A new species of Pristimantis (Anura: Strabomantidae) from white-sand forests of central Amazonia, Brazil
- Published
- Accepted
- Received
- Academic Editor
- John Measey
- Subject Areas
- Biodiversity, Taxonomy, Zoology
- Keywords
- Amphibia, Advertisement call, Campinarana, Integrative taxonomy, Phylogeny, Pristimantis unistrigatus species group
- Copyright
- © 2023 Mônico et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. A new species of Pristimantis (Anura: Strabomantidae) from white-sand forests of central Amazonia, Brazil. PeerJ 11:e15399 https://doi.org/10.7717/peerj.15399
Abstract
The white-sand ecosystems in the Solimões-Negro Interfluve are among the less studied in Amazonia. Recent herpetological surveys conducted west of Manaus, Brazil (central Amazonia) indicate that white-sand forests host a unique anuran fauna comprising habitat specialized and endemic species. In the present study we describe a new species of rain frog belonging to the Pristimantis unistrigatus species group from the white-sand forest locally called “campinarana” (thin-trunked forests with canopy height below 20 m). The new species is phylogenetically close to rain frogs from western Amazonian lowlands (P. delius, P. librarius, P. matidiktyo and P. ockendeni). It differs from its closest relatives mainly by its size (male SVL of 17.3–20.1 mm, n = 16; female SVL of 23.2–26.5 mm, n = 6), presence of tympanum, tarsal tubercles and dentigerous processes of vomers, its translucent groin without bright colored blotches or marks, and by its advertisement call (composed of 5–10 notes, call duration of 550–1,061 ms, dominant frequency of 3,295–3,919 Hz). Like other anuran species recently discovered in the white-sand forests west of Manaus, the new species seems to be restricted to this peculiar ecosystem.
Introduction
The genus Pristimantis Jiménez de la Espada, 1870 is the most species-rich among vertebrates. Nevertheless, its total species diversity remains highly underestimated as suggested by the numerous new species described each year (e.g., De Oliveira et al., 2020; Ron et al., 2020; Gete, Buckely & Ron, 2021; Fouquet et al., 2022a; Ortega, Brito & Ron, 2022; Roberto et al., 2022). Since 2020 around 60 species of Pristimantis were described (Frost, 2023). The Andes is the most species-rich region for Pristimantis (Pinto-Sanchez et al., 2012), hosting more than 60% of the nominal species of the genus (Meza-Joya & Torres, 2015). Many other species are distributed throughout Amazonia, and the rest occurs in the Cerrado, Atlantic Forest, Pantepui and in Trans-Andean forest areas (Frost, 2023). This species-richness disparity is at least partially explained by the evolutionary history of the genus that started to diversify in the Andes and dispersed many times to Amazonian lowlands (Mendoza et al., 2015) with subsequent dispersals back to the Andes (Fouquet et al., 2022b). However, such disparity is also a consequence of the large amount of poorly sampled areas in Amazonia, where many undescribed species of Pristimantis occur (e.g., Vacher et al., 2020; Fouquet et al., 2022a). Finally, the low number of taxonomists working in lowland Amazonia also contributes to this knowledge gap (Melo-Sampaio, Ferrão & Moraes, 2021).
Amazonia is composed mostly of tropical ombrophilous rainforests (Veloso, Rangel Filho & Lima, 1991), but many other types of habitats exist due to variation in edaphic, hydrological and climatic conditions (Prance, 1979; Terborgh & Andresen, 1998), notably the white-sand ecosystem (WSE). It is characterized by grasslands, shrublands or forests with low-stature canopies on nutrient-poor sandy soils (Eiten, 1978). In central Amazonia, the WSE can be subdivided in two main types (Adeney et al., 2016): campina (open grasslands and shrublands with canopy height below 10 m) and campinarana (closed-canopy, thin-trunked forests with canopy height below 20 m). Although still poorly documented (Adeney et al., 2016), recent studies have indicated that WSE harbors a high proportion of specialized species for several biological groups, like birds (e.g., Almeida, 2010; Borges et al., 2016; Matos et al., 2016; Capurucho et al., 2020), snakes (e.g., Fraga et al., 2018) and plants (e.g., Fine & Baraloto, 2016; Vicentini, 2016; Guevara et al., 2016; Costa et al., 2020). For anurans, data collection is still incipient, albeit WSE specialization has been hypothesized for three hylid frogs (Carvalho et al., 2018; Ferrão et al., 2019; Ferrão et al., 2022).
Anuran sampling in Amazonia is concentrated near urban areas, navigable rivers, roads and highways (Jenkins et al., 2015; see map in Vacher et al., 2020). This is especially true for the Negro-Solimões Interfluve (NSI), a region with the largest amount of WSE (see Adeney et al., 2016) and only a few documented anuran assemblages (Neckel-Oliveira & Gordo, 2004; Menin et al., 2017; Moraes et al., 2022a; Moraes et al., 2022b). Even regions geographically close to the largest city in Brazilian Amazonia (Manaus) and regions easy to access remain poorly sampled and studied. However, this picture is changing. The Reserva do Desenvolvimento Sustentável do Rio Negro (RDS Rio Negro)—a NSI reserve covered mainly by a mosaic of dense forests and WSE patches lying ca. 100 km west of Manaus—has recently become a research center for biodiversity studies focussing on this ecosystem. The first attempt to document the anuran communities of the RDS Rio Negro has notably resulted in the rediscovery of an overlooked spiny-backed treefrog (Ferrão et al., 2019) and the description of a new snouted treefrog (Ferrão et al., 2022).
Herpetological surveys conducted in the RDS Rio Negro and nearby WSE patches in 2018 and 2020 resulted in the discovery of an unknown Pristimantis species associated with campinarana. The external morphology and the advertisement call of this species indicated that it represents an unnamed species and preliminary molecular comparisons confirmed this assumption. Herein, we use an integrative taxonomy approach and describe this new species of Pristimantis as well as its phylogenetic position, geographic distribution and natural history.
Materials & Methods
Sampling
Twenty-two adults and two juveniles of the new species were manually collected in two localities in the municipality of Iranduba, state of Amazonas, Brazil. Among them, 20 specimens were collected at the Ramal Nova Esperança, Km 20 of the AM-070 Highway (3°09′14.5 ″S, 60°13′59.4″W, 83 m elevation) on 13 December 2020, and four specimens at the RDS Rio Negro (3°03′42.0″S, 60°45′02.1″W, 61 m elevation) on 14 September 2018. Specimens were anaesthetized and killed with topic 5% lidocaine. Muscle or liver tissue was preserved in 100% ethanol for posterior genetic analysis, whereas the specimens were fixed in 10% formalin and subsequently preserved in 70% ethanol. Specimens were sexed by the presence of vocal slits exclusively present in males and internally by the condition of the gonads. All males used in the type series were found calling, which ensures their reproductive status as adults, while gravid females (n = 4) and specimens with large SVL were also considered adults. Vouchers were deposited in the herpetological collection of the Instituto Nacional de Pesquisas da Amazônia—INPA-H (Manaus, Brazil) and Museu Paraense Emílio Goeldi—MPEG (Belém, Brazil). Protocols of collection and animal care follow the Brazilian Federal Council for Biology resolution number 148/2012 (Conselho Federal de Biologia—CFBio, 2012) and the Ethics Committee on the Use of Animals of the Instituto Nacional de Pesquisas da Amazônia—CEUA-INPA (Process n° 35/2020, SEI 01280.001134/2020-63). Specimens were collected under collection permit number 73647-3 issued by the Centro Nacional de Pesquisa e Conservação de Répteis e Anfíbios of the Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio.
Advertisement calls of nine males of the new species were recorded at the Ramal Nova Esperança on 13 December 2020. Recordings were made with a Sennheiser K6/ME66 unidirectional microphone (Sennheiser, Germany) coupled to a digital recorder Marantz PMD660 (Marantz, Japan). Air temperatures (24.6–26.2 °C) and humidity (86–93%) during call recording were measured with a thermohygrometer Incoterm 7663.02.0.00. Each calling male was recorded for three minutes using frequency rate of 44 kHz and 16 bits of resolution in the mono pattern. Recordings were deposited in the Fonoteca Neotropical Jacques Vielliard (FNJV) of the Universidade de Campinas (UNICAMP), Campinas, Brazil under access number FNJV 59105–59115.
Sequencing and phylogenetic analyses
Genomic DNA was extracted from tissues of five specimens from Ramal Nova Esperança and three from RDS Rio Negro using the kit PureLink™ Genomic DNA (Invitrogen by Thermo Fisher Scientific, Carlsbad, CA, USA). Fragments of two mitochondrial (16S rRNA and Cytochrome C Oxidase sub-unit 1—COI) and a nuclear gene (Recombination Activating 1—RAG1) were amplified through polymerase chain reaction (PCR) following the protocol described in Mônico et al. (2022). The 16S was amplified using primers 16Sar (5′-CGCCTGTTTATCAAAAACAT-3′) and 16Sbr (5′-CCGGTCTGAACTCAGATCACGT-3′) (Palumbi, 1996), COI using primers Chmf4f (5′-TYTCWACWAAYCAYAAAGAYATCGG-3′) and Chmr4r (5′-ACYTCRGGRTGRCCRAARAATCA-3′) (Che et al., 2012) and RAG1 using primers R182 (5′-GCCATAACTGCTGGAGCATYAT-3′) and R270 (5′-AGYAGATGTTGCCTGGGTCTTC-3′) (Heinicke, Duellman & Hedges, 2007). Amplicons were sequenced in an ABI PRISMI 3130XL (Thermo Fisher) using the forward and reverse primers of each gene. Sequences were manually edited with Geneious Pro 5.4.6 (Biomatters Ltd.), then subjected to BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi; Altschul et al., 1997) to compare with sequences of other Pristimantis. Newly generated sequences were deposited in GenBank. Accession numbers are available in Appendix 1.
According to BLAST, sequences of the new species were highly similar to species currently assigned to the Pristimantis unistrigatus species group. To infer the phylogenetic relationships among the new species and its close relatives, newly generated sequences were inserted into a data set retrieved from GenBank containing selected homologous sequences (Appendix 1). Sequence selection in GenBank was focused on specimens of the P. unistrigatus species group from the Andes, Pantepui and Amazonian lowlands. Additionally, sequences of two species of the genus Oreobates were retrieved to root the tree. In total, 265 sequences (16S = 136; COI = 70; RAG1 = 59) corresponding to 137 specimens were selected. We aligned sequences of each gene using MAFFT online server (https://mafft.cbrc.jp/alignment/server/) with default parameters, except by the use of the E-INS-i strategy for the 16S and G-INS-i strategy for protein-coding genes (Katoh & Standley, 2013). The final matrix was concatenated in Mesquite (Maddison & Maddison, 2021) and composed of 137 terminals with 1,827 bp (16S = 561 pb; COI = 636 pb; RAG1 = 630 pb).
Best-fit evolutionary models and partition schemes were determined through ModelFinder (Kalyaanamoorthy et al., 2017) using seven initial partitions: one for the 16S and one for each codon of protein-coding genes. The best evolutionary models for partitions in the concatenated matrix were: TIM2 + F + R5 for 16S, GTR + F + I +G4 for COI 1st and RAG 3rd codons, HKY + F +G4 for COI 2nd, RAG 1st and 2nd codons, and GTR + F +ASC + G4 for COI 3rd position. Phylogenetic relationships were reconstructed using Maximum Likelihood inference (ML). The ML tree was inferred with IQTREE (Nguyen et al., 2015) as implemented in the webserver (http://iqtree.cibiv.univie.ac.at; Trifinopoulos et al., 2016). Clade support was estimated with 10,000 ultrafast bootstrap replications (Hoang et al., 2018), 1,000 maximum iterations, and a minimum correlation coefficient of 0.99. We calculated pairwise genetic distances (p-distance and Kimura-two-parameter distance; Kimura, 1980) among the populations of new species and close relatives using MEGA 11 (Tamura, Stecher & Kumar, 2021). Genetic distances were calculated using pairwise deletion.
Morphology
Twenty five morphometric measurements were taken from 16 adult males and six adult females of the new species following Duellman & Lehr (2009) (eye diameter—ED, eye-nostril distance—EN, foot length—FL, interorbital distance—IOD, internarial distance—IND, head length—HL, head width—HW, snout-vent length—SVL, tibia length—TL, and tympanum diameter—TD), Caldwell, Lima & Keller (2002) (forearm length—FAL, hand length—HAND, snout length—SL, disc width of Finger III—WFD), Heyer et al. (1990) (tarsus length—TAL, thigh length—THL, upper arm length—UAL), Lima, Sanchez & Souza (2007) (hand length from proximal edge of palmar tubercle to tip of Finger I—HANDI, Finger II—HANDII, and Finger IV—HANDIV) and Mônico et al. (2022) (foot length from proximal edge of outer metatarsal tubercle to tip of Toe I—FLI, Toe II—FLII, Toe III—FLIII and Toe V—FLV, and disc width of Toe IV—WTD). Measurements were taken to the nearest 0.01 mm using a Leica stereomicroscope (model S8APO) coupled to a Leica DFC295 camera, except for SVL, measured to the nearest 0.01 mm with a digital caliper. Raw data are provided in Table S1.
Format of the description and terminology of morphological characters follow Kok & Kalamandeen (2008), Duellman & Lehr (2009), and Kok, Means & Bossuyt (2011). Color in life was described based on photographs taken in the field, following the color catalog provided by Köhler (2012).
Bioacoustics
Bioacoustic variables were analyzed with Raven Pro 1.6 (Bioacoustics Research Program, 2014) with the following configuration: window = Blackman, Discrete Fourier Transform = 2,048 samples and 3dB filter bandwidth = 80.0 Hz. The following temporal and spectral traits were measured: call duration—CD, number of notes per call—NN, note duration—ND, inter-note interval—SBN, and minimum—LF, maximum—HF and dominant frequency—DF. Inter-call interval was not measured because it is usually affected by microclimatic conditions at the time of recording (i.e., on rainy days, males call more often in a short period of time than on days without rain) and poorly informative. Dominant frequency was measured using the Peak frequency function; maximum and minimum frequencies were measured 20dB below the peak frequency to avoid background noise interference. Call description follows the call centered approach of Köhler et al. (2017). Spectrogram and oscillogram were generated in R environment (R Core Team, 2019) through the ‘seewave’ package 2.0.5 (Sueur, Aubin & Simonis, 2008) using a Hanning window, 256 points of resolution (Fast Fourier Transform) and an overlap of 85%. Bioacoustic raw data are provided in Tables S2.
Nomenclatural acts
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:F8ED54F6-9E18-49BD-8178-659BDFB79C65. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Phylogenetic relationships and genetic distances
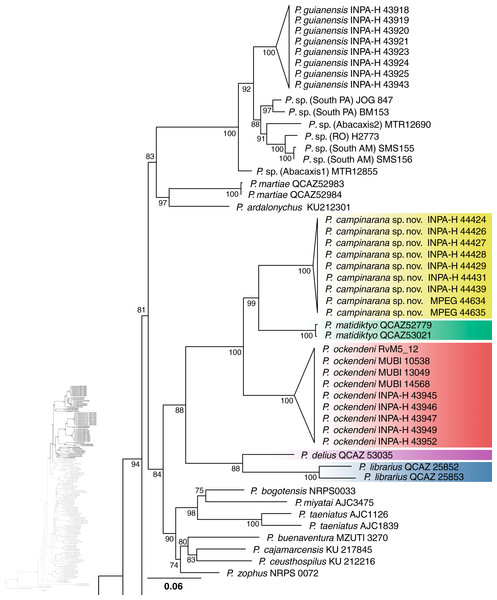
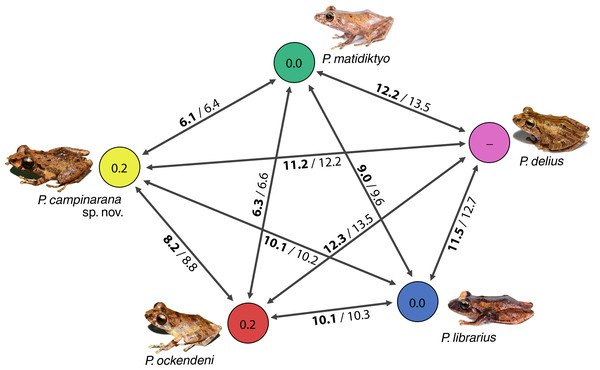
Individuals of the new species show low intraspecific genetic distances between populations (16S p-distance = mean 0.2%, maximum 0.4%). The new species is nested within a strongly supported clade grouping Pristimantis matidiktyo Ortega-Andrade & Valencia, 2012 and P. ockendeni (Boulenger, 1912) (Fig. 1, Appendix 2). This clade is sister to the clade formed by P. delius (Duellman & Mendelson III, 1995) and P. librarius (Flores & Vigle, 1994). Among the species mentioned above, mean interspecific p-distances range from 6.1 to 11.2% (Fig. 2). All these species occur in western Amazonian lowlands.
Figure 1: Part of the phylogenetic tree showing the position of Pristimantis campinarana sp. nov.
Maximum likelihood tree inferred with 16S, COI and RAG1. Non-parametric ultrafast bootstrap support is shown near the nodes. The species name is preceded by the specimen voucher number. The full phylogenetic tree is presented in Appendix 2.Figure 2: Genetic distances between Pristimantis campinarana sp. nov. and most closely related species (in percentage and based on 554 bp of 16S).
Mean interspecific distances are shown above or below arrows; p-distances are presented in bold numbers and followed by Kimura-2-parameters distance. Intraspecific p-distance is shown inside the circles. Photographs: S.R. Ron, PUC Ecuador, 2021 (P. delius, P. librarius and P. matidiktyo) and A.T. Mônico (P. campinarana sp. nov. and P. ockendeni).Taxonomic account
Pristimantis campinarana sp. nov.
LSID: urn:lsid:zoobank.org:act:61E81E96-5309-4FA0-AE8A-B34636CD5616
Pristimantis ockendeni: Lima et al. (2021).
Holotype
INPA-H 44426 (field number APL 23164), adult male, collected at Ramal Nova Esperança, Km 20 of the AM-070 Highway, municipality of Iranduba, state of Amazonas, Brazil (3°09′14.5″S, 60°13′59.4″W, 83 m elevation), on 13 December 2020 by A. T. Mônico and E. D. Koch.
Paratypes
Eighteen adult specimens collected at the same locality as the holotype on 12–19 December 2020 by A. T. Mônico, I. Y. Fernandes and E. D. Koch: twelve males INPA-H 44424, INPA-H 44427–29, INPA-H 44431–35 (field numbers APL 23162, 23165–67, 23169–72 and 23175 respectively) and MPEG 44637, MPEG 44639 and MPEG 44641 (field numbers APL 23181, 23183 and 23185, respectively); and six females INPA-H 44425, INPA-H 44436 and INPA-H 44437 (field numbers APL 23163, 23176 and 23177, respectively) and MPEG 44636, MPEG 44638 and MPEG 44640 (field numbers APL 23179, 23182 and 23184, respectively), and three adult males collected at Reserva do Desenvolvimento Sustentável do Rio Negro, municipality of Iranduba, state of Amazonas, Brazil(3°03′42.0″S, 60°45′02.1″W, 61 m elevation): MPEG 44634–35 and INPA-H 44439 (field numbers APL 22250–52, respectively), on 14 September 2018 by M. Ferrão, A. S. Ferreira and J. Moravec.
Referred material
Four specimens collected in the municipality of Iranduba, state of Amazonas, Brazil. One juvenile (INPA-H 44430, field number APL 23168), same data as the holotype. One juvenile (INPA-H 44440, field number APL 22253) and one adult male (INPA-H 44441, field number APL 22254), same data as the paratype INPA-H 44439. One adult male (INPA-H 44438, field number ATM 013) collected at the surroundings of Reserva do Desenvolvimento Sustentável do Rio Negro (3°06′36.5″S, 60°44′23.5″W), on 15 October 2022 by A. T. Mônico and I. Y. Fernandes. Two females (INPA-H 44698 and INPA-H 44699, field number ATM 49 and ATM 50, respectively) collected at Reserva do Desenvolvimento Sustentável do Rio Negro (2°55′16.6″S, 60°49′18.3″W), on 11 and 12 January 2023 by A. T. Mônico, I. Y. Fernandes, E. D. Koch, B. C. Martins, S. Dantas and A. P. Lima.
Diagnosis
This new species is characterized by the following combination of characters: (1) dorsal skin shagreen; (2) tympanum visible, tympanic membrane poorly differentiated, tympanum diameter 29–40% of eye diameter and annulus partially visible externally; dark supratympanic band; (3) snout moderately long (SL 37–43% of HL), subacuminate in dorsal view and truncated in lateral view, loreal region concave, lips not flared; (4) upper eyelid tubercles present; with or without dark bar between the eyes, and one or two oblique black streaks below the eye; postrictal tubercles absent; cranial crests absent; three scapular tubercles, less visible in specimens having dark dorsal coloration; (5) nostril ovoid, slightly protuberant, directed laterally; internarial distance 73–87% of interorbital distance; (6) tongue ovoid, longer than wide; (7) dentigerous processes of vomers present, small, oblique and positioned posterior to level of choanae, one or two on each side, ill-defined; (8) males with vocal slits; vocal sac small, subgular; nuptial pads absent; (9) Finger II longer than I; finger discs small, rounded (Finger I and II) to expanded (Finger III and IV); (10) fingers without lateral fringes; (11) ulnar tubercles aligned, barely visible in fixed specimens; (12) tibia length 46–54% of SVL; (13) heel tubercle absent; tarsal tubercles aligned, small and barely visible; tarsal fold absent; (14) thenar tubercle ovoid; palmar tubercle bifid, twice the width of the thenar tubercle; (15) inner metatarsal tubercle ovoid; outer metatarsal tubercle small, longer than wide, less than 1/3 of the thenar tubercle; (16) toes III–V with lateral fringes, webbing basal between toes IV–V; Toe I smaller than Toe II, not reaching the edge of disc on Toe II; Toe V longer than Toe III; (17) belly skin weakly areolate, and ventral region of the femur areolate; (18) in life, groin translucent, without brightly colored blotches or marks; posterior surfaces of thighs uniformly brown; (19) in life, iris dichromatic, pale bronze upper and lower parts with dark brown vermiculation and broad median mahogany-red stripe through pupil; (20) SVL in adult males of 17.3–20.1 mm (n = 16) and in females of 23.2–26.5 mm (n = 6); and (21) advertisement call with 5–10 notes and average call duration of 694 ± 115 ms, inter-note interval of 82.7 ± 11 ms, minimum frequency of 2,260–3,176 Hz, maximum frequency of 3,756–5,280 Hz and dominant frequency of 3,295–3,919 Hz.
Comparisons with other species
The new species is compared to all other currently recognized rain frogs of the Pristimantis unistrigatus species group occurring in Amazonian lowlands: P. aaptus (Lynch & Lescure, 1980); P. academicus (Lehr, Moravec & Gagliardi-Urrutia, 2010); P. altamazonicus (Barbour & Dunn, 1921); P. brevicrus (Andersson, 1945); P. carvalhoi (Lutz & Kloss, 1952); P. crepitaculus (Fouquet et al., 2022a; Fouquet et al., 2022b); P. croceoinguinis (Lynch, 1968); P. delius (Duellman & Mendelson III, 1995); P. diadematus (Jiménez de la Espada, 1875); P. divnae (Lehr & Von May, 2009); P. espedeus (Fouquet et al., 2013); P. eurydactylus (Hedges & Schlüter, 1992); P. grandoculis (Van Lidth de Jeude, 1904); P. guianensis (Mônico et al., 2022); P. inguinalis (Parker, 1940); P. kichwarum (Elmer & Cannatella, 2008); P. librarius (Flores & Vigle, 1994); P. luscombei (Duellman & Mendelson III, 1995); P. martiae (Lynch, 1974); P. matidiktyo (Ortega-Andrade & Valencia, 2012); P. miktos (Ortega-Andrade & Venegas, 2014); P. ockendeni (Boulenger, 1912); P. orcus (Lehr, Catenazzi & Rodríguez, 2009); P. variabilis (Lynch, 1968); and P. ventrimarmoratus (Boulenger, 1912).
Pristimantis campinarana sp. nov. differs from P. aaptus, P. diadematus, P. divnae, P. espedeus and P. orcus by having smaller male SVL of 17.3–20.1 mm [SVL 22.9 mm in P. aaptus (Lynch & Lescure, 1980); 20.0–27.4 mm in P. diadematus (Duellman & Mendelson III, 1995); 22.8–23.4 mm in P. divnae (Lehr & Von May, 2009); 20.7–24.8 mm in P. espedeus (Fouquet et al., 2013), and 20.0–25.1 mm in P. orcus (Lehr, Catenazzi & Rodríguez, 2009)] and from P. academicus, P. carvalhoi, P. martiae and P. grandoculis by larger male SVL [SVL 14.9 mm in P. academicus (Lehr, Moravec & Gagliardi-Urrutia, 2010); 13.5–14.8 mm P. carvalhoi (Lynch & Lescure, 1980; Duellman & Mendelson III, 1995); 14.7–17.9 mm in P. grandoculis (Fouquet et al., 2022a); 13.2–16.8 mm in P. martiae (Lynch, 1974)]; from P. aaptus, P. altamazonicus, P. brevicrus, P. delius, P. diadematus, P. eurydactylus, P. miktos, P. ockendeni, P. orcus, and P. ventrimarmoratus by smaller female SVL of 23.2–26.5 mm [SVL 29.9–34.8 mm in P. aaptus (Lynch & Lescure, 1980); 28.4–30.1 mm in P. altamazonicus (Ortega-Andrade et al., 2017); 27.2–35.0 mm in P. brevicrus (Ortega-Andrade et al., 2017); 30.9 mm in P. delius (Duellman & Mendelson III, 1995; Duellman & Lehr, 2009); 35.4–44.5 in P. diadematus (Duellman & Lehr, 2009); 29.4 mm in P. espedeus (Fouquet et al., 2013); 33.5–35.3 mm in P. eurydactylus (Hedges & Schlüter, 1992); 26.7–29.2 mm in P. miktos (Ortega-Andrade & Venegas, 2014); 30.4–30.6 mm in P. ockendeni (Mônico et al., 2022); 32.6–36.5 mm in P. orcus (Lehr, Catenazzi & Rodríguez, 2009), and 33.3–43.8 mm in P. ventrimarmoratus (Duellman & Lehr, 2009)].
Pristimantis campinarana sp. nov. can be easily distinguished from most of the lowland species (n = 16) by presence of vocal slits in males [absent in P. altamazonicus (Ortega-Andrade et al., 2017), P. brevicrus (Ortega-Andrade et al., 2017), P. carvalhoi (Duellman & Lehr, 2009), P. croceoinguinis (Lynch, 1968), P. diadematus (Duellman & Lehr, 2009), P. divnae (Lehr & Von May, 2009), P. eurydactylus (Hedges & Schlüter, 1992), P. grandoculis (Fouquet et al., 2022a), P. miktos (Ortega-Andrade & Venegas, 2014), P. orcus (Lehr, Catenazzi & Rodríguez, 2009), and P. ventrimarmoratus (Duellman & Lehr, 2009)], presence of eyelid tubercles [absent in P. carvalhoi (Duellman & Lehr, 2009), P. delius (Duellman & Mendelson III, 1995; Duellman & Lehr, 2009), P. diadematus (Duellman & Lehr, 2009), P. lythrodes (Lynch & Lescure, 1980), P. variabilis (Duellman & Lehr, 2009) and P. ventrimarmoratus (Duellman & Lehr, 2009)], presence of tympanum [absent in P. brevicrus (Ortega-Andrade et al., 2017), P. carvalhoi (Duellman & Lehr, 2009), P. croceoinguinis (Lynch, 1968; Duellman & Lehr, 2009), P. grandoculis (Fouquet et al., 2022a), P. martiae (Lynch, 1974) and P. ventrimarmoratus (Duellman & Lehr, 2009)], presence of dentigerous processes of vomers [absent in P. delius (Duellman & Mendelson III, 1995; Duellman & Lehr, 2009) and P. guianensis (Mônico et al., 2022)], and by presence of tarsal tubercles in males [absent in P. diadematus (Duellman & Lehr, 2009), P. librarius (Flores & Vigle, 1994), P. lythrodes (Lynch & Lescure, 1980), P. kichwarum (Elmer & Cannatella, 2008), P. martiae (Lynch, 1974; Duellman & Lehr, 2009), P. matidiktyo (Ortega-Andrade & Valencia, 2012) and P. ventrimarmoratus (Duellman & Lehr, 2009)].
Furthermore, the absence of brightly colored blotches or marks in the groin distinguishes the new species from P. aaptus (black groin; Lynch & Lescure, 1980), P. academicus (yellow groin; Lehr, Moravec & Gagliardi-Urrutia, 2010), P. altamazonicus (red to bright orange groin with black mottling; Ortega-Andrade et al., 2017), P. brevicrus (bluish white to yellowish white groin with black mottling; Ortega-Andrade et al., 2017), P. carvalhoi (yellow to yellowish white groin; Duellman & Lehr, 2009), P. crepitaculus (dark grey groin; Fouquet et al., 2022a), P. croceoinguinis (yellow or orange groin; Lynch, 1968; Elmer & Cannatella, 2008; Duellman & Lehr, 2009), P. diadematus (bluish white, yellowish tan or pink groin; Duellman & Lehr, 2009), P. divnae (yellow groin with brown marks; Lehr & Von May, 2009), P. espedeus (red-orange groin; Fouquet et al., 2013), P. eurydactylus (pale tan groin with dark brown vertical or diagonal bars; Hedges & Schlüter, 1992), P. grandoculis (dark grey groin; Fouquet et al., 2022a), P. inguinalis (bright yellow groin; Fouquet et al., 2013), P. librarius (reddish orange paler groin; Flores & Vigle, 1994; Elmer & Cannatella, 2008), P. lythrodes (black with yellowish white groin; Lynch & Lescure, 1980), P. martiae (pale brown groin; Lynch, 1974), P. matidiktyo (pale yellowish white groin; Ortega-Andrade & Valencia, 2012), P. miktos (yellowish-tan groin; Ortega-Andrade & Venegas, 2014), and P. orcus (black groin with white or whitish blue blotches; Lehr, Catenazzi & Rodríguez, 2009). Moreover, dichromatic iris differs P. campinarana sp. nov. from the species having monochromatic iris: P. academicus (golden to bronze iris; Lehr, Moravec & Gagliardi-Urrutia, 2010); P. altamazonicus (coppery red iris; Ortega-Andrade et al., 2017); P. brevicrus (coopery red to silver iris; Ortega-Andrade et al., 2017); P. carvalhoi (pale gray iris; Duellman & Lehr, 2009); P. croceoinguinis (gray to dull bronze iris; Lynch, 1968; Duellman & Lehr, 2009); P. divnae (silver iris; Lehr & Von May, 2009; P. lythrodes (grayish brown iris; Duellman & Lehr, 2009); P. miktos (deep orange iris; Ortega-Andrade & Venegas, 2014); P. orcus (dark gray to gold with cupper tint; Lehr, Catenazzi & Rodríguez, 2009); in P. ventrimarmoratus (pale bronze iris; Duellman & Lehr, 2009).
The advertisement call of Pristimantis campinarana sp. nov. is relatively similar with the calls of P. espedeus, P. guianensis and P. ockendeni. Nevertheless, calls of the new species differ in temporal and spectral characteristics [P. campinarana sp. nov.: call duration 694 ± 115 ms (550–1061 ms), inter-note interval 82.7 ± 11.9 ms (64–109 ms), and dominant frequency 3,587 ± 204 Hz (3,295–3,919 Hz) from P. espedeus (call duration 330 ms (240–500 ms), dominant frequency 2,700 Hz (2,680–2,840 Hz); Fouquet et al., 2013), P. guianensis (call duration 232 ± 42 ms (158–371 ms), inter-note interval 44 ± 5 ms (14–56 ms); Mônico et al., 2022) and P. ockendeni (dominant frequency 2,864 ± 202 Hz (2,519–3,143 Hz); Mônico et al., 2022)]. Furthermore, the advertisement call of P. campinarana sp. nov. easily differs from P. inguinalis and P. orcus by having multi-noted calls (single note in both mentioned species: Fouquet et al., 2013; López-Rojas et al., 2013).
Description of holotype
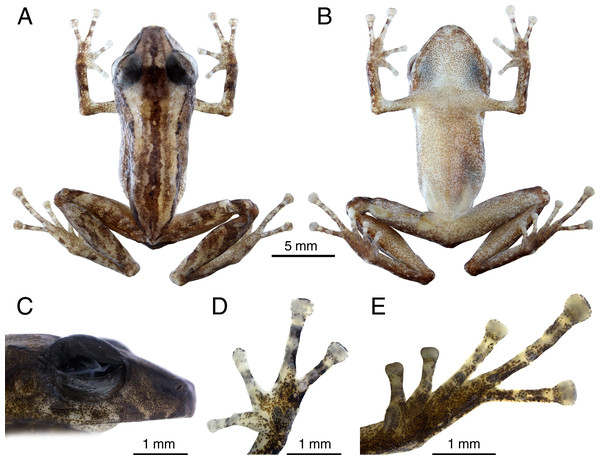
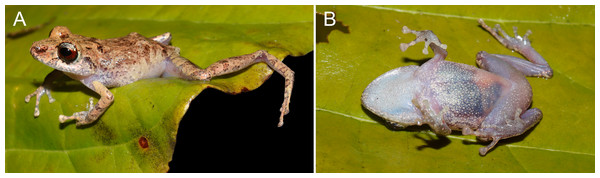
INPA-H 44426 (field number APL 23164). Morphometric measurements are presented in Table 1. An adult male (Fig. 3), SVL 19.1 mm; head slightly longer than wide (HL 103% of HW); head width 35.9% of SVL; head length 36.9% of SVL; cranial crest absent. Snout moderately long(SL 131% and 106% of EN and ED, respectively), subacuminate in dorsal view (Fig. 3A) and moderately truncated in lateral view (Fig. 3C); nostril ovoid, slightly protuberant, directed dorsolaterally; IND 87.3% of IOD; internarial region almost straight; canthus rostralis almost straight in dorsal view, slightly angular in profile; loreal region concave; lips not flared; one small tubercle on upper eyelid; interorbital region straight, IOD 32.1% of HW; eyes large (ED/TD = 2.7), pupil horizontally elliptical; supratympanic fold slightly distinct, extending from posterior margin of eyelid angling posteroventrally behind tympanic annulus; tympanum visible and rounded, TD 36.5% of ED; tympanic membrane poorly prominent, directed laterally; tympanic annulus poorly distinct, obscured dorsally by the supratympanic fold; one small postrictal tubercle, poorly visible; choanae of moderate sized, rounded to ovoid, not concealed by palatal sheath of maxilla; dentigerous processes of vomers present, with two ill-defined teeth, small, oblique and positioned posterior to level of choanae; tongue ovoid, longer than wide; short vocal slits, located in posterior half of mouth floor between tongue and margin of jaw; vocal sac small, simple and subgular.
Figure 3: Preserved holotype (INPA-H 44426) of Pristimantis campinarana sp. nov.
(A) Dorsal and (B) ventral views of body, (C) lateral view of head, (D) ventral view of hand, (E) ventral view of foot. Photographs: A.T. Mônico.Forearm shorter than hand (FAL 81.2% of HAND), notched posteriorly, nearly 78% free; three small ulnar tubercles ill-defined and aligned, almost indistinct after preservation; relative length of fingers I <II <IV <III (Fig. 3D); discs small and rounded on fingers I and II, expanded on fingers III and IV, with circumferential grooves; thenar tubercle poorly distinct, ovoid; palmar tubercle distinct, bifid; subarticular tubercles ill-defined, most prominent on fingers III and IV, rounded in dorsal and lateral view; small supernumerary tubercles present, but poorly visible; ventral pads well-defined on fingers III and IV.
Hindlimbs slender; tibia length 49% of SVL; heel without tubercles; tarsus with a row of small, poorly defined tubercles; tarsal fold absent; foot length 41% of SVL; relative length of toes I <II <III <V <IV (Toe III reaches the second subarticular tubercle of the Toe IV; Fig. 3E); toes with lateral fringes, more developed on toes III–V, webbing basal between toes IV–V; discs small and rounded on toes I and II, expanded on toes III–V; inner metatarsal tubercle large and ovoid, more than two times the size of ovoid outer metatarsal tubercle; subarticular tubercles large, protuberant, single, round on toes I–III and elliptical on toes IV and V; small supernumerary tubercles more visible on toes II-IV; ventral pads well-defined on toes III–V.
Dorsal skin shagreen (Fig. 3A), with longitudinal stripes and an irregular dorsolateral fold composed of spaced tubercles; small tubercles on scapular region; upper eyelid shagreen, with small tubercles; skin on flanks and chest smooth; skin on belly slightly areolate; upper and posterior surfaces of hindlimbs smooth, with small flat tubercles on thigh; dorsolateral folds absent; pectoral and discoidal folds not visible; cloaca protuberant, cloacal region without tubercles.
In life, dorsum yellow ocher (color 14 by Köhler, 2012) with dark brown mid-dorsal stripe running from snout to cloaca. Canthal stripe present, black, running from the tip of snout to anterior margin of eyelid. Dorsolateral stripe present, irregular, formed by a series of small, dark brown dots and blotches, running from posterior eye to cloacal region (Fig. 4A). Upper lip with dark brown subocular and dark brown supratympanic bar. Dark brown transversal bars on forearms; light brown transversal bars on thigh and tibia. Posterior surfaces of thigs uniformly brown. Groin white, translucent, with sparse brown melanophores. Throat, chest, belly and ventral surfaces of legs white, translucent, densely covered by tiny brown melanophores (Fig. 4B). Iris pale bronze with dark brown vermiculation and broad horizontal median mahogany-red (color 34 by Köhler, 2012) stripe through pupil.
Figure 4: Coloration in life of the holotype (INPA-H 44426, SVL 19.1 mm) of Pristimantis campinarana sp. nov.
(A) Dorsolateral and (B) ventral views of body. Photographs: A.T. Mônico.In alcohol, color pattern faded (Fig. 3), upper lip with five dark bars (Fig. 3C), bars on dorsal surfaces of forearms, thighs and tibias dark brown; venter whitish with dense dark brown melanophores.
Variation in the type series
SVL ranges from 17.3 to 20.1 mm in males (n = 16) and from 23.2 to 26.5 mm in females (n = 6) (Table 1). Canthus rostralis almost straight in dorsal view (e.g., Fig. 3B, holotype) to slightly curved in some individuals (e.g., Figs. 5E and 5F). Three to four ulnar tubercles are present in males (barely visible in 63% of them), ulnar tubercles absent in females. Dorsal skin texture varies from shagreen to granular, small tubercles are present or absent (character of skin texture probably depends on individual activity at the moment of sampling; see Guayasamin et al., 2015; Kok et al., 2018).
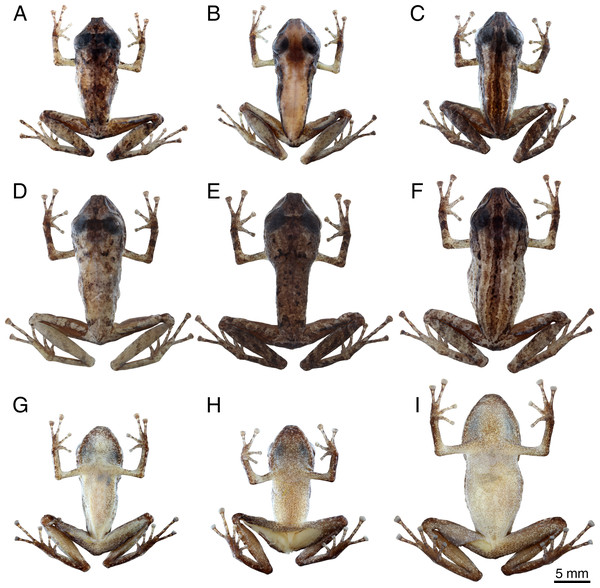
Figure 5: Preserved specimens of Pristimantiscampinarana sp. nov.
Dorsal view: (A) MPEG 44639, (B) MPEG 44641, (C) INPA-H 44424, (D) INPA-H 44436, (E) INPA-H 44437 and (F) MPEG 44638. Ventral view: (G) MPEG 44641, (H) INPA-H 44424, and (I) MPEG 44638. Males (A–C, G–H). Females (D–F, I). Photographs: A.T. Mônico.In preservative, three types of basic dorsal color patterns can be detected in the type series of Pristimantis campinarana sp. nov.: dorsum with irregular dark brown markings (68%; Figs. 5A, 5D and 5E), dorsal coloration sharply outlined against the flanks (9%; Fig. 5B), dorsum with dark brown stripes as in the holotype (23%; Figs. 5C and 5F). In addition, a dark brown interorbital bar is present in 73% of the specimens (Figs. 5A, 5D and 5E); a dark brown W-shaped mark is presented on the scapular region of 18% of the individuals, in some of them more conspicuous (Figs. 5A and 5D) than in others (Figs. 5D and 5E). Dark brown bars and blotches are present on the upper lip of all type specimens, dark and distinct in 73% of the specimens, faded or less conspicuous in the others. Obvious transversal dark brown bars are present on the arms and hands of 63% of the individuals, less conspicuous or absent in 23% and 14% of the specimens, respectively. Distinct transversal dark bars are present on the thigh and tibia of 32% of the specimens (Figs. 5C and 5F), poorly conspicuous in 54% (Fig. 5A) and absent in 14% of the individuals (Fig. 5B). Dark supratympanic stripe present in all specimens. Ventral surface is whitish cream to yellowish white, with a moderate amount of melanophores in 59% of individuals (Fig. 5I), small and large amount in 32% (Fig. 5G) and 9% (Fig. 5H) of individuals, respectively.
In life, dorsal background coloration is widely variable, from yellow ocher (color 14 by Köhler, 2012; Figs. 6A, 6D and 6J) and light chrome orange (color 76 by Köhler, 2012; Figs. 6D, 6G and 6J) to antique brown (color 24 by Köhler, 2012; Figs. 6C, 6G, 6I and 6L). Dorsolateral stripe present in some individuals (Figs. 6H and 6I, 6L), but absent in others (Figs. 6A–6G, 6J–6K). Canthal stripe present in some individuals (Figs. 6D, 6H, 6I and 6L), but absent in others (Figs. 6A–6C, 6E–6G, 6J–6K). The iris of most specimens is similar to that of the holotype, but some specimens show a small portion of the lower iris pale bronze to gold (Figs. 6A, 6C, 6I and 6K). At the day, the groin and ventral surface are platt’s-payne’s-gray (color 293 by Köhler, 2012) with brown melanophores (Fig. 5B); at night, when melanophores are less expanded, the groin (Fig. 7A) and ventral surface (Fig. 7B) becomes lighter, almost white.
Figure 6: Paratypes of Pristimantis campinarana sp. nov. in life.
Males: (A) INPA-H 44427 (SVL 19.5 mm), (B) INPA-H 44429 (SVL 18.4 mm), (C) INPA-H 44435 (SVL 18.9 mm), (D) INPA-H 44433 (SVL 18.7 mm), (E) INPA-H 44432 (SVL 18.6 mm), (F) INPA-H 44431 (SVL 19.8 mm), (G) INPA-H 44428 (SVL 17.3 mm), (H) MPEG 44641 (SVL 19.1 mm) and (I) MPEG 44637 (SVL 19.2 mm). Females: (J) INPA-H 44425 (SVL 24.4 mm), (K) INPA-H 44437 (SVL 23.5 mm) and (L) MPEG 44638 (SVL 24.7 mm).Figure 7: Nocturnal coloration of the groin and ventral surface of Pristimantis campinarana sp. nov. in life.
(A) Lateral and (B) ventral view of the female (INPA-H 44699, SVL 24.4 mm) at Reserva do Desenvolvimento Sustentável do Rio Negro, municipality of Iranduba, state of Amazonas, Brazil. Photographs: A.T. Mônico.Advertisement call
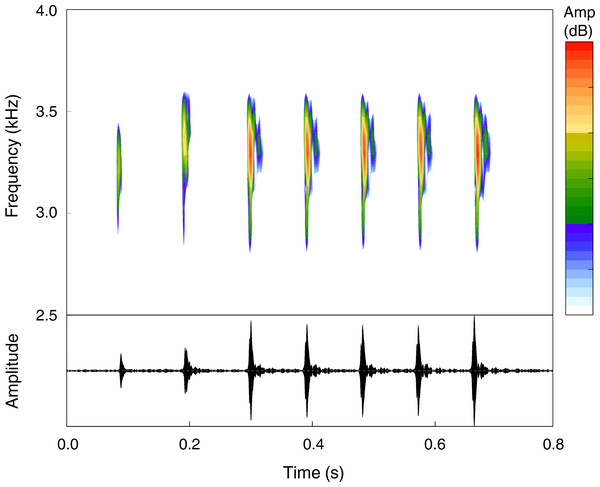
The advertisement call of Pristimantis campinarana sp. nov. (n = 9 males) is composed of 5–10 notes (n = 26 calls)—most commonly of 6–8 notes (n = 21)—and has a call duration of 694 ± 115 ms (550–1,061 ms). Notes are tonal, have duration of 26.5 ± 7.1 ms (13.2–39.8 ms) and an inter-note interval of 82.7 ± 11.9 ms (64.4–109.4 ms). Calls are emitted with a minimum frequency of 2,853 ± 141 Hz (2,260–3,176 Hz), a maximum frequency of 4,490 ± 413 Hz (3,756–5,280 Hz) and a dominant frequency of 3,587 ± 204 Hz (3,295–3,919 Hz) (Fig. 8). Temporal and spectral traits summarized according to individual call arrangement are presented in Table 2.
Figure 8: Advertisement call of the holotype (INPA-H 44426, FNJV 59105) of Pristimantis campinarana sp. nov. recorded at the Ramal Nova Esperança, municipality of Iranduba, state of Amazonas, Brazil.
Air temperature 25.9°C.Etymology
The specific epithet ‘campinarana’ is used as a noun in apposition and refers to the word in Portuguese that defines the type of forest that the new species occupies: the white-sand forest campinarana.
Distribution, natural history and conservation
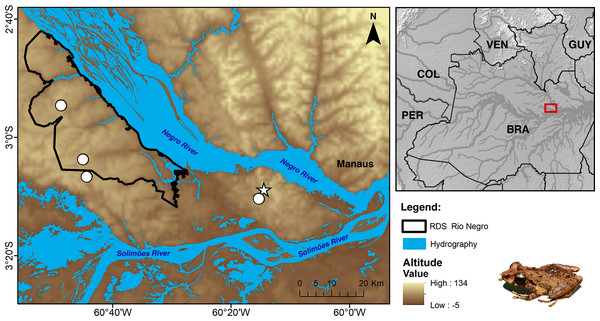
Currently, Pristimantis campinarana sp. nov. is known only from primary and slightly anthropized forests at two localities in the municipality of Iranduba, state of Amazonas, Brazil (Fig. 9). All individuals were recorded in WSE characterized as campinarana (a forest with canopy height below 20 m; Fig. 10A), where the species is locally abundant. However, none was found in campina, a white-sand forest with canopy height below 10 m and large patches of exposed white sandy soils.
Figure 9: Geographic distribution of Pristimantis campinarana sp. nov.
Symbols represent the type (star) and paratype (circle) localities. Country acronyms: BRA, Brazil; COL, Colombia; GUY, Guyana; PER, Peru; and VEN, Venezuela.| Morphometric measurements | Pristimantis campinarana sp. nov. | ||
|---|---|---|---|
| Holotype | Males* (n = 16) | Females (n = 6) | |
| SVL | 19.1 | 18.9 ± 0.7 (17.3–20.1) | 24.5 ± 1.2 (23.2–26.5) |
| HW | 6.8 | 6.7 ± 0.3 (6.1–7.0) | 8.8 ± 0.6 (8.0–9.8) |
| HL | 7.0 | 7.1 ± 0.2 (6.6–7.3) | 9.2 ± 0.5 (8.5–10.1) |
| SL | 2.9 | 2.8 ± 0.1 (2.6–3.1) | 3.6 ± 0.2 (3.2–3.9) |
| IND | 1.9 | 1.8 ± 0.1 (1.7–1.9) | 2.3 ± 0.2 (2.0–2.6) |
| EN | 2.2 | 2.2 ± 0.1 (2.0–2.4) | 2.8 ± 0.3 (2.7–3.2) |
| IOD | 2.2 | 2.3 ± 0.1 (2.1–2.4) | 2.9 ± 0.1 (2.7–3.1) |
| ED | 2.7 | 2.8 ± 0.1 (2.6–3.0) | 3.2 ± 0.1 (3.0–3.3) |
| TD | 0.9 | 0.9 ± 0.1 (0.8–1.0) | 1.2 ± 0.1 (1.2–1.4) |
| UAL | 5.2 | 5.0 ± 0.3 (4.4–5.4) | 6.7 ± 0.3 (6.3–7.0) |
| FAL | 4.3 | 4.2 ± 0.2 (3.8–4.7) | 5.8 ± 0.4 (5.4–6.3) |
| HAND | 5.2 | 4.8 ± 0.2 (4.6–5.3) | 6.4 ± 0.4 (6.0–7.3) |
| HANDI | 2.6 | 2.5 ± 0.2 (2.2–2.8) | 3.4 ± 0.3 (3.2–3.9) |
| HANDII | 3.4 | 3.3 ± 0.2 (3.0–3.6) | 4.2 ± 0.4 (3.8–4.9) |
| HANDIV | 4.2 | 4.1 ± 0.2 (3.7–4.3) | 5.3 ± 0.4 (4.9–6.2) |
| WFD | 0.8 | 0.8 ± 0.1 (0.7–1.0) | 1.1 ± 0.1 (0.9–1.2) |
| THL | 9.4 | 9.4 ± 0.2 (9.1–9.7) | 11.7 ± 0.8 (11.2–13.2) |
| TL | 9.7 | 9.8 ± 0.3 (9.3–10.4) | 12.5 ± 0.4 (11.8–13.7) |
| TAL | 4.9 | 4.9 ± 0.3 (4.6–5.5) | 6.4 ± 0.4 (5.9–7.1) |
| FL | 7.9 | 7.8 ± 0.3 (7.4–8.3) | 10.0 ± 0.7 (9.5–11.1) |
| FLI | 2.2 | 2.3 ± 0.2 (2.1–2.8) | 3.2 ± 0.2 (3.0–3.6) |
| FLII | 3.4 | 3.3 ± 0.2 (3.1–3.7) | 4.5 ± 0.3 (4.2–4.9) |
| FLIII | 5.5 | 5.2 ± 0.3 (4.7–5.6) | 6.8 ± 0.5 (6.4–7.6) |
| FLV | 6.4 | 6.3 ± 0.3 (6.0–7.1) | 8.2 ± 0.6 (7.6–9.2) |
| WTD | 0.9 | 0.8 ± 0.1 (0.7–1.0) | 1.2 ± 0.1 (1.0–1.4) |
Notes:
| Call arrangement | CD | ND | INI | LF | HF | DF | |
|---|---|---|---|---|---|---|---|
| 5 notes (n = 2) | mean | 605 | 35 | 92 | 2,941 | 4,546 | 3,553 |
| SD | 12 | 5 | 7 | 332 | 2.8 | 274 | |
| min | 596 | 31 | 98 | 2,706 | 4,544 | 3,359 | |
| max | 613 | 38 | 87 | 3,176 | 4,548 | 3,747 | |
| 6 notes (n = 9) | mean | 624 | 24 | 87 | 2,888 | 4,528 | 3,637 |
| SD | 47 | 9 | 12 | 139 | 503 | 267 | |
| min | 550 | 13 | 72 | 2,682 | 3,785 | 3,316 | |
| max | 691 | 39 | 109 | 3,064 | 5,241 | 3,919 | |
| 7 notes (n = 8) | mean | 679 | 30 | 77 | 2,778 | 4,321 | 3,486 |
| SD | 57 | 5 | 6 | 95 | 375 | 176 | |
| min | 601 | 23 | 69 | 2,686 | 3,756 | 3,295 | |
| max | 748 | 40 | 84 | 2,947 | 4,773 | 3,768 | |
| 8 notes (n = 5) | mean | 749 | 24 | 79 | 2,832 | 4,397 | 3,630 |
| SD | 25 | 2 | 6 | 126 | 66 | 78 | |
| min | 710 | 22 | 70 | 2,660 | 4,287 | 3,531 | |
| max | 779 | 27 | 86 | 2,977 | 4,456 | 3,725 | |
| 9 notes (n = 1) | – | 974 | 23 | 95 | 2,904 | 5,053 | 3,725 |
| 10 notes (n = 1) | – | 1,065 | 21 | 95 | 3,019 | 5,279 | 3,661 |
Notes:
- SD
-
standard deviation
- CD
-
call duration
- ND
-
note duration
- NN
-
number of notes per call
- INI
-
inter-note interval
- LF
-
minimum frequency
- HF
-
maximum frequency
- DF
-
dominant frequency
Figure 10: Natural history and breeding aspects of Pristimantis campinarana sp. nov.
(A) An example of “campinarana” environment inhabited by the new species at Ramal Nova Esperança. (B) Habitat of P. campinarana sp. nov. with terrestrial bromeliads at RDS Rio Negro, municipality of Iranduba, Amazonas, Brazil. (C) An unvouchered active calling male perched horizontally on a leave. (D) An amplectant couple (female MPEG 44640, SVL 26.52 mm; male MPEG 44641, SVL 19.15 mm) at Ramal Nova Esperança. Photographs: A.T. Mônico (A, D), J. Moravec (B) and E.D. Koch (C).Pristimantis campinarana sp. nov. is a crepuscular and nocturnal species, with peak activity at crepuscule. Its breeding activity takes place in the rainy season (November to February). In the dry season, we found males sheltering among the leaves of terrestrial bromeliads of the genus Guzmania (Fig. 10B). In the rainy season, males start calling at dusk (∼18:00 h) and are very active until ∼20:00 h. Then their activity decrease and end usually around 22:00 h. In rainy days, the vocalization is sustained throughout the night. Males were observed calling perched on the vegetation (Fig. 10C) usually 1 m above the ground, rarely above 3 m. Calling males aggregate in groups of up to ten individuals, separated from each other by ∼4–5 m, but it is not uncommon to observe smaller groups of three to four calling individuals that are spatially more spaced. The amplexus (n = 3) is axillary (Fig. 10D). Clutches were not found in situ, but females produce about 14–17 large oocytes (n = 4). The new species occurs in sympatry with a candidate species closely related to Pristimantis orcus (AT Mônico, 2022, unpublished data). We do not have sufficient data to categorize the new species following the criteria of the International Union for Conservation of Nature (IUCN), it should be thus considered Data Deficient (DD).
Discussion
The new species described herein is currently known only from white-sand forests from the Negro-Solimões interfluve. In Amazonia most of WSE is found within this interfluve, peppered within a matrix of dense forest (Adeney et al., 2016). Although long-term herpetological surveys were conducted in dense ombrophilous forests east of Negro River and in the Purus-Madeira Interfluve, the new species was never found there. Therefore, we assume that the new species is endemic of the Negro-Solimões interfluve and only found in WSE. This distributional pattern is possibly similar to the ones of other anuran species (Trachycephalus venezolanus [Mertens, 1950], Osteocephalus vilarsi [Melin, 1941] and Scinax albertinae Ferrão et al., 2022) recently discovered from the Negro-Solimões interfluve (Ferrão et al., 2019; Ferrão et al., 2022). Moreover, additional undescribed species (e.g., species of Adenomera; M Ferrão, 2022, unpublished data; Phyllomedusa AP Lima, 2022, unpublished data; Pristimantis aff. orcus, AT Mônico, 2022, unpublished data) are also found associated to WSE, thus totaling at least seven frog species sharing this habitat specialization and distribution pattern endemic to the Negro-Solimões interfluve. In fact, the Jaú region was recently defined as an additional independent area of endemism in Amazonia due to the coocurrence of six bird species (Borges & Da Silva, 2012). Our finding, thus, strengthen the idea that white-sand ecosystems of the Negro-Solimões interfluve harbors a unique biodiversity that deserves effective protection.
Pristimantis campinarana is nested within a clade formed by species otherwise restricted to western Amazonia lowlands: P. matidiktyo, P. ockendeni, P. delius and P. librarius. This nested position of P. campinarana suggests that the speciation occurred after a dispersal from the west. A possible scenario involves historical changes in the Amazon basin drainage (Hoorn et al., 2010; Albert, Val & Hoorn, 2018) such as the disappearance of a riverine barrier that connected the Japurá River to Negro River (Ruokolainen et al., 2019), in the Jaú region, that could have favored the dispersal P. campinarana ancestors. Relatively recent eastward dispersals have been reported for other anuran groups, from small leaflitter toads (i.e., Allobates caeruleodactylus and A. trilineatus clades, Réjaud et al., 2020; Ameerega, Roberts et al., 2006) to arboreal treefrogs(i.e., Osteocephalus taurinus and O. buckleyi groups, Ortiz et al., 2022). Alternative scenarios involve dispersal across rivers (Smith et al., 2014; Moraes et al., 2016; Pirani et al., 2019). Both hypotheses, however, involve subsequent isolation by river and eventually speciation (Wallace, 1852; Haffer, 1974; e.g., Ribas et al., 2012; Rojas et al., 2018).
Despite large genetic divergence and consistent diagnosis characters, the species forming this clade remain very similar in morphology (e.g., SVL of males, ventral skin texture and iris in life) illustrating the trend in the genus of highly conserved morphology and the challenge that describing its diversity (Padial & De la Riva, 2009; Padial et al., 2009; Waddell et al., 2018; De Oliveira et al., 2020). This phenotypic conservatism have been discussed for other Amazonian frogs (Gonzalez-Voyer et al., 2011; Kaefer et al., 2013; Guayasamin et al., 2015; De Oliveira et al., 2020), as well as its implications for taxonomy (Funk et al., 2007; Kaefer et al., 2013; Waddell et al., 2018).
New species are being described recurrently in Amazonia, even from areas close to large urban and research centers. For example, in the last years, several new species were described from the Reserva Florestal Adolpho Ducke (Manaus, Brazil), an intensively studied area (e.g., Amazophrynella manaos Rojas-Zamora et al., 2014; Atelopus manauensis Jorge, Ferrão & Lima, 2020; Synapturanus ajuricaba Fouquet et al., 2021; and Pristimantis guianensis Mônico et al., 2022). The lowlands in the Negro-Solimões interfluve are also close to Manaus, and also harbor newly described species notably the discovery of a new lizard genus (i.e., Marinussaurus Peloso et al., 2011). This illustrates how far we are from understanding the species diversity in Amazonia particularly for small and cryptic species like the P. unistrigatus group (Fouquet et al., 2022a; Mônico et al., 2022).
Conclusions
Using morphology, bioacoustics and molecular data from three markers (16S, COI and RAG-1), we described a novel species of rain frog (genus Pristimantis) from an unexplored environment in the Amazonia: the white sand ecosystems. Description of Pristimantis campinarana sp. nov. reaffirms that the species richness of the west Amazonian anurans remains considerably underestimated even near to the largest and dynamically developing Amazonian metropole—Manaus. The congruence of seven frog species sharing a WSE specialization and distribution pattern likely endemic to the Negro-Solimões interfluve seem to reinforce the area of endemism proposed by Borges & Da Silva (2012) in the Jaú region. Also, the vertebrate fauna of white sand forests is unique and recent studies have revealed notable discoveries, reinforcing the need for effective protection of these environments.
Supplemental Information
Morphometric measurements (in mm) of adults of the type series of Pristimantis campinarana sp. nov
Measurement acronyms are defined in the text. Abbreviations: INPAH, Instituto Nacional de Pesquisas da Amazônia; MPEG, Museu Paraense Emílio Goeldi; FN, field numbers; M, male; F, female.
Acoustic parameters of advertisement call of Pristimantis campinarana sp. nov
Abbreviations: vouchers: INPAH, Instituto Nacional de Pesquisas da Amazônia; MPEG, Museu Paraense Emílio Goeldi; FNJV, Fonoteca Neotropical Jacques Vielliard; AT, air temperature (ºC); NN, number of notes per call; CD, call duration (ms); ND, note duration (ms); INI, inter-note interval (ms); LF, minimum frequency (Hz); HF, maximum frequency (Hz); and DF, dominant frequency (Hz).