Trade-offs between aquatic and terrestrial locomotion and functional parallelism in Desmognathus salamanders
- Published
- Accepted
- Received
- Academic Editor
- Laura Brannelly
- Subject Areas
- Evolutionary Studies, Zoology
- Keywords
- Ecomorph, Escape, Fast start, Performance, Kinematics, Black-bellied salamander, Shovel-nosed salamander, Swimming-running dilemma, Trade-off, Habitat partitioning
- Copyright
- © 2025 Fitzpatrick
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Trade-offs between aquatic and terrestrial locomotion and functional parallelism in Desmognathus salamanders. PeerJ 13:e20111 https://doi.org/10.7717/peerj.20111
Abstract
A trade-off between aquatic and terrestrial locomotion is self-evident at broad phylogenetic scales. While the effects of more subtle trade-offs in the evolution of closely related species are less clear, they are hypothesized to drive ecological speciation and adaptive radiation. Amphibious animals strike a balance between aquatic and terrestrial activity, and the need to maintain performance in one medium is hypothesized to constrain evolution of high performance in the other (the running-swimming dilemma). Closely related species of Desmognathus salamanders partition local habitats along a gradient from mid-stream to stream edge to completely terrestrial. The trade-off hypothesis predicts that these species will differ in relative running vs. swimming performance depending on the relative importance of each mode of locomotion in their niches. Here, I show that primarily aquatic Desmognathus ecomorphs are superior swimmers and inferior runners relative to semi-aquatic ecomorphs using paired escape performance trials in aquatic and terrestrial arenas. I measured performance as the velocity of the fast-start response to simulated predator attack. I tested two species of each ecomorph, representing two divergent clades with parallel evolution of aquatic and semi-aquatic species. Notably, the different southern clade ecomorphs have been genetically isolated for millions of years, whereas the northern clade ecomorphs share a recent common ancestor and interbreed regularly. My results showed a negative correlation between aquatic and terrestrial performance, with aquatic ecomorphs being faster swimmers and semi-aquatic ecomorphs being faster runners. While there was a possible trend consistent with faster swimming speeds of northern forms relative to their southern counterparts, the functional differences between ecomorphs were similar in both clades. These results are consistent with the hypothesis that trade-offs between aquatic and terrestrial locomotion have contributed to a consistent pattern of habitat partitioning during parallel speciation.
Introduction
According to the ecological theory of adaptive radiation, diversity is produced by divergent selection for disparate phenotypes in different environments (Schluter, 2000). Species produced by divergent selection are ecologically differentiated because of performance trade-offs: The phenotypic syndrome that is optimal in one environment or niche is suboptimal elsewhere and vice versa. Examples include feeding on different sized seeds in Galapagos finches (Grant & Grant, 2006), benthic vs. limnetic foraging in three-spined sticklebacks (Schluter, 1995), and locomotion on different substrates in lizards (Garland & Losos, 1994). However, some studies concluded that functional trade-offs were absent or weak, particularly in within-species comparisons or groups with similar generalist phenotypes (e.g., Gvozdik & Van Damme, 2006; Nauwelaerts, Ramsay & Aerts, 2007; Zamora-Camacho, 2023; Peron, 2024).
Aquatic and terrestrial locomotion present different challenges (Blob et al., 2016; Kawano & Blob, 2022; Axlid et al., 2023), and amphibians such as salamanders are often regarded as examples of general-purpose phenotypes that are not particularly good at either because the requirement to maintain adequate function in one medium constrains the evolution of high performance in the other (Bonett & Blair, 2017). Gvozdik & Van Damme (2006) labeled this constraint the ‘running-swimming dilemma,’ but found no evidence to support it among Triturus salamanders (family Salamandridae). That is, species that were faster on land were not necessarily slower in water. Desmognathus salamanders (family Plethodontidae) provide an opportunity to test the hypothesis in a different ecological and evolutionary context.
The dusky salamanders (genus Desmognathus) are hypothesized to be an adaptive radiation with variation in habitat use along an aquatic-terrestrial microhabitat gradient (Tilley & Bernardo, 1993; Kozak et al., 2005; Bruce, 2011). Once, each adaptive zone along the gradient was considered the province of one or two species (Dunn, 1917; Dunn, 1926). Several decades of behavioral and molecular research have revealed that the group is composed of dozens of species, with repeated evolutionary transitions among ecomorphs (e.g., Tilley, Verrell & Arnold, 1990; Kozak et al., 2005; Kozak, Mendyk & Wiens, 2009; Tilley et al., 2013; Camp & Wooten, 2016; Pyron et al., 2022; Pyron et al., 2025). An ‘ecomorph’ describes a syndrome of similar habitat, morphology, and behavior shared by species that are not necessarily closely related (Williams, 1972).
The two largest and most aquatic ecomorphs are the semi-aquatic ‘black-bellied’ form and the aquatic ‘shovel-nosed’ form (Dunn, 1917; Martof, 1962; Petranka, 1998). They were formerly classified as two species D. quadramaculatus and D. marmoratus, respectively. However, molecular phylogenetic studies demonstrated that both ecomorphs occur in each of two divergent clades that are paraphyletic with respect to most other species of Desmognathus (Jackson, 2005; Kozak et al., 2005; Jones & Weisrock, 2018; Pyron et al., 2022). In the southern clade, the aquatic (D. aureatus) and semi-aquatic (D. amphileucus, D. gvnigeuswotli, and D. folkertsi) species are genetically distinct and reproductively isolated (Camp et al., 2002; Jackson, 2005; Pyron et al., 2022). However, in the northern clade, there is either a single polymorphic species (Jackson, 2005; Jones & Weisrock, 2018), or a complex of closely related hybridizing species (Pyron & Beamer, 2022; Pyron & Beamer, 2023; Pyron et al., 2025) with little correlation between genomic similarity and ecomorph. These studies show that the evolutionary dynamics of the aquatic and semi-aquatic forms have been different between the northern and southern clades, but there is no evidence as to whether or not there are functional differences between species of the same ecomorph.
Both ecomorphs studied here are stream-dwelling and have keeled tails, hardened toe-tips and a prolonged larval phase, features characteristic of the most aquatic Desmognathus species (Petranka, 1998). However, the aquatic ecomorphs, initially described as a separate genus (Moore, 1899), have more broadly finned tails (Fig. 1) and features of the head and internal nares thought to be associated with their aquatic habit (Martof, 1962). While they are capable of survival and locomotion on land, they rarely leave large streams, feed primarily on aquatic prey (Martof & Scott, 1957), and appear reluctant to feed when removed from water (Deban & Marks, 2002). The semi-aquatic ecomorphs are more often found on stream banks, breed in both large and small streams, and forage primarily on terrestrial prey as adults (Martof & Scott, 1957; Davic, 1991). Thus, the relative importance of aquatic vs. terrestrial locomotion differs between the two forms. I hypothesized that the aquatic and semi-aquatic ecomorphs would differ in relative performance according to the running-swimming dilemma (Gvozdik & Van Damme, 2006), but that the northern and southern representatives of each ecomorph would be functionally equivalent despite their disparate histories.
Figure 1: Representative specimens of semi-aquatic and aquatic Desmognathus ecomorphs.
(A) Dorsal views show the slightly smaller and narrower head of the aquatic ecomorph (lower specimen, closest to the ruler). (B) Lateral views show the more spatulate tail of the aquatic ecomorph (lower specimen, closest to the ruler. Collection data from the University of Tennessee Knoxville Vertebrate Zoology Collection are as follows. Specimen 00308 Desmognathus amphileucus: 05 July 1976; West Fork of Mill Branch, NW Slope of Big Fodderstack Mtn, Cherokee Nat. Forest, Monroe Co. TN; 84 02 45 W, 35 25 19 N; 750 m elevation; Map Ref: Whiteok Flats; Collector R. L. Jones. UTKVZC Cat # 00308, Field Number 00116. Specimen 01138 Desmognathus marmoratus: 06 May 1977; Tom’s Branch, just below mouth of Twin Springs Branch, 5.7 air miles south of town of Roan Mtn. Carter Co.; TN. 82 05 26 W, 36 07 26 N; 3840 ft elevation; Map Ref: Carver’s Gap; Collectors W. H. Redmond, R. L. Jones, J. Wojtowicz. UTKVZC Cat # 00831, Field Number 01138. Photo credit: Ben Fitzpatrick.Materials & Methods
Experimental subjects
Ten adult semi-aquatic (D. amphileucus) and 10 adult aquatic (D. aureatus) morphs were collected from a stream near DeSoto Falls Recreational Area, Lumpkin Co., Georgia. These represent distinct species in the southern clade. Twelve adult semi-aquatic (D. sp.) and 12 adult aquatic (D. marmoratus) morphs were collected from Little Laurel creek along Whitetop Rd., Smyth Co. Virginia. These represent the ecomorphs in the northern clade. The northern semi-aquatic taxa cannot be distinguished visually and either D. mavrokoilius or D. kanawha could be present at the Virginia locality (Pyron & Beamer, 2022). Collections were made in 2010, before the taxonomic revision of Pyron & Beamer (2022). Based on the locality and genetic data of Pyron et al. (2022), the Virginia locality is in or near a contact zone between D. mavrokoilius and D. kanawha, and it is likely that they interbreed where they co-occur (Jones & Weisrock, 2018; Pyron et al., 2022). As a pragmatic matter for this analysis, they are lumped collectively as the northern semi-aquatic ecomorph, Desmognathus sp. Aquatic ecomorphs were collected by looking under rocks underwater in stream riffles and semi-aquatic ecomorphs were collected by looking under rocks along the edge of the stream or above water in the stream.
All 44 individual salamanders were evaluated for fast-start performance after 8–12 weeks in captivity. The sexes of the individuals were not known. Based on previous studies (Azizi & Landberg, 2002; Fitzpatrick, Benard & Fordyce, 2003), we intended to test 10–20 individuals per species. In practice, we let the less common ecomorph dictate the sample size for each collection (the aquatic morph in both cases). Individuals with tail damage were not collected. Individuals smaller than 50 mm snout-vent length were assumed to be juveniles and not collected (Martof, 1962). The average and standard deviation of the snout-vent lengths for each group, measured at the time of capture, were as follows: 66.5 ± 0.69 mm for southern aquatic, 74.9 ± 1.52 for southern semi-aquatic, 72.2 ± 1.23 for northern aquatic, and 60.6 ± 0.89 for northern semi-aquatic.
Salamanders were maintained at 15 °C and 70% r.h. in Sterilite 6-quart (5.7 L) plastic shoeboxes (34.5 × 20.3 × 12.7 cm). Semi-aquatic individuals were housed on a damp paper towel substrate, while aquatic individuals were housed in two L of de-chlorinated and well-aerated water. Bedding was changed weekly and water changed every three to four days. All salamanders were provided with a terra cotta refuge and were fed either crickets or California blackworms ad libitum. Food was withheld for two days prior to performance assessment to minimize effects of recent feeding on locomotion (Yan et al., 2015; Zhao et al., 2020). Salamanders were monitored daily for signs infection, loss of appetite, or impaired mobility. There were no adverse events.
Experiments were carried out in accordance with the University of Tennessee Institutional Animal Care and Use Committee protocol # 1870-0510, a scientific collection permit from the Virginia Department of Game and Inland Fisheries (# 37891) and the Georgia Department of Natural Resources. All animals were alive and healthy at the end of the experiment and were retained for additional research on mating behavior.
Escape performance trials
Salamander escape performance was assessed as fast-start velocity in response to a threat stimulus (Domenici & Blake, 1997; Walker et al., 2005; Fitzpatrick, 2008). A few studies have demonstrated that swimming and running velocity are good predictors of survival in amphibians and reptiles (Watkins, 1996; Walker et al., 2005; Husak, 2006). The Desmognathus species studied here are more active at night, but can be observed moving about at any time of day (Martof, 1962; Huheey & Stupka, 1967; Valentine, 1974; Dodd, 2004). They are not chemically defended and adults are preyed upon by large aquatic predators such as trout (Salvelinus fontinalis, Oncorhyncus mykiss), and semi-aquatic/terrestrial predators such as snakes (e.g., Nerodia and Thamnophis), river otters (Lutra canadensis), raccoons (Procyon lotor), and other medium sized mammals and birds (Martof, 1962; Petranka, 1998; Sánchez-Hernández, 2020; Dempsey, Roden & Bidwell, 2021). Thus, the velocity of the initial burst of swimming or running in response to attack is likely an important component of ecological performance. Moreover, sprinting speed can be correlated with other components of performance requiring physical vigor, including foraging and territory defense (e.g., Peterson & Husak, 2006).
Escape response was recorded three times for each individual salamander in both a terrestrial and an aquatic arena. The terrestrial arena consisted of a glass container with a gravel substrate and water level with the substrate. The aquatic arena consisted of a glass container with enough water to fully submerge a salamander. Salamanders were given at least 30 min to acclimate to room temperature because the camera setup was in a different room from where the salamanders were housed. The temperature in the camera room was 21–22 C and preliminary tests indicated that 200g of water equilibrated within 30 min. Previous work demonstrated that salamanders typically equilibrate to a 6–7 C change in temperature within 15 min (e.g., Hutchison, 1961; Lunghi et al., 2016).
After acclimating, a salamander was placed in an arena and allowed to acclimate until it ceased moving. A gentle pinch at the base of the tail with forceps was used to stimulate an escape response. I had no way of measuring the strength of the stimulus. Animals were always approached from above and behind, and presumably they could see the forceps approaching. If they moved before being touched, the forceps were withdrawn and the stimulus was attempted again when the animal stopped moving. On the rare occasions when this occurred three times in a row, the trial was stopped and the animal was moved to the end of the list of animals to be tested that day. Variation in the stimulus and/or motivation may affect escape responses (Barry & Harrison, 1957), but I assume this variation was unbiased with regard to the comparisons of interest for this study.
Escape responses were recorded at a rate of 500 frames per second using a high speed digital camera (NAC Memrecam Ci digital system). Trials were repeated only after at least a 30 min break to reduce stress on the animals and to prevent habituation to the stimulus. Azizi & Landberg (2002) found no evidence of fatigue across five escapes during 15 min trials with salamanders, so I assumed a 30 min break between single escapes was more than enough to avoid fatigue accumulation. If fatigue affected performance, I assumed the effects were unbiased with regard to the comparisons of interest for this study. The order of the trials was randomized to eliminate confounding treatment (aquatic vs. terrestrial) with ecomorph, species, or individual experience.
Kinematic analysis
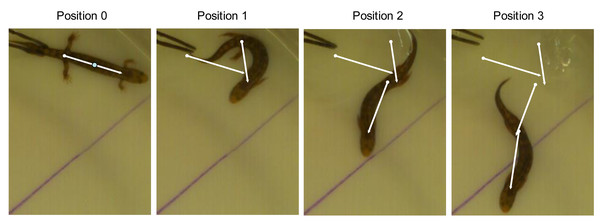
Fast start performance data were collected using ImageJ software. Each video was analyzed independently by two technicians without prior knowledge of the species or ecomorphological hypothesis. Head, trunk, and tail measurements were taken once from each video when the animal was stationary, prior to the stimulus. Four timepoints were recorded from each trial. The initial position (position 0) was defined as the frame just prior to the first perceived movement. The subsequent three positions (positions 1–3) were the frames in which the trunk experienced maximum curvature during each of the first three lateral undulations (Fig. 2). The frame of maximum trunk curvature was determined by finding the frame with the minimum distance between shoulder and pelvis. Salamanders generate forward motion by generating a traveling wave down the trunk and tail when swimming or a standing wave in the trunk when walking (Frolich & Biewener, 1992). By recording the salamander’s position at each maximum curvature of the trunk, we can determine the velocity generated by each wave. Here I focus on the net velocity generated by the first three waves, estimated as the linear distance between positions 0 and 3 (cm) divided by the time in seconds.
I used the mean net velocity across trials and observers as a single fast start performance metric for each individual in each medium. My goal was not to estimate maximal performance, but rather to compare performance between ecomorphs. Mean velocity across trials is highly correlated with the maximum of many trials, and unlike the sample maximum, the mean is unbiased and yields more reliable correlations with explanatory variables (Adolph & Pickering, 2008; Head, Hardin & Adolph, 2011).
For the purposes of this study, I assumed that velocity (distance from the attacker over time, regardless of direction) is a good indicator of ecological performance. Other components of escape behavior, such as changes in direction or speed, can be important for survival (e.g., Brodie III, 1992). By focusing on the velocity of the initial burst, I intended to isolate basic physical ability from behavioral tactics. While direction was not quantified, most salamanders in this study tended to turn toward the center of the arena during aquatic trials, but moved directly forward during terrestrial trials.
Figure 2: Measurement of fast start performance.
Video frames corresponding to each position in an aquatic escape trial were identified as the moment of maximum curvature of each locomotor wave. Total distance covered was measured between the midpoint of the line (axilla to groin) in positions 0 and 3. The specimen pictured is Desmognathus marmoratus, an aquatic ecomorph.Statistical analysis
To test for an overall signal of a trade-off, I estimated the Pearson correlation between terrestrial running velocity and aquatic swimming velocity expressed as body lengths per second. Further, to test whether the strength of the trade-off differs between northern and southern clades, I estimated separate correlation coefficients for each regional sample (Northern and Southern clades) and compared their 95% confidence intervals estimated via the normal approximation with the stats package in R (R Core Team, 2024).
To test whether the semi-aquatic and aquatic ecomorphs differed in performance, I used a mixed-effect linear model (Fox & Weisberg, 2019) with velocity as the dependent variable, species and trial habitat (aquatic or terrestrial) as factors, body length (measured from the snout to the groin), head length, and tail length as covariates, and individual as a random effect (because each individual was tested for both aquatic and terrestrial performance). I included all interaction terms, particularly a species by environment interaction term to test whether a performance difference between ecomorphs depended on the environmental context (aquatic vs. terrestrial). I then extracted the marginal mean velocity for each species x habitat combination and four planned contrasts to compare the species (different ecomorphs) within each clade under both aquatic and terrestrial conditions (Lenth, 2025).
Neither visual inspection of bivariate scatter plots nor the Shapiro–Wilk test of normality on the residuals indicated that any variable transformations were warranted (W = 0.9856, p-value = 0.4418). I used the variance inflation factor, VIF (Fox & Weisberg, 2019) to check for multicollinearity and found that head and tail length were highly correlated with body length. VIFs were acceptable and the model fit improved after eliminating head and tail length.
Results
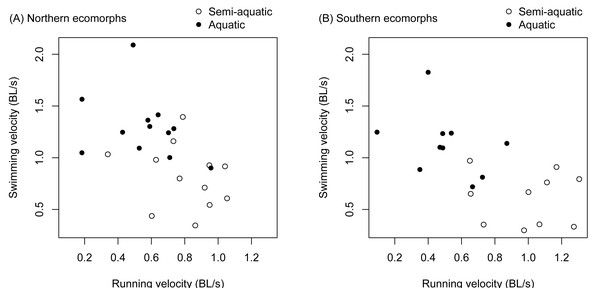
There was a significant negative correlation between an individual salamander’s fast start velocity in the terrestrial arena and its velocity in the aquatic arena (r = − 0.571, t 42 = −4.507, p < 0.0001). The relationship was similar for the northern and southern clades (Fig. 3), and separately estimated correlation coefficients were not significantly different (the 95% confidence interval was −0.759 to −0.139 for the northern clade, and −0.834 to −0.245 for the southern clade).
Figure 3: Trade-offs between terrestrial and aquatic escape performance.
Terrestrial (running velocity) and aquatic (swimming velocity) fast start speed expressed as body lengths per second were negatively correlated and different between ecomorphs (aquatic vs. semi-aquatic). The relationship was similar for ecomorphs in both the northern clade (A) and southern clade (B) of the Desmognathus quadramaculatus-marmoratus complex.There was a significant interaction between environment and species for velocity during a fast start escape response (Table 1). The semi-aquatic ecomorphs were faster in the terrestrial arena, while the aquatic ecomorphs were faster in the aquatic arena (Fig. 3, Tables 2 and 3). Body length (snout to groin) and environment (aquatic vs. terrestrial) also had significant main effects (Table 1). Larger salamanders tended to be faster, and swimming velocity was faster on average than running velocity (Fig. 3, Table 2).
| Variable | χ2 | Df | P-value |
|---|---|---|---|
| Test habitat (aquatic vs. terrestrial) | 18.95 | 1 | <0.0001 |
| Species | 3.74 | 3 | 0.2904 |
| Body length | 28.24 | 1 | <0.0001 |
| Habitat:Species interaction | 72.19 | 3 | <0.0001 |
| Habitat:Body Length interaction | 0.40 | 1 | 0.5249 |
| Species:Body Length interaction | 0.58 | 3 | 0.9016 |
| Habitat:Species:Body Length | 7.14 | 3 | 0.0675 |
Notes:
Each variable was evaluated while accounting for all others (Type II tests) via the Wald χ2 Analysis of Deviance (Fox & Weisberg, 2019). Each salamander of each species was tested in both aquatic and terrestrial conditions (swimming vs. running). The marginal r2 for the fixed effect portion of the model was 0.621.
| Clade | Species | Ecomorph | Habitat | Velocity | 95% CI |
|---|---|---|---|---|---|
| Southern | D. amphileucus | Semi-aquatic | Terrestrial | 5.76 | 4.67–6.84 |
| Aquatic | 3.72 | 2.63–4.80 | |||
| D. aureatus | Aquatic | Terrestrial | 2.90 | 1.89–3.91 | |
| Aquatic | 6.70 | 5.69–7.71 | |||
| Northern | D. sp | Semi-aquatic | Terrestrial | 5.10 | 3.88–6.33 |
| Aquatic | 4.28 | 3.06–5.51 | |||
| D. marmoratus | Aquatic | Terrestrial | 3.25 | 2.30–4.20 | |
| Aquatic | 7.58 | 6.63–8.52 |
| Habitat | Comparison | Difference | SE | tdf=72 | p-value |
|---|---|---|---|---|---|
| Terrestrial | D. amphileucus–D. aureatus | 2.86 | 0.745 | 3.841 | 0.0006 |
| Aquatic | D. amphileucus–D. aureatus | −2.98 | 0.745 | −4.003 | 0.0006 |
| Terrestrial | D. sp–D. marmoratus | 1.85 | 0.776 | 2.383 | 0.0198 |
| Aquatic | D. sp–D. marmoratus | −3.29 | 0.776 | −4.240 | 0.0004 |
Notes:
A positive difference means the semi-aquatic ecomorph was faster, and a negative difference means the aquatic ecomorph was faster. T-tests use the degrees of freedom determined by the Kenward-Roger method (Lenth, 2025). P-values were adjusted using the Holm (1979) sequential method to control family-wise error.
Discussion
The negative correlation between aquatic and terrestrial fast start velocity supports the hypothesis of a functional trade-off in Desmognathus salamanders. Moreover, performance differences between previously defined aquatic and semi-aquatic ecomorphs are consistent with a predictable evolutionary response to differences in the relative importance of aquatic and terrestrial locomotion that accompany shifts along the ecological gradient from large-stream habitat use to terrestrial foraging. This pattern is similar in evolutionarily distinct clades of Desmognathus, reinforcing the interpretation that parallel evolution of form, behavior, and function has accompanied adaptive diversification in the genus.
The running-swimming dilemma might be more evident in Desmognathus than in Triturus salamanders for two reasons. First, Gvozdik & Van Damme (2006) evaluated locomotor performance by chasing newts along a 200 cm runway and recording the maximum velocity per 25 cm interval. This is different from fast start velocity and measured over much longer interval than the data in this study (Fig. 2). Thus, we have measured different components of locomotor performance, and our different conclusions might reflect use of different variables rather than different animals. Second, Triturus are chemically defended by potent neurotoxins and when threatened often adopt a static warning posture (the Unken reflex) rather than flight (Brodie Jr, 1977; Telea, Stanescu & Cogalniceanu, 2021). Thus, maximum speed might have little survival value for Triturus. On the other hand, Desmognathus are palatable and vulnerable to many predators (Martof, 1962; Petranka, 1998; Sánchez-Hernández, 2020; Dempsey, Roden & Bidwell, 2021), making escape ability an important contributor to fitness.
The significance of the functional parallelism between northern and southern clades depends to some extent on the resolution of ongoing efforts to understand the diversity and species boundaries in the northern clade. Parallel evolution in concert with ecological speciation is well understood (Schluter & Nagel, 1995; Schluter, 2000; Nosil, 2012). However, functional divergence between long-isolated species is not often matched by hybridizing species complexes or within-species polymorphisms because recombination disrupts coordinated inheritance of multiple loci affecting complex traits (Dobzhansky, 1937; Felsenstein, 1981; Schluter & Rieseberg, 2022). In this study, the functional differences within the northern clade, maintained in the face gene flow, appear to match those between long-isolated species in the southern clade.
Conclusions
Trade-offs between aquatic and terrestrial locomotion are self-evident in broad comparisons (Gvozdik & Van Damme, 2006; Kawano & Blob, 2013; Blob et al., 2016), but not always relevant within species or ecologically conservative clades where other considerations dominate the observable variation (Gvozdik & Van Damme, 2006; Nauwelaerts, Ramsay & Aerts, 2007; Zamora-Camacho, 2023). Desmognathus salamanders are sometimes regarded as morphologically and ecologically conservative, but also as a textbook example of niche partitioning (Hairston, 1994). This study demonstrates that the ecological differences between the two most aquatic of the Desmognathus ecomorphs are accompanied by differences in fast start performance consistent with a functional trade-off forcing them into different evolutionary solutions to the running-swimming dilemma. This supports the general hypotheses that functional trade-offs contribute to ecological speciation and coexistence.