Study on the effect of chlorogenic acid on the antimicrobial effect, physical properties and model accuracy of alginate impression materials
- Published
- Accepted
- Received
- Academic Editor
- Nagendran Tharmalingam
- Subject Areas
- Bioengineering, Microbiology, Dentistry, Drugs and Devices
- Keywords
- Chlorogenic acid, Alginate oral impression, Disinfection effect, Physical property, Dimensional accuracy
- Copyright
- © 2024 Jiang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Study on the effect of chlorogenic acid on the antimicrobial effect, physical properties and model accuracy of alginate impression materials. PeerJ 12:e18228 https://doi.org/10.7717/peerj.18228
Abstract
Background
Dental impressions are essential for accurately capturing the detailed anatomy of teeth and surrounding oral structures. However, these impressions often become contaminated with saliva and blood, making proper disinfection necessary. The application of chemical disinfectants has been associated with negative side effects, leading to suboptimal disinfection practices in clinical settings.
Objective
The purpose of this study was to evaluate the effectiveness of chlorogenic acid (CA) as a disinfectant for alginate impression materials, the impact of CA disinfection on the physical properties and dimensional accuracy of alginate impressions was also investigated.
Methods
The physical properties of alginate impression materials, such as elastic recovery, strain-in-compression, initial setting time, and fluidity, were assessed after mixing the alginate impression materials with three different concentrations of CA solution (10 mg/mL, 15 mg/mL, 20 mg/mL). To evaluate the antimicrobial effect of CA, alginate impressions mixed with a 10 mg/mL CA solution and impressions mixed with distilled water (control group) were contaminated with four types of microorganism: Escherichia coli, Staphylococcus aureus, Candida albicans, and Streptococcus pneumoniae. Following a five-minute incubation period, a CA solution at a concentration of either 50 mg/mL, 55 mg/mL, or 60 mg/mL was sprayed on the samples for disinfection. Samples were collected at different time intervals (10 min, 20 min, 30 min) and cultured to determine the number of colony-forming units (CFU/mL), providing insight into the antimicrobial efficacy of these CA solutions. The dimensional accuracy of alginate impressions was assessed in three groups: one with alginate impressions mixed with distilled water, another with alginate impressions sterilized with available chlorine (2,000 mg/L) mixed with distilled water, and the last group consisting of alginate impressions mixed with 10 mg/mL CA solution and sprayed with 60 mg/mL CA solution. Both the standard model and the plaster model underwent 3D scanning, and the data were processed and compared by software. The root mean square (RMS) was used as a parameter to evaluate the deviation between models.
Results
All alginate impression materials mixed with either 10 mg/mL, 15 mg/mL, or 20 mg/mL concentrations of CA solution met the ISO 21563 standard for elastic recovery, strain-in-compression, and fluidity. However, only the material mixed with a concentration of 10 mg/mL CA had an initial setting time within the range specified by the T-6505 Japanese industrial standard. The application of CA solution by mixing or spraying showed significant antimicrobial effects on Staphylococcus aureus, Escherichia coli, Candida albicans, and Streptococcus pneumoniae. There was no significant difference in the dimensional accuracy of the alginate impressions between the group of the CA solution applied, the blank group, or the chlorine intervention group.
Introduction
Taking impressions is a basic, important step for dental prosthesis and orthodontic treatment (Punj, Bompolaki & Garaicoa, 2017). Alginate impression material is a type of irreversible elastic impression material with good fluidity, elasticity, plasticity, and dimensional accuracy. It has become the most widely-used impression material because of its low price and ease of use (Cervino et al., 2018). During impression making, dental impression materials invariably come in contact with the patient’s saliva and blood, which can contain potentially pathogenic microorganisms such as Streptococcus, Staphylococcus, Escherichia coli, Mycobacterium tuberculosis, in addition to hepatitis B and C viruses, human immunodeficiency virus (HIV), and herpes simplex virus (Chidambaranathan & Balasubramanium, 2019; Flanagan et al., 1998). Until 1991, the recommended disinfection method was simply rinsing the dental impressions under running tap water (Oosthuysen, Potgieter & Fossey, 2014). However, this method only eliminates about 40% of microorganisms, leaving a significant risk of infection (McNeill, Coulter & Hussey, 1992). Proper disinfection protocols are necessary to prevent cross-infection between clinicians, patients, and laboratory personnel (Ganavadiya et al., 2014). Recognizing this, the American Dental Association (ADA) and the Centers for Disease Control (CDC) now recommend immediate disinfection of impression materials after removal from the mouth to mitigate the spread of infectious diseases (Aeran et al., 2015).
Currently, there is no universally accepted “gold standard” method for disinfecting dental impressions (Cervino et al., 2018). Due to the nature of alginate, sterilization by heat is not feasible, leaving cold chemical disinfection as the only available option. Immersing or spraying impressions with chemical disinfectants is commonly practiced in clinical settings, and studies have demonstrated that disinfectants can effectively reduce the microbial count on alginate impressions (Al-Enazi & Naik, 2016). However, immersion disinfection may result in dimensional changes (Guiraldo et al., 2012; Iwasaki et al., 2016) and affect the accuracy (Ud Din et al., 2022) of the dental cast prosthesis due to alginate imbibition and synergistic effects. To address this issue, spray disinfection using chemical disinfectants has gained popularity for disinfecting alginate impression materials. Commonly used chemical disinfectants include chlorhexidine, alcohol, glutaraldehyde, and sodium hypochlorite (Lagla Abata et al., 2024; Qiu et al., 2023). However, the aerosols generated during the spray disinfection process can release harmful substances into the air, potentially causing irritation and occupational exposure (Slaughter et al., 2019). Long-term use of bleach has been linked to non-allergic adult-onset asthma symptoms in some individuals (Matulonga et al., 2016). Extensive or prolonged exposure to chemical disinfectants can cause skin irritation, hypersensitivity, and damage (Chia Shi Zhe et al., 2016). Moreover, the by-products of chemical disinfection are harmful to the environment (Li et al., 2023), and these disinfectants can corrode metal trays (Nagamatsu et al., 2016). Therefore, a disinfectant is needed that can effectively eliminate microorganisms on dental impressions without compromising their physical properties and dimensional accuracy while also ensuring occupational safety.
In recent years, there has been a growing focus on the identification and development of new medicines derived from natural products. Many studies have reported that a significant number of drugs in the development stage are extracted from natural sources, with approximately 80% originating from plants (Coy-Barrera, Ogungbe & Schmidt, 2023; Liang, Luo & Luesch, 2019). This search for natural medicines not only promotes human health but also supports economic development and habitat conservation.
This study explores the use of pure, natural extract compounds as a disinfectant for alginate impression material (Wu et al., 2014), based on previous research efforts. This study specifically investigates chlorogenic acid (CA), a phenolic compound belonging to the hydroxycinnamic acid family (Naveed et al., 2018). CA is commonly found in various foods and medicines, including traditional Chinese herbal medicines, like “honeysuckle” (Santana-Gálvez, Cisneros-Zevallos & Jacobo-Velázquez, 2017). CA possesses a broad range of biological activities, such as antioxidant, anti-inflammatory, and blood pressure-lowering effects (Gupta et al., 2022; Tajik et al., 2017), making it a safe naturally-derived compound. Recent studies have demonstrated that CA has potential as an effective antimicrobial agent by directly targeting bacterial cell wall and cell membranes to cause irreversible osmotic damage (Liu et al., 2019; Lou et al., 2011; Lu et al., 2020). It has shown promising results as an anti-inflammatory agent in different chronic and acute inflammatory conditions, such as mastitis (Feng et al., 2023) and osteoarthritis (Liu et al., 2017). CA plays a therapeutic role in periodontal inflammatory diseases by influencing proteasome activity and the growth of Porphyromonas gingivalis (Tsou et al., 2019). CA has also been used for disinfection purposes in polydimethylsiloxane microfluidic devices (Ren et al., 2015), showing superior antimicrobial effects against both gram-positive and gram-negative bacteria compared to gentamicin. A recent study on the dental unit waterlines demonstrated CA’s significant inhibitory effect on opportunistic pathogens such as Mycobacterium (Li et al., 2024). Another previous study demonstrated CA’s antioxidant and anti-diabetes benefits in humans, and found that CA would not harm the human body (Faria et al., 2020).
Alginate impression material is a special functional material that replicates the complex oral environment. Alginate impression material disinfectants must have good antimicrobial efficacy without affecting the physical properties of the impression material or the accuracy of the gypsum model. If the disinfectant changes the physical properties of the material, affecting the accuracy of the final restoration system, then the disinfectant’s antimicrobial properties are meaningless. Therefore, any study of disinfectants for impression materials must assess the physical properties of impression materials and the accuracy of the plaster model after use. In this study, four physical properties—elastic recovery, compressive strain, initial setting time, and fluidity—were selected for assessment, according to the ISO 21563 standard (Standardization, 2021) and the American National Standards Institute (ANSI)/ADA specification No. 18 for irreversible hydrocolloid impression material (Dental Materials, 1992). Gram-positive bacteria, gram-negative bacteria, and fungi were used to evaluate the antimicrobial effect of CA on different microorganisms. CA has not been used for the disinfection of any hydrocolloid impression material in dentistry prior to this study. This study provides valuable insights on CA as a safe and effective alginate impression disinfectant, and as a traditional Chinese medicine impression disinfectant.
Materials & Methods
Ethical approval and informed consent were not required for this study since it did not involve human subjects. This study performed in vitro laboratory testing of dental alginate impression material (V514450, Dentsply, Charlotte, NC, USA). To prepare the experimental materials, a CA solution (CJS 20220620, XI’AN FOREVIEW Bio-tech Co., Ltd., China), at concentrations of 10 mg/mL, 15 mg/mL, and 20 mg/mL, along with dental alginate impression powder, was mixed into a uniform paste using an automatic alginate mixer (GX300, Xianyang Holy Medical Co, Ltd, Shaanxi, China). The liquid to powder ratio used was 23 mL:10 g, as specified in the instructions of the dental alginate impression material. The prepared paste material was immediately used for subsequent experiments.
Physical properties: elastic recovery and strain-in-compression
To evaluate the elastic recovery and strain-in-compression, cylindrical samples were prepared using a split-mold specimen with fixation ring following the ISO 21563 standard (Standardization, 2021). For this procedure, the mold was placed on a glass plate and the impression mixture was poured into the mold. The glass plate with the mold was then placed in a constant temperature water bath at 35 °C for two minutes and 30 s to ensure proper setting of the alginate material. The final alginate specimens had a diameter of 12.5 mm and a height of 20 mm.
Immediately after the specimen was separated from the forming assembly, it was placed on the base of the well-tuned universal material testing instrument (AGS-10KN, SHIMADZU, Kyoto, Japan). The initial height of the sample (h_1) was then recorded. To assess elastic recovery, a deforming force was gradually released over a five-second period. After the deformation force was completely removed from the specimens, the sample height (h_2) was measured again after 40 s.
The elastic recovery, represented as a percentage (K), was evaluated by calculating the change in sample height before and after the deformation force, using the following Eq. (1): (1)
Here, h_0 denotes the measured height (in mm) of the split mold used in the experiment.
To evaluate strain-in-compression, five samples per material were tested. An initial force of 1.2 ± 0.1 N was applied to the specimen, and the sample height was recorded after 30 s (h_1). The pressure was then increased to 12.2 ± 0.1 N for 10 s, and the sample height (h_2) was recorded again after 30 s under the total load. The percentage of strain-in-compression, E, was calculated by measuring the change in sample height before and after the application of pressure, using the following Eq. (2): (2) Here, h_0 represents the height (in mm) of the split mold used for preparing the samples.
Initial setting time
The initial setting time test involved the preparation of cylindrical samples measuring 16 mm in length and 30 mm in diameter using a rigid ring mold, in accordance with the ISO 21563 standard (Standardization, 2021). The mold was placed on a flat glass plate measuring 50 mm by 50 mm, and the prepared impression mixture material was injected into the mold.
To determine the initial setting time, one end of the test rod (measuring 100 mm in length and 6 mm in diameter) was briefly brought into contact with the unset specimen material. Any excess material on the rod was carefully removed before repeating the contact/withdrawal and rod clearing steps at intervals of 10 s. This process was repeated until the test rod cleanly separated from the material, and the time at which this separation occurred was recorded as the initial setting time.
Fluidity
The experimental method used for testing the fluidity of the material followed the guidelines set by the ANSI/ADA Specification No. 18 for irreversible hydrocolloid impression material (Dental Materials, 1992). To begin the test, an open syringe was used to quickly measure and dispense 0.5 mL of the impression mixture into the center of a clean glass plate. After mixing the material for 60 s, another clean glass plate was gently placed on top of the mixture with a vertical light pressure of 1.5 kg. After applying the pressure for five seconds, the load was released, and the diameter of the solidified specimen was measured at five different locations using a digital vernier caliper. The average of these measurements was then taken as the measurement value for the fluidity of the impression material mix.
Antimicrobial effect
Based on the results obtained from the physical performance test experiment, a solution of 10 mg/mL CA was selected as an autologous disinfectant for the alginate printing material. Four pathogenic bacteria, namely Escherichia coli ATCC 25922 (provided by the Oral Biology Laboratory of the Key Laboratory of the Ministry of Education), Staphylococcus aureus ATCC 6538, Candida albicans ATCC 10231, and Streptococcus pneumoniae ATCC 49619 (all provided by the Jiangxi Clinical Laboratory Center), were cultured, isolated, and chosen for further testing.
For the experiment, Escherichia coli and Staphylococcus aureus were inoculated on nutrient agar medium, with Streptococcus pneumoniae cultured on blood agar medium and Candida albicans cultured in Sabouraud’s agar medium. Bacterial suspensions were prepared at a concentration of 107 cfu/mL. Standard dentition model (Nobel BIOCARE, Sweden) was disinfected with ultraviolet lamp (ZYW-170Z, Zhong Yi, China) for 1 h before impression preparation. The alginate material was mixed with the CA solution (10 mg/mL) to prepare a total of 244 pairs of impressions on a standardized maxillary dentition model, four disinfection methods, four bacteria, three time periods, five replicates per group, required a total of 240 CA-mixed impressions, and four blank impressions are required to observe the effect of bacterial contamination. An additional 64 pairs of impressions were prepared using distilled water, four strains, three time periods, five replicates per group, required a total of 60 impressions mixed with distilled water, also required four blank impressions. Resulting in a total of 308 pairs.
The impressions were divided into four groups based on the four different types of bacteria, and 300 µL of bacterial suspension was inoculated onto the surface of each impression in a clean laboratory setting. After five minutes of bacterial inoculation, the impressions mixed with CA solutions were sprayed with distilled water, then with a CA solution at a concentration of either 50 mg/mL, 55 mg/mL, or 60 mg/mL. The impressions mixed with distilled water were sprayed with a 60 mg/mL CA solution. Each impression was sprayed with 40 mL of solution. The remaining six pairs (three pairs of distilled water mix and three pairs of CA mix) were not disinfected, and only samples were taken to observe the effect of infection. At 10 min, 20 min, and 30 min, sterile cotton swabs were used to sample the same area of each impression with consistent applied force. The sampled cotton swabs were collected in test tubes containing five mL of sterile phosphate-buffered saline (PBS) under sterile conditions and vigorously shaken using an IKA MS3 Basic Vortex (IKA, STAUFEN, Germany) for three minutes. Following this, 200 µL of the PBS containing the sampled material was inoculated onto the appropriate agar plates (blood agar for Streptococcus pneumoniae and Sabouraud’s agar for Candida albicans). The plates were then incubated at 36 ± 1 °C for 48 h to allow for the recovery of any contaminating microorganisms. In the case of Streptococcus pneumoniae, incubation occurred in a 36 ± 1 °C carbon dioxide incubator for 48 h. Lastly, the colony-forming units (CFU) on each plate were counted and recorded.
Dimensional accuracy
Based on the experimental results obtained from the physical property detection and bacteriostatic effect detection, a 10 mg/mL CA solution was chosen as an appropriate self-disinfectant for the alginate impression material, while a 60 mg/mL CA solution was selected as the spray disinfectant. For this experiment, a total of 36 pairs of impressions were prepared: 24 pairs using distilled water and 12 pairs using a 10 mg/mL CA solution. The impressions prepared with CA solutions were further sterilized by spraying them with a 60 mg/mL CA solution. Another 12 pairs of impressions were made with distilled water and underwent no further treatment, while the remaining 12 pairs were sterilized by spraying them with a disinfectant containing 2,000 mg/L of available chlorine. The impressions were then poured with gypsum product (E.50102, HERAEUS, China) after 30 min of disinfection, and the molds were released after two hours. The standard model was first scanned using the SHINING3D dental desktop scanner (AutoScan-DS-EX Pro(H), SHINING 3D, China). Subsequently, the plaster models were scanned in 3D and the digital models were output in standard triangular language (STL) format, with each one being named and saved. Geomagic Quality 13.0 software was used for data analysis, digital model trimming, and preservation of dental morphological data. The digitized superanhydrite models were compared with the standard model using the “best fitting alignment” algorithm based on dental surface and subsequently assessed in a 3D comparison. The root mean square (RMS) was employed as a parameter to measure the deviation between the gypsum model and the standard model, while a 3D chromatogram of the deviation was generated and saved for visual analysis.
Statistical analysis
A statistical analysis of the obtained results was conducted using GraphPad Prism 9.1.2 software (GraphPad Software, San Diego, CA, USA). A minimum of n ≥ 5 measurements were performed for each group, and the results were presented as mean ± standard deviation. Parametric one-way ANOVA with post hoc Tukey HSD test or nonparametric Kruskal–Wallis with post hoc Dunn’s test was used to assess differences between the disinfection methods and the non-disinfected control. Statistical significance was determined at p < 0.05. For the comparison of colony counts at different time points among the five groups, a repeated measures ANOVA analysis was performed using SPSS software. Mean results for each procedure were compared using pairwise tests, with a post hoc Bonferroni adjustment for multiple comparisons at a confidence level of 95%.
Results
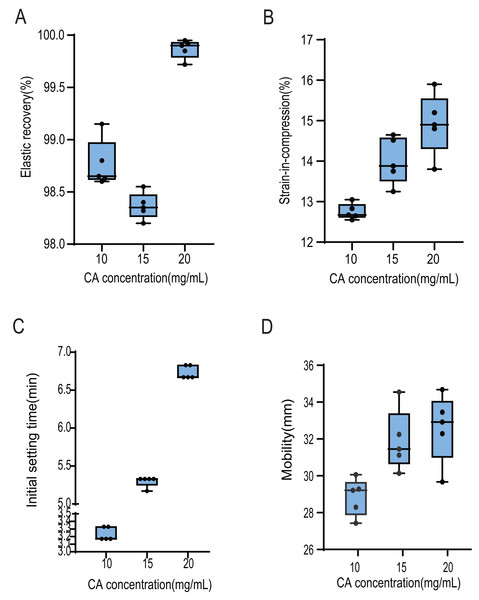
Figure 1 and Table 1 present the physical properties of alginate impression materials mixed with a 10 mg/mL, 15 mg/mL, or 20 mg/mL CA solution. The concentration of CA solution used in the mixing process significantly impacted the deformation elastic recovery and strain-in-compression of the alginate dental impression material (P < 0.01). However, all measurements fell within the standard range of ISO 21563 (Standardization, 2021) for alginate impression materials. The initial setting time of alginate impressions mixed with different CA solution concentrations also differed significantly (P < 0.01). Only the impressions mixed with 10 mg/mL CA solution fell within the initial setting time range of 1–5 min specified by the T-6505 Japanese Industrial Standard (Kitamura & Kawai, 2015). The fluidity of the oral model was affected by the various CA solution concentrations (P < 0.01), but all three materials complied with the ANSI/ADA Specification No. 18-1992 (Dental Materials, 1992) regarding the fluidity of alginate impression materials.
Figure 1: Effect of alginate impression material mixed with three chlorogenic acid liquids of different concentrations on its physical properties.
(A) The elastic recovery of alginate impression material changes with the concentration of chlorogenic acid. (B) The strain-in-compression of alginate impression material increases with the increase of chlorogenic acid concentration. (C) The initial setting time of alginate impression material becomes longer with the increase of chlorogenic acid concentration. (D) The mobility of alginate impression material is enhanced with the increase of chlorogenic acid concentration.| Chlorogenic acid concentration (mg/mL) | F (P-value) | |||
|---|---|---|---|---|
| 10 | 15 | 20 | ||
| Elastic recovery (%) | 98.76 ±0.23 | 98.36 ±0.13 | 99.86 ±0.09 | 119.00 (P < 0.01) |
| Strain-in-compression (%) | 12.75 ±0.20 | 14.01 ±0.58 | 14.92 ±0.76 | 18.79 (P < 0.01) |
| Initial setting time (min) | 3.23 ±0.088 | 5.29 ±0.071 | 6.73 ±0.088 | 2,267.11 (P < 0.01) |
| Mobility (min) | 28.86 ± 1.01 | 31.90 ± 1.66 | 32.60 ± 1.86 | 8.21 (P < 0.01) |
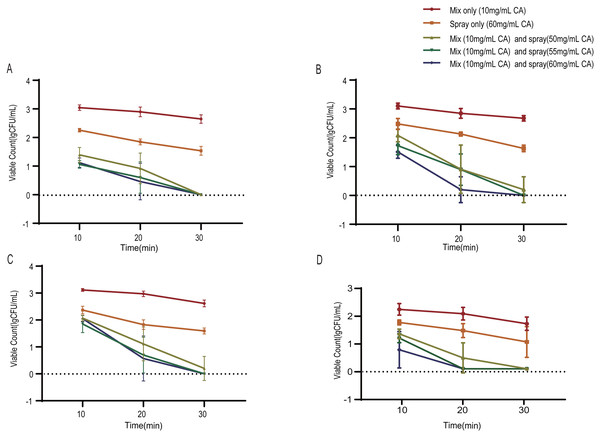
Figure 2 illustrates the changes in viable microorganism populations on impressions during CA intervention for up to 30 min. Values are presented as means ± SD (n = 5). Analysis of the repeated measurements indicated that different intervention methods had varying disinfection effects on the four types of bacteria (P < 0.01), with the number of viable bacteria decreasing as the intervention time increased. After 30 min, only those receiving disinfection through mixing a CA solution with the impression material (10 mg/mL) or through spray disinfection with only a CA solution (60 mg/mL) had high colony counts for Staphylococcus aureus, Escherichia coli, Candida albicans, and Streptococcus pneumoniae. However, when self-disinfection with a CA solution was combined with spray disinfection, there was a significant reduction in colony count for all four bacteria (P < 0.01).
Figure 2: Antimicrobial effect of chlorogenic acid solution intervention on alginate impression materials.
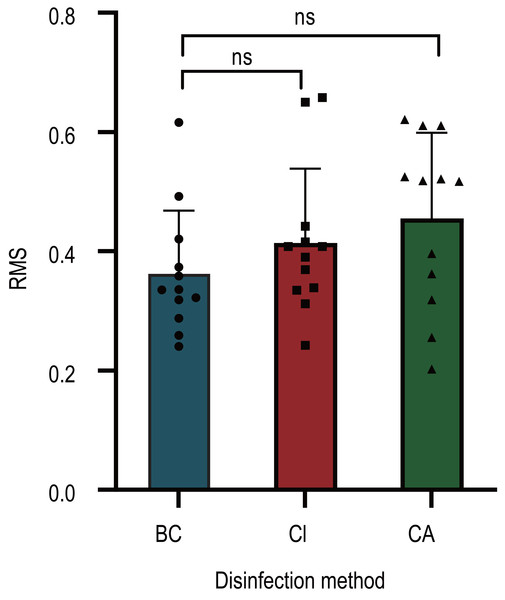
(A) Staphylococcus aureus (B) Escherichia coli (C) Candida albicans (D) Streptococcus pneumoniae. The error bar represents the standard deviation; Dashed lines indicate detection limits.The dimensional accuracy test results for three different oral impression methods are displayed in Fig. 3. These methods include pure distilled water mixing, disinfection with 2,000 mg/L available chlorine after distilled water mixing, and spray disinfection with 60 mg/mL CA solution after mixing with a 10mg/mL CA solution. When comparing these three alginate dental impression processing methods, there was no statistically significant difference in the impact of available chlorine spray disinfection and CA solution treatment on the dimensional accuracy of dental impressions (P > 0.05).
Figure 3: Effect of chlorogenic acid intervention on the precision of gypsum injection of alginate impression material.
The deviation between the plaster model and the standard model of different intervention methods is used as the parameter of root mean square (RMS) after fitting overlap. (BC, Blank control: The impression was prepared by mixing distilled water without any other treatment. CL, Available chlorine intervention group: The impression was prepared by mixing distilled water, 2,000 mg/L available chlorine spray disinfection. CA, Chlorogenic acid intervention group: The impression was prepared by mixing 10 mg/L chlorogenic acid, 60 mg/L chlorogenic acid spray disinfection).Discussion
This study investigated the potential use of CA, a natural substance renowned for its broad-spectrum antimicrobial properties, in the disinfection of alginate dental impression materials. The effect of CA disinfection was comprehensively verified from the following three aspects: physical properties, antimicrobial effect, and influence on the accuracy of gypsum model. The study yielded three significant findings. Firstly, the elastic recovery, compressive strain, fluidity, and initial setting time of alginate impression material mixed with 10 mg/mL CA were all within the standard range. Secondly, this study demonstrated that CA had a significant bactericidal effect on the microorganisms on the surface of alginate-stained bacterial impressions, and thirty minutes after mixing and spraying disinfection, no viable bacteria was detected on the four bacteria-stained alginate impressions. Lastly, there was no statistically significant difference between the CA intervention group, the traditional chemical disinfectant intervention group, and the distilled water mixing group on the accuracy of the plaster model made by the impression. These research findings provide guidance for the clinical application of alginate impression disinfection and establish the groundwork for the potential use of CA as a disinfectant for alginate impressions.
In our previous study on the disinfection effect of CA sprayed on silicone rubber impressions, CA was found to have a significant disinfection effect on pathogenic bacteria. However, another study found that after the same disinfection procedure, the microbial load on the surface of alginate impressions was higher than that of silicone rubber impressions (Divya Dharshini, Somasundaram & Muralidhara, 2020; Demajo et al., 2016). This may be because the water absorption properties of alginate materials result in a lower amount of disinfectant remaining on the impression’s surface after spraying (Ginjupalli et al., 2018), making disinfection procedures for alginate impressions more demanding than those for other types of impressions. In recent years, many scholars have studied the self-disinfection method of adding bacteriostatic agents directly to alginate impression materials (Ginjupalli et al., 2016; Hussian & Jassim, 2015). Self-disinfection not only ensures the disinfection of the entire impression material but also prevents the infiltration of alginate caused by prolonged immersion disinfection (Ahmed, Jawad & Nahidh, 2020). Self-disinfection also allows for long-term contact of the disinfectant with microorganisms. This paper studied CA as a self-disinfectant for alginate impressions because of its anti-inflammatory, antimicrobial, safe, and pollution-free properties.
The complexity of the oral structure requires impression materials to have corresponding properties, including good elasticity, plasticity, fluidity, and appropriate solidification time, which are reflected in the physical properties of elastic recovery, strain-in-compression, fluidity, and initial setting time (Dilip, Gupta & Geiger, 2023). The fluidity of alginate impression materials mixed with 10 mg/mL, 15 mg/mL, 20 mg/mL, 25 mg/mL, or 30 mg/mL CA solutions was explored in pre-experiments, which showed that alginate impression material mixed with more than 20 mg/mL CA was too mobile to meet the required standards. Based on this finding, this study explored the physical properties of alginate impression materials mixed with CA concentrations of 10 mg/mL, 15 mg/mL, and 20 mg/mL.
The elastic recovery of impression materials is crucial for achieving an accurate reproduction of the oral cavity. Elasticity refers to the ability of the material to recover after solidification, ensuring that the impression does not undergo permanent deformation, affecting dimensional accuracy, upon removal from the mouth (Wezgowiec et al., 2022). ISO 21563 (Standardization, 2021) specifies a minimum elastic recovery value of 95% for alginate impression materials.
Strain-in-compression is an important indicator that assesses the flexibility or rigidity of a material. It provides insights into several aspects of the impression, such as if it can be easily removed from the mouth without causing damage to oral tissues and if the cured plaster model can be successfully removed from the impression without any breakage. Strain-in-compression also evaluates the rigidity of the impression’s weak peripheral areas, ensuring they can withstand the pressure exerted during mold creation without deforming. According to ISO 21563 (Standardization, 2021) standards, the strain-in-compression of alginate impression materials should range between 5% and 20%. In this study, varying concentrations of CA solutions led to statistically significant differences in the elastic recovery and strain-in-compression of the mixed alginate impression materials (P < 0.01). This finding aligns with the studies conducted by Singer & Bourauel, (2023) and Dreesen et al. (2013), who found that modifications in filler content and mixing techniques can result in changes to the properties of elastic recovery and strain-in-compression. The research results of this study show that the elastic recovery, strain-in-compression, and average values of alginate dental impressions prepared using different concentrations of CA solution were all within the standard range.
The initial setting time of alginate impression materials refers to the time required from the start of mixing until the material attains the necessary elasticity and strength for separating and removing the impression. Initial setting time serves as an indicator of the curing speed of dental impression materials (Ramer, Gerhardt & McNally, 1993). An excessively long initial setting time can cause discomfort to the patient, while an overly short initial setting time may not provide enough time for the dentist to perform the procedure (Cervino et al., 2018). The T-6505 Japanese Industrial Standard (Kitamura & Kawai, 2015) specifies an initial setting time range of 1–5 min for alginate impression materials. In this experiment, only the alginate dental impressions mixed with 10 mg/mL CA solution had an initial setting time that fell within the 1–5-minute range. There was a positive correlation between the primary coagulation time and the concentration of CA solutions (P < 0.01). The solidification time of alginate material is determined by how fast the replacement reaction between calcium sulfate and sodium alginate occurs after mixing with water. Factors affecting the initial coagulation time include the amount of retardant used, the ratio of alginate to binder, and temperature (Cervino et al., 2018). The initial setting time of alginate material is greatly affected by CA concentration, which may be due to the interaction between calcium alginate and CA (Demircan & Oral, 2023).
It is essential for the fluidity of the impression material to fall within a specific range. According to ANSI/ADA Specification No. 18-1992 (Dental Materials, 1992), the average diameter of the solidification test piece for alginate impression materials should be between 27 mm and 36 mm. In this study, the concentration of the CA solution affected the fluidity of the alginate impression material (P < 0.01), with the fluidity of the impression material increasing alongside CA concentration. The average diameter of the solidification plate for CA self-disinfecting alginate impression materials ranged from 28.86 mm to 32.60 mm, thus meeting the relevant standards.
Based on the results of the experiment on physical properties, a 10 mg/mL CA solution was chosen as a suitable self-disinfecting solution for alginate impression materials. Previous studies have demonstrated the effectiveness of CA spraying at concentrations of 100 mg/mL, 150 mg/mL, and 200 mg/mL for disinfecting silicone rubber stain impressions. However, since this study focused on mixing disinfection, CA concentrations of 50 mg/mL, 55 mg/mL, and 60 mg/mL were chosen for spray disinfection. In the experimental group, the CA solution mixing protocol was combined with spray disinfection of infected impressions with 50 mg/mL, 55 mg/mL, or 60 mg/mL CA solutions. The control groups consisted of only a 10 mg/mL CA solution for mixing disinfection and only a 60 mg/mL CA solution for spraying disinfection. Maciel et al. (2023) performed an experiment using the inhibition zone method to measure the antimicrobial effect of a 2% chlorhexidine solution as an impression disinfectant. However, this method only demonstrated the killing effect of the disinfectant on the corresponding bacteria without providing insight into the disinfection effect of the procedure on the impression’s surface. In this experiment, contaminated impressions were disinfected and sampled, thereby maximizing the assessment of the disinfectant’s antimicrobial effect on the corresponding bacteria under clinical disinfection procedures.
In recent years, the antimicrobial effect of CA has been confirmed by many researchers. This effect occurs mainly through direct targeting of the bacterial cell wall and membrane, leading to irreversible permeability damage (Feng et al., 2023; Liu et al., 2019). The results of this experiment also showed the powerful antimicrobial effect of CA. As intervention time increased, the colony count of each group decreased significantly. The growth of Escherichia coli, Staphylococcus aureus and Candida albicans on the impression surface was completely inhibited after 30 min of intervention in the experimental group. Escherichia coli, is a gram-negative bacterium, which can cause gastrointestinal infection or urinary tract infection and impact other local tissues and organs of humans and animals under certain conditions (Yang, Lan & Xie, 2022). Staphylococcus aureus, a gram-positive coccus, can cause a variety of diseases including skin infection, respiratory infection, and bacteremia (Ahmad-Mansour et al., 2021; Plumet et al., 2022) and is one of the main causes of nosocomial infections (Konstantinovski et al., 2021). Candida albicans is an opportunistic pathogenic fungus, which can cause oral fungal infections in people with low immunity (Eichelberger et al., 2023) and is also an indicator strain for killing fungi in laboratory tests. It has been reported that pathogens such as Candida albicans and Staphylococcus aureus can still be detected on oral impressions and plaster models after disinfection (Egusa et al., 2008). A study on medical microfluidic devices also found that CA inhibited the growth of Escherichia coli on polydimethylsiloxane (PDMS) surfaces (Ren et al., 2015). The findings of this study align with the results of Yang, Lan & Xie (2022), who demonstrated that CA influences the antioxidant system and energy metabolism of Staphylococcus aureus by significantly reducing its ATPase and CAT activity. A study by Yun & Lee (2017) also discovered that CA induces apoptosis in Candida albicans by blocking K ion channels. This study showed that 10 mg/mL CA mixing combined with 55 mg/mL or 60 mg/mL CA spraying disinfection for 20 min can completely inhibit the growth of Streptococcus pneumoniae on the impression surface. This may be because Streptococcus pneumoniae is more sensitive to CA (Lou et al., 2011). However, the results of the control groups were less positive than those of the experimental groups; a significant number of live bacteria (1.6–2.77 lgCFU/mL) remained on the control group impressions even after 30 min of disinfection, indicating a poor disinfection effect. Even when the impression was sprayed with a high concentration CA solution for 30 min, viable bacteria still persisted on the impression surface, which may be attributed to the physical properties of alginate impression materials (Hardan et al., 2022). During the initial solidification stage, hydrocolloid impression materials exhibit continuous percolation, followed by condensation. This percolation and condensation process leads to the transfer of microorganisms from inside the material to the impression surface (Al Shikh & Milosevic, 2020). The results of this study show that CA has a similar disinfection effect to traditional chemical disinfectants. In addition, because of CA’s natural extraction, its use as an alginate impression disinfectant can help eliminate potential health damage caused by chemical disinfectants (Pimpley et al., 2020; Tajik et al., 2017).
The ANSI/ADA’s Specification No. 18-1992 and the International Organization for Standardization’s 21563:2021, which both specifically focus on dental alginate, do not provide specific requirements or limits on dimensional change values (Dental Materials, 1992; Standardization, 2021). Currently, there are two primary methods for evaluating the impact of disinfectants on impression dimensional accuracy. One method involves measuring linear changes at specific sites on standard impression materials or plaster models (Babiker, Khalifa & Alhajj, 2018; Singer et al., 2023). The other method uses model scanning technology, which has seen rapid development in recent years, to analyze three-dimensional deviations in the impression materials using software (Reich et al., 2023), thus reducing systematic errors caused by manual measurements (Parize et al., 2022). Due to the absence of standard feature points in the dentition, the “best fit alignment” algorithm was employed to align the two digital models in this study. The distilled water mixing group was used as a blank control to eliminate the influence of any systematic errors that may occur during the impression-making process. The systematic analysis results demonstrated that chlorine spray disinfection at a concentration of 2,000 mg/L and CA intervention had little effect on the dimensional accuracy of alginate dental impressions. Moreover, the difference in dimensional accuracy between these disinfection methods and the distilled water mixing group was not statistically significant. These findings align with studies conducted by Vrbova et al. (2020), Babiker, Khalifa & Alhajj (2018) and Singer & Bourauel, (2023), who modified alginate materials with hydrogen peroxide and silver ion powder and observed no changes in the dimensional accuracy of the tested groups.
Limitations
This study also had limitations that should be considered. This study did not apply the CA disinfection protocol to impressions taken from the oral cavity, which will occur in future studies. Secondly, due to the antioxidant properties of CA, the alginate impression turns from the original blue to yellow after adding CA, which may affect the mood of the patient when making the impression. In addition, CA is recognized as an important antioxidant with broad-spectrum antiviral activities against HSV-1 and HSV-2 (Adeosun et al., 2022), HIV (Fredsgaard et al., 2023), and adenovirus (Ma et al., 2017). However, this particular study does not investigate the antiviral activity of CA on dental impressions.
Conclusions
This study is the first to investigate the effect of CA disinfection on alginate impressions. The results demonstrate that four physical properties of alginate impression materials mixed with a CA solution of 10 mg/mL met industry standards. Additionally, alginate impression materials mixed with CA solutions of 10 mg/mL, 15 mg/mL, and 20 mg/mL were found to be within the acceptable range for elastic recovery, strain-in-compression, and fluidity.
The CA disinfection protocol exhibited a significant inhibitory effect on the four selected oral pathogenic bacteria, particularly Staphylococcus aureus. Importantly, the dimensional accuracy of alginate impressions was not affected by CA mixing and spraying disinfection. CA is natural and non-irritating, with broad-spectrum antimicrobial properties. It is environmentally friendly, does not cause bacterial resistance, and has no negative impact on the health of medical personnel. Therefore, using CA as an alginate mold disinfectant is highly feasible. This study also indicates that CA is a promising traditional Chinese medicine impression disinfectant. These findings offer an experimental foundation for the clinical application of CA in dental impressions. In the future, more clinical trials will be conducted to explore the potential of CA as a clinical alternative to alginate impression disinfectants. This study also promotes further application of CA in stomatology.
Supplemental Information
Physical properties of alginate impression mixed with chlorogenic acid
Experimental colony count
Antibacterial effect of chlorogenic acid solution intervention on alginate impression materials