Association between triglyceride glycemic index and ejection fraction preserved heart failure in hypertensive patients
- Published
- Accepted
- Received
- Academic Editor
- Prashanth Ravishankar
- Subject Areas
- Cardiology, Epidemiology
- Keywords
- Triglyceride glucose index, Essential hypertension, Heart failure with preserved ejection fraction, Insulin resistance
- Copyright
- © 2024 Shan et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Association between triglyceride glycemic index and ejection fraction preserved heart failure in hypertensive patients. PeerJ 12:e18220 https://doi.org/10.7717/peerj.18220
Abstract
Background
The triglyceride-glucose (TyG) index is regarded as an independent predictor of cardiovascular disease consequences and a reliable surrogate measure of insulin resistance (IR). However, the correlation analysis between triglyceride glucose index and heart failure with preserved ejection fraction in patients with essential hypertension remains unknown.
Methods
A single-center, retrospective study was conducted with patients diagnosed with essential hypertension at the First Affiliated Hospital of Xinjiang Medical University, from December 2018 to September 2020. Participants were selected based on specific inclusion and exclusion criteria, with their clinical data and laboratory tests collected. The study employed Spearman’s correlation analysis, logistic regression models, restricted cubic spline plots, and receiver operating characteristic (ROC) curves to investigate the relationships between the TyG index and HFpEF.
Results
Out of 1,602 enrolled hypertensive patients, 992 were included in the analysis after applying exclusion criteria. Patients were categorized into tertiles based on the TyG index, which showed that patients in the highest tertile had characteristics associated with a higher risk of HFpEF, including age, body mass index (BMI), systolic blood pressure (SBP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and left ventricular mass index (LVMI). A significant, independent association between the TyG index and HFpEF was confirmed, with an odds ratio (OR) of 5.127 (95% CI [3.894–6.856]). Furthermore, an S-shaped nonlinear relationship was observed between the TyG index and the incidence of HFpEF (nonlinear p < 0.001). TyG index (AUC: 0.824, 95% CI [0.795–0.854]), NT-proBNP (AUC: 0.840, 95% CI [0.816–0.864]), and LVMI (AUC: 0.847, 95% CI [0.820–0.875]) showed good predictive ability for HFpEF. In addition, the TyG+LVMI combination demonstrated the strongest predictive ability (AUC: 0.907, 95% CI [0.887–0.927]).
Conclusion
The study underscores a significant association between IR, as indicated by the TyG index, and the development of HFpEF in hypertensive patients. It highlights the critical role of metabolic dysfunction in the pathophysiology of HFpEF, advocating for a broader perspective on cardiovascular risk management.
Introduction
Hypertension is a major risk factor for cardiovascular diseases, affecting over 1 billion individuals globally and 27.9% of adults in China, accounting for significant morbidity and mortality (Mills et al., 2016; Wang et al., 2018). Hypertension increases cardiac afterload and peripheral arterial resistance, leading to cardiac hypertrophy and eventually heart failure (HF) (Díez, 2014).
Heart failure with preserved ejection fraction (HFpEF), characterized by an ejection fraction greater than 50%, accounts for approximately half of all HF cases (Metra et al., 2017; Zhang, Jin & Li, 2021). Despite the prevalence, effective pharmacological treatments for HFpEF are lacking, unlike heart failure with reduced ejection fraction (HFrEF) (Cilia et al., 2019; Jeong & Dudley, 2015; Simmonds et al., 2020). The etiology of HFpEF is commonly linked to hypertension, diabetes, obesity, and ischemia, all of which contribute to ventriculo-arterial stiffening and diastolic dysfunction. Early identification and prediction of HFpEF are crucial for better patient management (Cilia et al., 2019; Hall et al., 2015; Jia & Sowers, 2021).
Recent studies have emphasized that the triglyceride-glucose (TyG) index, derived from fasting glucose and triglyceride levels, can effectively represent insulin resistance (IR) (Lopes et al., 2020). Research has found that an elevated TyG index is significantly associated with increased risks of coronary heart disease, acute myocardial infarction, and stroke (Hou et al., 2024; Liang et al., 2023; Yang et al., 2023). Additionally, the TyG index can be used to predict the risk of developing metabolic syndrome and type 2 diabetes (Tahapary et al., 2022; Zhang et al., 2022). In summary, the TyG index has been widely validated in multiple clinical studies as a simple and effective tool for metabolic risk assessment. However, existing studies have mostly focused on the association between the TyG index and overall cardiovascular risk, with few studies specifically examining its relationship with HFpEF. Therefore, this study aimed to investigate the relationship between the TyG index and the occurrence of HFpEF in patients with essential hypertension. We aimed to elucidate the potential of the TyG index as a novel reference point for managing this challenging clinical condition, paving the way for earlier and more precise interventions.
Materials and Methods
Study design and participants
This is a single-center retrospective study of patients diagnosed with essential hypertension and treated at the Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University from December 2018 to September 2020. This is a sub-study of a larger study aimed at elucidating the pathogenesis of cardiovascular disease. We retrospectively analyzed data from that study to determine whether there is an association between insulin resistance and the development of heart failure with preserved ejection fraction in hypertensive patients. Written informed consent was obtained from all participants. This project is in line with the Declaration of Helsinki, and the study protocol was approved by the Human Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (approval number: S220722-25). Participants’ information is recorded in the JiaHe eCase system.
Inclusion criteria
Participants were included if they met the standard definition of the International Society of Hypertension Global Hypertension Practice Guidelines 2020 (Unger et al., 2020). The criteria were: (1) Essential hypertension was diagnosed as repeated blood pressure measurements with office systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or taken anti-hypertensive treatment. (2) Complete clinical data collection.
Exclusion criteria
All participants with one of the following conditions were excluded. (1) White coat hypertension is defined as an elevated office blood pressure in the presence of a normal out-of-office blood pressure, typically with an office blood pressure of at least 140/90 mmHg and a 24-h average blood pressure of less than 130/80 mmHg (Nuredini et al., 2020). (2) Secondary hypertension; (3) other structural heart disease including congenital, valvular and pericardial diseases; (4) hypertrophic cardiomyopathy; (5) HF due to other cardiac disease rather than hypertension (6) abnormal thyroid function; (7) Mental illness or psychological disorders. (8) Severe liver and renal dysfunction; (9) unwilling to participate in the study or refused to sign the informed consent form.
General clinical data collection and laboratory test
Upon admission, detailed patient demographics, medical history, and records were meticulously documented by a physician specializing in cardiovascular medicine. This comprehensive data collection included gender, age, height, weight, history of smoking and alcohol consumption, systolic and diastolic blood pressure (SBP and DBP), past medical history detailing diabetes mellitus or coronary artery disease (CAD), and current medication usage. Eight milliliters of fasting venous blood was drawn from each patient early in the morning of the day following hospitalization (after at least 8 h of fasting) for assessment of fasting blood glucose (FBG), triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid (UA), creatinine, and N-terminal pro-B-type natriuretic peptide (NT-proBNP). The TyG index was calculated using the formula: TyG = ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] (Tao et al., 2022). Body Mass Index (BMI) was calculated as weight (kg) divided by height (m) squared. Patients who smoked regularly within the past 6 months were classified as current smokers. Alcohol consumption was defined as drinking at least 100 g of alcoholic beverages per week in the past month. Diabetes mellitus was defined as having a history of or suffering from diabetes mellitus, and/or having at least two fasting plasma glucose levels >7.0 mmol/L, or at least one random blood glucose value >11.1 mmol/L, or a glycosylated hemoglobin (HbA1c) >6.5%, or a 2-h OGTT blood glucose ≥11.1 mmol/L before the current admission, or being on antidiabetic medication.
Echocardiographic evaluation
Transthoracic echocardiography was performed on all patients within 24 h of admission utilizing the Vivid 7 Ultrasound System (GE Medical Systems, Chicago, IL, USA). This examination, conducted by a specialized sonographer, meticulously recorded a series of echocardiographic parameters. These parameters included: (1) Left atrial diameter (LAD): indicative of atrial size and potentially atrial pressure. (2) LV end-diastolic diameter (LVEDD): reflecting LV size and volume status at the end of diastole. (3) Interventricular septum thickness (IVS): measuring the thickness of the septum separating the left and right ventricles. (4) LV posterior wall thickness (LVPWT): assessing the thickness of the LV’s posterior wall. (5) E/A ratio: the ratio of peak velocity flow from early LV filling (E wave) to late filling (A wave) via the mitral valve, offering insight into diastolic function. (6) E/e′ ratio: the ratio of peak E wave velocity at the mitral valve orifice to peak velocity of early diastolic mitral annulus motion (e′), providing an estimation of LV filling pressure. (7) LV mass index (LVMI): a quantification of LV mass relative to body surface area (Ren et al., 2020). (8) LV ejection fraction (LVEF): indicating the percentage of blood volume ejected from the LV with each heartbeat, calculated using the biplane method of disks (modified Simpson’s rule) (Brooks et al., 2016).
Diagnosis of heart failure with preserved ejection fraction
The diagnosis of heart failure with preserved ejection fraction (HFpEF) adheres to the criteria set forth by the 2016 European Society of Cardiology guidelines, encompassing a comprehensive approach that mandates the fulfillment of all the following points: (1) clinical signs or symptoms of heart failure. (2) elevated N-terminal pro-B-type natriuretic peptide (NT-proBNP) > 125 pg/ml) (3) evidence of structural changes in the heart (left atrial volume index ≥ 34 ml/m2 or LVMI ≥ 115 g/m2 for men and (≥95 g/m2 for women); (4) normal or mildly abnormal LVEF ≥ 50% (Ponikowski et al., 2016).
Statistical analyses
All statistical analyses in this study were conducted using R software (version 4.2.3). Initially, participants were stratified into three groups according to the tertiles of the triglyceride glucose (TyG) index to compare baseline characteristics. Continuous variables with normal distribution were presented as mean ± standard deviation (SD), while those not normally distributed were expressed as the median along with the 25th and 75th percentiles. For the comparison between groups, one-way Analysis of Variance (ANOVA) was employed for normally distributed variables, whereas the Kruskal-Wallis H test was utilized for variables without a normal distribution. Count data were denoted as frequencies (percentages), with inter-group comparisons facilitated by the chi-square test. The relationship between the TyG index and various functional and structural cardiac parameters was assessed using Spearman’s correlation analysis. Furthermore, logistic regression models were implemented to explore the association between HFpEF and different variables among patients with hypertension. To investigate the potential non-linear relationships between the TyG index and HFpEF, restricted cubic spline (RCS) plots were utilized. The diagnostic efficiency of various indices for HFpEF in the hypertensive patient cohort was evaluated through receiver operating characteristic (ROC) curves. Statistical significance was determined at a two-sided p-value of less than 0.05 across all statistical tests conducted.
Results
Baseline characteristics of hypertensive patients grouped by TyG index tertiles
Initially, 1,602 patients with essential hypertension were enrolled. Of these, 422 patients were excluded because they did not fulfill the inclusion criteria, and 188 patients were excluded because they were missing relevant clinical data. Finally, 992 patients (634 males and 358 females) who completed the study were included in the data analysis (Fig. 1).
Figure 1: Flowchart of the study design.
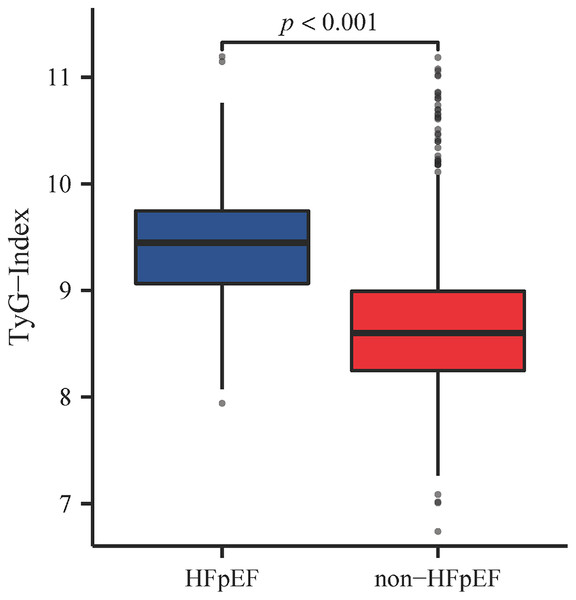
Patients were categorized into three tertiles based on the percentile of the TyG index: Tertile 1 (TyG ≤ 8.483), Tertile 2 (8.483 < TyG ≤ 9.058), and Tertile 3 (TyG > 9.058), and their baseline characteristics were compared (Table 1). In Tertile 3, the median age of patients was 61 years, and there were higher proportions of BMI, SBP, male gender, prevalence of diabetes, as well as the use of ACEI/ARB and diuretics. Additionally, this group exhibited lower levels of HDL-C, and higher levels of serum creatinine, uric acid, and NT-pro BNP. In terms of echocardiographic data, Tertile 3 also had higher LVMI and MVE/e′. Regarding the incidence of HFpEF, there were nine cases (2.7%) in Tertile 1, 41 cases (12.4%) in Tertile 2, and 152 cases (45.9%) in Tertile 3. Additionally, we further compared TyG index levels between hypertensive patients with and without HFpEF, finding that TyG index levels were significantly higher in patients with both hypertension and HFpEF (9.446 [9.064, 9.747] vs. 8.599 [8.250, 8.993], p < 0.001) (Fig. 2).
| Characteristic | Total | Tertile 1 | Tertile 2 | Tertile 3 | Statistic | p |
|---|---|---|---|---|---|---|
| n = 992 | n = 331 | n = 330 | n = 331 | |||
| TyG ≤ 8.483 | 8.483 < TyG ≤ 9.058 | TyG > 9.058 | ||||
| Demographic information | ||||||
| Age, years | 58.00 (50.00, 67.00) | 57.00 (47.00, 65.00) | 56.00 (49.00, 66.00) | 61.00 (53.00, 70.00) | 27.136 | <0.001 |
| BMI, kg/m2 | 26.03 (23.72, 29.07) | 24.86 (23.14, 27.76) | 26.35 (23.81, 29.39) | 26.54 (24.22, 29.41) | 29.924 | <0.001 |
| SBP, mmHg | 141.00 (125.00, 156.00) | 140.00 (124.00, 153.00) | 141.00 (124.00, 156.00) | 145.00 (129.00, 158.00) | 7.04 | 0.030 |
| DBP, mmHg | 79.00 (70.00, 89.00) | 79.00 (69.00, 88.00) | 80.00 (69.00, 90.00) | 79.00 (72.00, 90.00) | 1.706 | 0.426 |
| Sex | ||||||
| Female | 358 (36.1) | 112 (33.8) | 107 (32.4) | 139 (42.0) | 7.653 | 0.022 |
| Male | 634 (63.9) | 219 (66.2) | 223 (67.6) | 192 (58.0) | ||
| Ethnicity | ||||||
| Han | 637 (64.2) | 220 (66.5) | 217 (65.8) | 200 (60.4) | 7.271 | 0.296 |
| Uygur | 198 (20.0) | 66 (19.9) | 65 (19.7) | 67 (20.2) | ||
| Kazak | 91 (9.2) | 22 (6.6) | 31 (9.4) | 38 (11.5) | ||
| Other | 66 (6.7) | 23 (6.9) | 17 (5.2) | 26 (7.9) | ||
| Smoking | 356 (35.9) | 133 (40.2) | 110 (33.3) | 113 (34.1) | 4.028 | 0.133 |
| Drinking | 281 (28.3) | 104 (31.4) | 89 (27.0) | 88 (26.6) | 2.353 | 0.308 |
| Diabetes mellitus | 188 (19.0) | 65 (19.6) | 50 (15.2) | 73 (22.1) | 5.278 | 0.071 |
| Family history of hypertension | 374 (37.7) | 148 (44.7) | 134 (40.6) | 92 (27.8) | 21.945 | <0.001 |
| Drug utilization | ||||||
| ACEI/ARB | 451 (45.5) | 133 (40.2) | 144 (43.6) | 174 (52.6) | 10.907 | 0.004 |
| CCB | 393 (39.6) | 139 (42.0) | 135 (40.9) | 119 (36.0) | 2.871 | 0.238 |
| Beta-blockers | 315 (31.8) | 93 (28.1) | 112 (33.9) | 110 (33.2) | 3.104 | 0.212 |
| Diuretics | 43 (4.3) | 11 (3.3) | 10 (3.0) | 22 (6.6) | 6.437 | 0.04 |
| Laboratory indicators | ||||||
| FBG, mmol/L | 5.01 (4.38, 6.13) | 4.41 (4.02, 4.78) | 4.95 (4.42, 5.62) | 6.41 (5.61, 8.31) | 374.079 | <0.001 |
| TG, mmol/L | 1.55 (1.08, 2.34) | 0.94 (0.78, 1.15) | 1.57 (1.37, 1.81) | 2.68 (2.22, 3.33) | 738.833 | <0.001 |
| TC, mmol/L | 3.78 (3.09, 4.53) | 3.78 (3.09, 4.57) | 3.75 (3.13, 4.40) | 3.80 (3.07, 4.59) | 0.434 | 0.805 |
| HDL-C, mmol/L | 0.98 (0.84, 1.16) | 1.09 (0.94, 1.28) | 0.98 (0.86, 1.12) | 0.91 (0.75, 1.06) | 88.64 | <0.001 |
| LDL-C, mmol/L | 2.47 (1.84, 3.13) | 2.49 (1.85, 3.10) | 2.48 (1.90, 3.23) | 2.44 (1.78, 3.13) | 1.114 | 0.573 |
| Cr, mmol/L | 69.40 (59.90, 82.40) | 66.00 (58.00, 77.00) | 69.40 (60.26, 83.60) | 74.10 (62.00, 86.00) | 22.702 | <0.001 |
| UA, mmol/L | 318.00 (265.10, 375.54) | 296.30 (244.00, 355.00) | 330.10 (283.70, 380.00) | 330.00 (270.00, 384.00) | 31.052 | <0.001 |
| NT-pro BNP, pg/ml | 118.00 (51.90, 274.00) | 107.00 (55.50, 243.00) | 91.90 (46.90, 174.00) | 172.00 (61.00, 447.00) | 34.879 | <0.001 |
| Echocardiography information | ||||||
| LVMI | 105.37 (93.29, 127.78) | 101.68 (88.66, 115.48) | 103.13 (91.69, 127.78) | 113.88 (97.61, 138.08) | 36.446 | <0.001 |
| LVEF, % | 62.68 (61.23, 64.04) | 62.95 (61.90, 64.73) | 62.95 (61.68, 64.36) | 62.14 (60.43, 63.69) | 24.017 | <0.001 |
| E/e′ | 7.93 (6.92, 9.57) | 7.75 (6.75, 9.33) | 7.74 (6.82, 9.14) | 8.27 (7.12, 10.14) | 9.452 | 0.009 |
| LAD, mm | 35.00 (33.00, 39.00) | 34.00 (32.00, 37.00) | 35.00 (33.00, 39.00) | 37.00 (33.00, 41.00) | 38.877 | <0.001 |
| LVEDD cm | 4.90 (4.60, 5.10) | 4.80 (4.60, 5.00) | 4.90 (4.60, 5.10) | 5.00 (4.70, 5.30) | 23.588 | <0.001 |
| IVS, cm | 0.90 (0.90, 1.10) | 0.90 (0.90, 1.00) | 0.90 (0.90, 1.10) | 1.00 (0.90, 1.10) | 31.112 | <0.001 |
| HFpEF | 202 (20.4) | 9 (2.7) | 41 (12.4) | 152 (45.9) | 209.702 | <0.001 |
Note:
Categorical variables are expressed as frequencies (percentages), and continuous variables are expressed as medians (P25,P75). Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, Calcium channel blockers; FPG, fasting plasma glucose; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; UA, uric acid; Cr, creatinine; NT-pro BNP, N-terminal pro-B-type natriuretic peptide; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction; E/e′, E/e′ Ratio; LAD, left atrial diameter; LVEDD, left ventricular end‐diastolic diameter; IVS, interventricular septal; HFpEF, heart failure with preserved ejection fraction.
Figure 2: TyG index levels in HFpEF and non-HFpEF groups.
Correlation analysis of TyG index with important cardiac indices in hypertensive patients
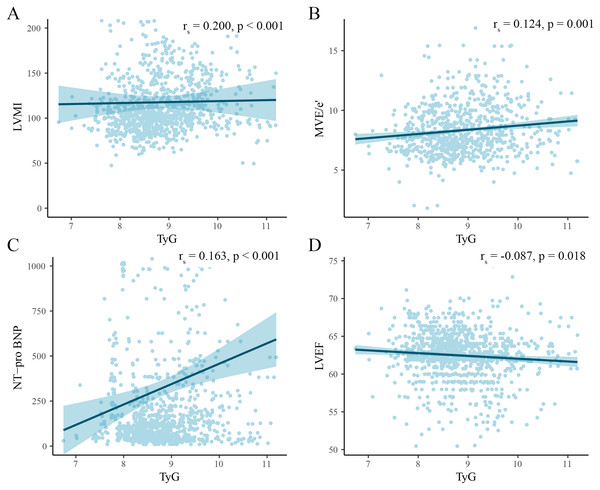
We analyzed the association of the TyG index with several key ultrasound indices and NT-pro BNP using Spearman correlation analysis and plotted scatter plots (Fig. 3). The analysis revealed that the TyG index had a positive correlation with LVMI (rs = 0.200, p < 0.001), MVE/e′ (rs = 0.163, p < 0.001), and NT-pro BNP (rs = 0.163, p < 0.001), and a negative correlation with LVEF (rs = −0.087, p < 0.018).
Figure 3: Scatter plot of correlation between TyG index and cardiac injury indicators.
Association between TyG index and occurrence of HFpEF in patients with essential hypertension
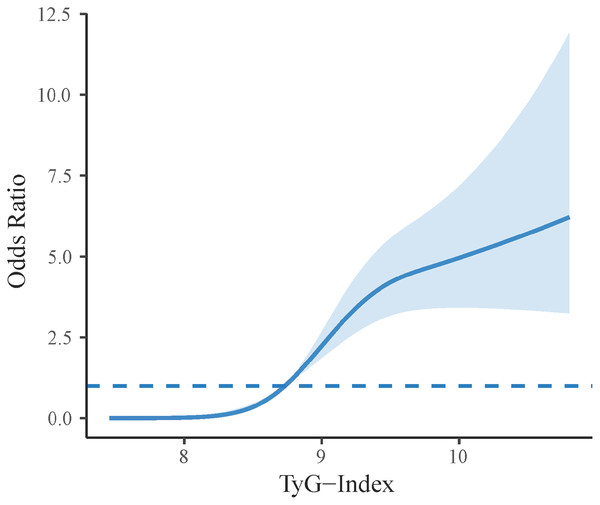
Logistic regression analysis revealed an association between the TyG index and the occurrence of HFpEF in hypertensive patients (Table 2). The base model showed an OR of 4.739 (95% CI [3.738–6.082], p < 0.001). In Model 1, adjusted for demographic details such as age and gender, the adjusted OR was 4.842 (95% CI [3.747–6.344], p < 0.001). Model 2, further adjusted for laboratory indices in addition to the adjustments in Model 1, confirmed that the TyG index remained an independent predictor with an OR of 5.127 (95% CI [3.894–6.856], p < 0.001). After standardization and categorization of the TyG index data, it was found that the standardized TyG index still independently associated with HFpEF in the fully adjusted Model 2, with an OR of 3.267 (95% CI [2.677–4.031], p < 0.001). Moreover, compared to Tertile 1, the OR was 5.355 (95% CI [2.587–12.271], p < 0.001) for Tertile 2 and 34.214 (95% CI [17.025–77.350], p < 0.001) for Tertile 3, indicating a significant increase in risk across tertiles. We conducted an in-depth analysis of the nonlinear relationship between the TyG index and HFpEF incidence using a restricted cubic spline plot (RCS). This analysis revealed a significant nonlinear association between the TyG index and HFpEF occurrence in hypertensive patients, with a nonlinear p value of less than 0.001. Notably, as the TyG index increased, the incidence of HFpEF also rose significantly, delineating a roughly S-shaped curve (Fig. 4).
| Characteristic | Crude model | Model 1 | Model 2 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| TyG-index | 4.739 [3.738–6.082] | <0.001 | 4.842 [3.747–6.344] | <0.001 | 5.127 [3.894–6.856] | <0.001 |
| TyG-index (standardization) | 3.085 [2.598–3.697] | <0.001 | 3.134 [2.603–3.811] | <0.001 | 3.267 [2.677–4.031] | <0.001 |
| TyG-index (categorize) | ||||||
| Tertile 1 | Ref | Ref | Ref | |||
| Tertile 2 | 5.076 [2.534–11.325] | <0.001 | 5.276 [2.585–11.912] | <0.001 | 5.355 [2.587–12.271] | <0.001 |
| Tertile 3 | 30.381 [15.981–65.572] | <0.001 | 32.825 [16.751–72.396] | <0.001 | 34.214 [17.025–77.350] | <0.001 |
| p for trend | <0.001 | <0.001 | <0.001 | |||
Note:
TyG-index (standardization) means that the mean of the TyG index is changed to 0 and the variance is changed to 1. Crude model adjusted for none. Model 1 adjusted for sex, age, BMI, diabetes, family history of hypertension, diuretics, ACEI/ARB. Model 2 adjusted for Model 1 and laboratory indicators (HDL-C, uric acid, creatinine, TC).
Figure 4: Restricted cubic spline plot of TyG index and the occurrence of HFpEF in patients with essential hypertension.
Predictive power of TyG index for HFpEF
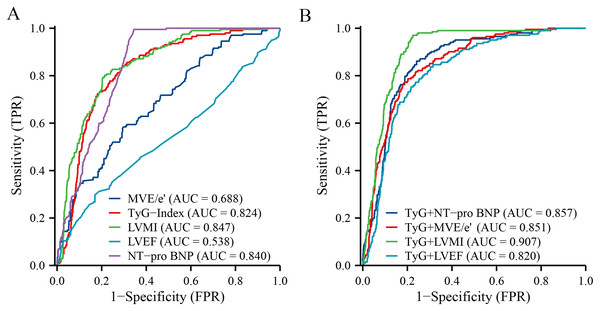
In evaluating the predictive performance for HFpEF among hypertensive patients, our ROC analysis revealed distinct efficacies across various markers and their combinations (Table 3). The TyG Index alone showed a promising AUC of 0.824 (95% CI [0.795–0.854]), indicating its substantial role in risk identification. Similarly, NT-proBNP demonstrated excellent sensitivity, with an AUC of 0.840 (95% CI [0.816–0.864]), while LVMI also presented strong predictive power, marked by an AUC of 0.847 (95% CI [0.820–0.875]). On the other hand, MVE/e′ and LVEF displayed moderate and relatively low predictive values, with AUCs of 0.688 (95% CI [0.648–0.729]) and 0.538 (95% CI [0.490–0.586]), respectively. The analysis of combined markers significantly enhanced predictive accuracy; notably, the TyG+LVMI combination emerged as the most potent predictor, achieving the highest AUC of 0.907 (95% CI [0.887–0.927]) (Fig. 5).
| Characteristic | AUC | 95% CI | Sensitivity | Specificity |
|---|---|---|---|---|
| Individual indicators | ||||
| TyG-Index | 0.824 | [0.795–0.854] | 0.842 | 0.706 |
| NT-pro BNP | 0.840 | [0.816–0.864] | 0.995 | 0.656 |
| MVEe | 0.688 | [0.648–0.729] | 0.579 | 0.706 |
| LVMI | 0.847 | [0.820–0.875] | 0.792 | 0.796 |
| LVEF | 0.538 | [0.490–0.586] | 0.297 | 0.830 |
| Combined indicators | ||||
| TyG+NT-pro BNP | 0.857 | [0.830–0.883] | 0.861 | 0.758 |
| TyG+MVE/e′ | 0.851 | [0.824–0.878] | 0.767 | 0.818 |
| TyG+LVMI | 0.907 | [0.887–0.927] | 0.970 | 0.775 |
| TyG+LVEF | 0.820 | [0.789–0.850] | 0.752 | 0.784 |
Note:
Abbreviations: AUC, area under the curve; CI, confidence interval; TyG-Index, triglyceride-glucose index; NT-pro BNP, N-terminal pro-B-type natriuretic peptide; MVE/e', mitral valve E-wave to early diastolic mitral annulus velocity ratio; LVMI, left ventricular mass index; LVEF, left ventricular ejection fraction
Figure 5: Receiver-operated characteristic curves of the TyG index and the combined index as predictors of HFpEF in patients with essential hypertension.
(A) Individual predictive capacity for each indicator. (B) Predictive capacity of TyG to combine multiple indicators.Discussion
This study highlights the significant association between the TyG index and the occurrence of HFpEF in hypertensive patients. Our primary findings reveal that patients with higher TyG index levels exhibit a substantially increased risk of developing HFpEF. Specifically, the TyG index was independently associated with HFpEF, with a significant nonlinear S-shaped relationship observed, indicating a marked increase in HFpEF incidence above certain TyG index thresholds. Additionally, the combination of the TyG index with the left ventricular mass index (LVMI) demonstrated the strongest predictive ability for HFpEF, underscoring the critical role of metabolic dysfunction in the pathogenesis of HFpEF in hypertensive patients.
HFpEF accounts for about half of all heart failure cases, and its prevalence is rising, in part due to an aging population and rising rates of hypertension, diabetes, and obesity (Gorter et al., 2018). Ejection fraction is a measure of the percentage of blood that flows out of the heart with each contraction, and an ejection fraction of 50% or more is generally considered to be within the normal range (Wang, Gareri & Rockman, 2018). Although patients with HFpEF have normal or near-normal ejection fractions, the heart is unable to relax and fill properly during diastole (DeVore et al., 2017). Thus, in patients with HFpEF, the heart pumps efficiently during systole, but the stiff ventricles prevent adequate filling during diastole, leading to congestion and the typical symptoms of heart failure (Hieda et al., 2020). Hypertension is considered to be one of the most important risk factors for HFpEF. Some of the possible mechanisms are probably as follows: Chronic hypertension leads to left ventricular hypertrophy (LVH) as the myocardium thickens to counteract the increased vascular resistance (Lee & Park, 2021). Over time, this adaptive mechanism becomes maladaptive, leading to changes in cardiac structure and function that are typically characterized by HFpEF. These changes include increased left ventricular stiffness, impaired relaxation (diastolic dysfunction), and a subsequent decrease in diastolic ventricular filling efficiency (Tsioufis et al., 2017). Hypertension increases the pressure at which the heart pumps blood, known as afterload. Chronic elevation of afterload also induces compensatory myocardial hyperplasia, which, when exceeded, induces myocardial fibrosis and hypertrophy, leading to myocardial stiffness and impaired relaxation. Hypertension is also associated with changes in the microcirculation, including decreased capillary density and impaired microvascular reactivity (Myhre, Selvaraj & Solomon, 2021). These changes impair myocardial perfusion, especially during stress, leading to diastolic dysfunction and HFpEF.
Insulin resistance (IR) signifies a metabolic dysfunction characterized by a diminished capacity of the body to transport and utilize glucose (Roberts, Hevener & Barnard, 2013). Under healthy conditions, insulin facilitates glucose absorption and utilization by cells to maintain stable blood sugar levels. However, in the state of IR, tissues respond less effectively to insulin, leading to inadequate glucose absorption by cells and compelling the pancreas to produce more insulin in an attempt to lower blood sugar levels. This prolonged state of abnormal blood sugar and insulin levels not only can lead to diabetes but is also closely associated with an increased risk of cardiovascular diseases (Artunc et al., 2016). The etiology of IR involves multiple factors, including genetics, diet, activity levels, and obesity. The global rise in obesity rates has turned IR and its resulting spectrum of metabolic abnormalities into a prominent public health issue (Ighbariya & Weiss, 2017). These abnormalities, such as hypertension, hyperglycemia, lipid metabolism disorders, and central obesity, are collectively referred to as metabolic syndrome, a primary risk factor for cardiovascular diseases (Hill et al., 2021). Our results similarly support this notion, with higher BMI and SBP levels, and higher proportions of men and diabetes in the Tertile 3 group (TyG > 9.058) than in the other groups. Given the close relationship between IR and cardiovascular diseases, early identification and intervention of IR are crucial. However, traditional methods of detecting IR, such as the high insulin-normal glucose clamp technique and insulin sensitivity testing (HOMA-IR), are limited in their application in routine clinical practice due to their complexity and high cost (Alizargar et al., 2020). The need for precisely controlled experimental conditions and specialized operators makes these methods unsuitable as standard tests for widespread screening or primary healthcare. In recent years, the TyG index, as a novel marker for assessing IR, has garnered widespread attention. By simply calculating fasting blood glucose and triglyceride levels, the TyG index provides a solution that overcomes the limitations of traditional methods, offering a low-cost, easily operable tool for clinical assessment of IR (Huang et al., 2022). Numerous studies have confirmed the strong correlation between the TyG index and IR, and in some cases, its ability to predict cardiovascular disease risk even surpasses traditional HOMA-IR indicators. Research specifically targeting the hypertensive population further underscores the clinical value of the TyG index. Findings indicate that a high TyG index is not only associated with the progression of atherosclerosis in hypertensive patients but also correlates with increased risks of hyperuricemia and stroke (Ormazabal et al., 2018; Wu et al., 2021). More importantly, this indicator is also associated with impaired left ventricular diastolic function and structural abnormalities, providing essential evidence for assessing cardiac health status (Sun et al., 2022; Yu et al., 2022). Our results similarly confirmed a positive correlation between TyG index and cardiac injury-related indices (e.g., LVMI, NT-pro BNP). In addition, the TyG index was negatively correlated with LVEF. This is even though LVEF is usually higher in patients with HFpEF. However, it is important to recognize that LVEF varies even among patients with HFpEF and is not completely normal in all cases. HFpEF is characterized by diastolic dysfunction and increased ventricular stiffness rather than a markedly reduced LVEF (Gevaert et al., 2022). The slight negative correlation observed may reflect the wider range of ejection fraction in our study population, including those with mildly reduced LVEF still above the 50% threshold but below the optimal value. This can be attributed to the interaction between IR and the renin-angiotensin system (RAS), as well as to intramyocardial lipid deposition resulting from the action of IR and excess fatty acids, leading to subsequent myocardial fibrosis and contractile dysfunction (Chiu et al., 2021; Zhou, Schulman & Zeng, 2012).
Our study confirmed that TyG index was an independent correlate of the development of HFpEF in hypertensive patients (OR: 5.127 (95% CI [3.894–6.856])). In addition, the present study found an S-shaped nonlinear relationship between TyG index and the incidence of HFpEF in hypertensive patients by restricted triple spline analysis, which further emphasizes that the risk of HFpEF is significantly increased above a certain TyG index value. ROC analysis showed an AUC of 0.824 (95% CI [0.795–0.854]) for the TyG index alone, demonstrating its important role in risk identification. Notably, the TyG+LVMI combination was the most effective predictor, achieving the highest AUC of 0.907 (95% CI [0.887–0.927]). All these results confirm the significant association of IR with the development of HFpEF in hypertensive patients, indicating that IR may play a pivotal role in the pathophysiology of HFpEF. This association highlights the importance of metabolic health in cardiovascular disease and suggests that interventions aimed at improving insulin sensitivity could potentially have a positive impact on preventing or managing HFpEF in this patient population (Lopaschuk et al., 2021).
Our study has several strengths. First, the large sample size of 992 hypertensive patients enhances the statistical power and reliability of our findings. Second, the focus on the TyG index provides a simple and cost-effective tool for assessing insulin resistance and its association with HFpEF, potentially facilitating early identification and intervention in clinical practice. Despite these strengths, our study has limitations. The population had a greater proportion of males (634) than females (358), and a higher proportion of younger patients. HFpEF is known to be more prevalent in older females, which may limit the generalizability of our findings to the broader population. Additionally, the cross-sectional design precludes the establishment of causality, underscoring the need for longitudinal research to delineate the direction of this relationship. Reliance on a single measurement for the TyG index and other biomarkers may not fully capture the nuances of metabolic status over time, suggesting the value of repeated measurements. Furthermore, unmeasured confounders, including lifestyle factors, genetic predispositions, and other comorbidities, could also influence the observed associations.
Conclusion
In conclusion, there is a significant association between TyG index and the development of HFpEF in hypertensive patients, and our study further emphasizes the critical role of insulin resistance in the pathogenesis of HFpEF in hypertensive patients, advocating a broader perspective on cardiovascular risk management, including metabolic health.