A new firefly genus from South America, with seven new species, a new combination, and notes on the phylogeny of Lampyrinae: Lucidotini (Coleoptera: Lampyridae)
- Published
- Accepted
- Received
- Academic Editor
- Viktor Brygadyrenko
- Subject Areas
- Biodiversity, Entomology, Taxonomy, Zoology
- Keywords
- Photinus, Zoiudo, Ybytyramoan, Neotropical Lampyridae
- Copyright
- © 2025 Viana et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. A new firefly genus from South America, with seven new species, a new combination, and notes on the phylogeny of Lampyrinae: Lucidotini (Coleoptera: Lampyridae) PeerJ 13:e18967 https://doi.org/10.7717/peerj.18967
Abstract
Background
Lucidotini is a diverse tribe of lampyrine fireflies present throughout the New World, Europe, and Asia. Most of the over 30 genera have overlapping diagnoses, largely due to a lack of revisionary and phylogenetic studies. Widespread convergence in sensory morphology, traditionally used in genus-level diagnoses, further compounds the taxonomic issues surrounding the Lucidotini. Recent work has cast light on the value of terminalia and genitalic traits for Lucidotini taxonomy and called for a more thorough screening of morphological characters. Of special interest are basal outgrowths of the phallus (i.e., ventrobasal processes)—currently only known in Alychnus Kirsch and Photinus Laporte–that can be quite informative at the species level, but its variation within Lucidotini remains poorly studied. Most Lucidotini species remain only superficially described, while internal characters—including those of terminalia and genitalia—which could inform species identification and phylogenetic relatedness, remain unknown. Upon studying eight Lucidotini species superficially looking like Photinus and Photinoides McDermott—all of which bearing long ventrobasal processes–we raised the hypothesis that they belonged to a genus yet to be recognized.
Methods
Here, we analyzed 97 morphological characters of 32 lampyrid species spanning 17 of 30 Lucidotini genera under Bayesian Inference.
Results
We found evidence for the recognition and description of Saguassu gen. nov. to include seven new species (Saguassu acutum sp. nov., Saguassu grossii sp. nov., Saguassu manauara sp. nov., Saguassu rebellum sp nov., Saguassu roura sp. nov., Saguassu serratum sp. nov. and Saguassu sinuosum sp. nov.), in addition to Photinus dissidens Olivier ((transferred herein, thus generating Saguassu dissidens comb. nov.), for which we also designate a lectotype and two paralectotypes). This previously neglected lineage of Lucidotini spans four South American biomes: Amazon, Atlantic Rainforest, Cerrado, and Pampa. Interestingly, Saguassu species span a gradient of morphologies related to signaling: from Lampyris-style ventrally bulging eyes, tiny antennae and no lanterns; intermediate eyes and antennae, with complete lanterns as in Photinus; to small eyes and long antennae and small lanterns as in many Lucidota Laporte. Saguassu gen. nov. was consistently found closely related to the three other Lucidotini taxa with ventrobasal processes (i.e., Alychnus, Photinoides, and Photinus). We provide an occurrence map of and a dichotomous key to Saguassu species, thoroughly compare this genus with co-occurring Lucidotini genera, and suggest steps towards a revision of the Lucidotini tribe.
Introduction
Lucidotini Lacordaire 1857—a senior synonym of Photinini LeConte, 1881 (see Bouchard et al., 2011) overlooked in some recent works—is one of the largest firefly lineages, with over 820 species and 33 genera (Gisseth-Ladino, Botero & Silveira, 2022; Roza, Mermudes & Silveira, 2022; see also The Lampyridae of the World Database (Keller, 2024)), i.e., nearly a third of Lampyridae at large. Lucidotini is unique among Lampyrinae Rafinesque, 1815 tribes in its adult morphology by the long, overlapping and often robust adult mandibles (typically reduced in other lampyrine groups) and dorsally placed abdominal spiracles (usually ventrally placed in the other Lampyrinae except Cratomorphini Olivier, 1911) (but see Silveira et al., 2019). Current genus-level diagnoses of Lucidotini genera are largely overlapping and rely on few external, homoplastic characters, which has led to taxonomic confusion.
Recent research focusing on Neotropical fireflies has led to an accumulation of new species and genera of Lucidotini (Silveira & Mermudes, 2014; Silveira et al., 2016; Zaragoza-Caballero & Navarrete-Heredia, 2014; Zaragoza-Caballero, 2015; Zaragoza-Caballero, 2017; Zaragoza-Caballero & Carranza, 2018; Campello-Gonçalves et al., 2019; Zaragoza-Caballero et al., 2020; Gutiérrez-Carranza et al., 2023; Gutiérrez-Carranza, Zaragoza-Caballero & Domínguez-León, 2023; Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023; Gutiérrez-Carranza & Zaragoza-Caballero, 2024), sometimes supported by phylogenetic analyses (Silveira et al., 2021; Vaz et al., 2020; Roza, Mermudes & Silveira, 2022; Silveira et al., 2022; Zaragoza-Caballero et al., 2023) and taxonomic revisions (Gisseth-Ladino, Botero & Silveira, 2022; Zeballos et al., 2023). However, the genus-level taxonomy of Lucidotini remains unstable.
The value of genitalic characters has long been recognized in firefly taxonomy, across levels (e.g., Gorham, 1884; Green, 1956; McDermott, 1962), but only more recently they were included in phylogenetic analysis (e.g., Jeng, 2008). For example, genitalic traits supported the revalidation of Alychnus Kirsch, 1865 (Gisseth-Ladino, Botero & Silveira, 2022), the description of Costalampys Silveira et al., 2021 and Zoiudo Roza, Mermudes & Silveira, 2022, and several new combinations (e.g., Silveira et al., 2021; Vaz et al., 2020; Zeballos et al., 2023), thus helping to stabilize Lucidotini taxonomy. Likewise, genitalic traits also supported the synonymization of Macrolampis Motschulsky, 1853 and Ellychnia Motschulsky, 1853 with Photinus Laporte, 1833 (Zaragoza-Caballero et al., 2020). This synonymization was based on the shared presence of basal processes of the dorsal plate of the phallus (therein referred to as dorsal excrescences (sic), but see Gisseth-Ladino, Botero & Silveira (2022) for a discussion; we from now on call it ventrobasal processes), but also by the findings of DNA-based or integrative phylogenies that found Ellychnia Motschulsky, 1853 nested with Photinus (Stanger-Hall, Lloyd & Hillis, 2007; Stanger-Hall & Lloyd, 2015; Martin et al., 2017, 2019).
The current state of Lucidotini genera calls for a more comprehensive and thorough comparative study to consolidate their diagnoses and stabilize their taxonomy to facilitate identification. For example, ventrobasal processes are also present in Alychnus, but this genus can be easily distinguished from Photinus by the number of tibial spurs on each leg: zero on the proleg (vs one in Photinus) and one on meso- and metalegs (vs two on Photinus) (Gisseth-Ladino, Botero & Silveira, 2022). While searching for Lucidotini specimens with similar leg traits across collections, we identified eight species (seven of which were undescribed) with Alychnus-like tibial spurs but otherwise very disparate external morphologies (Fig. 1). Nevertheless, all of them share unique genitalic traits, while sharing a pair of ventrobasal processes on the dorsal plate with Photinus. Here, we included these eight species in a phylogenetic analysis to explore their relationship to other Lucidotini taxa and test the hypothesis that they form a monophyletic group not nested in Photinus.
Figure 1: Diversity of Saguassu gen. nov.
This illustration highlights the diversity in sensor morphology (eyes and antennae) and lantern structures, as observed in the habitus, with dorsal views (A, C, E, G, I, K, M, O) and ventral views (B, D, F, H, J, L, N, P). (A, B) S. acutum sp. nov. (C, D) S. sinuosum sp. nov. (E, F) S. serratum sp. nov. (G, H) S. manauara sp. nov. (I, J) S. roura sp. nov. (K, L) S. rebellum sp. nov. (M, N) S. grossii sp. nov. (O, P) S. dissidens (Olivier, 1894) comb. nov. Scale bar: 1 mm.Materials and Methods
Morphology and terminology
Specimens were obtained from the following institutions, with curators’ names listed in parentheses:
BYU–USA, Utah, Provo, Brigham Young University, Monte L. Bean Life Science Museum (M. Whiting & S. Bybee).
CERPE–Brazil, Pernambuco, Recife, Coleção Entomológica da Universidade Federal Rural de Pernambuco (P. Grossi)
DZUP–Brazil, Paraná, Curitiba, Universidade Federal do Paraná, Departamento de Zoologia, Museu de Entomologia Pe. Jesus Santiago Moure (C. Ribeiro-Costa & N. Ganho).
FSCA–USA, Florida, Gainesville, Division of Plant Industry, Florida State Collection of Arthropods (P. Skelley & K. Schnepp).
IFML–Argentina, Tucumán, San Miguel de Tucumán, Instituto Fundación Miguel Lillo (C. Perez).
INPA–Brazil, Amazonas, Manaus, Instituto Nacional de Pesquisas da Amazônia, Coleção de Invertebrados (C. R.Vasconcellos da Fonseca).
MNHN–France, Paris, Muséum National d’Histoire Naturelle (A. Taghavian).
MPEG–Brazil, Pará, Belém, Museu Paraense Emilio Goeldi (O. Tobias).
MUSM–Peru, Lima, Universidad Nacional Mayor de San Marcos, Museo de Historia Natural (L. Figueiroa).
MSNG–Italy, Genova, Museo Civico di Storia Naturale "Giacomo Doria" (M. Tavano).
MZUF–Italy, Firenze, Museo di Storia Naturale ("La Specola") (L. Bartolozzi).
NCSU–USA, North Carolina, Raleigh, North Carolina State University Insect Collection (G. Powell & B. Blinn).
NHMUK–United Kingdom, London, the Natural History Museum [formerly British Museum (Natural History)] (Max Barclay & Michael Geiser).
UGCA–USA, Georgia, Athens, University of Georgia Collection of Arthropods (J. McHugh).
USNM–USA, Washington D.C., Smithsonian National Museum of Natural History (M. Branham).
WCCA–USA, North Carolina, Cullowhee, Western Carolina University Collection of Arthropods (L. Silveira).
Where dissections were permitted by the curators, one or more whole specimens were relaxed in warm water, then placed in 10% KOH for 24 h, fully dissected, and imaged under a stereomicroscope. Stacked images were prepared using Leica Application Suite X, and plates were assembled in Adobe Photoshop 2023. For the MPEG specimens (S. sinuosum sp. nov. and S. serratum sp. nov.—see below), only the abdomen was dissected. Terminology follows Roza, Mermudes & Silveira (2022). For the material examined, we transcribed the labels verbatim, and eventually added comments between brackets.
For the higher-level Lampyridae classification, we followed The Lampyridae of the World Database (Keller, 2024), which considers (i) the observations of Bouchard et al. (2011) in correctly using Lucidotini, the valid senior synonym of a still widely used name Photinini, (ii) the Lampyrinae classification as in Martin et al. (2019), with posterior additions (e.g., Alychnus, Costalampys, Zoiudo).
The species identification key provided below was based only the males because most females remain unkonwn.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:55E09EC0-1F67-436F-9674-0B47868ACFC7. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE and CLOCKSS.
Phylogenetic analysis
Our matrix expanded on the morphological characters listed in recent works of our group (Zeballos et al., 2023; Roza, Mermudes & Silveira, 2022). Our taxon sampling was chosen to maximize the morphological and taxonomic diversity of Lucidotini, with an emphasis on taxa superficially similar to the eight focal species (examined material other than the eighth focal species can be found in Supplemental Material S1). Character coding followed the logical basis of Sereno (2007). The character matrix was constructed in Mesquite v3.2 (Maddison & Maddison, 2022). The character matrix is provided as a nexus file in Material S2, and a list of characters and states is provided in S3.
We ran phylogenetic analyses based on Bayesian inference (BI) using MrBayes 3.2.7a (Huelsenbeck & Ronquist, 2001) (available at The CIPRES Science Gateway V. 3.3 (https://www.phylo.org, accessed on 13 September 2022; Miller, Pfeiffer & Schwartz, 2012). A model selection ran with ModelFinder in IQTREEE2 selected the MKV model with equal state frequencies, four gamma categories, and correction for ascertainment bias (i.e., MK+FQ+ASC+G4) (Lewis, 2001; Kalyaanamoorthy et al., 2017; Minh et al., 2020) (Supplemental File S4) as the best model of evolution. We ran 10 × 106 generations, saving trees every 2,000 generations and discarding the first 25% as burn-in, and checked for convergence using Tracer v1.6 (Rambaut et al., 2018) (MrBayes input file can be found in Supplemental File S5). The evolution of unambiguous characters under maximum parsimony was optimized on the majority consensus tree using WinClada (Nixon, 2022).
Trees were read in FigTree version 1.4.4 (obtained at https://github.com/rambaut/figtree/releases; accessed on 2 January 2021) and edited in Adobe Photoshop 2023.
Distribution map
We built an occurrence map in R for the focal species of this work based on specimen collection labels and historical records when available (see Saguassu dissidens comb. nov. below). We colored the map by Neotropical dominions sensu Morrone (2014), based on the shapefile given in Morrone et al. (2022). The following packages were used for the generation of the map: “sf” (Pebesma, 2018), “dplyr” (Wickham et al., 2023), and “ggplot2” (Wickham, 2016).
Results
Phylogenetic analysis
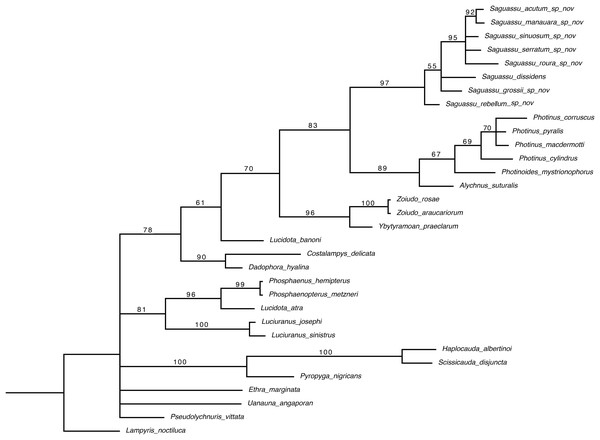
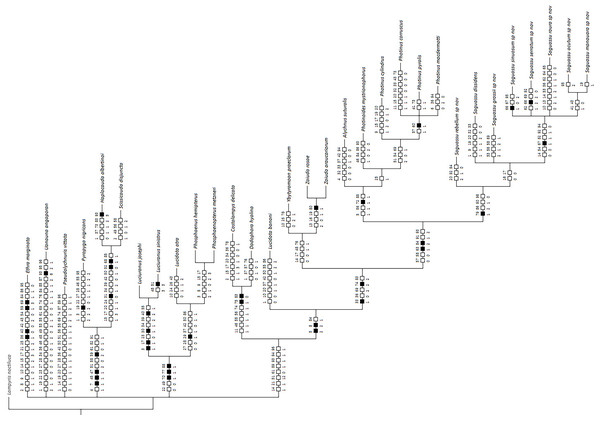
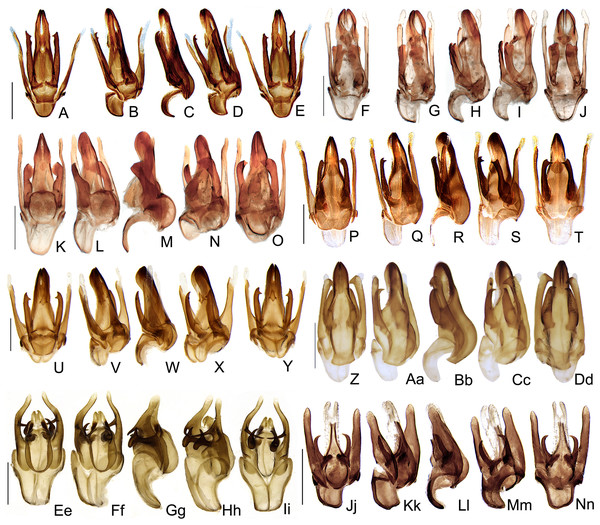
Bayesian inference (BI) majority consensus (Fig. 2; Supplemental File S6) recovered Saguassu gen. nov. as monophyletic with high support (PP = 97). This lineage is supported by one uncontroverted and three controverted (i.e., homoplastic) synapomorphies (Fig. 3): phallus, dorsal plate, basal 1/2 with dorsoventral depth at least 1/6 deeper than paramere (97:1, not homoplastic); phallus, endosac with clefted opening (86:2, homoplastic); paramere with subapical ventral spike elongate (90:1, homoplastic); and paramere with basal lobe absent (96:0, homoplastic). Saguassu gen. nov. was recovered with moderate support (PP = 83, Fig. 2) as sister to a clade (PP = 89) formed by Alychnus (Photinoides + Photinus). This relationship is supported by two uncontroverted and four controverted synapomorphies (Fig. 3): phallobase, sagittal line extending throughout the phallobase (57:0, homoplastic); phallobase, apical margin slightly emarginate (58:0, homoplastic); phallus, dorsal plate with basal protuberances (63:1, not homoplastic); phallus, endosac slightly shorter than phallus (84:2, not homoplastic); paramere, base laterally (coplanar) orientated in relation to phallus (91:1, homoplastic); and paramere, apex evenly curved inwards in lateral view (93:2, homoplastic).
Figure 2: Majority consensus of a Bayesian inference (see Material & Methods for more details) of 32 species and 97 traits. The number above each node is the bayesian inference (BI) posterior probabilities (pp).
The resulting phylogenetic tree found strong support for a monophyletic Saguassu gen. nov. upon the transfer of Photinus dissidens Olivier, 1894. Saguassu gen. nov. was found closer to other taxa bearing ventrobasal processes (i.e., Alychnus, Photinus, and Photinoides), forming the “Photinus” lineage discussed in this article.Figure 3: Maximum parsimony optimization of unambiguous character state changes plotted on the majority rule Bayesian consensus tree.
Black and white squares represent uncontroverted and controverted (i.e., homoplastic) synapomorphies, respectively.This larger clade was recovered, with low support (PP = 70), as sister to Ybytyramoan Silveira & Mermudes, 2014 + Zoiudo (a strongly supported clade; PP = 96), a relationship supported by two uncontroverted and three controverted synapomorphies (Fig. 3): sternum VII with lantern (33:1, not homoplastic); sternum VIII, posterior margin almost straight (36:0, homoplastic); phallus, dorsal plate as long as the parameres (69:2, homoplastic); phallus, dorsal plate slightly curved ventrally in lateral view (74:2, not homoplastic); and phallus with ventral plate rudimentary (83:2, homoplastic). The clades neighboring this most inclusive node are mostly weakly supported or polytomic (Fig. 2), except for the following relatively more inclusive nodes: (i) Pyropyga Motschulsky, 1852 (Haplocauda Silveira, Lima, Da Fonseca & McHugh, 2021 + Scissicauda McDermott, 1964) (PP = 100); and (ii) Luciuranus Silveira, Khattar & Mermudes, 2016 (Lucidota atra (Olivier, 1790) (Phosphaenus Laporte, 1833 + Phosphaenopterus Schaufuss, 1870) (PP = 81)). These findings are discussed below (see Discussion).
Taxonomy
Lampyrinae Rafinesque, 1815
Lucidotini Lacordaire, 1957
Photinina LeConte, 1881
Saguassu gen. nov. Viana, Roza, Vaz, and Silveira
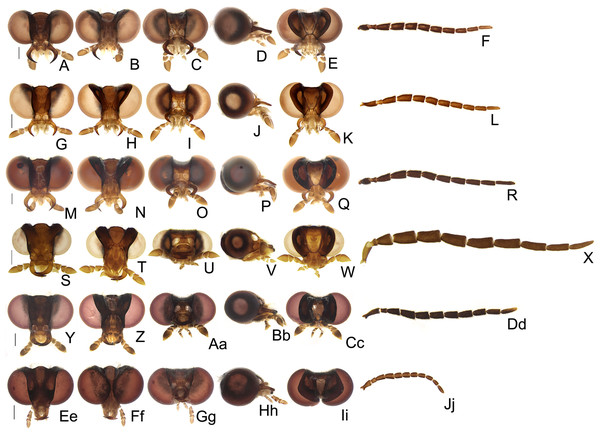
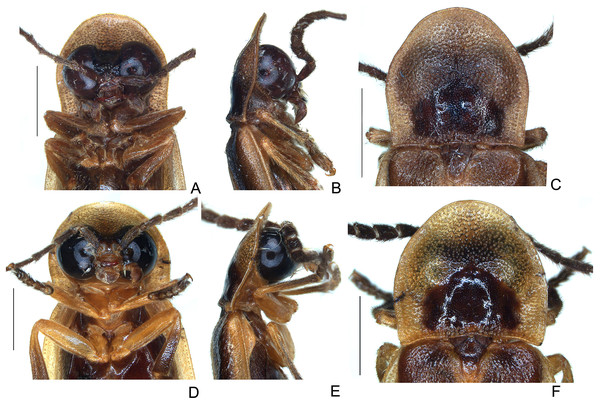
Figure 4: Diversity of head morphologies in Saguassu gen. nov.
(A–F) S. acutum sp. nov. (G–L) S. manauara sp. nov. (M–R) S. roura sp. nov. (S–X) S. rebellum sp. nov. (Y–Dd) S. grossii sp. nov. (Ee–Jj) S. dissidens (Olivier, 1894) comb. nov. Head, dorsal (A, G, M, S, Y, Ee), ventral (B, H, N, T, Z, Ff), frontal (C, I, O, U, Aa, Gg), lateral (D, J, P, V, Bb, Hh), occipital (E, K, Q, W, Cc, Ii) views. Left antenna, frontal (F, L, R, X, Dd, Jj) view. Scale bars: A–F, G–L, M–R, S–X, Y–Dd, Ee–Jj: 0.5 mm.Figure 5: Diversity of prothoracic morphologies in Saguassu gen. nov.
(A–E) S. acutum sp. nov. (F–J) S. manauara sp. nov. (K–M) S. roura sp. nov. (P–T) S. rebellum sp. nov. (U–Y) S. grossii sp. nov. (Z–Dd) S. dissidens (Olivier, 1894) comb. nov. Prothorax, dorsal (A, F, K, P, U, Z); ventral (G, G, L, Q, V, Aa), lateral (C, H, M, R, W, Bb), (D) frontal (D, I, N, S, X, Cc); (E) posterior views (E, J, O, T, Y, Dd). Scale bars: A–F, F–J, K–O, P–T, U–Y, Z–Dd: 0.5 mm.Figure 6: Head and thoracic morphology of Saguassu serratum sp. nov. (A–C) and S. marajoara sp. nov. (D–F).
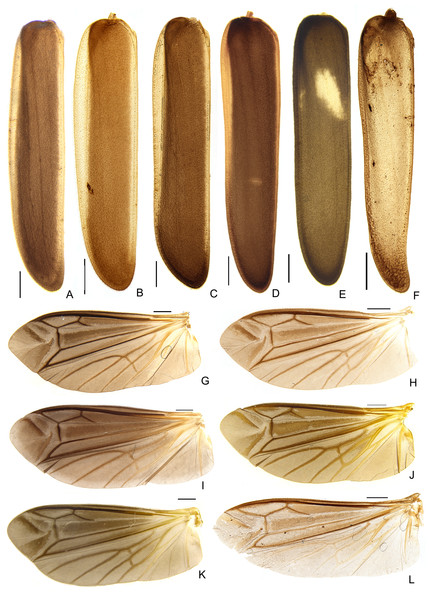
Ventral (A and D), lateral (B and E), and dorsal views. Scale bars: A–B and D–E: 1 mm; C and F: 1 mm.Figure 7: Diversity of elytron and wing morphologies in Saguassu gen. nov. S. grossii sp. nov.
(A–E) Left elytra, dorsal view. (G–L) Left wings, dorsal view. (A and G) S. acutum sp. nov. (B and H) S. manauara sp. nov. (C and I) S. roura sp. nov. (D and J) S. rebellum sp. nov. (E and K) S. grossii sp. nov. (F and L) S. dissidens (Olivier, 1894) comb. nov. Scale bars: 1 mm.Figure 8: Thoracic morphology of male Saguassu grossii sp. nov.
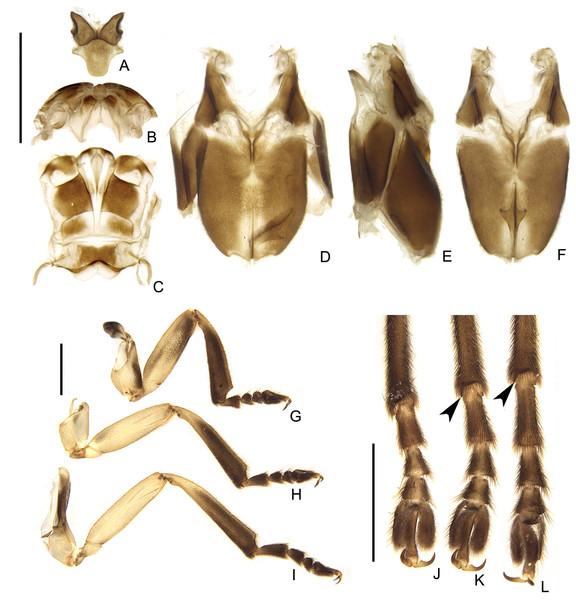
(A) Mesonotum, dorsal view. Alinotum, (B) anterior and (C) dorsal views. (D–F) Pterothoracic in (D) ventral, (E) lateral, and (F) ventral views. (G–I) Are pro, meso and hindlegs, respectively. (J–L) Are pro, meso and hindlegs, respectively. Black arrowheads show tibial spurs. Scale bars: A–F 2 mm; G–I and J–L 1 mm.Figure 9: Diversity of terminalia in Saguassu gen. nov.
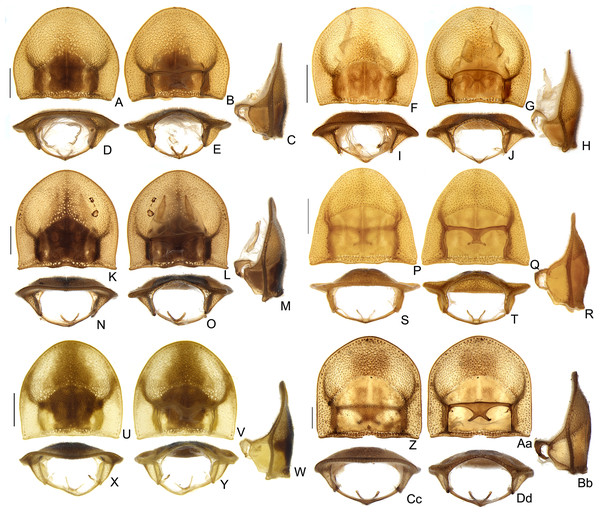
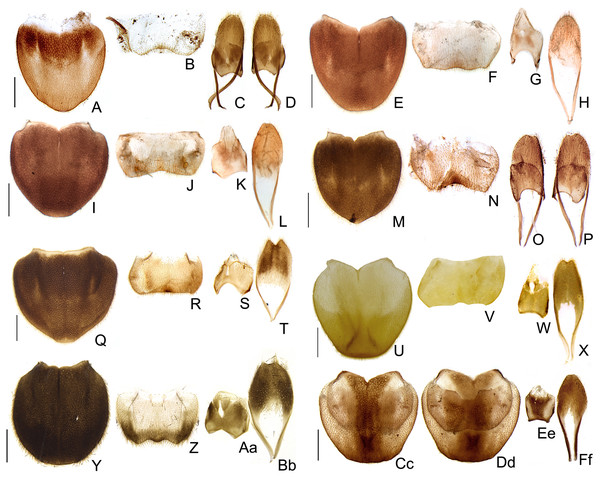
(A–D) S. acutum sp. nov., (E–H) S. sinuosum sp. nov., (I–L) S. serratum sp. nov. (M–P) S. manauara sp. nov. (Q–T) S. roura sp. nov. (U–X) S. rebellum sp. nov. (Y–Bb) S. grossii sp. nov. (Cc–Ff) S. dissidens (Olivier, 1894) comb. nov. Pygidium, dorsal view (A, E, I, M, Q, U, Y, Cc). Sternum VIII, ventral view (B, F, J, N, R, V, Z, Dd). Aedeagal sheath, syntergite, dorsal view (C, G, K, O, S, W, Aa, Ee), sternum IX, ventral view (D, H, L, P, T, K, Bb, Ff). Scale bar: 0.5 mm.Figure 10: Aedeagal diversity in Saguassu gen. nov.
(A–E) S. acutum sp. nov. (F–J) S. sinuosum sp. nov. (K–O) S. serratum sp. nov. (P–T) S. manauara sp. nov. (U–Y) S. roura sp. nov. (Z–Dd) S. rebellum sp. nov. (Ee–Ii) S. grossi sp. nov. (Jj–Nn) S. dissidens (Olivier, 1894) comb. nov. Aedeagus dorsal (A, F, K, P, U, Z, Ee, Jj), dorso-lateral (B, G, L, Q, V, Aa, Ff, Kk), lateral (C, H, M, R, W, Bb, Gg, Ll), latero-ventral (D, I, N, S, X, Cc, Hh, Mm); ventral views (E, J, O, T, Y, Dd, Ii, Nn). Scale bar: 0.5 mm.Figure 11: Morphology of Saguassu grossii sp. nov. female.
Habitus: (A) dorsal, (B) ventral view. (C) Abdominal tergites (left) and ventrites (right). (D) Pygidium, dorsal view. (E) Sternum VIII, ventral view. Genitalia: dorsal (F and H), and ventral (G and I) views. Scale bar: A–C: 2 mm; D–E: 1 mm; F–I: 0.5 mm.Type-species: Saguassu grossii sp. nov. Viana, Roza, Vaz, and Silveira; by original designation.
Diagnosis (based on males): Antenna filiform, compressed (slightly serrate only in S. rebellum sp. nov. Fig. 4X). Mandible stout and almost right-angled. Pronotum with disc strongly convex, anterior margin rounded, anterior expansion very long (at least 2/3 as long as disc), lateral expansions narrow (~1/3 as wide as disc). Tibial spur formula (0-1-1). Sternum VIII about 1/5 shorter than VII. Pygidium as wide as long, lacking posterolateral corners. Parameres completely separated in dorsal view, often bearing subapical membranous appendages. Phallus with dorsal plate of apically clefted, arms contiguous; ventral plate present, rudimentary; ventrobasal processes elongate and apically convergent, variably ornate apically.
Etymology. Saguassu is a singular gender neutral in the nominative case. Saguassu (saguaçú) comes from the Tupi-Guarani origin (Brazilian Indian languages), meaning “those with big eyes” (Barbosa, 1951). This name refers to the huge male eyes observed in species of this genus.
Immature stages. Unknown.
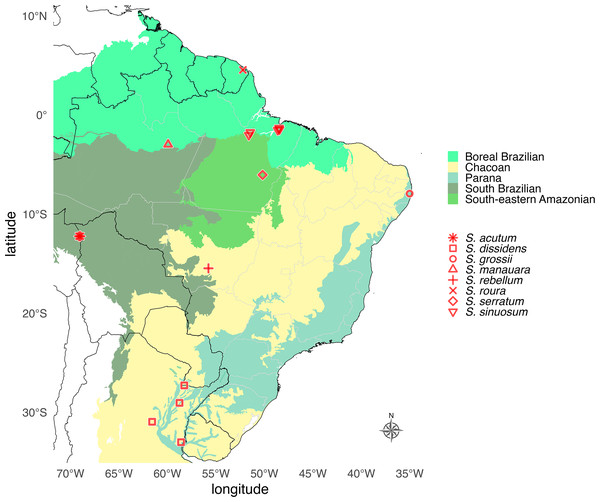
Distribution. South America East of the Andes (Argentina, Brazil, Peru, French Guiana, and Uruguay) from Northern Amazon to Southern Atlantic Forest and Pampas, including the Cerrado (Fig. 12).
Figure 12: Geographic distribution map of Saguassu gen. nov.
Species of Saguassu gen. nov. are found in all Neotropical dominions completely enclosed in South America. This new genus is more diverse in the Amazonian dominions (Boreal Brazilian, South Brazilian, South-eastern Amazonian), and all its species are allopatric.Biology. Field records and roughly documented flash patterns are available for Saguassu acutum sp. nov. and S. grossi sp. nov. (see below). Both species are nocturnal (active after dusk).
Remarks. Saguassu gen. nov. is overall similar to several Lucidotini: Photinina taxa (remarkably Photinus, Ybytyramoan, and Zoiudo) due to the presence of well-developed lanterns and eyes, and filiform antennae. These taxa cannot be easily distinguished from each other based on external characters alone due to broad variation within Photinus.
S. grossii sp. nov. is particularly similar to Photinus, due to its complete lanterns. However, Saguassu gen. nov. can be easily distinguished from these three genera by the very distinctive phallic dorsal plate, which is basally swollen leading to complete separation of the parameres in dorsal view. These are connected by a bridge in Photinus, Ybytyramoan, and Zoiudo. The presence of ventrobasal processes on the dorsal plate is shared between Photinus and Saguassu gen. nov., and separates these two from Ybytyramoan and Zoiudo. Finally, Saguassu gen. nov. can be distinguished from Photinus by the elongate, apically oriented ventrobasal processes (growing outwards, globose, vestigial, or absent in Photinus (see for example Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023)).
In contrast, S. rebellum sp. nov. is most similar to Lucidota, due to the presence of small eyes and lanterns, but long serrate antennae. The question of the poor definition of Lucidota Laporte, 1833 has been addressed by multiple recent works (e.g., Vaz et al., 2020; Zeballos et al., 2023; note also that Lucidota has been consistently recovered as polyphyletic in recent phylogenetic analyses, including both morphological, molecular, and integrative phylogenetic analyses (e.g., Martin et al., 2017)). Comparing S. rebellum sp. nov. to the type species of Lucidota, L. banoni Laporte, 1833, striking similarities on external morphologies arise, including the prothorax shape and color, tibial spur formula (0-1-1), rounded lanterns on sterna VI and VII, and emarginate posterior margin of sternum VIII. However, the aedeagal morphology is radically different: the dorsal plate is basally produced, and much longer than the phallobase, the ventral base is rudimentary, and the parameres are co-planar with the phallus and dorsally separated by the phallus in S. rebellum sp. nov. (whereas the dorsal plate is nearly flat and shorter than phallobase, the ventral plate is very well developed and basally widened, and the parameres are dorsal to the phallus and connected by a bridge in L. banoni).
Saguassu dissidens comb. nov. is similar to, and partly sympatric with, Photinus cylindrus (Olivier, 1905). Both species have large eyes accommodated by the prothorax with anterior bulgings of the hypomeron, very short, cylindrical antennae, an anterior margin of the disc strongly curved, and moderately dehiscent elytra. However, S. dissidens comb. nov. can be distinguished from Photinus cylindrus by the absence of lanterns, tibial spur formula (0-1-1), and aedeagal morphology (dorsal plate basally produced and separating the parameres, and much longer than the phallobase, ventrobasal processes falciform and apically sharp) of the former (lanterns present and complete on sterna VI and VII, tibial spur formula Photinus-like (1-2-2)), and aedeagal morphology Photinus-like (dorsal plate basally connected to the parameres by a thin bridge, shorter than the phallobase, ventrobasal processes short, projecting outwards and apically blunt in Photinus cylindrus).
The remaining four species (Saguassu manauara sp. nov., S. sinuosum sp. nov., S. acutum sp. nov., and S. serratum sp. nov.) are superficially similar to Photinoides mystrionophorus McDermott, 1963 due to the intermediate-sized eyes and antennae, curved anterior margin of pronotal disc, well developed lanterns on sterna VI and VII, and sternum VII barely covering the anterior margins of VIII, vs broadly covering sternum VIII in most Photinus). However, these Saguassu species can be distinguished from Photinoides mystrionophorus based on the pronotal morphology (anterior expansion slightly longer than disc in Saguassu gen. nov. vs slightly shorter in Photinoides) tibia spur formula (0-1-1, vs 1-2-2 in Photinoides), in addition to aedegal morphology.
Checklist of Saguassu gen. nov. species:
– Saguassu acutum sp. nov
– Saguassu dissidens (Olivier, 1894) comb. nov.
– Saguassu grossii sp. nov.
– Saguassu manauara sp. nov.
– Saguassu rebellum sp nov.
– Saguassu roura sp. nov.
– Saguassu serratum sp. nov.
– Saguassu sinuosum sp. nov.
Key to Saguassu gen. nov. species based on males
2 – Antennae long (~3× as long as greater head width) (Fig. 1L); vertex plane; pronotum lateral margins divergent posteriorly (Figs. 5P–5Q); sternum VII with an quadrangular, lantern, with posterior margin emarginate and covering the longitudinal medial third and up to two thirds of the sternum length; sternum VI and VII with faint lanterns, lacking a reflector layer (Fig. 1L)…………………………………… Saguassu rebellum sp. nov.
3’ – Elytral disc brown, lateral expansion pale yellow; lanterns not reaching outer margins; dorsal plate longer than parameres, ventrobasal processes without a basal projection; parameres without a subapical spike4
4 – Dorsal plate distinctly globose at basal 1/3 (abruptly curved in lateral view), with a dorsolateral keel at basal third……………………………………5
4’ – Dorsal plate less globose at basal 1/3 (more evenly tapered towards apex), without a dorsolateral keel at basal third……………………………………6
6’ – Basal 1/5 of tibia testaceous, then progressively more dark-brown towards apex; ventrobasal processes apically acute, with a subapical spike7
Saguassu acutum sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1A, 1B, 4A–4F, 5A–5E, 7A, 7G, 9A–9D, 10A–10E)
Diagnostic description. Antennal sockets separated by less than half of socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally moderately approximate, distance between eyes around three times submentum greatest width. Labrum with anterior margin strongly emarginate. Pronotum lateral margins rounded. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII with lanterns covering almost the whole sternal area, not reaching outer margins. Sternum VIII with emarginate posterior margin. Sternum IX with feebly emarginate posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and rounded acuminate apex. Syntergite with anterior margin slightly emarginate, subparallel lateral margins up to two thirds of its length. Dorsal plate distinctively longer than parameres, without apical membranous projection, as wide as phallobase at midlength, slightly globose at basal third, without a dorsolateral keel at basal third. Ventrobasal processes almost as long as core paramere, without basal projection and with apex acute with two spikes. Ventral plate diamond-shaped. Parameres with a subapical membranous appendage, without medioventral projection.
Etymology. Acutum is the Latin word for sharp, and it refers to the apex of the ventrobasal processes of the male aedeagus in this species. The name is in the nominative neutral singular case.
Biology. Males were seen by G. Powell in Peru, Madre de Dios, Finca Las Piedras, in January 2022. Males produce very long (2–3 s) green flashes followed by a dark phase of 5–8 s. The glow seemed to be overly faint for their size.
Material examined. HOLOTYPE. PERU: Madre de Dios, Finca Las Piedras, BYU-PE-2022 (−12.2255, −69.1147), 16/01/2022, SM Bybee & GS Powell col., ♂ (MUSM).
33 PARATYPES: Madre de Dios, Finca Las Piedras, BYU-PE-2022 (−12.2255, −69.1147), 16/01/2022, SM Bybee & GS Powell col., 20♂ (BYU), idem 9♂ (USNM). Idem, Concession Bilca, −12.2248, −68.9699, 14/01/2022, SM Bybee & GS Powell col, 2♂ (NCSU). Idem, 2♂ (UGCA)
Saguassu manauara sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1G–1H, 4G–4L, 5F–5J, 7B, 7H, 9E–9H, 10U–10Y)
Diagnostic description. Antennal sockets separated by less than half of socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally moderately approximate, distance between them around three times submentum greatest width. Labrum with anterior margin straight. Pronotum lateral margins rounded. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length, evanescence on elytral apex. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII with lanterns covering almost the whole sternal area, not reaching outer margins. Sternum VIII with emarginate posterior margin. Sternum IX with feebly emarginate posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and acuminate apex. Syntergite with anterior margin deeply emarginate, subparallel lateral margins up to two thirds of its length. Dorsal plate as long as parameres, without apical membranous projection, one-fifth wider than phallobase at midlength, slightly globose at basal third, without a dorsolateral keel at basal third. Ventrobasal processes as long as two thirds of core paramere, without basal projection and with apex acute with two spikes. Ventral plate subcircular. Parameres with a subapical membranous appendage, without medioventral projection.
Etymology. Manauara is the gentilic word for “born in Manaus” in Portuguese, the main language spoken in Brazil. Name in apposition.
Material examined. HOLOTYPE: Brasil, AM, Manaus, Reserva Ducke, Malaise 5, Plot B, III/1995, 1 ♂ (BMNH).
15 PARATYPES: Brasil, AM, Manaus, Reserva Ducke, Malaise 2, Plot A, III/1995, 2♂, MGV Barbosa col. Idem, Malaise 2, Plot B, III/1995, 1♂, MGV Barbosa col. (MNRJ). Idem, Malaise 3, Plot B, III/1995, 2♂, MGV Barbosa col. (INPA). Idem, Malaise 5, Plot A, III/1995, 2♂, MGV Barbosa col. (MPEG). Idem, Malaise 4, Plot A, II/1995, 1♂, MGV Barbosa col. (BMNH). Idem, Malaise 4, Plot A, III/1995, 1♂, MGV Barbosa col. (BMNH). Idem, Malaise 4, Plot A, I/1996, 2♂, MGV Barbosa col. (MZSP). Idem, Malaise 4, Plot B, III/1995, 3♂, MGV Barbosa col. Idem, MGV Barbosa col. (BMNH). Idem, no date, MGV Barbosa col., 1♂ (BMNH).
Saguassu dissidens (Olivier, 1894) comb. nov.
(Figs. 1O–1P, 4Ee–4Jj, 5Z–5Dd, 7F, 7L, 9Cc–9Ff, 10Jj–10Nn)
= Photinus dissidens Olivier, 1894
= Heterophotinus dissidens (Olivier, 1894)
Diagnostic description. Antennal sockets separated by almost the entire socket width. Antennae short (~1× as long as greater head width), antennomere III lateral margins subparallel, slightly longer than IV. Vertex slightly concave. Eyes ventrally approximate, distance between eyes narrower than submentum greatest width. Labrum with anterior margin straight. Pronotum lateral margins subparallel. Elytron moderately dehiscent, brown without any stripe or spot. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII without lanterns. Sternum VIII with emarginate posterior margin. Sternum IX with truncate posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and almost straight apex. Syntergite with anterior margin slightly emarginate, subparallel lateral margins up to two thirds of its length. Dorsal plate shorter than parameres, with apical membranous projection, as wide as phallobase at midlength, distinctly globose at basal third, without a dorsolateral keel at basal third. Ventrobasal processes as long as half of core paramere, without basal projection and with apex acute with one spike. Ventral plate absent. Parameres without a subapical membranous appendage, without medioventral projection.
Material examined. LECTOTYPE (designated herein). Banda Orienta[l] [URUGUAY] // Heterophotinus dissidens, Ern. Oliv., 1 ♂ (MNHN). 3 PARALECTOTYPES. Banda Oriental, 2 ♂, 1 ♀ (MNHN).
Additional material examined. URUGUAY: Banda Oriental (Montevideo?), 1 ♂, 1 ♀ (MNHN). Banda Oriental (Montevideo?) 1 ♂ (MNHN). Idem, Cornell U. lot 631, Sub 248, 1 ♂ (CUIC). Uruguay, 1 ♀ (MNHN). ARGENTINA: Corrientes, I/1972, 3 ♂ (WCU). Sunchales, (Córdoba) (sic, currently Santa Fé), XI.1898, F. Silvestri, 1 ♂ (MSNG).
Remarks. Olivier (1894) described the species from both males and a female. We infer that four specimens in Olivier’s collection at MNHN are syntypes because they have the same label data (“Banda Oriental”). In addition, the author gave a range (10–12 mm) of male sizes, implying that he saw more than one male specimen. Even though this species was described as a Photinus, Olivier (1894) proceeded to say that it would constitute a section that he would call Heterophotinus Olivier, 1894, consistent with the label on the specimen designated here as lectotype.
Saguassu dissidens (Olivier, 1894) comb. nov. is similar to a few Lucidotini species from Southern South America with large eyes, short cylindrical antennae, elytra, convex pronotum, and dehiscent elytra, including Photinus cylindra (Olivier, 1905) and Heterophotinus porrectus Olivier, 1908. However, it can be distinguished from these by the tibial spur formula (0-1-1 vs 1-2-2 in P. cylindra and H. porrectus) and the absence of lanterns (in P. cylindra and H. porrectus).
Saguassu rebellum sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1K–1L, 4S–4X, 5P–5T, 7D, 7J, 9U–9X, 10Z–10Dd)
Diagnostic description. Antennal sockets separated by almost the entire socket width. Antennae long (~3× as long as greater head width), antennomere III lateral margins apically divergent, two thirds of IV length. Vertex plane. Eyes ventrally separated, distance between eyes around five times submentum greatest width. Labrum with anterior margin emarginate. Pronotum lateral margins divergent posteriorly. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI without lantern, VII with a roughly quadrangular lantern, with posterior margin emarginate and covering the longitudinal medial third and up to two thirds of the sternum length. Sternum VIII with emarginate posterior margin. Sternum IX with emarginate posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and truncated apex. Syntergite with anterior margin slightly emarginate, subparallel lateral margins up to half of its length. Dorsal plate slightly longer than parameres, without apical membranous projection, as wide as phallobase at midlength, distinctly globose at basal half, without a dorsolateral keel at basal third. Ventrobasal processes as long as half of core paramere length, without basal projection and with apex acute with a single spike. Ventral plate absent. Parameres with a subapical membranous appendage, without medioventral projection.
Etymology. Rebellum is a Latin word for rebel, in the nominative singular, gender neutral form. The name refers to the unusual morphology of this species among its congenerics.
Material examined. HOLOTYPE: Brasil, MG, Chapada dos Guimarães, PI. 1, 12-18.X.2013, Malaise trap, G. Melo col., ♂ (DZUP).
2 PARATYPES: Brasil, MG, Chapada dos Guimarães, PI. 1, 12-18.X.2013, Malaise trap, G. Melo col., 2♂ (DZUP).
Saguassu roura sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1I–1J, 4M–4R, 5K–5M, 7C, 7I, 9Q–9T, 10U–10Y)
Diagnostic description. Antennal sockets separated by less than half of socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally moderately approximate, distance between eyes around three times submentum greatest width. Labrum with anterior margin strongly emarginate. Pronotum lateral margins rounded. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length, evanescence in the apex. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII with lanterns covering almost the whole sternal area, not reaching outer margins. Sternum VIII with straight posterior margin. Sternum IX with distinctly emarginate posterior margin. Pygidium with anterior margin straight, lateral margins convergent and rounded acuminate apex. Syntergite with anterior margin distinctly emarginate, subparallel lateral margins up to two thirds of its length. Dorsal plate slightly longer than parameres, without apical membranous projection, as wide as phallobase at midlength, distinctly globose at basal third, with a dorsolateral keel at basal third. Ventrobasal processes as long as two thirds of core paramere, without basal projection and with apex acute with two spikes. Ventral plate diamond-shaped. Parameres with a subapical membranous appendage, without medioventral projection.
Etymology. Roura is a commune in French Guyana and the type locality of this new species. Name in apposition.
Material examined. HOLOTYPE. FRENCH GUIANA: 30 km SE Roura on Kaw Rd., 11-ii-2010, N04°33.882′, W052°12.404′, J.E.Eger, coll., at night, 323 m, ♂ (FSCA).
4 PARATYPES. FRENCH GUIANA: 21 km SE Roura on Kaw Rd., 12-ii-2010, N04°36.115’, W052°15.972′, J.E.Eger, coll., MV Light, voucher code PIE150, 1 ♂ (NCSU). Amazone Nature lodge, 30 km SE Roura on Kaw Rd., 5-19-ii-2010, N04°33.570′, W052°12.433′, J.E.Eger, coll., UV light trap, 300 m, 1 ♂ (MZSP). idem, 1 ♂ (USNM). FRENCH GUIANA: 21 km SE Roura on Kaw Rd., 6-7-ii-2010, N04°36.115′, W052°15.972′, J.E.Eger, coll., MV Light, voucher code PIE150, 1 ♂ (FSCA).
Saguassu grossii sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1M, 1N, 4Y–4Dd, 5U–5Y, 7E, 7K, 8A–8L, 9Cc–9Ff, 10Ee–10Ii, 11A–11I)
Diagnostic description. Antennal sockets separated by around half of the socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally separated, distance between eyes around five times submentum greatest width. Labrum with anterior margin emarginate. Pronotum lateral margins rounded. Elytron subparallel, dark brown with a pale yellow spot at anterior half, of variable length but usually from suture to two thirds of elytron width. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII with lanterns covering the whole sternal area. Sternum VIII with tridentate posterior margin. Sternum IX with emarginate posterior margin. Pygidium with anterior margin emarginate, lateral margins rounded and rounded apex. Syntergite with anterior margin distinctly emarginate, subparallel lateral margins up to half of its length. Dorsal plate shorter than parameres, with apical membranous projection, one fifth thinner than phallobase at midlength, distinctly globose at basal two thirds, without a dorsolateral keel at basal third. Ventrobasal processes as long as one third of core paramere length, with basal projection and with apex acute with a single spike. Ventral plate absent. Parameres without a subapical membranous appendage, with an inner medioventral tooth-like projection.
Etymology. The epithet is in honor of the former naturalist and professor Paschoal Grossi, who is, up to now, the only collector of this species. Name in the genitive masculine case.
Biology. P. Grossi (2024, personal communication) reported seeing them swarming deep into the woods on several occasions, males were flashing (and definitely not emitting long glows), but Grossi could not determine the exact flash pattern.
Material examined. HOLOTYPE: Brasil, PE, Paulista, Aldeia Granja do Delegado, Km 14, −7.919426°S, −35.019929°W, 128 m, 04-16.III.2022, Ponto Mata, Malaise trap, Grossi & Galdino col., ♂ (CERPE).
238 PARATYPES. Brasil, PE, Paulista, Aldeia Granja do Delegado, Km 14, −7.919426°S, −35.019929°W, 128 m, 04-16.III.2022, Ponto Mata, Malaise trap, Grossi & Galdino col., 5♂, (MZSP); idem, 17.II-04.III.2022, Ponto Mata, Malaise trap, Grossi & Galdino col., 33♂ (DZRJ); idem, 04-18.III.2022, Ponto Riacho, Malaise trap, Grossi, Costa & Galdino col., 4♂ (INPA); Brasil, PE, Paulista, Aldeia Granja do Delegado, 15-30.III.2022, Malaise trap, Grossi & Galdino col., 16♂ (MNRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 15-30.III.2022, Ponto Mata, Malaise trap, Grossi & Galdino col., 2♂ (MPEG); Brasil, PE, Paulista, Aldeia Granja do Delegado, 15-30.III.2022, Ponto Riacho, Malaise trap, Grossi & Galdino col., 16♂ (DZUP); Brasil, PE, Paulista, Aldeia Granja do Delegado, 15-30.III.2022, Malaise trap, Grossi & Galdino col., 15♂ (DZRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 30.III-11.IV.2022, Ponto Mata, Malaise trap, Grossi & Costa col., 19♂ (1 completely dissected), 1♀ (abdomen dissected) (DZRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 30.III-11.IV.2022, Ponto Riacho, Malaise trap, Grossi & Costa col., 4♂ (DZRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 11-28.IV.2022, Ponto Riacho, Malaise trap, Grossi & Costa col., 2♂ (DZRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 11-28.IV.2022, Ponto Mata, Malaise trap, Grossi & Costa col., 22♂, 1♀ (DZRJ); Brasil, PE, Paulista, 28.IV-10.V.2022, Ponto Mata, Malaise trap, Grossi & Costa col., 15♂ (DZRJ); Brasil, PE, Paulista, Aldeia Granja do Delegado, 28.IV-10.V.2022, Ponto Riacho, Malaise trap, Grossi & Costa col., 6♂ (DZRJ). Idem, 04-16.III.2022, Malaise trap, 40♂ (CERPE); idem, 17.ii-04.iii.2022, Malaise trap, Costa, Galdino & Grossi col., 37♂ (CERPE).
Saguassu sinuosum sp. nov. Viana, Roza, Vaz, and Silveira
Diagnostic description. Antennal sockets separated by less than half of socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally moderately approximate, distance between eyes around three times submentum greatest width. Labrum with anterior margin straight. Pronotum lateral margins rounded. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length. Legs evenly brown. Sternum VI and VII with lanterns covering almost the whole sternal area, not reaching outer margins. Sternum VIII with sinuouse posterior margin. Sternum IX with rounded posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and rounded apex. Syntergite with anterior margin distinctly emarginate, subparallel lateral margins up to half of its length. Dorsal plate as long as parameres, without apical membranous projection, as wide as phallobase at midlength, slightly globose at basal third, without a dorsolateral keel at basal third. Ventrobasal processes as long as half of core paramere, without basal projection and with sinuous apex, without spikes. Ventral plate diamond-shaped. Parameres with a subapical membranous appendage, without medial projection.
Etymology. The epithet refers to the sinuose shape of the apex of the ventrobasal processes in this species. Name in the nominative neutral form.
Material examined. HOLOTYPE. Brasil, Pará, Melgaço, Caxiuanã-Grade, PPBio (Programa de Pesquisa em Biodiversidade), 22.III.2006, Silva S. S. & J. Dias, Malaise trap, ♂, (MPEG).
23 PARATYPES: Five specimens with same labels, 3♂, 1♀, 1 of indeterminate sex (lacking abdomen) (MPEG); idem, 2♂ (MZSP); Brasil, Pará, Melgaço, Caxiuanã-Grade PPBio, 27.III.2006, Silva S. S. & J. Dias, Malaise trap, 1♂, (MPEG); Brasil, Pará, Melgaço, Caxiuanã-ECFPn, 23.III.1998, O. Oliveira & J. Pena col, S. Malaise trap, 1♂, 1♀ (MNRJ); Brasil, Pará, Melgaço, Caxiuanã-ECFPn, 28.III.1998, O. Oliveira & J. Pena col, S. Malaise, 1♂, 1♀ (MPEG); Brasil, Pará, Barcarena, Capoeira, 20 a 23.I.1984, Armadilha suspensa, 2m.a.s.l., 1♂ (MPEG); Brasil, Pará, Barcarena, 22.I.1984, Tadaieswky V. 1♂ (MPEG); Brasil, Pará, Belém, Icoraci, 19.XII.1961, J. & B. Bechyné, 7♂, 1♀ (MPEG); Brasil, Pará, Belém, Mocambo, 10.I.1978, Mata terra firme, Armadilha Malaise trap, 1♂ (MPEG).
Saguassu serratum sp. nov. Viana, Roza, Vaz, and Silveira
(Figs. 1E, 1F, 6A–6C, 9I–9L, 10K–10O)
Diagnostic description. Antennal sockets separated by less than half of socket width. Antennae moderately long (~2× as long as greater head width), antennomere III lateral margins subparallel, almost as long as IV length. Vertex concave. Eyes ventrally moderately approximate, distance between eyes around three times submentum greatest width. Labrum with anterior margin emarginate. Pronotum lateral margins rounded. Elytron subparallel, brown with an inner and an outer pale-yellow stripes throughout its length. Legs with coxae and tibia yellowish brown, femur and tarsus brown. Sternum VI and VII with lanterns covering almost the whole sternal area, not reaching outer margins. Sternum VIII with emarginate posterior margin. Sternum IX with truncate posterior margin. Pygidium with anterior margin emarginate, lateral margins convergent and rounded apex. Syntergite with anterior margin slightly emarginate, subparallel lateral margins up to a third of its length. Dorsal plate as long as parameres, without apical membranous projection, as wide as phallobase at midlength, distinctly globose at basal third, with a dorsolateral keel at basal third. Ventrobasal processes as long as half of core paramere, without basal projection and with serrate apex with an inconspicuous spike. Ventral plate diamond-shaped. Parameres without a subapical membranous appendage, without medial projection.
Etymology. The epithet refers to the serrate shape of the apex of the ventrobasal processes in this species. Name in the nominative neutral form.
Material examined. HOLOTYPE. Brasil, Pará, Serra Norte, 15 a 18.II.1985, Armadilha Malaise, ♂ (MPEG). PARATYPE. Brasil, Pará, Serra Norte, 15 a 18.II.1985, Armadilha Malaise, ♂ (MPEG).
Discussion
Our results warranted the description of a new genus of Lucidotini: Photinina fireflies, based on a Bayesian phylogenetic analysis of 32 taxa and 97 characters. Our finds bear implications for the biology and taxonomy of Lucidotini fireflies, as discussed below.
Sensory morphology and lantern diversity within Saguassu gen. nov. mirrors, at a smaller scale, the range seen for the family at large.
Fireflies have evolved using a combination of visual and olfactory cues for mate finding and selection (Stanger-Hall et al., 2018; Powell et al., 2022; Novák & Jakubec, 2024). Visual cues include both continuous glows and rhythmic flashes (see Faust, 2017 for an excellent overview of bioluminescence in North American species). A few main “mating syndromes” across firefly lineages result from the differential role each signal (light—flashes and glows; and pheromones) plays. Where males follow female glows, they usually have ventrally bulging eyes, and short filiform antennae (Stanger-Hall et al., 2018). In such systems, males may (e.g., Phausis LeConte, 1851, Lamprohiza Motschulsky, 1853, Diaphanes Motschulsky, 1853) or may not (Lampyris Geoffroy, 1762, Pelania Mulsant, 1860, Pleotomodes Green, 1948) have lanterns; when present, the lanterns never occupy the whole sterna. Where males and females use flashes to communicate, males have intermediate-sized eyes, and well developed lanterns that occupy the whole sterna VI and VII, which may be longer than preceding sterna. In contrast, when only pheromones are available, males have long and more elaborate (serrate, flabellate) antennae, smaller eyes, and, if present, rudimentary lanterns (e.g., Costalampys, Lucidota banoni).
Saguassu gen. nov. is an interesting case in which closely related species have widely different sensor and lantern morphology (Fig. 1). The spectrum of diversity seen on those structures mirrors that seen for the family at large (except for antennal lamellae, unknown in Saguassu gen. nov.). It is, to our knowledge, the firefly genus with the broadest variation in these external structures. In contrast, the aedeagal morphology is remarkably stable, comparatively. While females of most species remain unknown, it is very likely that these male traits reflect their different modes of comunication with their respective females.
Saguassu grossii sp. nov. is a textbook example of a “flasher” morphology, whereas S. dissidens comb. nov. has a “glower” morphology—the latter is indeed the first South American species with “Lampyris-like” morphology (enlarged eyes, short antennae, and no lanterns). Meanwhile, sensor and lantern morphology in S. rebellum sp. nov. matches those seen in many diurnal Lucidotini, and the remaining four species look like an intermediate between “flashers” and “glowers”. Consistent with that observation, S. acutum sp. nov. males have been seen producing very long and faint flashes, which could be seen as an intermediate between short, very bright flashes and more or less continuous glows. However, given the long dark phases and the faint light it produces, it could also be seen as a pathway to a loss of light, as in many males of glowing species (e.g., Lampyris). Yet, the direction of eye growth is mainly ventral in S. dissidens comb. nov., as in many other lampyrid “glowers”, in contrast to the roughly globular or elliptical (evenly bulging dorsally and ventrally) eyes of the flashers.
Other cases of closely related species with widely different sensory and lantern morphology—all in Lampyrinae—include species in Photinus (see Stanger-Hall, Lloyd & Hillis, 2007; Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023), Dilychnia Motschulsky, 1853 (Vaz et al., 2020), Diaphanes (Li & Liang, 2007), and Pyrocoelia Gorham, 1880 (Jeng, Lai & Yang, 1999; Zhu, Xu & Zhen, 2022). “Dark” (i.e., lanternless) Photinus species were found indeed nested with (Stanger-Hall, Lloyd & Hillis, 2007 (including “Ellychnia” species, but also Photinus cookii Green, 1956 and Photinus indictus LeConte, 1881)) or very close to (Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023) flashing species of Photinus, which ultimately led to a redefinition of this genus by Zaragoza-Caballero, Zurita-García & Ramírez-Ponce (2023). Dilychnia is another genus with species with greater eyes, smaller filiform antennae, and complete lanterns (e.g., D. dumasi Vaz et al., 2020, D. propinqua (Olivier, 1909), D. cavicollis Olivier, 1912), but also species with longer serrate antennae, intermediate-sized eyes, and rudimentary lanterns (e.g., D. disparilis Olivier, 1911, D. guttula (Fabricius, 1801), D. ruficollis Motschulsky, 1854) (Vaz et al., 2020).
A wide variation in sensory and lantern morphology is also seen in both Diaphanes and Pyrocoelia. For example, Diaphanes pectinealis Li & Liang, 2007 males have flabellate antennae and intermediate-sized eyes, unlike all other Diaphanes, which have enlarged eyes and filiform antennae. Despite important similarities between D. pectinealis and diurnal Pyrocoelia species, the former was confirmed as a Diaphanes by molecular phylogenetics and morphology (Li, Yang & Liang, 2006; Li & Liang, 2007; Zhu, Xu & Zhen, 2022; but see Martin et al., 2019). The overlap between diagnostic sensory traits between Diaphanes and Pyrocoelia led Li & Liang (2007) to call for updated diagnoses of these genera. Likewise, Pyrocoelia species feature a broad range of variation in sensor and antennal morphology, including completely dark species with long, elaborate antennae, small eyes, and species with well developed lanterns, enlarged eyes and comparatively smaller, simpler antennae (see, for example, Jeng, Lai & Yang, 1999). The close relationship of species with such disparate external morphologies, independently confirmed by molecular phylogenies (e.g., Suzuki, 1997; Zhu, Xu & Zhen, 2022), also suggests that other traits be used at the genus-level taxonomy of the Lampyrinae.
Given the wide variation in sensor morphology in Saguassu gen. nov., our study echoes these claims for updated diagnoses of Lucidotini taxa, and suggests that diversification in mating signals and sensor morphology played a key role in the diversification of this lineage.
Steps towards a revision of the Lucidotini genera and subtribes
Our study corroborates the value of traits not traditionally used in lampyrid taxonomy for reconstructing evolutionary relationships of Lucidotini. It also reiterates the unsuitability of traditionally used traits in genus-level diagnoses, such as overall sensor and lantern traits (e.g., whether the antenna is serrate, flabellate or filiform; or the presence/absence or size of lanterns). For example, many Saguassu gen. nov. and Photinus species are incredibly similar based on traditional characters, and yet both lineages show parallel variations on these. Nevertheless, they can be easily separated by the tibial spur formula (0-1-1 and 1-2-2, respectively), or aedegal morphology (phallobase longer than dorsal plate, dorsal plate basally produced, and parameres completely separated in Saguassu gen. nov.; phallobase shorter than dorsal plate, dorsal plate not basally produced and connected to parameres by a thin bridge in Photinus). We note that many of the most useful traits require dissections, and future genus-level diagnoses of Lucidotini taxa may be dominated by such traits, given the overall lability of traditionally used external characters. Below, we discuss character variation in Lucidotini, with an emphasis on taxa most similar to Saguassu gen. nov.
Saguassu gen. nov. is one of four valid genera bearing ventrobasal processes, along with Alychnus, Photinus, and Photinoides. Two junior synonyms of Photinus, Ellychnia and Macrolampis, were synonymized based on the shared presence of ventrobasal processes (Zaragoza-Caballero et al., 2020), which was later supported by phylogenetic analyses (Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023). Several Photinus species exist which do not have such processes (e.g., genus Paraphotinus Zaragoza, 1995, synonymized with Photinus in Zaragoza-Caballero, Zurita-García & Ramírez-Ponce, 2023), supposedly due to a secondary loss. However, the presence of these processes in closely related but reciprocally monophyletic (i.e., Saguassu gen. nov.) or neighboring taxa (i.e., Alychnus and Photinoides), that also happen to be morphologically very distinctive, calls for future studies with denser taxon and character sampling to revisit the condition of Ellychnia and Macrolampis. We believe that splitting (i.e., pulling monophyletic taxa out of) Photinus and providing authoritative species-level keys and illustrated atlases is sorely needed to facilitate identification of Lucidotini taxa. However, the shared presence of ventrobasal processes, in combination with other traits, may be used in the future to improve the diagnoses of higher-level taxa in Lucidotini.
Lucidotini has four subtribes: Photinina LeConte, 1881, Lucidotina Lacordaire 1857, Dadophorina Olivier, 1907, and Phosphaenina McDermott, 1964. In addition, there are several genera currently placed as “incertae sedis”. While revising the subtribal classification of Lucidotini goes beyond the scope of this work, the amount of phylogenetic information recently accumulated yielded important information that we summarize below in hopes of fostering future revisionary work on this tribe.
Three groups within Lucidotini have started to emerge in phylogenetic analyses, both based on morphology and DNA. We will refer to them as “Photinus lineage”, “Scissicauda lineage”, and “Phosphaenus lineage” (note that these could serve as a basis for a revised Photinina and Phosphaenina in the future). Defining traits of these lineages are mostly based on features that require dissection (e.g., terminalic, genitalic, or otherwise cryptic (e.g., submentum)). These three lineages are consistent with and the most recent DNA-based phylogenetic analysis (Martin et al., 2019) and have also been recovered in most recent phylogenetic analyses based on morphology (Roza, Mermudes & Silveira, 2022; Zeballos et al., 2023). We refrain from taking nomenclatural acts until a more comprehensive and thorough study of Lucidotini taxa is undertaken.
“Photinus lineage” as defined here include those taxa with submentum anteriorly membranous and usually rounded or straight, pygidium as wide as long or wider, posterior corners barely visible or absent; syntergite evenly sclerotized, and aedeagi with a dorsal plate entire and variably clefted, bearing ventrobasal processes, struts absent (not seen through phallobase), ventral plate rudimentary or absent, and paramere tips distinctly membranous and/or bearing subapical membranous appendages. The parameres are co-planar to the phallus, but never broadly fused at the base; instead, they are connected by a thin bridge. “Photinus lineage” include Alychnus, Photinoides, Photinus, and Saguassu gen. nov.
The “Scissicauda lineage” include those taxa with submentum well sclerotized and notched anteriorly, pygidium usually longer than wide, sometimes as wide as long in some Pyropyga, posterior corners barely visible or absent, syntergite medially longitudinally split forming two plates, and aedeagi with a dorsal plate entire and variably clefted, without ventrobasal processes, struts absent (not seen through phallobase), ventral plate usually well-developed (except in Haplocauda), and parameres evenly sclerotized. The parameres are co-planar and broadly fused to the dorsal plate at the base. The “Scissicauda lineage” includes Haplocauda, Scissicauda, Pyractonema Solier, 1849, and Pyropyga. See Zeballos et al. (2023) for a more detailed account on this lineage. Illustrations and descriptions of Heterophotinus, Robopus Motschulsky, 1853, and Rufolychnia Kazantsev, 2007 suggest they may also belong here (see Kazantsev & Zaitsev, 2008; L Silveira, 2024, personal observation).
Lastly, the “Phosphaenus lineage” includes those taxa with submentum anteriorly membranous, pygidium usually as wide as long but longer than wide in some Luciuranus, posterior corners well developed but absent in some Luciuranus, syntergite evenly sclerotized, and aedeagi with a dorsal plate longitudinally split forming two stylets or bars, often with a membranous endossac, without ventrobasal processes, struts present and with a unique joint, and parameres evenly sclerotized. The parameres are dorsal to the dorsal plate, and connate to this unique joint. “Phosphaenus lineage” includes Phosphaenus, Phosphaenopterus, and Luciuranus, in addition to a few Lucidota species that are very different from the type, L. banoni, such as L. atra and L. punctata (LeConte, 1852). Illustrations and descriptions (e.g., Kawashima, 2021) suggest that Lucidina Gorham, 1883 may also belong to this lineage.
Together, these observations highlight the value of thorough comparative studies in search of new traits, and the need for updated revisions of higher taxa in Lucidotini.
Conclusions
Our work includes the description of seven new species of Lucidotini fireflies. Our phylogenetic analysis supported the description of Saguassu gen. nov., the transfer of a former Photinus species, S. dissidens comb. nov., and the polyphyly of Lucidota. In addition, our work contributes to a revision of the higher-level taxonomy of the Lucidotini by providing a thorough comparison of the closely allied Photinina, and by pointing out key traits supporting a few inclusive nodes in the Lucidotini phylogeny.