Tribe reassessment of the subhimalayan leafhopper genus Pseudosubhimalus (Homoptera: Cicadellidae) based on molecular phylogeny
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Entomology, Taxonomy
- Keywords
- Phylogeny, Leafhopper, Tribe, Molecular, Deltocephalinae, Cicadellidae
- Copyright
- © 2019 Niranjana et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Tribe reassessment of the subhimalayan leafhopper genus Pseudosubhimalus (Homoptera: Cicadellidae) based on molecular phylogeny. PeerJ 7:e7162 https://doi.org/10.7717/peerj.7162
Abstract
The phylogeny of the Pseudosubhimalus were investigated using of two different data sets, including 91 taxa and 3853 aligned nucleotide positions from the histone H3, 28S rDNA (D2 & D9–10 region). The results suggest the placement of genus in the tribe Ciacadulini, as it was clustered with Cicadulini genera. Relationships between genera of the Cicadulini were strongly supported and leads placement to tribe Cicadulini from Athysanini. Along with this, genus Pseudosubhimalus Ghauri is revised, and P. trilobatus sp. nov. (Himachal Pradesh: Katrain) is added, described from Indian subcontinent and deposited to National Pusa Collection, IARI, New Delhi, with repository number RRS1.
Introduction
Genus Pseudosubhimalus are medium sized, ochraceous to dark brown with black spots on crown, distributed in Himalayan and Subhimalayan region. The species of genus were established by Ghauri (1974), with type species Ophiola bicolor Pruthi (Pruthi, 1936), as a replacement name for Ophiola Edwards. This genus was replaced to present name due to its general coloration which superficially resembles Subhimalus Ghauri (Ghauri, 1971). This genus was earlier placed in the tribe Deltocephalini (Oman, Knight & Nielson, 1990) of Deltocephalinae. Recently, Zahniser & Dietrich (2013), based on the molecular and morphological aspects of Deltocephalinae, placed it in the tribe Athysanini. Pseudosubhimalus is recorded from higher altitudes (8,000–12,000 ft), and mostly feed and breeds on grasses. This genus is distinguished from its related genera by aedeagal shaft with a pair of subapical processes; subgenital plates triangular, apophysis of style slender, tapering distally. Pruthi (1936) described the two species P. bicolor from India and P. yatungensis from Tibet.
In spite of the colour patterns and male genitalia character that characterize Pseudosubhimalus, the composition and placement of the genus have been problematic. Molecular data potentially provide numerous additional characters useful for phylogenetic hypotheses. Zahniser & Dietrich (2013) revised the classification of Deltocephalinae based on the molecular and morphological data and provided a revised interpretation of tribe Athysanini and placed this genus under this tribe. Here, we replaced Pseudosubhimalus to the tribe Cicadulini based on histone H3, 28S rDNA (D2 & D9–10 region). Along with this morphological characterization of Pseudosubhimalus with a new species from India is provided.
Material and Methods
Collection of samples & morphological study
Collections were not done from any national park or other protected area of land or sea, or on any private land, hence no permission was required. No specific permissions were required for any of the collection localities/activities, as the collections were done in and around ICAR research institutes. The field studies did not involve any endangered or protected species. Specimens were collected through mercury vapour lamp light traps from different location in Himachal Pradesh, India were processed by a series of steps such as sorting, cleaning, and mounting. Male genitalia dissections were carried out as given by Oman (1949) and Knight (1965). The abdomen was removed by inserting a sharp pin between the abdomen and thorax and with gentle piercing. The abdomen will be treated in 10% KOH for 2–4 h to remove unsclerotized material by gently prodding the abdomen with the head of a pin. Afterwards, the abdomen was rinsed thoroughly in water. The internal structures were then removed by a hooked pin, before being stored in glycerol vials for study.
Photographs were taken with a Leica DFC 425C digital camera on the Leica M205FA stereozoom automontage microscope.
Type material is deposited in National Pusa Collection, IARI, New Delhi, with repository number RRS1.
New Taxon LSID. Pseudosubhimalus: urn: lsid:zoobank.org:act:766C2292-F762-4F2E-93A2-8745C84D7BCA, Pseudosubhimalus trilobatus: urn: lsid:zoobank.org:act:9A9DA3CD-A577-41DB-8059-28D57E38F875.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:DB2CE2D9-1238-4199-96A4-A7FDCD98DD8B. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
DNA extraction and PCR amplification
Total genomic DNA, from the legs of each specimens, were extracted with the help of the DNA Sure Tissue Mini Kit, following the manufacturer protocol. The extracted DNA was stored at −20 °C for further processing. The amplification of the desired product was done with the help diagnostic PCR reactions, using universal Histone H3 primers (HEX-AF 5′-ATGGCTCGTACCAAGCAGACGGC-3′ and HEX-AR 5′-ATATCCTTGGGCATGATGGTGAC-3′) (Ogden & Whiting, 2003) and 28S rDNA primers (for D2 region 5′-AGTCGKGTTGCTTGAKAGTGCAG-3′ & 5′-TTCGGGT CCCAACGTGTACG-3′) and for D9–D10 region 5′-GTAGCCAAATGCCTCGTCA-3′ & 5′-CACAATGATAGGAAGAGCC-3′ (Dietrich et al., 2001). The PCR protocol for Histone H3 followed Zahniser & Dietrich (2010) and 28S gene was amplified in 25 µl reactions using DNA polymerase (Fermentas GmBH, St. Leon- Rot, Germany) under the following cycling protocol: 4 min. hot start at 94 °C, 35 cycles of denaturation for 30 s at 94 °C, annealing for 60 s at 47 °C, elongation for 50 s at 72 °C and a final extension was carried out at 72 °C for 8 min in a C1000™ Thermal cycler (Meshram, Shashank & Sinha, 2017). The reactions were combined (as described by KOD FX puregene™ manufacturer protocol) of DNA template 4 µl, 2× PCR buffer 12.5 µl, 2 mM dNTP 10 µl, TAQ (KODFX) enzyme 1 unit, and forward and reverse primers were 0.3 µM each at final concentration. The products were checked on 1% agarose gel and visualized under UV using Alphaview® software version 1.2.0.1. The amplified products were sequenced at AgriGenome Pvt. Ltd. (Cochin, India). The quality sequences were assembled with BioEdit version 7.0.0 and deposited in NCBI GenBank (Table 1).
Alignment and phylogenetic analyses
Sequences were aligned with the CLUSTAL W application in MEGA 6 (Tamura et al., 2013), the alignment was imported into BIOEDIT 7.0.9.0 (Hall, 1999), and minor changes were subsequently made by hand. NEXUS data block for combined analysis of histone H3 & 28S rDNA (D2 & D9–10 region), were prepared with following commands: #NEXUS begin data; dimensions ntax = 91 nchar = 3853; format datatype = dna interleave gap = −missing = N; matrix; end; for analysis through PAUP*4.0b10.
| #S.No. | Species | Tribe | Accession number | |
|---|---|---|---|---|
| 28S | Histone H3 | |||
| 1. | Xestocephalus desertorum | Aphrodinae | AF304619 | GU123892 |
| 2. | Acinopterus acuminatus | Acinopterini | JX845484 | GU123790 |
| 3. | Acostemma sp | Acinopterini | GU123696 | GU123791 |
| 4. | Acostemmini gen. sp. | Acostemmini | JF835026 | JN177306 |
| 5. | Arrugada affinis | Arrugadini | GU123699 | GU123795 |
| 6. | Atanus sp | Athysanini | GU123700 | GU123796 |
| 7. | Brazosa picturella | Athysanini | GU123709 | GU123806 |
| 8. | Cerrillus sp | Athysanini | GU123711 | GU123808 |
| 9. | Chimaerotettix ochrescens | Athysanini | JX845489 | JX845530 |
| 10. | Colladonus lineatus | Athysanini | GU123718 | GU123815 |
| 11. | Dagama forcipata | Athysanini | GU123720 | GU123817 |
| 12. | Euscelis seriphidii | Athysanini | GU123729 | GU123830 |
| 13. | Eutettix pictus | Athysanini | GU123730 | GU123831 |
| 14. | Eusama amanda | Athysanini | AF304590 | GU123829 |
| 15. | Idioceromimus delector | Athysanini | GU123740 | GU123844 |
| 16. | Loralia sp | Athysanini | GU123746 | GU123851 |
| 17. | Napo sp | Athysanini | GU123751 | GU123856 |
| 18. | Nesothamnus sanguineus | Athysanini | GU123755 | GU123860 |
| 19. | Neohegira breviceps | Athysanini | GU123753 | GU123858 |
| 20. | Neohegira sp | Athysanini | GU123786 | GU123891 |
| 21. | Orientus sp | Athysanini | GU123757 | GU123862 |
| 22. | Pachytettix sp | Athysanini | GU123761 | GU123865 |
| 23. | Platymetopius obsoletus | Athysanini | GU123771 | GU123875 |
| 24. | Renonus rubraviridis | Athysanini | JX845524 | JX845552 |
| 25. | Thamnotettix confinis | Athysanini | GU123783 | GU123888 |
| 26. | Twiningia sp | Athysanini | GU123785 | GU123890 |
| 27. | Bahita sp | Bahitini | GU123702 | GU123798 |
| 28. | Kinrentius sp | Bahitini | JX845523 | JX845549 |
| 29. | Bonaspeia sp | Bonaspeiini | JX845521 | GU123804 |
| 30. | Cerus goudanus | Bonaspeiini | GU123712 | GU123809 |
| 31. | Renosteria waverena | Bonaspeiini | GU123772 | GU123878 |
| 32. | Chiasmus sp | Chiasmini | GU123713 | GU123810 |
| 33. | Gurawa minorcephala | Chiasmini | X845495 | JX856131 |
| 34. | Listrophora styx | Chiasmini | JX845500 | JX845539 |
| 35. | Nephotettix modulatus | Chiasmini | GU123754 | GU123859 |
| 36. | Cicadula quadrinotata | Cicadulini | GU123717 | GU123813 |
| 37. | Elymana acuma | Cicadulini | GU123726 | GU123826 |
| 38. | Proceps acicularis | Cicadulini | JX845511 | JX845550 |
| 39. | Cochlorhinus pluto | Cochlorhinini | AF304586 | GU123814 |
| 40. | Deltocephalus sp | Deltocephalini | GU123721 | GU123819 |
| 41. | Paramesodes sp | Deltocephalini | GU123764 | GU123868 |
| 42. | Dorycephalus baeri | Dorycephalini | JX845491 | JX845532 |
| 43. | Drabescus sp | Drabescini | GU123724 | GU123824 |
| 44. | Bhatia satsumensis | Drabescini | GU123706 | GU123803 |
| 45. | Drakensbergena retrospina | Drakensbergenini | GU123725 | GU123825 |
| 46. | Eupelix cuspidata | Eupelicini | AF304644 | GU123828 |
| 47. | Paradorydium lanceolatum | Eupelicini | AF304637 | GU123877 |
| 48. | Hecullus bracteatus | Faltalini | GU123737 | GU123841 |
| 49. | Tenucephalus sp | Faltalini | GU123781 | GU123886 |
| 50. | Fieberiella florii | Fieberiellini | AF304594 | GU123834 |
| 51. | Goniagnathus guttulinervis | Goniagnathini | GU123736 | GU123838 |
| 52. | Glossocratus sp | Hecalini | GU123735 | GU123837 |
| 53. | Attenuipyga vanduzeei | Hecalini | AF304653 | GU123822 |
| 54. | Hecalus viridis | Hecalini | AF304596 | GU123840 |
| 55. | Hypacostemma sp | Hypacostemmini | GU123739 | GU123843 |
| 56. | Koebelia grossa | Koebeliini | AF304599 | GU123846 |
| 57. | Limotettix striola | Limotettigini | GU123745 | GU123850 |
| 58. | Balclutha neglecta | Macrostelini | GU123704 | GU123800 |
| 59. | Dalbulus gelbus | Macrostelini | AF304587 | GU123818 |
| 60. | Magnentius clavatus | Magnentiini | JX845503 | JX845541 |
| 61. | Agrica arisana | Mukariini | GU123779 | GU123884 |
| 62. | Mukaria maculata | Mukariini | GU123750 | GU123855 |
| 63. | Occinirvana eborea | Occinirvanini | JX845507 | JX845545 |
| 64. | Neoaliturus carbonarius | Opsiini | GU123752 | GU123857 |
| 65. | Pseudophlepsius binotatus | Opsiini | JX845512 | JX845551 |
| 66. | Hishimonus phycitis | Opsiini | GU123738 | GU123842 |
| 67. | Japananus hyalinus | Opsiini | JX845499 | JX845538 |
| 68. | Nesophrosyne maritima | Opsiini | JX845506 | JX845544 |
| 69. | Opsius sp | Opsiini | GU123756 | GU123861 |
| 70. | Orosius orientalis | Opsiini | JX845509 | JX845547 |
| 71. | Aflexia rubranura | Paralimnini | GU123698 | GU123793 |
| 72. | Laevicephalus monticola | Paralimnini | GU123744 | GU123849 |
| 73. | Bandaromimus parvicauda | Pendarini | GU123705 | GU123802 |
| 74. | Tropicanus chiapasus | Pendarini | GU123784 | GU123889 |
| 75. | Jafar javeti | Penthimiini | JX845498 | JX845537 |
| 76. | Penthimidia eximia | Penthimiini | JX845510 | JX845548 |
| 77. | Penthimiola sp | Penthimiini | GU123766 | GU123871 |
| 78. | Excultanus conus | Phlepsiini | GU123732 | GU123833 |
| 79. | Phlepsius intricatus | Phlepsiini | GU123768 | GU123873 |
| 80. | Anoplotettix fuscovenosus | Scaphoideini | JX845486 | JX845527 |
| 81. | Scaphoideus sp. n. | Scaphoideini | JX845513 | JX845553 |
| 82. | Scaphytopius frontalis | Scaphytopiini | JX845514 | JX845555 |
| 83. | Adama elongata | Selenocephalini | GU123694 | GU123788 |
| 84. | Selenocephalus sp | Selenocephalini | GU123777 | GU123881 |
| 85. | Pachymetopius decoratus | Stegelytrini | GU123760 | GU123864 |
| 86. | Kinonia elongata | Stenometopiini | GU123741 | GU123845 |
| 87. | Stirellus catalinus | Stenometopiini | AF304614 | GU123882 |
| 88. | Tetartostylus parabolatus | Tetartostylini | GU123782 | GU123887 |
| 89. | Stymphalus rubrolineatus | Vartini | GU123778 | GU123883 |
| 90. | Pseudosubhimalus bicolor | Cicadulini | (a) MK680069 (D2) | MH172175 |
| (b) MK680065 (D9–10) | ||||
| 91. | Pseudosubhimalus trilobatasp.nov. | Cicadulini | (a) MK680071 (D2) | MH172179 |
| (b) MK680067 (D9–10) | ||||
Maximum Parsimony Bootstrap Analysis (MPBS)
Maximum parsimony (MP) analyses were run in PAUP*4.0b10 (Swofford, 1998) Analyses were run with the following search commands: ‘log file = mp-log; set autoclose = yes; set maxtrees = 100 increase = no; set criterion = parsimony; outgroup; hsearch addseq = random nreps = 100 multrees = yes hold = 1 swap = tbr; showtrees; describetrees 1/plot = phylogram brlens = yes; pscores ALL/tl = yes ci = yes ri = yes rc = yes hi = yes; savetrees file = mp-all.tre root = yes brlens = yes; bootstrap nreps = 1000 keepall = yes/AddSeq = random nreps = 100 savereps = yes; savetrees file = mp-boot.tre from = 1 to = 1 savebootp = both maxdec = 0 root = yes brlens = yes; pscores ALL/tl = yes ci = yes ri = yes rc = yes hi = yes; log stop’.
Results
Taxonomy
Pseudosubhimalus Ghauri
Pseudosubhimalus Ghauri, 1974: 553. Type species Ophiola bicolor Pruthi, 1936, by replacement.
Diagnosis. Male: Small leafhoppers, 3.0–4.0 mm long, dark brown to pale yellow. Crown, pronotum and scutellum marked with irregular dark brown spot. Pleurite black, spine on the legs pale yellow. Face black to ohraceous with irregular transeverse dark brown to yellow stripe. Forewings ochraceous to dark brown with hyaline venations with dark brown mottling.
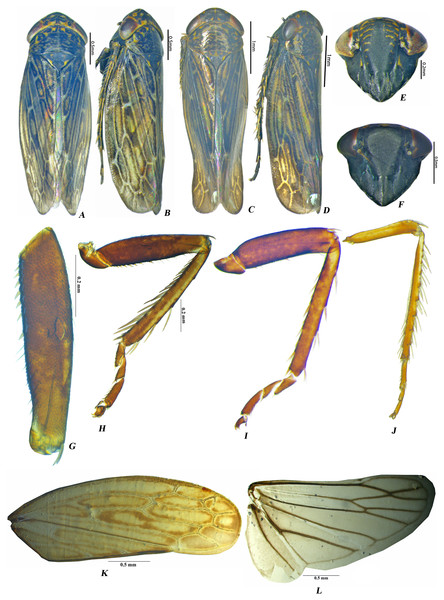
Description. Head conical to subconical, as broad as pronotum or broader, crown broad; frontoclypeus longer than wide, lateral margins broadly convex; clypeal sulcus distinct; clypellus elongate with sides parallel over basal 0.66, narrowed at apex, apically slightly exceeding the facial margin; genal margins concave beneath eyes; ocelli small, situated near anterior margin of crown by a distance equal to its own diameter, eyes large, occupying less than of entire dorsal area of head. Pronotum short, median length about equal to median length of crown, almost three times as wide as its median length, surface knobbed, combined length of mesoscutum and scutellum longer than median length of pronotum, scutellum shorter than mesoscutum. Forewing (Fig. 1K) with distinct venation, with three anteapical cells, outer anteapical and middle cells closed; outer apical cell elongate, appendix narrowed not well developed. Hindwing venation complete with one anal vein; m-cu crossvein subapical (Fig. 1L). Prothoracic femur, with AV (anteroventral) stout setae present, AV1 setae long, hairlike, (Fig. 1G). Prothoracic tibia with dorsal surface rounded but not expanded (Fig. 1H); AD (anterodorsal) and PV (posteroventral) setae sparse; PD (posterodorsal) setae very dense; AV setae dense and long (Fig. 1H). Mesothoracic femur with sparse AV setae (Fig. 1I). Metathoracic femur with setal formula 2 + 1 + 1 (Fig. 1J); lateral surface broadened distally with dense setae distally (Fig. 1J). Metathoracic tibia flattened, tibial row PD with long macrosetae, AD with macrosetal bases spine like, AV with macrosetae, PV with numerous long tapered setae, tarsomere II less than 1/2 length of tarsomere I& II (Fig. 1J).
Figure 1: Male Pseudosubhimalus species (A) P. bicolor dorsal habitus. (B) P. bicolor lateral habitus. (C) P. trilobatus sp.nov. dorsal habitus. (D) P. trilobatus sp.nov. lateral habitus. (E) P. bicolor face. (F) P. trilobatus sp.nov. face. (G–L) P. bicolor male (G) Fore Femur (H) Fore leg (I) Middle leg (J) Hind leg (K) Forewing (L) Hind wing.
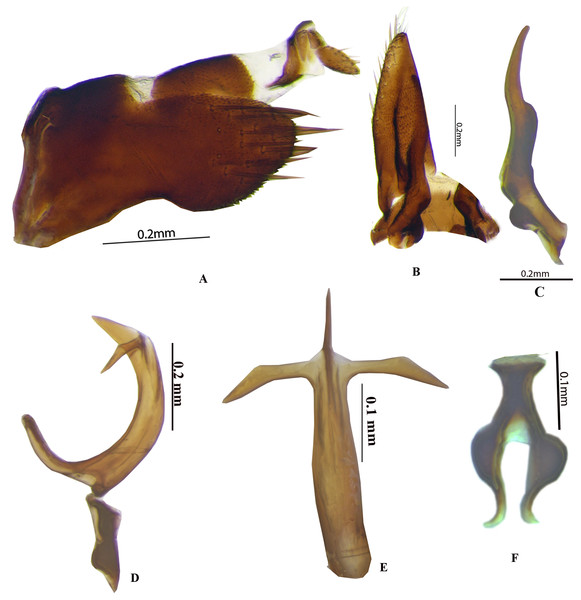
Photo credit: Naresh M. Meshram.Genitalia. Male: Pygofer in lateral view (Fig. 2A) broad anteriorly and narrowed posteriorly, with long setae on posterior half, dorsal and ventral posterior margin with or without minute serrations. Anal tube elongate, long, well sclerotised. Subgenital plate (Fig. 2B) triangular in shape with wide base, sharply narrowed posteriorly, with long setae laterally and hair like long setae posteriorly. Connective (Fig. 2F) Y-shaped with stem shorter than arm. Style broad at base (Fig. 2C) with well-developed preapical lobe, apophysis long, slender, with blunt apex, >0.25 of total style length. Aedeagus with two or four processes, gonopore subapical.
Figure 2: Genitalia male P. bicolor (Pruthi).
(A) Pygofer lateral. (B) Subgenital plate with valve. (C) Style. (D) Aedeagus lateral. (E) Aedeagus ventral. (F) Connective. Photo credit: Stuti.Female genitalia. Adult ♀: VII sternite hind margin broadly concave (Fig. 3D). Valvulae I (Figs. 3E–3F), in lateral view, with apex acute; dorsal sculptured area extending from base portion to apex of blade, formed mostly by scale-like processes arranged in oblique lines. Valvulae II (Figs. 3G–3H), in lateral view, moderately expanded beyond basal curvature; dorsal margin concave in the middle with dorsal hyaline area and convex apically on toothed areas, dorsal margin with 17 teeth with irregular reticulation on distal 1/3rd, dorsal margin.
Figure 3: Female P. bicolor (Pruthi) (A–H).
(A) Habitus dorsal. (B) Habitus lateral. (D) Seventh sternite. (E–F) Valvulae I (GH) Valvulae II. Photo credit: Naresh M. Meshram.Key to species of Pseudosubhimalus (Males)
| 1. | Pygofer ventral margin with dentations, aedeagus acute apically.............P. bicolor (Pruthi) |
| - | Pygofer ventral margin smooth.......................................................................................2 |
| 2. | Aedeagus bulbous at base with pair of very small subapical processes (Pruthi, 1936: Figs. 136a, b)..............................................................................................P. yatungensis (Pruthi) |
| - | Apex of aedeagus trilobed, subapical processes long (Figs. 4D and 4E).................... P. trilobatus sp. nov. |
Pseudosubhimalus bicolor (Pruthi) [Figs. 1A, 1B, 1E, 1G–1L, 2A–2F, 3A–3H]
| Ophiola bicolorPruthi, 1936: 123 |
| Pseudosubhimalus bicolor (Pruthi): Ghauri, 1974: 554 |
Diagnosis: Coloration, structure of the specimens studied agrees well with the description of the species by Pruthi (1936), except that the head is ochraceous, leg ochraceous. Male genitalia structure also shows some variations. Pygofer 1.4× longer than broad, with long setae on posterior half, dorsal and ventral posterior margin with minute serrations.
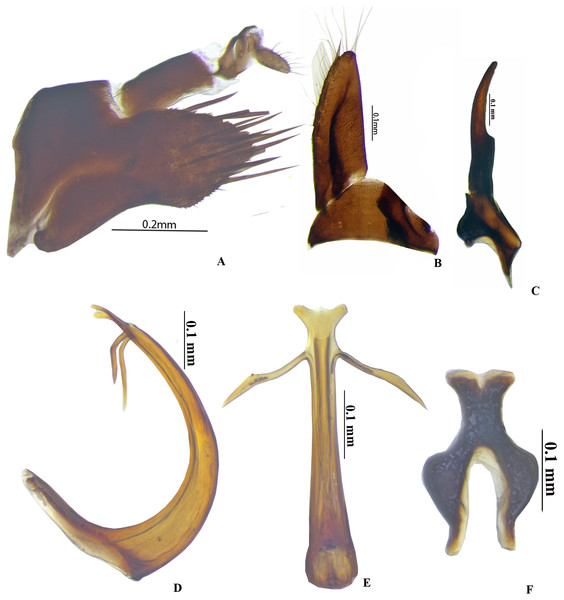
Figure 4: Genitalia male P. trilobatus sp. nov.
(A) Pygofer lateral. (B) Subgenital plate with valve. (C) Style. (D) Aedeagus lateral. (E) Aedeagus ventral. (F) Connective. Photo credit: Niranjana GN.Description. Male: Dark brown color, Crown, pronotum and scutellum marked with irregular dark brown spot. Face black to ochraceous with irregular transeverse dark brown stripe. Forewings dark brown with hyaline venations with dark brown mottling.
Head including eyes 1.09× width of pronotum (Fig. 1A), in dorsal view obliquely rounded in front, crown length 0.35× width across eyes; face length 0.9× width of face. Ocelli near anterior margin of crown and distance between eye and ocellus equal to diameter of ocellus. Pronotum 0.5× as long as width and 0.8× length of scutellum.
Genitalia. Male. Pygofer (Fig. 2A) is longer 1.4× than broad, with long setae on posterior half, dorsal and ventral posterior margin with minute serrations. Subgenital plate (Fig. 2B) triangular with wide base, sharply narrowed posteriorly, with setae 10 along lateral margin. Connective Y-shaped (Fig. 2F) with stem shorter 0.6× than arm. Style (Fig. 2C) broad at base with well-developed preapical lobe, apophysis long, slender, with blunt apex, 0.33 of total style length. Aedeagus in lateral view (Fig. 2E) narrowed basally, pointed apically and moderately broad medially with two small traingluar subapical processes. Gonopore subapical at the base of the processes.
Female. Adult ♀: Seventh sternite (Fig. 2D) hind margin broadly concave. Valvulae I (Figs. 2E–2F), in lateral view, with apex acute; dorsal sculptured area extending from base portion to apex of blade, formed mostly by scale-like processes arranged in oblique lines. Valvulae II (Figs. 2G–2H), in lateral view, moderately expanded beyond basal curvature; dorsal margin concave in the middle with dorsal hyaline area and convex apically on toothed areas, dorsal margin with 17 teeth, with irregular reticulation on distal 1/3rd, dorsal margin.
Measurement (mm): Total Length: 7.1; Crown Length: 0.5; Width of Head: 1.9; Width of Pronotum: 2.4.
Matrial examined. INDIA: 11 ♂ & 15 ♀ Himachal Pradesh, Katrain, (76°59′N, 32°30′E and 3,300 msl) 23.ix.2016, sweep net Coll. Niranjan. Himachal Pradesh: Palchau, 11 ♂ & 8 ♀ 07.vi.1987, Coll. V.R.S. Rao (NPC); Uttarakhand: 11 ♂ & 8 ♀, Tehri Garhwal, 12.x.1988, Coll. V.V. Ramamurthy (NPC).
Pseudosubhimalus trilobatus sp. nov. Meshram & Niranjana [Figs. 1C, 1D, 4A–4F]
Diagnosis. P. trilobatus sp. nov. resembles P. bicolor (Pruthi) in coloration and external morphology but can be distinguished by certain male genitalia characters like pygofer dorsal and ventral posterior margin without serrations. Aedeagal shaft narrowed apically, with trilobed apex in dorsal view. Gonopore subapical placed above base of the processes.
Description. Male: Dark brown color, Crown, pronotum and scutellum marked with irregular dark brown spot. Face completely black without any marking. Compound eyes black with reddish tinge and ocelli orange color. Forewings dark brown with hyaline venations with dark brown mottling.
Head including eyes 1.1× width of pronotum (Fig. 1C), in dorsal view obliquely rounded in front, crown length 0.35× width across eyes; face length 0.87× width of face. Ocelli near anterior margin of crown and distance between eye and ocellus equal to diameter of ocellus. Pronotum 0.5× as long as width and 0.8× length of scutellum.
Genitalia. Male: Pygofer is longer than broad (Fig. 4A), posterior margin conically rounded, macrosetae confined to posterior half, dorsal and ventral posterior margin without serrations. Valve broadly triangular (Fig. 4B), subgenital plate long (Fig. 4B), triangular uniseriate macrosetae and fringe of very long fine setae along. Connective Y-shaped (Fig. 4F); stem narrowed, 0.6× smaller than arms. Style broad basally (Fig. 4C), apophysis long, slender, with blunt apex, 0.33 of total style length. Aedeagus, in lateral view (Fig. 4D), broadly C-shaped, shaft narrowed apically, with trilobed apex in dorsal view, with two subapical processes, length of process is 0.33 of total length in dorsal view (Fig. 4E). Gonopore subapically placed above base of the processes.
Measurement (mm). Total Length: 7.1; Crown Length: 0.5; Width of Head: 1.9; Width of Pronotum: 2.4.
Etymology. Species name refers to the three lobed apex of aedeagus.
Type Material. Holotype: 1 ♂ INDIA: Himachal Pradesh, Dalang maidan, (32.6192°N, 77.3784°E and 3,300 msl) 24.ix.2016, sweep net Coll. Niranjan. Paratype 6 ♂ same data as on Holotype.
Holotype Submitted to NPC- Repository number: RRS1.
New Taxon LSID. Pseudosubhimalus: urn: lsid:zoobank.org:act:766C2292-F762-4F2E-93A2-8745C84D7BCA, Pseudosubhimalus trilobatus: urn: lsid:zoobank.org:act:9A9DA3CD-A577-41DB-8059-28D57E38F875.
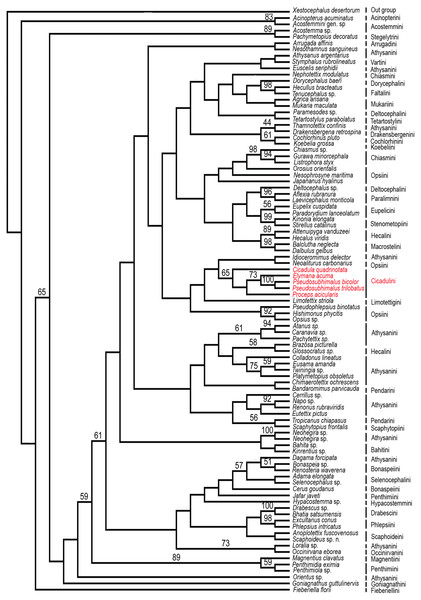
Figure 5: Strict consensus parsimonious tree resulting from combined parsimony analysis of histone H3 and 28S rDNA gene (D2 & D9–10 region).
Molecular analysis
Maximum Parsimony Bootstrap analysis of the 91 taxa and 3853 aligned nucleotide positions from the histone H3 and 28S rDNA gene (D2 & D9–10 region) by PAUP*4.0b10 was done and yielded strict consensus tree (Fig. 5). The genus Pseudosubhimalus, which was sister to Elymana well-supported clade (>50 MPBS). This suggest the placement of the genus in the tribe Cicadulini. Our combined analysis resulted to be the most closely (Cicadula, Proceps, and Elymana), which resolved the placement of the genus to the tribe Ciacaulini from Atthysanini.
Discussion
The systematic position of Pseudosubhimalus (Ghaurii), as suggested by morphological evidence, is ambiguous. This genus can be distinguished from its closely associated genera by the following combination of characters: 1. Prothoracic femur, with AV stout setae present, AV1 setae long, hairlike, (Fig. 1G). 2. Prothoracic tibia with dorsal surface rounded but not expanded (Fig. 1H); AD and PV setae sparse; PD setae very dense; AV setae dense and long (Fig. 1H). Mesothoracic femur with sparse AV setae (Fig. 1I). Metathoracic femur with setal formula 2 + 1 + 1(Fig. 1J); lateral surface broadened distally with dense setae distally (Fig. 1J). Metathoracic tibia flattened, tibial row PD with long macrosetae, AD with macrosetal bases spine like, AV with macrosetae, PV with numerous long tapered setae, tarsomere II less than 1/2 length of tarsomere I & II (Fig. 1J).
In Pseudosubhimalus anal tube elongate, long, well sclerotized which strongly suggest the placement of this genus to tribe Cicadulini. To confirm actual phylogenetic position of the Pseudosubhimalus in the context of the subfamily Deltocephalinae, we performed a preliminary molecular analysis (Maximum Parsimony Bootstrap analysis) by using available material of a series of taxa belonging to different tribes of Deltocephalinae from NCBI GenBank (Table 1). Our molecular results exclude Pseudosubhimalus from a close relationship among the genera of the previously placed tribe Athysanini (Zahniser & Dietrich, 2013) and placed it in the tribe Cicadulini. Our histone H3, 28S rDNA (D2 & D9–10 region) bases combined analysis resulted to be the most closely (Cicadula, Proceps and Elymana) or the most distantly related (Xestocephalus desertorum) (Fig. 5). The final data matrix of our preliminary phylogenetic analysis (Table 1) included 91 terminals (90 ingroup taxa of Deltocephalinae and 1 outgroup taxon). Overall, these results improved our understanding of the systematic position of Pseudosubhimalus.
Conclusion
Previously Pseudosubhimalus placed within the tribe Athysanini and our combined gene phylogenetic analysis leads its placement to the tribe Cicadulini. Present study reassessed the taxonomic position of genus Pseudosubhimalus with a more robust and well-resolved phylogeny. Which will help ongoing evolutionary and taxonomic work. Our molecular analysis based on Maximum Parsimony Bootstrap Analysis (PAUP) leads the placement of the Pseudosubhimalus to the tribe Cicadulini from Athysanini based on histone H3, 28S rDNA (D2 & D9–10 region). Overall, these results improved our understanding of the systematic position of Pseudosubhimalus; however, more rigorous evaluations with larger numbers of genes are still necessary in the future.