Cholecystectomy and risk of cardiovascular disease, all-cause and cause-specific mortality: a systematic review and updated meta-analysis
- Published
- Accepted
- Received
- Academic Editor
- Yoshinori Marunaka
- Subject Areas
- Cardiology, Epidemiology, Evidence Based Medicine, Internal Medicine
- Keywords
- Cholecystectomy, Cardiovascular disease, Mortality, Systemic review, Meta-analysis
- Copyright
- © 2024 Song et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Cholecystectomy and risk of cardiovascular disease, all-cause and cause-specific mortality: a systematic review and updated meta-analysis. PeerJ 12:e18174 https://doi.org/10.7717/peerj.18174
Abstract
Objective
Questions remain about the association among cholecystectomy, cardiovascular disease, all-cause and cause-specific mortality. We performed a systematic review and meta-analysis to clarify these associations.
Methods
PubMed, Web of Science, Embase, and Cochrane Library databases were searched up to February 2024. Summary relative risks (RRs) and 95% confidence intervals (CIs) were calculated using a DerSimonian–Laird random effects model.
Results
We screened 16,595 articles and included 14 studies. No significant association was found between cholecystectomy and cardiovascular disease (CVD), with RR being 1.03 (95% CI [0.77–1.37], p = 0.848, I2 = 99.6%), even in results with high heterogenous studies excluded (RR 1.20, 95% CI [0.97–1.49], p = 0.095, I2 = 77.7%). Same result was proved in its subtype, coronary heart disease (RR 1.06, 95% CI [0.84–1.33], p = 0.633, I2 = 96.6%). Cholecystectomy increased CVD risk compared with healthy controls without gallstones (RR 1.19, 95% CI [1.05–1.35], p = 0.007, I2 = 83.3%) and lowered CVD risk compared with gallstone carriers (RR 0.62, 95% CI [0.57–0.67], p < 0.001, I2 = 82.1%). As for mortality, increase in the risk for all-cause (RR 1.17, 95% CI [1.03–1.34], p = 0.020, I2 = 51.6%) and cardiovascular (RR 1.24, 95% CI [1.06–1.47], p = 0.009, I2 = 20.7%) mortality, but not for cancer mortality (RR 1.18, 95% CI [0.95–1.47], p = 0.131, I2 = 0.0%), were observed after cholecystectomy.
Conclusion
Cholecystectomy may not be associated with the overall development of CVD, as well as CHD. Cholecystectomized patients showed increased CVD risk compared with healthy controls without gallstones, but decreased CVD risk compared with gallstone patients. Increased risk for all-cause and cardiovascular, but not cancer mortality was observed following cholecystectomy.
Introduction
Gallstones represent a major health problem in general populations worldwide (Stinton & Shaffer, 2012). During follow-up period, approximately less than one fourth developed symptomatic gallstones which needed hospitalization, leaving most of gallstones remaining clinically silent (Shabanzadeh, 2023; Shabanzadeh, Sorensen & Jorgensen, 2016). Cholecystectomy is presently an appropriate and frequently-used treatment for symptomatic gallstones, as well as other gallbladder diseases. However, several persistent symptoms and clinical conditions have been reported following cholecystectomy (Lamberts et al., 2013), including pain (Wennmacker et al., 2018), carcinoma (Kharazmi et al., 2023; Mu et al., 2023; Wang, Xie & Lin, 2019), liver diseases (Konyn et al., 2023; Luo et al., 2023), etc.
It has been demonstrated that gallstones share similar risk factors with cardiovascular disease (CVD), including age, hypertension, obesity, diabetes, hyperlipidemia, fatty liver disease, etc (Ata et al., 2011; Tsai et al., 2004). Those similar risk factors could be explained by cholesterol, the main component of gallstones (Di Ciaula, Wang & Portincasa, 2018) and atherosclerotic plaque in CVD (Momiyama et al., 2014), as well as shared genetic architecture between the two diseases (Zhang et al., 2023). Previous meta-analyses have demonstrated that a remarkable increase in the risk of CVD among patients with gallstone disease (GSD) (Fairfield, Wigmore & Harrison, 2019; Fan, Chen & Dai, 2017; Upala, Sanguankeo & Jaruvongvanich, 2017). However, only one meta-analysis reported the association between cholecystectomy and CVD (Fairfield, Wigmore & Harrison, 2019), with only three studies included. The results indicated that cholecystectomy exerted an increased risk of CVD compared with controls without gallstones, but neutral effect compared with gallstone subjects without cholecystectomy (Fairfield, Wigmore & Harrison, 2019). No meta-analysis has focused on all-cause and cause-specific mortality following cholecystectomy. These unsolved issues raised more population-based cohort studies on this issue (Chen, Lin & Kao, 2021; Kim et al., 2021; Park et al., 2022; Wei et al., 2019) recently. A more comprehensive meta-analysis was needed considering with the publication of more population-based new research. Thus, we conducted a systematic review and meta-analysis of the current evidence to investigate these associations.
Materials and Methods
Search strategy
This work was registered with ID number CRD42024499426 on PROSPERO. We did not publish the protocol online or elsewhere. The article was prepared in compliance with the newest PRISMA statement. We searched case-control and cohort studies aiming to investigate the association among cholecystectomy, CVD, all-cause and cause-specific mortality up to February 2024 in the following databases, PubMed, Web of Science, Embase, and Cochrane Library databases. Only articles written in English were searched and the key words were displayed as follows: (“cholecystectomy” OR “cholecystectomies”) AND (“cerebrovascular disease” OR “cardiovascular disease” OR “carotid atherosclerosis” OR “angina” OR “ischaemic heart disease” OR “myocardial infarction” OR “coronary artery disease” OR “stroke” OR “mortality” OR “all-cause mortality”).
Selection criteria
Studies meeting the following criteria were included: (a) population-based research with calculable data; (b) the intervention strategy was cholecystectomy; and (c) the outcome contained at least one of the following outcomes: CVD, all-cause, cardiovascular and cancer mortality. The identification of CVD was in accordance with previous studies, which included coronary heart disease (CHD) and stroke, defined as International Classification of Diseases 10th version (ICD-10) codes of I20-I25 and I60-I69 (Moon et al., 2023). Studies with one of the following items were excluded: (a) reviews, letters, case reports/series; (b) guidelines, protocols, and replies; (c) no original data or incalculable data; (d) follow-up time less than 90 days or deaths due to acute complications; and (e) studies limited to specific populations, such as people with diabetes, hemodialysis, etc.
The titles, abstracts and full texts of the selected literature were reviewed by two authors (Yang Song and Haishu Wang) leaving discrepancies confirmed by Dr.Yaowen Xu. Relevant articles which met the inclusion criteria were added during the reviewing process.
Data extraction and quality assessment
The data extraction process was conducted by two researchers (Yang Song and Yaowen Xu). The final confirmation of data extraction results was verified by Dr. Yaowen Xu. The detailed characteristics of the included studies were recorded, including the author and publication year, number of participants and follow-up time, method of cholecystectomy identification, type of outcome, number of cases for outcome, and adjustment. The Newcastle–Ottawa Scale (NOS) (Wells et al., 2013) was utilized to assess the study quality. Studies with a score of 7 to 9 were considered as high quality, studies with a score of 4 to 6 of moderate quality, and studies with a score of 0 to 3 of low quality.
The certainty of the evidence was rated using the online GRADEpro software (https://www.gradepro.org/) (Atkins et al., 2004) by Dr. Yang Song and Yaowen Xu. Five aspects, including study limitations, consistency of effect, imprecision, indirectness, and publication bias (Guyatt et al., 2008) were evaluated in the GRADE system. The certainty of evidence in each dimension was categorized as high, moderate, low, or very low quality.
All data analyses were conducted using STATA 17.0 (College Station, TX, USA: StataCorp LLC). Pooled relative risk (RR) and 95% CI was calculated from RR, hazard ratio (HR), etc, using the DerSimonian–Laird method and p value for the pooled analysis was provided. Heterogeneity was assessed using the I2 statistic (Greenland, 1987). I2 value > 50% was identified as significant heterogeneity. Publication bias was identified by a funnel plot and Egger’s test (Harbord, Egger & Sterne, 2006). A sensitivity analysis was also conducted performed to find the possible source of heterogeneity. The significance level was α = 0.05.
Results
Study selection and characteristics
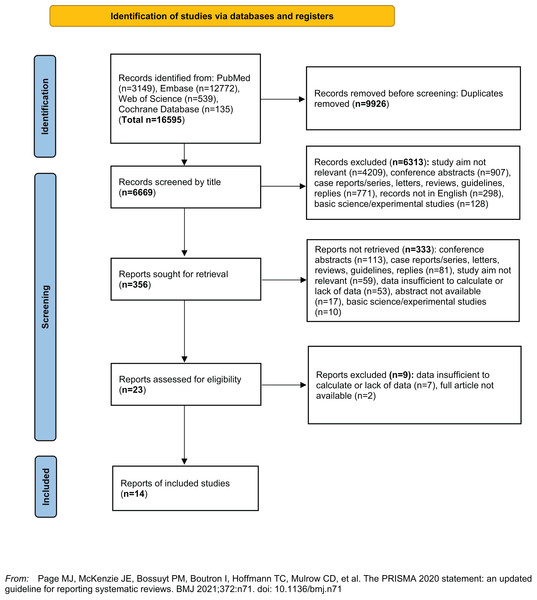
Figure 1 showed the literature selection process. Totally the initial search yielded 16,595 articles, of which 9,926 repetitive articles were removed. After further review for title, articles were excluded due to the following reasons: study aim not relevant (n = 4,209), conference abstracts (n = 907), case reports/series, letters, reviews, guidelines, replies (n = 771), records not in English (n = 298), basic science/experimental studies (n = 128). After further revision for abstracts and full articles, a total of 14 articles were finally included in the meta-analysis (Andersen et al., 1995; Bortnichak et al., 1985; Chen, Lin & Kao, 2021; Kim et al., 2021; Konyn et al., 2023; Park et al., 2022; Rosenmuller et al., 2007; Ruhl & Everhart, 2011; Shabanzadeh et al., 2017; Shabanzadeh, Sorensen & Jorgensen, 2017; Strom et al., 1986; Su et al., 2022; Wei et al., 2019; Wirth et al., 2015).
Figure 1: Flow diagram of literature search.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.Table 1 shows the number of studies, cases, participants and other main characteristics of included studies. Five studies were from Europe, four were from the USA, and five were from Asia. Eight studies reported outcome of CVD and six studies reported mortality, including all-cause, cardiovascular and cancer mortality. Cases from most studies derived from medical records in hospitals or data from health care programs. NOS scoring analysis were indicated in Table S1. The mean NOS score was 6.64.
| Authors, Year | Study characteristics | Identification of CS | Type of outcome | No. of cases for outcome | Adjustment |
|---|---|---|---|---|---|
| Bortnichak et al. (1985) | Cohort study: 5,209 subjects at baseline (367 patients with CS) Follow up period: 26 years |
Medical records | CVD (CHD) | 706 | Sex, diabetes, left ventricular hypertrophy, serum cholesterol, age, length of follow-up, systolic blood pressure, Framingham Relative Weight, cigarette smoking |
| Strom et al. (1986) | Case-control study: Women (550 MI cases and 1,658 controls), Men (1,511 MI cases and 3,837 controls) | Questionnaires | CVD (MI) | 2,061 | Age and geography |
| Andersen et al. (1995) | Cohort study: 11,123 CS patients at baseline Follow up period: 6 years |
Medical records | All-cause mortality | 869 | Age differences |
| Rosenmuller et al. (2007) | Cohort study: 44,084 CS patients at baseline Follow up end: the date of death or the end of follow-up (December 31st 2004), whichever occurred first |
Medical records | All-cause mortality | 275 | None |
| Ruhl & Everhart (2011) | Cohort study: 14,228 participants (12,210 non-GSD subjects and 2018 GSD subjects, including CS) Follow up period: 18 years |
Medical records | All-cause, cardiovascular, and cancer mortality | 2017 (all-cause) 737 (cardiovascular) 550 (cancer) |
Age, sex, race/ethnicity, education, BMI, waist-to-hip ratio, glucose status, total serum cholesterol, high-density lipoprotein cholesterol, smoking, drinking, caffeine, physical activity, C-reactive protein |
| Wirth et al. (2015) | Cohort study: 46,468 participants free of CVD and diabetes at baseline Follow up period: 8 years |
Questionnaires | CVD (stroke or MI) | 919 | Sex, age, study center, educational achievement, physical activity, smoking habits, alcohol intake, BMI, waist circumference, hypertension and hyperlipidemia |
| Shabanzadeh et al. (2017) (mortality) | Cohort study: 5,928 participants at baseline (gallstones at ultrasound: n = 402; CS: n = 189; no gallstones: n = 5,337) Follow up period: 24.7 years |
Medical records | All-cause, cardiovascular, and cancer mortality | 2,428 (all-cause) 652 (cardiovascular) 750 (cancer) |
Age, sex, BMI, social group I–V, smoking, alcohol consumption, diabetes, SBP > 140 mmHg, DBP > 90 mmHg, non-HDL, HDL |
| Shabanzadeh et al. (2017) (CVD) | Cohort study: 5,496 participants at baseline (gallstones at ultrasound: n = 346; CS: n = 158; no gallstones: n = 4,992) Follow up period: 32 years |
Abdominal ultrasound | CVD (CHD, cerebrovascular disease, and peripheral artery disease) | 1,892 | Age, sex, cohort number, BMI, SBP > 140 mmHg, DBP > 90 mmHg, non-HDL, HDL, smoking, alcohol consumption, diet, physical activity level, social group |
| Wei et al. (2019) | Cohort study: 155,356 CS and 155,356 control subjects in the study cohort Follow up end: until diagnosis of stroke of the end of 2013 |
Medical records | CVD (stroke) | 19,098 | Age, sex, and major comorbidities |
| Chen, Lin & Kao (2021) | Cohort study: 122,421 CS and 122,421 control subjects in the cohort Follow up period: until the diagnosis of AMI or the end of 2011 |
Medical records | CVD (AMI) | 4,093 | Age, sex, occupation, urbanization level, comorbidity of atrial fibrillation, hypertension, hyperlipidemia, diabetes, stroke, heart failure, and COPD |
| Kim et al. (2021) | Cohort study: 146,928 CS subjects and 268,502 control subjects at baseline Follow up period: 2.56 years (median) |
Medical records | CVD (MI and heart failure) | 8,924 | Age, sex, income, place of residence, diabetes, hypertension, dyslipidemia, smoking, drinking, regular exercise, BMI |
| Park et al. (2022) | Cohort study: 491,267 gallstone patients (179,321 with CS) and controls (n = 4,912,670) Follow up period: 15 years |
Medical records | CVD (MI and cerebral infarction) | 15,691 | Visit frequency as an outpatient |
| Su et al. (2022) | Cohort study: 13,975 ACS patients at baseline (12,265 without GSD and 1,710 with GSD, of which 511 had CS) Follow up period: 2.96 years (mean) |
Medical records | All-cause and cardiovascular mortality | 1,015 (all-cause) 540 (cardiovascular) |
Age, sex, BMI, smoking habits, alcohol intake, classification of ACS, PCI therapy, previous myocardial infarction, hypertension, diabetes, and hyperlipidemia |
| Konyn et al. (2023) | Cohort study: 11,153 individuals were included (9,521 without GSD and 1,632 with GSD, of which 717 had CS) Follow up period: 23 years |
Abdominal ultrasound | All-cause, cardiovascular, and cancer mortality | Not mentioned | Seasonality |
Note:
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; COPD, chronic obstructive pulmonary disease; CS, cholecystectomy; CVD, cardiovascular disease; CHD, coronary heart disease; DBP, diastolic blood pressure; GSD, gallstone disease; HDL, high-density lipoprotein; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
Cholecystectomy and CVD
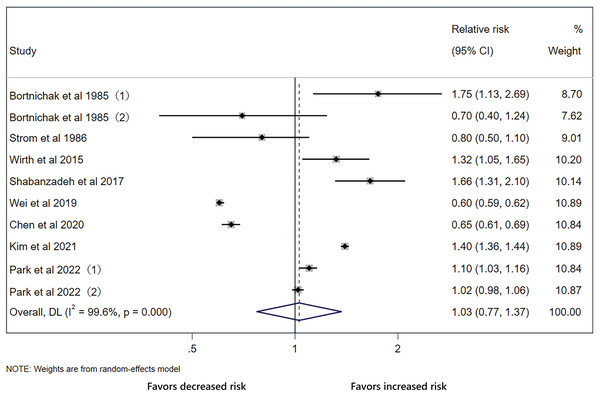
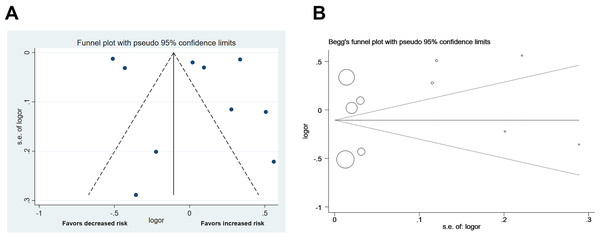
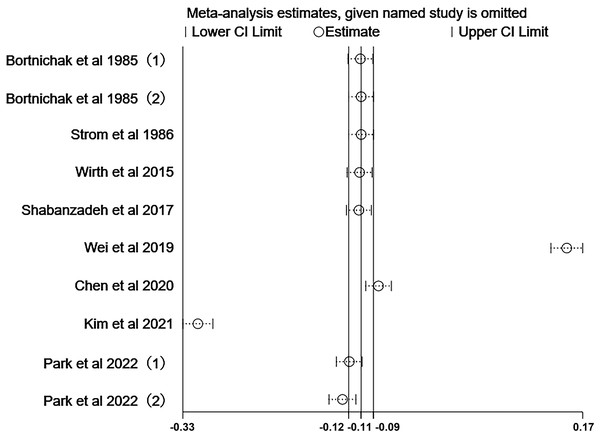
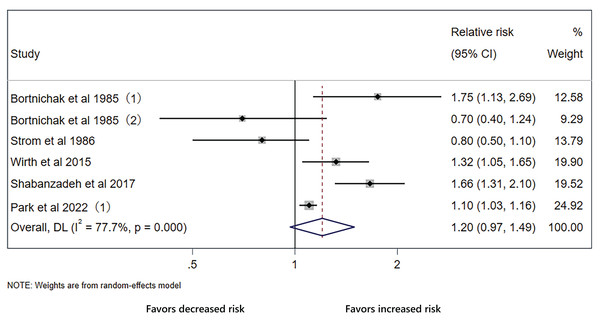
The pooled effect on the development of CVD in patients with cholecystectomy was 1.03 (95% CI [0.77–1.37], p = 0.848, I2 = 99.6%, Fig. 2), with heterogeneity between studies. We did not detect significant publication bias through funnel plot and Egger’s tests (Fig. 3). Sensitivity analysis indicated that four studies may be the main source of heterogeneity (Chen, Lin & Kao, 2021; Kim et al., 2021; Park et al., 2022; Wei et al., 2019) (Fig. 4). After removal of the four studies, cholecystectomy still showed no association with CVD (RR 1.20, 95% CI [0.97–1.49], p = 0.095, I2 = 77.7%, Fig. 5).
Figure 2: Pooled analysis of the effect of cholecystectomy on cardiovascular disease.
Forest plot for cholecystectomy and risk of cardiovascular disease with all studies.Figure 3: Funnel plot of the analysis.
Publication bias of the included studies. (A) Funnel plot for publication bias. (B) Egger’s test results.Figure 4: Sensitivity analysis.
Sensitivity analysis of all included studies for cholecystectomy and cardiovascular disease.Figure 5: Analysis with stable results still shows no association between cholecystectomy and cardiovascular disease.
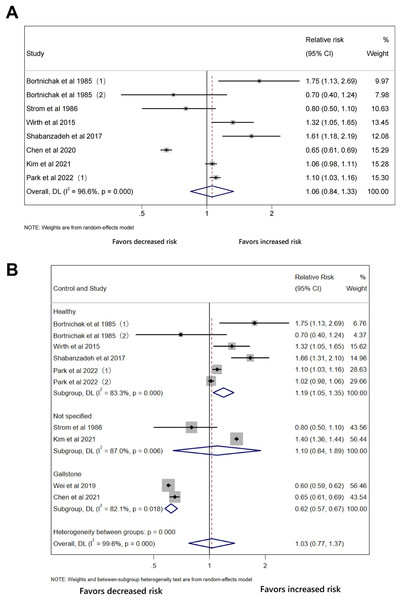
Forest plot of relative risk between cholecystectomy and cardiovascular disease with stable results.The outcomes of the original analysis include CHD and stroke, which may be a possible explanation of heterogeneity. In the initial analysis, only two studies reported outcome of stroke and they were the major sources of heterogeneity. We conducted subgroup analysis of CHD. There was still no significant association between cholecystectomy and CHD (RR 1.06, 95% CI [0.84–1.33], p = 0.633, I2 = 96.6%, Fig. 6A). As for the type of control group, cholecystectomy increased CVD risk compared with healthy controls without gallstones (RR 1.19, 95% CI [1.05–1.35], p = 0.007, I2 = 83.3%, Fig. 6B) and lowered CVD risk compared with gallstone carriers (RR 0.62, 95% CI [0.57–0.67], p < 0.001, I2 = 82.1%, Fig. 6B).
Figure 6: No association between cholecystectomy and coronary heart disease.
Forest plot of relative risk between cholecystectomy and CVD in subgroup analysis. (A) Cholecystectomy in coronary heart disease. (B) Stratified by type of control group.Cholecystectomy, all-cause, cardiovascular and cancer mortality
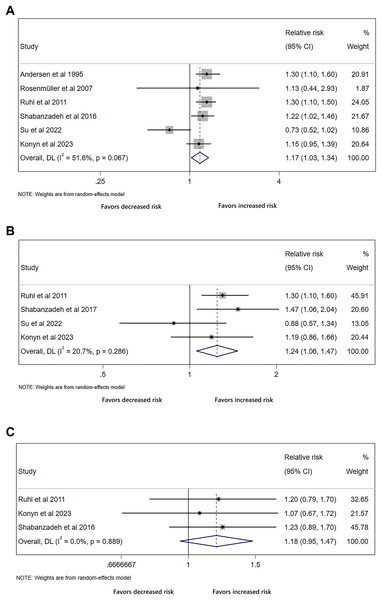
The analysis of all-cause mortality included six studies, with four studies for cardiovascular mortality and three studies for cancer mortality. The results showed that cholecystectomy conferred an increased risk of all-cause mortality (RR 1.17, 95% CI [1.03–1.34], p = 0.020, I2 = 51.6%, Fig. 7A), and cardiovascular mortality (RR 1.24, 95% CI [1.06–1.47], p = 0.009, I2 = 20.7%, Fig. 7B). However, we did not detect significant difference in cancer mortality (RR 1.18, 95% CI [0.95–1.47], p = 0.131, I2 = 0.0%, Fig. 7C).
Figure 7: Cholecystectomy shows increased risk for all-cause and cardiovascular mortality.
Forest plot of relative risk between cholecystectomy and mortality. (A) All-cause mortality. (B) Cardiovascular mortality. (C) Cancer mortality.Discussion
In our study, cholecystectomy was not associated with the overall development of CVD, as well as CHD. In subgroup analysis, cholecystectomy increased CVD risk compared with non-gallstone controls but lowered risk compared with gallstone patients. Besides, increase in risk were observed for all-cause and cardiovascular mortality, but not for cancer mortality. These results raised concern for surveillance strategies following cholecystectomy.
Several studies have reported significant association between GSD, especially cholesterol stone and CVD (Upala, Sanguankeo & Jaruvongvanich, 2017; Zhang et al., 2023; Zheng et al., 2016). One of the potential mechanisms is the established common risk factors between the two diseases, including hypertension, diabetes, insulin resistance, obesity, hyperlipidemia, etc (Misciagna et al., 2000; Targher & Byrne, 2015; Tsai et al., 2004; Volzke et al., 2005; Wang, Cohen & Carey, 2009), which is still significant even after adjustment of common risk factors. Besides, similar alterations in microbiota and microbiota-derived metabolites are also observed in the two diseases, with decreased abundance of Faecalibacterium (van den Munckhof et al., 2018; Wu et al., 2013) and increased concentration of trimethylamine-N-oxide (TMAO) (Amrein et al., 2020; Chen et al., 2019; Zhu et al., 2021). It seems that the above mechanisms focus more on gallstone, especially cholesterol stone itself, rather than cholecystectomy. Our study also demonstrated that cholecystectomy showed no association with the overall development of CVD, as well as CHD, based on current evidence. Partially in accordance with our results, a recent study demonstrated that the association between GSD and CVD might be identifies as sharing a similar biological mechanism, but not a direct causal effect (Zhang et al., 2023), characterized by multiple pleiotropic loci identified in cross-phenotype association study and shared gene-tissue pairs detected by transcriptome-wide association study (Zhang et al., 2023).
One of the interesting findings was that cholecystectomy increased CVD risk compared with healthy controls without gallstones, while lowered CVD risk compared with gallstone carriers. Besides, the reasons for cholecystectomy of the included studies in “cholecystectomy vs healthy controls” group were all due to gallstones. With the established conclusions that GSD imposed an increased risk on CVD (Fairfield, Wigmore & Harrison, 2019; Upala, Sanguankeo & Jaruvongvanich, 2017), it was logically anticipated that cholecystectomy in patients with gallstone could partially neutralize the detrimental effects of the cardiovascular system caused by gallstone itself. However, it could still increase CVD risk significantly. With limited included studies in our meta-analysis, more population-based studies are still needed to verify these conclusions.
Our study also proved that cholecystectomy increased all-cause and cardiovascular mortality with little heterogeneity, but not cancer mortality. Currently, the underlying mechanisms are still uncertain. It is postulated that GSD shares similar features with the mechanisms of cardiovascular and other common causes of death (Ruhl & Everhart, 2011), including gallbladder hypomotility (Portincasa, Moschetta & Palasciano, 2006), insulin resistance (Cortes, Barrera & Nervi, 2020; Jornayvaz, Samuel & Shulman, 2010) and dysregulation of lipid metabolism in the liver (Targher, Day & Bonora, 2010). The above mechanisms, except for gallbladder hypomotility, are also verified in cholecystectomy (Cortes et al., 2017; Latenstein et al., 2020; Shi et al., 2020). One study pointed out that all-cause mortality for gallstones and cholecystectomy was nearly the same, but was higher compared with non-GSD controls (Ruhl & Everhart, 2011). However, the studies to investigate the association between gallstone and mortality is insufficient. Whether the increased all-cause and cardiovascular mortality for cholecystectomy is due to gallstone formation or other independent mechanisms still needs further investigation.
Our study has several limitations. Although most of the included studies were adjusted for major confounders such as age, sex, diabetes, hypertension, there were likely to be residual or unmeasured confounding factors. In addition, the high heterogeneity in this study arouse the need for more studies to confirm these associations. Although we conducted analysis to reduce heterogeneity according to sensitivity or subgroup analysis, the heterogeneity was still significant in some results. The heterogeneity may be ascribed to subtype of CVD, reasons for cholecystectomy, ethics, genetic differences and environmental and lifestyle-related factors, etc. Besides, the certainty of the evidence was low according to the assessment of limitations, indirectness, and imprecision.
Conclusions
Currently, there is no enough solid evidence on the association between cholecystectomy and CVD, including CHD. It should be noted that cholecystectomy may increase CVD risk compared with non-gallstone controls but lower risk compared with gallstone patients. Besides, we demonstrate an increased risk of all-cause and cardiovascular mortality following cholecystectomy. The current findings provide evidence for surveillance strategies for cholecystectomy. More population-based studies are needed for to confirm these associations.