696
Abstract
When the evolution of Euarthropoda is discussed, often the lineage of Chelicerata s. str. is assumed to be the more ‘primitive’ or ‘basal’ part of the tree, especially when compared to the other major lineage, Mandibulata. This claimed primitiveness is (at least partly) based on the assumption that different morphological structures are still in an ancestral state and did not evolve any further. One of these sets of structures is the feeding apparatus, which has been stated to be highly advanced in Mandibulata, but not ‘properly’ developed, or at least not to such a high degree, within Chelicerata s. str. In this study, I reinvestigate the feeding apparatus of different ingroups of Euchelicerata, with a focus on assumed ‘primitive’ groups such as Eurypterida and Trigonotarbida. The basis of this study is a large amount of material from different museum collections, with fossils with the entire feeding apparatuses being exceptionally well preserved. Based on high-resolution micro-photography and three-dimensional imaging, it is possible to resolve fine details of the feeding apparatuses. The results make clear that the feeding apparatuses of different ingroups of Euchelicerata are highly specialised and often possess morphological structures comparable to those of the feeding apparatuses of representatives of Mandibulata, apparently convergently evolved. Though the reconstruction of the evolution of the feeding apparatus within Euchelicerata is to a certain degree hampered by unclear phylogenetic relationships, there was clearly a shortening of the feeding apparatus from posterior (i.e. only the anterior appendages being involved in the feeding apparatus), probably linked to the colonisation of land in Arachnida.
Introduction
The origin of Euarthropoda, including the extant groups Myriapoda, Insecta, Eucrustacea (sensu Walossek, 1999) and Chelicerata s. str. (sensu Maas et al., 2004) and different fossil representatives, lies more than half a billion years ago (e.g. Daley et al., 2018 and references therein). In this long time, an enormous species richness and morphological diversity evolved in the different ingroups. Within Euarthropoda, the lineage of Chelicerata s. str. is often somehow treated as the more ‘basal’ or ‘primitive’ side of the tree (e.g. Damen, 2002; Dunlop, 2011; see the discussion in Krell & Cranston (2004) or Omland, Cook & Crisp (2008) on the topic why there is nothing like a more ‘basal’ side of the tree) and is thought to be more ancestral, especially in comparison to Mandibulata, the other major lineage within Euarthropoda (Damen, 2002). This view is most extremely applied to Xiphosurida (horseshoe ‘crabs;’ for example Sekiguchi, 1988; Malakhov, 2010; Williams, 2019), but also to now extinct groups such as Eurypterida (sea scorpions) or the spider-like group of Trigonotarbida (Resh & Cardé, 2003).
Especially horseshoe ‘crabs’ (though the old fashioned ‘sword tails’ would be less ambiguous) have often been treated as ‘living fossils’ (a very unscientific term, see also discussion in Wagner et al. (2017a); for attempts of more scientific approaches, see Kin & Błażejowski (2014) and Bennett, Sutton & Turvey (2018)). They have also been assumed to be a kind of proxy for the early terrestrialisation within Euchelicerata (Martin, 2017). Yet, this interpretation is most likely incorrect. It is unlikely that the stem species of Euchelicerata was already amphibious, hence the terrestrial behaviour of modern representatives of Xiphosurida has most probably evolved independently (see Lamsdell (2016) on independent evolution of non-marine life habits; see also recent discussion about the phylogenetic position of Xiphosurida and the consequences on the evolution of terrestrialisation: Ballesteros & Sharma, 2019; Giribet & Edgecombe, 2019; Lozano-Fernandez et al., 2019). It needs to be emphasised that modern representatives of Xiphosurida are not direct proxies for the ancestor of Euchelicerata, but possess their own specialisations (as all living groups do).
One reason why the lineage of Chelicerata s. str. is assumed to be primitive might be the organisation of the feeding apparatus in most representatives. In different textbooks, the impression is given that these forms lack ‘proper’ mouthparts, while ingroups of Mandibulata have a ‘full’ set of mouthparts. Gruner (1993, as an example for an important German text book) states that representatives of Chelicerata s. str. bear structures for acting in feeding but would lack true antagonistic jaws. He also states that mostly only the second pair of appendages and rarely the third and fourth one is incorporated in the feeding apparatus in most forms. This statement most likely refers to the state in scorpions and other arachnids, but appears to ignore the state in Xiphosurida where appendage pairs 1–7 are involved in the feeding apparatus.
Also in other cases the description of the feeding apparatuses appears to refer to only certain ingroups. For example the statement that there are always three pairs of mouthparts in Mandibulata (Gruner, 1993) is clearly correct for Insecta, but ignores maxillipeds in Chilopoda or Decapoda, or complex thoracic feeding apparatuses as for example in Branchiopoda (e.g., in fairy shrimps).
Hence, such textbook statements are at best oversimplified and tend to be based more on general assumptions than on direct observations. To improve this situation, I provide here functional morphological interpretations of the feeding apparatuses (= all external structures involved in feeding) of two supposedly ‘primitive’ chelicerate groups, namely of Eurypterida and of Trigonotarbida and present them in the context of evolution in Euchelicerata. With this, I aim at providing a framework to evaluate the presumed ‘primitiveness’ of the feeding apparatuses of modern representatives of Euchelicerata.
Materials and Methods
Material
For this study, fossil material from different museum collections was investigated, including the invertebrate palaeontology collection of the Yale Peabody Museum of Natural History, New Haven (YPM IP), the Natural History Museum, London (NHM), the Naturhistoriska riksmuseet, Stockholm (NRM), the Harvard Museum of Comparative Zoology, Cambridge (MCZ) and the Royal Ontario Museum, Toronto (ROM). Every specimen of Eurypterida and Trigonotarbida in these collections was briefly inspected, and those preserving morphological details of interest for this study were documented (see “Methods” below).
The specimens of Eurypterida in the YPM IP collections had been collected by a private collector, Samuel J. Ciurca, from late Silurian deposits in New York State, USA, and southern Ontario, Canada, and donated by him to the YPM; it is by far the largest collection of sea scorpions worldwide. The sea scorpions in the NHM collections originate from different late Silurian deposits: few specimens come from New York State and from Scotland. Also one sea scorpion from the MCZ collections comes from the Silurian of Scotland. Most specimens in the NHM collections come from Estonia, more precisely from the village Rootsiküla on the island of Saaremaa (also called Ösel). From the same Estonian locality, also many specimens of Eurypterida in the NRM collections and most in the MCZ collections originate, as well as from the island of Gotland, Sweden. The specimens from Saaremaa/Ösel and Gotland are insofar exceptional as of most of these the surrounding limestone matrix had been dissolved already in the 19th century by Holm (1896, 1898), resulting in isolated specimens with only their cuticle being preserved. Subsequently, the specimens had either been mounted between two large cover slips, or between a microscopic slide and a cover slip (dry or with Canada balsam), or they have been fully embedded in resin. All these methods allow to access dorsal as well as ventral structures (Holm, 1898; Selden, 1981).
The specimens of Trigonotarbida in the ROM collections come from the Upper Carboniferous Mazon Creek, Carbondale Formation, IL, USA. The NHM collections houses specimens of Trigonotarbida preserved in Rhynie Chert, Lower Devonian of Scotland, the rest of the material stems from the British Coal Measures, Upper Carboniferous.
Comparative material of extant species came from the former teaching collection of the University of Ulm (now at the University of Rostock) and the teaching collection of LMU Munich.
Methods
For the documentation of the specimens different macro- and microphotographic methods were used. All specimens were documented in their corresponding museum collections.
The Rhynie Chert specimens were documented on a Nikon Eclipse microscope at the Imaging and Analysis Centre of the NHM. Of each specimen an image stack with shifting focus was recorded to overcome the limitations in depth of field. For larger specimens several image stacks of adjacent and partly overlapping areas of the specimens were recorded to overcome limitations in field of view. From the image stacks, stereo images were recorded according to the protocol of Haug et al. (2012a).
The other fossil specimens were documented with a macro-photographic setup with a Canon Rebel T3i and an EF-S 18–55 mm lens or an MP-E 65 mm macro lens, depending on the size of the structures of interest. For illumination, a Canon MT 24-EX Macro Twin Flash or two Yongnuo Digital Speedlite YN560 flash lights were used. To enhance the contrast between fossil and matrix and reduce reflections, a polarisation filter was placed in front of the lens, and perpendicular filter foils were placed in front of the flashlights, resulting in cross-polarised light (Haug et al., 2011a; Kerp & Bomfleur, 2011). Specimens mounted on cover slips or microscopic slides were placed at some distance to a white background to avoid shadows. Also of the non-Rhynie Chert specimens image stacks were recorded; of these, high-resolution, entirely sharp images were calculated with Combine ZM/ZP and Photoshop CS3 (for details, see Haug, Haug & Ehrlich, 2008; Haug et al., 2011a). Specimens with high relief were additionally documented from different angles by moving the camera around the specimen; from matching image pairs, stereo images were produced.
Of some images, additional colour-marked versions were produced. For this purpose, the images were desaturated and the histogram was optimised before the important structures were colour-marked. For the Rhynie Chert specimens, the red channel of the stereo images was deleted; afterwards, the images were processed in the same way as those from non-Rhynie Chert specimens.
The extant specimens were documented with different macro- and micro-photographic methods, including photography under cross-polarised light and under fluorescence conditions.
Results
In the following, the feeding apparatuses of different representatives of Eurypterida and Trigonotarbida are described. The aim of these descriptions is not to provide an in-depth species-diagnostic description, but instead to present the general characteristics of the feeding apparatuses of each group.
Feeding apparatus of representatives of Eurypterida
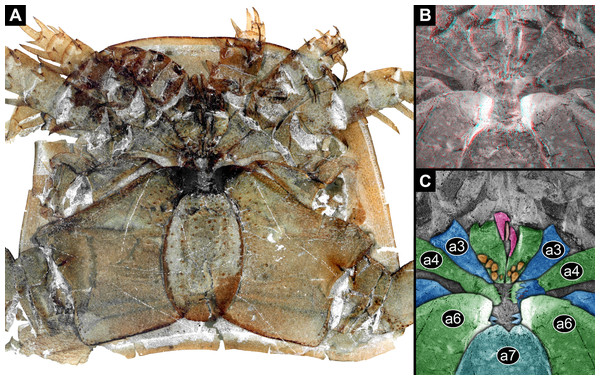
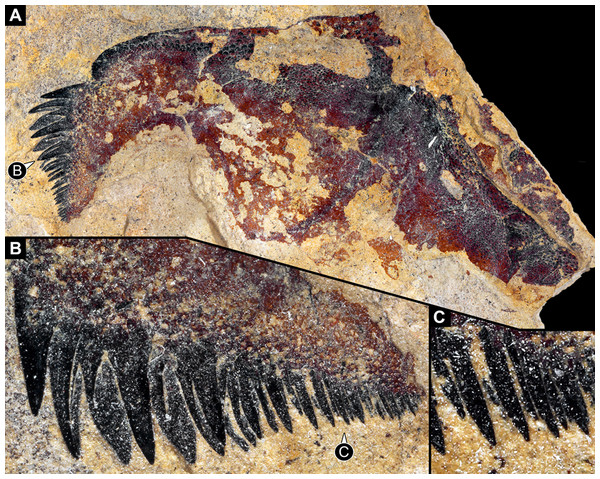
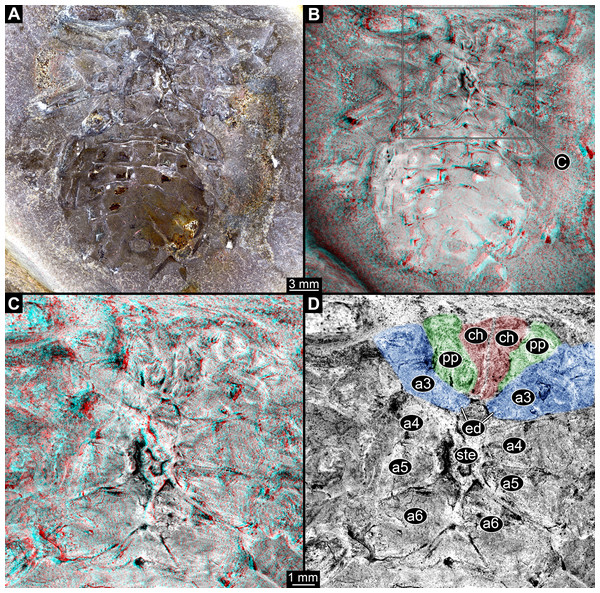
The appendages of seven segments clearly contribute to the feeding apparatus in Eurypterida: chelicerae, five subsequent pairs of legs (often called walking legs, but see discussion), and the plate-like appendage pair of the seventh post-ocular segment, the metastoma (Figs. 1A, 2A–2C and 3A; for the origin of the metastoma, see also Holm, 1898; Dunlop, 1998, his fig. 4; Dunlop & Selden, 1998; Jeram, 1998). The appendages are arranged in a circle around the central area of the feeding apparatus, with their median parts being very close together (Figs. 2B, 2C, 3B and 4A). The basipods (= coxae in chelicerate terminology) of the appendages of post-ocular segment 6 (the leg pair right in front of the metastoma) are much larger than those of the other appendages, forming roughly a rectangle, but additionally with a pronounced endite (Fig. 2A). The metastoma covers part of these basipods as well as the median gap between the left and right appendage (Figs. 2B and 2C).
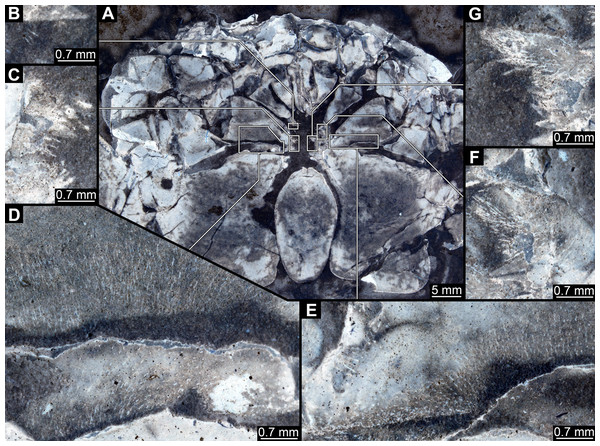
Figure 1: Feeding apparatus of YPM_IP_216689, Eurypterus lacustris (Eurypteridae, Eurypterida), Ontario, Welland County; images colour-inverted to enhance contrast.
(A) Overview. (B)–(G) Close-ups. (B) Stout spine on appendage 2. (C) Spines and setae on appendages 3 and 4. (D) and (E) Surface ornamentation on basipods. (F) Setae on appendages 2 and 3. (G) Spines and setae on (presumably) appendages 4 and 5.Figure 2: Feeding apparatus of NHM I3406_2, Eurypterus fischeri (Eurypteridae, Eurypterida), Rootsiküla, Saaremaa, Estonia.
(A) Overview. (B) Stereo image of central area, colour-inverted; use red-cyan glasses to view. (C) Colour-marked version of one half image of B; pink = chelicerae and setae; blue and green = basipods of appendages 2–6; cyan = metastoma (appendage 7); orange = spines. Abbreviations: a3–a7 = post-ocular appendages 3–7.Figure 3: Feeding apparatus of NRM Ar 35344, Eurypterus fischeri (Eurypteridae, Eurypterida), Rootsiküla, Saaremaa, Estonia.
(A) Overview. (B) Close-up of median edges of basipods. (C) Further close-up of the differentiated armature of the basipods.Figure 4: Feeding apparatus of Eurypterus fischeri (Eurypteridae, Eurypterida), Rootsiküla, Saaremaa, Estonia.
(A) and (B) NRM Ar35343. (A) Overview over central area. (B) Close-up of median edges of basipods with differentiated armature and very strongly sclerotised edges of post-ocular appendage 6. (C) and (D) NRM Ar48883. (C) Overview over anterior part of feeding apparatus; posterior part not preserved. (D) Close-up of differentiated armature on basipods, including broader and blunt teeth, pointed spines of different sizes and thin setae.Post-ocular appendages 2–6, that is all post-cheliceral legs, bear a more or less pronounced armature on the endites protruding from the median edges of their basipods (Figs. 1B, 1C, 1F, 1G, 2C, 5A, 5B and 5C). The armature differs between the appendages. The spines on some of the basipods (probably of the further anterior ones, but there appear to be species-specific differences) are thinner than on others (Figs. 5A and 5B). The basipods of post-ocular appendage 6 bear the stoutest spines, the entire basipodal median edge appearing strongly sclerotised, recognisable from its very dark colour (Figs. 2A, 3B, 4B, 5D and 5E).
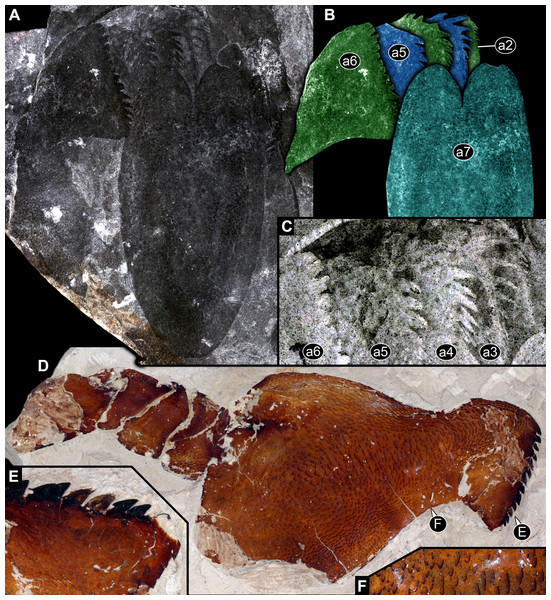
Figure 5: Parts of feeding apparatuses of Eurypterida.
(A)–(C) MCZ PALI 131326, Erettopterus bilobus (Pterygotidae, Eurypterida), Lesmahagow, Lanarkshire, Scotland. (A) Overview of a series of basipods with pronounced armature and metastoma. (B) Colour-marked version of (A). (C) Close-up of basipod armature with differentiation between different basipods; colour-inverted to enhance contrast. (D)–(F) Isolated leg of post-ocular segment 6 of MCZ PALI 185687, Erettopterus osiliensis (Pterygotidae, Eurypterida), Saaremaa, Estonia. (D) Overview; note pronounced endite with spines. (E) Close-up of spines. (F) Close-up of surface structure of basipod.Also the armature on the same basipod is differentiated (also here with species–specific differences). The spines closer to the anterior edge of the basipod (in relation to the orientation of the body of the animal) are stouter and partly also longer, while those closer to the posterior edge of the basipod are thinner and smaller (Figs. 5B, 5C, 6A and 6B). This differentiation can be very pronounced, with very stout and large teeth, elongate and pointed spines, and thin setae on the same basipod (Figs. 3C, 4C and 4D). The armature on the median edge of the basipods can occur in two (or possibly three?) rows (Figs. 6B and 6C). The remaining surface of the basipods can be covered with setae of other surface structures (Figs. 1D, 1E and 5F).
Figure 6: Isolated basipod, presumably of leg of post-ocular segment 6, of NRM Ar31829, undetermined representative of Eurypterida, Visby, Gotland, Sweden.
(A) Overview. (B) Close-up of spines; note different spine sizes and arrangement in different rows. (C) Further close-up of smaller spines with apparent row arrangement.Feeding apparatus of representatives of Trigonotarbida
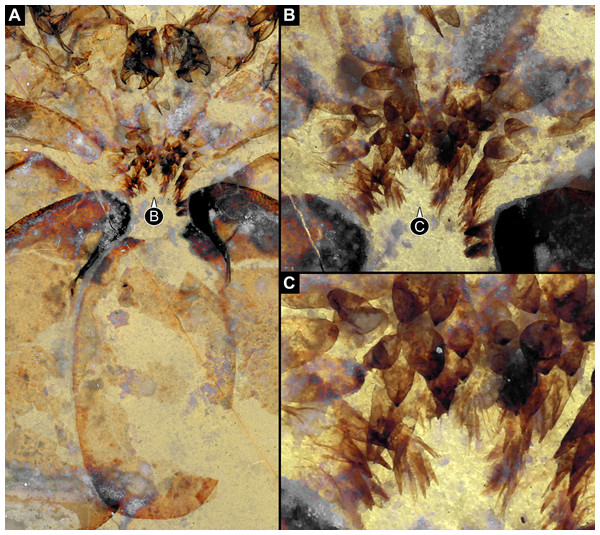
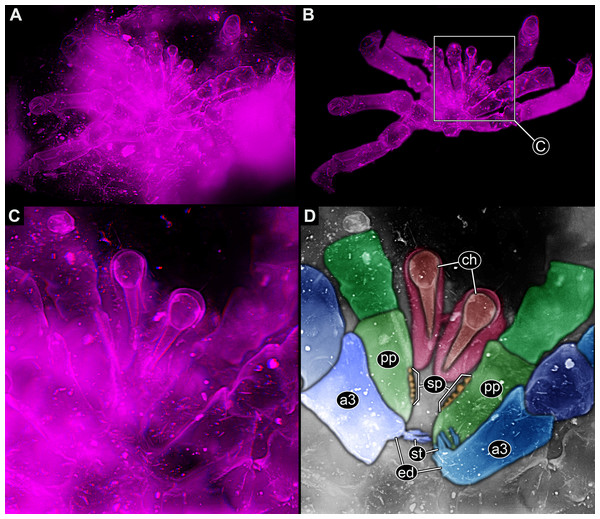
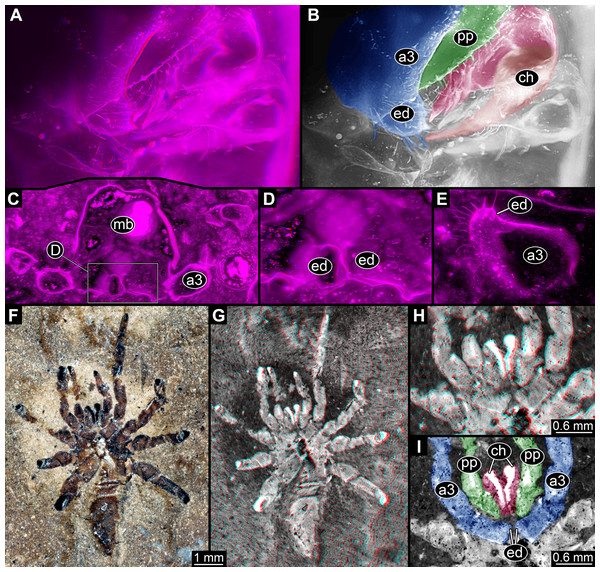
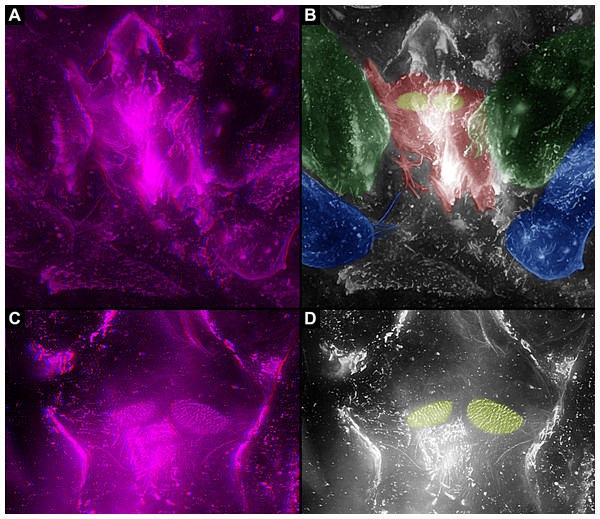
In Trigonotarbida, the appendages of three segments contribute to the feeding apparatus: chelicerae, pedipalps and first pair of walking legs (Figs. 7A–7D, 8A, 8B, 8F–8I and 9A–9D). The proximal parts of these appendages sit close together. The pedipalps are proximally armed with a row of short spines (Fig. 7D). The basipods of the first pair of walking appendages bear a prominent endite medially, which again bear setae (Figs. 7D, 8A, 8B, 8E, 8H, 8I, 9C, 9D, 10A and 10B). The endites are in some fossils positioned very closely together (Figs. 8C, 8D, 8H and 8I), in others further apart (Figs. 9C, 9D, 10A and 10B), which may point to a high movability during life. Additionally, there are two distinct oval fields of densely arranged short setae next to each other in the area between chelicerae and pedipalps (Figs. 10A–10D). Another less distinct field of similar setae appears to be positioned slightly posteriorly to the two oval fields (Figs. 10C and 10D). Based on the three-dimensional position information, these fields are probably situated on the hypostome (though often termed ‘labrum’ by different authors).
Figure 7: Feeding apparatus of NHM In24671, Palaeocharinus sp. (Trigonotarbida), Rhynie Chert, Scotland.
(A)–(C) Stereo images, use red-cyan glasses to view. (A) Overview of entire specimen. (B) Same as A, but with background virtually removed. (C) Close-up of feeding apparatus. (D) Colour-marked version of one half image of (C). Abbreviations: a3, appendage of post-ocular segment 3; ch, chelicera; ed, endite; pp, pedipalp; st, setae.Figure 8: Parts of feeding apparatuses of Trigonotarbida.
(A)–(E) Palaeocharinus sp., Rhynie Chert, Scotland. (A) and (B) NHM RC_019; ventro-lateral view on feeding apparatus. (B) Colour-marked version of one half image of (A). (C) and (D) NHM In27357; cross section through body at position of first walking appendages (appendages of post-ocular segment 3) with prominent endites. (D) Close-up of endites of (C). (E) NHM In24687; close-up of endite of first walking appendages. (F)–(I) NHM In31241, Trigonotarbus johnsoni, Coal Measures, UK. (F) and (G) Overview of entire specimen. (H) Stereo image of feeding apparatus. (I) Colour-marked version of one half image of H. Images (A), (C)–(E), (G) and (H) are stereo images, use red-cyan glasses to view. Abbreviations: a3, appendage of post-ocular segment 3; ch, chelicera; ed, endite; pp, pedipalp.Figure 9: Feeding apparatus of ROMIP45532, undetermined trigontarbidan, Mazon Creek, USA.
(A) and (B) Overview of entire specimen. (B) and (C) Stereo images, use red-cyan glasses to view. (C) and (D) Close-up of feeding apparatus and further walking appendages. (D) Colour-marked version of one half image of (C). Abbreviations: a3–6, appendages of post-ocular segments 3–6; ch, chelicera; ed, endite; pp, pedipalp; ste, sternum.Figure 10: Feeding apparatus of NHM In24702, Palaeocharinus sp. (Trigonotarbida), Rhynie Chert, Scotland.
(A) and (B) Overview. (A) Stereo image. (B) Colour-marked version of one half image of (A). (C) and (D) Close-up of fields with setae. (C) Stereo image. (D) Colour-marked version of one half image of (C); red = chelicerae; green = pedipalps; blue = first walking appendages; yellow = fields with setae.Discussion
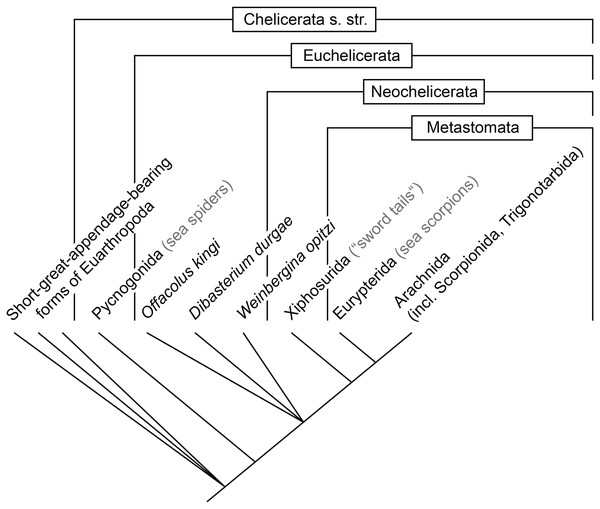
While this study focuses on the feeding apparatus of different groups of Euchelicerata, a proper character polarisation demands an outgroup comparison (see overview in Fig. 11). For this purpose it is especially important to include now extinct groups as those may exhibit character states no longer present in the extant fauna (Donoghue et al., 1989; Rust, 2007; Edgecombe, 2010).
Figure 11: Coarse phylogenetic overview of the groups discussed in this article.
For more details, see Haug, Wagner & Haug (2019).Reconstructing the original chelicerate feeding apparatus
The supposed sister group to Euchelicerata is Pycnogonida (Waloszek & Dunlop, 2002; Dunlop, 2010; Haug et al., 2012b; Sharma et al., 2014 and references therein), together forming Chelicerata s. str.. While being quite speciose today, the general body organisation is the same in all extant species of Pycnogonida, with a highly reduced posterior body area (the terms pro- and opisthosoma cannot be applied here yet; see discussion in Haug, Wagner & Haug (2019)) and a strongly modified feeding apparatus adapted to suction feeding (Fahrenbach & Arango, 2007; Wagner et al., 2017b). The fossil record of Pycnogonida dates back to the Cambrian (Waloszek & Dunlop, 2002), so more than half a billion years ago. However, as already at this time the morphology appears derived (and partly also as the Cambrian representatives of Pycnogonida are exclusively larvae), it does not provide relevant information about the evolution of the feeding apparatus in the lineage towards Euchelicerata. It is necessary to take a look at the feeding apparatus in supposed early representatives of Megacheira, the so-called ‘short great-appendage arthropods,’ among which the sister group to Chelicerata s. str. is assumed by some authors (Chen, Waloszek & Maas, 2004; Maas et al., 2004; Haug et al., 2012b; Tanaka et al., 2013; Edgecombe & Legg, 2014; see also recent review by Dunlop & Lamsdell (2017)).
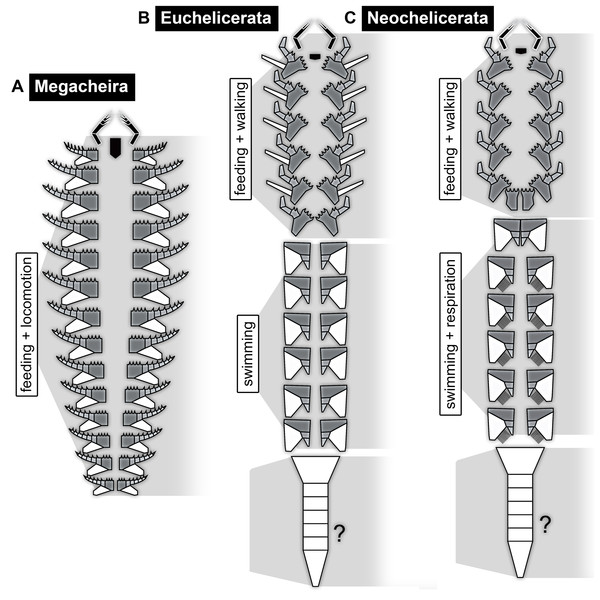
Recent reinvestigations of different of these species as well as older publications provide a sound basis for the reconstruction of the feeding apparatus in the ground pattern of Megacheira (Bruton & Whittington, 1983; Chen, Waloszek & Maas, 2004; Haug et al., 2012b; Haug, Briggs & Haug, 2012; Yang et al., 2013). At this evolutionary stage, the entire set of appendages was included into the feeding apparatus (possibly including the hypostome; Fig. 12A). While the first pair of appendages was specialised for grasping the prey, the second to last pair of appendages all exhibit the same general morphology, serving for swimming and feeding at the same time. While slight modifications in the size of the second appendage pair of Leanchoilia superlata might indicate a starting specialisation to a mouthpart (Haug, Briggs & Haug, 2012), the ‘whole-body-feeding’ method represents the original condition in Megacheira and presumably in Chelicerata s. str.
Figure 12: Schemes of feeding apparatuses and general body organisation in the ground pattern of different evolutionary levels.
(A) Megacheira. (B) Euchelicerata. (C) Neochelicerata. Grey background shadings mark different tagmata. Colour coding: black = appendages of first post-ocular segment (great appendages resp. chelicerae) and hypostome (‘labrum’); dark grey = basipod; light grey = endopod; white = exopod and (possibly) limbless segments. Presence of specific respiratory structures not known for the ground patterns of Megacheira and Euchelicerata.This also holds true if assuming that short great-appendage arthropods branched off earlier along the evolutionary lineage (as suggested, for example by Legg (2013)). The polarisation of characters remains the same. Also several other early representatives of Euarthropoda possess similar characters in their feeding apparatuses, most prominently opposing basipods with strong spines (Legg, 2014; Bicknell et al., 2018a; Aria & Caron, 2017, 2019), but will not be discussed in detail here.
Shortening of the feeding apparatus in Euchelicerata
As already mentioned above, the feeding apparatus of Pycnogonida is adapted to suction feeding and highly derived already in the fossil forms. This specialised feeding apparatus is an autapomorphy of Pycnogonida (see also Waloszek & Dunlop, 2002 for character evolution in the lineage towards and within Pycnogonida).
In Euchelicerata the systematic affinities are still in a certain state of flux and the feeding apparatus cannot be properly reconstructed for all early fossil representatives. The feeding apparatus and in general the tagmosis pattern can be reconstructed to a certain extent for the species Offacolus kingi (Sutton et al., 2002), Dibasterium durgae (Briggs et al., 2012) and Weinbergina opitzi (Stürmer & Bergström, 1981; Moore, Briggs & Bartels, 2005), successively splitting off the evolutionary lineage towards the remaining representatives of Euchelicerata (= Prosomapoda sensu Lamsdell, 2013, though unclear if also including W. opitzi depending on the presence of exopods on the walking appendages; see also Selden, Lamsdell & Qi, 2015; Lamsdell, 2016; Dunlop & Lamsdell, 2017).
At the level of Euchelicerata, a characteristic division into an anterior and a posterior tagma usually termed prosoma and opisthosoma appears for the first time, supposedly as an autapomorphy for this group (for problems with the correspondence of prosoma and opisthosoma in different groups of Euchelicerata, see Haug, Wagner & Haug, 2019). The subdivision into these two distinct tagmata is characterised by dorsal and ventral structural changes (see Haug et al. (2012c) and Haug, Wagner & Haug (2019) for characteristics of tagmata).
Dorsally, the segments of the anterior tagma form a uniform shield without any visible segmental borders. The segments of the posterior tagma possess separate tergites in the ground pattern of Euchelicerata. Ventrally, the tagmatisation is characterised by a ‘division of labour’ between the different appendages. While in short great-appendage arthropods all appendages were still involved in feeding, in the ground pattern of Euchelicerata the feeding apparatus becomes shorter; only the appendages of the anterior tagma contribute to the feeding apparatus, in addition to their locomotory (walking) function (Fig. 12B). The more posterior appendages serve for swimming; if they also possessed structures for oxygen exchange remains currently unclear (Sutton et al., 2002; Dunlop & Lamsdell, 2017).
The appendage of post-ocular segment 7 (the pre-genital segment) differs morphologically in O. kingi, D. durgae and W. opitzi, being significantly smaller in the first two species (Sutton et al., 2002; Briggs et al., 2012). Yet, as W. opitzi possesses an appendage on this segment similar to the preceding ones with apparently locomotory function (Lehmann, 1956; Stürmer & Bergström, 1981; Moore, Briggs & Bartels, 2005), this was presumably the ground pattern condition for Euchelicerata. Hence, the feeding apparatus in the ground pattern of Euchelicerata most likely consists of (possibly the hypostome and) chelicerae and six pairs of walking appendages.
Further modification of the feeding apparatus in Neochelicerata
Neochelicerata is an ingroup of Euchelicerata, the ‘crown group’ (most inclusive group with extant representatives). It has been characterised by Haug, Wagner & Haug (2019) as certain aspects of body organisation have not evolved in Euchelicerata yet, but are present in the ground pattern of Neochelicerata. The ground pattern condition of Neochelicerata is mainly reconstructed based on the morphology of Xiphosurida (sensu Lamsdell, 2016).
The body organisation in general and the feeding apparatus in particular are very similar in the ground pattern of Neochelicerata to that of Euchelicerata. Also here the anterior tagma dorsally bears a uniform shield. The tergites of the posterior tagma are fused into an entire dorsal shield, the thoracetron, in Xiphosurida (Anderson & Selden, 1997; Lamsdell, 2016). However, this condition appears to be an autapomorphy of Xiphosurida, while in the ground pattern of Neochelicerata the tergites were most probably still separate.
Also ventrally, the ground pattern condition of Neochelicerata is largely the same as that of Euchelicerata (Fig. 12C). The appendages of the anterior tagma, (possibly the hypostome and) chelicerae and the following six pairs of appendages, are incorporated into the feeding apparatus. However, the last of these appendage pairs, the chilaria (appendages of the pre-genital segment or post-ocular segment 7), consists of only the shovel-shaped most proximal part (basipod in neutral euarthropodan terminology, usually called coxa in chelicerate terminology); the walking part (endopod) is lacking. With this, the appendages of post-ocular segment 7 no longer perform a combined feeding-and-walking function, but instead close the feeding apparatus from its posterior end to reduce the loss of food in Xiphosurida (e.g. Patten, 1894; see Manton, 1964 for a detailed functional explanation of the feeding process). This condition is possibly already present earlier as it looks very similar in a species splitting off the evolutionary lineage before the node of Neochelicerata, Venustulus waukashensis (Moore et al., 2005, their fig. 3.3 + 3.4). Therefore, in the ground pattern of Neochelicerata (or slightly earlier) the feeding apparatus became further specialised, consisting of (possibly the hypostome,) chelicerae, five pairs of walking appendages and chilaria.
The specialised feeding apparatus of Eurypterida
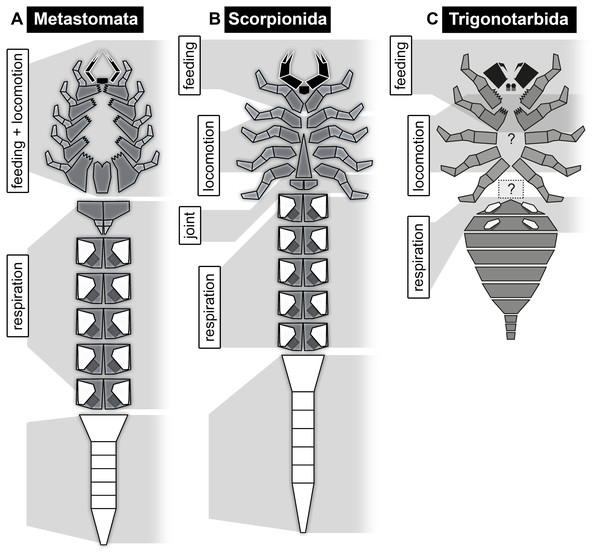
The exact relationships of Eurypterida, Arachnida, Xiphosurida, and other exclusively fossil groups such as Chasmataspidida are still not entirely resolved (e.g. Dunlop, 2010; Garwood & Dunlop, 2014a and references therein). The feeding apparatus of Eurypterida (and possibly already of Metastomata if this is a natural group; see Haug, Wagner & Haug, 2019) shows a stronger specialisation than that in the ground pattern of Neochelicerata, but is still composed of (possibly the hypostome and) the appendages of the same segments (chelicerae, following five pairs of appendages, metastoma, see below; Fig. 13A). The further posterior segments are also here not involved in feeding or locomotion; the corresponding appendages most probably became (partly) internalised and fulfilled respiratory function (Waterston, 1975; Braddy et al., 1999).
Figure 13: Schemes of feeding apparatuses and general body organisation in the ground pattern of different evolutionary levels, continued.
(A) Metastomata. (B) Scorpionida. (C) Trigonotarbida. Grey background shadings mark different tagmata. Colour coding: black = appendages of first post-ocular segment (chelicerae) and hypostome (‘labrum’); dark grey = basipod; light grey = endopod; white = exopod and (possibly) limbless segments. Question marks indicate uncertainties about origin of sternum and transition area.Also in sea scorpions, feeding and locomotion appears to be performed by the same appendages. However, Selden (1981), who described the functional morphology of Eurypterus tetragonophthalmus in great detail, assumed that only the posterior appendages were used for locomotion to avoid coordination problems between the differently long appendages (though this assumption does not have to account for all species of Eurypterida due to their morphological differences). He also assumed that there is a task differentiation in food handling between the anterior and posterior legs, the anterior ones gathering food, while especially the last pair crushed hard food particles (Selden, 1981). This differentiation is corroborated by the specimens investigated in this study, as the basipods of the different appendages are equipped with different types of teeth on their median edges (see Bicknell et al. (2018a) for microstructure of these teeth, and Bicknell et al. (2018b) for similar studies on Limulus polyphemus), best visible when (almost) the entire feeding apparatus is preserved in situ (Figs. 5A and 5B). In some specimens the teeth on the basipods of the further anterior appendages are thinner and appear less robust than those on the basipods of the fifth (last) pair of walking legs (appendage pair of post-ocular segment 6). The antero-median edges of the latter are equipped with strong teeth and reach under the metastoma (Figs. 2A and 3A; Holm, 1898; Selden, 1981). They are also significantly elongated in anterior-posterior axis (Figs. 1A and 2A; based on its position in the fossil, not on its evolutionary origin), probably to achieve a larger biting force. With this, sea scorpions possessed fully functional antagonistic jaws (in contrast to earlier statements; for example Gruner, 1993) comparable to the condition in many crustaceans (Manton, 1964), but convergently evolved.
In addition to the differentiation of armature on the basipods of different appendages, on the same basipod the armature is also differentiated. The teeth further anterior on the median edge of the basipod appear stronger than the further posterior ones on the same basipod. Selden (1981) describes this differentiation for E. tetragonophthalmus, and discusses that some of the teeth or spines would have been movable while others were not. The presence of movable teeth may be species specific based on the observations in this study, but the general pattern of differentiated armature on the same basipod apparently occurs in different species of Eurypterida. This differentiation appears very similar to the situation in Mandibulata, in which the mandibles bear the pars incisivus and the pars molaris, that is two regions with rather different armature (Edgecombe, Richter & Wilson, 2003). Apparently, also this specialisation evolved convergently.
The metastoma (appendage of pre-genital segment or post-ocular segment 7) in representatives of Eurypterida basically fulfils the same function as the chilaria in representatives of Xiphosurida, it closes the feeding apparatus from posteriorly (Selden, 1981). Yet, in sea scorpions this is only a single plate (but which can have various shapes; for example Tollerton, 1989), so most probably the conjoined basipods of formerly free appendages (see Holm, 1898; Dunlop, 1998, his fig. 4; Dunlop & Selden, 1998; Jeram, 1998). Some species show a notch in the anterior area of the metastoma (Holm, 1896), which may represent a remnant of the not completed fusion process. Unfortunately, no extremely early ontogenetic stages are preserved (late embryos to hatchlings; but see Lamsdell & Selden (2013) for fairly small stages), at least not well enough to allow investigation of the development of the metastoma (see below for development of the corresponding structure in scorpions).
In addition to the closing of the feeding apparatus, the metastoma appears to provide a kind of guide rail for the movements of the basipods of the appendage pair right in front of it (Selden, 1981). This function is comparable to that of the paragnaths in eucrustaceans, which are elevations of the sternite of the mandible segment and guide the movement of the mandibles (Haug et al., 2011b; Rötzer & Haug, 2015). Again, this morphological similarity evolved most likely convergently.
The condition of the metastoma as a single plate and its covering of the proximal area of the posterior appendages leads to a more tightly closed feeding apparatus in comparison to that in Xiphosurida and the ground pattern condition of Euchelicerata. Considering that representatives of Eurypterida may have had an amphibious lifestyle (Lamsdell & Braddy, 2009), a more closed feeding apparatus could have been a predisposition for going on land, as food may get lost more easily on land than in the water, where it sinks slower when it is not grabbed tightly enough. The specialised feeding apparatus of sea scorpions in its highly differentiated morphology is probably best interpreted as an autapomorphy of Eurypterida or even of an ingroup.
Further shortening of the feeding apparatus in Arachnida
In the following, scorpions are taken as a first example for Arachnida. Scorpions have been assumed to be the sister group to the remaining groups of Arachnida (Weygoldt & Paulus, 1979). Yet, in recent studies, scorpions resulted as sister group to Megoperculata (Sharma et al., 2014; Klußmann-Fricke & Wirkner, 2016; Lozano-Fernandez et al., 2019); in these analyses sea scorpions were not included (which was mostly not possible due to the type of analysed characters). This deep ingroup position of scorpions may be an artefact resulting from the lack of proper character polarisation due to the absence of Eurypterida, but this problem cannot be further discussed here.
In Arachnida, the posterior border of the feeding apparatus lies further anteriorly than in the previously discussed groups. In modern scorpions (Scorpiones), only the first four appendage-bearing segments (and possibly the hypostome) are involved in the feeding apparatus: chelicerae, pedipalps, and two pairs of walking appendages. The basipods of these two pairs of walking appendages are antero-medially elongated into a pronounced endite, which reaches far anteriorly, closing the feeding apparatus from the posterior end (Snodgrass, 1948). The median areas of the basipods of walking appendage pairs 3 and 4 are oriented almost anteriorly, but apparently not included into the feeding apparatus. Also the sternum, most likely the embryonically fused appendages of the seventh post-ocular (pre-genital) segment (see Dunlop & Webster (1999); Farley (2005); discussion in Haug, Wagner & Haug (2019)), is oriented anteriorly, but not involved in the feeding apparatus. Dorsally, the shield extends further posteriorly, including the segments bearing chelicerae, pedipalps, all walking appendages and possibly also the sternum-bearing segment (see discussion in Haug, Wagner & Haug (2019)). Hence, the posterior border of the feeding apparatus in Scorpiones no longer corresponds to the posterior border of the dorsal shield, in contrast to the presumed ground pattern condition in Euchelicerata, Neochelicerata, and Metastomata.
However, the condition of the feeding apparatus in modern scorpions differs from that in early fossil scorpions. In early scorpions, the feeding apparatus extends posteriorly only to the pedipalps (Kjellesvig-Waering, 1986; Waddington, Rudkin & Dunlop, 2015). The walking appendages do not bear enditic protrusions, hence do not appear to have been involved in the feeding process (Fig. 13B). If this condition is the ground pattern condition of Scorpionida, the group including Scorpiones and different fossil scorpions, the feeding apparatus in modern scorpions would be an autapomorphy of Scorpiones.
In Trigonotarbida, the chelicerae, pedipalps and first pair of walking appendages contribute to the feeding apparatus (Fig. 13C). The basipods of the pedipalps and especially of the first pair of walking appendages possess endites medially, which were probably used for food manipulation (e.g. Garwood, Dunlop & Sutton, 2009; Garwood & Dunlop, 2014b, their fig. 1.5; Dunlop & Garwood, 2017). This condition is unknown from other arachnids.
The phylogenetic position of Trigonotarbida is still unclear (Garwood & Dunlop, 2014a). In different analyses, they have already resolved, for example, as sister group to Ricinulei (Dunlop, 1996; Dunlop, Kamenz & Talarico, 2009) or as closely related to Megoperculata (Shear & Selden, 1986; Selden, Shear & Bonamo, 1991). Similarities to the one respectively the other group occur, for example, in the filtering structures on the mouthparts (Dunlop, 1994; Haug, 2017), details on the pedipalps (Dunlop, Kamenz & Talarico, 2009), or the general body organisation.
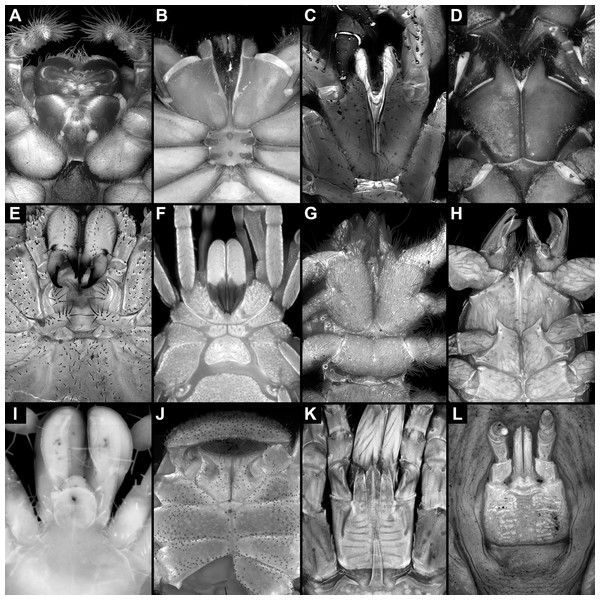
The feeding apparatus does not provide clear arguments for placing Trigonotarbida near the one or the other group of Arachnida. In most representatives of Arachnida, the feeding apparatus only consists of (possibly a hypostome,) chelicerae and pedipalps (Figs. 14B–14D, 14G, 14H and 14J). In some of them, the basipods of the pedipalps are more or less closely connected medially (Figs. 14D and 14H). The basipods of the pedipalps are in many web spiders involved in the feeding process, using median projections for mastication (sometimes referred to as gnathocoxae or maxillae; for example Williams, 1979; Sierwald, 1988; Żabka, 2001; J. Dunlop, 2020, personal observations). In certain cases, a lower lip contributes to the feeding apparatus from posteriorly (Figs. 14A and 14I). In some groups, all these structures form a single, tightly connected unit (Figs. 14K and 14L). If this short condition of the feeding apparatus would be present in all representatives of Arachnida besides Trigonotarbida, two options for the ground pattern of Arachnida would be possible: either the feeding apparatus of Trigonotarbida would represent the ground pattern condition of Arachnida, or the very short condition of the other representatives of Arachnida would be the ground pattern condition and Trigonotarbida autapomorphically elongated the feeding apparatus.
Figure 14: Feeding apparatuses of different extant representatives of Arachnida.
(A) Araneae. (B) Amblypygi. (C) Schizomida. (D) Thelyphonida. (E) Phalangida (Opiliones). (F) Cyphophthalmi (Opiliones). (G) Solifugae. (H) Pseudoscorpiones. (I) Palpigradi. (J) Ricinulei. (K) Mesostigmata (Acari). (L) Ixodoidea (Acari).However, the entire situation is more complicated due to the feeding apparatus of Opiliones (harvestmen). In harvestmen, the feeding apparatus includes also the first pair of walking legs, which bears endites on the basipods (Fig. 14E). Also the second pair of walking legs bears endites, at least in certain harvestmen, which may have a supporting function in the feeding process (Shultz & Pinta-da-Rocha, 2014). The presence of a preoral chamber, stomotheca, formed by all these endites in Opiliones and Scorpionida led Shultz (2000, 2007) to suggest a sister group relationship between these two groups (together forming Stomothecata). The endites of the first pair of walking appendages in harvestmen look rather similar to those in Trigonotarbida, and in general also the composition of the feeding apparatus would be similar between the two groups (Garwood, Dunlop & Sutton, 2009). However, the morphology in harvestmen is highly variable (Figs. 14E and 14F), and it is unclear how the feeding apparatus in the ground pattern of Opiliones looks. This together with the still unresolved phylogenetic position of Opiliones (Garwood & Dunlop, 2014a; Lozano-Fernandez et al., 2019) does not allow to make a reliable assumption about the feeding apparatus in the ground pattern of Arachnida.
Conclusions
(1) The feeding apparatus in different groups of Euchelicerata is far from primitive, but is in fact a highly specialised system in each group.
(2) During evolution, the feeding apparatus became progressively shorter in Euchelicerata, though the evolution within Arachnida remains still unclear due to unresolved phylogenetic relationships. The shortness of the feeding apparatus is not an ancestral character, but in fact a highly derived one, probably evolved in adaptation to new requirements resulting from habitat changes such as terrestrialisation.
(3) Sea scorpions possess true antagonistic mouthparts with differentiated armature and a guide rail system. These characters are all similar to the condition in the mandibles of Mandibulata, apparently as result of convergent evolution.
(4) Representatives of Trigonotarbida show similar specialisations concerning their feeding apparatuses to harvestmen.
(5) In conclusion, the supposedly ‘primitive’ groups Eurypterida and Trigonotarbida are astonishingly specialised.