Effects of exercise combined with brain stimulation on hand function in children with cerebral palsy: a meta-analysis of randomized controlled trials
- Published
- Accepted
- Received
- Academic Editor
- Faiza Farhan
- Subject Areas
- Neurology, Pediatrics, Translational Medicine, Rehabilitation, Sports Medicine
- Keywords
- Brain stimulation, Hand function, Cerebral palsy, Children
- Copyright
- © 2026 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2026. Effects of exercise combined with brain stimulation on hand function in children with cerebral palsy: a meta-analysis of randomized controlled trials. PeerJ 14:e20670 https://doi.org/10.7717/peerj.20670
Abstract
Background
Cerebral palsy (CP) is a paediatric condition generally characterized by persistent motor disabilities in hand function. This review examined the impact of exercise with and without brain stimulation on hand function in children with CP.
Methodology
A systematic literature search was conducted from January 2010 to June 2025 across four electronic databases: Web of Science, Scopus, PubMed, and EBSCO. This review established the inclusion criteria as follows: 1. Children with CP; 2. Randomised controlled trial; 3. Exercise with and without brain stimulation; 4. Measurements included gross motor function (GMF), fine manual control (FMC) and grip strength (GS) evaluated at pre- and post-intervention. The quality of the included studies was assessed using the Cochrane Risk of Bias tool. For data analysis, the standardized mean difference (SMD) was selected as the appropriate effect size index, and RevMan 5.4 software was employed to analyze the mean differences in the data extracted from the included articles. (Registration number: CRD420251106181).
Results
The results showed that exercise with brain stimulation comprising more than 16 sessions could notably improve GS (SMD, 1.38 (0.88, 1.88), p < 0.05, I2 = 0%), whereas that comprising fewer than 10 sessions did not demonstrate a statistically significant effect (SMD, 0.19 (−0.29, 0.67), p = 0.44, I2 = 0%). Consequently, brain stimulation intervention could substantially enhance FMC (SMD, 0.46 (0.15, 0.76), p < 0.05, I2 = 47%). Subgroup analysis also presented that exercise with transcranial direct current stimulation (tDCS) resulted in a significant improvement in FMC (SMD, 0.71 (0.29, 1.14), p < 0.05, I2 = 49%) compared to exercise with repetitive transcranial magnetic stimulation (SMD, 0.19 (−0.25, 0.63), p = 0.09, I2 = 47%).
Conclusion
This review demonstrated that exercise with brain stimulation could significantly enhance hand function in children with CP. Specifically, more than 16 sessions has greater benefits for GS, and the tDCS may confer benefits for FMC.
Introduction
Cerebral palsy (CP) is a paediatric condition generally denoted by enduring motor disabilities. Children with CP may exhibit neuromuscular issues, which are contingent upon the type and severity of the condition. Examples of these issues include impaired coordination, contractures, muscle weakness, and spasms. Consequently, daily activity and motor function-related deficits can occur due to these issues (Ostensjø, Carlberg & Vøllestad, 2004). This observation indicates that hand function constitutes a critical component of motor function, encompassing several activities. Examples of these activities are reaching targets, manipulating, grasping, and releasing objects. Although children with CP must integrate their visual perception skills and movement sequences to execute these tasks efficiently, several challenges are constraining the upper limb function in these children. These challenges include a limited range of motion in the joints, muscle tone changes, and joint contractures (Hanna et al., 2003). The identified constraints may then augment the dependence of children with CP in their everyday activities by hindering play and self-care activities (Figueiredo et al., 2020).
Hand function rehabilitation is usually employed to foster independence, avert additional deformities, enhance the overall quality of life, and improve manual capabilities among individuals (Burgess et al., 2021). This rehabilitation can also enhance upper limb function within children with CP, encompassing numerous brain stimulation and exercise therapies. Notably, exercise therapy is a widely utilised treatment approach among clinical practitioners and researchers, underscoring the integration of affected upper limb and task-related exercises (Ilieva & Ilieva, 2020). Multiple studies have reported that children with CP can significantly benefit from this type of therapy, potentially facilitating motor function and neural remodelling. Two frequently employed strategies of brain stimulation therapy are transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS). These techniques are non-invasive methods for brain stimulation that deliver low-intensity stimulation to targeted brain regions via magnetic poles or electrodes positioned on the scalp. The tDCS enhances neuronal membrane potential through weak DC polarization, resulting in anodic enhancement and cathodic inhibition of cortical excitability; The rTMS induces induced currents through pulsed magnetic fields, which bidirectionally regulate cortical activity according to frequency, both of which work by regulating neural plasticity (Chiara et al., 2019). Hence, neural network activity and neurotransmitter release in specific brain regions are positively impacted (Brunoni et al., 2022). Furthermore, repetitive brain stimulation can lead to enduring alterations in cortical, potentially improving neurological and musculoskeletal disorder-related impairments (Hilderley et al., 2023; Zhong et al., 2025).
Currently, the correlation between exercise-brain stimulation (exercise combined with brain stimulation) and hand function in children with CP remains ambiguous. Certain studies have suggested that exercise-brain stimulation enhances fine manual control (FMC) and grip strength (GS) in children with CP compared to brain stimulation alone (Salazar Fajardo et al., 2022; Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024). Nonetheless, no notable difference between the two groups has been recorded in several studies (Wu et al., 2022; Gupta et al., 2023). At present, there is no meta-analysis to specifically study the synergistic effect of combined exercise and brain stimulation on different components of hand function in children with CP. Thus, this review examined the impact of exercise (in the presence and absence of brain stimulation) on hand function in children with CP using meta-analysis.
Survey Methodology
Data sources and study selection
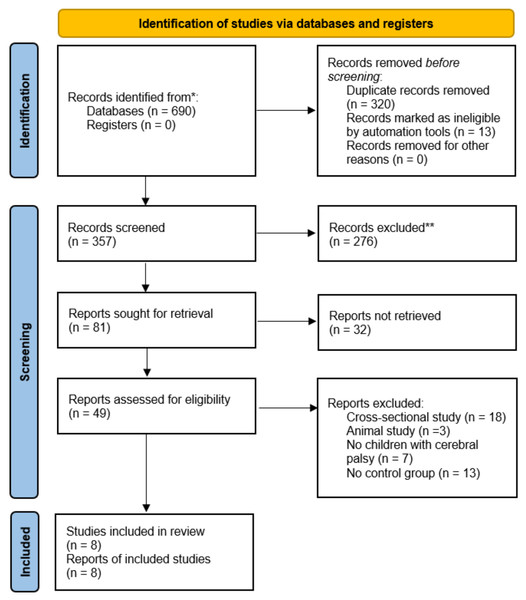
A systematic literature search was performed between January 2010 and June 2025 across four electronic databases: (i) Web of Science, (ii) Scopus, (iii) PubMed, and (iv) EBSCO. The most recent search update occurred on 29th June 2025, utilising two keywords: (i) “Cerebral palsy” and (ii) “Stimulate”. Information specialists initially developed the search strategies, with outcomes and comprehensive protocols and outcomes recorded (Appendix A). Subsequently, two independent researchers (S.L. and S.G.) conducted an initial screening of abstracts and titles obtained from the databases. The full-text articles were then assessed according to established inclusion and exclusion criteria. These same reviewers conducted quality assessment and data extraction. In instances of disagreement among reviewers, a third investigator (H.L.) was consulted to achieve consensus. The reference lists were then manually examined for further relevant articles upon confirming the included publications. This review protocol was also recorded in the international Prospective Register of Systematic Reviews (PROSPERO) (CRD420251106181). Figure 1 presents the process of study selection and preparation following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) requirements.
Figure 1: Flow diagram of the search results using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Inclusion and exclusion criteria
This review established the inclusion criteria as follows:
-
Randomised controlled trials.
-
Children with CP.
-
Articles comparing exercise-brain stimulation as the experimental (EXP) group and exercise with sham-brain stimulation as a control (CON) group.
-
Measurements included gross motor function (GMF), GS, and FMC evaluated at pre- and post-intervention.
-
Test indicators were documented as mean (standard deviation) and/or mean (95% confidence interval (CI)).
-
Full-text articles were documented in English language.
In contrast, the exclusion criteria involved excluding conference proceedings, abstracts, and poster presentations from this review.
Quality assessment
The methodological quality of the included articles was investigated through the Cochrane risk of bias assessment tool (Higgins et al., 2019). Several processes could be accomplished by employing this tool, including examining blind outcome, generating random sequences, introducing additional biases, handling incomplete outcome data, concealing allocation, selective reporting, and participant with personnel blinding (Li et al., 2023). Items were then evaluated as “yes”, “no”, or “unclear” to assess the quality of the articles.
Data extraction
Each article contained relevant information, which was extracted and organised into a table (see Table 1). The gathered variables involved brain stimulation protocol, exercise protocol, duration, age, gender, and equipment.
| Study | Age (y) | Gender | Duration | Exercise program | Brain stimulation program | Equipment | Index |
|---|---|---|---|---|---|---|---|
| Ebrahimabadi, Ghaderian & Askary Kachoosangy (2024) | EXP: 7.6 ± 3.2 CON: 6.8 ± 2.5 |
28M/22F | 4 weeks; 5x/week | Occupational therapy exercises, hand function exercises such as stretching and grasping, 45 min |
EXP: tDCS, 1.5 mA, Anode O1 area, cathode O2 area, 20 min CON: Sham tDCS, 20 min |
tDCS (Active Tek, USA) | GMF, GS, FMC |
| Salazar Fajardo et al. (2022) | EXP: 4.9 ± 2.3 CON: 5.8 ± 1.7 |
EXP: 5M/9F CON: 7M/3F |
5 weeks 3x/week |
Neurodevelopmental treatment, 30 min | EXP: tDCS, 1 mA, Anode M1 region, cathode contralateral orbital region, 20 min CON: Sham tDCS, 20min |
tDCS (Ybrain Inc., Korea) | GMF, FMC |
| Fan et al. (2024) | EXP: 4.6 ± 0.7 CON: 4.7 ± 0.5 |
EXP: 9M/7F CON: 13M/3F |
12 weeks; 5x/week | Action observation training, 6 body movements, 30 min |
EXP: rTMS, 1 Hz, 30 min unaffected M1 area, 80% RMT CON: Sham rTMS, 30min |
NA | GMF |
| Gillick et al. (2018) | EXP: 13.2 ± 3.7 CON: 12.3 ± 4.5 |
EXP: 5M/5F CON: 4M/6F |
10 days | CIMT, shaping functionality and developing motor skills, 120 min | EXP: tDCS, 0.7 mA, Anode SO area, cathode M1 area, 20 min CON: Sham tDCS, 20min |
tDCS (Soterix Medical, USA) | GS, FMC |
| Gupta et al. (2023) | EXP: 8.7 ± 2.8 CON: 8.6 ± 3.2 |
EXP: 13M/10F CON: 17M/6F |
4 weeks 2x/week |
CIMT, shaping functionality and developing motor skills, 120 min | EXP: rTMS, 1 Hz, 20 mins unaffected M1 area, 90% RMT CON: Sham rTMS, 20min |
rTMS (Magventure, Denmark) | GS, FMC |
| Hassan et al. (2024) | EXP: 11.0 ± 2.0 CON: 12.2 ± 2.1 |
EXP: 6M/8F CON: 3M/11F |
4 weeks; 4x/week | WBVT, Stand barefoot, with both feet parallel and bent 30 degrees. 18 Hz, amplitude of 12 mm, 20 mins | EXP: tDCS 2 mA, Anode M1 region, cathode contralateral orbital region,20 mins CON: Sham tDCS, 20min |
NA | GS |
| He et al. (2024) | EXP: 6.5 ± 2.4 CXP:7.4 ± 2.9 |
EXP: 12M/3F CON: 11M/5F |
4 weeks; 5x/week | Routine rehabilitation treatment, 30 min | EXP: rTMS, 1 Hz, 20 mins, unaffected M1 area, 90% RMT CON: Sham rTMS, 20min |
rTMS (Youde Medical Company, China) | GMF |
| Wu et al. (2022) | EXP: 4.2 ± 0.9 CON: 3.7 ± 1.05 |
EXP: 6M/11F CON: 8M/10F |
10 days | CIMT, Tug of war, shooting competition, balloon passing, 180 min | EXP: rTMS, 1 Hz, unaffected M1 area, 20 mins, 90% RMT CON: Sham rTMS, 20 mins |
rTMS (Yiruide Company, China) |
FMC |
Notes:
- EXP
-
experimental group
- CON
-
Control group
- M
-
Male
- F
-
Female
- CIMT
-
Constraint-induced movement therapy
- tDCS
-
Transcranial direct current stimulation
- rTMS
-
Repetitive transcranial magnetic stimulation
- GMF
-
Gross motor function
- GS
-
Grip strength
- FMC
-
Fine manual control
Data analysis
Data analyses were conducted utilising RevMan 5.4 Cochrane Collaboration (2020). Likewise, mean difference (MD) or standardised mean difference (SMD) were applied for analysis involving continuous variables. In instances where various measurement tools or units were employed for the similar outcome, the aggregated statistic chose the SMD with a 95% CI. Otherwise, the MD with a 95% CI was computed in instances where identical measurement tools or units were utilised across articles. An inverse method for data processing was also used in cases of continuous data exhibiting opposite directions of effect (higher or lower scores indicated improved outcomes in certain articles). This approach was achieved by reversing the mean and standard deviation prior to the recombination of results.
The I2 statistic was implemented for evaluating the heterogeneity among study results, in which high and low heterogeneity thresholds were concluded for the I2 statistic values of 50%, respectively (Li et al., 2023). Particularly, a random effects model was utilised if I2 > 50%. This outcome implied that the sources of heterogeneity should be examined using a sensitivity analysis. Nevertheless, heterogeneity among the articles was deemed acceptable if I2 ≤ 50% (Li et al., 2023). The meta-analysis also established a significance level of p < 0.05. In studies that provided the standard error, mean, and sample size, the standard error was converted to standard deviation following data extraction. Subsequently, the subgroups of GS and FMC were analyzed according to the intervention duration and brain stimulation type. The authors of the articles were also contacted through email to acquire the missing data. Only the reported data were assessed in cases in the absence of missing data.
Results
Eligibility of studies
This review collected 690 articles from Web of Science, Scopus, PubMed, and EBSCO databases. The articles were then imported into Mendeley Desktop software (version 1.19.8), resulting in 357 articles after the removal of duplicates. A preliminary screening of titles and abstracts also identified 49 articles, with 41 articles excluded following a full-text review. Exclusions were then applied for four primary reasons: (i) seven articles did not include children with CP, (ii) 13 articles lacked a CON group, (iii) 18 articles were cross-sectional studies, and (iv) three articles were for animals. Consequently, the final analysis comprised eight articles. Figure 1 presents the comprehensive literature selection flowchart. All selected articles obtained ethical approval from their respective institutions for their protocols. Two investigators (S.L. and S.G.) also conducted the calibration activity to verify the consistency of their assessments, in which the Cohen kappa coefficient (k) calculated among the two investigators was 0.917. Overall, the final eight articles comprised 147 male and 119 female participants, with 134 and 132 children in the EXP and CON groups, respectively. This review also determined that four articles applied tDCS and rTMS, respectively. Meanwhile, the minimum and maximum durations were 10 days and 12 weeks, respectively.
Quality assessment
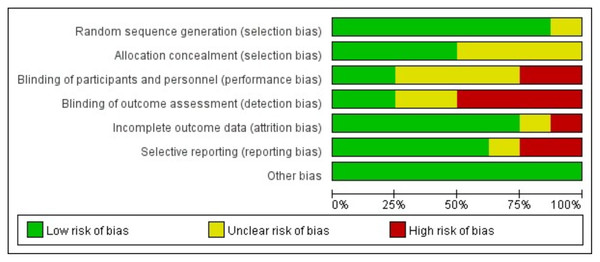
Figure 2 displays an evaluation of the methodological quality and potential bias risk of the selected articles, in which the overall quality was comparatively high. The proportions of low, high, and unclear risk of bias were also 60.72%, 16.07%, and 23.21%, respectively. Moreover, this figure illustrated the risk of bias associated with each article chosen in the analysis.
Figure 2: Analysis of risk of bias according to Cochrane Collaboration guideline.
Sensitivity analysis
Sensitivity analysis was performed by excluding studies one by one and modifying the type of analysis model. The sensitivity analysis then indicated no significant changes in the outcomes across the groups in the meta-analysis, confirming the reliability of the findings.
Quantitative synthesis
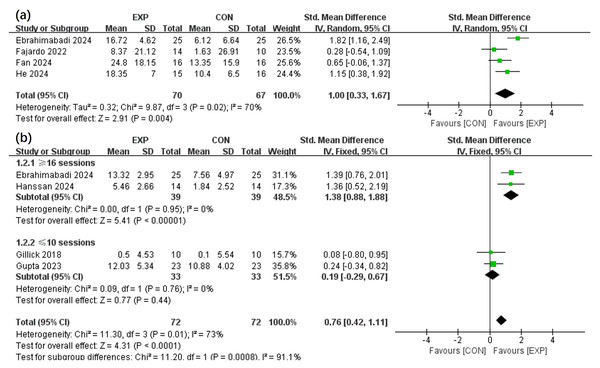
The effects of the intervention on GMF (Salazar Fajardo et al., 2022; Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024; Fan et al., 2024; He et al., 2024) and GS (Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024; Gupta et al., 2023; Gillick et al., 2018; Hassan et al., 2024) in children with CP within the EXP and CON groups were analysed across four selected articles. Figure 3 portrays that the EXP group exhibit a greater improvement in GMF relative to the CON group (SMD, 1.00 (0.33, 1.67), p < 0.05, I2 = 70%). The findings also implied that EXP significantly enhanced GS compared to CON (SMD, 0.76 (0.42, 1.11), p < 0.05, I2 = 73%). Subgroup analysis is based on clinical practice and data-driven results to address heterogeneity. Furthermore, subgroup analysis signified that interventions comprising above 16 sessions could notably improve GS (SMD, 1.38 (0.88, 1.88), p < 0.05, I2 = 0%), whereas those below 10 sessions did not demonstrate a significant effect (SMD, 0.19 (−0.29, 0.67), p = 0.44, I2 = 0%).
Figure 3: Forest plot illustrates the effects of EXP versus CON group on gross motor function (A) and grip strength (B).
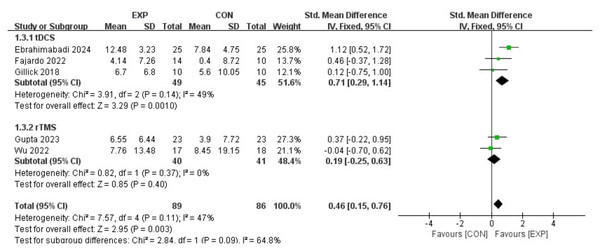
Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024; Salazar Fajardo et al., 2022; Fan et al., 2024; Gillick et al., 2018; Gupta et al., 2023; Hassan et al., 2024; He et al., 2024.Figure 4 illustrates a forest plot depicting the impact of brain stimulation and motor node on FMC in children with CP by incorporating data from five articles (Salazar Fajardo et al., 2022; Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024; Wu et al., 2022; Gupta et al., 2023; Gillick et al., 2018). Consequently, brain stimulation intervention could substantially enhance FMC (SMD, 0.46 (0.15, 0.76), p < 0.05, I2 = 47%). Subgroup analysis also presented that exercise-tDCS resulted in a significant improvement in FMC (SMD, 0.71 (0.29, 1.14), p < 0.05, I2 = 49%) compared to exercise-rTMS (SMD, 0.19 (−0.25, 0.63), p = 0.09, I2 = 47%).
Figure 4: Forest plot illustrates the effects of EXP versus CON group on fine manual control.
Ebrahimabadi, Ghaderian & Askary Kachoosangy, 2024; Salazar Fajardo et al., 2022; Gillick et al., 2018; Gupta et al., 2023; Wu et al., 2022.Analysis of publication bias
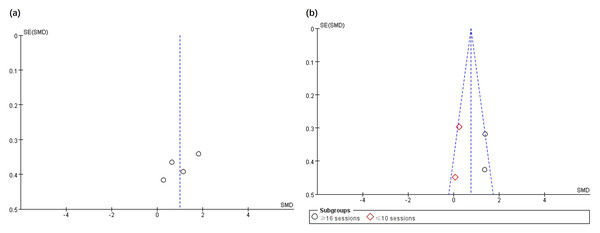
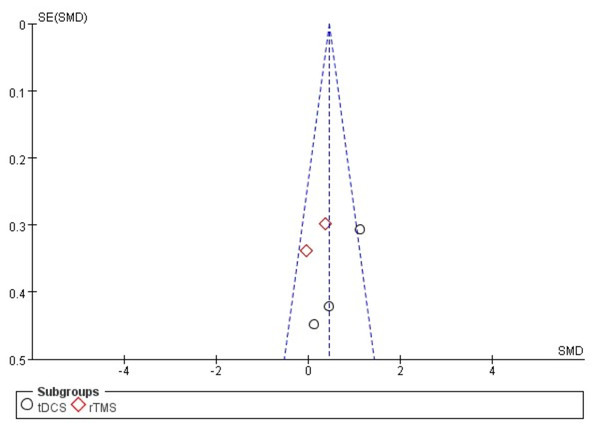
The funnel plot was applied to assess the publication bias of the selected articles in the analysis. Specifically, the total number of selected articles approached the minimum threshold for using a funnel plot, indicating that publication bias could be assessed to a certain extent (Lu et al., 2020). Figs. 5 and 6 show no significant asymmetry, but the small number of studies limits the interpretation.
Figure 5: Funnel plot of publication bias for gross motor function (A) and grip strength (B) in the EXP versus CON group.
Figure 6: Funnel plot of publication bias for fine manual control in the EXP versus CON group.
Discussion
This review examined the impact of exercise-brain stimulation on hand function in children with CP. The primary indicators for assessing hand function were GMF, GS, and FMC. For results with high heterogeneity, the reasons for heterogeneity were evaluated by adjusting variables such as age, gender, intervention duration, intervention frequency, exercise time, type, intensity, and duration of brain stimulation. Despite exercise-brain stimulation substantially enhancing GMF, GS, and FMC, a framework involving fewer than 10 sessions did not demonstrate a significant impact on GS. Subgroup analysis also revealed a differential effect of tDCS and rTMS on FMC. Generally, brain plasticity serves as the theoretical foundation for physical therapy in children with CP, in which these children primarily exhibit hand dysfunction. The reduction in fine motor control and hand strength significantly impacts the daily activities and educational experiences in children with CP (Das & Ganesh, 2019). Hence, prompt and efficient rehabilitation treatment is pivotal to address this issue. Even though current physical therapy for children with CP primarily involves different movements and techniques, the functional areas of the cerebral cortex are often not directly stimulated. Therefore, external brain stimulation equipment is beneficial to address the limitations of traditional therapy. Examples of these advantages are promoting balance recovery, restoring cerebral hemisphere equilibrium, and enhancing motor function in children with CP (Tang et al., 2022).
This review demonstrated that exercise-brain stimulation could selectively enhance GMF, which aligned with the outcomes reported by previous studies. Salazar Fajardo et al. (2022) integrated neurodevelopmental treatment and tDCS, which was administered to children with CP over a period of five weeks (three times weekly). The study also employed the current intensity and duration of brain stimulation of 1 mA and 20 min, respectively. Consequently, the brain stimulation group exhibited a notable enhancement in the GMF score in comparison to the sham stimulation group. This phenomenon may be elucidated by the influence of anodal tDCS on the γ-aminobutyric acid (GABA) ergic system. Anodal tDCS may also result in a reduction of GABA levels in M1. Specifically, anode tDCS significantly reduced the GABA concentration in M1 region by inhibiting the activity of GABA synthase, which lasted for at least 60 minutes (Kim et al., 2014). Magnetic resonance spectroscopy study confirmed that the decrease of GABA concentration in M1 region after anode tDCS was significantly positively correlated with motor learning ability, that is, the greater the decrease of GABA, the better the performance of motor adaptation task and the stronger the retention of motor memory (Kim et al., 2014; Stagg, Bachtiar & Johansen-Berg, 2011). Buch et al. (2017) demonstrated that lower GABA levels within the primary motor cortex during anodal tDCS were associated with the extent of motor learning, as GABA reduction was essential for the occurrence of long-term potentiation (LTP).
The findings of this review presented the positive effects of exercise-tDCS on FMC, which agreed with several studies. Ebrahimabadi, Ghaderian & Askary Kachoosangy (2024) combined occupational therapy and tDCS, which was administered to children with CP over a period of four weeks (five times weekly). The tDCS current intensity was established at 1.5 mA, with the anode positioned in the O1 region of the left occipital lobe and the cathode in the O2 region of the right occipital lobe. Following the intervention, the real tDCS group produced a substantial improvement in FMC compared to the sham tDCS group. Typically, the interaction between exercise and tDCS in enhancing FMC can be attributed to the modulation of excitability within the primary motor cortex, which facilitates neural plasticity. Anodal tDCS can then enhance the neuronal discharge in the primary motor cortex, lower the action potential threshold, and improve the accuracy of motor commands. The study also noted that employing anodal tDCS-occupational therapy for children with unilateral CP notably increased the Fugl Meyer upper limb function score, with improvements observed to be twice that of the control group. Concurrently, a substantial enhancement was observed for hand grasping speed and coordination based on the box and block test. This effect pertained to the enhanced conduction efficiency of the cortical spinal pathway (immediately and 24 h post-stimulation). Furthermore, the tDCS could facilitate synaptic remodelling through the activation of the brain-derived neurotrophic factor/tropomyosin receptor kinase B pathway. In addition, exercise and tDCS seem to have a cumulative effect, and the simultaneous application can bring better benefits to FMC. tDCS creates a neural plasticity window for exercise training by regulating cortical excitability. The synergy of TDCS and sports training is based on the theory of “neural plasticity window” (Marchiotto, Cambiaghi & Buffelli, 2025; Steinberg, Pixa & Fregni, 2018). Anode tDCS creates a highly plastic state in the motor cortex by reducing GABA inhibition, enhancing BDNF expression and promoting LTP (Marchiotto, Cambiaghi & Buffelli, 2025). Subsequent exercise training, as an intensive stimulus, repeatedly activates specific neural pathways during this window period, significantly enhancing the training effect. Studies have shown that this synergistic effect magnifies the effect of neural plasticity by 2–3 times, far more than using tDCS or exercise training alone (Steinberg, Pixa & Fregni, 2018). Anodic stimulation depolarizes M1 neurons and triggers LTP to improve synaptic transmission efficiency; Cathodic stimulation can induce long-term depression through GABA inhibition and correct the hemispheric imbalance. Exercise training uses this window to repeatedly strengthen action memory, and the two cooperate to optimize the accuracy of neuromuscular regulation and further improve FMC (Reis et al., 2009; Madhavan, Weber & Stinear, 2011).
A study (Liang et al., 2022) documented that tDCS-exercise training could enhance the maturation of newborn neurons in the hippocampus, which was a process closely associated with the release of brain-derived neurotrophic factor. This review also indicated that an optimal level of exercise and brain stimulation positively influenced GS (Zhang et al., 2022). Meanwhile, a significant relationship has been suggested between GS and hand function for children with CP (Jesunathadas et al., 2010). Enhanced neuroplasticity can then optimise motor unit recruitment and coordination among hand muscle groups. The regulation of cerebello cortical circuit by tDCS can also increase temporal accuracy of force control. Conversely, stimulation of the dorsolateral prefrontal cortex (DLPFC) improves executive function and motor planning, optimising GS stability and accuracy (Gbadeyan et al., 2016; Schmitter & Straube, 2022). Overall, the combination of exercise and tDCS, especially in the program with sufficient intervention duration, seems to be a promising non drug rehabilitation method for children with cerebral palsy.
This review presented multiple limitations. Initially, the sample size was limited (comprising only eight articles), and the units of measurement were inconsistent. Notably, the main analyses exhibited high heterogeneity, which limits the strength of the overall conclusions. Consequently, only SMDs were applicable for comparison. Another constraint was that this review only included children with CP, limiting the applicability of the results to other populations. Although the intervention of less than 10 sessions did not show a statistically significant impact on GS index, it could not exclude the clinically meaningful effect. The intervention protocol in tDCS for this review also exhibited inconsistencies between low current and high current protocols, restricting cross-sectional comparisons among articles. Additionally, the number of studies in each subgroup was small, which limits the statistical power and generalizability of the subgroup findings. In addition, the mechanism through which exercise-brain stimulation enhanced hand function in children with CP remained unclear. Therefore, the impact of exercise-brain stimulation across various populations warrants further investigation in future studies, and the underlying mechanisms should be analysed in greater detail.
Conclusion
This review demonstrated that exercise-brain stimulation could significantly enhance hand function in children with CP. Specifically, more than 16 sessions has greater benefits for GS, and tDCS may confer benefits for FMC. Therefore, a rehabilitation protocol combining exercise with tDCS, delivered in more than 16 sessions, represents a viable non-pharmacological strategy to enhance hand function in children with CP.