The impact of maternal serum biomarkers on maternal and neonatal outcomes in twin pregnancies: a retrospective cohort study conducted at a tertiary hospital
- Published
- Accepted
- Received
- Academic Editor
- Fanglin Guan
- Subject Areas
- Gynecology and Obstetrics, Pediatrics, Women’s Health
- Keywords
- Twin pregnancies, Preeclampsia, Serum biomarker, Risk factor, Multivariable logistic model
- Copyright
- © 2024 Wu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. The impact of maternal serum biomarkers on maternal and neonatal outcomes in twin pregnancies: a retrospective cohort study conducted at a tertiary hospital. PeerJ 12:e18415 https://doi.org/10.7717/peerj.18415
Abstract
Background
Prior prediction models used for screening preeclampsia (PE) in twin pregnancies were found to be inadequate. In singleton pregnancies, various maternal biomarkers have been shown to be correlated with negative pregnancy outcomes. However, the impact of these biomarkers in twin pregnancies remained uncertain.
Methods
A retrospective cohort study was carried out on 736 twin pregnancies at a tertiary hospital in Hangzhou, China. Multivariable logistic models were employed to examine the association between levels of serological markers and the likelihood of adverse pregnancy outcomes. The final logistic model was formulated as a user-friendly nomogram. The primary outcome assessed was the occurrence of PE. Results were presented as odds ratios (ORs) with corresponding 95% confidence intervals (CIs).
Results
The prevalence of PE in the study was 10.3%. When comparing women diagnosed with PE to those without, it was evident that the former group experienced a significantly higher risk of unfavorable maternal and neonatal outcomes. A multivariable logistic regression analysis revealed notable associations between various factors including maternal age, parity, gestational weight gain, a family history of hypertension, as well as levels of cholesterol, albumin, and creatinine and the risk of developing PE, with a significance level of P < 0.05. The concordance index for the constructed nomogram was determined to be 0.792 (95% CI: [0.739–0.844]). Furthermore, an increment of 1 * 1012/L in red blood cell (RBC) count was associated with more than a two-fold increase in the odds of experiencing adverse maternal outcomes (OR 2.247, 95% CI: [1.229–4.107]). However, no significant correlations were identified between any of the examined variables and neonatal outcomes.
Conclusions
In this study, we developed a user-friendly predictive model that achieves notable detection rates by incorporating maternal serum biomarker levels alongside maternal characteristics and medical history. Our findings indicate that the probability of adverse maternal outcomes increases with elevated levels of RBCs. Obstetricians should consider intensifying surveillance for these women in clinical practice.
Introduction
With the widespread use of assisted reproductive technology, the incidence of multiple pregnancies has been steadily increasing (Sunderam et al., 2022; Deng et al., 2019; Smith et al., 2014). Multiple pregnancies have been found to have a higher occurrence of hypertensive disorders compared to singleton pregnancies (Aviram et al., 2021; Francisco et al., 2017a). Preeclampsia (PE) is a significant contributor to maternal and perinatal morbidity and mortality, with estimated costs reaching $2.18 billion within the first year post-delivery in the United States (MacDonald et al., 2019; Huang et al., 2022; von Dadelszen et al., 2023; Stevens et al., 2017). Therefore, it is crucial to develop a model that can identify high-risk women early in pregnancy to implement preventive measures and close monitoring.
Many predictive models for PE integrate maternal characteristics, medical history, ultrasound vascular indices, and serum biomarkers such as placental growth factor and pregnancy-associated plasma protein-A. Certain models have demonstrated high detection rates accompanied by low false-positive rates, and as a result, they have been effectively implemented in clinical settings (Benkő et al., 2021; Francisco et al., 2017b; Chen et al., 2020; Kosinska-Kaczynska et al., 2020; Nzelu et al., 2020). Nevertheless, the accessibility of these biomarkers is limited in developing countries or remote regions, which poses a challenge to the universal applicability of these models. When PE screening relies solely on maternal risk factors and medical history in twin pregnancies, the efficacy of such a model tends to be relatively inadequate (Benkő et al., 2019). Recent studies have assessed the predictive value of various novel maternal biomarkers and echocardiographic parameters; however, the detection rates in these investigations have been found to be lower than those of earlier models (Xiong et al., 2022; Xiang et al., 2023).
Multiple serum biomarkers have been identified as being associated with unfavorable pregnancy outcomes, for instance, high hemoglobin (Hb) levels in the first trimester have been associated with an increased risk of small for gestational age or low birth weight (Peng et al., 2022). Additionally, both low and high Hb levels in the first 20 weeks of pregnancy have been linked to elevated risks of adverse outcomes (Randall et al., 2019). Studies have also shown that maternal anemia can elevate the risk of low birth weight, preterm birth, and perinatal mortality (Rahman, Khan & Rahman, 2020). Other maternal biomarkers such as serum albumin (ALB), serum uric acid (UA), and serum lipid levels have also been linked to maternal and perinatal outcomes, including PE (Yang et al., 2016; Xiong et al., 2022; de Mendonça et al., 2022; Zheng et al., 2022; Okala et al., 2020). However, existing studies have primarily focused on singleton pregnancies, leaving a gap in understanding the impact of these biomarkers on twin pregnancies. We hypothesize that these indicators are associated with the incidence rate of PE and may serve as a foundation for developing a predictive model for PE.
Therefore, this study aims to investigate the association between various maternal serum biomarkers in the second trimester and the risk of PE and other adverse pregnancy outcomes in twin pregnancies. Furthermore, the study seeks to assess the predictive value of these biomarkers in determining the risk of PE in twin pregnancies.
Methods
Study population and data collection
The present retrospective study was carried out at Hangzhou Women’s Hospital. The study cohort consisted of twin pregnancies delivered after 26 weeks of gestation from 2019 to 2023. Exclusion criteria included women who had terminated pregnancies or fetal reduction, as well as those with pre-existing diabetes mellitus or chronic hypertension.
All data were collected from the electronic medical records system following approval from the medical ethics committee of Hangzhou Women’s Hospital (No. 2023-A-130). Demographic characteristics (such as maternal age, parity, interpregnancy interval, pre-pregnancy body mass index (BMI), gestational weight gain, level of education, history of autoimmune disease and family history of hypertension), obstetric characteristics (including mode of conception, chronicity, aspirin use during pregnancy, corticosteroid treatment for fetal maturation, presence of gestational diabetes mellitus and fetal sex), and laboratory data (levels of maternal serum biomarkers) were gathered by trained researchers. For women diagnosed with PE, data regarding blood pressure levels and the presence of proteinuria were systematically gathered. Pre-pregnancy maternal BMI was calculated using the WHO standard classification, and gestational weight gain was categorized according to Institute of Medicine (IOM) recommendations for twin pregnancies (World Health Organization, 2023; Institute of Medicine, 2009). Women with a body weight below the recommendations set by the Institute of Medicine were classified according to guidelines from a comprehensive population-based cohort study (Lin et al., 2022).
Levels of red blood cells (RBC), platelets (PLT), Hb, hematocrit (Hct), cholesterol (CHO), triglycerides (TG), ALB, UA, creatinine (Cr), alanine aminotransferase (ALT), and lactate dehydrogenase (LDH) were measured between 24–28 weeks of gestation after a minimum 8-h fast. Blood cell count were measured by Automatic Blood cell analyzer (XN9000; Sysmex, Kope, Japan) while biochemical parameters were measured using Automatic biochemical analyzer (Cobas 8000; Roche, Basel, Switzerland). In instances where the automated results warranted further scrutiny, a manual peripheral blood smear was prepared. A drop of the blood sample was applied to one end of a glass slide, and a second glass slide was positioned at a 45-degree angle. Subsequently, the blood was spread evenly across the surface of the slide in a single, uniform motion. After the smear had dried, it was subjected to Leishman’s stain. Following an incubation period of 5 min, the stain was diluted with distilled water and mixed on the slide. The slide was then allowed to absorb the stain for a duration of 15 min before being rinsed gently under a stream of water. Finally, the smear was examined under a high-power microscope for the enumeration of blood cells.
Outcome definition
The primary focus of our study was the occurrence of PE, which was identified by elevated blood pressure in addition to the presence of protein in the urine or damage to other maternal organs after the 20th week of pregnancy (American College of Obstetricians and Gynecologists, 2020). Fetal parameters were not considered indicative of PE, as conditions such as twin-to-twin transfusion syndrome or selective fetal growth restriction could also lead to fetal growth restriction or alterations observed in uterine Doppler ultrasound measurements. Given the high rates of preterm birth and cesarean section within our study population, we defined a composite maternal outcome as the occurrence of any of the following events: postpartum hemorrhage, blood transfusion, admission to the intensive care unit, chorioamnionitis, and postpartum infection. Similarly, a composite neonatal outcome was defined as the occurrence of any of the following events: neonatal asphyxia, need for ventilator support, neonatal hypoglycemia, sepsis, blood transfusion, and hemorrhage. Further details regarding the definitions of these outcomes can be found in Data S1.
Statistical analysis
The baseline characteristics of the study cohort were described using mean ± standard deviation (SD) or frequency with its corresponding percentage depending on the type of variable. The study cohort was categorized into three distinct groups: the normal control group, the early-onset preeclampsia (EOPE) group, and the late-onset preeclampsia (LOPE) group. Continuous variables that exhibited a Gaussian distribution were assessed using One-way analysis of variance, whereas those that did not conform to this distribution were evaluated using the Kruskal-Wallis H test. Categorical variables were assessed using Chi-square test or Fisher’s exact test. Variables that yielded a P-value of less than 0.1 in the univariate analysis were subsequently incorporated into a multivariate logistic regression model. Covariates were systematically removed in a stepwise manner until all remaining covariates in the final model had a P-value of less than 0.05.
A nomogram was developed utilizing coefficients derived from a logistic regression model implemented in R. The internal validation of the nomogram involved assessments of both discrimination and calibration. The model’s discriminative capability was evaluated using the area under the receiver operating characteristic (ROC) curve, which ranges from 0.5, indicating no discrimination, to 1, indicating perfect discrimination (Zhou et al., 2019). Calibration was visually assessed by plotting the observed rates against the probabilities predicted by the nomogram. Furthermore, the nomogram underwent 1,000 bootstrap resampling for internal validation to evaluate its predictive accuracy.
In order to investigate the association between the onset timing of PE and the variables included in the multivariate logistic regression model, a time-event analysis was conducted employing the Log Rank test. The cut-off age was established at 35 years, while for other continuous variables, the median value was utilized as the cut-off point.
Statistical analyses were carried out using SPSS version 25.0 and RStudio version 2023.09.1 Build 494 (RStudio Team, 2023) within the R environment version 4.3.3 (2024-02-29; R Core Team, 2024), and results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). A two-tailed P-value <0.05 was considered statistically significant.
Results
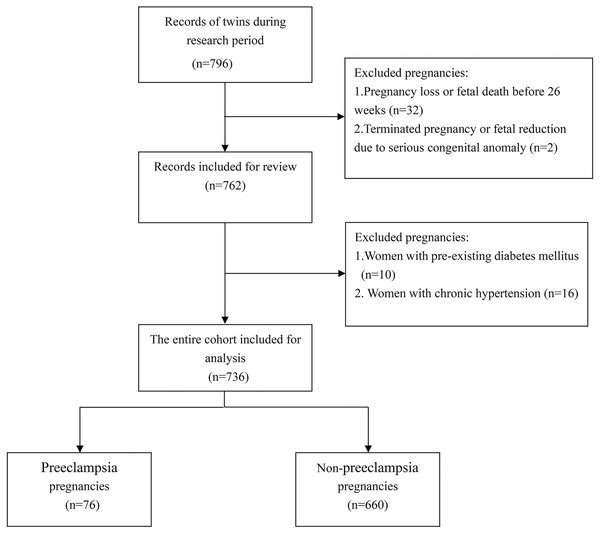
The study included 736 women with twin pregnancies, of whom 76 were diagnosed with PE, resulting in a prevalence of 10.3% (Fig. 1). Baseline characteristics of the cohort are presented in Table 1. Women diagnosed with both EOPE and LOPE were found to be older, with average ages of 33.0 ± 2.8 years and 32.2 ± 4.5 years, respectively, compared to an average age of 30.6 ± 3.7 years for women without PE (P = 0.004 and P = 0.042, respectively). Additionally, a higher proportion of women with EOPE (31.6%) and LOPE (29.8%) reported a family history of hypertension, in contrast to only 15.2% of women without PE. Aside from the percentage of women receiving corticosteroid treatment for fetal maturation, which was associated with their earlier gestational age, no significant differences were observed across the various variables between the EOPE and LOPE groups.
Figure 1: Selection of eligible participants.
| Characteristics | NC (n = 660) | EOPE (n = 19) | LOPE (n = 57) | LOPE vs. EOPE |
EOPE vs. NC |
LOPE vs. NC |
P-value |
|---|---|---|---|---|---|---|---|
| Maternal age (year) | 30.6 ± 3.7 | 33.0 ± 2.8 | 32.2 ± 4.5 | 0.604 | 0.004 | 0.042 | 0.001 |
| Parity | |||||||
| Multiparous | 146 (22.1) | 4 (21.1) | 6 (10.5) | 0.257 | 1.000 | 0.040 | 0.110 |
| Interpregnancy interval (years) | |||||||
| <5 | 60 (41.1) | 0 (0) | 1 (16.7) | 0.190 | 0.208 | 0.040 | 0.031 |
| 5–10 | 55 (37.7) | 3 (75) | 1 (16.7) | ||||
| ≥10 | 31 (21.2) | 1 (25) | 4 (66.6) | ||||
| Pre-pregnancy BMI (kg/m2) | |||||||
| Underweight (<18.5) | 101 (15.3) | 5 (26.3) | 7 (12.3) | 0.184 | 0.242 | 0.008 | 0.012 |
| Optimum (18.5–24.9) | 494 (74.8) | 11 (57.9) | 42 (73.7) | ||||
| Overweight (25.0–29.9) | 61 (9.2) | 3 (15.8) | 4 (7.0) | ||||
| Obese (≥30.0) | 4 (0.6) | 0 (0) | 4 (7.0) | ||||
| Gestational weight gain | |||||||
| Above recommendations | 377 (57.1) | 8 (42.1) | 18 (31.6) | 0.333 | 0.345 | <0.001 | <0.001 |
| Comply with recommendations | 255 (38.6) | 10 (52.6) | 28 (49.1) | ||||
| Below recommendations | 28 (4.2) | 1 (5.3) | 11 (19.3) | ||||
| Highest level of education | |||||||
| Primary or secondary school | 115 (17.4) | 5 (26.3) | 10 (17.5) | 0.800 | 0.494 | 1.000 | 0.891 |
| University | 498 (75.5) | 13 (68.4) | 43 (75.4) | ||||
| Higher professional education | 47 (7.1) | 1 (5.3) | 4 (7.0) | ||||
| History of autoimmune disease | 13 (2.0) | 2 (10.5) | 0 (0) | 0.060 | 0.063 | 0.614 | 0.058 |
| Gestational diabetes mellitus | 139 (21.1) | 8 (42.1) | 18 (31.6) | 0.416 | 0.043 | 0.065 | 0.021 |
| Family history of hypertension | 100 (15.2) | 6 (31.6) | 17 (29.8) | 1.000 | 0.099 | 0.004 | 0.005 |
| Mode of conception | |||||||
| Spontaneous | 335 (50.8) | 14 (73.7) | 36 (63.2) | >0.05 | >0.05 | >0.05 | 0.033 |
| ART | 325 (49.2) | 5 (26.3) | 21 (36.8) | ||||
| Chorionicity of twin pregnancy | |||||||
| Dichorionic | 155 (23.5) | 4 (21.1) | 11 (19.3) | 1.000 | 1.000 | 0.472 | 0.798 |
| Monochorionic | 505 (76.5) | 15 (78.9) | 46 (80.7) | ||||
| Fetal sex | |||||||
| Identical | 437 (66.2) | 13 (68.4) | 37 (64.9) | 1.000 | 0.841 | 0.842 | 0.979 |
| Aspirin prescribed during pregnancy | 56 (8.5) | 7 (36.8) | 9 (15.8) | 0.100 | 0.001 | 0.065 | <0.001 |
| Corticosteroid treatment for fetal maturation | 267 (40.5) | 18 (94.7) | 31 (54.4) | <0.05 | <0.05 | >0.05 | <0.001 |
| Onset gestational age of PE (weeks) | — | 30.7 ± 2.7 | 35.5 ± 1.0 | <0.001 | — | — | |
| Highest systolic blood pressure (mmHg) | — | 154.4 ± 14.9 | 152.2 ± 9.6 | 0.444 | — | — | |
| Highest diastolic blood pressure (mmHg) | — | 97.0 ± 6.2 | 94.6 ± 6.5 | 0.168 | — | — | |
| Proteinuria (g/24 h) | — | 1.7 ± 1.4 | 1.2 ± 1.1 | 0.108 | — | — |
Note:
Data are presented as mean ± SD or n (%). Abbreviations: NC, normal control; EOPE, early-onset preeclampsia; LOPE, late-onset preeclampsia.
Variations in the levels of RBC, Hb, Hct, CHO, TG, ALB, Cr and ALT were noted among the three study groups. With the exception of ALB levels, no statistically significant differences were found in the other serological markers when comparing EOPE group with the LOPE group (see Table 2). Furthermore, women diagnosed with PE exhibited an increased risk of adverse maternal and neonatal outcomes in comparison to those without PE. When evaluating maternal outcomes, no significant differences emerged between the EOPE and LOPE groups. Additionally, the discrepancies in the risk of neonatal asphyxia or the need for ventilator support can be attributed to variations in the gestational age at delivery (refer to Table S1).
| Serological markers | NC (n = 660) | EOPE (n = 19) | LOPE (n = 57) | LOPE vs. EOPE |
EOPE vs. NC |
LOPE vs. NC |
P-value |
|---|---|---|---|---|---|---|---|
| Red blood cell (1012/L) | 3.62 ± 0.33 | 3.83 ± 0.34 | 3.73 ± 0.34 | 0.795 | 0.039 | 0.143 | 0.008 |
| Platelet (109/L) | 215.52 ± 50.10 | 214.53 ± 39.44 | 228.61 ± 53.59 | — | — | — | 0.336 |
| Hemoglobin (g/L) | 114.00 ± 10.24 | 119.42 ± 14.09 | 117.48 ± 11.05 | 0.765 | 0.066 | 0.049 | 0.007 |
| Hematocrit | 0.34 ± 0.03 | 0.36 ± 0.04 | 0.35 ± 0.03 | 0.676 | 0.033 | 0.030 | 0.002 |
| Cholesterol (mmol/L) | 6.73 ± 1.17 | 7.57 ± 1.37 | 7.06 ± 1.05 | 0.371 | 0.004 | 0.048 | <0.001 |
| Blood triglyceride (mmol/L) | 2.93 ± 1.25 | 3.72 ± 1.65 | 2.95 ± 0.82 | 0.234 | 0.029 | 1.000 | 0.025 |
| Serum albumin (g/L) | 34.38 ± 2.01 | 32.81 ± 2.42 | 34.17 ± 2.03 | 0.031 | 0.005 | 1.000 | 0.006 |
| Creatinine (umol/L) | 49.61 ± 7.55 | 55.05 ± 6.69 | 51.09 ± 7.02 | 0.144 | 0.005 | 0.438 | 0.003 |
| Uric acid/creatinine | 5.17 ± 1.21 | 5.42 ± 1.58 | 4.95 ± 1.06 | — | — | — | 0.312 |
| Alanine aminotransferase (U/L) | 14.41 ± 10.16 | 16.42 ± 9.67 | 15.36 ± 6.75 | 1.000 | 0.534 | 0.020 | 0.012 |
| Lactate dehydrogenase (U/L) | 153.60 ± 22.46 | 160.33 ± 20.17 | 157.72 ± 33.51 | — | — | — | 0.339 |
Note:
Abbreviations: NC, normal control; EOPE, early-onset preeclampsia; LOPE, late-onset preeclampsia.
In the univariate analysis, most variables demonstrated a level of P < 0.1 and were subsequently included in the multivariate logistic regression modeling (Table 3). The following variables were omitted from the analysis: interpregnancy interval, history of autoimmune disease, TG levels, and ALT levels. A multivariable logistic regression analysis revealed significant associations between maternal age, parity, gestational weight gain, family history of hypertension, CHO, ALB, and Cr levels with the risk of PE (P < 0.05). Specifically, for each additional year in maternal age, the odds of developing PE increased by 20.5% (OR 1.205, 95% CI [1.108–1.312]). Women with family history of hypertension exhibited more than a two-fold increase in the risk of PE compared to those who without (OR 2.193, 95% CI [1.194–4.026]). Furthermore, an increase of 1 g/L in serum ALB was linked to a 14.9% decrease in the likelihood of PE, with an OR of 0.851 and a 95% CI of 0.744 to 0.973. Conversely, a rise of 1 umol/L in creatinine was associated with a 5.1% increase in the odds of PE, reflected by an OR of 1.051 and a 95% CI of 1.010 to 1.093.
| Characteristics | Unadjusted OR (95%CI) | P | Adjusted OR (95%CI) | P |
|---|---|---|---|---|
| Maternal age (year) | 1.127 [1.059–1.199] | <0.001 | 1.205 [1.108–1.312] | <0.001 |
| Parity | ||||
| Multiparous | 0.533 [0.268–1.064] | 0.074 | 0.231 [0.094–0.572] | 0.002 |
| Interpregnancy interval (years) | 0.109 | |||
| <5 | Ref | — | — | |
| 5–10 | 4.364 [0.473–40.243] | — | — | |
| ≥10 | 9.677 [1.083–86.501] | — | — | |
| Pre-pregnancy BMI, kg/m2 | 0.023 | 0.381 | ||
| Underweight (<18.5) | Ref | Ref | ||
| Optimum (18.5–24.9) | 0.903 [0.466–1.751] | 0.924 [0.430–1.987] | ||
| Overweight (25.0–29.9) | 0.966 [0.361–2.586] | 0.490 [0.145–1.653] | ||
| Obese (≥30.0) | 8.417 [1.860–38.085] | 3.576 [0.307–41.631] | ||
| Gestational weight gain | <0.001 | <0.001 | ||
| Above recommendations | Ref | Ref | ||
| Comply with recommendations | 0.161 [0.073–0.353] | 0.137 [0.054–0.346] | ||
| Below recommendations | 0.348 [0.163–0.741] | 0.420 [0.174–1.014] | ||
| History of autoimmune disease | 1.345 [0.298–6.077] | 0.700 | — | — |
| Gestational diabetes mellitus | 1.949 [1.171–3.244] | 0.010 | 1.825 [0.959–3.473] | 0.067 |
| Family history of hypertension | 2.430 [1.425–4.144] | 0.001 | 2.193 [1.194–4.026] | 0.011 |
| Mode of conception | 0.014 | 0.705 | ||
| Spontaneous | Ref | Ref | ||
| ART | 1.866 [1.134–3.069] | 0.883 [0.465–1.679] | ||
| Aspirin prescribed | 2.876 [1.554–5.324] | 0.001 | 1.572 [0.753–3.280] | 0.228 |
| Red blood cell (1012/L) | 3.111 [1.529–6.329] | 0.002 | 1.919 [0.424–8.689] | 0.397 |
| Hemoglobin (g/L) | 1.037 [1.013–1.062] | 0.002 | 0.969 [0.882–1.065] | 0.517 |
| Hematocrit * 10 | 4.005 [1.774–9.043] | 0.001 | 5.000 [0.120–208.122] | 0.398 |
| Cholesterol (mmol/L) | 1.392 [1.138–1.704] | 0.001 | 1.392 [1.089–1.779] | 0.008 |
| Blood triglyceride (mmol/L) | 1.118 [0.956–1.307] | 0.164 | — | — |
| Serum albumin (g/L) | 0.876 [0.779–0.984] | 0.026 | 0.851 [0.744–0.973] | 0.018 |
| Creatinine (umol/L) | 1.047 [1.013–1.083] | 0.007 | 1.051 [1.010–1.093] | 0.014 |
| Alanine aminotransferase (U/L) | 1.010 [0.990–1.030] | 0.321 | — | — |
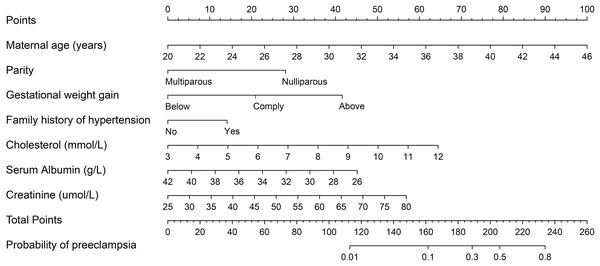
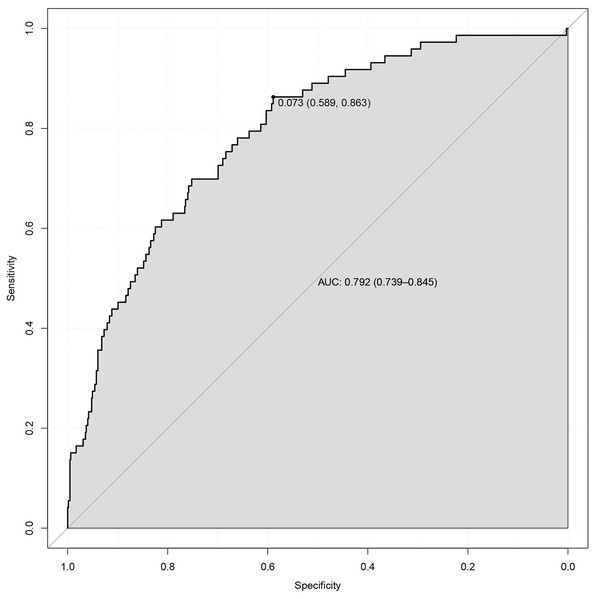
The final logistic model was formulated as a user-friendly nomogram, as illustrated in Fig. 2. The calibration plot for the nomogram, presented in Fig. S1, indicated a strong correlation between the observed and predicted risks of PE, with a mean absolute error of 0.011. The concordance index for the nomogram was calculated to be 0.792 (95% CI [0.739–0.844]), and the corresponding ROC curve along with its Area Under the Curve (AUC) is shown in Fig. 3. Time-event analyses indicated that there were no significant differences in the timing of PE onset among the various subgroups of variables in the multivariate logistic regression model (see Fig. S2).
Figure 2: Nomogram predicting risk of preeclampsia in twin pregnancies.
For a given women, points are assigned to each of the variables using the point axis at the top of the figure and a total score is derived. The total points correspond to a predicted probability of PE.Figure 3: ROC curve for prediction of preeclampsia in twin pregnancies.
The multivariable logistic regression analysis conducted on composite maternal outcomes revealed that only RBC count exhibited a significant association with the risk of adverse maternal outcomes. Specifically, an increase of 1 × 1012/L in RBC count was associated with more than a two-fold increase in the odds of experiencing adverse maternal outcomes (OR 2.247, 95% CI [1.229–4.107], see Table S2). The ROC curve is illustrated in Fig. S3, with an AUC of 0.553 (95% CI [0.492–0.614]). No significant relationships were identified between any of the variables and neonatal outcomes.
Discussion
In this study, the occurrence of PE in twin pregnancies was found to be 10.3%. Women diagnosed with PE demonstrated a greater likelihood of encountering negative maternal and neonatal outcomes. A comparative analysis of EOPE and LOPE revealed no significant differences in maternal outcomes. The levels of CHO, ALB, and Cr were found to be associated with the risk of developing PE. When these biomarkers were integrated with maternal characteristics and medical history, the logistic regression model achieved an approximate detection rate of 80% for PE. Furthermore, women exhibiting elevated RBC counts were at an increased risk of adverse maternal outcomes; however, the predictive value of this marker was relatively low.
In a study conducted by Liu et al. (2023), the prevalence of hypertensive disorders during pregnancy in twin pregnancies was reported to be 9.2%, which closely resembled the incidence of PE in our study. We observed that women who developed PE had a higher incidence of gestational diabetes mellitus, which was consistent with the findings of Liu et al. (2023). Wu et al. (2010) investigated the risk factors for adverse pregnancy outcomes in women with early onset severe PE and determined that a higher RBC count was linked to negative pregnancy outcomes. Their findings align with ours, indicating that increased RBC counts may serve as a predictor for the occurrence of adverse outcomes.
The predictive capacity of our logistic regression model was found to be on par with contemporary prediction models (Xiang et al., 2022, 2023). In contrast to prior research, our investigation encompassed over 700 twin pregnancies, significantly surpassing the sample sizes of previous studies. Our model utilized commonly assessed serological markers that are routinely measured during clinical prenatal examinations, thereby eliminating the necessity for additional testing to assess the risk of PE. From an economic perspective, our model demonstrates enhanced feasibility and superior cost-effectiveness. It is important to recognize that the prediction of PE is ideally conducted during the early gestational weeks, which facilitates the preventive administration of aspirin (American College of Obstetricians and Gynecologists, 2020). However, in instances where early prediction is unattainable, or where certain women may decline further testing, our model has the capability to identify those at elevated risk for PE who may benefit from increased monitoring.
Cr is a key indicator of renal function, providing a direct reflection of kidney health. Previous research has established a strong association between Cr levels and an increased risk of hypertension (Chen et al., 2023; Almobarak et al., 2020). The present investigation identified an increased risk of PE among individuals exhibiting elevated Cr levels, corroborating the findings of previous research. A recent study indicated that the serum uric acid to serum creatinine (UA/Cr) ratio is linked to adverse pregnancy outcomes and the onset of PE (Piani et al., 2023; Zhang et al., 2024). This ratio effectively distinguished between subgroups of hyperuricemic patients, thereby facilitating the screening of women at high risk for PE (Furuhashi, 2020). To date, there have been no studies that have specifically examined this ratio within a cohort of twin pregnancies. In our study, we observed no significant correlation between the UA/Cr ratio and the risk of developing PE. We hypothesize that in women who ultimately develop PE, the UA/Cr ratio during the second trimester may not have been influenced for an extended period prior to the onset of PE. Similarly, in their investigation, Piani et al. (2023) found that a higher UA/Cr ratio in the second trimester did not correlate with an increased likelihood of PE development.
An increasing body of literature has emphasized the connection between maternal and fetal hemodynamics, as well as the association between the maternal cardiovascular profile and adverse outcomes for both the mother and fetus, including fetal growth restriction and hypertensive disorders during pregnancy (Ferrazzi et al., 2017; Di Martino et al., 2023). It is posited that changes in maternal serological markers are indicative of a hemodilution state, which plays a critical role in the regulation of maternal hemodynamics. In the context of PE, glomerular endotheliosis leads to renal impairment due to the damage of the endothelium and injury to podocytes. This condition is characterized by a reduction in the glomerular filtration rate, the presence of protein in the urine, and an elevation in serum Cr levels (Cornelis et al., 2011; Taşkömür & Erten, 2021).
The current study had several strengths. Firstly, we included a larger number of relevant confounding factors in our multivariable logistic regressions compared to previous similar studies, which increased the reliability of our findings. Secondly, we gathered data on laboratory results during the same gestational period, allowing us to explore the relationship between maternal serum biomarkers and pregnancy outcomes. However, there are some limitations to consider in our study. Firstly, the research was carried out at a single specialized hospital in China, which may limit the generalizability of our results to other populations. Secondly, the limited sample size of participants in our study precluded the possibility of effectively predicting EOPE. Furthermore, considering the timing of blood sample collection during gestation, predicting adverse events may prove to be more beneficial than merely diagnosing PE itself. However, we were unable to identify significant biomarkers that could facilitate the prediction of pregnancy-related complications.
While aspirin was shown to be effective in preventing PE in twin pregnancies, the level of evidence was deemed to be low (D’Antonio et al., 2022). A study conducted in a real-world setting involving 2,946 twin pregnancies found that prescribing aspirin at a dosage of 75 to 100 mg did not lead to a significant reduction in PE incidence (Zhou et al., 2023). Therefore, early identification of pregnant women at high risk of developing PE was considered crucial in order to improve pregnancy outcomes. Our study presented precise evidence supporting the use of routine blood tests to identify high-risk women in the second trimester, enabling more rigorous monitoring in clinical practice.
Conclusions
In this study, we developed a straightforward predictive model that exhibits relatively high detection rates by incorporating maternal serum biomarker levels alongside maternal characteristics and medical history. Our findings indicate that the probability of adverse maternal outcomes increases in correlation with elevated levels of RBC. Obstetricians are advised to enhance monitoring for these individuals in their clinical practice.
Supplemental Information
Time-event analyses of the timing of PE onset among the various subgroups of variables in the multivariate logistic regression model.
Secondary outcomes of the participants.
Data are presented as n (%). Abbreviations: NC, Normal control; EOPE, early-onset preeclampsia; LOPE, late-onset preeclampsia.