Biochemical, anatomical, and histochemical characterization of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps: Metteniusaceae) seeds exposed to different thermal treatments
- Published
- Accepted
- Received
- Academic Editor
- Rogerio Sotelo-Mundo
- Subject Areas
- Biochemistry, Food Science and Technology, Plant Science
- Keywords
- Metteniusaceae, Nutrient concentrations, Seed biochemistry, Seed anatomy, Histochemistry
- Copyright
- © 2024 Hernández-Mora et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Biochemical, anatomical, and histochemical characterization of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps: Metteniusaceae) seeds exposed to different thermal treatments. PeerJ 12:e16663 https://doi.org/10.7717/peerj.16663
Abstract
Background
Cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps: Metteniusaceae) is an arboreal species native to the Misantla mountain range, Veracruz, Mexico, whose fruit contains an edible seed with potential nutraceutical properties. Basic biochemical analyses have been performed, though the effects of thermal treatments on the concentration of vital molecules, the seed structure and the histochemistry have yet to be elicited. Herewith we determined the effect of different thermal treatments on the concentrations of total sugars; glucose and fructose; proteins; and amino acids; as well as the impact of such treatments on the anatomy and histochemistry of seeds.
Methods
Biochemical, anatomical, and histochemical characterizations of the cachichín seed were carried out in raw form (T1) and under three thermal treatments: boiled (T2), commercial toasting (T3), and controlled toasting (T4). The biochemical variables evaluated were total and reducing sugars, total proteins, and total amino acids. Observations of the seed structure were performed by scanning electron microscopy (SEM), while histochemical staining was carried out to identify starch, lipids, tannins, polysaccharides and proteins under compound light microscopy.
Results
Concentration of total sugars was reduced in boiled (T2) and commercial toasting (T3) seeds as compared to raw (T1) and controlled toasting (T4) seeds; boiled seeds (T3) displayed the lowest concentration of total sugars. An increase in the concentrations of glucose and fructose was observed in T4. As compared to T1, all other treatments did not change protein concentrations in the seed; the only significant difference observed was between T2 and T3, with commercial toasting displaying the highest mean for this variable. Amino acid concentrations decreased in T3 and T4 compared to T1, while in T2 the concentration of these molecules increased. The anatomic analysis of (T1) revealed a well-organized structure, compared to applied thermal treatments, where degradation of anatomical structures was observed. In general, the thermal treatments tested modified the concentrations and distribution of starch, lipids, tannins, polysaccharides and proteins as compared to raw seeds. The raw cachichín seed has a well-defined anatomical and cellular compartmental organization, while the application of the thermal treatments caused a loss of its structural organization and degradation of vital biomolecules.
Conclusion
The cachichín seed can be considered a good source of proteins and lipids. Thermal treatments can improve its organoleptic properties, though they negatively impact its nutritional value and anatomical structure. Among thermal treatments tested, the controlled toasting can maintain or even improve some nutraceutical properties with a few structural and biochemical modifications.
Introduction
Maintenance of food security, health and nutrition at a global scale are paramount for achieving overall human well-being, as well as for securing livelihoods and sustainable development worldwide. Importantly, biodiversity is critical for safeguarding global food security, underpinning healthy and nutritious diets (FAO, 2019), and thus representing the basis of sustainable agriculture and food systems. Mexico is categorized as a megadiverse country since between 10 and 12% of the world’s species can be found in its territory. Interestingly, up to 7,000 plant species (equivalent to 23% of the total Mexican flora) have a traditional use, with nearly 4,000 species of medicinal plants (Vidal & Brusca, 2020) and more than 1,500 wild edible plants that may contribute with up to 17% of the annual diet of Mexican peasant families (Lascurain et al., 2010). Unfortunately, an important number of those plants are still considered neglected and underutilized species, since their contributions to sustainable food systems are severely under-valued due to a general lack of awareness and information. This may be the case of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.), a fruit tree belonging to the family Metteniusaceae, that produces an edible seed with potential nutraceutical properties.
The genus Oecopetalum is endemic to the Neotropics, distributed in North America (Chiapas, Oaxaca, Tabasco, and Veracruz in Mexico) and Central America (Guatemala, Nicaragua, and Costa Rica) (Hernández-Urban et al., 2019). In Veracruz, cachichín is naturally abundant as a primary species in the transition between perennial tropical forests and cloud forests, at elevations between 400 and 1,100 m within the Misantla mountain range (Mapes & Basurto, 2016; Lascurain, López-Binnqüist & López, 2018). Furthermore, the tree is cultivated as shade for coffee plantations, orchards and semi-monoculture cachichín plantations (Lascurain-Rangel et al., 2013; Lascurain, López-Binnqüist & López, 2018) in various municipalities nearby Misantla.
The cachichín tree produces a walnut-shaped fruit, wrinkly and glabrous, of 2–3 cm in length, green when unripe, hard and dark-brown when ripe. The seeds are white when raw, with an approximate size of 1.66 cm long, 1.23 cm wide and 1.18 cm thick (Hernández-Mora et al., 2021). Once ripe, fruits fall to the ground, and they are subsequently gathered or collected by local peasants as part of their local traditions and customs. These activities are performed yearly in April and May. Though the seed can be preserved for as long as a year if well-dried and managed, it is recommended to consume it as fresh as possible. As walnuts, cachichín fruits have a dehiscent exocarp (husk) that has to be removed before consumption of the seeds. The use of this edible seed is part of the everyday life of about 100,000 inhabitants of the Misantla mountain range region who are involved directly or indirectly in its collection, processing, consumption and sale (Lascurain et al., 2012). Seeds are usually eaten as a treat or snack after they are toasted, although they are also consumed either raw, cooked or boiled with both nutritional and medicinal purposes. Seeds have a bitter taste, with an aroma and consistency similar to that of peanuts (Lascurain-Rangel et al., 2013). However, since local cachichín producers process the fruits under non-standardized conditions (no control of moisture, temperature and time of exposure to heat), one may expect variations in the final nutritional value of the seeds expended in the local markets.
Cachichín may be considered a multipurpose plant species since it provides not only a seed as a source of food and bioactive compounds, but also wood for house construction, fish traps, and firewood, while cachichín orchards represent important carbon sinks crucial for climate change mitigation (Lascurain, López-Binnqüist & Emery, 2016; Lascurain, López-Binnqüist & López, 2018).
Some studies on the biochemical composition of this seed have already been carried out (i.e., Ballinas et al., 2009; Chávez-Quiñones, 2010; Hernandez et al., 2013; Ovando-Chacón et al., 2018a, 2018b). Those studies have demonstrated that the cachichín seed contains considerable levels of vital biomolecules and bioactive compounds such as proteins, sugars, saponins, sterols, coumarins, and unsaturated fatty acids, including oleic acid, linoleic acid, and α-linolenic acid, which may have nutritional and health benefits to the consumers. Nevertheless, detailed analyses evaluating the effect of different heat or thermal treatments and the concomitant Maillard reaction on seed anatomy and nutraceutical properties of this seed are not yet available. The Maillard reaction encompasses a series of nonenzymatic chemical reactions during the thermal processing of foodstuffs, in which the carbonyl groups of reducing sugars react with the nucleophilic groups of amino acids (Lund & Ray, 2017; ALjahdali & Carbonero, 2019). While the Maillard reaction may result in improved food color, organoleptic properties (i.e., taste, aroma, texture), protein functionality, and protein digestibility, if not properly managed, this reaction may also produce toxic products and some foods lose their nutritional value (Tamanna & Mahmood, 2015), especially that of compounds with bioactive properties (Alkaltham et al., 2020). Consequently, the Maillard reaction during thermal processing of food has important implications for food quality, safety, and health effects.
The objective of this study was to carry out more in-depth biochemical, anatomical, and histochemical characterizations of the cachichín seed in response to different thermal treatments (boiled, commercial toasting and controlled toasting) as compared to raw seeds collected in orchards established in the Misantla mountain range region in Veracruz, Mexico.
Materials and Methods
Sample collection
Cachichín seeds were subjected to the following treatments: T1, Raw (control); T2, Boiled; T3, Commercial toasting; and T4, Controlled toasting. The seeds were obtained from local merchants in the municipality of Misantla, Veracruz, Mexico. In general, for commercial toasting, local producers use clay pots heated over wood-burning stoves for approximately 15–20 min. The controlled toasting thermal treatment was performed with raw seeds from the same sites, in a homemade aluminum pan and a heating rack (SP131015Q; Thermo Fisher Scientific, Waltham, MA, USA) at 134 °C for 25 min (Hernández-Mora et al., 2017).
For the main analyses of this study, the seed samples were dried for 72 h in a forced-air oven at 72 °C (HCF-125; Riossa, Guadalajara, Mexico). Subsequently, the samples were ground in a mortar and sieved to mesh No. 1, in order to obtain a homogeneous sample for each treatment.
Determination of total sugars
The determination of total sugars was made using the anthrone method according to the protocol developed by Yemm & Willis (1954), with some modifications (Kejla et al., 2023). In 125 mL Erlenmeyer flasks, 1 g of previously ground cachichín seed sample was placed, and 50 mL of 80% ethanol and four boiling beads were added. The flasks were placed on a heating plate (SP131015Q; Thermo Fisher Scientific, Waltham, MA, USA) at 85 °C until a volume equal to or less than 20 mL was obtained. The sample was cooled, filtered with short-stemmed plastic funnels placed in 20 mL flasks brought to a volume of 20 mL with 80% ethanol, and left to stand overnight at −20 °C.
The following day, 1 mL of predigested solution was added to 25 mL Erlenmeyer flasks and dried in a water bath. Subsequently, 20 mL of distilled water was added and then a 1 mL aliquot of the previous solution was taken and placed in a test tube, adding 2 mL of distilled water and 6 mL of anthrone (0.60 g of anthrone dissolved in 100 mL of sulfuric acid). Then, it was shaken vigorously in a bucket with ice and then the tubes were placed for 3 min at 85 °C in a water bath. Once the reaction time was over, the tubes were withdrawn and placed again in the bucket with ice to stop the anthrone reaction. A volume of 1 mL of the above solution was added to a spectrophotometer cell. The readings were obtained at a wavelength of 600 nm using a spectrophotometer (6715 UV/Vis; Jenway, Staffordshire, UK). A calibration curve with sucrose (purity ≥ 95%) (Sigma-Aldrich, St. Louis, MO, USA) was performed and the results were expressed in micrograms of total sugars per gram of cachichín seed (μg g−1).
Determination of reducing sugars
The quantification of reducing sugars was carried out following the protocol developed by Wight & Niekerk (1983), with some modifications. We used an HPLC Dionex Ics-3000 Ion chromatography system (Poway, CA, USA) equipped with an electrochemical detector and a CarboPac PA1 2 × 250 mm column (Thermo Fisher Scientific, Waltham, MA, USA) with an isocratic flow rate of 0.5 mL min−1, using HPLC grade water as the mobile phase and 300 mM NaOH for the post-column electrochemical reaction. For the extraction, 30 mg of previously ground cachichín seed was weighed and 50 mL of 1 N hydrochloric acid was added. The solution was kept at 92 °C for 2.5 h and stirred every 30 min. Subsequently, it was brought to 100 mL volume, and a 5 mL aliquot was taken and brought to 100 mL volume. A 10 mL aliquot was taken and passed through a filter with a 0.45 μm membrane. The injection volume was 25 μL. The results were expressed as milligrams of reducing sugar per gram of cachichín seed (mg g−1). A calibration curve was performed with glucose and fructose standards (purity ≥ 95%) (Sigma-Aldrich, St. Louis, MO, USA).
Determination of total proteins
The determination of total protein concentration was performed following the Lowry assay (Lowry et al., 1951), with some modifications according to Waterborg & Matthews (1994) and Kielkopf, Bauer & Urbatsch (2020). First, 500 mg of cachichín seeds was weighed and placed in previously cooled mortars. Next, 2 mL of homogenization buffer containing 20 μL of phenylmethylsulfonyl fluoride (PMSF), 8 μL of 1,4-dithiothreitol (DDT), 0.75% (w/v) of polyvinylpyrrolidone-40 (PVP-40), and 0.15% (w/v) sodium dithionite were added, and they were macerated until obtaining a liquid extract. Subsequently, it was transferred to a 2 mL microcentrifuge tube and centrifuged at 10,000 rpm for 15 min at 4 °C. A volume of 1 mL of the obtained liquid phase, 500 μL of phenol, and 20 μL of β-mercaptoethanol was placed in a 2 mL microcentrifuge tube and incubated on ice for 30 min, with vigorous vortexing every 10 min. After 30 min, the tube was centrifuged at 14,000 rpm for 5 min at 4 °C and the liquid phase (upper part) was removed. Then 1.2 mL of ethanol-glycerin and 13 μL of 75 mM ammonium acetate solution were added to the remaining phase, after which it was vigorously vortexed and stored at −20 °C for 12 h.
The following day, the samples were centrifuged at 14,000 rpm for 15 min at 4 °C; the resulting upper phase was removed and 1 mL of ethanol-glycerin was added to the remaining one. We proceeded to centrifuge again at 14,000 rpm for 15 min at 4 °C, discarding the liquid phase repeatedly and, finally, the sample in the tube was dried with absorbent paper. We added 500 μL of poly (fluorene-alt-phenylene) derivative (PFBx) and 2 μL of iodoacetamide to the solid phase with vigorous shaking between adding reagents and incubated it in a water bath for 5 min at 100 °C. Subsequently, 5 μL of DDT was added and then it was incubated at 100 °C for 1 min.
To carry out protein quantification, a gridded nitrocellulose membrane, 1.5 × 1.5 cm on each side, was used, applying 5 μL of sample in each square that was later deposited in a container with 50% methanol (covering the membrane) and with continuous shaking on a reciprocating shaker (Heavy Duty Shaker Cat. 6000; Eberbach, Van Buren Charter Township, MI, USA) for 5 min. After this, the methanol was removed and amido black was added (covering the membrane) for 5 min in a reciprocating shaker (Heavy Duty Shaker Cat. 6000; Eberbach); later the amido black was decanted and three rinses with 50% methanol were carried out for 5 min in each repetition. The results were expressed as milligrams of proteins per gram of cachichín seed (mg g−1). In order to have comparable results, we also determined protein concentrations according to the Kjeldahl method (AOAC, 2001).
Determination of total amino acids
Total amino acids were determined by the ninhydrin method (Moore & Stein, 1954), with some modifications as described by Sun et al. (2006). First, 20 mg of previously ground cachichín seed was weighed and placed in a 2 mL microcentrifuge tube. Then 500 μL of the triple extraction with ethanol was taken and mixed with 500 μL of the sodium citrate (16 mM)-ascorbic acid (34 mM) buffer solution at 0.2% (w/v), pH 5.2 and 1,000 μL of ninhydrin (added 1%; w/v) in 70% (v/v) ethanol. After incubating the samples at 95 °C for 20 min and letting them cool at room temperature, they were read on a 6715 UV/Vis spectrophotometer (Jenway, Staffordshire, UK) at 570 nm. The results were expressed as nanograms of amino acids per gram of cachichín seed (ng g−1). The calibration curve was done with a leucine standard ≥95% (Sigma-Aldrich, St. Louis, MO, USA).
Seed size, weight and shape
Measurements of the length, width, and thickness of the raw cachichín seed were performed using a Stemi 305 stereomicroscope (Zeiss; Heidelberg, Germany), according to the protocol described by Arapa-Carcasi & Padrón-Pereira (2014). For image capture, O. mexicanum seeds were placed in a Petri dish on millimeter paper (observation distance at discretion) under a 1x magnification for photography. A professional SLT A37 digital camera (Sony, Tokyo, Japan) was used to record the images captured, which were saved as .jpg files during image processing. Seed weight was recorded in an AV213C analytical balance (OHAUS, Parsippany, NJ, USA).
Seed anatomy
The anatomy of the seed was explored by scanning electron microscopy (SEM) according to the protocol described by Sandoval-Molina et al. (2018). Longitudinal sections of seeds of each treatment (raw, boiled, toasted) were fixed in glutaraldehyde solution (2.5% glutaraldehyde in Sorensen’s 0.1 M phosphate buffer at pH = 7.2) for 2 h and a vacuum was provided to facilitate fixation. The tissues were post-fixed in 1% osmium tetroxide in water at 22 °C for 2 h. After two washes (1 h each) with deionized water, the tissues were dehydrated in an ethanol series and dried to critical point using a Samdari-780® critical point dryer (Tousimis Research Corporation, Rockville, MD, USA). Then the samples were coated with gold-palladium (80:20%) in a JFC-1100 fine coat ion sputter (JEOL, Tokyo, Japan), and observed at the scanning electron microscope JSM-6390 (JEOL, Tokyo, Japan) at 15 kV. The results were documented digitally.
Histochemical stains
For the histochemical study, an average of 10 seeds of cachichín per treatment was used, without employing any prior pre-treatment for staining and keeping them at −10 °C. Cross sections (40 µm) were obtained from these samples with a hand microtome (R. Jung AG Heidelberg 20175®; R. Jung AG, Heidelberg, Germany). Specific staining for detection of starch, lipids, tannins, polysaccharides and proteins was performed following the protocols developed by Zavaleta-Mancera & Engleman (1991). Starch was detected using the potassium iodide-iodine (0.5% I2KI) reaction. Lipid identification was performed with oil red “O” (0.5% w/v oil red “O”, 25% v/v 1-butanol, 75% v/v ethylene glycol). Tannins were identified with vanillin (2% in ethanol) and HCl (50%) (Ruzin, 1999). Polysaccharides were detected with a periodic acid-Schiff (PAS) staining system. Proteins were stained with naphthol blue black. The sections were observed in a compound light microscope (Axioskop 2 Plus®; Zeiss, Heidelberg, Germany) and photographed with a digital camera (AxioCam Mrc 5®; Zeiss, Heidelberg, Germany).
Experimental design and statistical analysis
The treatments were distributed completely at random in the experiment. The data were processed using RStudio version 1.2.5033 statistical software (RStudio Team, 2020). Once the assumptions of normality (Shapiro-Wilk test) and homogeneity (Levene test) of the variances were proven, a one-way analysis of variance (ANOVA) was performed to determine significant differences with a confidence level of 95% (P ≤ 0.05) using the Tukey test.
Results
Concentration of total and reducing sugars
For total sugars, the highest means were observed in raw seeds (T1; mean 487.50 µg g−1) and controlled toasting (T4; 490.83 µg g−1), with these means being statistically similar to each other, and higher than those observed in commercial toasting (T3; 391.50 µg g−1) and boiling (T2; 160.300 µg g−1), by more than 30%. For glucose, the highest mean was recorded in seeds subjected to controlled toasting (T4; 2.97 mg g−1), while the lowest means were observed in commercial toasting (T3; 1.80 mg g−1) and raw seeds (T1; 1.67 mg g−1), while that observed in boiled seeds (T2; 2.27 mg g−1) was statistically similar to commercial toasting (T3). A similar behavior was observed for fructose, since the highest means was observed in controlled toasting (T4; 2.57 mg g−1), and the lowest in raw seeds (T1; 1.20 mg g−1) and commercial toasting (T3; 1.47 mg g−1), while boiled seeds (T2) displayed an intermediate value (1.93 mg g−1) that was statistically different to the rest of the treatments (Table 1).
| Treatment | Total sugars | Reducing sugars | Proteins | Amino acids | |
|---|---|---|---|---|---|
| Glucose | Fructose | ||||
| µg g−1 | mg g−1 | ng g−1 | |||
| T1: Raw | 487.50 ± 28.53 a | 1.67 ± 0.08 c | 1.20 ± 0.06 c | 95.46 ± 2.15 ab | 430.71 ± 20.13 b |
| T2: Boiled | 160.00 ± 9.56 c | 2.27 ± 0.14 b | 1.93 ± 0.09 b | 86.80 ± 2.38 b | 534.84 ± 29.54 a |
| T3: Commercial toasting | 391.50 ± 28.12 b | 1.80 ± 0.06 bc | 1.47 ± 0.09 c | 100.67 ± 0.13 a | 408.04 ± 27.59 b |
| T4: Controlled toasting | 490.83 ± 12.69 a | 2.97 ± 0.14 a | 2.57 ± 0.14 a | 91.68 ± 1.59 ab | 258.86 ± 15.32 c |
Note:
Mean ± SE with a different letter in the column indicates statistical difference (Tukey, P ≤ 0.05). FBW: Fresh biomass weight, DBW: Dry biomass weight.
Concentrations of proteins and amino acids
Regarding protein concentrations (Table 1), numerically the highest mean was recorded in commercial toasting (T3; 100.67 mg g−1), which was statistically similar to those observed in raw seeds (T1; 95.46 67 mg g−1) and controlled toasting (T4; 91.68 mg g−1); the only statistical difference was found between commercial toasting (T3; 100.67 mg g−1) and boiled seeds (T2; 86.80 mg g−1). Interestingly, all thermal treatments (T2, T3 and T4) resulted in means statistically similar to raw seeds (T1). These results obtained using the Lowry assay were comparable to those obtained by the Kjeldahl method (data not shown).
For amino acids, the highest mean was recorded in boiled seeds (T2; 534.84 ng g−1), followed in descending order by raw seeds (T1; 430.71 ng g−1), commercial toasting (T3; 408.04 ng g−1) and controlled toasting (T4; 258.86 ng g−1). Seeds subjected to boiling (T2) showed an increase of 24% in amino acid concentration, while controlled toasting (T4) had a decrease of 40%, both with respect to raw seeds (T1).
Seed size, weight and anatomy
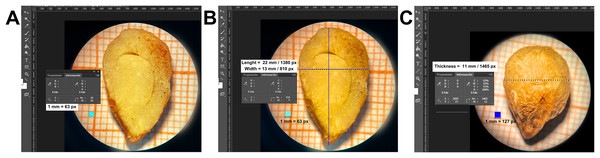
The average dimensions of the raw seeds were as follows: length 22 mm, width 13 mm, and thickness 11 mm. The average weight was approximately 1 g. Once the seed coat is removed, the seed exhibits an ovoid shape, with a smooth texture (Fig. 1).
Figure 1: Dimensions (length and width and thickness) of a raw cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seed under a longitudinal section.
The dimensions were calculated using the Adobe® Photoshop CS6 image editor and a millimeter grid as a reference frame of 1 mm = 63 px for length and width, and 1 mm = 127 px for thickness.The internal structure of the raw seed (T1) exhibits a small embryo with bumps on the surface. The embryo is formed by a hypocotyl, radicle and two cotyledons, and a differentiated plumule, located parallel to the cotyledons. The cotyledons are unequal in size, flat, leafy, oval and curved. A poorly differentiated ovoid embryo with bumps on the surface was observed. Next to the hypocotyl is the end of the radicle opposite the ends of the cotyledons, and a differentiated plumule parallel to the cotyledons. The seed has two elliptical, curved, flat and leafy cotyledons, unequal and free from each other. The seed has a large fleshy endosperm that covers approximately 80% of the seed, and there is an integument or episperm around the endosperm (Fig. 2A).
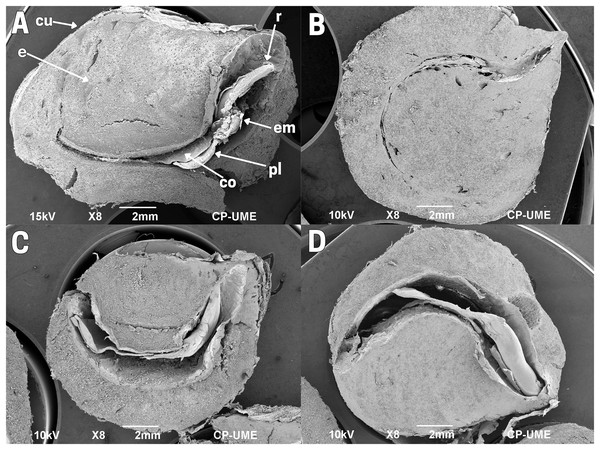
Figure 2: Structure of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images are longitudinal sections of seeds, obtained through scanning electron microscopy (SEM) at a magnification of ×8. (A) A raw seed showing the following structures: embryo (em), radicle (r), plumule (pl), cotyledons (co), endosperm (e), cuticle (cu); (B) a cachichín seed subjected to boiling; (C) a cachichín seed subjected to commercial toasting; (D) a cachichín seed subjected to controlled toasting. CP-UME: Colegio de Postgraduados-Electron Microscopy Unit.The structure of the cachichín seed was affected by the thermal processes evaluated. Boiled seeds (T2) exhibit an endosperm with a soft texture and expanded by the absorption of water that occurred during the process; a normal cuticle with slight folds is evident, and the internal organs (embryo, radicle and plumule) were preserved despite the treatment (Fig. 2B), although it is not observable due to the extended endosperm compared to the raw seed. In seeds subjected to commercial toasting (T3; Fig. 2C) and controlled toasting (T4; Fig. 2D), a drastic loss of moisture content is observed, reflected in a reduction in the uniformity of the endosperm. In seeds subjected to commercial toasting (T3), a moderately damaged cuticle is evident, in addition to thin cotyledons with a folded-rough texture, a detached plumule with deterioration, a poorly differentiated embryo and radicle, and an affected hypocotyl with noticeable differences with respect to the raw seed. In seeds subjected to controlled toasting (T4), an endosperm with a low moisture content and a smooth texture can be observed, with a homogeneous, preserved hypocotyl (cotyledons, plumule, embryo and radicle) and a cuticle perfectly covering the endosperm.
Internal structure of the endosperm
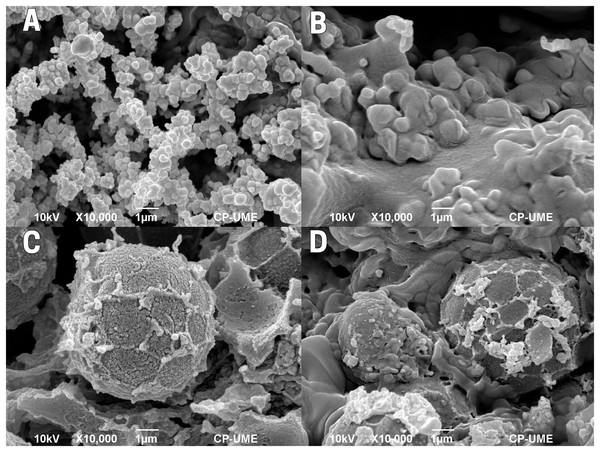
With a focus of ten times its original size, the endosperm of the raw cachichín seed (T1; Fig. 3A) shows well-defined starch grains with a homogeneous distribution and particles that differ in size. In the boiled treatment (T2; Fig. 3B), the effect of the thermal process is noticeable, with agglomerations of particles with a gelatinized texture caused by high moisture and due to the hygroscopic and solubility characteristics of starch. Seeds subjected to commercial toasting (T3; Fig. 3C) show an absence of moisture within the endosperm, and possible changes in the biomolecular organization, forming clumps of starch of considerable size due to the addition of heat. Similarly, in controlled toasting (T4; Fig. 3D) the absence of moisture within the endosperm is evident, with an increase in the size of clumps of starch and a rough texture, maybe caused by the evaporation of water molecules due to the addition of heat during the process of toasting; behind the rough particles, gelatinized molecules can be seen as in boiled seeds (Fig. 3B).
Figure 3: Endosperm of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained through scanning electron microscopy (SEM) at a magnification of ×10,000. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed. CP-UME: Colegio de Postgraduados-Electron Microscopy Unit.External integument or testa of the seed
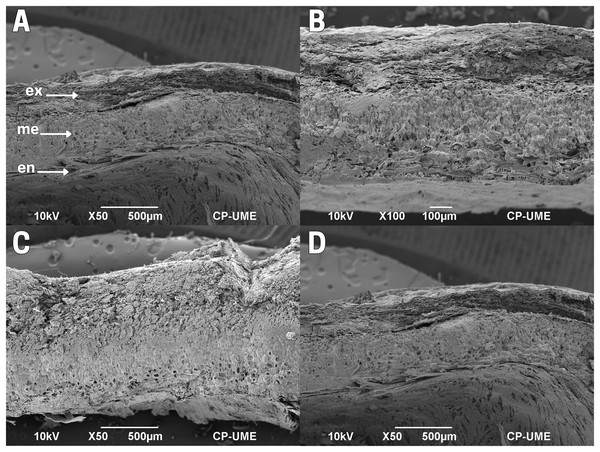
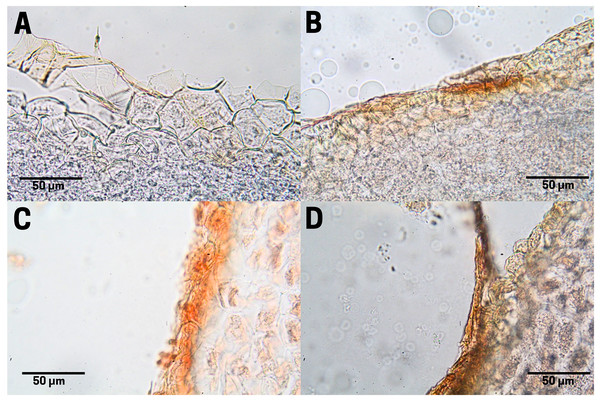
The outer integument or testa of the cachichín seed lacks flexibility; it is robust, hard and firm. The testa is delimited by three layers: exotesta, mesotesta and endotesta, formed by thickened sclerenchymatous tissue, organized by sclereids (Barclay, 2015; Jiménez-López, 2017; Crang, Lyons-Sobaski & Wise, 2018; Fitzgerald, 2020). Specifically, this seed structure shows horizontally positioned tissue organization in the exotesta, irregular for the mesotesta, and well-organized tissues throughout the endotesta (Fig. 4A).
Figure 4: Cross-section of the testa of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
The image was obtained through scanning electron microscopy (SEM) at a magnification of ×50. (A) A raw seed showing the following structures: exotesta (ex); mesotesta (me); endotesta (en). (B) Boiled seed; (C) commercially toasted seed; (D) controlled toasted seed. CP-UME: Colegio de Postgraduados-Electron Microscopy Unit.In raw (T1; Fig. 4A) and boiled (T2; Fig. 4B) cachichín seeds, a testa with a moist texture and degradation in the exotesta was observed. In seeds subjected to commercial toasting (T3; Fig. 4C), the exotesta appears with a dry texture and broken structures. In seeds subjected to commercial toasting (T4; Fig. 4C), the mesotesta and the endotesta are observable, and complete testa in controlled toasting (Fig. 4D), without showing tissue degradation.
Histochemical staining and effect of treatments on the biochemical composition of the seeds
Starch staining in endosperm
Raw seeds (T1; Fig. 5A) exhibit organized and uniform starch grains (black color) within the endosperm cells. It is noteworthy how these molecules are located in specialized compartments for use as reserve energy. When the seed is boiled (T2; Fig. 5B) an increase in moisture and loss of organization is observed both in the cells and in the starch molecules. The seed subjected to commercial toasting (T3; Fig. 5C) shows a loss of starch contained in the endosperm cells caused by the rise in temperature during the thermal process, which in turn causes a breaking of the glycosidic bonds during the Maillard reaction, assuming an uncontrolled thermal treatment of the seed. The controlled toasting (T4; Fig. 5D) exerted similar effects as the commercial one (T3), with the singularity that the degradation of starch molecules was less extended, conserving a higher content of internal carbohydrates.
Figure 5: Starch distribution in cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained using light microscopy. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed.Lipid staining in endosperm
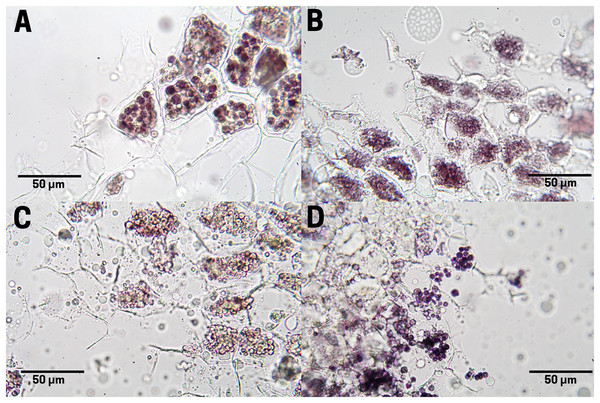
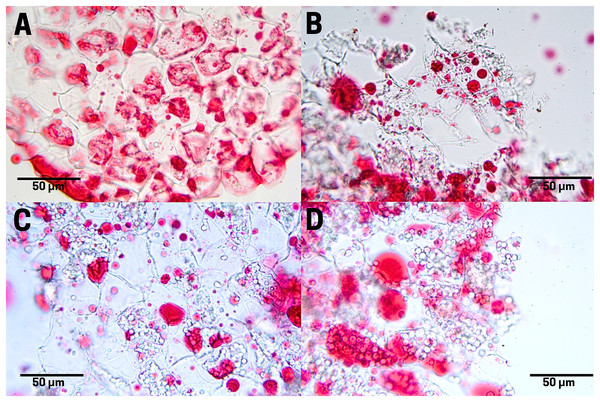
The raw seeds (T1) show a high content of lipids (red pigments) in the endosperm cells as large and small drops (Fig. 6A). In boiled seeds (T2) the lipids appeared as smaller drops and very small drops denominated spherosomes (Fig. 6B). In commercial toasting seeds (T3), the rupture of spherosomes and the appearance of scattered lipids throughout the extracellular space were noticeable (Fig. 6C). In controlled toasting seeds (T4) a more extended rupture of oil drops and spherosomes was observed, but unlike commercial toasting, the lipids appeared in large drops (Fig. 6C).
Figure 6: Lipid distribution in cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained using light microscopy. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed.Tannin staining in endosperm
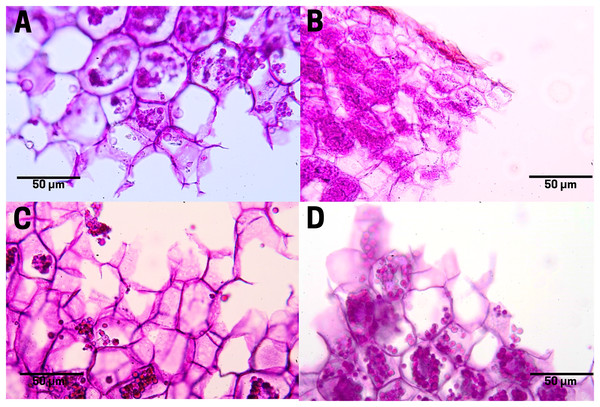
In raw seeds (T1;), there was a low presence of tannins (bright red pigment) located at the end of the endosperm, where a thin residual cuticle was noticeable due to the detachment of the endotesta of the seed (Fig. 7A). Seeds subjected to boiling (T2; Fig. 7B), commercial toasting (T3; Fig. 7C), and controlled toasting (T4; Fig. 7D) present tannins located in the residual cuticle of the endotesta. Interestingly, as observed in the corresponding tissue staining, the toasting treatments increased the concentrations of tannins as compared to raw and boiled seeds.
Figure 7: Tannin distribution of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained using light microscopy. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed.Polysaccharide staining in endosperm
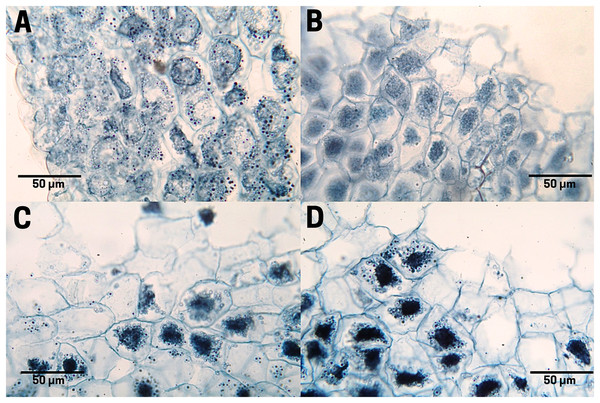
The staining of polysaccharides (purple pigmentation) in the endosperm cells of the raw cachichín seed (T1; Fig. 8A) appears with a normal distribution. Polysaccharides found in seeds may include cellulose, hemicellulose, lignin, pectin and resistant starch (Popoola-Akinola, Raji & Olawoye, 2022). The boiled seed (T2; Fig. 8B) presents the staining of these same carbohydrates, although the contact between boiling water and pectins may have activated the gelling properties of this polysaccharide visible in a close-up in Fig. 8B. In seeds subject to commercial toasting (T3; Fig. 8C), the polysaccharide content is preserved in the membrane, but the staining reveals a possible degradation of starches due to the effect of the Maillard reaction when adding a high temperature. In seeds subjected to controlled toasting (T4; Fig. 8D), there was a thermal process similar to that in commercial toasting (T3), but it preserves the starch content and a stable structure like in raw seeds (T1).
Figure 8: Polysaccharide distribution of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained using light microscopy. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed.Protein staining in endosperm
The histochemical staining of proteins revealed an even distribution of these biomolecules in the endosperm of raw cachichín seeds (T1; Fig. 9A). In boiled seeds (T2), a different organization of proteins may be appreciated (Fig. 9B). When adding heat in commercial toasting (T3; Fig. 9C) and controlled toasting (T4; Fig. 9D), the presence of protein agglomerations is noticeable, which is indicative of a possible structural change in the organization of these biomolecules caused by the thermal treatments applied.
Figure 9: Protein distribution of cachichín (Oecopetalum mexicanum Greenm. & C.H. Thomps.) seeds subjected to different treatments.
Images were obtained using light microscopy. (A) Raw seed; (B) boiled seed; (C) commercially toasted seed; (D) controlled toasted seed.Discussion
The thermal treatments to which the cachichín seeds were subjected differentially affected their biochemical and structural composition, at least in part as a result of the Maillard reaction. Indeed, the Maillard reaction leads to major compositional, structural, and functional changes to food components, including proteins, amino acids, and sugars, and has potentially significant implications for food color, taste, protein functionality, and digestibility of foods (Lund & Ray, 2017).
Under our experimental conditions, the lowest total sugar concentration was observed in boiled seeds (T2; Table 1). This significant reduction may be attributed to a possible dissolution of sugars in contact with water, causing their loss from the seeds (McMurry, 2014). During commercial toasting (T3) the total sugar concentration decreased as compared to raw seeds (T1), whereas in controlled toasting the total sugar concentration was similar to that observed in raw seeds. The final concentration of these biomolecules will depend on precursors, thermal processing parameters, pH, and the quantitative ratio of amino nitrogen to reducing sugar (Martins, Jongen & van Boeke, 2001).
The concentrations of the reducing sugars glucose and fructose, as well as that of total amino acids, are affected during toasting (Nooshkam, Varidi & Bashash, 2019). In cachichín seeds, glucose and fructose increased in controlled toasting (T4), which may be attributed to a controlled breakdown of the soluble carbohydrates contained in the seed (Liu et al., 2022).
Amino acid concentrations increased in boiled seeds (T2), but decreased in both toasting treatments (T3 and T4), suggesting an effect of the Maillard reaction (Nooshkam, Varidi & Bashash, 2019). The Maillard reaction may lead to a loss of essential amino acids, a destruction of vitamins and a reduction in the bioavailability of certain trace elements (Hurrell, 1990; Kathuria, Gautam & Thakur, 2023). In boiled seeds (T2), the increase in amino acid concentration may be due to the release of these molecules by protein degradation (Table 1). The boiling temperature in T2 was not sufficient to initiate the Maillard reaction and thus the amino acid concentration increased in this treatment (Jaiswal, 2020).
In its natural state (raw), the cachichín seed has a well-differentiated structure (Figs. 1 and 2). It presents a rigid testa and sclerenchyma (Figs. 2–4) that represents an important protection for resistance to environmental factors (Dickison, 2000). The endosperm contains polysaccharides, mainly starch (Fig. 5), which serve as an energy reserve (Crang, Lyons-Sobaski & Wise, 2018).
Both biochemical and tissue staining analyses showed that the seed contains abundant biomolecules distributed in different structures. Histochemical staining revealed starch stored in specialized cell structures. In seeds subjected to toasting (T3 and T4), variables such as biomolecule content, organization, and structure in seeds are affected, while boiling (T2) modifies agglomerations possibly due to the hygroscopic characteristic of starches (Cutler, Botha & Stevenson, 2008; Roman-Benn et al., 2023). Within the polysaccharides, pectic compounds may cause gelation in boiled seed endosperm, while in toasted seeds there was a loss of these biomolecules. The Maillard reaction that took place in the toasting treatments may have reduced the energy and fiber values of the food, but, on the other hand, may have improved its organoleptic properties (Wang, Qian & Yao, 2011).
The high lipid content is a characteristic of oilseeds, and the cachichín seed has shown a high content of these biomolecules (Hernandez et al., 2013; Hernández-Mora et al., 2017; Ovando-Chacón et al., 2018a; 2018b; Hernández-Mora et al., 2021). Lipid staining (Fig. 6) revealed the presence of spherosomes where lipids are stored within endosperm cells (Kochhar & Gujral, 2020). When the seed is boiled, the spherosomes are degraded, which generates an accumulation of lipids as a result of the addition of water during treatment and the immiscibility between fatty acids and water molecules (Sharma et al., 2022). When boiling the seeds, the lipid extraction leads to pectins with gelling power, which implies an additional step in the purification of oils (Osadchuk et al., 2021). When toasting, the breaking of the lipid bodies is evident, which in turn may provoke a concomitant release of fatty acids that may facilitate their extraction (Hernández-Mora et al., 2017; 2021).
Phenolic compounds such as tannins interact with carbohydrates forming hydrogen bonds due to the hydrophilic affinity between them (McMurry, 2014; Caleja et al., 2017). Through histochemical staining, these studies also identified tannins in the cachichín seeds analyzed (Fig. 7). Although their presence was scarce, the interactions between polyphenols and carbohydrates have effects on seed metabolism, which depend on the size of the phenolic compound, hydrophilic property, and carbohydrate structure (Dueñas-Martín, Iriondo-DeHong & del Castillo-Bilbao, 2018). Tannins exhibit antioxidant, apoptotic, antitumor, anti-bacterial, anti-viral, and anti-plasmin inhibitory activities, in addition to their fundamental activities related to binding to proteins, large molecular compounds and metallic ions (Okuda & Ito, 2011). Importantly, the toasting treatments increased the concentration of tannins, which is in full agreement with Quintero-Hilario et al. (2019), who reported that roasting increased the tannin concentration in breadnut seeds.
When the seed is subjected to thermal processing, the testa undergoes slight changes at the microscopic level, although it does not deform, preserving the initial structure and offering protection to the seed. However, the internal structure shows endosperm degradation. When there is no strict control over time and temperature factors during toasting (T3), moisture dissipates and the natural structure of the seed is broken, with a concomitant degradation of polysaccharides (Fig. 8) (Nooshkam, Varidi & Bashash, 2019). When these factors are controlled (T4), it is possible to maintain a structure similar to the raw state in a macroscopic view, although there will still be a slight degradation as a result of the thermal process. When the seed is subjected to boiling (T2), the moisture content increases, which can be attributed to the hygroscopic action of the starches and a gelation reaction of the pectins contained in the cell membrane, thus increasing flexibility and creating a rubbery texture (Roman-Benn et al., 2023). Similar results have been observed in sesame (Sesamum indicum L.) (Liu et al., 2020; Yao et al., 2021).
All thermal treatments resulted in protein concentrations statistically similar to raw seeds (Table 1); however, the commercial toasting (T3) exhibited higher concentrations of these biomolecules as compared to boiled seeds (T2). Likewise, thermal treatments did not affect protein concentrations in breadnut (Brosimum alicastrum Sw) (Quintero-Hilario et al., 2019), which means that both underutilized seeds can maintain their protein content after toasting or roasting. Interestingly, the images obtained after histochemical protein staining (Fig. 9) revealed changes in protein organization. Therefore, thermal treatments may induce changes in the organization of proteins in the cachichín seeds (Jaiswal, 2020), though the concentrations of these biomolecules are not significantly affected by the toasting process as compared to raw seeds.
During the Maillard reaction a wide range of intermediate low molecular mass compounds (i.e., organic acids, aldehydes, 1,2-dicarbonyl, and heterocyclic compounds), are formed, which indeed leads to changes in flavor, taste, and texture of food. Moreover, high molecular mass melanoidins are formed as end products of the reaction (Wang, Qian & Yao, 2011; Langner & Rzeski, 2014). These products can be responsible for the color development in food. They are formed through polymerization of sugar degradation products to high molecular mass polymers. The mass of these molecules depends on the reaction conditions and the amino compound involved. In particular, the structures and the reaction pathways of melanoidin metabolism are largely unknown, being highly dependent on the food chemical composition (simple sugars/polysaccharides, protein/peptides/amino acids, phenolic compounds, etc.) and the variety of different products formed during the Maillard reaction (Bruhns et al., 2019). In recent years, melanoidins have gained great attention, because of their effects on human health and the chemical characterization of the beneficial components they may contain (Nunes, Del Castillo & Carbonero, 2022). Furthermore, the laboriousness in purifying and identifying them makes a thorough analysis of melanoidins a challenging scientific endeavor. For our research group, the characterization of melanoidins in toasted cachichín seeds remains a daunting task.
Although significant progress has been made in the study of the effects of thermal treatments on cachichín seed, there are still several nutritional attributes of the seed to be explored, such as the quantification of organic matter, ash, minerals, and phenolic compounds, among others. These additional studies could contribute to determining the possible benefits of cachichín for food and human health. In addition, the determination of the effects of thermal treatments on the concentrations of antioxidants and bioactive compounds could shed light on the development of a nutraceutical food with potential hypoglycemic power. Therefore, it is necessary to continue exploring the properties of the cachichín seed to determine its nutritional value in an integral way and its potential as a source of nutraceutical and bioactive compounds beneficial to human health.
Conclusions
This study represents a significant contribution to our knowledge of the nutritious, anatomical and histochemical characteristics of cachichín seed, an underutilized and neglected edible plant species with high nutritional and nutraceutical properties. We demonstrated that the treatments tested (raw seeds: T1; boiling: T2; commercial toasting: T3; and controlled toasting: T4) differentially affected the concentrations of total sugars, reducing sugars, proteins and amino acids. Interestingly, controlled toasting increased the concentrations of glucose and fructose, while the protein concentration did not change in the thermal treatments as compared to the raw seeds. The highest concentration of total free amino acids was found in boiled seeds. The anatomical and histochemical analyses revealed that the raw seed has a well-defined internal and external structure, which is differentially affected by the treatments tested. Controlled toasting retains a structure similar to the raw state in a macroscopic view, although it also results in slight degradation of some components. The findings contribute to our knowledge of how thermal treatments affect the composition and structure of the cachichín seed. Finally, the cachichín seed, currently underutilized, could be considered a functional food because of its nutritional quality.