Physiological and anatomical differentiation of two sympatric weed populations
- Published
- Accepted
- Received
- Academic Editor
- Mike Thiv
- Subject Areas
- Ecology, Evolutionary Studies, Plant Science, Population Biology
- Keywords
- Shepherd’s Purse, Capsella, Photosynthetic capacity, Sympatric evolution, Anatomy, Flowering time, Spe mutant
- Copyright
- © 2020 Neuffer et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Physiological and anatomical differentiation of two sympatric weed populations. PeerJ 8:e9226 https://doi.org/10.7717/peerj.9226
Abstract
In the vineyards of Rhineland-Palatinate (Germany), two different types of Shepherd’s Purse (Capsella bursa-pastoris) coexist: (1) the common type called ‘wild type’, and (2) the decandric type called Capsella apetala or ‘Spe’ with four stamens in place of the four petals. In this study, we compare the anatomical and physiological characters of rosette leaves of the respective types. Progeny of individual plants was cultivated in growth chambers under low- and high-light conditions. Under low-light conditions, the stomata densities of the adaxial and abaxial epidermis did not differ between the two types. When grown under high-light conditions, wild type and Spe, both exhibited increased stomata densities compared to low-light conditions, but Spe to a lesser extent than the wild type. The maximal photosynthetic capacity of Spe was lower in both, low-light and high-light conditions compared to wild-type plants. Under all CO2 concentrations, Spe seemed to be less productive. The less effective CO2 assimilation of the Spe mutant C. apetala was accompanied by later flowering. This fact prolonged the vegetative phase of Spe by about two weeks and was sufficient for the maintenance of both populations stably over years.
Introduction
In the vineyards of southwestern Germany (Rhineland-Palatinate) two morphologically distinct types of the common annual weed Shepherd’s Purse (Capsella bursa-pastoris (L.) Medik., Brassicaceae) occur side by side (Reichert, 1998, Fig. 1). The two populations can readily be differentiated by their characteristic flower structure. Wild type (wt) C. bursa-pastoris flowers exhibit the conserved body plan of Brassicaceae: it comprises four sepals in the first, four petals in the second, six stamens in the third, and two fused carpels in the fourth, the innermost whorl (Fig. 1).
Figure 1: Flower morphology.
Typical wild-type flower (A: arrow points to the petal) and its typical pollinator, a wild bee (D). Typical decandric flower (C) and its typical pollinator, a hoverfly (E). Proof for a rare hybrid between both flower types with a developing petal and pollen sacs beneath (B). In the field, both types are growing in mixed populations (F). Fotos: Gitta Schüttler, Birgit von Höveling.In contrast, the flowers of the floral variant, also referred to as Spe (Hameister & Neuffer, 2017; Nutt et al., 2006), can easily be identified by four additional stamens replacing the wild-type petals thus giving it its unique decandric phenotype (Fig. 1). This floral variant of C. bursa-pastoris has initially been described as C. apetala Opiz (Opiz, 1821), a terminology which we follow here to differentiate between both types. Notably, the decandric flower shape does not affect floral symmetry, but the lack of petals might have an impact on pollination success (Hameister & Neuffer, 2017). Recent field surveys in vineyards revealed that the wild type is the predominant taxon with tens of thousands of individuals, and the Spe occurs with a stable frequency of about 10% (Hameister, Neuffer & Bleeker, 2009). Intriguingly, potential pollinators, especially wild bees, and hoverflies, tend to prefer one over the other type, probably due to differing volatile emissions of the two flower types (Ziermann et al., 2009; Hameister & Neuffer, 2017, Fig. 1). Recent investigations by Hameister & Neuffer (2017) demonstrated that flower induction is delayed in Spe when compared with wild type in both, field and controlled greenhouse conditions. Notably, the authors could show that in an overlapping period both types are fertile. However, both taxa exhibit a high “selfing“ rate and a low crossing rate between the two flower types has been found (Hameister & Neuffer, 2017), Fig. 1). Furthermore, pollen tubes of C. apetala self-pollen grew faster compared with C. apetala crossed with wild-type pollen (spe ×wt, Neuffer & Paetsch, 2013). Field experiments in the Botanical Garden of the University of Osnabrueck revealed that the two types differ in the physiological investments into their respective offspring. Wild-type individuals produced more siliques per plant, whereas the Spe generated a higher number of seeds in the siliques (Hameister & Neuffer, 2017). The total seed output per plant was balanced among the two flower types (Hameister & Neuffer, 2017) indicating that the fitness-related factor seed production remains unaffected.
Taken together, the previous investigations lead to the assumption that C. bursa-pastoris and C. apetala are separated by several smooth to strong isolation barriers. Thus, they form two morphologically distinct types that grow sympatrically in the vineyards of southwestern Germany (Reichert, 1998). Further support for this hypothesis can be extracted from genetic work by Hameister, Neuffer & Bleeker (2009). Here, the authors showed that wild type and Spe differed in their isozyme genotype. Furthermore, the genetic difference was confirmed by creating a mapping population between a wild-type individual and a Spe individual followed by comparative linkage analysis (Hameister et al., 2013).
In this study, we focus on the properties of the leaves of the two types in the vegetative stage when the plant accumulates assimilates which then are used for building up the inflorescence and production of seeds. Rosette leaves provide the basis for an individual to withstand harsh environmental conditions, e.g., cold temperatures, high irradiation, and drought stress. When the apical meristem switches from the vegetative to the reproductive state, no further rosette leaves are generated. The question was: what is the contribution of leaf differentiation to the ecotypic variation between sympatrically growing individuals with normal (wild-type) flowers in contrast to individuals with decandric (Spe) flowers? Here, we studied the ecotypic differentiation of the rosette leaf after growth under stress (high-light) and non-stress (low-light) conditions using anatomical and physiological indicators.
Material and Methods
Plant material and cultivation of plants
Seeds of individual plants of Capsella bursa-pastoris (wild type) and Capsella apetala Opiz (Spe) have been collected in the vineyards located near to Gau-Odernheim (Rhineland-Palatinate, 25 km southwest of Mainz, 49.7847N, 8.1942E, elevation 150 m, Germany) and subsequently stored at minus 20 °C in the seed gene bank of the Botanical Garden of Osnabrueck. The material refers to the gene bank numbers 1951/19 and 1960/25 for the wild type and 1956/5 and 1961/4 for the decandric type. Note that plant material subjected to photophysiological and anatomic characterization in this publication is material derived from seeds of two independent population subgroups of each flower morphological type. Plants were initially cultivated under controlled conditions programmed with a 12-h photoperiod per day, resulting in four subgroups with six individuals each. The temperature was adjusted to 15 °C during day time and 5 °C during the night phase (initial growth conditions for seedlings). After six weeks, the plants were divided into two experimental groups. Three individuals of each subgroup were transferred into two different light conditions in an 8-h light period at 20 °C: (i) high-light setting (600 µmol quanta m−2s−1); (ii) low-light setting (100 µmol quanta m−2s−1) (Fig. 2). Later on sister individuals have been planted in the experimental field of the Botanical Garden.
Morphology and anatomy
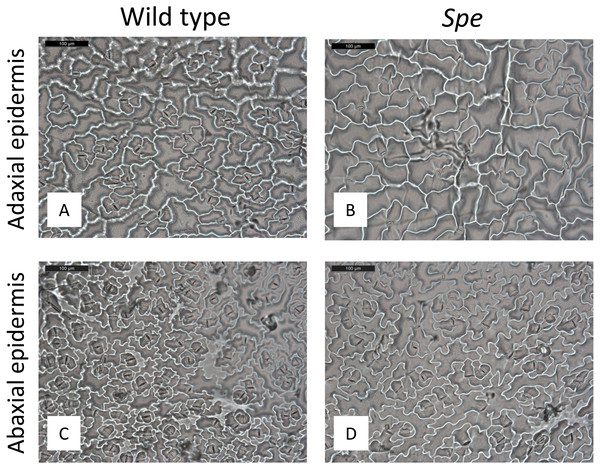
Leaf characterization was performed on three-months-old leaves. Epidermal leaf tissue was prepared for microscopic examination (Fig. 3) by a customized technique applying nail polish. After solidification of the nail polish, the epidermal cell layer was removed by transparent adhesive tape (Tesafilm). The nail polish imprint, including adhesive tape, was observed with a microscope. Subsequently, stomata were quantified in a representative area of 400 × 400 µm2 on one rosette leaf of every individual plant (Fig. 4).
Photosynthetic characterization
All measurements were performed using one fully expanded rosette leaf from plants two months after sowing when they did not yet flower. The CO2 gas exchange was measured using the LI-6400/XT Portable Photosynthesis System (LI-COR Environmental, Lincoln, NE, USA). All measurements were conducted under saturating light intensities of 800 µmol quanta m−2 s−1 at 20 °C. The relative humidity was adjusted to approximately 50%. The rate of CO2 assimilation (A) was measured as a function of sub-stomatal CO2 concentration (Ci). Ci values were applied in the following order: 400 ppm; 200 ppm; 100 ppm; 50 ppm; 0 ppm; 400 ppm; 600 ppm; 1,000 ppm; 1,750 ppm. Here, the initial decrease in Ci concentration was chosen to ensure a sufficient leaf conductance. The leaf conductance is widely considered as an indicator of effective gas exchange of the leaf with the surrounding environment and is predominantly determined by the extent to which the stomata are opened. By applying decreasing CO2 concentrations in the first half of a photosynthesis measurement, a conductance of at least 0.2 was maintained throughout the entire measurement (Fig. S1). In this way, a possible limitation of CO2 assimilation due to inefficient gas exchange was avoided. Data points were logged within 2 min after the start of each Ci concentration when infrared gas analyzer (IRGA) parameters reached a steady-state value (raw data for 22 individuals see Table S2).
Determination of flowering time under field conditions
Seventy days after sowing, sister plants were planted into the experimental field of the Botanical Garden Osnabrueck and flowering time was determined by observation of the emerging inflorescence bud.
Results
Stomata density on both sides of rosette leaves
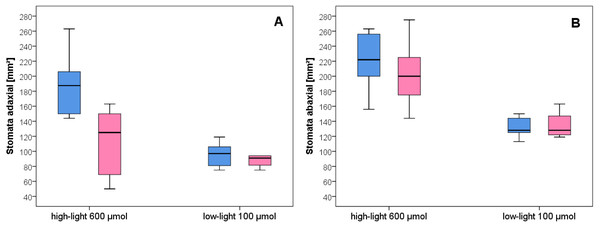
After growth for three months in the climate chambers, epidermal peel from upper and lower leaf surfaces was prepared and inspected by light microscopy (Fig. 3). The number of stomata was determined for wild type and Spe after growth in low light and high light. As expected, the stomata density of the lower epidermis was found to be higher compared to the upper surface for both types in all light conditions (Fig. 4). When grown under low-light conditions, no obvious differences could be detected between the two flower types. In contrast, after growth under high-light conditions, both types exhibited a significantly increased stomata density on both leaf surfaces (Figs. 3 and 4, Table S1). Interestingly, the number of stomata on the adaxial (upper) surface of the leaves of Spe was significantly less increased when compared with wild-type individuals after growth in high-light conditions (Fig. 4). Notably, this trend could not be observed for the abaxial (lower) epidermis under the same conditions.
Figure 2: Habitus of individual plants cultivated under different light conditions.
Habitus of individual plants of the same age (8th to 9th week after sowing) that had been cultivated under different light conditions. (A) Wild-type individuals under low-light conditions, (B) Spe individuals under low-light conditions, (C) wild-type individuals under high-light conditions, (D) Spe under high-light conditions. In C, the inflorescence shoot was distorted since the light source was mounted 20 cm above the rosettes.Photosynthetic characteristics of rosette leaves
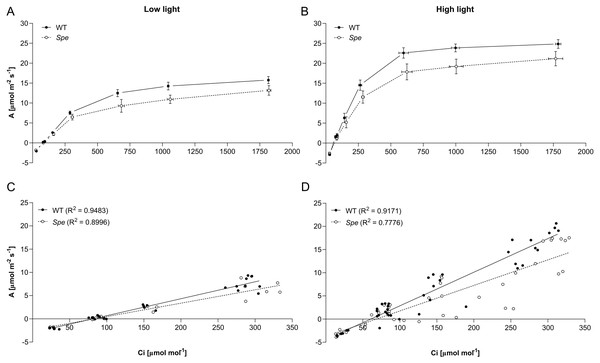
To further elucidate the impact of the differentially increased stomata density observed in plants grown under high-light conditions, the photosynthetic efficiency was determined under saturating light (800 µmol quanta m−2 s−1) using gas-exchange measurements. The rate of carbon assimilation (A) in response to various externally applied CO2 concentrations given as internal CO2 (Ci) was monitored for wild type and Spe. The resulting A/Ci plots show the typical saturation behavior for all plants grown under both light conditions (Fig. 5).
Figure 3: Stomata density of plants grown under high-light conditions.
Microscopical views of stomata of wild type (A adaxial, C abaxial) and Spe (B adaxial, D abaxial) epidermis. Nail polish tracks of upper and lower leaf surfaces were taken. Black scale bar corresponds to 100 µm.Figure 4: Stomata density of wild-type C. bursa-pastoris versus C. apetala (= Spe) grown under different light conditions.
(A) Adaxial. (B) Abaxial leaf surface. Blue bars represent wild-type and pink bars Spe individuals. For significant differences between experimental groups see Table S1.Figure 5: CO2 -concentration dependence of the rate of CO2 assimilation.
Wild type (WT, five individuals low light, seven individuals high light) and C. apetala (Spe, three individuals low-light, seven individuals high light) had been grown either under low-light (A and C) or under high-light conditions (B and D), respectively. Determination of the rates of CO2 assimilation was performed under saturating light using one leaf for each individual as described in Material and Methods. A and B: After application of descending CO2 concentrations, the internal CO2 concentration (Ci) was calculated in each case, and similar Ci values were clustered (horizontal error bars). Stomata aperture was recorded for each measurement and is shown in Fig. S1. (C and D) Single measurements in the linear range up to 350 ppm were plotted against Ci.Photosynthesis was limited by the availability of CO2 until a calculated internal CO2 concentration (Ci) of approximately 350 ppm was reached. The initial linear increase of CO2 assimilation reflects the maximal carboxylation rate of RubisCO (Figs. 5C and 5D). Above 350 ppm, the curves enter saturation as net photosynthesis becomes limited by other factors such as the electron transport and, at higher CO2 concentrations, the utilization of triose phosphate. At saturating CO2 concentrations, maximum net-photosynthesis rates of Spe were always lower than of the wild type when cultivated under both, low- and high-light conditions (Figs. 5A, and 5B). However, the initial carboxylation rate was rather similar for the two types. At ambient CO2 concentrations of approximately 400 ppm which more accurately reflect the photosynthetic efficiency of the plants, the rates of CO2 assimilation are higher when the plants have been grown under high light, and significantly lower when grown under low-light conditions (Figs. 5C, and 5D). No premature stomatal closure had limited gas exchange as shown by plotting the stomatal conductance (gs) against the Ci values for each measurement (Fig. S1).
Flowering time
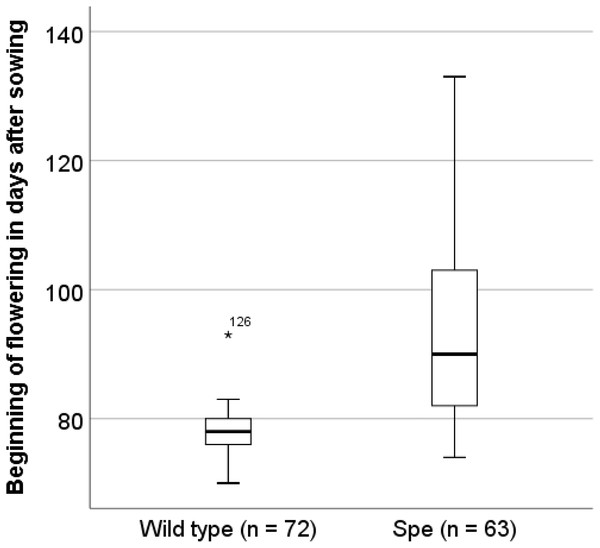
In order to identify traits of Spe, which explain the observed stable co-existence in the field even with a lower photosynthetic performance, the flowering time was analysed. After 70 days sister plants (wt 72 individuals, Spe 63 individuals) were transferred from the initial growth conditions for seedlings to the experimental field in the Botanical Garden of Osnabrueck. Under these field conditions, wild-type individuals in average flowered about two weeks earlier (x = 77.64 ± 3.562 days after sowing) than Spe individuals (x = 91.75 ± 12.618 days after sowing (Fig. 6).
Figure 6: Onset of flowering in days after sowing.
Sister individuals of the plants that had been used for the physiological and anatomical analyses have been planted into the experimental field of the Botanical Garden Osnabrück on day 70 after sowing.Discussion
Finely scaled ecotypic differentiation for Shepherd’s Purse is well known and quite substantial regarding germination, the onset of flowering, and growth forms (Linde, Diel & Neuffer, 2001; Neuffer & Linde, 1999, reviewed in Neuffer et al., 2011). Already at the beginning of the 20th century, Almquist (1907) determined several elementary species based on fruit and rosette-leaf morphology. This variable leaf morphology attracted the interest of the geneticist Shull (1909) and Shull (1911). Aksoy, Hale & Dixon (1999) included the system of Shull in their attempt towards a simplified taxonomy of C. bursa-pastoris and Ianetta et al. (2007) widened the system of Shull.
Combining anatomical and physiological traits provides the first insight into the value of leaf differentiation other than the morphology of leaf types after Shull (Neuffer et al., 2018). The ecotypic differentiation of the leaf in various habitats and conditions at geographically and climatically different places of origin of many populations is an obvious character that somehow serves adaptation and persistence in new growth conditions. The occurrence of a specific flower morphology of C. bursa-pastoris individuals in the vineyards south of Mainz in southwestern Germany is known for quite some time (Reichert, 1998). At first sight, this natural mutant is predominantly important for studies of plant geneticists (Nutt et al., 2006; Ziermann et al., 2009; Hintz et al., 2006; Hameister et al., 2013). With a more detailed analysis, several traits became apparent, and population studies led to the insight that the wild type of C. bursa-pastoris and its variant C. apetala even form two different ecotypes, sympatrically occurring at one place over many years (Hameister & Neuffer, 2017; Hameister, Neuffer & Bleeker, 2009). This differentiation is also evident from genetic differences (Isozyme Genotypes, AFLPs, see Hameister, Neuffer & Bleeker (2009).
As a typical leaf characteristic, stomata density was determined in the experimental populations (Fig. 4). In general, all leaves had developed more stomata under high-light growth conditions than in low-light as was already observed for C. bursa-pastoris provenances from Morocco and Norway, but not from Russia (Neuffer et al., 2018). Furthermore, stomatal density on the abaxial epidermis was about twice as high as in the adaxial leaf surface both in wild type and Spe after growth under high light. However, as an exception, Spe had not increased its stomata density on the upper leaf surface to the same extent as did the wild type. It had developed about 35% fewer stomata per area unit on the upper surface of its rosette leaves as compared to the wild type (Fig. 4A). A possible explanation for this phenomenon might lie in the fact that the incident light primarily impacts the adaxial surface of the rosette leaves and is exposed to adverse conditions more than the abaxial surface.
In our study, wild type individuals compared to Spe exhibited a higher maximal photosynthetic capacity both when grown under low light as well as high light. At saturating CO2 and light, the rates of CO2 assimilation were always higher for the wild type compared to C. apetala (Figs. 5A and 5B). This points to a limitation at the level of electron transport and would cause a disadvantage for Spe. However, as can be taken from the initial slopes of the A/Ci curves, under ambient conditions with limiting CO2 concentrations up to 400 ppm, only slight differences were apparent when both types were compared (Figs. 5C and 5D).
One way to explain the coexistence of wild type and Spe in the same habitat is based on the fact that the flowering phenology of both types differs. Wild-type plants flower earlier by several days after sowing under greenhouse conditions (Hameister, Neuffer & Bleeker, 2009; Hameister et al., 2013) as well as under field conditions at the Botanical Garden of Osnabrueck (Hameister & Neuffer, 2017). The appearance of the inflorescences was monitored also for sister plants after transfer to field conditions in the Botanical Garden in Osnabrück (Fig. 6). On average, flowering started two weeks later in Spe individuals as compared to wild type. This might be explained by the disadvantage of Spe over the total growth period due to somewhat lower photosynthetic capacity and consequently a lower growth rate resulting in a delay of biomass production in the vegetative stage. Either due to the difference in the nutritional status or due to environmental signals, flowering induction occurs significantly later in Spe thus leading to a temporal niche for successful pollination and seed set. A small but stable population of C. apetala can apparently coexist in the presence of the large wild-type population. Both types might be adapted to slight differences in their growth properties and the environment by growing in the rosette stage for a longer or shorter time resulting in a difference in flowering time of about two weeks. Taken together, the selection pressure for both types is apparently similar leading to stable populations growing side by side.
Conclusions
In this study, we were able to analyse two independent sympatrically occurring populations of Shepherd’s Purse. These two populations (wild type and Spe) occur intermingled with each other in stable frequencies of 9:1 over the years. This situation is rather specific and the question arose: What are the characters enabling these populations to coexist as they actually do? Both populations are isolated by strong (selfing) and smooth (flowering, reduced pollen growth activity between taxa) barriers. We have been able here to quantify anatomical and physiological traits which, with all probability, stay under selection pressure. Of course, many more characters are included in the ecotypic differentiation and already have been studied elsewhere (e.g., seed production). Here, in Spe, photosynthetic capacity appears to be lower, while growing for a longer time in the vegetative stage and later flowering induction. As both populations occur in stable frequencies, the selection pressure on single traits might be different, however, when combined, they seem to be neutralized. The specific combination of characters in each taxon is linked due to predominantly selfing.
Supplemental Information
Dependence of stomata aperture on external CO2 concentration
Wild type and Spe plants that had been grown under low or high light were examined for photosynthetic capacity (Fig. 5). In order to prove that stomata had not closed due to any CO2 response, the value of leaf conductance was taken as a measure for the fraction of open stomata (mol H2O m−2 s−1) and calculated with 1 for completely opened and 0 for closed stomata.
Significance of differences between experimental groups compared in Fig. 4
As data were not normally distributed (Kolmogorov-Smirnov-test) we used parameter-free Mann-Whitney test. * = significant, ** highly significant differences between experimental groups.