Comparative proteome analysis reveals VPS28 regulates milk fat synthesis through ubiquitylation in bovine mammary epithelial cells
- Published
- Accepted
- Received
- Academic Editor
- Cong-Jun Li
- Subject Areas
- Agricultural Science, Cell Biology, Genetics, Molecular Biology
- Keywords
- VPS28, Milk fat synthesis, iTRAQ, Proteome, Ubiquitylation
- Copyright
- © 2020 Liu and Zhang
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Comparative proteome analysis reveals VPS28 regulates milk fat synthesis through ubiquitylation in bovine mammary epithelial cells. PeerJ 8:e9542 https://doi.org/10.7717/peerj.9542
Abstract
In our previous study, we found that VPS28 (vacuolar protein sorting 28 homolog) could alter ubiquitylation level to regulate milk fat synthesis in bovine primary mammary epithelial cells (BMECs). While the information on the regulation of VPS28 on proteome of milk fat synthesis is less known, we explored its effect on milk fat synthesis using isobaric tags for relative and absolute quantitation assay after knocking down VPS28 in BMECs. A total of 2,773 proteins in three biological replicates with a false discovery rate of less than 1.2% were identified and quantified. Among them, a subset of 203 proteins were screened as significantly down-(111) and up-(92) regulated in VPS28 knockdown BMECs compared with the control groups. According to Gene Ontology analysis, the differentially expressed proteins were enriched in the “proteasome,” “ubiquitylation,” “metabolism of fatty acids,” “phosphorylation,” and “ribosome.” Meanwhile, some changes occurred in the morphology of BMECs and an accumulation of TG (triglyceride) and dysfunction of proteasome were identified, and a series of genes associated with milk fat synthesis, ubiquitylation and proteasome pathways were analyzed by quantitative real-time PCR. The results of this study suggested VPS28 regulated milk fat synthesis was mediated by ubiquitylation; it could be an important new area of study for milk fat synthesis and other milk fat content traits in bovine.
Introduction
VPS28 is a member of the class E VPS proteins, and also is a major component of ESCRT І (endosomal sorting complexes required for transport І). ESCRT-0, ESCRT-І, ESCRT-ІІ, ESCRT-III and some auxiliary components constitute ESCRTs, play crucial roles in concentration and sorting of ubiquitinated proteins of the multivesicular body for incorporation into intralumenal vesicles (Teo et al., 2006; Pineda-Molina et al., 2006; Saksena et al., 2007). The importance of ESCRTs was demonstrated by Raymond et al. (1992), who showed that disruption of ESCRTs resulted in an accumulation of membrane proteins and no longer degraded in the vacule. Recent studies showed that ESCRTs play a critical role in degradation of ubiquitinated proteins through lysosome and proteasom (Ciechanover, 1994; Katzmann, Babst & Emr, 2001). Particularly, VPS28 is localized to ubiquitin-rich endosomes during ligand-induced receptors internalization and contributes directly to receptor trafficking (Bishop, Horman & Woodman, 2002).
In previous studies, we found that a SNP in the 5′ UTR region of VPS28 showed a very strong association with milk fat percentage, and that its expression level significantly affected milk fat synthesis in Chinese Holstein (Liu et al., 2018; Liu & Zhang, 2019; Jiang et al., 2010, 2014). Based on the structural feature and function of ESCRTs, we believed that VPS28 could regulate milk fat synthesis through engaging ESCRTs complexes to affect the ubiquitin-mediated degradation of proteins, which has been proved in our previous study (Liu et al., 2018; Lily Liu, 2019). However, the molecular mechanisms of VPS28 response to milk fat synthesis still remain unclear. Thus, in present study, to better understand the mechanisms, we used isobaric tags for relative and absolute quantitation (iTRAQ) technology that allows quantitative comparisons of protein abundance to much greater insight into the regulation of VPS28 on milk fat synthesis in Chinese Holstein. After performing an RNAi experiment in bovine primary mammary epithelial cells (BMECs), we compared the knockdown BMECs groups with the control groups to identify differentially expressed proteins by iTRAQ. Changes in the expression patterns of the proteins could provide a basis for clarifying the molecular mechanisms for VPS28 regulating milk fat synthesis in Chinese Holstein, which may be a forward step for milk fat synthesis regulation, In addition, this study can provide a reference for elucidating the molecular mechanisms of milk fat traits.
Materials and Methods
Animals
The procedures of collecting BMECs from the mammary tissues of Chinese Holstein cows according to the Animal Welfare Committee of Shandong Agricultural University (Permit Number is SDAUA-2018-022).
Cell culture
Cell culture experiments were performed using primary BMECs. Chemicals were purchased from Life Technologies (Carlsbad, CA, USA) unless noted otherwise. Primary BMECs were kept in our laboratory. BMECs were plated in serum-containing medium DMEM-F12 supplemented with 10 kU/mL penicillin, 10 mg/mL streptomycin, 10% fetal bovine serum and 1% ITS-G (1 mg/mL Insulin, 0.55 mg/mL Transferrin, 0.67 mg/L Selenium Solution). All cells were cultured on plastic cell culture plates at 37 °C in a humidified atmosphere containing 5% CO2.
Knockdown of VPS28 via RNAi in BMECs
Stealth RNAi™ siRNAs targeting the bovine VPS28 gene open reading frame were designed and synthesized by GenePharma Corporation (Shanghai, China). One day prior to transfection, BMECs were seeded without antibiotics. When cells reached 80% confluence, VPS28-siRNAs (GUCCAGGGCUCAGAAAUCATT and GACGUGGUCUCGCUCUUUATT) as tandem constructs, were transfected in to BMECs using X-treme GENE siRNA Transfection Reagent (Roche, Penzberg, Germany) at a 1:10 molar ratio. Cells were harvested at 72 h after transfection for mRNA analysis via real-time quantitative PCR (RT-qPCR).
Sample preparation
Six BMECs samples from two groups (control and VPS28 knockdown) were incubated in lysis buffer (7M urea, 2M thiourea and 0.1% CHAPS) for 30 min on ice and sonicated (80W, ultrasonic 0.2 s, intermittent 2 s, a total 60 s) on ice. Cells debris was pelleted by centrifugation at 15,000×g for 20 min at 4 °C. The supernatants were collected and stored at −80 °C. The protein concentration was determined using Bradford assay (Sigma-Aldrich, St. Louis, MO, USA).
Protein digestion and iTRAQ labeling
Protein digestion was performed using the filter aided sample preparation method. Each protein extract (200 µg) was mixed with 4 µL reducing reagent (AB Sciex, Redwood City, CA, USA) for 1 h at 60 °C and 2 µL cysteine-blocking reagent for 10 min at room temperature, the alkylated protein solution was added to 10 K ultrafiltration tube and discarded the filtrate after centrifuging at 12,000×g for 20 min. Then 100 µL dissolution buffers were added to the filtered unit and the solution was centrifuged again at 12,000×g for 20 min and repeated three times. After incubating overnight, the units were transferred to new collection tubes, and then adding 4 µg trypsin (protein to enzyme ratio 50:1 w/w) and mixed them at 37 °C for overnight. The units were centrifuged at 12,000×g for 20 min discarded the filtrate, then added 50 µL dissolution buffer 5 and centrifuged 12,000×g for 20 min incubated at room temperature. Finally, the extracted peptides were collected from bottom.
The iTRAQ labeling was performed according to the manufacturer’s protocol (AB Sciex, Redwood City, CA, USA). After trypsin digestion, the peptides were transferred to vials containing individual iTRAQ regents by incubation at room temperature for 2 h, which was thawed and reconstituted in 150 μL isopropanol per one unit. The three knock-down VPS28 groups were labeled with iTRAQ 115, 116 and 117; the three WT groups were labeled with iTRAQ 118, 119 and 121, respectively.
Peptide fractionation with strong cation exchange chromatography
The iTRAQ labeled peptides were fractionated by SCX using RIGOL L-3000 HPLC system (RIGOL, Beijing, China). The dried peptide was dissolved with 100 μL buffer A (98% ddH2O, 2% acetonitrile) and the solution was centrifuged at 14,000×g for 20 min, the supernatants were collected. The peptides were eluted at a flow rate of 0.7 mL/min with a buffer B (98% acetonitrile, 2% H2O) gradient of 5% at 0–5 min, 8% at 5–35 min, 18% at 35–62 min, 32% at 62–64 min, 95% at 64–68 min, 5% at 72 min. The elution was monitored by absorbance at 214 nm.
Quantitative analysis of proteins by iTRAQ LC-MS/MS
Each collected component of the processed SCX fractions was redissolved with 20 µL 2% methanol and 0.1% formic acid, and the solution was centrifuged at 12,000×g for 10 min, the supernatants were collected. 10 μL solution was trapped on a precolumn (100 μm × 2 cm) and then eluted on an analytical column (75 μm × 12 cm) for separation. The precolumn was packed with Acclaim PepMap-C18 5 μm and analytical column was packed with EASY-Spray-C18 3 µm. The peptides were separated over 90 min and eluted at a flow rate of 350 nL/min. The MS analysis was performed using an Applied Biosystems Q-Exactive mass spectrometer.
The BMECs iTRAQ identification and quantification analysis were obtained using Proteome Discoverer1.3 (Thermo, Waltham, MA, USA). Proteome Discoverer1.3 was set up to search the NCBI Bos taurus major database assuming the digestion enzyme trypsin. The differential expressed proteins were accepted if they have been identified with greater than 95% confidence in all iTRAQ preparations, and have ≥1.2 or ≤0.83 fold changes (iTRAQ ratios (VPS28 knockdown)-115+116+117: (control)-118+119+121) in addition to P ≤ 0.05. Gene ontology (GO) was used to annotate the proteins under the biological progress (BP), molecular function (MF) and cellular components (CC) GO categories (DAVID, https://david.ncifcrf.gov/) in the BMECs.
Microscopy analysis
The control and VPS28 knockdown BMECs were collected and fixed with 2.5% glutaraldehyde at 4 °C for overnight, and washed by PBS (pH 7.0, 0.1M) for three times. And then the BMECs was fixed with 1% osmium tetroxide for 1–2 h, washed by sodium cacodylate buffer, and then dehydrated with gradient alcohol until complete, finally embedded in Epon 812. The fixed BMECs were cut into 1-um-thick sections and stained with uranyl acetate and lead citrate. The ultrathin sections were examined under JEM-1400 electron microscope (JEOL, Tokyo, Japan).
Measurement of cellular TG content and proteasome activities
The control and VPS28 knockdown BMECs were collected and broken by ultrasonication. The total lipids were extracted using the TG assay Kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China) and monitored with Infinite M200 Reader (Tecan, Männedorf, Switzerland) according to the manufacturer’s instructions.
The proteasome activities (Chymotrypsin-Like, Caspase-Like and Trypsin-Like) were measured using the Proteasome-Glo™ Cell-Based Assays (Promega, Mannheim, Germany) according to the manufacturer’s instructions, and the fluorescence intensity was monitored with Infinite M200 Reader (Tecan, Männedorf, Switzerland).
Real-time quantitative PCR analysis
The primers of selected genes for RT-qPCR were designed with Primer 5.0 and synthesized by The Beijing Genomics Institute Co., Ltd. The glyceraldehyde-3-phosphate dehydrogenase gene was used as the control. The primer sequences are listed in Table 1. RNA extraction, cDNA synthesis and RT-qPCR were performed according to the manufacturer’s instructions, and were repeated three times. The relative expression of genes was computed using the 2−ΔΔCt method.
| Genes | Primer sequences (5′→3′) | Relative expression |
|---|---|---|
| GAPDH | AGATGGTGAAGGTCGGAGTG CGTTCTCTGCCTTGACTGTG |
/ |
| VPS28 | GGAAACAAGCCGGAGCTGTA CTGGATCTCGTCCATGGCTC |
0.22 |
| CD36 | GACGGATGTACAGCGGTGAT GAAAAAGTGCAAGGCCACCA |
16.00 |
| ACACA | AGTGTTCTGATCAGGTCTTCTTGT GGGAGGCAAAAACCTCCAGA |
0.67 |
| FASN | AGGCGTGCGTGACACTT AATACAGTTGGCCGTCACCA |
6.85 |
| SCD | TCCTGATCATTGGCAACACCA CCAACCCACGTGAGAGAAGAA |
1.48 |
| DGAT1 | TACCCCGACAACCTGACCTA GGGAAGTTGAGCTCGTAGCA |
2.06 |
| ADFP | GCGTCTGCTGGCTGATTTC AGCCGAGGAGACCAGATCATA |
2.95 |
| PSMG1 | GGGAAGAAGTCGGTTGTGCT AAAAAGCCTCTGTGGGGGAC |
2.87 |
| UBE2L | CTGGCACAGTATATGAAGACCTGA GGTAGCAGGGTGTGAGGAAC |
1.28 |
| RPS29 | TGTTTCCGCCAGTATGCGAA GCTGGATGAGCCATCTAAGGAA |
2.13 |
| ISG15 | CCATCCTGGTGAGGAACGAC GTCTGCTTGTACACGCTCCT |
19.02 |
Statistical Analysis
R-package (R v3.02) was conducted to evaluated changes between VPS28 knockdown BMECs groups and the control groups. And differences were declared significant at P ≤ 0.05.
Results
VPS28 knockdown alters expression of multiple proteins in BMEC
VPS28 expression in BMEC were down-regulated by 78% with tandem constructs (as shown in Fig. 1A), and then, to obtain a whole picture of the proteomic changes in VPS28 knockdown BMEC, we conducted iTRAQ experiment in combination with LC-ESI-MS/MS analysis to investigate differentially expressed proteins in VPS28 knockdown BMECs groups (labeled iTRAQ-115, 116 and 117) and the control groups (labeled iTRAQ-118, 119 and 121). At a false discovery rate of 1.2%, a total of 2,773 proteins were identified from 14,031 peptides. The peptides of all proteins are provided in Table S1.
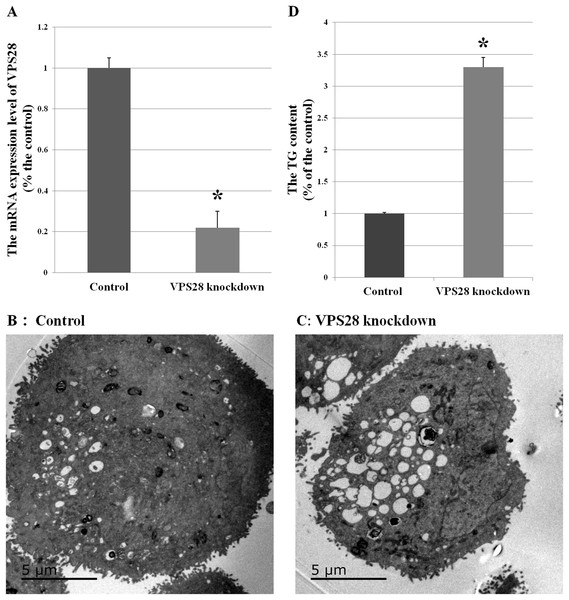
Figure 1: Effects of VPS28 knockdown on BMECs.
(A) The mRNA expression of VPS28 was decreased by tandem siRNAs constructs. (B) and (C) Electron micrographs of BMECs. (D) The TG content was significantly increased in VPS28 knockdown BMEC. Data are averages of three replicates. The error or bars denote SEM. *Indicates the difference is significant (P ≤ 0.05).To further understand the differentially expressed proteins after knocking down VPS28 in BMECs and basing on standard of the differentially expressed proteins, a total of 203 distinct proteins were identified by iTRAQ analysis in VPS28 knockdown BMECs (Detailed gene information and fold-change following VPS28 knockdown were provided in Table 2). A total of 92 proteins were significantly up-regulated (≥1.2-fold) while 111 proteins were significantly down-regulated (≤0.83-fold) when compared with the control BMECs.
| Accession | Description | Ratio |
|---|---|---|
| 300796460 | Pescadillo homolog | 0.79 |
| 333440457 | Immortalization up-regulated protein | 1.38 |
| 528937065 | PREDICTED: fragile X mental retardation syndrome-related protein 1 isoform X6 | 0.83 |
| 741896620 | PREDICTED: bifunctional coenzyme A synthase isoform X2 | 1.25 |
| 741972182 | PREDICTED: serpin B8 isoform X1 | 1.33 |
| 359069079 | PREDICTED: apoptotic chromatin condensation inducer in the nucleus isoform X5 | 0.74 |
| 528978576 | PREDICTED: lysosomal acid phosphatase isoform X3 | 0.8 |
| 77736117 | Actin, alpha cardiac muscle 1 | 1.33 |
| 741976470 | PREDICTED: actin filament-associated protein 1-like 2 isoform X5 | 0.82 |
| 156120791 | A-kinase anchor protein 8 | 0.62 |
| 155371939 | Putative N-acetylglucosamine-6-phosphate deacetylase | 1.23 |
| 741911242 | PREDICTED: AP-2 complex subunit sigma-like | 0.82 |
| 115496866 | AP-3 complex subunit beta-1 | 1.22 |
| 75832056 | Apolipoprotein A-I preproprotein | 0.46 |
| 114052298 | Apolipoprotein A-II precursor | 0.55 |
| 741944057 | PREDICTED: apolipoprotein B-100 isoform X3 | 0.71 |
| 27806739 | Apolipoprotein E precursor | 0.34 |
| 51491835 | Ovarian and testicular apolipoprotein N precursor | 0.61 |
| 528973530 | PREDICTED: ADP-ribosylation factor GTPase-activating protein 1 isoform X3 | 0.8 |
| 300798482 | Rho GTPase-activating protein 35 | 1.36 |
| 329664977 | AT-rich interactive domain-containing protein 1A | 0.83 |
| 529009701 | PREDICTED: acid ceramidase isoform X1 | 1.62 |
| 329663402 | ATPase family AAA domain-containing protein 1 | 0.76 |
| 741980112 | PREDICTED: atlastin-3 isoform X1 | 0.83 |
| 60101829 | ATP synthase subunit 8 (mitochondrion) | 1.24 |
| 28603752 | ATP synthase subunit e, mitochondrial | 0.63 |
| 116004323 | Ataxin-10 | 0.83 |
| 41386683 | Beta-2-microglobulin precursor | 0.82 |
| 84000125 | B-cell receptor-associated protein 29 | 1.34 |
| 27806229 | 2-oxoisovalerate dehydrogenase subunit alpha, mitochondrial precursor | 0.83 |
| 741929024 | PREDICTED: uncharacterized protein C4orf3 homolog isoform X1 | 1.35 |
| 741945468 | PREDICTED: calcium-binding protein 39-like isoform X1 | 0.79 |
| 45439308 | CD63 antigen | 1.36 |
| 78042548 | CD81 antigen | 1.25 |
| 741967799 | PREDICTED: LOW QUALITY PROTEIN: serine/threonine-protein kinase MRCK beta isoform X2 | 1.37 |
| 529002260 | PREDICTED: CUB domain-containing protein 1 | 0.8 |
| 77735577 | CCR4-NOT transcription complex subunit 7 | 0.77 |
| 741922497 | PREDICTED: collagen alpha-3(VI) chain isoform X7 | 0.81 |
| 114052042 | COMM domain-containing protein 1 | 1.23 |
| 741945876 | PREDICTED: COMM domain-containing protein 6 isoform X2 | 0.68 |
| 528966533 | PREDICTED: COP9 signalosome complex subunit 2 isoform X1 | 0.82 |
| 149642865 | COP9 signalosome complex subunit 3 | 0.74 |
| 330688478 | Crooked neck-like protein 1 | 1.6 |
| 262073106 | Cathepsin D precursor | 1.23 |
| 118151448 | CUGBP Elav-like family member 2 | 1.25 |
| 741917150 | PREDICTED: cytochrome P450 20A1 isoform X1 | 1.27 |
| 164420721 | Dynactin subunit 5 | 1.29 |
| 528937089 | PREDICTED: DCN1-like protein 1 isoform X1 | 0.67 |
| 149642575 | ATP-dependent RNA helicase DDX24 | 1.54 |
| 114051872 | Density-regulated protein | 0.81 |
| 157427916 | H/ACA ribonucleoprotein complex subunit 4 | 1.22 |
| 115497846 | deoxyhypusine hydroxylase | 1.48 |
| 528989517 | PREDICTED: developmentally-regulated GTP-binding protein 1 isoform X1 | 1.47 |
| 114051994 | Dysbindin | 1.35 |
| 329663806 | Cytoplasmic dynein 1 light intermediate chain 2 | 1.23 |
| 56710336 | Dynein light chain 1, cytoplasmic | 0.83 |
| 77735949 | 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase | 1.24 |
| 62751595 | Translation initiation factor eIF-2B subunit beta | 0.71 |
| 300794424 | Eukaryotic translation initiation factor 5 | 0.78 |
| 329664532 | Ephrin type-A receptor 2 precursor | 0.74 |
| 77735625 | Enhancer of rudimentary homolog | 0.81 |
| 27806943 | Coagulation factor V precursor | 0.81 |
| 528957418 | PREDICTED: protein FAM114A2 isoform X1 | 1.29 |
| 329663573 | Protein FAM134A | 0.75 |
| 359069460 | PREDICTED: protein FAM98B | 0.8 |
| 29135293 | Farnesyl pyrophosphate synthase | 0.77 |
| 77736507 | Mitochondrial fission 1 protein | 1.27 |
| 156718120 | Fat storage-inducing transmembrane protein 2 | 1.23 |
| 27806621 | Ferritin heavy chain | 0.8 |
| 114051796 | Glucosylceramidase precursor | 0.81 |
| 84000253 | Glutamate--cysteine ligase regulatory subunit | 1.21 |
| 114051291 | GDP-L-fucose synthase | 0.78 |
| 741919465 | PREDICTED: lysosomal protein NCU-G1 isoform X2 | 0.81 |
| 115496402 | Glucosamine-6-phosphate isomerase 2 | 0.83 |
| 297488836 | PREDICTED: histone H1x | 0.82 |
| 116812902 | Hemoglobin subunit alpha | 0.55 |
| 17985949 | Hemoglobin subunit beta-1 [Rattus norvegicus] | 1.23 |
| 741905547 | PREDICTED: host cell factor 1 isoform X9 | 1.26 |
| 114052627 | Hepatocyte growth factor-regulated tyrosine kinase substrate | 1.21 |
| 134085671 | Histone H1.2 | 0.55 |
| 155371863 | Histone H1.3 | 0.54 |
| 741971316 | PREDICTED: histone H2A type 1-J | 1.29 |
| 157785601 | Histone H2B | 0.82 |
| 115496175 | High mobility group protein HMG-I/HMG-Y | 0.8 |
| 77736489 | Non-histone chromosomal protein HMG-14 | 0.79 |
| 297477251 | PREDICTED: heterogeneous nuclear ribonucleoprotein A0 | 0.83 |
| 375364520 | HCLS1-binding protein 3 | 1.48 |
| 41386699 | Heat shock-related 70 kDa protein 2 | 1.23 |
| 529014943 | PREDICTED: immunoglobulin-binding protein 1 isoform X2 | 1.23 |
| 27805955 | Ubiquitin-like protein ISG15 | 0.83 |
| 157427772 | Involucrin | 1.25 |
| 195539527 | Keratin 15 | 1.24 |
| 77736483 | Ragulator complex protein LAMTOR1 | 0.82 |
| 741894288 | PREDICTED: galectin-7 | 1.26 |
| 528952868 | PREDICTED: LIM and calponin homology domains-containing protein 1 isoform X5 | 1.36 |
| 115497506 | LIM and cysteine-rich domains protein 1 | 1.23 |
| 686713724 | PREDICTED: LOW QUALITY PROTEIN: collagen alpha-4(VI) chain-like, partial [Pongo abelii] | 0.72 |
| 741878073 | PREDICTED: N-acylneuraminate cytidylyltransferase | 1.96 |
| 741946731 | PREDICTED: ankyrin repeat domain-containing protein 26-like isoform X2 | 0.35 |
| 62460494 | Hemoglobin fetal subunit beta | 0.56 |
| 84000167 | WD repeat-containing protein 61 | 1.36 |
| 741960002 | PREDICTED: protein arginine N-methyltransferase 1 isoform X2 | 0.76 |
| 297483902 | PREDICTED: apolipoprotein R | 0.61 |
| 155372051 | Tropomyosin alpha-4 chain | 0.83 |
| 78369240 | U6 snRNA-associated Sm-like protein LSm4 | 0.7 |
| 122692397 | Latexin | 0.79 |
| 77735445 | Protein mago nashi homolog | 1.22 |
| 741939300 | PREDICTED: dual specificity mitogen-activated protein kinase kinase 1 isoform X1 | 1.21 |
| 528995215 | PREDICTED: dual specificity mitogen-activated protein kinase kinase 4 isoform X2 | 1.21 |
| 741898851 | PREDICTED: MAP/microtubule affinity-regulating kinase 3 isoform X1, partial | 0.83 |
| 528957564 | PREDICTED: methionine adenosyltransferase 2 subunit beta isoform X1 | 0.81 |
| 528966905 | PREDICTED: protein max isoform X2 | 0.76 |
| 741957547 | PREDICTED: mediator of RNA polymerase II transcription subunit 15 isoform X3 | 0.79 |
| 300794942 | DNA mismatch repair protein Msh6 | 0.74 |
| 27806841 | interferon-induced GTP-binding protein Mx1 | 0.73 |
| 528936325 | PREDICTED: N-alpha-acetyltransferase 50 isoform X1 | 1.3 |
| 375065860 | NAD kinase 2, mitochondrial | 1.29 |
| 300795748 | NEDD8-activating enzyme E1 regulatory subunit | 0.77 |
| 331284195 | Nucleolin | 1.38 |
| 78369204 | Protein NDRG2 | 1.24 |
| 28372495 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 11 | 1.23 |
| 75812936 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 11, mitochondrial precursor | 1.32 |
| 28603776 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 5, mitochondrial precursor | 0.72 |
| 528944090 | PREDICTED: nexilin isoform X5 | 0.76 |
| 300794221 | Nuclear protein localization protein 4 homolog | 0.82 |
| 83035119 | Nuclear transport factor 2 | 0.79 |
| 741958202 | PREDICTED: prolyl 3-hydroxylase OGFOD1 isoform X1 | 0.65 |
| 27807193 | Platelet-activating factor acetylhydrolase IB subunit beta | 1.27 |
| 75812940 | Phosphatidylethanolamine-binding protein 1 | 1.27 |
| 528913445 | PREDICTED: presequence protease, mitochondrial isoform X2 | 1.21 |
| 329664500 | Pyruvate kinase PKM | 1.25 |
| 528961976 | PREDICTED: pyruvate kinase PKM isoform X1 | 1.26 |
| 741932605 | PREDICTED: perilipin-3 isoform X3 | 0.82 |
| 116004039 | Peptidyl-prolyl cis-trans isomerase C precursor | 1.22 |
| 741957590 | PREDICTED: protein phosphatase 1F | 1.53 |
| 115497768 | RelA-associated inhibitor | 1.24 |
| 528943961 | PREDICTED: cAMP-dependent protein kinase catalytic subunit beta isoform X8 | 1.22 |
| 741948151 | PREDICTED: pre-mRNA-processing factor 6 isoform X1 | 0.73 |
| 115496548 | Proteasome assembly chaperone 1 | 1.26 |
| 741926509 | PREDICTED: prostaglandin E synthase 3 isoform X1 | 0.83 |
| 157428086 | Ras-related protein Rab-8A | 0.8 |
| 77736231 | Ras-related protein Ral-A | 1.2 |
| 56118252 | RING finger protein 113A | 1.27 |
| 741937627 | PREDICTED: ribosome production factor 2 homolog isoform X1 | 0.77 |
| 27807465 | 60S ribosomal protein L10 | 0.79 |
| 62751646 | 60S ribosomal protein L13 | 0.7 |
| 116004215 | 60S ribosomal protein L13a | 0.81 |
| 118150852 | 60S ribosomal protein L15 | 0.7 |
| 62751887 | 60S ribosomal protein L26 | 0.77 |
| 77404275 | 60S ribosomal protein L27 | 0.79 |
| 77735585 | 60S ribosomal protein L36a | 0.8 |
| 62460480 | 60S ribosomal protein L4 | 0.74 |
| 114053031 | 39S ribosomal protein L48, mitochondrial precursor | 0.81 |
| 72534798 | 60S ribosomal protein L6 | 0.72 |
| 62460552 | 60S ribosomal protein L7 | 0.77 |
| 77736197 | 60S ribosomal protein L8 | 0.78 |
| 164420694 | 60S ribosomal protein L9 | 1.21 |
| 70778762 | 60S acidic ribosomal protein P1 | 1.2 |
| 66792924 | 40S ribosomal protein S11 | 0.76 |
| 77735975 | 28S ribosomal protein S26, mitochondrial precursor | 1.23 |
| 528994013 | PREDICTED: 28S ribosomal protein S23, mitochondrial isoform X3 | 1.23 |
| 27807381 | 40S ribosomal protein S29 | 1.23 |
| 70778956 | 40S ribosomal protein S8 | 0.79 |
| 155372029 | 40S ribosomal protein S9 | 0.69 |
| 741947465 | PREDICTED: ribosome-binding protein 1 isoform X2 | 1.31 |
| 300798287 | Sec1 family domain-containing protein 1 | 0.83 |
| 115497454 | Protein SEC13 homolog | 1.28 |
| 300794266 | SEC23-interacting protein | 1.3 |
| 741978352 | PREDICTED: protein transport protein Sec24C isoform X2 | 1.31 |
| 115497008 | Protein transport protein Sec61 subunit beta | 0.82 |
| 70778796 | Splicing factor 3B subunit 5 | 1.3 |
| 77736509 | S-phase kinase-associated protein 1 | 1.35 |
| 82617542 | Monocarboxylate transporter 1 | 1.24 |
| 288557348 | SWI/SNF complex subunit SMARCC2 | 0.8 |
| 115496404 | U1 small nuclear ribonucleoprotein C | 0.78 |
| 329664862 | S1 RNA-binding domain-containing protein 1 | 0.73 |
| 741921253 | PREDICTED: serine/arginine-rich splicing factor 11 isoform X4 | 1.49 |
| 329664840 | synaptopodin | 0.82 |
| 84000143 | T-complex protein 1 subunit alpha | 1.21 |
| 114051768 | Tudor domain-containing protein 3 | 1.36 |
| 300797062 | Tudor domain-containing protein 6 | 1.52 |
| 529000498 | PREDICTED: THUMP domain-containing protein 3 isoform X1 | 1.3 |
| 114326224 | Tight junction protein ZO-3 | 1.25 |
| 300794719 | E3 ubiquitin-protein ligase TRIP12 | 0.78 |
| 27806789 | Transthyretin precursor | 1.29 |
| 529005013 | PREDICTED: thioredoxin-like protein 1 isoform X2 | 1.21 |
| 83035103 | Ubiquitin-conjugating enzyme E2 H | 1.24 |
| 528979920 | PREDICTED: ubiquitin conjugation factor E4 B isoform X1 | 1.22 |
| 114050863 | Ubiquitin-like domain-containing CTD phosphatase 1 | 0.72 |
| 529012185 | PREDICTED: UBX domain-containing protein 1 isoform X1 | 0.78 |
| 62751620 | Ubiquitin-fold modifier-conjugating enzyme 1 | 0.82 |
| 529006388 | PREDICTED: ubiquitin carboxyl-terminal hydrolase 7 isoform X2 | 1.37 |
| 115496338 | Vesicle-associated membrane protein-associated protein A | 1.2 |
| 78369492 | Vacuolar protein sorting-associated protein 28 homolog | 0.79 |
| 78045497 | Vitronectin precursor | 0.43 |
| 741916372 | PREDICTED: xin actin-binding repeat-containing protein 2 isoform X2 | 0.72 |
| 126723764 | Cap-specific mRNA (nucleoside-2′-O-)-methyltransferase 1 | 0.8 |
| 78042540 | Synaptobrevin homolog YKT6 | 0.82 |
| 148224064 | Transcriptional repressor protein YY1 | 0.76 |
| 84370039 | Zinc finger protein ZPR1 | 0.77 |
| 528942220 | PREDICTED: rho guanine nucleotide exchange factor 2 isoform X5 | 1.2 |
| 528962021 | PREDICTED: geranylgeranyl transferase type-2 subunit alpha isoform X1 | 0.83 |
| 528979380 | PREDICTED: glyoxylate reductase/hydroxypyruvate reductase | 1.35 |
The DEPs were categorized into 53 clusters (P < 0.05, as shown in Table 3) according to their biological processes (BPs), cellular components (CCs) and molecular functions (MFs). The top 6 GO terms for BPs were enriched in cytoplasmic translation (GO:0002181), translation (GO:0006412), cholesterol homeostasis (GO:0042632), cholesterol efflux (GO:0033344), positive regulation of cholesterol esterification (GO:0010873) and high-density lipoprotein particle assembly (GO:0034380). These biological processes were involved in the lipid metabolism and transportation. The top 5 GO terms for CCs were cytosolic large ribosomal subunit (GO:0022625), extracellular exosome (GO:0070062), focal adhesion (GO:005925), membrane (GO:0016020) and very-low-density lipoprotein particle (GO:0034361). These cellular components were response to the ubiquitin system. The top 5 GO terms for MFs were mainly enriched in structural constituent of ribosome (GO:0003735), RNA binding (GO:0003723), cholesterol transporter activity (GO:0017127), phosphatidylcholine-sterol O-acyltransferase activator activity (GO:0019843). These results showed that the DEPs following VPS28 knockdown were mainly involved in the functions of transport and metabolism of lipid, lipoprotein and lipoprotein receptor binding, and ribosome translation.
| GO ID | Term | P-value |
|---|---|---|
| Biological process | ||
| GO:0002181 | Cytoplasmic translation | 3.60E−07 |
| GO:0006412 | Translation | 8.90E−07 |
| GO:0042632 | Cholesterol homeostasis | 2.20E−03 |
| GO:0033344 | Cholesterol efflux | 2.70E−03 |
| GO:0010873 | Positive regulation of cholesterol esterification | 2.80E−03 |
| GO:0034380 | High-density lipoprotein particle assembly | 3.70E−03 |
| GO:0000463 | Maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) | 4.70E−03 |
| GO:0043691 | Reverse cholesterol transport | 7.10E−03 |
| GO:0033700 | Phospholipid efflux | 8.40E−03 |
| GO:0098779 | Mitophagy in response to mitochondrial depolarization | 1.00E−02 |
| GO:0042157 | Lipoprotein metabolic process | 1.30E−02 |
| GO:0019433 | Triglyceride catabolic process | 1.50E−02 |
| GO:0006904 | Vesicle docking involved in exocytosis | 2.30E−02 |
| GO:0000027 | Ribosomal large subunit assembly | 2.30E−02 |
| GO:0018158 | Protein oxidation | 2.30E−02 |
| GO:0006403 | RNA localization | 2.30E−02 |
| GO:0010628 | Positive regulation of gene expression | 2.60E−02 |
| GO:0001843 | Neural tube closure | 3.10E−02 |
| GO:0006695 | Cholesterol biosynthetic process | 3.20E−02 |
| GO:0051028 | mRNA transport | 3.20E−02 |
| GO:0042921 | Glucocorticoid receptor signaling pathway | 3.50E−02 |
| GO:0010903 | Negative regulation of very-low-density lipoprotein particle remodeling | 3.50E−02 |
| GO:0006046 | N-acetylglucosamine catabolic process | 3.50E−02 |
| GO:1901998 | Toxin transport | 3.70E−02 |
| GO:0006888 | ER to Golgi vesicle-mediated transport | 3.80E−02 |
| GO:0018206 | Peptidyl-methionine modification | 4.60E−02 |
| GO:0042159 | Lipoprotein catabolic process | 4.60E−02 |
| GO:0042158 | Lipoprotein biosynthetic process | 4.60E−02 |
| Cellular component | ||
| GO:0022625 | Cytosolic large ribosomal subunit | 4.00E−12 |
| GO:0070062 | Extracellular exosome | 2.70E−08 |
| GO:0005925 | Focal adhesion | 1.30E−05 |
| GO:0016020 | Membrane | 1.70E−04 |
| GO:0034361 | Very-low-density lipoprotein particle | 3.60E−04 |
| GO:0005840 | Ribosome | 8.30E−04 |
| GO:0022627 | Cytosolic small ribosomal subunit | 9.90E−04 |
| GO:0042627 | Chylomicron | 3.30E−03 |
| GO:0072562 | Blood microparticle | 9.70E−03 |
| GO:0034363 | Intermediate-density lipoprotein particle | 3.30E−02 |
| GO:0005730 | Nucleolus | 3.80E−02 |
| GO:0008180 | COP9 signalosome | 4.60E−02 |
| GO:0005737 | Cytoplasm | 4.70E−02 |
| Molecular function | ||
| GO:0003735 | Structural constituent of ribosome | 5.20E−10 |
| GO:0003723 | Poly(A) RNA binding | 2.90E−09 |
| GO:0017127 | Cholesterol transporter activity | 3.30E−04 |
| GO:0019843 | rRNA binding | 5.20E−04 |
| GO:0060228 | Phosphatidylcholine-sterol O-acyltransferase activator activity | 1.40E−03 |
| GO:0003729 | mRNA binding | 6.90E−03 |
| GO:0005543 | Phospholipid binding | 1.70E−02 |
| GO:0003743 | Translation initiation factor activity | 2.10E−02 |
| GO:0003723 | RNA binding | 3.50E−02 |
| GO:0008035 | High-density lipoprotein particle binding | 3.50E−02 |
| GO:0070653 | High-density lipoprotein particle receptor binding | 3.50E−02 |
| GO:0015485 | Cholesterol binding | 4.60E−02 |
Effect of VPS28 knockdown on morphology of BMECs
Electron micrographs could observe the morphological changes in BMECs. Compared with the control BMECs groups, the VPS28 knockdown groups showed containing more and strikingly large lipid droplets and many luminal spaces were completely filled with aggregated lipid (as shown in Figs. 1B and 1C). And in parallel, the content of TG was increased by 3.3-fold above the control BMECs groups (Fig. 1D).
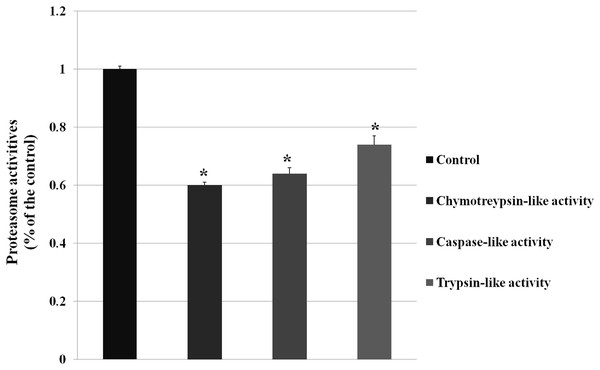
The GO analysis demonstrated DEPs enriched in ubiquitylation singaling, and ubiquitylation mediates the degradation of membrane proteins and intracellular proteins, which plays an crucial role in receptor-mediated signaling pathways and quality control of intracellular proteins. And then we examined the proteasome activity (chymotreypsin-like activity, caspase-like activity, trypsin-like activity) after knocking down VPS28 (as shown in Fig. 2). The results showed that VPS28 knockdown could significantly decrease the three activites of proteasome, the relative activities of chymotreypsin-like, caspase-like and trypsin-like are 0.60, 0.64, 0.74, respectively. And we also found the level of ubiquitinated proteins was increased by VPS28 knockdown (the data has published) (Lily Liu, 2019). These results indicated that VPS28 could regulate ubiquitylation-proteasome system.
Figure 2: The proteasome activity was decreased by VPS28 knockdown.
Chymotreypsin-like activity, caspase-like activity and trypsin-like activity are the three activities in proteasome. An asterisk (*) indicates the difference is significant (P ≤ 0.05).Validation of gene expression by RT-qPCR
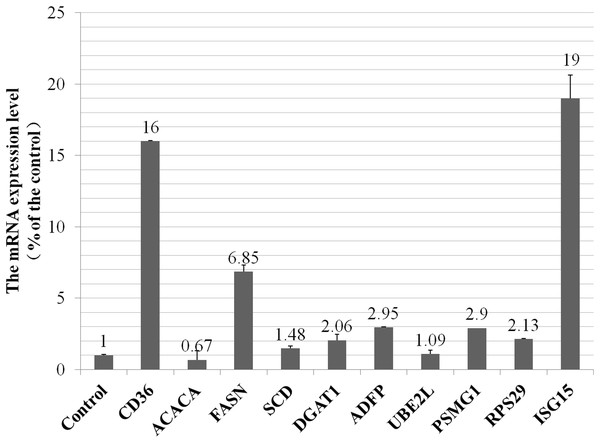
To investigate whether the alteration of proteins expression level were the result of transcriptional regulation, we detected mRNA levels of five selected proteins and five genes that were related to metabolism of fatty acids, ubiquitylation and proteasome pathways. The RT-qPCR results showed high qualitative and quantitative concordance (correlation coefficient > 0.95). As shown in Fig. 3, CD36 (cluster of differentiation 36) in fatty acids taken up process, FASN (fatty acid synthase), SCD (stearoyl-CoA desaturas) and DGAT1 (diacylglycerol acyltransferase 1) in fatty acids synthesis pathway, ADFP (adipose differentiation-related protein) in lipid droplet secretion process, were all up-regulated by VPS28 knockdown. ACACA (acetyl-CoA) in de novo fatty acids synthesis pathway was down-regulated in VPS28 knockdown BMECs. PSMG1 (proteasome assembly chaperone 1) in proteasome system, RPS29 (ribosomal protein S29) in ribosome translation pathway, UBE2L (ubiquitin-conjugating enzyme E2L), ISG15 (interferon-stimulatory gene ISG15) in ubiquitylation pathway, were all up-regulated by VPS28 knockdown. The results showed that the mRNA expression levels of genes were generally corresponded with the changes in the morphology of BMECs and proteins expression detected by the iTRAQ approach.
Figure 3: The mRNA expression of selected genes in VPS28 knockdown BMECs.
Discussion
Milk fat synthesis is a complex process. Numerous types of molecular and chemical relationships exist which directly or indirectly could affect protein activity and regulate milk fat synthesis, such as ubiquitylation and protein–protein interaction. Ubiquitylation is an important post-translational modification and it can mediate the intercellular proteins degradation which plays a crucial role in receptor-mediated signaling pathways. VPS28 as a subunit of ESCRTs is crucial for ubiquitin-mediated degradation of proteins, and we found VPS28 could alter the ubiquitylation level to regulate milk fat synthesis in previous studies (Liu et al., 2018; Lily Liu, 2019). However, much less is understood regarding the molecular mechanisms of VPS28 regulating milk fat synthesis through ubiquitylation. In this study, iTRAQ technology were performed to accurately identify the peptides and precisely quantify the iTRAQ labels. Subsequently, cluster and pathways analysis were devoted to obtain consistent results to further elucidate the regulation pathways of VPS28 on the milk fat synthesis.
The ubiquitin system is a protein degradation pathway, dedicates to the ubiquitylation of cellular targets and the subsequent control of numerous cellular functions and plays an important role in metabolism regulation (Hoeller & Dikic, 2009). The deregulation of components of this elaborate network leads to an accumulation of membrane proteins and no longer degraded in the vacule. Numerous studies indicated that, as one subunit of ESCRTs, VPS28 played a crucial role in ubiquitin-mediated degradation of membrane proteins (Ciechanover, 1994; Katzmann, Babst & Emr, 2001) and cytoplasmic proteins (Smith et al., 2008). In this study, BMECs sections showed that the form of fat droplets was affected after knocking down VPS28, and we found an accumulation of ubiquitinated proteins and a dysfunction of proteasome activity in VPS28 knockdown BMECs groups. The proteomic analysis indentified many differentially expressed proteins that were considerably enriched in extracellular exosome (GO:0070062) and membrane (GO:0016020). These GO categories were associated with the ubiquitylation system. These indicated that VPS28 knockdown played a crucial role in ubiquitylation.
In BMECs, fatty acids taken up and de novo fatty acids synthesis are involved in milk fat synthesis. In our previous study, by knocking down VPS28 in BMECs, we found ubiquitinated CD36 level was increased significantly which is the main protein involved in long-chain fatty acids uptake, and the mRNA expression of other milk fat-related genes were also up-regulated. These results indicated the process of long-chain fatty acids taken up was promoted by VPS28 knockdown in BMECs. In parallel, the expression of ADFP was found increased in VPS28 knockdown BEMCs. ADFP as a specific marker for lipid droplet, its expression level is in keeping with with abundance of lipid droplets in cell (Chang & Chan, 2007). The proteomic analysis also indentified many differentially expressed proteins enriched in lipid metabolism. These data confirmed VPS28 knockdown could facilitate milk fat synthesis in BMECs.
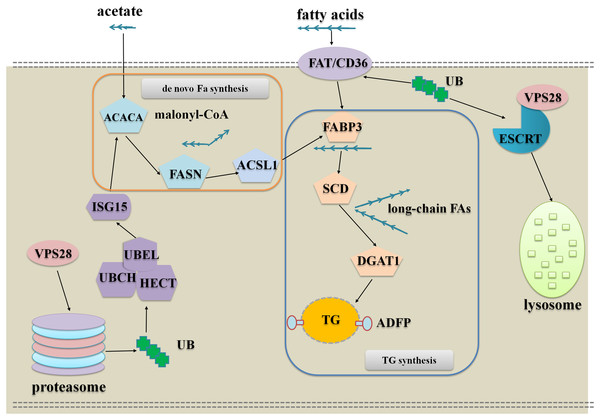
The DEPs analysis indicates that VPS28 could regulate milk fat synthesis in two approaches, the one is VPS28 directly regulates milk fat synthesis through ubiquitylation and the other one is VPS28 mediates ubiquitin-proteasome system to regulate milk fat synthesis. To further understand these, we used the key interact proteins and genes to generate the pathway networks, following is the description of the model presented in Fig. 4:
Figure 4: The network of VPS28 knockdown regulates milk fat synthesis in BMECs.
VPS28 knockdown leads an accumulation of ubiquitinated membrane proteins to promote fatty acids taken up to synthesize TG: In this regulation, CD36 appears to be the most important protein, and the other enzymes involved in milk fat synthesis could be increased through allosteric effect. CD36 as a membrane scavenger receptor was identified as a receptor of fatty acid and ubiquitinated CD36 facilitates long-chain fatty acids uptake (Liang et al., 2004; Schrader, Harstad & Matouschek, 2009; Lamb et al., 2010). Following VPS28 knockdown, the accumulation of ubiquitinated CD36 could import more long-chain fatty acids into BMECs, and the imported long-chain fatty acids are combined and transported to endoplasmic reticulum by fatty acid binding proteins. Subsequently, SCD and DGAT1 are induced to utilize fatty acids to synthesize TG. Therefore, VPS28 knockdown could promote long chain fatty acids taken up to synthesize TG.
VPS28 knockdown leads an accumulation of ubiquitinated cytoplasmic proteins to promote de novo biogenesis, activation and channeling of fatty acids to synthesize TG: In this regulation, proteasome plays the most important role. Following VPS28 knockdown, proteasome activity and the expression of ISG15 (interferon-stimulatory gene ISG15) were decreased. ISG15 is an ubiquitin-like protein that mediates the conjugation of different proteins through its ISGylation enzymes UBE2L6 (ubiquitin conjugating enzyme E2 L6) (Haq et al., 2016), and we also found UBE2L6 was down regulated. Previous studies have suggested that down-regulation of ISG15 and UBE2L6 can counteract degradation of triglyceride lipase (Zhou et al., 2015; Kim et al., 2004; Zhao et al., 2004), and ISG15 conjugation regulates exosome secretion (Villarroya-Beltri et al., 2016). The accumulation of ACACA (Acetyl-CoA) (Emery, 1973) and the other allosteric affected enzymes promote the de novo biogenesis, activation and channeling of fatty acids to synthesize TG in BMECs.
Conclusions
In this study, iTRAQ technology was used to demonstrate proteome spectrum changes in the BMECs after knocking down VPS28. It was concluded that VPS28 knockdown promotes milk fat synthesis in BMECs which might be attributed to differentially expressed proteins. The DEPs enriched in GO categories associated with ubiquitylation likely played an important role in the TG synthesis in BMECs. The dysfunctional proteasome, accumulation of TG might explain the regulation of VPS28 on milk fat synthesis was mediated by ubiquitylation. Our results provide a comprehensive dataset of ubiquitylation regulating milk fat synthesis, and also provide a reference for the further study of ubiquitination in dairy breeding.