Combining citizen science, phylogenetics, and bioacoustics to inform taxonomy and conservation of the Near Threatened Proceratophrys paviotii (Anura, Odontophrynidae)
- Published
- Accepted
- Received
- Academic Editor
- Andrew Gregory
- Subject Areas
- Animal Behavior, Genetics, Molecular Biology, Taxonomy, Zoology
- Keywords
- Amphibia, Atlantic Forest, Hotspot, Santa Teresa
- Copyright
- © 2024 Lacerda et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Combining citizen science, phylogenetics, and bioacoustics to inform taxonomy and conservation of the Near Threatened Proceratophrys paviotii (Anura, Odontophrynidae) PeerJ 12:e17990 https://doi.org/10.7717/peerj.17990
Abstract
Herein, basel on novel data gathered by citizens scientists and specialists, we contribute to the improvement of scientific knowledge and conservation of the Near Threatened Proceratophrys paviotii in order to: 1) test for the first time the phylogenetic position and a species delimitation of P. paviotii through a molecular approach; 2) describe a larger sample of its advertisement call to properly encompass the species intraspecific variation; 3) describe for the first time the P. paviotii release call; and 4) provide novel insights on the species conservation status. Our 16S tree confidently grouped P. paviotii with P. cururu, P. renalis, and P. laticeps. The average sequence divergence between P. paviotii and its congeners ranged from 2.2% (P. laticeps) to 9.1% (P. redacta). Advertisement calls consisted of a single note with duration of 0.26–0.58 s, 17–41 pulses emitted at rate of 54.19–77.49 pulses/s and peak frequency of 775.19–947.46 Hz. Release calls consisted of a single note with duration of 0.04–0.43 s, 2–13 pulses emitted at rate of 21.17–81.58 pulses/s and peak frequency of 689.1–1,722.6 Hz. Additionally, our study strongly supports the notion that Citizen Science approaches can yield invaluable information concerning species’ geographic distribution and conservation.
Introduction
Amphibians are among the most endangered animal groups on the planet, with 41% of their species classified as threatened with extinction, including many Neotropical lineages under some risk (Luedtke et al., 2023). The Neotropical genus Proceratophrys Miranda-Ribeiro, 1920 occurs in Brazil, Argentina, Paraguay and possibly Bolivia, and currently includes 43 species (Frost, 2024). Eight species of Proceratophrys are threatened with extinction, classified as Critically Endangered (CR), Vulnerable (VU), or Endangered (EN). Two species of Proceratophrys are classified as Near Threatened (NT). A taxon is classified as Near Threatened (NT) when it is close to or likely to qualify for a threatened category in the near future (IUCN, 2024). Therefore, these species require special attention and focus from both the scientific community and conservation initiatives.
Evolutionary studies have played an important role in anuran taxonomy. Several populations recovered as independent evolutionary units have supported the description of new taxa (e.g., Ferreira et al., 2023; Folly et al., 2024). Conversely, taxa that have not been recovered as reciprocally monophyletic have led to synonymizations (e.g., Faivovich et al., 2021; Pereira et al., 2022). Among Proceratophrys, the same scenarios are observed with species that have been recently described (Santana et al., 2021a, 2021b; Mângia et al., 2022) and synonymized (Mângia et al., 2020) supported by phylogenetic approaches. These studies have great potential to impact conservation status, given that we protect nominal species.
Citizen Science (CS) approaches have great potential in providing information on several biological groups, such as fungi, plants and animals, even on data deficient, rare, or threatened species (e.g., Mori & Menchetti, 2014; Campanaro et al., 2017; Irga, Barker & Torpy, 2018; Heard, Chen & Wen, 2019; Pirotta et al., 2020; Deacon, Govender & Samways, 2023; Farquhar et al., 2023; Krueger et al., 2023; Lacerda et al., 2023), once such projects have encouraged the participation of the public in different stages of scientific projects, such as gathering and/or analyzing biological data (Bonney et al., 2009).
Citizen Science projects focused on anurans have contributed to a vast range of issues in science and conservation (e.g., Pittman & Dorcas, 2006; Price & Dorcas, 2011; Cosentino et al., 2014; Westgate et al., 2015; Sterrett et al., 2019; Antúnez-Fonseca et al., 2021; Ceríaco et al., 2021; Lee et al., 2021; Forti et al., 2022a, 2022b; Glorioso et al., 2022; Forti & Szabo, 2023). The public usually contributes by sending pictures and thus providing data on species occurrence via digital platforms, such as the iNaturalist (e.g., Forti et al., 2022a, 2022b; Forti & Szabo, 2023). This active involvement of the public in sharing visual data enables researchers to gain insights into the distribution and presence of anuran species. However, while the pictures are invaluable for assessing anuran’s identity, CS projects often overlook a critical aspect: a vast vocal repertoire of this group, emitted depending on its social context, such as reproductive, aggressive, defensive, and feeding calls (Köhler et al., 2017). In anurans, advertisement calls are heritable and usually species-specific (Duellman & Trueb, 1994; Wells, 2007). Despite the importance of bioacoustics for taxonomic and conservation purposes, only a few CS projects focus on receiving anurans audio files from the public. For instance, the FrogID project gathers audio files containing anuran vocalization from Australian citizens (https://www.frogid.net.au/) (Rowley et al., 2019). This project has contributed to several scientific and conservation issues, such as: fire effect (Rowley, Callaghan & Cornwell, 2020), invasive species (Rowley & Callaghan, 2022), species geographic distribution (Cutajar et al., 2022), behavior (Thompson et al., 2022), urban impact (Liu et al., 2022), among others.
Since 2020, we have been conducting a CS project entitled Cantoria de Quintal (translated as “Songs from the Back Yard”) in the municipality of Santa Teresa (Lacerda et al., 2023; Lacerda, Santos & Lima, 2023). We have encouraged the public to record anurans from their yards and/or surroundings using smartphones. The Cantoria de Quintal project aims to investigate scientific and conservation issues, such as species distribution, whether threatened species also occur in unprotected areas, urban and deforestation impacts, among others. However, species identification through audio files is not always easy and can be hampered when dealing with rare or poorly known species.
During 2020–2023 we received 42 audio files as part of the Cantoria de Quintal project from 10 citizen scientists. These files contained calls that we initially categorized as belonging to Proceratophrys sp.. After assessing literature information (Cruz, Prado & Izecksohn, 2005; Ferreira et al., 2019a), we assigned those records to Proceratophrys sp. (cf. paviotii). We were unable to confirm its identification to the species level due to two main reasons: 1) the vocalization of P. paviotii is not available for comparison in any existing public audio collections; and 2) the call description provided by Cruz, Prado & Izecksohn (2005) in the species’ original description is based on only seven calls from a single male, which certainly do not properly encompass the species’ intra-specific variation. Motivated by the challenge of properly identifying the citizen scientists’ records, we initiated an integrative investigation on the Proceratophrys paviotii taxonomy.
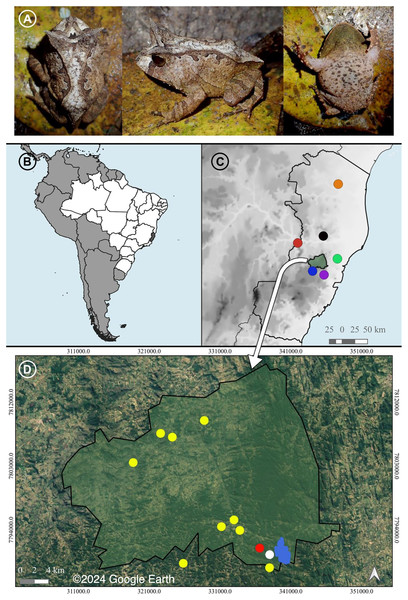
Proceratophrys paviotii (Fig. 1A) is a Near Threatened species (IUCN, 2024) that occurs in the central and northern State of Espírito Santo (Prado & Pombal, 2008; Almeida, Gasparini & Peloso, 2011; Peres & Simon, 2011; Figs. 1B–1D). This species has never been included in phylogenetic or molecular species delimitation approaches so far (see Mângia et al., 2020; Santana et al., 2021a, 2021b; Mângia et al., 2022). Therefore, its phylogenetic position has not been properly tested.
Figure 1: A recorded male and the distribution of Proceratophrys paviotii.
(A) A recorded male of Proceratophrys paviotii (MBML 12369; snout-vent-length, SVL 44.1 mm): dorsal, lateral and ventral views. (B) State of Espírito Santo (shaded) in Southeastern Brazil. (C) Municipality of Santa Teresa (shaded) and the known distribution of Proceratophrys paviotii in the State of Espírito Santo: Municipality of Aracruz (green circle; Prado & Pombal (2008)), Municipality of Baixo Guandu (red circle; Prado & Pombal (2008)), Municipality of Santa Leopoldina (purple circle; Prado & Pombal (2008)), Municipality of Santa Maria do Jetibá (blue circle; Almeida, Gasparini & Peloso (2011)), Municipality of Marilândia (black circle; Almeida, Gasparini & Peloso (2011)), and Municipality of Pinheiros (red circle; Peres & Simon (2011)). (D) Municipality of Santa Teresa, type locality of P. paviotii Estação Biológica Santa Lucia (blue); records of P. paviotii made through audio files sent by citizen scientists (yellow); records of P. paviotii made by formal scientists while visiting a citizen scientists’ property (red); and records of P. paviotii made by formal scientists (white). Map builded using the Google Satellite tool from QGis 3.22.2: source IBGE (https://www.ibge.gov.br/geociencias/organizacao-do-territorio/malhas-territoriais/15774-malhas.html). © 2024 Google Earth.Efficient conservation predictions and strategies depend on filling knowledge gaps such as on its taxonomic identity (Linnean shortfall), geographic distribution (Wallacean shortfall), and evolution (Darwinian shortfall) (see Hortal et al., 2015). Therefore, combining data provided by citizen scientists and specialists, in this study we: 1) test for the first time the phylogenetic position and a species delimitation of Proceratophrys paviotii through a molecular approach; 2) describe a larger sample of its advertisement call to properly encompass the species intraspecific variation; 3) describe for the first time the P. paviotii release call; and 4) provide novel occurrence data and insights on the species conservation status. Thus, herein we contribute to the improvement of scientific knowledge and conservation of the Near Threatened Proceratophrys paviotii.
Materials and Methods
Study area
The Municipality of Santa Teresa, State of Espírito Santo, located within the Atlantic Forest of Southeastern Brazil (Fig. 1), is known for its rich biodiversity across various biological groups (e.g., Thomaz & Monteiro, 1997; Brown & Freitas, 2000; Passamani, Mendes & Chiarello, 2000; Wendt et al., 2010; Gatti et al., 2014; Novaes et al., 2016). With 109 amphibian species, Santa Teresa stands out for harboring one of the highest diversities of this group in the world (Ferreira et al., 2019a; Lacerda et al., 2021). Notably, Santa Teresa is the only place where six species of Proceratophrys can be found in sympatry (Prado & Pombal, 2008; Ferreira et al., 2019a): Proceratophrys boiei (Wied-Neuwied, 1824); P. laticeps Izecksohn & Peixoto, 1981; P. moehringi Weygoldt & Peixoto, 1985; P. paviotii Cruz, Prado & Izecksohn, 2005; P. phyllostomus Izecksohn, Cruz & Peixoto, 1998; and P. schirchi Miranda-Ribeiro, 1937.
Phylogenetic inference and genetic distances
We extracted DNA from tissue samples (liver) using the QIAGEN DNeasy Blood and Tissue Kit (Valencia, California, USA) following the manufacturer’s protocol. Next, we amplified a fragment of the mitochondrial 16S gene using primers 16Sar and 16Sbr (Palumbi et al., 1991). The PCR protocol was configured with one initial phase of 94 °C for 3 min, followed by 35 cycles of 94 °C for 20 s, 50 °C for 20 s, 72 °C for 40 s, and a final extension phase of 72 °C for 5 min. Purification of PCR products and sequencing were performed by ACGTene Análises Moleculares Ltda. (Alvorada, Rio Grande do Sul, Brazil).
We combined our newly generated 16S sequences with comparable 16S sequences of Proceratophrys individuals available on GenBank, and also included the outgroups Odontophrynus spp., Macrogenioglottus alipioi, Cycloramphus acangatan and Thoropa miliaris, which are available in GenBank (Document S1). We aligned 16S mtDNA gene fragments using the MAFFT algorithm (Katoh & Toh, 2008) in Geneious v9.0.5 with default settings. The final dataset comprised 84 sequences of a 498 base-pair (bp) fragment of the 16S gene. All GenBank accession numbers and genetic voucher samples used here are listed in the Supplemental Material. We used the Bayesian Information Criterion in jModelTest (Darriba et al., 2012) to determine that GTR+I+G was the best model of nucleotide substitution for our 16S data set. To estimate phylogenetic relationships, we used Bayesian Inference (BI) in BEAST v2.6.6 (Bouckaert et al., 2019) for 20 million generations, sampling every 2,000, using a Yule speciation prior, implementing a relaxed clock log normal. We checked for stationarity by visually inspecting trace plots and ensuring that all effective sample sizes were above 200 in Tracer v1.7.1 (Rambaut et al., 2018). The first 10% of sampled genealogies were discarded as burn-in, and the maximum clade credibility tree with median node ages was calculated with TreeAnnotator v2.6.6 (Bouckaert et al., 2019). We calculated sequence divergences (uncorrected p-distances) among species/individuals using MEGA v10.1.1 (Kumar et al., 2018). In order to explore the relationship among haplotypes, we estimated haplotype networks among species closely related to the P. paviotii for the 16S mtDNA gene in POPART (Leigh & Bryant, 2015) using the median-joining network method. We identified each species using different colors in the haplotype network. All molecular protocols follow previous studies on Proceratophrys taxonomy (e.g., Santana et al., 2021a, 2021b; Mângia et al., 2022).
Bioacoustics
Records (Table 1) made by specialists were performed with Tascam DR-40 and Tascam DR-05 recorders at a sampling rate of 44.1 kHz and 24 bits resolution (FNJV 60377–60383, 60384–60387). One male (MBML 12366) emitted release calls when handled (FNJV 60382). The citizen scientists’ files were recorded using smartphones: Motorola Moto G7 plus (FNJV 60388, 60391), Samsung Galaxy A21s (FNJV 60397) and other two unspecified models (FNJV 60389–60390, 60392–60396). These files were sent via Whatsapp app (.ogg format) and then converted to .wav format. Bioacoustic analyses were performed using Raven Pro 1.5 software (Bioacoustics Research Program, 2014). Spectrograms were produced using Hann window type, DFT 256 sample size, and time grid overlap of 80.1%. Figures were built using seewave and tuneR packages in R (R Core Team, 2017), with window length = 512, overlap = 80.1%. Bioacoustic terminology follows the call-centered approach proposed by Köhler et al. (2017). We calculated call duration (s), number of pulses per call (pulses/call), pulse emission rate per call (pulses/s), pulse duration (s), interval between pulses (s), peak frequency (Hz), frequency 5% (Hz), and frequency 95% (Hz). Quantitative parameters are presented as minimum–maximum (mean ± standard deviation; n = sample size). Low frequencies up to 200 Hz (safely below the minimum frequency reached by P. paviotii) were high-pass filtered to decrease background noise in the recording files using Raven Pro 1.5. Recordings were then compared to the literature available for other Proceratophrys species. Vocalization recordings were deposited at Fonoteca Neotropical Jacques Vielliard (FNJV; https://www2.ib.unicamp.br/fnjv/).
| Municipality | Voucher | Coordinates | Area description |
|---|---|---|---|
| Santa Teresa | FNJV 60391 | 19°56′20″S; 40°36′46″W | A wetland inside the urban area |
| FNJV 60388* | 19°56′19″S; 40°36′45″W | A street located at the border of the urban area (rainwater stored by the sidewalk channel) | |
| FNJV 60397 | 19°56′38″S; 40°35′17″W | A vacant lot located at the border of the urban area | |
| FNJV 60396 | 19°49′10″S; 40°41′37″W | A yard located at a rural area | |
| FNJV 60389*, 60390*, 60392–60395 | 19°49′27″S; 40°40′40″W | A yard located at a rural area | |
| – | 19°48′03″S; 40°38′01″W | A yard located at a rural area | |
| – | 19°51′21″S; 40°43′39″W | A yard located at a rural area | |
| – | 19°55′54″S; 40°35′42″W | A street located at the border of the urban area | |
| FNJV 60377–60383* | 19°58′00″S; 40°33′41″W | A yard located at a rural area | |
| FNJV 60384–60387* | 19°58′30″S; 40°32′53″W | Rainwater stored at a roadside | |
| Santa Leopoldina | – | 19°59′37″S; 40°32′41″W | A yard located at a forest edge |
| Santa Maria de Jetibá | – | 19°59′11″S; 40°39′58″W | A yard located at a rural area |
We examined the variability of acoustic parameters in male calls, focusing on both intra-individual and inter-individual levels. For assessing the intra-individual coefficient of variation (CVintra), we utilized a dataset of 105 calls from 11 individuals and computed the mean () and standard deviation (SD) of individual calls using the formula CV = (SD/) × 100. To determine inter-individual variation (CVinter), we evaluated the mean and standard deviation of parameter values across all individuals, including two cases where only one call was available for each. Parameters with CV values ≤ 5% are considered static, while those with CV values ≥ 12% are considered dynamic (Gerhardt, 1991). We calculated both CVintra and CVinter for six parameters (note duration, peak frequency, 5% frequency, 95% frequency, pulse number, and pulses/second). Additionally, to discern whether intra-individual variation was greater than inter-individual variation, we computed the CVintra/CVinter ratio. If CVintra/CVinter is >1, it means a greater variation between males (inter-individual) than an intra-individual variation (Gerhardt, 1991; Moser et al., 2022).
Occurrence data through Citizen Science and by specialists
We received 42 audio files (.ogg format) through the Cantoria de Quintal project containing calls emitted by Proceratophrys paviotii. Files were sent by eight citizen scientists from eight distinct localities in the Municipality of Santa Teresa, one in Santa Maria de Jetibá, and one in Santa Leopoldina, State of Espírito Santo (Fig. 1; Table 1). The Cantoria de Quintal activities include visiting citizen scientists to provide environmental education and enhance their engagement in the project. During one of these visits (October 23rd, 2020), we found a population of Proceratophrys paviotii inhabiting the citizen scientist’s yard and surroundings with several males in calling activity (FNJV 60377–60383). This property (19°58′00″S; 40°33′41″W) is located about 2.2 km straight line from Estação Biológica de Santa Lúcia (type locality of P. paviotii; Fig. 1). Males were calling from the banks of an anthropized sandy stream. Recordings took place from 06:00 to 08:30 pm, during a light rain, air temperature ca. 20 °C. Additionally, we found a second population of Proceratophrys paviotii (February 19th, 2021) with several males calling from the rainwater stored at a roadside about 800 m from the species type locality (Fig. 1). We recorded five of these males (FNJV 60384–60387). Recordings took place from 9:00 to 10:00 pm, during a light rain, air temperature ca. 20 °C.
Voucher specimens
Specimens collected as vouchers were anesthetized and killed with lidocaine 2%, fixed in formaldehyde 10%, and preserved in 70% ethanol at Museu de Biologia Prof. Mello Leitão (MBML) from Instituto Nacional da Mata Atlântica (INMA) (MBML 12366–12370). Before fixation, specimens had tissue (liver) samples extracted and stored in 96% ethanol for whole genomic DNA extraction. We have followed CONCEA (Jared et al., 2023) for guidelines for the ethical treatment. The Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio #63575-5) and animal research ethics committee of the Universidade de Vila Velha (CEUAUVV #491-2018) provided sampling permits.
Results
Phylogenetic inference and genetic distances
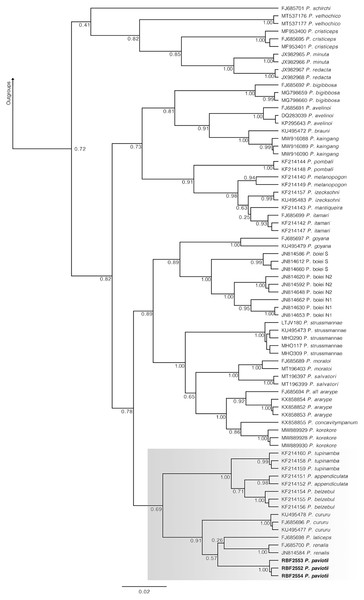
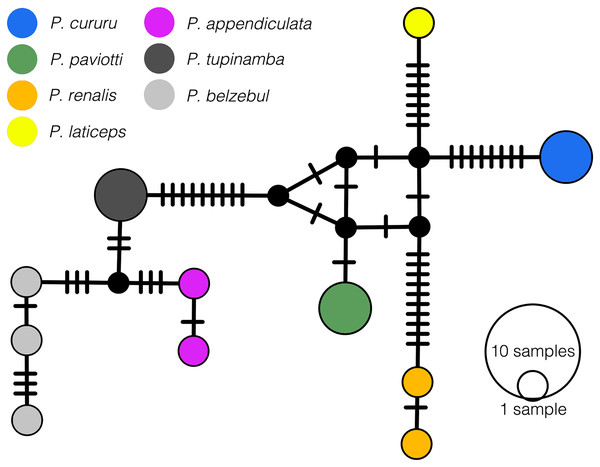
In our 16S tree analysis, Proceratophrys paviotii was grouped with P. cururu, P. renalis, and P. laticeps with a high posterior probability (PP = 0.91), forming a distinct clade (Fig. 2). Additionally, P. appendiculata, P. belzebul, and P. tupinamba formed a sister clade to this group (PP = 1.00). However, the relationship between these clades was not strongly supported (PP = 0.69). Due to the utilization of a single mtDNA locus for species barcoding, our tree had low posterior probabilities for several nodes within Proceratophrys, which is expected. The average sequence divergence between P. paviotii and its congeners ranged from 2.2% (P. laticeps) to 9.1% (P. redacta) (Table 2). Furthermore, the mitochondrial haplotype network, based on a fragment of the 16S gene of P. paviotii and its closely related species (see Fig. 3), shows seven distinct mitochondrial lineages, with no haplotype sharing between them.
Figure 2: Phylogenetic analysis of the 16S mtDNA gene for the Proceratophrys spp.
Nodes are labeled with the Bayesian posterior probability. Scale bar is substitutions/site. Gray box indicates the lineage of P. paviotii and closely related species used on the haplotype network. DJ Santana prepared the tree figure using FigTree v1.4.4.| Species | p-distance | Species | p-distance |
|---|---|---|---|
| P. aff. ararype | 0.076 | P. kaingang | 0.051 |
| P. appendiculata | 0.028 | P. korekore | 0.077 |
| P. ararype | 0.081 | P. laticeps | 0.022 |
| P. avelinoi | 0.059 | P. mantiqueira | 0.038 |
| P. belzebul | 0.035 | P. melanopogon | 0.041 |
| P. bigibbosa | 0.046 | P. minuta | 0.080 |
| P. boiei N1 | 0.056 | P. moratoi | 0.068 |
| P. boiei N2 | 0.064 | P. pombali | 0.049 |
| P. boiei S | 0.037 | P. redacta | 0.091 |
| P. brauni | 0.060 | P. renalis | 0.030 |
| P. concavitympanum | 0.086 | P. salvatori | 0.067 |
| P. cristiceps | 0.087 | P. schirchi | 0.073 |
| P. cururu | 0.027 | P. strussmannae | 0.069 |
| P. goyana | 0.036 | P. tupinamba | 0.024 |
| P. itamari | 0.034 | P. velhochico | 0.082 |
| P. izecksohni | 0.033 |
Figure 3: Median-joining haplotype network based on mtDNA 16S of Proceratophrys pavitotii.
Each haplotype circle is proportional to its frequency (indicated in legend). Each color represents distinct species, and black dots represent inferred unsampled or extinct haplotypes. Mutational steps between alleles are represented by lines. Proceratophrys cururu (blue), P. paviotii (green), P. renalis (orange), P. laticeps (yellow), P. appendiculata (pink), P. tupinamba (dark grey), P. belzebul (light grey). DJ Santana prepared the Haplotype network using POPART.Bioacoustics
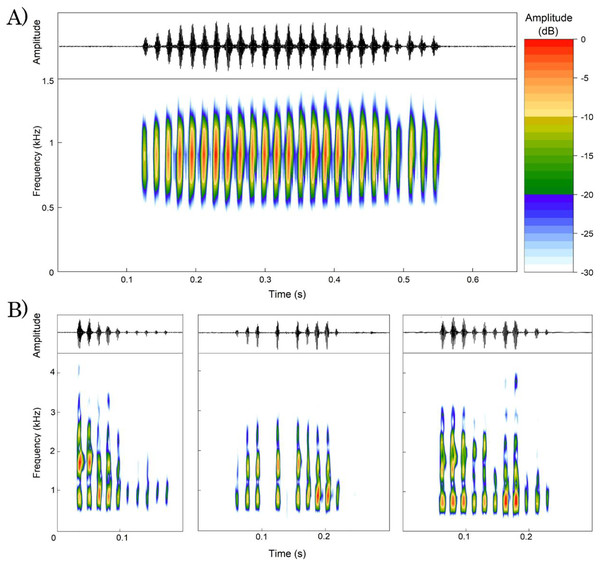
We analyzed a total of 107 advertisement calls emitted by 13 males of Proceratophrys paviotii (Table 3). Calls consisted of a single note with duration of 0.26–0.58 s (0.46 s ± 0.07; n = 107 calls), 17–41 pulses (31.17 pulses ± 5.23; n = 104 calls), pulse duration of 0.007–0.026 s (0.012 s ± 0.002; n = 755 pulses), pulses emitted at rate of 54.19–77.49 pulses/s (68.32 pulses/s ± 6.64; n = 104 calls). Calls had peak frequency of 775.19–947.46 Hz (861.85 Hz ± 33.09; n = 101 calls), frequency 5% of 656.25–861.33 Hz (718.85 Hz ± 45.51; n = 101 calls), and frequency 95% of 937.5–1,119.73 Hz (1,001.91 Hz ± 54.84; n = 101 calls). Calls have an ascendant amplitude modulation on its beginning and descendant amplitude modulation on its ending (Fig. 4A).
| Species | Notes/call | Note duration (s) | Pulses/ note | Pulses/s | Dominant frequency (kHz) | Source |
|---|---|---|---|---|---|---|
| P. paviotii | 1 | 0.26–0.58 | 17–41 | 54.2–77.5 | 0.775–0.947 | Present study |
| P. paviotii | 1 | 0.34–0.43 | 26–32 | x | 0.660–1.280 | Cruz, Prado & Izecksohn (2005) |
| P. appendiculata | 1 | 1.32–2.41 | 51–129 | 30.0–65.4 | 0.562–0.656 | Dias et al. (2013) |
| P. ararype | 1 | 0.37–0.65 | 38–65 | 95.7–102.7 | 1.033–1.378 | Mângia et al. (2018) |
| P. avelinoi | 1 | 0.22–0.75 | 23–70 | 64.0–72.0 | 1.050–2.300 | Kwet & Baldo (2003) |
| P. bigibbosa | 1 | 1.60–1.90 | 40–45 | 23.0–27.0 | 1.050 | Kwet & Faivovich (2001) |
| P. boiei | 1 | 0.70–0.80 | 30–35 | 45.0 | 0.350–1.350 | Heyer et al. (1990) |
| P. brauni | 1 | 0.70–0.90 | 24–28 | 35.0–40.0 | 1.350 | Kwet & Faivovich (2001) |
| P. carranca | 1–10 | 0.04–0.19 | 5–21 | 95.2–131.5 | 1.033–1.378 | Godinho et al. (2013) |
| P. concavitympanum | 1 | 0.18–0.50 | 19–51 | 100.0–119.7 | 754.6–1,186.3 | Santana et al. (2010) |
| P. cristiceps | 1 | 0.52–0.79 | 33–69 | 78.5–91.8 | 0.860–1.030 | Nunes & Juncá (2006), Nunes et al. (2015) |
| P. cururu | 1 | 1.20 | x | 45.0 | 0.600–1.000 | Eterovick & Sazima (1998) |
| P. goyana | 1–34 | 0.06–0.24 | 7–23 | 83.3–120.5 | 0.937–1.125 | Martins & Giaretta (2013) |
| P. huntingtoni | 1 | 0.2–0.3 | 19–25 | x | 1.095–1.3445 | Ávila, Pansonato & Strüssmann (2012) |
| P. itamari | 1 | 0.4–0.8 | 20–42 | 49.0–55.0 | 1.033–1.205 | Mângia et al. (2014) |
| P. korekore | 1 | 0.16–0.33 | 18–32 | 96.4–111.1 | 0.861 | Santana et al. (2021b) |
| P. laticeps | 1 | 0.49–1.57 | 28–94 | 44.0–74.4 | 0.340–0.680 | Araújo et al. (2021), Martins & Giaretta (2021), Sichieri et al. (2021) |
| P. mantiqueira | 1 | 0.17–0.48 | 12–41 | 68.0–96.0 | 0.999–1.274 | Mângia, Santana & Feio (2010) |
| P. melanopogon | 1 | 0.40–0.80 | 20–38 | 40.0–55.0 | 0.831–1.033 | Mângia et al. (2014) |
| P. minuta | 1 | 0.40–0.72 | 30–52 | 66.8–75.2 | 1.980–2.070 | Nascimento et al. (2019) |
| P. moehringi | 1 | 3.50–4.00 | x | 33.0–40.0 | 0.200–0.700 | Weygoldt & Peixoto (1985) |
| P. moratoi | 1 | 0.14–0.33 | 12–26 | 65.0–103.0 | 1.153–1.594 | Brasileiro, Martins & Jim (2008), Martins & Giaretta (2012), Magalhães et al. (2020) |
| P. palustris | 1 | 0.33–0.75 | 12–26 | 32.0–37.0 | 1.464 | Martins & Giaretta (2012) |
| P. pombali | 1 | 0.30–0.60 | 30–49 | 76.2–88.8 | 1.312–1.550 | Malagoli, Mângia & Haddad (2016) |
| P. redacta | 1 | 0.15–0.85 | 13–74 | 84.8–106.7 | 1.697–2.142 | Simões et al. (2020) |
| P. renalis | 1 | 0.15–0.46 | 13–30 | 61.5–86.1 | 0.689–1.033 | Santana et al. (2011) |
| P. rotundipalpebra | 1–24 | 0.04–0.33 | 4–32 | 78.1–130.4 | 1.125–1.453 | Martins & Giaretta (2013) |
| P. salvatori | 1 | 0.19–0.42 | 15–25 | 54.0–61.0 | 1.572–1.875 | Bastos et al. (2011), Magalhães et al. (2020) |
| P. sanctaritae | 1 | 0.20–0.90 | 31–94 | 102.3–142.4 | 0.950–1.290 | Cruz & Napoli (2010) |
| P. schirchi | 1–6 | 0.18–0.45 | 15–31 | 66.0–93.4 | 0.861–1.810 | Nascimento et al. (2019), Sichieri et al. (2021) |
| P. velhochico | 1 | 0.32–0.51 | 29–46 | 85.4–96.5 | 1,312.5 | Mângia et al. (2022) |
| P. vielliardi | 3–20 | 0.04–0.30 | 4–30 | 95.6–118.8 | 1.022–1.291 | Martins & Giaretta (2011) |
Figure 4: Oscillogram and spectrogram of Proceratophrys paviotii recorded at Santa Teresa, State of Espírito Santo, Southeastern Brazil.
(A) Advertisement call; and (B) three release calls showing different patterns of amplitude and frequency modulation. The scale of amplitudes is decibels (dB), where colors represent different amplitude levels. The scale ranges from 0 to 30 dB, with warm colors indicating lower amplitudes and cool colors indicating higher amplitudes. The colors are distributed as follows: Red for 0 dB, Orange for 5 dB, Yellow for 10 dB, Light Green for 15 dB, Dark Blue for 20 dB, Light Blue for 25 dB, and White for 30 dB.We analyzed a total of 55 release calls emitted by a single male of Proceratophrys paviotii. Release calls consisted of a single note with duration of 0.04–0.43 s (0.15 s ± 0.07; n = 55 calls), 2–13 pulses (7.78 pulses ± 3.22; n = 55 calls) with 0.003–0.023 s (0.008 s ± 0.002; n = 427 pulses) emitted at rate of 21.17–81.58 pulses/s (51.56 s ± 11.9; n = 55 calls). Release calls had peak frequency of 689.1–1,722.6 Hz (1,227.8 Hz ± 424.3; n = 55 calls), frequency 5% of 516.8–861.3 Hz (667.1 Hz ± 81.6; n = 55 calls), and frequency 95% of 1,205.9–3,445.3 Hz (2,211.7 Hz ± 322.7; n = 55 calls). Release calls had irregular patterns of amplitude and frequency modulation. Isolated pulses were sporadically emitted between calls (Fig. 4B).
The CVintra demonstrated a static variation in peak frequency, 5% frequency, 95% frequency and pulse rate, and an intermediate variation in note duration and number of pulses per call. On the other hand, in a CVinter approach, note duration and number of pulses per call were both considered dynamic; pulse rate was considered intermediate; and peak frequency, 5% frequency, 95% frequency were considered static (Table 4). Additionally, the ratio between CVinter and CVintra was greater than one (>1) in all parameters, so it was considered a dynamic variation.
| Acoustic signal | CVintra | CVinter | CVinter/CVintra |
|---|---|---|---|
| Note duration | 9.3 | 14.6 | 1.5 |
| Peak frequency | 1.6* | 3.8* | 2.3 |
| 5% frequency | 2.8* | 5.9* | 2.1 |
| 95% frequency | 3.9* | 5.4* | 1.4 |
| Pulses/call | 8.6 | 16.8 | 2.0 |
| Pulse rate | 2.1* | 9.7 | 4.4 |
The advertisement call of Proceratophrys paviotii differs from those of P. carranca, P. goyana, P. rotundipalpebra, and P. vielliardi by its single note call pattern (multi noted call pattern in these species). The advertisement call of P. paviotii differs from those of P. appendiculata, P. bigibbosa, P. boiei, P. brauni, P. cururu, and P. moehringi by its shorter notes. The advertisement call of P. paviotii differs from those of P. carranca and P. goyana by its longer notes. The advertisement call of P. paviotii differs from those of P. appendiculata and P. cristiceps by its lower number of pulses per note. It differs from those of P. bigibbosa, P. boiei, P. brauni, P. cururu, P. moheringi, and P. palustris by its higher pulse emission rate. It differs from those of P. ararype, P. carranca, P. concavitympanum, P. cristiceps, P. goyana, P. korekore, P. redacta, P. rotundipalpebra, P. sanctaritae, P. velhochico, and P. vieliardi by its lower pulse emission rate. The advertisement call of P. paviotii differs from those of P. brauni, P. minuta, P. moratoi, P. palustris, P. pombali, P. redacta, P. salvatori, and P. velhochico by its lower peak of frequency. The advertisement call of P. paviotii differs from those of P. appendiculata and P. moehringi by its higher peak of frequency. For detailed comparison and literature sources, see Table 3.
Discussion
Data gathered by citizen scientists were crucial in discovering new populations of Proceratophrys paviotii, enabling valuable systematic evaluations. Our phylogenetic analysis grouped Proceratophrys paviotii with P. cururu, P. renalis, and P. laticeps (PP = 0.91) in a clade, and P. appendiculata, P. belzebul, and P. tupinamba in a sister clade (PP = 1.00). Sequence divergence ranged from 2.2% to 9.1%. Advertisement calls consisted of single-note calls lasting 0.26–0.58 s with 17–41 pulses emitted at a rate of 54.19–77.49 pulses/s, and peak of frequency at 775.19–947.46 Hz. Additionally, the analysis of 55 release calls from a single male showed durations of 0.04–0.43 s with 2–13 pulses emitted at a rate of 21.17–81.58 pulses/s, and peak of frequency at 689.1–1,722.6 Hz. Variation analysis indicated dynamic traits.
The genus Proceratophrys currently encompasses 43 species with occurrences in Brazil, Argentina, Paraguay, and possibly in Bolivia (Frost, 2024). Historically, most Proceratophrys species have been arranged into four morphological groups, currently considered non-monophyletic: the P. appendiculata complex, the P. bigibbosa group, the P. boiei complex, and the P. cristiceps group (Izecksohn, Cruz & Peixoto, 1998; Giaretta, Bernarde & Kokubum, 2000; Kwet & Faivovich, 2001; Prado & Pombal, 2008). We did not recover the monophyly of these phenotypic arrangements in accordance with previous studies (Mângia et al., 2020; Santana et al., 2021a, 2021b; Mângia et al., 2022).
Among anurans, genetic distance of 3% (16S rDNA) between taxa is commonly used as threshold to consider a lineage as candidate species (Fouquet et al., 2007) (i.e., genetic distance below this threshold is commonly observed intraspecifically). Among Proceratophrys spp., four species showed genetic distance below this threshold compared to P. paviotii: P. appendiculata, P. cururu, P. laticeps, and P. tupinamba. In spite of this low genetic distance, we reinforce that P. paviotii can be easily diagnosed from these species based on the following combination of characters: presence of palpebral appendages and lack of rostral appendage (Prado & Pombal, 2008). In addition, other Proceratophrys were proven to be morphological, genetic, and geographically distinguished, and have been previously described or validated with values lower than 3% of genetic distance (Dias et al., 2013; Magalhães et al., 2020; Santana et al., 2021a).
Bioacoustic characters have commonly shown to be relevant in diagnosing species of Proceratophrys (e.g., Cruz & Napoli, 2010; Martins & Giaretta, 2011; Godinho et al., 2013; Mângia et al., 2014, 2018, 2022). However, 12 species of Proceratophrys still lack call description. Among the 31 species of Proceratophrys with described vocalization, P. paviotii stands out due to its notably subsampling. The description provided by Cruz, Prado & Izecksohn (2005) is based on seven calls emitted by a single male. Such a sample limitation certainly makes taxonomic comparisons questionable because it does not properly encompass intra-specific variation. Herein we increased samples of both the number of analyzed calls (seven calls in Cruz, Prado & Izecksohn (2005) and 107 in the present study) and recorded males (one in Cruz, Prado & Izecksohn (2005) and 13 in the present study). Nevertheless, the advertisement call of P. paviotii distinguishes it from 22 out of 30 Proceratophrys spp. (Table 3). Given that the advertisement call of P. paviotii differentiates it from the majority of species within the genus based on various parameters, such as note duration, pulse emission rate per call, and dominant frequency, we reinforce the importance of using bioacoustics characters in diagnosing Proceratophrys species.
Spectral parameters and pulse rate are commonly considered static in amphibians while temporal parameters are classified as dynamic (Gerhardt, 1991; Bee et al., 2001). Similar pattern was observed for Proceratophrys paviotii. The ratio between CVinter and CVintra observed indicates that there is greater variation between males than the individual variation itself, as also observed in other species of anurans (e.g., Morais et al., 2012; Forti, Lingnau & Bertoluci, 2017). Furthermore, all advertisement call properties examined in this study exhibited greater variability inter-individuals than intra-individuals, suggesting the possibility of individual recognition (Bee et al., 2001; Pettitt, Bourne & Bee, 2013; Moser et al., 2022).
A taxon is classified as Near Threatened (NT) when is close to or likely to qualifying for a threatened category in the near future (IUCN, 2024). However, this does not appear to apply to P. paviotii. In several studies conducted across its distribution range in the central and northern parts of the State of Espírito Santo (Fig. 1) (Cruz, Prado & Izecksohn, 2005; Prado & Pombal, 2008; Almeida, Gasparini & Peloso, 2011; Peres & Simon, 2011; Zocca, Tonini & Ferreira, 2014; Silva et al., 2018; Ferreira et al., 2019a, 2019b), the species has been consistently observed within protected areas (Cruz, Prado & Izecksohn, 2005), as well as in disturbed areas such as coffee plantations (see Prado & Pombal (2008) comment on Teixeira & Coutinho (2002)), and even urban areas (present study). Thus, our results suggest that Proceratophrys paviotii should be classified as Least Concern regarding its conservation status in future evaluations. Additionally, we emphasize that, despite suggesting the classification as Least Concern, assessing the species under the Green Status framework can aid in monitoring the effectiveness of the conservation actions presented herein. This methodology aids in tracking how close the species is to being “Fully Recovered” and in devising more efficient conservation strategies (IUCN, 2024).
The Cantoria de Quintal CS project stands as a pioneering initiative in Brazil, with specific focus on frog vocalizations. Although the project is limited to the State of Espírito Santo, it has managed to gather calls from more than 40 anuran species, including the Near Threatened Proceratophrys paviotii. Our study strongly supports the notion that Citizen Science approaches can yield invaluable information concerning species’ geographic distribution and taxonomy. The data obtained through this participatory approach have provided important insights for conservation efforts, underscoring the potential significance of involving the public in scientific research and conservation initiatives.
Despite the many new studies using Citizen Science, this approach for Neotropical amphibians is still incipient. The approach used in “Cantoria de Quintal” was paramount in discovering new individuals of Proceratophrys paviotii. The genus Proceratophrys includes opportunistic breeders that call only after heavy rains (Prado & Pombal, 2005; Godinho et al., 2013; Mângia et al., 2014; Malagoli, Mângia & Haddad, 2016), and many species in the genus have been described based on limited type series due its rarity (e.g., Prado & Pombal, 2008; Mângia et al., 2014). Therefore, initiatives such as “Cantoria de Quintal,” which utilize a contributive Citizen Science approach, should be encouraged in future studies, as they have demonstrated their effectiveness in targeting rare or explosively breeding species.
Conclusions
We conducted the first molecular analysis to determine the phylogenetic position and species delimitation of the Near Threatened Proceratophrys paviotii. Our findings did not support the monophyly of phenotypic arrangements within Proceratophrys, consistent with previous studies. The 16S tree confirmed P. paviotii as a valid species, grouping it with P. cururu, P. renalis, and P. laticeps in a clade. The advertisement call of P. paviotii distinguishes it from 22 other species within the genus based on various parameters, highlighting the importance of bioacoustic characters in diagnosing Proceratophrys species.
Although Near Threatened species are close to qualifying for higher risk categories, P. paviotii has been observed in both protected and disturbed areas, including coffee plantations and urban regions. Our results suggest that P. paviotii should be classified as Least Concern in future conservation assessments. We also emphasize the value of Citizen Science in gathering information on species distribution, taxonomy, and conservation, contributing to the scientific knowledge and conservation of Proceratophrys paviotii.