Effect of oral tryptamines on the gut microbiome of rats—a preliminary study
- Published
- Accepted
- Received
- Academic Editor
- Marcello Iriti
- Subject Areas
- Microbiology, Molecular Biology, Neuroscience, Gastroenterology and Hepatology
- Keywords
- Psilocybin, Norbaeocystin, Gut microbiome, Proteobacteria, Verrucomicrobia, Actinobacteria, Rat
- Copyright
- © 2024 Xu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Effect of oral tryptamines on the gut microbiome of rats—a preliminary study. PeerJ 12:e17517 https://doi.org/10.7717/peerj.17517
Abstract
Background
Psilocybin and related tryptamines have come into the spotlight in recent years as potential therapeutics for depression. Research on the mechanisms of these effects has historically focused on the direct effects of these drugs on neural processes. However, in addition to such neural effects, alterations in peripheral physiology may also contribute to their therapeutic effects. In particular, substantial support exists for a gut microbiome-mediated pathway for the antidepressant efficacy of other drug classes, but no prior studies have determined the effects of tryptamines on microbiota.
Methods
To address this gap, in this preliminary study, male Long Evans rats were treated with varying dosages of oral psilocybin (0.2 or 2 mg/kg), norbaeocystin (0.25 or 2.52 mg/kg), or vehicle and their fecal samples were collected 1 week and 3 weeks after exposure for microbiome analysis using integrated 16S ribosomal DNA sequencing to determine gut microbiome composition.
Results
We found that although treatment with neither psilocybin nor norbaeocystin significantly affected overall microbiome diversity, it did cause significant dose- and time-dependent changes in bacterial abundance at the phylum level, including increases in Verrucomicrobia and Actinobacteria, and decreases in Proteobacteria.
Conclusion and Implications
These preliminary findings support the idea that psilocybin and other tryptamines may act on the gut microbiome in a dose- and time-dependent manner, potentially identifying a novel peripheral mechanism for their antidepressant activity. The results from this preliminary study also suggest that norbaeocystin may warrant further investigation as a potential antidepressant, given the similarity of its effects to psilocybin.
Introduction
In addition to its hallucinogenic properties, psilocybin has gained recent interest as a potential fast-acting treatment for depression (Nichols, 2004). A growing number of clinical studies have suggested that when paired with talk therapy, a single dose of psilocybin can have strong and persistent effects that may be equal to or greater than traditional antidepressants, including widely used selective serotonin reuptake inhibitors, such as fluoxetine (Prozac), sertraline (Zoloft), and escitalopram (Lexapro), for treating major depressive disorder (Becker et al., 2022; Carhart-Harris et al., 2021; Carhart-Harris et al., 2018; Davis et al., 2021; Goodwin et al., 2022; Goodwin et al., 2023a, 2023b; Griffiths et al., 2016; Gukasyan et al., 2022). The mechanisms of these effects have not yet been fully understood. Previous findings have implied that psilocybin’s psychedelic and hallucinogenic properties depend on a mixture of actions on excitatory and inhibitory neuronal circuits, with substantial evidence pointing to its activation of 5-HT2A receptors as a likely mechanism (De Gregorio et al., 2021; González-Maeso et al., 2007; Presti & Nichols, 2004). However, the mechanisms of its therapeutic effects are still unclear and may be independent from its hallucinogenic effects. In animal models, co-treatment with ketanserin, a 5-HT2A antagonist, has been shown to block both the psychedelic effects of psilocybin and its therapeutic efficacy (Hesselgrave et al., 2021; Slocum, DiBerto & Roth, 2022). Additionally, animal models have shown that selective activation of 5-HT2A receptors by other compounds can recapitulate many of the psychedelic effects of psilocybin (Hanks & González-Maeso, 2013). This mechanism differs from conventional antidepressants such as selective serotonin reuptake inhibitors (SSRIs) that block serotonin transporters and elevate serotonin levels in neuronal synapses. With these traditional antidepressants, despite acutely increasing in serotonin levels, phenotypic depressive symptoms are usually not relieved until 4–6 weeks later (Stahl, 2021).
Much of the research investigating the mechanisms of psilocybin and other antidepressants has focused on their interaction with central nervous system processes (Chen et al., 2023; Meccia, Lopez & Bagot, 2023), including those reviewed above. However, a growing body of research has pointed to alternative mechanisms for antidepressant action, through peripheral processes. Specifically, significant research indicates that modulation of gut processes such as gut motility, permeability, and gut microbiome composition may be important contributors to the antidepressant efficacy of serotonin modulating compounds. Serotonin receptors, including 5-HT2A, are widely distributed throughout the gut and peripheral tissues (Mawe & Hoffman, 2013). Moreover, the serotonin produced in the gut accounts for more than 60% of peripheral serotonin in mice and more than 95% in humans (Yano et al., 2015). Enteric serotonin is predominantly secreted by enterochromaffin cells that line the gut. Thus, psychedelic drugs such as psilocybin have strong potential to influence enteric processes.
In addition to enteric factors, there is also substantial evidence that the gut microbiome has bi-directional effects on a variety of psychological disorders through a combination of neural, endocrine, and metabolic signals of the gut-brain axis (Burokas et al., 2015; Carabotti et al., 2015). The gut microbiome refers to the diverse array of microscopic organisms that exist in the gastrointestinal tract and their genomes. These microorganisms collectively contain a number of genes 150 times greater than that of the human genome (Weinstock, 2012). Gut bacteria such as Bacilli, Bifidobacterium, Candida, Enterococcus, Escherichia coli (E. coli), Lactobacillus, Streptococcus, and Serratia secrete serotonin, acetylcholine, dopamine, gamma-aminobutyric acid, glycine, and catecholamine (Yano et al., 2015), which can promote serotonin production and release within the lining of the colon, affecting gut motility and permeability. Thus, gut microbes secrete a wide array of neurotransmitters and neuroactive molecules that regulate various complex cognitive processes, including mood, memory, learning, and cognition (Yano et al., 2015). Both preclinical and clinical studies have observed that gut microbiota affect the symptoms of mood disorders (Cruz-Pereira et al., 2020). Most prominent are studies using germ-free rodents, which have found that, when compared with specific pathogen free (SPF) counterparts free of certain infectious pathogens but not completely free of all microbes, germ-free rats develop anxiety-like behavior and germ-free mice develop exaggerated stress responses (Crumeyrolle-Arias et al., 2014; Sudo et al., 2004). These changes have been shown to be reversible upon recolonization of the gut through dietary probiotics (Wallace & Milev, 2017). These studies provide a basis for the idea that altering the gut microbiome could be an alternative therapeutic strategy to treat mood disorders. The exact relationships between bacterial populations and host remain relatively unknown due to the complexity of the gut-brain axis (Dinan & Cryan, 2017; Sharon et al., 2016).
Recent work on the importance of the gut microbiome suggests a need for more understanding of how therapeutics alter these microbe populations, potentially modulating a vast array of signaling and metabolic pathways. Prior studies of treatment with various traditional antidepressants have found inconsistent changes of gut microbiota diversity, richness, and composition (Donoso et al., 2023). Additionally, a study on ketamine, a novel fast-acting antidepressant, has reported dose-dependent relationships between drug treatment and shifts in gut microbe compositions in relatively short time periods (Getachew et al., 2018). Specifically, 7 days after a single ketamine treatment, some bacteria have an over 90-fold increase in abundance at the family level (Getachew et al., 2018). Combined, these studies suggest that psilocybin may also, in part, exert its antidepressant effects through similar mechanisms. This is particularly likely, since 5-HT2A receptors are an essential component of the gut-brain axis (Fiorica-Howells et al., 2002). Although gut microbiome has been proposed as a potential mechanism that psychedelics act upon (Kelly et al., 2023; Kuypers, 2019), no prior studies have investigated the effects of any psychedelics on gut microbe populations. Norbaeocystin is structurally similar to psilocybin and is also found in Psilocybe mushrooms. Prior studies have shown it does not cause head twitch behaviors in rats (Adams et al., 2022), a proxy for 5-HT2A activation and possibly hallucinations. Thus, by comparing psilocybin’s effects to those of norbaeocystin, findings could contribute to the understanding of the role of 5-HT2A receptors in psilocybin’s effects on the gut microbiome. Additionally, should norbaeocystin cause similar effects on the gut microbiome, this might suggest it also possesses therapeutic potential. Therefore, the primary objective of this study was to determine if psilocybin dose-dependently modulates gut bacterial composition. To accomplish this, animals were treated orally with varying dosages of psilocybin, vehicle, or norbaeocystin (a psilocybin precursor).
Materials and Methods
Production of psilocybin and precursors from E. coli
Psilocybin and norbaeocystin-containing cell broths, acquired from Dr. J. Andrew Jones’ lab at Miami University, were produced using a genetically modified E. coli biosynthetic production pathway (Adams et al., 2022; Adams et al., 2019). Concentrations of target metabolites in filtered cell broths were analyzed using HPLC. HPLC results indicated that the psilocybin containing broths had high levels of psilocybin (approx. 1 g/L), trace levels of norbaeocystin (<20 mg/L) and aeruginascin (<1 mg/L), and low levels of baeocystin (approx. 150 mg/L); while the norbaeocystin containing broths had high levels of norbaeocystin (approx. 1.5 g/L) only, with no baeocystin, psilocybin, or aeruginascin due to the lack of the methyltransferase responsible for the synthesis of the latter metabolites. The cell culture media broth contained none of the aforementioned metabolites.
Animals, housing condition, treatment, and fecal collection
Thirty-nine adult male Long Evans rats (90–120 days of age) were bred in-house. Rats were housed individually in standard cages with water and a standard rodent chow (Purina Rodent Chow No. 5001, St. Louis, MO, USA) provided ad libitum and were kept on a 12 h:12 h light/dark cycle (lights on 0700) throughout the study. All the rats were SPF animals, as the cage bedding and materials were tested periodically for certain designated pathogens to ensure safety of researchers and research quality.
Rats were randomly assigned to one of 5 groups, which received oral gavage of low (0.2 mg/kg) or high (2 mg/kg) dosages of psilocybin (P-low and P-high, respectively), low (0.25 mg/kg) or high (2.52 mg/kg) dosages of norbaeocystin (N-low and N-high, respectively), or an equivalent volumetric amount of cell culture media broth as a negative control, with the order of administration randomized. As published previously, “low” dosages show no observable head twitch responses and “high” dosages cause observable head twitches in male Long Evans rats (Adams et al., 2022). This study was to determine if psilocybin or norbaeocystin (a psilocybin precursor) at different doses modulates gut bacterial composition. To accomplish this, gut bacterial composition caused by varying dosages of psilocybin or norbaeocystin with or without induced behavior were compared.
Fresh fecal samples were collected in the morning, in the order of defecation, but was random among groups. Collections occurred from 19 rats 1 week after treatment (control: n = 7; low dosage psilocybin: n = 3; high dosage psilocybin: n = 2; low dosage norbaeocystin: n = 5; and high dosage norbaeocystin: n = 2) and from 20 rats 3 weeks after treatment (control: n = 8; low dosage psilocybin: n = 3; high dosage psilocybin: n = 4; low dosage norbaeocystin: n = 3; and high dosage norbaeocystin: n = 2). All the collected fecal samples were snap frozen immediately and stored at −80 °C until processing. Each fecal sample was provided with a unique sample identification number. Thus, although researchers were aware of group allocation during drug administration and fecal sample collection, they were unaware of group allocation when samples were processed for gut microbiome analysis using 16S rDNA sequencing (see below for details).
All experimental interventions, including oral gavage and fresh fecal sample collection, were carried out in conscious rats without any anesthesia by experienced researchers. No rats showed any sign of distress throughout the study; thus, no analgesia was given. Consequently, all rats were included in this study and their results were reported. Criteria were established for euthanizing animals prior to the planned end of the experiment, but this was not needed. At the conclusion of the experiment, rats were euthanized with one IP injection of Euthasol (200 mg/kg body weight; a sodium pentobarbital-based drug). These euthanasia methods comply with AVMA standards. The research question, groups, fecal sample collection, and gut microbiome analysis using 16S rDNA sequencing were discussed before the study among involved researchers. All procedures were approved by Miami University’s Institutional Animal Care and Use Committee (IACUC Project Number: 1033_2023_Apr).
16S rDNA sequencing and analysis
Genomic DNA was isolated and purified from the fecal samples via a commercialized kit (MPBio FastDNA™ Spin Kit for Feces SKU116570200, Santa Ana, CA, USA), and underwent PCR amplification of the 16S ribosomal DNA (rDNA) V4 region using the 515f/806r primer set (Earth Microbiome Project; http://www.earthmicrobiome.org/). Gel electrophoresis was then used to check the quality and size of the amplicons. Amplified 16S rDNA samples were purified by the SequalPrep Normalization Plate kit (Thermo Fisher, Waltham, MA, USA). Purified products were quantified by KAPA Library Quantification Kit Illumina Platforms (Kapa Biosystems, Wilmington, MA, USA), and used the Illumina Next Generation Sequencing MiSeq platform for amplicon sequencing at Miami University’s Center for Bioinformatics and Functional Genomics for 16S rDNA sequencing based on our established protocol (Xu et al., 2020). The project is registered with the BioProject database (BioProjectID: PRJNA1054120). Raw sequencing data are accessible via http://www.ncbi.nlm.nih.gov/bioproject/1054120. Raw sequencing data were processed and cleaned. The sequencing reads of all samples were demultiplexed based on the associated GOLAY indices on the reverse primer. The generated sequences were analyzed using QIIME2 (Xu, Yang & Zhu, 2020). QIIME 2 is capable of analyzing MiSeq data with two or more biological replicates and tends to be conservative in revealing statistical significance (Bolyen et al., 2019), and has been used to analyze studies with sample sizes of 1 and 2 (McKenzie et al., 2017).
The sequences were grouped into 97% identity clusters via an operational taxonomic unit (OTU) selection method against the Greengenes reference database. Subsequently, the resultant feature tables were transformed into tables displaying relative abundance. The number and relative abundance of bacterial phylum level were exported and analyzed by GraphPad™ Prism 10. The taxonomic composition at the phylum level provides a broad framework for understanding the taxonomic diversity and organization of biological communities in ecological terms. Shannon diversity index considers the number of species indicating richness and their relative abundance indicating evenness, thus estimates the diversity of species in a community. The relative abundance refers to the percentage of one microbial phylum in relation to the total number of phyla in the community, which was analyzed to indicate the population size of specific phyla and their commonalities with other phyla in the fecal samples.
Statistical analysis
Significant differences in diversity at the phylum level and relative abundance of different bacterial populations between groups were determined by comparing the means of these variables between groups using a two-way analysis of variance (ANOVA). The False Discovery Rate (FDR) of all post hoc comparisons was controlled using the two-stage linear step-up method of Benjamini, Krieger & Yekutieli (2006) (GraphPad™ Prism 10, San Diego, CA, USA). Alpha levels for all comparisons were set at p < 0.05.
Results
Effects of psilocybin and norbaeocystin on microbial ecology and diversity
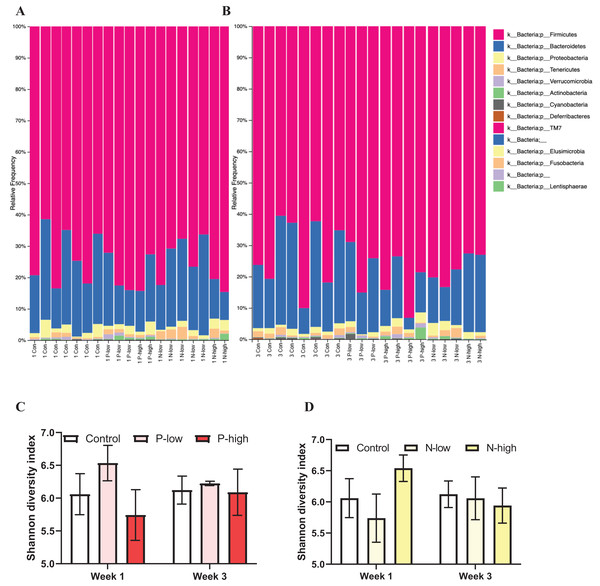
Analysis of gut microbial taxonomic composition of bacterial communities at the phylum level across all treatment groups at both Week 1 and Week 3 timepoints revealed Firmicutes and Bacteroidetes as the dominant phyla, with Proteobacteria, Actinobacteria, Tenericutes, and Verrucomicrobia as sub-dominant phyla. Additionally, Cyanobacteria and Deferribacteres were present in some, but not all, samples. Furthermore, a few phyla, including TM7, Elusimicrobia, Fusobacteria, and Lentisphaerae contributing to the rare biosphere occurred at low abundance level within the community (Figs. 1A and 1B).
Figure 1: Effects of psilocybin and norbaeocystin treatment on microbial diversity.
Effects of low dosage 0.2 mg/kg of psilocybin (P-low), high dosage 2 mg/kg of psilocybin (P-high), low dosage 0.25 mg/kg of norbaeocystin (N-low), and high dosage 2.52 mg/kg of norbaeocystin (N-high) on taxonomic composition of bacterial communities at the phylum level 1 week after treatment (A) or 3 weeks after treatment (B). Effects of P-low and P-high (C) and N-low and N-high (D) on microbial diversity, measured by Shannon diversity index. Significant differences in diversity at the phylum level between groups were determined by a two-way ANOVA followed by FDR-corrected post hoc comparisons analysis. p < 0.05 was considered statistically significant.Microbial diversity measured by Shannon diversity index and analyzed by a two-way ANOVA (treatment × time) did not reveal any significant effect of psilocybin treatments (F(2, 21) = 0.7496; p = 0.4848), time (F(1, 21) = 0.01407; p = 0.9067), or their interaction (F(2, 21) = 0.3673; p = 0.6970) (Fig. 1C). Similarly, a separate ANOVA found that microbial diversity was not significantly affected by norbaeocystin treatment (F(2, 21) = 0.3363; p = 0.7182), time (F(1, 21) = 0.05253; p = 0.8209), or their interaction (F(2, 21) = 0.5505; p = 0.5848) (Fig. 1D). Therefore, neither psilocybin nor norbaeocystin at either dose resulted in any significant change in microbial ecology or diversity compared to the vehicle control (Fig. 1).
Effects of psilocybin and norbaeocystin on microbial abundance of dominant phyla
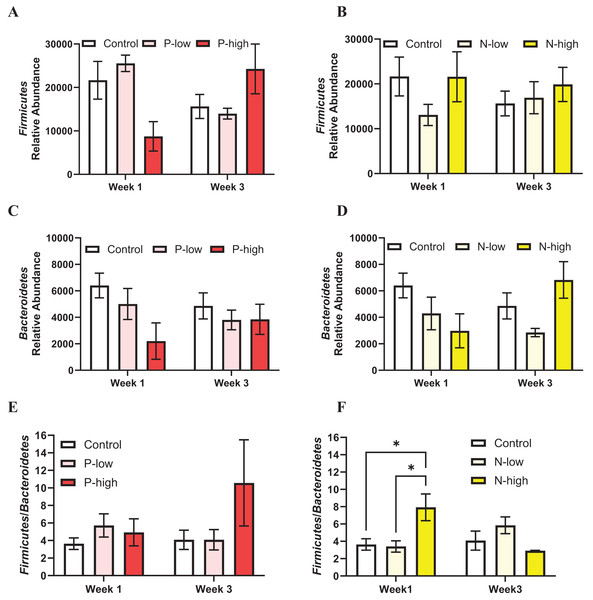
The populations of two major gut microbiota phyla, Firmicutes and Bacteroidetes representing ~80% of gut microbiota (Arumugam et al., 2011; Ley et al., 2008), were analyzed.
Firmicutes abundance was analyzed by a two-way ANOVA (treatment × time), which revealed a significant interaction between psilocybin treatment and time (F(2, 21) = 3.797; p = 0.0391), but Firmicutes abundance was not affected by main effects of psilocybin treatment (F(2, 21) = 0.1963; p = 0.8233) or time (F(1, 21) = 0.0323; p = 0.8591) (Fig. 2A). Post hoc comparisons indicated trends toward decreased Firmicutes abundance after high dose psilocybin treatment compared to the control group (p = 0.0847) and compared to low dose psilocybin treatment (p = 0.0512) at the Week 1 timepoint. This trend did not persist at the Week 3 timepoint, instead the trend reversed, showing increased Firmicutes abundance after high dose psilocybin treatment compared to the control (p = 0.1282) and low dose psilocybin treatment (p = 0.1451) (Fig. 2A). Firmicutes population was not significantly affected by norbaeocystin treatment (F(2, 21) = 0.7270; p = 0.4951), time (F(1, 21) = 0.1179; p = 0.7347), or their interaction (F(2, 21) = 0.8594; p = 0.4378) (Fig. 2B). Therefore, psilocybin or norbaeocystin at either dose did not result in any significant changes in Firmicutes population compared to the vehicle control (Figs. 2A, 2B).
Figure 2: Effects of psilocybin and norbaeocystin treatment on abundance of major microbial phyla at the phylum level and their ratio.
Effects of low dosage 0.2 mg/kg of psilocybin (P-low) and high dosage 2 mg/kg of psilocybin (P-high) (A), low dosage 0.25 mg/kg of norbaeocystin (N-low) and high dosage 2.52 mg/kg of norbaeocystin (N-high) (B) on Firmicutes abundance. Effects of P-low and P-high (C) and N-low and N-high (D) on Bacteroidetes abundance. Effects of P-low and P-high (E) and N-low and N-high (F) on the Firmicutes/Bacteroidetes ratio. Significant differences in relative abundance of Firmicutes or Bacteroidetes bacterial population and the Firmicutes/Bacteroidetes ratio between groups were determined by a two-way ANOVA followed by FDR-corrected post hoc comparisons analysis. p < 0.05 was considered statistically significant. *Indicated statistical significance.Bacteroidetes population was not significantly affected by psilocybin treatment (F(2, 21) = 2.430; p = 0.1124), time (F(1, 21) = 0.1239; p = 0.7283), or the interaction of treatment and time (F(2, 21) = 0.8893; p = 0.4259) (Fig. 2C). Similarly, Bacteroidetes population was not significantly affected by norbaeocystin treatment (F(2, 21) = 1.732; p = 0.2013), time (F(1, 21) = 0.06842; p = 0.7962), or their interaction (F(2, 21) = 1.992; p = 0.1613) (Fig. 2D).
The Firmicutes/Bacteroidetes ratio holds substantial clinical relevance, as its change has been implicated in various diseases, such as obesity, metabolic syndrome, systemic inflammation, cardiovascular disease, and inflammatory bowel disease (Ley et al., 2006; Spychala et al., 2018; Stojanov, Berlec & Štrukelj, 2020); thus, it is considered a critical factor influencing health. The Firmicutes/Bacteroidetes ratio was not significantly affected by psilocybin treatment (F(2, 21) = 1.562; p = 0.2331), time (F(1, 21) = 0.6156; p = 0.4414), or the interaction of treatment and time (F(2, 21) = 1.056; p = 0.3656) (Fig. 2E). Similarly, the overall effect of norbaeocystin treatment on the Firmicutes/Bacteroidetes ratio was not significant (F(2, 21) = 0.8673; p = 0.4346), nor was the effect of time (F(1, 21) = 0.4964; p = 0.4888). However, a significant interaction between treatment and time was found (F(2, 21) = 3.651; p = 0.0436). Post hoc comparisons indicated significantly increased Firmicutes/Bacteroidetes ratio after high dose norbaeocystin treatment compared to control (p = 0.0140) and after low dose norbaeocystin treatment (p = 0.0140) at Week 1 timepoint; such change did not persist at the Week 3 timepoint (Fig. 2F).
Effects of psilocybin and norbaeocystin on microbial abundance of sub-dominant phyla
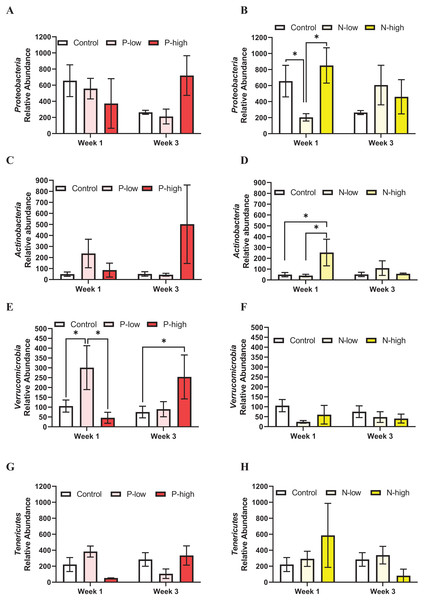
The populations of four minor gut microbiota phyla, Proteobacteria, Actinobacteria, Tenericutes, and Verrucomicrobia were analyzed.
Proteobacteria population at the phylum level was not affected by psilocybin treatment (F(2, 21) = 0.2846; p = 0.7552), time (F(1, 21) = 0.6990; p = 0.4125), or their interaction (F(2, 21) = 2.165; p = 0.1397) (Fig. 3A). Analysis of Proteobacteria population revealed a significant interaction between norbaeocystin treatment and time (F(2, 21) = 3.946; p = 0.0351), but not main effects of norbaeocystin treatment (F(2, 21) = 0.7901; p = 0.4668) or time (F(1, 21) = 0.7484; p = 0.3968) (Fig. 3B). Post hoc multiple comparisons indicated significantly decreased Proteobacteria abundance after low dose norbaeocystin treatment compared to control (p = 0.0283) and high dose norbaeocystin treatment (p = 0.0282) at Week 1 timepoint, but not at Week 3 timepoint (Fig. 3B).
Figure 3: Effects of psilocybin and norbaeocystin treatment on abundance of minor microbial phyla at the phylum level.
Effects of low dosage 0.2 mg/kg of psilocybin (P-low) and high dosage 2 mg/kg of psilocybin (P-high) (A), low dosage 0.25 mg/kg of norbaeocystin (N-low) and high dosage 2.52 mg/kg of norbaeocystin (N-high) (B) on Proteobacteria abundance. Effects of P-low and P-high (C) and N-low and N-high (B) on Actinobacteria abundance. Effects of P-low and P-high (E) and N-low and N-high (F) on Verrucomicrobia abundance. Effects of P-low and P-high (G) and N-low and N-high (H) on Tenericutes abundance. Significant differences in relative abundance of bacterial population were determined by a two-way ANOVA followed by FDR-corrected post hoc comparisons analysis. p < 0.05 was considered statistically significant. *Indicated statistical significance.Actinobacteria population at the phylum level was not affected by psilocybin treatment (F(2, 21) = 1.399; p = 0.2701), time (F(1, 21) = 0.3574; p = 0.5567), or their interaction (F(2, 21) = 1.653; p = 0.2167) (Fig. 3C). However, a significant main effect of norbaeocystin treatment (F(2, 21) = 3.631; p = 0.0452) and interaction between norbaeocystin treatment and time (F(2, 21) = 4.977; p = 0.0176) were revealed with no effect of time (F(1, 21) = 1.846; p = 0.1893) (Fig. 3D). Post hoc comparisons indicated significantly increased Actinobacteria abundance after high dose norbaeocystin treatment compared to control (p = 0.0014) and low dose norbaeocystin treatment (p = 0.0014) at Week 1 timepoint. This change did not persist at the Week 3 timepoint (Fig. 3D). Verrucomicrobia population was significantly affected by the interaction of psilocybin treatment and time (F(2, 21) = 4.027; p = 0.0331); but there were not main effects of treatment (F(2, 21) = 1.646; p = 0.2167) or time (F(1, 21) = 0.04451; p = 0.8349) (Fig. 3E). Post hoc comparisons indicated a significant increase in Verrucomicrobia abundance after low dose psilocybin treatment compared to control (p = 0.0332) and high dose psilocybin treatment (p = 0.0355) at the Week 1 timepoint; and a significant increase in Verrucomicrobia abundance after high dose of psilocybin treatment compared to control (p = 0.0288) at Week 3 timepoint (Fig. 3E). Verrucomicrobia population at the phylum level was not changed by norbaeocystin treatment (F(2, 21) = 1.735; p = 0.2007), time (F(1, 21) = 0.07775; p = 0.7831), or their interaction (F(2, 21) = 0.3985; p = 0.6763) (Fig. 3F).
Tenericutes population at the phylum level was not significantly affected by psilocybin treatment (F(2, 21) = 0.1573; p = 0.8554), time (F(1, 21) = 0.05945; p = 0.8097), or their interaction (F(2, 21) = 2.551; p = 0.1019) (Fig. 3G); nor was it affected by norbaeocystin treatment (F(2, 21) = 0.2521; p = 0.7795), time (F(1, 21) = 1.386; p = 0.2523), or their interaction (F(2, 21) = 2.142; p = 0.1424) (Fig. 3H).
Discussion
The gut-brain axis is a highly complex system, the importance of which is not yet fully understood. Although neither psilocybin nor norbaeocystin treatments significantly impacted gut microbe diversity (Fig. 1), some significant changes in microbial abundance at the phylum level were observed. The Shannon diversity index was selected to assess species diversity due to its robust, interpretable, and widely applicable nature, reflecting both species richness and evenness within a community, which is well-suited for the objectives of this study. A study comparing various commonly used diversity indices for analyzing 16S gene sequencing data has reported that the Shannon diversity index is the most effective measure (Feranchuk et al., 2018). Furthermore, the Shannon diversity index is a widely used measure for quantifying species diversity within communities, making it particularly valuable for comparing diversity levels among different studies. It is noteworthy that alternative indices for assessing species diversity, such as Fisher’s alpha and abundance-based coverage estimator, could be explored in future studies to comprehensively understand community diversity.
The human gut is predominantly composed of Bacteroidetes and Firmicutes, complemented by sub-dominant Actinobacteria, Proteobacteria, and Verrucomicrobia (Qin et al., 2010). Interestingly, neither Firmicutes nor Bacteroidetes, which together represent ~80% of gut microbes (Ley et al., 2008), was significantly impacted (Fig. 2); whereas three of the four sub-dominant bacterial phyla analyzed, Proteobacteria, Verrucomicrobia, and Actinobacteria (Ley et al., 2008), were significantly impacted by psilocybin or norbaeocystin treatments at different timepoints. Specifically, low dose psilocybin treatment increased Verrucomicrobia abundance at the Week 1 timepoint and high dose psilocybin treatment increased Verrucomicrobia abundance at the Week 3 timepoint. Additionally, while low dose norbaeocystin decreased Proteobacteria abundance, high dose norbaeocystin increased Actinobacteria abundance, both of which occurred at the Week 1 timepoint (Figs. 3B, 3D). It is noteworthy that the rare biosphere encompasses a diverse array of microbial taxa. The specific composition of rare phyla may vary depending on factors such as environmental conditions, treatments, and the microbial community under study (Hernandez et al., 2021). Additionally, advancements in sequencing technologies and analytical methods may lead to the discovery of new rare taxa and provide further insights into the rare biosphere (Jousset et al., 2017; Lynch & Neufeld, 2015). In future investigations, with a large sample size and sufficient power for statistical analysis, a microbial network analysis could be pursued to elucidate microbial co-occurrence patterns within the community (Banerjee, Schlaeppi & van der Heijden, 2018). Such an analysis may yield valuable insights into the intricate relationships within microbiome data, enhancing our understanding of microbial interactions and their impact on community dynamics and function.
Emerging evidence supports the microbiota-gut-brain axis in regulation of physiology and behavior, and suggests that disturbance of the gastrointestinal microbiota could affect the immune system and psychiatric functioning (Cruz-Pereira et al., 2020). The bidirectional communication between gastrointestinal microbiota and immune system mediates many neural processes, such as neurogenesis, neurotransmission, neuroinflammation, and neurochemical functions such as activation of stress responses, depression, and other mental health disorders (Cryan et al., 2019; Dinan & Cryan, 2015; Sarkar et al., 2018). An interesting finding is that high dose norbaeocystin treatment increased the Firmicutes/Bacteroidetes ratio at the Week 1 timepoint (Fig. 2F). Findings from previous human and animal studies (Bäckhed et al., 2004; Turnbaugh et al., 2009) suggest the increased Firmicutes/Bacteroidetes ratio as a hallmark for obesity. However, all the rats in the current study were fed a standard rodent chow and were lean. Thus, findings from this study are not directly comparable to obesity studies in the literature. It has been reported that Firmicutes more effectively extract energy from food than Bacteroidetes (Krajmalnik-Brown et al., 2012). Thus, high dose norbaeocystin treatment may promote efficient absorption of calories, which awaits further investigation. In the current study, phyla Verrucomicrobia and Actinobacteria were increased by psilocybin and norbaeocystin, respectively. Phylum Verrucomicrobia are mucin-degrading bacteria, constitutes 3–5% of the bacterial community mainly residing in the intestinal mucosa that forms an interface between host and gut microbiome. Low abundance of Verrucomicrobia has been reported in prediabetic and type 2 diabetic patients (Zhang et al., 2013), in patients with inflammatory gut diseases such as Crohn’s disease, ulcerative colitis, and inflammatory bowel disease (Papa et al., 2012; Png et al., 2010), and in populations with poorer sleep quality or disrupted sleep (Anderson et al., 2017). In contrast, abundance of Verrucomicrobia increases following dieting and Roux-en-Y gastric bypass in diabetic patients accompanied with many beneficial metabolic outcomes (Barlow, Yu & Mathur, 2015). Thus, a low level of Verrucomicrobia has been associated with metabolic disorders and weakened immune system, while a high abundance of Verrucomicrobia is considered as a potential biomarker of a healthy gut status (Anderson et al., 2017; Barlow, Yu & Mathur, 2015; Papa et al., 2012; Png et al., 2010; Zhang et al., 2013). Phylum Actinobacteria contributes to the maintenance of gut homeostasis and supports immune system (Binda et al., 2018). In contrast to beneficial phyla Verrucomicrobia and Actinobacteria that were increased following treatment of psilocybin and norbaeocystin, respectively, high abundance of phylum Proteobacteria is considered as a microbial signature of disease (Rizzatti et al., 2017) and was decreased by low dose norbaeocystin treatment. Thus, it is possible that psilocybin and norbaeocystin could be candidates for alleviating gut dysbiosis and producing positive effects in disease conditions.
The findings of the current study are promising, as significant alteration of the gut microbiome may provide a possible explanation as to why psilocybin users report a reduction of depressive symptoms after treatment (Chen et al., 2023; Meccia, Lopez & Bagot, 2023). It has been proposed that psychedelics may affect gut microbiome to influence their treatment responses (Kelly et al., 2023; Kuypers, 2019). To our knowledge, the current study is the first study that investigated the effects of the tryptamines, psilocybin and norbaeocystin, on gut microbe populations. Unlike conventional mood modulating drugs that require chronic doses over a long timeline, it is possible that psilocybin in part works by altering microbe populations within the gut, potentially targeting a component of the disease state rather than treating the symptoms. These results also suggest that norbaeocystin, a psilocybin precursor with limited study in the peer reviewed literature (Adams et al., 2022), may warrant further investigation as a potential antidepressant. Additionally, in future investigations, the exploration of microbial and functional biomarkers in the microbiome affected by psilocybin or norbaeocystin treatments could be pursued. For example, conducting linear discriminant effect size analysis, a methodology that facilitates the inference of functional and metabolic potential from microbial community metagenome (Segata et al., 2011) may offer valuable insights.
The limitation of this study is low sample sizes analyzed for some groups. Although QIIME 2 is capable of analyzing MiSeq amplicon data with two or more biological replicates and tends to be conservative in revealing statistical significance (Bolyen et al., 2019), as shown in a publication with some sample sizes of 1 and 2 (McKenzie et al., 2017), we should interpret findings with caution. The lack of statistical significance in diversity and some phylum abundance, along with high variability of the relative abundance of some phyla, could be due to the small sample size of this preliminary study. Consequently, the findings from this preliminary study have limited generalizability, and would require further validation with larger sample sizes and comparing across routes of drug delivery.
One caveat to the current work is that it was conducted in normal, healthy rats. It is likely that rats modeling a disease-state, such as chronic stress, anxiety, and depression, may respond differently to psilocybin and/or norbaeocystin. Previously, we have reported the impact of chronic stress on gut microbiome diversity and composition, leading to gut dysbiosis (Xu et al., 2020). Rats with disturbed microbiome may react very differently to these drugs. As such, findings from healthy rats in this study may not generalize to other animals or treatment conditions. Further research is needed using disease models, where multiple physiological, biochemical behavioral and microbiome outcomes are evaluated, such that biological mechanisms can be elucidated. Additionally, there is still little information on how psilocybin and norbaeocystin interact with the body, and continued study is needed in order to inform potential side effects in human trials. Altogether, psilocybin and norbaeocystin stand as strong candidates for managing gut dysbiosis.
Conclusions
In order to use psilocybin to treat mood disorders, it is critical to better understand its efficacy and safety. However, the schedule I status of psilocybin has greatly hindered advancements in research. Psilocybin may also be used to treat other diseases, such as those related to gut health. For example, the FDA recently approved a Phase 2A clinical trial for the treatment of irritable bowel syndrome with psilocybin. Although our study does not use a paradigm that induces stress or depression, nor does it determine psilocybin’s ability to modulate mood via the gut-brain axis, it does begin to probe the mechanisms by which psilocybin affects body physiology and behavior. The observed alterations to the gut microbiome show promise for the ability of psilocybin and norbaeocystin to affect the gut microbiome in a positive manner and establish a path for future research to investigate how psilocybin, or other related tryptamines, could be used to modulate the gut microbiota to treat dysbiosis as well as other disorders. Prior to this study the potential effects of psilocybin and its biosynthetic precursor, norbaeocystin, on gut microbe populations was unknown. Further investigations building upon this work could open the door to a new potential avenue for pharmaceuticals which target the gut-brain axis.
Supplemental Information
Shannon diversity index.
Shannon diversity index was done utilizing QIIME 2 for differentiation of bacteria at the phylum level.
Relative abundance of microbial phylum.
Relative abundance refers to the percentage of one microbial phylum in relation to the total number of phyla in the community.