The use of silver diamine fluoride to prevent/treat enamel carious lesions: a narrative review
- Published
- Accepted
- Received
- Academic Editor
- Ajinkya Pawar
- Subject Areas
- Dentistry, Drugs and Devices
- Keywords
- Silver diamine fluoride, SDF, Enamel carious lesions, Tooth staining
- Copyright
- © 2024 AlSheikh
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. The use of silver diamine fluoride to prevent/treat enamel carious lesions: a narrative review. PeerJ 12:e17897 https://doi.org/10.7717/peerj.17897
Abstract
This comprehensive literature review examines the use of silver diamine fluoride (SDF) for the prevention and treatment of enamel carious lesions. SDF has been approved by different international drug associations as a caries-preventing agent to be used on deep carious lesions (dentin). However, SDF can cause staining of exposed tooth structures. Furthermore, the effect of SDF on the bond of adhesives to the tooth structure is still being determined. This review explores various studies on the use of SDF to treat enamel carious lesions, highlighting its effectiveness and preventive action. The literature suggests that SDF inhibits bacterial growth, promotes remineralization, and does not negatively affect adhesive retentions. Potassium iodide (KI) or glutathione (GSH) can reduce staining and discoloration. However, the reviewed studies have limitations. Further research, including well-designed clinical trials, is necessary to validate the findings and evaluate the long-term implications of SDF treatment. Conclusion: Despite the above-mentioned limitations, SDF shows potential as a therapy for enamel caries prevention, remineralization, and use as an adjuvant to other dental treatments, warranting further investigation and the refinement of application methods.
Background
Silver compounds have been used for centuries in treating infections, such as ocular, surgical, oral, and other diseases. Over the last 40+ years, several in vitro and in vivo studies have investigated using a silver fluoride regimen in dentistry. In vitro studies found that silver diamine fluoride (SDF) inhibits plaque metabolic activity, the growth of Streptococcus mutans, and carious lesion progression (Thibodeau, Handelman & Marquis, 1978; Ostela & Tenovuo, 1990; Craig, Powell & Cooper, 1981). Likewise, in vitro studies noted that using silver compounds to treat caries in permanent or primary teeth inhibits carious lesions’ spread and progression and plaque accumulation (Oppermann & Johansen, 1980; Oppermann & Rölla, 1980; McDonald & Sheiham, 1994).
Recent bibliometric studies have demonstrated an increased interest in the use of SDF globally since 2014, with a peak from 2016 to the present; this can be attributed to the global acceptance of SDF as an adjunct agent to treat tooth sensitivity and arrest and prevent dental carious lesions (cavitated or not) (Jiang et al., 2021). However, the increase in interest in using SDF from 2019 to the present can be attributed to the COVID-19 pandemic and the ensuing limitation of available dental care.
In 2014, the US Food and Drug Administration approved SDF for treating tooth sensitivity, and experienced dentists have used SDF for caries arrest (U.S. Food & Drug Administration, 2017), for which it was approved by the ADA (code D-1354) (Horst, Ellenikiotis & Milgrom, 2016; Horst, Ellenikiotis & Milgrom, 2017). In 2017, SDF was also approved by Health Canada for use in dental care (Lamberghini, 2017; American Dental Association, 2017), and in 2020, SDF was found to have fulfilled the United States Institute of Medicine and the World Health Organization criteria. By 2021, the British Association of Pediatric Dentistry supported using SDF in caries treatment (Jiang et al., 2021).
Dental caries continues to be of high concern globally reflecting a high burden on health institutes (Ndagire et al., 2020; Kutesa et al., 2015). The prevalence of caries lesions both enamel and dentine continue to increase among primary and permanent teeth. A study stated that the prevalence of white spot lesions (enamel caries) was as high as 31% among children and young adults (Massignan et al., 2016) and another study stated a high provenance of enamel caries lesions as high as 99% in Finnish adults (Laajala et al., 2019). Coronal caries starts with an initial sub-clinical enamel lesion with the start of mineral loss through bacterial acid-byproducts if no prevention regimen is applied caries will progress with clinical manifestations beginning with a white lesion, followed by minor cavitation, and later reaching dentin and definite cavitation. With the latest oral health institute recommendation, it is favorable to prevent the lesion in the earliest stages possible to limit tissue loss and the need for drilling and restorations (Featherstone, 1999; Sakulratchata et al., 2024).
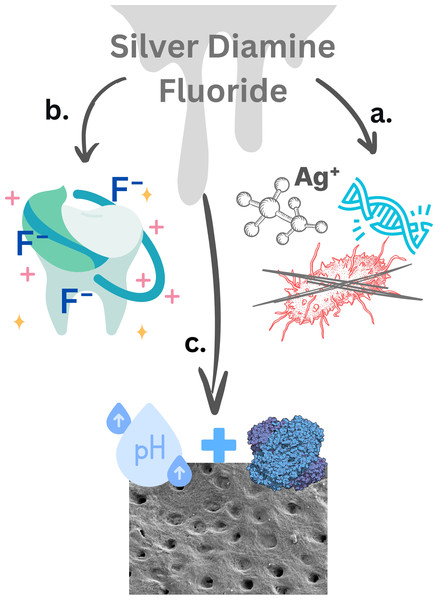
Silver diamine fluoride is a minimally invasive, safe, effective, and economical agent that prevents and arrests dental caries in primary and permanent teeth (Rosenblatt, Stamford & Niederman, 2009). SDF interacts with cariogenic bacteria and the tooth structure (Fig. 1). Different modes of action of the silver compounds with these bacteria have been proposed: silver interacting with DNA and sulfhydryl groups of proteins, DNA unwinding, cell wall synthesis, altering hydrogen bonding and inhibiting respiratory processes, and cell division. The interaction of silver with thiol groups is thought to occur through the following mechanism (Rosenblatt, Stamford & Niederman, 2009; Liau et al., 1997):
Figure 1: The effects of fluoride, silver nitrate, and silver diamine fluoride on bacteria and teeth.
Mechanism of action of SDF: (A) silver ion actions: Ag ions bind to bacterial DNA preventing bacterial replication. Additionally, Ag ions attached to bacterial cell walls lead to cell damage and lysis. (B) Fluoride ion action: fluoride ions enhance the remineralization of the tooth enamel by forming fluorapatite, which is more resistant to acid attack than hydroxyapatite. With calcium and phosphate ions in the saliva calcium fluoride-like deposits on the enamel and dentin act like fluoride reservoirs. (C) Protein coagulation and lesion stabilization: the high PH of SDF causes the proteins in the demineralized dentin tocoagulate and denature these proteins precipitate and block open dentinal tubules, thus act as barriers to protect soft dentin and reduce sensitivity (Rosenblatt, Stamford & Niederman, 2009; Liau et al., 1997). Created using Canva (https://www.canva.com).A/N –SH + AgX A/N –S –AgX + HX
where A, amino acid; N, nucleic acid; SH, thymol group; Ag, silver; and X, anion.
Regarding the tooth structure, the initial interaction is through the formation of silver oxides, calcium nitrate, and silver phosphates, and, further to that, the reaction of sodium fluoride with calcium phosphate occurs to form fluorapatite and sodium hydroxide (Rosenblatt, Stamford & Niederman, 2009).
Yu, Zhou & Zheng (2020) discovered that SDF’s antibacterial characteristics successfully inhibited cariogenic bacterial development and biofilm formation, mainly those of S. mutans. SDF can remineralize demineralized enamel and dentin, further arrest carious lesions, reduce mineral loss, and create a highly mineralized surface rich in calcium and phosphate. SDF also guards dentin collagen against deterioration by preventing collagenase formation. Furthermore, SDF can potentially offer a successful therapy for enamel carious lesions because it functions as a bactericidal agent, inhibits demineralization, and encourages remineralization (Yu, Zhou & Zheng, 2020).
Though there is an increase in the SDF literature with some focusing on its use with enamel caries, there are limited reviews summarizing the findings and suggesting guidelines to the use of SDF for enamel lesions given that the common standard use of SDF is with dentin and primary lesions. The primary objective of this review was to comprehensively assess the existing literature on the application of silver diamine fluoride (SDF) as a treatment modality for enamel caries. The intended audience for this literature review are dentists concerned with caries prevention and oral public health, restorative dentists, and pediatric dentists as well as researchers in cariology and caries management.
Survey Methodology
-
The literature search was conducted in MEDLINE and PubMed for articles published in WoS and/or Scopus-indexed journals after the year 2018, and to ensure a thorough review, the following search strategies were employed:
-
Electronic databases: Comprehensive searches were conducted using academic databases: PubMed, Scopus, Web of Science, MEDLINE, articles with relevance and significance and were used in this article review.
-
Keywords: A combination of keywords—“silver diamine fluoride,” “enamel caries,” “treatment,” and “effectiveness”—was used to identify relevant articles.
-
Inclusion criteria: Studies in English published in the last ten years were included. In vitro studies, clinical trials, reviews, and observational studies were targeted.
-
Exclusion criteria: Studies with no enamel samples, those with insufficient data, case reports, and studies not directly related to the topic were excluded.
All collected articles were inserted into endnote to review the title and abstract of the publication and accordingly, duplicates were removed. After ensuring the relevance of the abstract, each publication was retrieved and inspected thoroughly. The initial search generated 285 articles. After eliminating duplicates, articles not in English or those with English translations, non-retrievable articles, and other unrelated articles, the number was reduced to 30. Table 1 summarizes the articles ultimately included. Table 1 summarizes the type of publications/study, year of publication, type of SDF, and if any other treatment was compared to SDF as well as the article conclusion.
| Ref. | Author | Year | Type of SDF used | Comparison | Study design | Sample size | Aging process | Duration of study | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Studies investigating enamel remineralization, mineral content, or hardness | |||||||||
| 51 | Akyildiz & Sönmez (2019) | 2019 | Nano silver fluoride (experimental solution) Silver diamine fluoride |
Sodium fluoride |
In vitro human enamel blocks |
180 divided into 4 groups | pH-cycling model | 7 days | There were statistically significant differences among all groups. Microhardness: NaF>SDF>NSF>Control. Additional investigation is needed. |
| 24 | Abdelaziz et al. (2022) | 2022 | Silver diamine fluoride 38% (Advantage Arrest™; Elevate Oral Care) |
Number of SDF applications: 1 vs. 2 |
In vitro bovine enamel and dentin blocks |
46 samples per group | None | 12 weeks | SDF application showed significant changes in reflectivity, OP, and permeability in both sound and demineralized dentin. |
| 49 | Alcorn et al. (2022) | 2022 | 38% SDF (Advantage Arrest; Elevate Oral Care) |
FV Prevident 5% NaF Varnish (Colgate), KF (Sigma–Aldrich), AgNO3 (Sigma–Aldrich), and deionized water |
In vitro bovine enamel blocks |
180, 10 groups each with 18 samples |
pH-cycling model | 2 weeks | FV may be better suited than SDF for treating early, incipient, non-cavitated, white-spot, enamel carious lesions. |
| 48 | Hamdi et al. (2022) | 2022 | 38% silver diamine fluoride followed by potassium iodine (SDF-KI) | CPP-ACP (amorphous calcium phosphate-nanocomplexes), and TCS (tricalcium silicate paste) | In vivo | 45 patients in the age range of 15–50 years (in total, 90 teeth) | In vivo study | 24 months | Annual application of SDF-KI compared to twice-daily application of CPP-APC or TCS showed a significant remineralization effect after 24 months (p < 0.001). There was a significant difference between (SDF-KI and CPP-ACP) and (SDF-KI and TCS) at the different follow-up periods of 3, 6, 12, and 24 months (p < 0.001). |
| 52 | Heukamp et al. (2022) | 2022 | Riva Star (silver diamine fluoride) | Bifluorid 12 (NaF, CaF2), Cervitec F (CHX, CPC, NH4F), and negative control |
In vitro human enamel | 50 human tooth samples | pH cycling | 28 days | The fluorescence behavior of SDF varnish on smooth surfaces with artificial initial enamel lesions was significantly lower compared to Cervitec F varnish after short-term use (SDF showed the lowest fluorescence values). |
| 15 | Hiraishi et al. (2022) | 2022 | 38% SDF | – |
In vitro bovine enamel and dentin blocks |
None | Fluoride-substituted carbonate groups were detected in the SDF-treated enamel and dentin. In particular, the formation of FHAp indicated that SDF is effective in reducing the risk of tooth decay. | ||
| 20 | Jabin et al. (2021) | 2021 | 38% SDF | Acidulated phosphate fluoride and sodium fluoride |
In vitro 40 caries-free primary human molars |
10 samples per group (3 enamel blocks tested using SEM) | None | 7 days | Topical application of a 38% SDF solution inhibited the demineralization of enamel. |
| 32 | Kale et al. (2022a) | 2022 | Two SDF products: 38% SDF and FAgamin (FAgamin Silver Diamine Fluoride 38%) |
None |
In vitro human teeth |
32 extracted primary molars divided into two groups (n = 16 each: 8 tooth enamel, 8 tooth dentin) | Plaque bacterial model | 30 days | FAgamin and SDF showed similar cariostatic and remineralization potential for dental caries. |
| 33 | Kale et al. (2022b) | 2022 | Two SDF products: Advantage Arrest, and e-SDF |
None |
In vitro human teeth |
32 extracted primary molars divided into two groups (n = 16) | Thermo-cycling | 30 days | Advantage arrest and e-SDF showed similar cariostatic and remineralization potential for dental caries. The plaque bacterial model used in this study is an efficient method to induce artificial carious lesions in teeth. |
| 41 | Lee et al. (2022) | 2022 | 38% SDF, and 38% SDF with potassium iodide |
5% sodium fluoride varnish |
In vitro bovine teeth | 60 divided into 4 groups (n = 15) | None | 24 h | SDF/KI was effective for dental enamel remineralization even with KI, which reduced discoloration. |
| 47 | Luk et al. (2021) | 2021 | SDF and SDF following CO laser |
Co laser and negative control |
In vitro human enamel specimens |
40 divided into 4 groups (n = 10) | pH cycling | Using the CO laser or SDF separately enhanced the resistance of enamel to cariogenic challenge. Moreover, there was an additional effect of the combined use of the CO laser and SDF for preventing enamel demineralization. |
|
| 43 | Mashhour, Allam & Wassel (2023) | 2023 | 38% SDF | 5% sodium fluoride (Clinpro white varnish), enhanced fluoride varnish treatment with calcium and phosphate (MI varnish), and negative control |
In vitro human teeth | 48 molars divided into 4 groups (n = 12) | pH cycling | 24 h | In primary teeth, lesions treated with MI varnish displayed better resistance to demineralization compared to lesions treated with Clinpro white varnish and SDF. |
| 50 | Mohammadi & Farahmand Far (2018) | 2018 | 38% SDF | Fluoride varnish and negative control |
In vitro human teeth |
45 caries-free deciduous canine teeth (n = 15) | pH cycling | 24 h | SDF solution and fluoride varnish displayed similar effectiveness in preventing the demineralization of deciduous anterior teeth, and no significant difference was observed. |
| 25 | Phonghanyudh et al. (2022) | 2022 | 38% SDF | 5% sodium fluoride | Clinical trial | 960 active carious tooth surfaces in 137 children/group | 18 months | The semi-annual application of 38% SDF and 5% NaF varnish had comparable effectiveness in arresting enamel caries in primary teeth. | |
| 28 | Punhagui et al. (2021) | 2021 | Two different concentrations of SDF: 30% (Soforide) and 38% (Cariestop) Two sub-groups of application time: 1 or 3 min |
Two negative controls: sound enamel, demineralized enamel |
In vitro blocks of deciduous tooth enamel | 66 divided into 6 groups (n = 11) | pH cycling | The 1 min application time promoted enamel remineralization regardless of the SDF concentration (30% or 38%). | |
| 45 | Punyanirun et al. (2018) | 2018 | 38% SDF in adjunct to fluoride toothpaste | Control group: fluoride toothpaste only |
In vitro human premolars |
18 artificial caries slabs | bacterial pH cycling | 5 days | The adjunctive use of 38% SDF enhanced the remineralization of initial carious lesions based on mineral density, depth and remineralization percentage compared with the use of 1,000 ppm fluoride toothpaste alone. SDF might be used as an adjunct to fluoride toothpaste to remineralize incipient carious lesions on smooth tooth surfaces. |
| 31 | Romero & Lippert (2021) | 2020 | 38% SDF | Deionized water | In vitro bovine dentin sections | 48 divided into 4 groups (n = 12) | 24 and 48 h demineralization | 48 h | When applied to only demineralized teeth, in this chemical model, 38% SDF completely inhibited demineralization in adjacent untreated sound enamel. Demineralization prevention was observed to a lesser extent in adjacent pre-demineralized enamel but not in dentin. |
| 46 | Savas et al. (2015) | 38% SDF | Distilled water, acidulated phosphate fluoride, ammonium Hexaf-luoro-silicate (AHF), ammonium hexa-fluoro-silicate + cetylpyridinium chloride (AHF+CPC), and 0.2% chlorhexidine (CHX) |
In vitro saliva-coated enamel slabs | 48 | 2 days | SDF showed the highest antibacterial activity; none of the other fluoride agents used in this study, or 0.2 CHX agent, showed an antibacterial effect comparable to that of SDF. | ||
| 19 | Xue et al. (2022) | 2022 | 38% SDF | With or without 445 nm diode laser; deionized water control |
In vitro human enamel |
33 samples | pH cycling | 7 days | Superior caries-preventive effect of a combined treatment of the diode laser and SDF. Because the diode laser and SDF are affordable and readily available, clinicians can provide this treatment to their patients for caries prevention. |
| 16 | Yu et al. (2018) | 2018 | 38% SDF | 5% NaF and deionized water | In vitro human teeth | 48 | pH cycling | 48 h | The adjunctive application of SDF and NaF varnish had a similar remineralizing effect to that of SDF on enamel caries. |
| 44 | Zhao et al. (2018) | 2021 | 38% SDF | Carbon dioxide laser (CO2), and deionized water control | In vitro human teeth | 34 (n = 24) |
pH cycling | 8 days | Adding SDF to laser significantly increased the preventive effect and antibacterial ability. |
| Studies investigating the effect of SDF on bonding and adhesion | |||||||||
| 27 | Markham et al. (2020) | 2020 | 38% SDF (Advantage Arrest; Elevate Oral Care) |
In vitro human enamel and dentin preparations |
90 IBSS 20 FBS 20 SBS |
Dynamic fatigue testing | Until failure | The results suggest that SDF application on enamel and dentin reduces the bond stability of universal adhesives in self-etching mode. Spot application of SDF is recommended. | |
| 21 | Favaro et al. (2021) | 2021 | Nano silver fluoride (experimental solution), silver diamine fluoride (Cariestop Biodinâmica, Brazil) | Positive control: intact enamel (IE) Negative control: demineralized enamel (DE) |
In vitro human enamel blocks |
60 divided into 6 groups, each with 10 samples | pH-cycling model | 24 h | Anti-caries agents did not have a negative effect on the μ-SBS of composite resin when it was used on IE or DE. |
| 22 | Pérez-Hernández et al. (2018) | 2018 | 38% SDF followed by sealant | Negative control: no SDF | In vitro human enamel | 120 human molars (without caries and caries grade 1) | Thermo-cycling | 24 h | There was an improvement in the retention properties of a fissure sealant applied after treatment with silver diamine fluoride. The application of a fissure sealant to improve the esthetics of teeth treated with SDF is recommended. |
| 38 | Sorkhdini et al. (2021) | 2021 | SDF (38%), SDF followed by application of potassium iodide (SDF + KI) | Silver nitrate (silver control), potassium fluoride (fluoride control), and deionized water |
In vitro human teeth |
180 divided into 5 groups (n = 36) | pH-cycling model | 7 days | SDF and SDF + KI appeared to be effective options in preventing primary coronal caries. |
| 30 | Tiba et al. (2022) | 2022 | 38% SDF (Riva Star) followed by potassium iodide | 5% sodium fluoride solution (Mark3) Restoration: glass ionomer cement GC Fuji II LC capsules, and Filtek Z250 |
In vitro human teeth |
10 | None | 24 h | Mechanical and antimicrobial testing indicated Riva Star compared favorably with—and, in some cases, performed better in the laboratory than—a Mark3 NaF varnish. |
| Studies investigating tooth structure staining and color with the use of SDF | |||||||||
| 41 | Lee et al. (2022) | 2022 | 38% SDF, and 38% SDF with potassium iodide |
5% sodium fluoride varnish |
In vitro bovine teeth | 60 divided into 4 groups (n = 15) | None | 24 h | SDF/KI was effective for dental enamel remineralization even with KI, which reduced discoloration. |
| 39 | Rafiee, Memarpour & Benam (2022) | 2022 | SDF-treated enamel followed by 8 h/day application of 10% CP; SDF + KI-treated enamel followed by 8 h/day application of 10% CP |
None | In vitro | 96 teeth divided into 4 groups (n = 24) | Thermo-cycling aging | 2 weeks (2 groups); 3 weeks (the other 2 groups) |
SDF application on demineralized primary tooth enamel completely recovered enamel microhardness. 10% carbamide peroxide effectively bleached SDF stain without causing a significant decrease in EMH values. Color improvement was more evident with the use of KI immediately after SDF application. |
| 37 | Sayed et al. (2018) | 2018 | SDF only (control), SDF followed by application of a potassium iodide solution, and SDF mixed with 20% GSH | After treatment |
In vitro bovine teeth |
120: 60 enamel, 60 dentin (n = 20) |
Half the specimens were exposed to light and the remainder kept in the dark | 2 weeks | The SDF + GSH group was effective in decreasing the color changes under both light and dark conditions. The SDF + KI group showed an insignificant color change over time. |
| 40 | Sorkhdini et al. (2021) | 2021 | 38% SDF, and 38% SDF followed by potassium iodide (SDF+KI) | Silver nitrate (silver control), potassium fluoride (fluoride control), and deionized water |
In vitro human teeth |
180 divided into 5 groups (n = 36) | pH-cycling model | 7 days | KI did not interfere with the remineralization effect of SDF. |
| 38 | Sorkhdini et al. (2020) | 2020 | 38% SDF, and 38% SDF followed by potassium iodide | Silver nitrate (silver control), potassium fluoride (fluoride control), and deionized water |
In vitro human enamel specimens |
180 divided into 5 groups (n = 36) | None | 3 days | KI application after SDF treatment appeared to reduce dark discoloration but impaired SDF’s ability to prevent biofilm-mediated but not chemically induced demineralization. |
This review used published data, so ethical approval was not required.
The rationale for considering using sdf on enamel
Several factors are considered when evaluating a caries prevention agent, including the agent’s safety, method of action, and efficacy; all play a significant role in favoring one agent over another. SDF has been proven with acceptable scientific evidence to be efficient, cost-effective, and practical as an addition to routine or mass treatment, with a key benefit to its use being its ease of application. It has been approved and used to treat tooth sensitivity. Still, in addition to that application, the precipitation of silver compounds is believed to stop caries progression by enhancing remineralization and through a bactericidal effect.
SDF offers a non-surgical caries management alternative to traditional preventive and restorative dental treatment, particularly in at-risk populations with limited adaptive capacity. It has been proven to be effective in preventing and arresting dental caries in various populations, not only children with carious lesions but also medically compromised patients, elderly adults, and residents living in nursing homes. Additionally, SDF has been found to impact oral health-related quality of life and has the potential to address the epidemic of untreated decay in young children (Jiang et al., 2021). Furthermore, SDF has been used to control caries in large populations in various countries and has been suggested to become a key element of oral health promotion programs for children and medically compromised patients to meet the WHO Millennium Goals (Horst, Ellenikiotis & Milgrom, 2016; Horst, Ellenikiotis & Milgrom, 2017).
Hiraishi et al. (2022) showed that SDF has a stronger reactivity with dentin than with enamel. These researchers discovered fluorhydroxyapatite (FHAp) precipitations in both enamel and dentin, showing that SDF efficiently increases the enamel and dentin resistance to acid attacks and lowers the risk of caries progression. In addition, CaF2 and fluoride-substituted carbonate compounds were found in both enamel and dentin (Hiraishi et al., 2022). According to Yu et al. (2018) SDF exhibits more potent remineralizing effects than sodium fluoride (NaF) alone. The high fluoride concentration in SDF promotes enamel remineralization, while its alkaline nature aids in remineralization, neutralizes bacterial acids, and inhibits dentin collagenases. The silver in SDF adds to its ability to prevent enamel caries; it can integrate into hydroxyapatite crystals, resulting in silver-containing hydroxyapatite, which lowers bacterial adherence and tissue cytotoxicity against cariogenic bacteria (Yu et al., 2018).
The formation of structurally stable fluorohydroxyapatite after SDF application indicates its effectiveness in reducing the risk of tooth decay. FHAp forms a strong ionic bond with fluoride ions and contributes to caries prevention, according to Hiraishi et al. (2022) Other fluoride compounds, such as CaF2 and fluoride-substituted carbonate, are also detected, acting as fluoride reservoirs in the oral cavity and gradually releasing fluoride ions that inhibit carious lesion development. SDF, alone or combined with sodium fluoride varnish, has a remineralizing effect on enamel caries, attributed to the high fluoride concentration (448,000 ppm) in SDF and the formation of silver-containing hydroxyapatite (Hiraishi et al., 2022).
Zhao et al. (2018) highlighted the antibacterial properties of SDF against cariogenic bacteria, its ability to remineralize demineralized enamel, and its protective effect on dentin collagen. By inhibiting the growth of cariogenic bacteria, reducing mineral loss, and promoting remineralization, SDF offers a non-invasive, simple, and low-cost approach to arresting dental caries (Zhao et al., 2018). Furthermore, Xue et al. (2022) demonstrated that the combination of diode laser treatment and SDF application effectively prevented enamel demineralization. Hence, Xue et al. (2022) proposed that the combined use of a diode laser and SDF could offer an improved preventive effect against caries compared to the use of SDF only. Moreover, Jabin et al. (2021) demonstrated that when applied topically, SDF promotes remineralization and protects against demineralization, making it a viable approach for enamel remineralization and caries prevention.
Other investigators have justified the rationale of using SDF on enamel lesions before sealant or adhesive restoration application for a dual effect, as they believe SDF improves adhesion and bond strength. In the event of debonding or adhesive failure, the exposed enamel surface will be more resistant to caries due to the presence of silver compounds. Favaro et al. (2021) put forward the rationale for using SDF on enamel lesions, utilizing its anti-caries actions and ability to react with tooth mineral hydroxyapatite and form calcium fluoride and silver phosphate. This contributes to preventing and remineralizing carious lesions. Favaro et al. (2021) also suggested that SDF treatment can improve bond strength values, potentially enhancing the retention of resin composite restorations on decayed teeth. Pérez-Hernández et al. (2018) meanwhile, suggested that pre-treating the enamel with SDF improves the retention properties of the sealant, potentially enhancing its clinical performance and providing a minimally invasive treatment option for caries prevention.
Clinical concerns about using SDF
Initial enamel carious lesions are superficial lesions. However, sometimes, they are located on surfaces that affect the patient’s smile, esthetics, and appearance. The smile and esthetics significantly impact patients’ appreciation of the treatment provided, confidence, and quality of life (Baiju et al., 2017). SDF deposition reduces optical penetration in enamel and dentin, indicating the presence of silver metal, according to Abdelaziz et al. (2022). Additionally, SDF application reduces reflectivity and subsurface reflectivity in enamel, suggesting the formation of a surface barrier and hindered optical penetration (Abdelaziz et al., 2022). Thermal imaging showed alterations in permeability, as indicated by changes in ΔQ (temperature integrated over time), indicating deeper penetration of silver particles. The study also discussed the formation of silver compounds, contributing to greyish staining observed after SDF treatment. However, thermal imaging and optical coherence tomography (OCT) indicated that lesions ceased activity after SDF application (Abdelaziz et al., 2022).
According to Favaro et al. (2021) and Zhao et al. (2018), there is evidence of dark staining on the tooth surface when using silver diamine fluoride and silver nanoparticle (SNP) anti-caries agents, compromising esthetics. With SDF therapy, carious lesions frequently become blackened or stained; according to Phonghanyudh et al. (2022) the alteration in esthetic and teeth staining may affect how satisfied the patients and guardians are, especially in the case of children. However, in Phonghanyudh et al. (2022), there were no appreciable changes between the 38% SDF and 5% NaF varnish groups at 18 months, and parental satisfaction with the look of their children’s teeth remained constant; this might encourage the use of SDF as the discoloration might be comparable to other preventive agents/varnish in 18 months.
Clinically, there is a concern regarding gingival irritation and reaction when the tissues encounter SDF during application. Based on the recommended SDF use protocol, gingiva should be protected using a rubber dam or petroleum jelly (Yan et al., 2022).
Another consideration is whether SDF may significantly weaken the adhesive bond to enamel by reducing bond strength, a concern raised by to Markham et al. (2020). In their study, enamel staining confirmed the presence of silver, and reduced bond stability, by about one-third, which was attributed to surface alteration, the alkaline pH of SDF, and potential interference with adhesive etching. Markham et al. (2020) recommended avoiding using 38% SDF on the entire bonding surface, but they proposed that spot application to a caries-affected tooth structure may still be beneficial.
In what follows, each of the concerns mentioned here is discussed in detail.
SDF and composite bond strength
When choosing SDF to treat initial enamel carious lesions, the main purpose is to prevent caries progression. In some cases, further preventive measures are required, such as sealing the fissure to render it a non-retentive fissure. In other cases, further restoration, such as glass ionomer or resin composite restoration, is required either to restore tooth structure loss or improve esthetics and mask dark staining. Concerns have been raised regarding the presence of silver compounds and whether those will affect the ability to bond to the tooth structure and further jeopardize the bonding of adhesives or glass ionomers to the treated tooth structure.
Favaro et al. (2021) conducted an in vitro investigation to determine the effect of silver nanoparticles (SNPs) and SDF on the bonding strength of composite resin to intact and demineralized enamel after subjecting the samples to pH-cycling aging. The study found that using these anti-caries agents did not negatively affect the bond strength on intact or demineralized enamel. However, demineralized enamel treated with the agents showed lower bond strength than intact enamel treated with SDF or SNP. Nevertheless, the bond strength values surpassed the required thresholds for composite resin retention. SNPs were favored due to their esthetic advantages and lack of tooth staining. However, the researchers highlighted the need for further in situ and clinical studies to validate these findings in the oral cavity (Favaro et al., 2021).
To determine the effect of SDF on the adhesion and microleakage of pit and fissure sealants on tooth enamel, Pérez-Hernández et al. (2018) carried out an in vitro investigation. They found that pre-application of SDF improved sealant retention, with 100% of SDF-treated samples retaining the sealant compared to only 81.6% without SDF. Although there were no significant differences in the adhesion test, the SDF group exhibited slightly higher attachment values. Microleakage was also reduced in the SDF group. The researchers emphasized the importance of strong adhesion and complete sealant retention to prevent microleakage, recommending the use of resin-based sealants for optimal outcomes. The strengths of the study were that the researchers addressed contamination concerns by cleaning the tooth surfaces and assessing the effect of oral temperature changes on microleakage. However, the study was limited by the subjective nature of the adhesive remaining index (ARI) evaluation and the absence of significant differences in ARI scores between the groups (Pérez-Hernández et al., 2018).
Markham et al. (2020) inspected how 38% silver diamine fluoride affected the bond stability of universal adhesives to enamel and dentin in self-etching mode. The researchers used shear bond strength (SBS) and fatigue bond strength (FBS) tests to assess bond stability. This study’s results contradict the previous, they stated SDF treatment before bonding to both enamel and dentin surfaces considerably reduced the bond durability of universal adhesives. The study’s methodology was well-designed, with dynamic stress evaluation methodologies and the mold-enclosed approach for bonding testing. These strategies offered helpful information on bond stability. Although Markham et al. (2020) found a detrimental effect of SDF treatment on the bond durability of universal adhesives, the study’s limitations must be considered; The study results, highlight the need for more research and understanding of the impact of SDF treatment on bonding. However, the lack of thorough examination of existing studies on SDF application and bonding limits the context and comprehensibility of the findings (Markham et al., 2020). Furthermore, according to the clinical outcomes, the researchers advised against applying 38% SDF to the whole bonding surface of a prepared cavity, while remarking that spot treatment on caries-affected dentin may still be beneficial. However, it was noted that changes to the SDF application strategy are needed over time to improve its combination with restorative materials while keeping its benefits, but a limitation of their work was that the researchers did not investigate the long-term effects of SDF therapy on bond durability.
Camacho et al. (2018) investigated how pre-treatment with SDF affected the bonding strength of orthodontic brackets on non-carious permanent molar teeth. The control group received only phosphoric acid to etch, while the experimental group received SDF therapy between bracket bonding and phosphoric acid to etch. The researchers discovered no appreciable variation in the shear bond strengths between the two groups. However, the SDF group had more adhesive left on the enamel surface, and there was a significant difference in the bond failure characteristics. Meanwhile, no silver staining was found. These results led Camacho et al. (2018) to hypothesize that pre-treatment with SDF has no adverse effects on in vitro bond strength. These results indicate that the use of SDF can be beneficial if it is planned for use as a preventive measure to avoid caries initiation and white spot formation around the brackets, which is found to be a challenge when treating teenagers. However, we must highlight that this study investigated intact enamel and not carious enamel, as discussed already for other studies discussed earlier.
Tiba et al. (2022) compared the shear bond strengths of two dental restorative materials, Filtek Z250 and GC Fuji I, I LC, when combined with two fluoride treatments, Riva Star (SDR) and Mark3 NaF. According to the data, the Filtek Z250 and Riva S combination had a 16% greater SBS than Filtek Z250 with Mark3 NaF. According to the researchers, Riva Star enhances enamel and dentinal hardness and lowers oral biofilm production. These data indicate that SDF may be a promising therapeutic option for military dentistry applications, given it is suitability for mass application and for scenarios where there are limited resources and time. However, more research is required to replicate the findings in a clinical context and compare the effectiveness of Riva Star with various other restorative materials (Tiba et al., 2022).
Most of the reviewed articles stated that the SDF did not have a negative effect on the adhesive bond strength of the treated tooth structure, except Markham et al. (2020). All discussed studies were in vitro, where specimens were subjected to a pH-cycling aging process and tested within 24 h to 7 days. Only Markham et al. (2020) subjected the samples to dynamic fatigue until failure. In this area of the field, further investigation is required to state that SDF has no adverse effect confidently, and its spot application can be recommended when needed.
SDF and carious tooth enamel structures
Carious tooth structures refer to tissue that has been affected by dental caries, a bacterial infection that causes demineralization and destruction of the tooth’s hard tissues, such as enamel and dentin. If left untreated, caries can progress, leading to the formation of cavities. SDF has recently been recommended as a non-invasive treatment option for carious lesions, especially in children or individuals who may have difficulty undergoing traditional dental treatments. The application of SDF can arrest the progression of caries. It is beneficial when the affected tooth structure is minimal, and the decay has not progressed to a point where more extensive restorative measures are necessary.
Abdelaziz et al. (2022) utilized thermal imaging and optical coherence tomography to examine the effects of SDF on carious lesions. Their study highlighted the benefits of OCT and thermal imaging for the non-invasive monitoring of structural changes. The results showed notable changes in enamel and dentin after SDF application, suggesting silver deposition. Microscopy confirmed the presence of silver and fluoride on the surface and penetration into both sound enamel and lesion areas. However, limitations included the in vitro nature of the study, the lack of a control group, and the focus on structural changes rather than remineralization. Furthermore, the study did not address SDF treatment’s potential adverse effects or long-term implications (Abdelaziz et al., 2022).
Jabin et al. (2021) tested the efficacy of sodium fluoride, acidulated phosphate fluoride (APF), and SDF in stopping primary tooth enamel from demineralizing. Their findings showed that enamel treated with SDF exhibited inhibition of demineralization. The study utilized SEM, Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD) analysis to assess enamel characteristics. The results revealed smooth enamel surfaces with grooves and microporosities in all groups. Hydroxyapatite and carbonate apatite were identified as the main crystalline phases, with a higher concentration of type B carbonate. The study emphasizes the potential of SDF as a caries-preventing agent but underscores the need for further clinical trials to validate the findings, particularly in primary tooth enamel (Jabin et al., 2021).
Bovine enamel and dentin treated with silver diamine fluoride were studied by Hiraishi et al. (2022) using solid-state magic angle spinning–nuclear magnetic resonance (MAS NMR) spectroscopy to inspect the generation of fluoride compounds. In the 19F MAS NMR spectra, the study found fluorhydroxyapatite and other fluoride compounds, demonstrating SDF’s efficacy in lowering caries incidence. In contrast to enamel, SEM scans revealed the presence of more sediments and particles in dentin. According to Hiraishi et al. (2022) the structural variations in the crystals and the existence of lattice vacancies in the dentin were responsible for the distinct fluoride formation profiles between enamel and dentin. However, the study’s findings on bovine teeth may not directly apply to human teeth, and further research is needed to evaluate the clinical effectiveness of SDF in preventing enamel carious lesions and validate the identified fluoride compounds (Hiraishi et al., 2022).
Punhagui et al. (2021) explored how varied treatment periods and concentrations of silver diamine fluoride affected the remineralization of deciduous tooth enamel. The authors used microhardness tests, pH cycling, and micro-CT analysis to assess the remineralization and porosity of enamel samples treated with SDF. According to their results, there was no significant difference in enamel remineralization based on surface and cross-sectional microhardness, independent of SDF application duration or concentration. Regardless of SDF concentration, the 1-minute application duration was observed to improve enamel remineralization. The micro-CT data showed that 30% SDF for 3 min resulted in fewer pores, indicating higher mineralization. Yet, while the study offers valuable information about the effect of SDF on enamel remineralization, it has numerous drawbacks—for instance, Punhagui et al. (2021) employed deciduous enamel, which varies from permanent enamel, limiting the findings’ generalizability. Furthermore, the study was performed in vitro, which excluded in vivo variables such as salivary enzyme assaults and pH variations, which might impact the efficiency of enamel remineralization. In addition, the researchers did not directly evaluate fluoride and silver ion concentrations, critical determinants of SDF effectiveness (Punhagui et al., 2021).
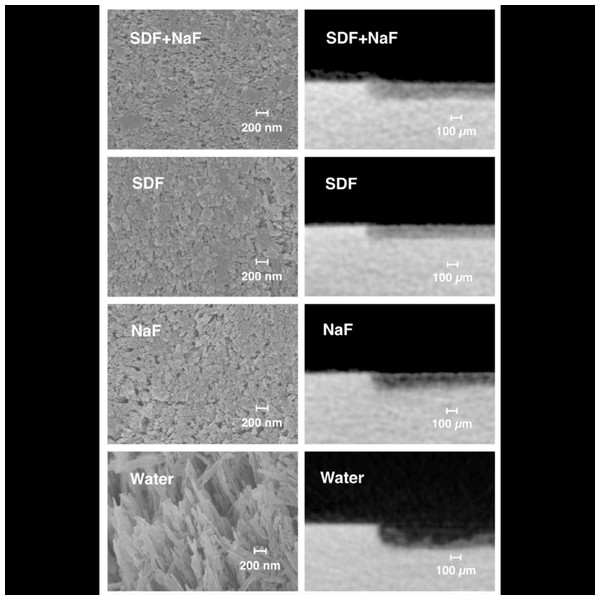
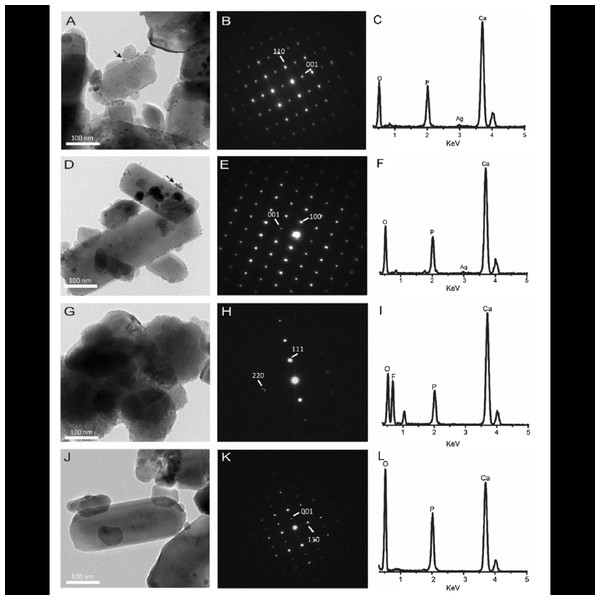
In another study, Yu et al. (2018) sought to determine the remineralizing effects of supplementary applications of 5% sodium fluoride varnish and 38% silver diamine fluoride solution on simulated enamel carious lesions. The surface morphology, fluoride content, lesion depth, crystal characteristics, and precipitates were evaluated using a variety of techniques, including scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS), micro-computed tomography, transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). According to Yu et al. (2018) the adjunctive application of SDF and NaF varnish exhibited a remineralizing impact on enamel caries comparable to that of SDF alone. As opposed to utilizing SDF alone, the results demonstrated that adding NaF did not lessen lesion depths (Figs. 2 and 3) (Yu et al., 2018). In the SDF-treated specimens, silver nanoparticles formed silver chloride and were incorporated into hydroxyapatite crystals. It was proposed that silver’s presence in SDF aided in the progression of caries arrest by lowering bacterial adherence and serving as an antibacterial agent. However, Yu et al. (2018) employed chemical models and artificial enamel carious lesions, which might not accurately reflect the clinical environment in real life. One risk is underestimating the function of silver due to the absence of a biological component in the chemical system. The SDF + NaF and SDF groups had equal fluoride absorption rates, showing that the extra fluoride from NaF did not enhance the amount of fluoride on the specimen surface. Additionally, Yu et al. (2018) pointed out that the specimens in the SDF group received less fluoride than those in the NaF group. The fluoride concentration and the short contact duration may have impacted the remineralization results (Yu et al., 2018).
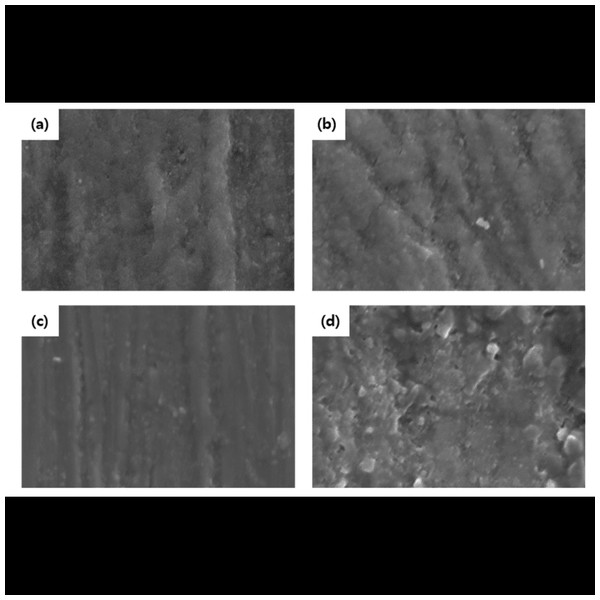
Figure 2: Scanning electron micrographs of the enamel surface morphology.
Representative scanning electron micrographs of the enamel surface morphology (images in the left column) and typical images of micro-computed tomography images (images in the right column) of the enamel caries lesions of the four treatment groups. SDF—silver diamine fluoride; NaF—sodium fluoride. Image credit: Yu et al. (2018), ©Elsevier.The mechanisms of action of SDF for preventing and treating enamel carious lesions were thoroughly reviewed by Zhao et al. (2018). According to the researchers, SDF has antibacterial qualities and can stop the growth of microorganisms that cause caries, notably S. mutans. Additionally, it prevents the development of cariogenic biofilms on teeth. SDF encourages the remineralization of demineralized enamel and dentin by preventing mineral loss and creating a highly mineralized surface rich in calcium and phosphate on carious lesions whose progression has been stopped. Additionally, SDF prevents collagenases from breaking down dentin collagen. However, quantitative analysis was complicated for Zhao et al. (2018) because of the studies’ methodological inconsistencies, which included differences in techniques and results evaluation amongst the included research data.
Romero & Lippert (2021) assessed the effectiveness of 38% SDF in preventing and treating enamel carious lesions. The effects of SDF on nearby untreated sound and pre-demineralized enamel and dentin were evaluated using a single-section model for digital transverse microradiography (TMR-D). According to the findings, SDF prevented demineralization in nearby sound enamel that had not been treated and, to a lesser extent, in enamel that had already undergone demineralization. SDF did not stop demineralization in dentin, although it did reduce the severity compared to the control group. Romero & Lippert (2021) recommended more research should be conducted so that we can better comprehend the processes and long-term effects of SDF in caries prevention, including clinical trials and comparisons with other fluoride treatments.
Using a bacterial plaque model, Kale et al. (2022a) examined the cariostatic and remineralizing effects of two commercial SDF preparations on enamel and dentinal caries: Advantage Arrest and e-SDF. The study employed confocal laser scanning microscopy (CLSM) and energy-dispersive X-ray spectroscopy–scanning electron microscopy (EDX-SEM) to assess the samples. Silver (Ag) and fluoride (F) concentrations in enamel and dentinal carious lesions were higher in both SDF preparations after treatment compared to before treatment. Under SEM, there was demineralization with exposed collagen. Both SDF formulations had equal remineralization capacities and decreased the depths of the lesions. According to Kale et al. (2022a) and Kale et al. (2022b), Advantage Arrest and e-SDF have equivalent cariostatic and remineralization abilities for dental caries, and the bacterial model employed was efficient in producing fake carious lesions.
Using a pH-cycling model, Romo et al. investigated the effects of several commercially available SDF products on the development of non-cavitated enamel carious lesions in primary teeth. The findings revealed that all tested SDF products reduced the depths of carious lesions compared to the control group. Higher concentrations of SDF showed a trend toward a more significant reduction, although statistical differences were not observed for some concentrations. According to the researchers, the mechanism of action of SDF is that it includes the development of mineral deposits on the enamel surface, such as fluorapatite and calcium-fluoride-like compounds, which stop mineral loss and dental caries from progressing. Romão et al. (2020) stressed SDF’s potential as a therapy for non-cavitated enamel carious lesions, especially in clinical settings with few resources or situations involving low patient compliance. Future research should confirm these results, explore various SDF concentrations using validated models, and examine how SDF affects oral biofilms (Romão et al., 2020).
Surendranath, Krishnappa & Srinath (2022) posited that SDF is a non-invasive, painless, and cost-effective treatment for preventing caries, especially in primary dentition and young children. In vivo and in vitro investigations, systematic reviews, and case reports were among the studies included in their review. The results revealed that SDF is a beneficial technique for treating caries in primary dentition, but its effectiveness in permanent molars needs to be improved and requires additional research. Even though the black staining created by SDF was highlighted as a potential downside, it was noted that parents/patients prefer SDF because it is painless and safe. Furthermore, SDF has acceptable bonding strength with composites, which helps to disguise the black stains (Surendranath, Krishnappa & Srinath, 2022).
Zaffarano et al. (2022) discovered considerable diversity in SDF administration techniques, which may impact research results. Despite this variation, the researchers proposed that SDF is effective at halting caries development, especially when given biannually. The study emphasized the importance of uniform treatment techniques and conducting more research on SDF effectiveness in posterior primary teeth. The DMFT index’s usage as an assessment tool was also criticized, with the ICDAS II index suggested to provide more accurate information. Furthermore, the presented data indicated the potential advantages of SDF therapy in primary molars, particularly in uncooperative children who may have difficulty with typical surgical methods (Zaffarano et al., 2022).
Based on the discussed articles, it seems that SDF can offer an effective tool in managing enamel caries. However, the use of SDF is a topic of ongoing research, and its application may vary depending on the specific clinical situation and the dentist’s judgment. In particular, SDF is a beneficial anti-cariogenic agent when the use scenario requires mass application, treatment of non-cooperative or non-compliant patients, or patients without access to regular dental care.
SDF and tooth structure staining
As mentioned earlier, treating enamel lesions sometimes involves anterior teeth, which play a significant role in a patient’s appearance and smile, and SDF is well-known for its staining effect. In this regard, a reasonable amount of research and literature has addressed different techniques or treatments to reduce SDF’s resultant staining and improve patient response.
Sayed et al. (2018) investigated the influence of glutathione (GSH) on enamel and dentin discoloration after applying a 38% silver diamine fluoride solution. The study divided bovine tooth specimens into three groups: SDF alone (control), SDF followed by application of a potassium iodide solution (KI), and SDF combined with 20% GSH. A spectrophotometer was used to assess color changes at different time intervals, and SEM/energy-dispersive X-ray spectroscopy analysis was performed on treated enamel and dentin. According to the findings, GSH significantly reduced color changes following SDF treatment, primarily on enamel and, to a lesser extent, on dentin. A similar effect was observed with the KI group, which had insignificant color changes over the examined time intervals. The study’s highlights were the systematic methodology, the use of a spectrophotometer for reliable color measurement, and SEM/EDS analysis for comprehensive evaluation of enamel and dentin (Sayed et al., 2018). However, the study used bovine teeth, and some argue that bovine teeth differ slightly from human teeth. In addition, including GSH as a viable remedy for reducing color changes in SDF-treated teeth led to valuable insights into how we can enhance esthetic outcomes, but the study focused mainly on color changes and ignored other significant elements, such as the effect of GSH on SDF’s bactericidal or remineralization characteristics. Furthermore, the difficulty in dissolving GSH in SDF limited the researchers’ ability to investigate greater concentrations, raising issues regarding the ideal GSH concentration for maximum effectiveness. Further studies on more accessible techniques for increasing the GSH concentration in SDF solutions are required (Sayed et al., 2018).
Conversely, shortly following SDF therapy, the application of KI dramatically decreased SDF-induced discoloration. The formation of photosensitive silver iodide (AgI) through the reaction of the iodine released by KI with the remaining unreacted silver, which can transform into metallic silver and iodine following light exposure, may explain why KI can prevent silver staining altogether after demineralization (Sorkhdini et al., 2020).
In their study, Rafiee, Memarpour & Benam (2022) addressed the need for a remedy to the problem of tooth discoloration by using potassium iodide. The study team discovered that applying SDF to primary tooth enamel that had undergone demineralization fully recovered the enamel microhardness (EMH) values. The SDF-treated samples had hardness comparable to sound enamel, indicating that remineralization had been effective (Rafiee, Memarpour & Benam, 2022). Similar results were found by Sorkhdini et al. (2021), who added that no significant difference was detected regarding hardness when using SDF, SDF+KI, or potassium fluoride (KF).
After using a 38% silver diamine fluoride solution, Sayed et al. (2018) investigated how combining SDF with biomolecule glutathione reduced enamel and dentin discoloration. According to the spectrophotometric data, the SDF group showed the greatest color change under both light-exposed and dark circumstances. In contrast, the SDF + GSH group showed significantly decreased color changes under both conditions, suggesting an improved esthetic compared to the SDF group (Sayed et al., 2018).
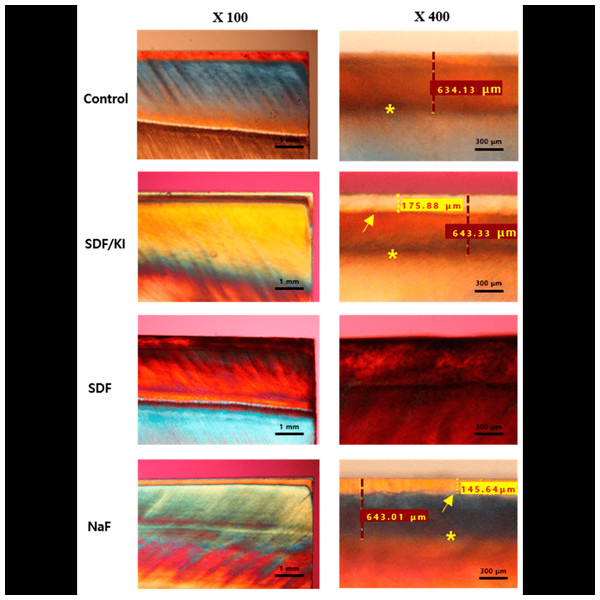
Lee et al. (2022) discovered that the administration of SDF/KI did not result in notable discoloration when compared to saline control and NaF groups. The lack of prominent discoloration showed that KI addition reduced tooth surface discoloration. The researchers proposed that silver iodide removes the excess silver ions and thereby helps lessen the resultant discoloration. From the extant research, we can surmise that clinical experiments and in vitro studies have shown KI works well in reducing SDF-induced discoloration and adult root cavities; however, it still has a chance of causing some tooth discoloration. The progression of caries may influence the amount of discoloration, based on whether it has simply impacted the enamel or reached the dentin (Figs. 4 and 5) (Lee et al., 2022).
Figure 3: Transmission electron microscopy data of experimental groups.
SDF + NaF group: (A) morphology, (B) SAED pattern, (C) EDS spectra. SDF group (D) morphology, (E) SAED pattern, (F) EDS spectra. NaF group (G) morphology, (H) SAED pattern, (I)EDS spectra. Water group (J) morphology, (K) SAED pattern, (L) EDS spectra. EDS—energy-dispersive X-ray spectroscopy; F—fluoride; O—oxygen; P—phosphorus; Ag—silver. SAED—selected-area electron diffraction; SDF—silver diamine fluoride; NaF—sodium fluoride. Image credit: Yu et al. (2018), ©Elsevier.Figure 4: Polarization light micrograph images of post-demineralization and remineralization.
Representative polarization light micrograph images of post-demineralization and remineralization at magnifications of 100 X and 400 X *: Margin of demineralization; arrow: margin of remineralization; red dashed line: the depth of demineralization; yellow dashed line: the depth of remineralization. The border of the SDF group could not be measured due to discoloration. Image credit: Lee et al. (2022).Figure 5: Scanning electron micrographs of enamel surfaces.
Scanning electron micrographs of enamel surfaces after remineralization. (A) Control group; (B) SDF/KI; (C) SDF; (D) NaF (magnification: 8000 X). Image credit: Lee et al. (2022).The effectiveness of SDF in preventing and curing enamel carious lesions was studied by Haiat et al. (2021). The antibacterial effect of an SDF/potassium iodide combination on cariogenic microorganisms and the potential of KI to decrease tooth discoloration associated with SDF treatment were the subjects of a systematic study. Twelve data points were included in the evaluation, all showing SDF/KI to have promising antibacterial effects against cariogenic microorganisms. However, there were conflicting data on the impact of SDF/KI on tooth color. According to Haiat et al. (2021) combining SDF with KI may reduce the chance of staining. However, additional well-planned clinical trials are required to provide solid data and overcome methodological shortcomings (Haiat et al., 2021).
The effects of applying fluoridated carbamide peroxide (CP) with or without potassium iodide on enamel surfaces treated with silver diamine fluoride in primary teeth were examined by Rafiee, Memarpour & Benam (2022). The study focused on enamel microhardness, surface topography, and color changes. Enamel penetration and mineral content were not evaluated, limiting the understanding that could be gained of the mechanisms involved. Furthermore, the study lacked a control group and did not consider long-term stability or potential adverse effects. Despite these limitations, Rafiee, Memarpour & Benam (2022) provided valuable insights into using SDF and fluoridated CP for enamel caries prevention and treatment. SDF application effectively restored enamel microhardness, while fluoridated CP improved color changes caused by SDF. Further research is needed to address the study’s limitations and provide a more comprehensive understanding of these interventions (Markham et al., 2020).
Based on the reviewed articles, using KI or GSH has been proven to reduce staining caused by SDF. Theoretically, removing the excess silver to reduce staining will reduce silver’s effect on bonding and acid attack resistance; however, further clinical investigation is needed to explore the long-term impacts of adding KI or GSH to SDF on color stability, the antibacterial effect, and adhesive bonds.
SDF and other preventive agents
Over the years, many caries-preventive agents have been introduced and utilized. Therefore, when recommending SDF as an anti-caries agent, it is logical to compare it with established, reliable, available preventive agents.
Mashhour, Allam & Wassel (2023) compared the efficiency of three topical treatments in preventing demineralization of white spot lesions (WSLs) in the enamel of primary teeth: Clinpro™ White varnish containing 5% sodium fluoride and functionalized tricalcium phosphate, MI varnish containing 5% NaF and casein phosphopeptide–amorphous calcium phosphate (CPP–ACP), and 38% silver diamine fluoride. All three treatments successfully remineralized demineralized enamel according to the authors. However, MI varnish and Clinpro white varnish were superior to SDF regarding calcium (Ca) and phosphate (P) contents and lesion depth. As such, Mashhour, Allam & Wassel (2023) offered insightful comparative information on the effectiveness of these three therapies for enamel demineralization. Nevertheless, some restrictions to their study must be considered (Mashhour, Allam & Wassel, 2023). First, the research was performed in a laboratory setting, which may not have accurately reflected the oral cavity’s dynamic and intricate biological processes. Because of this, care must be used when extending the results to clinical situations. Additionally, the antimicrobial properties of the treatments studied—which play a crucial role in the clinical remineralization of carious lesions—were not considered. To better simulate the oral environment, Mashhour, Allam & Wassel (2023) called for more studies incorporating the presence of microorganisms. Despite these drawbacks, Mashhour, Allam & Wassel (2023) noted the possible advantages of Clinpro white varnish and MI varnish in improving enamel remineralization and acid resistance. Additionally, they implied that SDF can be advantageous for patients who are uncooperative or have low finances. However, clinical studies are required to confirm the results of this in vitro research (Mashhour, Allam & Wassel, 2023).
Zhao et al. (2021) meanwhile, examined the use of a 9.3 mm carbon dioxide (CO2) laser combined with SDF on enamel to evaluate demineralization prevention and cariogenic bacterium inhibition. They found that the CO2 laser alone could prevent demineralization, reduce bacterial adhesion, and disrupt biofilm formation on enamel. The adjunctive use of SDF further enhanced these effects. Various techniques were employed to assess the outcomes, including pH cycling, SEM with EDS, and CFU and CLSM evaluations. The study demonstrated that the combined use of the CO2 laser and SDF showed promising results in preventing demineralization and inhibiting bacteria. The laser treatment improved enamel resistance through changes in hydrophobicity and crystallographic alterations. SDF contributed to preventing demineralization and inhibiting biofilm formation by disorganizing and disaggregating the biofilm and inhibiting its metabolism and growth. The findings provide insights into the potential of using a CO2 laser and SDF to improve enamel health and caries prevention (Zhao et al., 2021).
In research comparing the efficiency of 38% SDF and 5% sodium fluoride varnish in halting enamel caries in young children, Phonghanyudh et al. (2022) found that when administered semi-annually over 18 months to children aged 1–3 years with active carious surfaces, both therapies were equally effective in halting enamel caries in primary teeth. The study filled a gap in the literature by studying the use of 38% SDF in high-caries-risk toddlers in non-fluoridated settings. The study’s strengths included its low dropout rate, adequate sample size, and reliable examinations. However, limitations included the absence of a negative control group, reliance on visual inspection alone for caries assessment, and potential bias introduced by SDF staining. Furthermore, the findings applied to high-risk children in outreach settings, and further research is needed to explore the effectiveness of SDF in different populations (Phonghanyudh et al., 2022).
Using both biofilm and chemical models, Sorkhdini et al. (2020) investigated the efficiency of silver diamine fluoride and its components, silver (Ag+) and fluoride (F-), in preventing enamel demineralization. The study design was noteworthy, with appropriate controls and two models used to evaluate SDF’s action. It is also worth noting the addition of potassium iodide as a post-SDF application therapy to reduce staining caused by SDF. In all models, Sorkhdini et al. (2020) showed that SDF and SDF + KI were superior to silver nitrate (AgNO3) and deionized water (DIW) in preventing carious lesion development. In the biofilm model, however, SDF + KI was much less efficient in preventing demineralization than SDF alone. Sorkhdini et al. (2020) hypothesized that KI may reduce the bioavailability of silver ions or increase organic acid generation by bacteria, impairing the silver antibacterial activity. Nonetheless, it is critical to emphasize the study’s limitations, such as the absence of remineralization intervals and longitudinal studies of SDF’s capacity to prevent caries. Furthermore, the study concentrated on immediate impacts rather than long-term effectiveness. Future studies utilizing clinical models and monitoring lactic acid generation will extend our knowledge of SDF’s potential (Mashhour, Allam & Wassel, 2023).
Xue et al. (2022) investigated the effects of a 445 nm diode laser (L) and silver diamine fluoride (F) in terms of preventing enamel demineralization and suppressing cariogenic bacteria. They employed scanning electron microscopy, nano-hardness testing, micro-computed tomography, and biofilm evaluation to determine the efficiency of the treatments. Compared to other groups, the combination therapy of diode laser and silver diamine fluoride had a more significant caries-preventative effect. The utilization of different assessment methodologies by Xue et al. (2022) allowed for a complete investigation of the therapies’ impact on enamel. The SEM scans showed that the enamel treated with the diode laser and silver diamine fluoride had a smooth surface, indicating that the enamel was protected. Furthermore, nano-hardness measures and lesion depths supported the findings of decreased demineralization in the combined therapy group. The biofilm analysis also demonstrated that the combination therapy significantly reduced bacterial adhesion and growth (Xue et al., 2022).
However, Xue et al. (2022) employed enamel slices sectioned into blocks, which may not have adequately depicted the intricacy of regular teeth and caries progression. Furthermore, the sample size (33) could have been more extensive, which may have impacted the findings’ generalizability. Moreover, Xue et al. (2022) concentrated on the laboratory environment, and the practical application of combination therapy requires additional research mimicking the oral environment. Also, the mechanism behind the interaction between the diode laser and silver diamine fluoride remains unknown, according to Xue et al. (2022) More research is necessary to determine how the therapies reduce enamel demineralization and bacterial development. The possible esthetic concerns connected with employing the diode laser and silver diamine fluoride, such as black staining and decreased silver ion penetration depth, impact the willingness of dentists to use either treatment (Xue et al., 2022).
Lee et al. (2022) explored the remineralization of enamel carious lesions using 38% silver diamine fluoride and potassium iodide. The effects of SDF/KI varnish were compared with those of 38% SDF and 5% sodium fluoride varnish. Colorimetric analysis, Vickers microhardness tests, and microscopic exams were used to evaluate the remineralization capability of bovine incisors that had been demineralized for 120 h. According to Lee et al., SDF/KI did not induce substantial discoloration compared to the control and NaF groups, suggesting that adding KI minimized color alterations. According to polarized light microscopy (PLM) and scanning electron microscopy investigations, SDF/KI had the highest mean microhardness and the best remineralization capacity. Despite its limitations, the study emphasized the benefits of SDF as a productive, efficient, and cost-effective therapy option, and showed that adding KI alleviates the cosmetic problem of tooth discoloration caused by SDF usage. The findings added to the expanding body of evidence that supports the use of SDF as an alternate agent for caries prevention and enhancement (Lee et al., 2022).
The efficacy of 38% SDF as a supplement to fluoride toothpaste in remineralizing enamel carious lesions was investigated by Punyanirun et al. (2018). Artificial caries enamel slabs were used in the study and submitted to pH cycling and micro-CT analysis. The findings demonstrated that, compared to the control group, the SDF group displayed considerably better mineral density, depth of remineralization, and remineralization %. According to the study’s findings, using SDF and fluoride toothpaste improves the remineralization of early carious lesions. However, further clinical investigations are necessary, particularly in high-caries-risk patients, to validate these findings and assess the inhibition of lesion progression (Punyanirun et al., 2018).
Savas et al. (2015) assessed the efficiency of several antibacterial treatments, including silver diamine fluoride, to prevent and treat enamel carious lesions. The research used a biofilm model with S. mutans, the most potent cariogenic microorganism. The results indicated that SDF had the highest antibacterial activity compared to other fluoride agents and chlorhexidine. Savas et al. (2015) suggested that SDF effectively reduces viable microorganisms in the biofilm and demonstrates potential as an anti-caries agent. However, they also highlighted that the antibacterial effect of fluoride alone is lower than the synergistic effect of fluoride and silver ions present in SDF. Additionally, the study showed a correlation between plaque pH and viable microorganism count, implying the antibacterial impact of remineralization agents (Savas et al., 2015).
Luk et al. (2021) meanwhile, investigated the potential of carbon dioxide (CO2) laser irradiation followed by the application of SDF to enamel to prevent caries. Human enamel samples were divided into four groups for the study: SDF-treated, CO2-treated, CO2-treated then SDF-treated, and controlled untreated. The outcomes demonstrated that Group 3′s CO2 laser and SDF combination produced the lowest lesion depth and maximum microhardness compared to the other groups. Group 4 had the deepest lesions and the lowest microhardness since it received no treatment. According to Luk et al. (2021) combining SDF with CO2 laser increases enamel’s resistance to cariogenic challenge and prevents enamel demineralization.
Hamdi et al. (2022) examined the remineralization effects of casein phosphopeptide amorphous calcium phosphate, silver diamine with fluoride potassium iodide (SDF-KI), and tricalcium silicate paste (TCS) on early enamel lesions. There were three therapy groups for the 45 patients with early enamel lesions: SDF-KI, CPP-ACP, and TCS. The study discovered that after 24 months, both twice-daily treatment with CPP-ACP and TCS paste and a yearly application of SDF-KI significantly remineralized early enamel lesions. At various follow-up intervals, there were sizable differences between SDF-KI and CPP-ACP, as well as between SDF-KI and TCS. CPP-ACP and TCS, however, did not significantly vary from one another (Hamdi et al., 2022).
In their study, Alcorn et al. (2022) sought to assess the effects of SDF therapy on the surface microhardness of early enamel carious lesions. Bovine enamel samples with carious lesions were split into several treatment groups and put through pH cycling. The research discovered that SDF considerably outperformed deionized water (DI), silver nitrate, and 5% sodium fluoride varnish (FV) in terms of remineralization immediately after pH cycling. In comparison to FV, delayed cycling groups had increased remineralization. SDF showed reduced surface softening following a second demineralization test compared to AgNO3, DI, potassium fluoride, and FV. Based on the results, Alcorn et al. (2022) speculated that FV may be more effective than SDF for treating early enamel carious lesions.
Mohammadi & Farahmand Far (2018) compared the effects of SDF solution and fluoridated varnish on the primary tooth enamel’s resistance to demineralization. Three groups of enamel samples—control (distilled and deionized water), fluoride varnish (V), and SDF solution—were created from caries-free deciduous canine teeth. Surface microhardness (SMH) was assessed before and after the pH cycling and treatment of the samples. Although the difference between SDF and the other groups was not statistically significant, the study discovered that SDF led to the greatest resistance to mineral loss. According to Mohammadi & Farahmand Far (2018) fluoride varnish and SDF solution are equally efficient at preventing the demineralization of deciduous front teeth.
Akyildiz & Sönmez (2019) used an in vitro remineralization model to assess the remineralization impact of an experimental nano-silver fluoride (NSF) formulation compared to sodium fluoride varnish and SDF. Artificial lesions resembling caries were formed on enamel samples, and the remineralization agents were then administered. The samples were subjected to pH cycling, and their microhardness and surface morphology were evaluated. The demineralized enamel specimens responded to all remineralization treatments by rehardening statistically significantly more than the other samples. However, NSF was less successful in remineralizing artificial enamel carious lesions than sodium fluoride varnish and SDF. Before suggesting NSF as a substitute for regular fluoride treatments, Akyildiz & Sönmez (2019) recommended that more research be performed.
Heukamp et al. (2022) measured fluorescence behavior using human teeth samples and quantitative light-induced fluorescence (QLF). After 28 days, SDF exhibited a statistically significant decreased fluorescence value compared to Cervitec F and Bifluorid 12 varnishes. SDF showed promise in preventing demineralization and may offer a viable treatment option for reversible early carious lesions. However, the authors highlighted limitations to their methodology, such as the absence of cariogenic bacteria and biofilm in the demineralization solution and the cosmetic disadvantage of yellow discoloration associated with SDF. Despite these limitations, the data demonstrated SDF’s therapeutic importance in preventing demineralization development, particularly in individuals with fixed orthodontic appliances or poor dental hygiene. More research is required to overcome the reported limitations and investigate long-term consequences (Heukamp et al., 2022).
In sum, the majority of the articles mentioned above indicate that SDF exhibits a superior ability for remineralization, specifically in increasing enamel hardness compared to defective enamel when compared with other preventive agents. However, Alcorn et al. (2022) and Heukamp et al. (2022) deviated from this trend; both teams utilized bovine teeth in their studies and conducted investigations over two weeks, yielding different results from similar studies that employed human teeth and longer durations. This divergence in outcomes may thus be attributed to the study duration and/or variations in the histology or structure between bovine and human teeth.
In a noteworthy in vivo study by Hamdi et al. (2022), conducted over 24 months, it was concluded that annual application of SDF-KI, when compared to twice-daily application of CPP-APC or TCS, exhibited a significant remineralization effect. These finding positions SDF as a more convenient and cost-effective option.
Limitations
While the article highlighted several positive aspects and benefits of using SDF to prevent and treat enamel caries lesions, it is essential to consider the limitations of this article:
-
Lack of long-term clinical trials: the discussed studies showed promising results when using SDF in short-term caries arrest and remineralization, long-term clinical trials are essential to fully understand the SDF action durability and effect of caries recurrence.
-
Adverse reaction and side effects: although SDF is well tolerated initially, long-term clinical studies can help assess potential risks and side effects when using SDF, especially with diverse patient populations.
-
Scope of effectiveness: the scope of effectiveness of SDF can vary based on the targeted population, and understanding its limitations for certain populations is crucial for informed clinical decision-making.
-
Most of the published articles investigated the SDF under controlled laboratory environments with no regard to the oral environment changes and biofilm variation among the different populations. The same applies for the testing protocols; the studies are not in harmony with choosing the aging process or methods in testing enamel hardness or remineralization.
Conclusion
Overall, the reviewed literature showed that silver diamine fluoride may be beneficial in preventing and treating enamel carious lesions. SDF possesses antibacterial characteristics, preventing bacterial growth and biofilm formation while encouraging demineralized enamel and remineralization. In addition, there is not enough evidence to ensure that SDF can damage composite resin restorations or sealants bonding to enamel. Concerns about tooth staining caused by SDF therapy have been expressed, and it has been shown that adding potassium iodide or glutathione may help decrease discoloration. The dental community had accepted the use of SDF for treating teeth sensitivity as well as treating cavitated and nom-cavitated carious lesions. Although there is an increased interest in using SDF for caries treatment and prevention, it is important to recognize that the SDF is not approved for this use by the regulatory agencies.
Bibliometric studies exposed an increased interest globally in SDF since 2014, reaching a peak from 2016 to the present. The rush to use the SDF from 2019 onward can be attributed to the COVID-19 pandemic, the pandemic limited access to dental care leading to increasing reliance on alternative treatments like SDF.
Despite its limitations, the extant research shows that SDF has potential as a therapy for enamel caries prevention and remineralization and as an adjuvant to other dental treatments. Some showed superior results when using SDF compared to other preventive agents and other studies suggested the use of SDF in conjunction with other preventive agents. The factors that might influence the publicity of SDF are the ease of use and the required number of applications, while other agents require daily, or quarterly use SDF can be applied once to twice yearly. In limited cases such as deep retentive fissures, further preventive sealant might be indicated, in other cases when the patients require orthodontic treatment the effect of SDF on adhesive bonding should be considered.
Future studies should explain the specific processes through which SDF interacts with other therapies and investigate its potential in broader therapeutic applications. Dental professionals may leverage SDF’s benefits to enhance oral health outcomes by addressing uncertainties, answering outstanding questions, and improving SDF’s application methods.
Prospective developments
Based on this study limitations and further future investigating opportunities exist, such as the following:
-
Long-term clinical trials to assess SDF effectiveness, durability, and any possible side effects. Population diversity as well as acceptance and other factors like age and lesion depth should be considered.
-
Developing a standardized protocol for SDF application for various dental uses and concerns. The protocol should include application technique, duration, recall and expected outcome.
-
Educating and raising awareness among dental professionals, patients as well and caregivers about the SDF uses, benefits, and limitations.