Identification and biodiversity patterns of Aspergillus species isolated from some soil invertebrates at high altitude using morphological characteristics and phylogenetic analyses
- Published
- Accepted
- Received
- Academic Editor
- Héctor Mora-Montes
- Subject Areas
- Agricultural Science, Microbiology, Mycology
- Keywords
- Aspergillus spp., Mycotoxins, Armadillidium vulgare, Porcellio laevis, Millipedes
- Copyright
- © 2023 Awad et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Identification and biodiversity patterns of Aspergillus species isolated from some soil invertebrates at high altitude using morphological characteristics and phylogenetic analyses. PeerJ 11:e15035 https://doi.org/10.7717/peerj.15035
Abstract
Background
The carcinogenic, mutagenic, and teratogenic chemicals such as aflatoxin are a worldwide health problem. Aspergillus spp., responsible for most cases of aflatoxin contamination, are common in the environment and spread easily to many different types of food. The objectives of this study were to conduct a survey of fungi associated with three soil invertebrates in Taif, Saudi Arabia, identify these isolates and explore mycotoxins formation.
Methods
In total, 114 fungal isolates were collected from various soil invertebrates (millipedes, Armadillidium vulgare and Porcellio laevis) in Taif, Saudi Arabia, among them, 22 isolates were identified as Aspergillus spp. based on morphological and molecular characteristics followed by both Fusarium and Penicillium.
Results
The sequences of ITS 1 and ITS 4 were utilized. Using bootstrap analysis, phylogenetic tree was split into two distinct clusters. Five sub clusters were included inside the first major cluster, and their bootstrap value was 99%. While, there were two small clusters in the second major cluster. All the tested Aspergillus strains were able to have a single PCR fragment amplified using the primer AspTef. TEF-1 DNA sequence bootstrap analysis with 1,000 replicates revealed two distinct groups. Additionally, the Aspergillus isolates were grouped into two different clusters with about 65% genetic similarity using ISSR-PCR analysis. The standard polymerase chain reaction was used to effectively amplify the Aopks, afl-A and omt-A genes in aflatoxigenic Aspergillus strains. Four Aspergillus strains used in this investigation were shown to generate aflatoxin B1. While, three Aspergillus stains showed ochratoxin genes.
Conclusions
In conclusion, the results indicate significant differences in the fungal community between ecoregions and soil invertebrates. Moreover, mycotoxin detection and identification among Aspergillus isolates were elucidated. This study could shed light on the risk of mycotoxin contamination along the supply chain.
Introduction
The existence of invertebrates is an indicator that area is suitable for the long-term growth of nutritious plants or trees (Stork & Eggleton, 1992). Mycoflora constitutes an important part of the soil ecosystem, playing a fundamental role in the biotic and abiotic interactions in this environment, participating in the recycling of soil nutrients and decomposition of organic matter to make them available to plants (Val-Moraes et al., 2009). Therefore, soil fungi communities contribute to the alleviation of soil degradation and fertility (Jeffries et al., 2003; Treseder et al., 2014; Sterkenburg et al., 2015).
The fungal community of soil is an immensely diverse group of organisms. A recent study on the diversity of soil fungi revealed around 80,500 operational taxonomic units (OTUs) occurring in soils worldwide (Treseder et al., 2014; Rosas-Medina, Macia-Vicente & Piepenbring, 2020). Local environmental variables, such as physical and chemical soil features, influence the diversity of soil fungi (Tardy et al., 2015), which in turn strongly affects the current diversity of fungal communities (Requena et al., 2001). Aspergillus is a diverse genus followed by a large number of species, which covers all countries of the world and is found in different climatic conditions, as well as in different soil types (Burrough et al., 2012; Monmi, Nguyen & Lee, 2022). It is also known for its secretion of mycotoxins, as well as its ability to spoil food, in addition, it is known for high pathogenicity to humans and animals (Frisvad et al., 2011; Hussein, El-Said & Yassein, 2020; Wei et al., 2022). Moreover, many species of this fungus are used in the industrial field, biotechnology and used in food fermentation processes such as cheeses as well as the production of many antibiotics, organic acids, medicines, or enzymes (Mazrou et al., 2020).
Aspergillus species were classified and defined in the past on the basis of morphological characteristics, but recently the matter has changed when relying on molecular and chemical characterization, which gave a kind of accuracy and credibility in molecular characterization over morphological characterization (Samson et al., 2014; Gautier, Normand & Ranque, 2016). It is now possible to use morphological traits along with molecular identification, which is the case in most recent studies. The th phenotype-based classification of subspecies and divisions is largely consistent with currently published lineages. Recent phylogenetic studies have shed light on the relationships among Aspergillus species, but there is still a puzzling question about the relationship between the new nomenclature with a single name for fungi (Tamura, Kawahara & Sugiyama, 2000; Houbraken & Samson, 2011; Houbraken, Vries & de Samson, 2014; Hassan, Farid & Gaber, 2019; Hassan et al., 2022). It is also important to study and determine the evolutionary relationships of species in Aspergillus and closely related genera (McNeill et al., 2012; Vesth et al., 2018). Standardized procedures based on extrolite characterization, morphological traits, and multilocus DNA sequence studies are routinely used for identifying Aspergillus species (Geiser et al., 2008; Mazrou et al., 2020. Molecular markers used for the identification, characterization and evolution of Aspergillus species over time are increasingly being studied. Furthermore, molecular markers used in Aspergillus have included the internal transcription spacer sequence (ITS), calodulin (CaM), and β-tubulin (BenA), Tef1 gene and RNA polymerase II second largest subunit (RPB2) sequences. The molecular marker is employed for identifying Aspergillus species because of the extensive gene database available, the relative simplicity of locus amplification, and genetic polymorphism (Samson et al., 2014; Raja et al., 2017; Monmi, Nguyen & Lee, 2022). These locus are insufficient to identify all Aspergillus species correctly, thus secondary identification parameters such as the SCAR marker are needed (Hassan, Farid & Gaber, 2019). About 25 species of Aspergillus have been reported from Saudi Arabia (Kamel, 2014; Taha et al., 2021). A. koreanus, a previously unknown species of Aspergillus, has just been described (Ameen et al., 2022). Recently, six more species have been documented in Saudi Arabia, A. asperescens and A. flavips, A. ustus, A. egyptiacus, A. versiolor and A. terreus (El-Samawaty et al., 2012). Hence, the objectives of the present work were to isolate, identify, and molecularly characterize the mycological diversity of specific soil invertebrates (millipedes, Armadillidium vulgare and Porcellio laevis) in Taif, Saudi Arabia, and to explore the mycotoxin gene in the isolated Aspergillus species.
Materials and Methods
Fungal isolation
Twenty samples of each soil invertebrates (Armadillidium vulgare, Millipedes and Porcellio laevis) were collected from three regions in Taif Governorate (Hawia, Shafa, and Wady Ghazal). Spores or mycelium fragments on the cuticle surface of carcasses were resuspended by shaking the bodies in water containing 5% Tween, according to Samson et al. (2014). Standard PDA/chloramphenicol medium was used to plate 10 µl of dilutions of this solution. Single germinations (or mycelium regeneration) were moved to a new plate after 6 days in an incubator (25 ± 2 °C). Each insect had only one isolate chosen from a pool of thalli with identical appearance. The morphological stability of these isolates was monitored by repeated purification steps after each of their consecutive transfers. Single spore purification was performed on conidial species, including Aspergillus spp.
Morphological identification of fungal isolates
Pure cultures were grown on Czapek Agar (CZA) medium. Sporulation was induced by subjecting cultures to ultraviolet light. Isolates were characterized according to morphological features, cultural characteristics such as pigmentation of the mycelium and direction of hyphal growth, aerial or lateral, microscopic observation of structures involved in asexual and sexual reproduction (spores) and the presence of fruiting bodies. Identification was accomplished based on the morphology of fungi (Raper & Fennell, 1965; Samson & Pitt, 2000; Pitt & Hocking, 2009; Samson et al., 2014).
DNA extraction
Mycelia from certain pathogenic fungi were put into Czapex Dox broth and cultured for 5 days at 27 °C in order to harvest their DNA. Using the standard fungal genomic DNA extraction protocol, DNA was isolated from each fungal sample (Al-Samarrai & Schmid, 2000).
PCR amplification of ITS region and TEF gene
ITS and TEF genes were PCR amplified with the primers listed in Table 1. Reactions of ITS and TEF genes were carried out according to Hassan, Farid & Gaber (2019) and Druzhinina et al. (2005), in 50 µl volume containing 2 µl (20 ng) of genomic DNA, 1 µl of primer (20 p.mol), 25 µl of Go Taq® Green Master Mix (Promega, Madison, WI, USA) deionized distilled water (up to a total volume of 50 µl). The C1000TM Thermo Cycler from Bio-Rad (Hercules, CA, USA) was calibrated for DNA amplification at 94 °C for 10 min before adding Taq polymerase, then for 40 cycles. Each cycle included 1 min at 94 °C, 1.5 min at 58 °C, and 2.5 min at 72 °C, followed by a final extension time of 7 min at 72 °C. The results of DNA amplification were examined using electrophoresis on 2% agarose gel run in TBE. The gels were stained with ethidium bromide (5 µg ml−1). DNA Ladder RTU (100 bp), (Gene Direx®) was used as a standard. The Bio-Rad Gel Doc 2000 was used to photograph DNA under UV light for visualization.
| Primer name | Primer sequence (5′ → 3′) | Product size (bp) |
|---|---|---|
| ITS-1 | TCC GTA GGT GAA CCT GCG G | 600 |
| ITS-4 | TCC TCC GCT TAT TGA TAT GC | |
| Tef-F | CAT CGA GAA GTT CGA GAA GG | 700 |
| Tef-R | AAC TTG CAG GCA ATG TGG | |
| Omt-AF | GACCAATACGCCCACACAG | 300 |
| Omt-AR | CTTTGGTAGCTGTTTCTCGC | |
| Aopks-F | CAGACCATCGACACTGCATGC | 549 |
| Aopks-R | CTGGCGTTCCAGTACCATGAG | |
| AflA-F | GTAGGGTTCCTAGCGAGCC | 497 |
| AflA-R | GGAAAAAGATTGATTTGCGTTC | |
| Gdh-F | TTAACCGCGGCCTGCCTGG | 900 |
| Gdh-R | GGCTGCTGGCCGAACTGACTT |
Sequencing analysis of ITS region and TEF gene
To get the sequences, we used polymerase chain reaction (PCR) and sequenced the resulting amplicons directly according to Hassan et al. (2022) using a 3130 Genetic Analyzer (Applied Biosystems, Waltham, MA, USA) at Macrogen Co., Seoul, South Korea. Data from the sequencing experiment was compared to those in the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST/), using the nucleotide BLAST program to identify homology between the PCR fragments and sequences in the GenBank database. Using their assigned accession numbers, the sequences were submitted to GenBank at the NBCI (National Center for Biotechnology Information).
Molecular detection of aflatoxin and ochratoxin-producing genes
To detect the genes responsible for producing aflatoxin and ochratoxin, two published primers were used. Primers’ sequences were tabulated in Table 1. A 25 µl reaction volume was used for the PCR according to Hussein, El-Said & Yassein (2020). The C1000 was used to conduct the reactions. Denaturation at 94 °C for 5 min using a Thermo Cycler (BioRad, Hercules, CA, USA); 30 cycles of 1 min at 94 °C, 1 min at 58 °C, and 1 min at 72 °C; last step is extension at 72 °C for 10 min. PCR products were examined on an ethidium bromide-stained 1.3% agarose gel.
Inter simple sequence repeats (ISSR)-PCR analysis
For inter simple sequence repeats analysis, PCR of Aspergillus isolates was performed according to Mazrou et al. (2020). Reactions of PCR-ISSR were carried out in 25 µl volume and five ISSR primers (ISSR-4, ISSR-5, ISSR-8, ISSR-18, and ISSR-28) were used to amplify the genomic DNA. Primers (Macrogen Inc., Seoul, Korea) used in this analysis are listed in Table 1. An easy matching coefficient determined using Jaccard’s coefficient was used to provide an estimate for the similarity matrix (Rohlf, 2000).
Data analysis
Version 2 of ClustalW was used for the sequence alignment (Larkin et al., 2007). The haplotype diversity was examined using the DnaSP program (Librado & Rozas, 2009). Phylogenetic analysis and exploratory data were performed using the R Project for Statistical Computing (R Core Team, 2022). Using our own code, the haplotype sequence matrix was used to obtain the haplotype distance matrix (20 × 41). To elucidate the genetic relatedness between the Aspergillus isolates and molecular identification, a phylogenetic tree was constructed based on the sequence analysis of the ITS and TEF-1 α regions, using the neighbor-joining method in the MEGA 7.1 software.
Results
Isolation of Aspergillus species
In total, 114 fungal species belonging to 10 genera were isolated from some soil invertebrates (millipedes, Armadillidium vulgare and Porcellio laevis) in Taif Governorate, Saudi Arabia (Table 2). Twenty-two of them were identified as Aspergillus sp. Aspergillus was isolated from all soil invertebrate samples. The isolates swiftly expanded their colonies once grown on PDA, and although their mycelium used to start with white and floccose, it eventually became a dark color with black spores. The twenty-two Aspergillus isolates locations and the animal’s name is presented (Table 3). Nine Aspergillus isolates were isolated from millipedes, six of them (TU-1, TU-2, TU-3, TU-19, TU-20 and TU-23) were isolated from Shafa, Taif, and three of them (TU-14, TU-15 and TU-16) were isolated from Wady Ghazal, Taif, Saudi Arabia. On the other hand, eight Aspergillus isolates were isolated from A. vulgare. Five isolates (TU-4. TU-5, TU-6, TU-8 and TU-9) were isolated from Hawia, Taif. While, three Aspergillus isolates (TU-33, TU-35 and TU-36) were isolated from Shafa, Taif. Finally, five Aspergillus isolates (TU-13, TU-24, TU-25, TU-32 and TU-33) were isolated from Porcellio laevis in Hawia, Taif, Saudi Arabia.
| Fungi | Source of collection | No. isolates | ||
|---|---|---|---|---|
| Millipedes | A. vulgare | Porcellio laevis | ||
| Penicillium sp. | 7 | 2 | 6 | 15 |
| Alternaria sp. | 3 | 2 | 5 | 10 |
| Aspergillus sp. | 10 | 7 | 5 | 22 |
| Basidioascus sp. | 5 | 0 | 0 | 5 |
| Candida sp. | 4 | 8 | 3 | 15 |
| Curvularia sp. | 2 | 0 | 1 | 3 |
| Fusarium sp. | 9 | 7 | 5 | 21 |
| Trichoderma sp. | 4 | 6 | 2 | 12 |
| Geotrichum sp. | 5 | 3 | 2 | 10 |
| Talaromyces sp. | 1 | 0 | 0 | 1 |
| Total | 52 | 37 | 31 | 114 |
| Isolates | Species | Source | Locations |
|---|---|---|---|
| TU-1 | Aspergillus calidoustus | millipedes | Shafa, Taif |
| TU-2 | Aspergillus ochraceus | millipedes | Shafa, Taif |
| TU-3 | Aspergillus neoflavipes | millipedes | Shafa, Taif |
| TU-4 | Aspergillusvenezuelensis | A. vulgare | Hawia, Taif |
| TU-5 | Aspergillus caespitosus | A. vulgare | Hawia, Taif |
| TU-6 | Aspergillus caespitosus | A. vulgare | Hawia, Taif |
| TU-8 | Aspergillus venezuelensis | A. vulgare | Hawia, Taif |
| TU-9 | Aspergillus flavipes | A. vulgare | Hawia, Taif |
| TU-13 | Aspergillus caespitosus | Porcellio laevis | Hawia, Taif |
| TU-14 | Aspergillus caespitosus | millipedes | Wady Ghazal, Taif |
| TU-15 | Aspergillus flavipes | millipedes | Wady Ghazal, Taif |
| TU-16 | Aspergillus europaeus | millipedes | Wady Ghazal, Taif |
| TU-19 | Aspergillus ustus | millipedes | Shafa, Taif |
| TU-20 | Aspergillus aculeatus | millipedes | Shafa, Taif |
| TU-23 | Aspergillus oryzae | millipedes | Shafa, Taif |
| TU-24 | Aspergillus flavus | Porcellio laevis | Hawia, Taif |
| TU-25 | Aspergillus neoflavipes | Porcellio laevis | Hawia, Taif |
| TU-31 | Aspergillus arcoverdensis | Porcellio laevis | Hawia, Taif |
Morphological identification
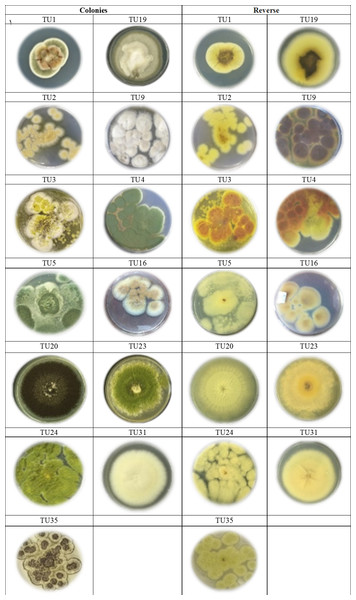
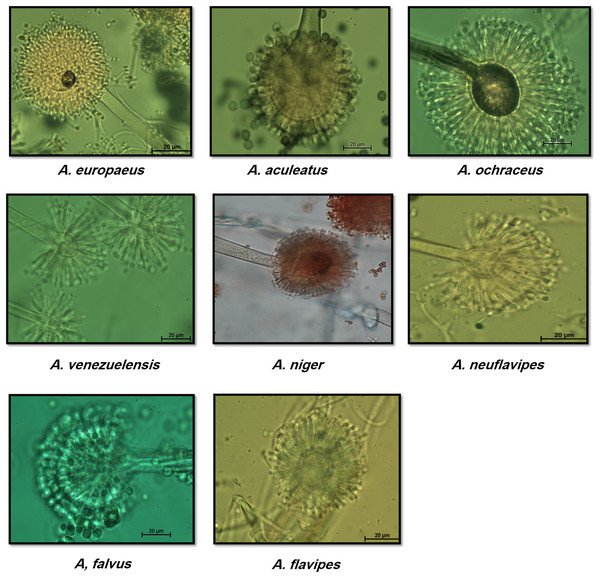
Two predominated Aspergillus sections were isolated, Flavipedes and Nidulantes (Table 3). The morphological characteristics of Aspergillus species are shown in Figs. 1 and 2. The colony of A. ochraceus TU2 belonging to Aspergillus section Circumdati was yellow-gold, granular and had a reddish-brown color on the reverse of the plate. Conidiophore was yellowish pale brown and coarsely rough. Vesicle was biseriate, globose and entirely Metula. While, the isolates A. europaeus TU16, 32 and 36 belonging to Aspergillus section Cremei were yellowish grey, velutinous to floccose, colorless, and light yellow on the reverse of the plate. Conidiophore was colorless and delicately roughened. Vesicle was biseriate, pyriform or globose and Metula covering 3/4 ∼the entire vesicle, globose, subglobose, elliptical conidia and coarsely roughened (Fig. 2). Sclerotia and Hülle cells were absent. A. flavus TU24 and A. oryzae TU24 were categorized under Aspergillus section Flavi. Their colonies were granular, floccose, yellowish green to olive green, and colorless to yellow, pale yellow color on the reverse of the plate, respectively. Conidiophore was pale brown, colorless and rough. Vesicle was biseriate and biseriate, rarely uniseriate, globose, subglobose and Cleistothecia present or not ascomata, respectively. Two isolates (A. flavipes TU9&15 and A. neoflavipes TU3&25) belong to Aspergillus section Flavipedes were floccose to cottony, velutinous, white to yellowish white, brightly to moderate yellow and pale yellowish orange and strong yellow to medium olive brown on the reverse of the plate, respectively. Conidiophore was hyaline to light brown, very pale brown and smooth. Vesicle was biseriate, rarely uniseriate and biseriate, spathulate, sometimes pyriform and subglobose, pyriform or spathulate and covering one-half to two-thirds of the vesicle. On the other hand, the colonies of Aspergillus arcoverdensis TU31&33, belonging to Aspergillus section Fumigati, were white to orange, white, floccose, and yellowish white to pale orange color on the reverse of the plate. Conidiophore was hyaline to pale yellowish brown and smooth. Vesicle was uniseriate, subglobose to hemispherical, and covering the upper half of the vesicle Metula. Aspergillus section Nidulantes was represented by A. caespitosus TU5,6,13&14 and A. venezuelensis TU4&8. Colony was velvety, graysih green, and floccose at center, velvety at edges or light yellow at center, white at edge and vinaceous buff to grey olivaceou, light yellow fading into cream white color on the reverse of the plate. Conidiophore was pale brown or brown to yellow and smooth. Vesicle was biseriate, hemisphere to subclavate and subglobose, pyriform, covering the upper half or two-thirds of the vesicle Metula. Conidia were globose or globose to subglobose and echinulate, spinulose. Ascomata, ascospores, globose and Hülle cells were present. On the other side, Aspergillus aculeatus TU20 and A. niger TU35, belonging to Aspergillus section Nigri, were dark brown/gray tones or dark brown to black, velvety, granular, and pale to yellow, colorless to light yellow color on the reverse of the plate. Conidiophore was slightly brown and smooth. Vesicle was uniseriate and biseriate, spherical, globose, and entirely Metula. Conidia were ellipsoid, spiny and globose or ellipsoid and very rough irregular. Fruiting bodies were absent. While, Aspergillus section Usti was represented by A. calidoustus TU1 and A. ustus TU19. Colony was floccose, blond/greyish yellow and yellow with beige or olive-brown center or yellow-olive edge with olive brown center color on the reverse of the plate. Conidiophore was brown and smooth. Vesicle was biseriate, pyriform to broadly spathulate and hemispherical to subglobose, covering the upper half to entire surface of the vesicle Metula. Conidia were globose, and very rough. Hülle cells were sparsely produced.
Figure 1: Cultures of Aspergillus. species isolated from soil invertebrates in Taif, Saudi Arabia on Czapek Agar (CZA) medium after 6 days at 25 ± 2 °C.
Figure 2: Morphological characteristics of of Aspergillus species isolated from soil invertebrates in Taif, Saudi Arabia on Czapek Agar (CZA) medium after 6 days at 25 ± 2 °C.
Molecular identification of Aspergillus species using 5.8S-ITS region sequence
To perform nucleotide sequencing, genomic DNA was successfully isolated from all Aspergillus isolates. All of the Aspergillus isolates utilized in this analysis had their rDNA internal transcribed spacer (ITS) regions amplified using the universal primers ITS 1 and ITS 4. The acquired sequences were submitted to NCBI for BLAST analysis in order to determine the identification of the isolates (accession numbers ON160831 –ON160852). The length of the ITS sequences varied greatly among Aspergillus isolates, from 550 to 650 bp. There was almost perfect nucleotide sequence homology between Aspergillus isolates (Table 4).
| Isolates | Species | Query coverage % |
E value | Identity% | Accession number |
|---|---|---|---|---|---|
| TU-1 | Aspergillus calidoustus | 98.00 | 0.00 | 99.00 | ON160831 |
| TU-2 | Aspergillus ochraceus | 100.00 | 0.00 | 100.00 | ON160832 |
| TU-3 | Aspergillusneoflavipes | 100.00 | 0.00 | 100.00 | ON160833 |
| TU-4 | Aspergillus venezuelensis | 100.00 | 0.00 | 100.00 | ON160834 |
| TU-5 | Aspergillus caespitosus | 99.00 | 0.00 | 99.00 | ON160835 |
| TU-6 | Aspergillus caespitosus | 100.00 | 0.00 | 100.00 | ON160836 |
| TU-8 | Aspergillus venezuelensis | 99.00 | 0.00 | 100.00 | ON160837 |
| TU-9 | Aspergillus flavipes | 100.00 | 0.00 | 100.00 | ON160838 |
| TU-13 | Aspergillus caespitosus | 100.00 | 0.00 | 99.00 | ON160839 |
| TU-14 | Aspergillus caespitosus | 100.00 | 0.00 | 100.00 | ON160840 |
| TU-15 | Aspergillus flavipes | 100.00 | 0.00 | 99.00 | ON160841 |
| TU-16 | Aspergillus europaeus | 100.00 | 0.00 | 99.00 | ON160842 |
| TU-19 | Aspergillus ustus | 100.00 | 0.00 | 99.00 | ON160843 |
| TU-20 | Aspergillus aculeatus | 99.00 | 0.00 | 100.00 | ON160844 |
| TU-23 | Aspergillus oryzae | 100.00 | 0.00 | 100.00 | ON160845 |
| TU-24 | Aspergillus flavus | 100.00 | 0.00 | 100.00 | ON160846 |
| TU-25 | Aspergillusneoflavipes | 99.00 | 0.00 | 100.00 | ON160847 |
| TU-31 | Aspergillus arcoverdensis | 100.00 | 0.00 | 100.00 | ON160848 |
| TU-32 | Aspergillus europaeus | 100.00 | 0.00 | 100.00 | ON160849 |
| TU-33 | Aspergillus arcoverdensis | 100.00 | 0.00 | 100.00 | ON160850 |
| TU-35 | Aspergillus niger | 100.00 | 0.00 | 100.00 | ON160851 |
| TU-36 | Aspergillus europaeus | 100.00 | 0.00 | 100.00 | ON160852 |
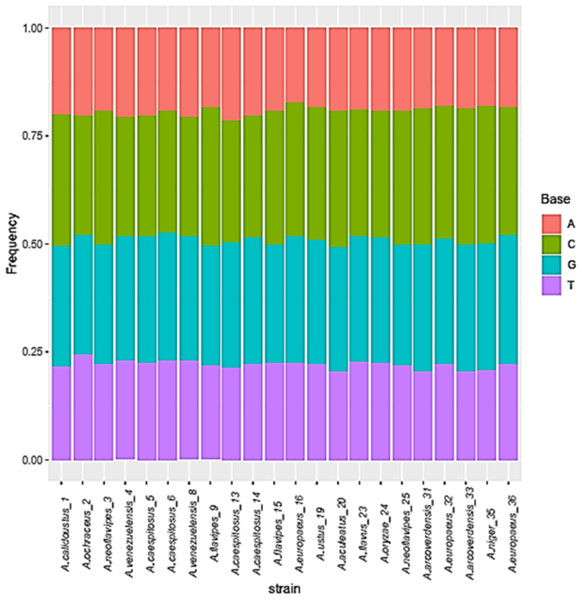
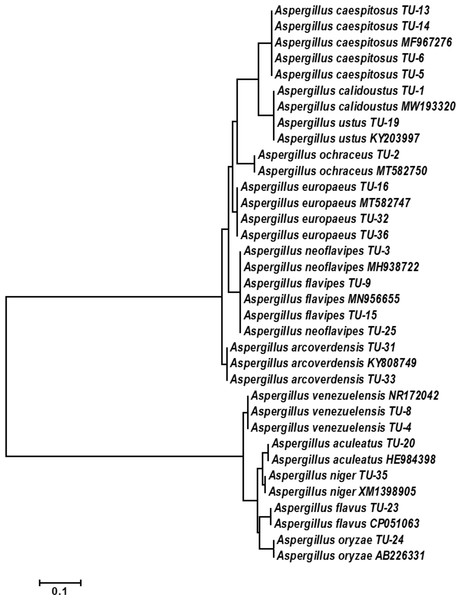
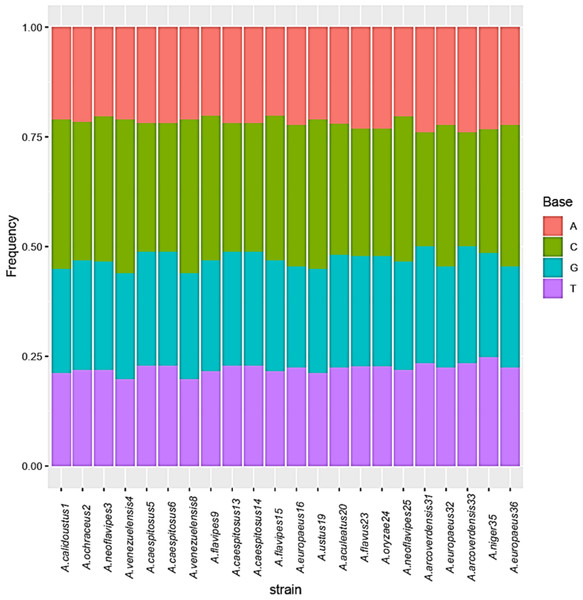
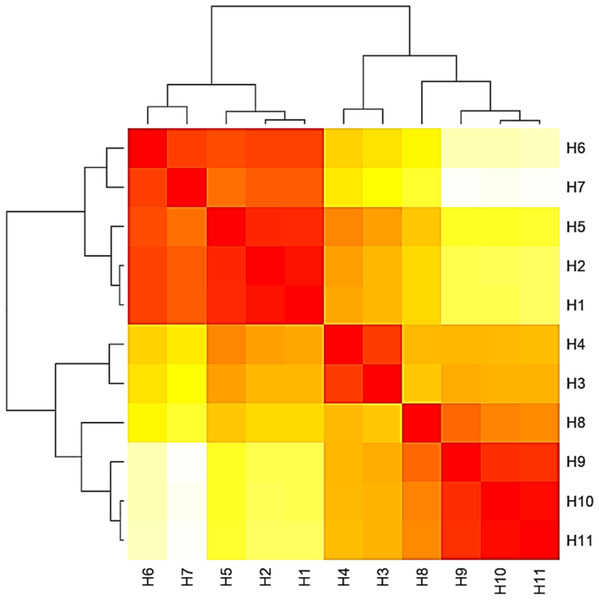
The base frequencies of all ITS sequenced isolates were shown in Fig. 3. A heat map using phylogenetic trees from the haplotype sequences matrix was shown in Fig. 4. The phylogenetic tree based on the 5.8S-ITS region sequence with 1,000 bootstrap repeats showed that the phylogeny tree contained two main clusters (Fig. 5 and Fig. S1). The first main cluster contained five sub-cluster with a bootstrap value of 99%. The first one contained Aspergillus europaeus TU-16, 32 and 36 with 99–100% similarity to A. europaeus MT582747 from NCBI GenBank. The second sub-cluster contained A. flavus TU-23 with 100% similarity to A. flavus MT635198, it contained also A. oryzae TU-24 with high similarity to A. oryzae MW331693. Moreover, the third sub-cluster contained A. arcoverdensis TU-31 and TU-33 which were homologous with A. arcoverdensis KY808749. The fourth sub-cluster contained A. aculeatus, A. niger, A. aculeatus TU-20 which were homologous with A. aculeatus MN795742 and A. niger TU-35 which was homologous with A. niger MT541880. The final sub-cluster contained A. neoflavipes and A. flavipes. A. flavipes TU-9 and TU-15 were homologous with A. flavipes MN956655, while A. neoflavipes TU-3 and TU-25 were homologous with A. neoflavipes MH938722. On the other hand, the second main cluster contained two subclusters. The first one contained A. ochraceus TU-2 with 100% similarity to A. ochraceus MT582750. The second sub-cluster contained three groups, the first one contained A. calidoustus TU-1 which was homologous with A. calidoustus MW193320 and A. ustus TU-19 which were homologous with KY203997. The second one contained A. venezuelensis TU-4 and TU-8 which were homologous with A. venezuelensis NR172042. The last group contained A. caespitosus TU-5, TU-6, TU-13 and TU-14 which were homologous with A. caespitosus MF967276 (Fig. 3).
Figure 3: Base frequencies of 22 Aspergillus ITS isolates.
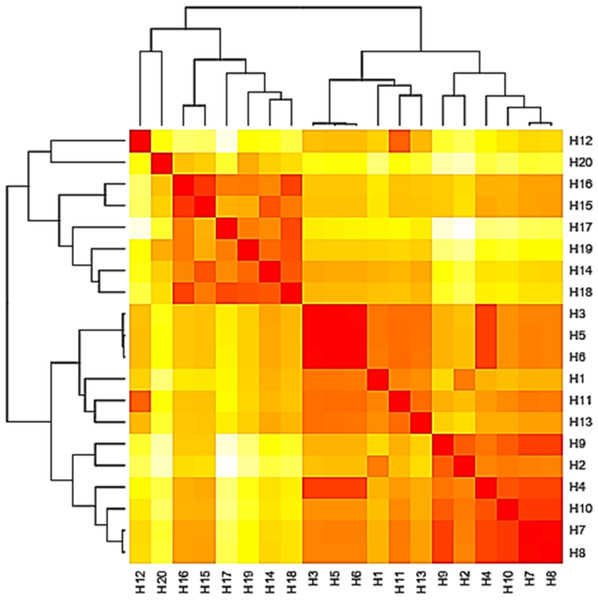
Figure 4: Heat map based on the number of nucleotide differences between the haplotypes.
Each branch of the phylogenetic tree represents the corresponding haplotype in the matrix. The close relationships were defined by a darker color and distant relationships by a lighter color.Figure 5: Phylogenetic tree and diversity of the 5.8S-ITS region with different Aspergillus species compared with reference Aspergillus strains.
Molecular identification of Aspergillus species based on TEF-1 α DNA gene
To evaluate the applicability of TEF-1 α DNA sequences for the differentiation and phylogenetic study of Aspergillus species, partial TEF-1 α genes were examined for 22 strains of Aspergillus (Table 5). Using the primer pair TEF-1 α F and TEF-1 α R, we were able to successfully amplify a single PCR fragment for all of the Aspergillus strains included in the study, and this was followed by analysis of the DNA sequences and a nucleotide-based phylogenetic analysis. Multiple alignment of sequences pertaining to TEF-1 α indicated a mean similarity of 92.6% between the species. A BLAST search was run on the acquired sequences to determine the identities of the isolates, and the results were deposited in the GenBank database (accession numbers ON456514 –ON456535) (Table 5).
| Isolates | Species | Query coverage % |
E value |
Ident % | Accession number |
|---|---|---|---|---|---|
| TU-1 | Aspergillus calidoustus | 100 | 0.00 | 100 | ON456514 |
| TU-2 | Aspergillus ochraceus | 100 | 0.00 | 99 | ON456515 |
| TU-3 | Aspergillus neoflavipes | 99 | 0.00 | 98 | ON456516 |
| TU-4 | Aspergillus venezuelensis | 100 | 0.00 | 99 | ON456517 |
| TU-5 | Aspergillus caespitosus | 100 | 0.00 | 99 | ON456518 |
| TU-6 | Aspergillus caespitosus | 99 | 0.00 | 99 | ON456519 |
| TU-8 | Aspergillus venezuelensis | 99 | 0.00 | 98 | ON456520 |
| TU-9 | Aspergillus flavipes | 99 | 0.00 | 98 | ON456521 |
| TU-13 | Aspergillus caespitosus | 99 | 0.00 | 98 | ON456522 |
| TU-14 | Aspergillus caespitosus | 99 | 0.00 | 99 | ON456523 |
| TU-15 | Aspergillus flavipes | 99 | 0.00 | 99 | ON456524 |
| TU-16 | Aspergillus europaeus | 99 | 0.00 | 99 | ON456525 |
| TU-19 | Aspergillus ustus | 100 | 0.00 | 100 | ON456526 |
| TU-20 | Aspergillus aculeatus | 100 | 0.00 | 100 | ON456527 |
| TU-23 | Aspergillus oryzae | 99 | 0.00 | 98 | ON456528 |
| TU-24 | Aspergillus flavus | 100 | 0.00 | 99 | ON456529 |
| TU-25 | Aspergillus neoflavipes | 100 | 0.00 | 99 | ON456530 |
| TU-31 | Aspergillus arcoverdensis | 100 | 0.00 | 100 | ON456531 |
| TU-32 | Aspergillus europaeus | 100 | 0.00 | 100 | ON456532 |
| TU-33 | Aspergillus arcoverdensis | 99 | 0.00 | 100 | ON456533 |
| TU-35 | Aspergillus niger | 99 | 0.00 | 100 | ON456534 |
| TU-36 | Aspergillus europaeus | 99 | 0.00 | 100 | ON456535 |
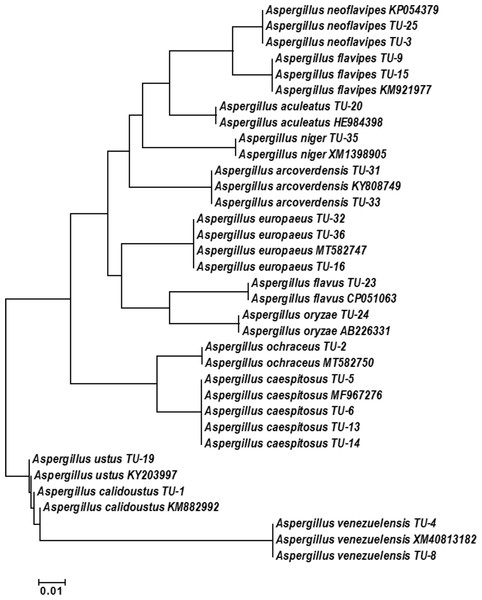
The base frequencies of all TEF-1 α sequenced isolates were explained (Fig. 6). From the matrix of haplotype sequences, phylogenetic trees were generated and used to create a heat map (Fig. 7). The phylogenetic tree based on the TEF-1 α DNA sequence with 1,000 bootstrap repeats showed that the phylogeny tree contained two main clusters (Fig. 8 and Fig. S2). The first main cluster contained eight sub-cluster with a bootstrap value of 99–100%. The first sub-cluster contained A. neoflavipes and A. flavipes. A. flavipes TU-9 and TU-15 were found to be homologs with A. flavipes MN956655, while A. neoflavipes TU-3 and TU-25 were found to be homologs with A. neoflavipes KM921977. The second sub-cluster contained A. aculeatus TU-20 which was homologs with A. aculeatus HE984398. The third one contained A. ochraceus TU-2 with 100% similarity to A. ochraceus MT582750. The fourth sub-cluster contained A. arcoverdensis TU-31 and TU-33 with 100% similarity to A. arcoverdensis KY808749. The fifth sub-cluster contained A. niger TU-35 which was found to be homologs with A. niger XM1398905. The sixth sub-cluster contained A. europaeus TU-16, 32 and 36 with 100% similarity to A. europaeus MT582747. The seventh subcluster contained A. flavus TU-23 with 100% similarity to A. flavus CP051063, it contained also A. oryzae TU-24 with high similarity to A. oryzae AB226331. The final sub-cluster contained A. caespitosus TU-5, TU-6, TU-13 and TU-14 which was found to be homologs with A. caespitosus MF967276. On the other hand, the second main cluster contained two sub-clusters. The first one contained A. calidoustus TU-1 which was found to be homologs with A. calidoustus KM882992 and A. ustus TU-19 which was found to be homologs with KY203997. Finally, the second sub-cluster contained A. venezuelensis TU-4 and TU-8 which were homologs with A. venezuelensis XM813182.
Figure 6: Base frequencies of 22 TEF Aspergillus isolates.
Figure 7: Heat map based on the number of nucleotide differences between the haplotypes.
Each branch of the phylogenetic tree represents the corresponding haplotype in the matrix. The close relationships were defined by the darker color and distant relationship by the lighter color.Figure 8: Phylogenetic tree and diversity of the TEF-1 α gene with different Aspergillus species compared with reference Aspergillus strains.
The phylogenetic tree was generated using parsimony neighbor-joining and maximum likelihood analysis.ISSR-PCR analysis
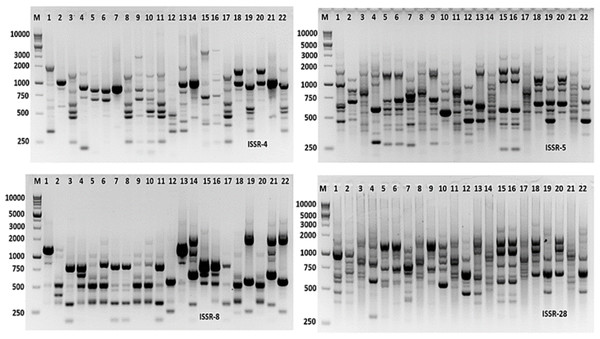
Molecular markers are effective tools for molecular characterization and correlation estimation through DNA fingerprinting. Aspergillus species were molecular characterized using ISSR-PCR markers. The ISSR-PCR results were summarized in Table 6 and shown in Fig. 9. The polymorphic and monomorphic bands were produced from the PCR amplification. About 92 bands resulted from the five ISSR-PCR primers. Out of them, 32 bands were monomorphic with a monomorphism average of 34.8%, while 60 bands were polymorphic bands with a polymorphism average of 65.2%. The number of total bands varied from 14 to 22 bands, with primers ISSR-8 and ISSR-5, respectively. The band’s size ranging from 220 to 2,800 bp with primer ISSR-5. The highest polymorphism among Aspergillus species was revealed by ISSR-4 primer (76.2%), followed by ISSR-5 primer (68.2%). However, the lowest polymorphism was 57.82% resulting from the application of ISSR-28 primer.
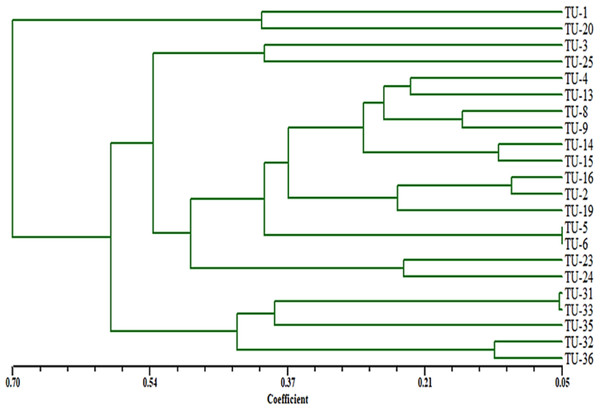
Genetic distances and the cluster dendrogram
A total of 92 fragments from all ISSR-PCR analyses were enough to determine genetic similarities and design the phylogenetic tree for these Aspergillus isolates. According to a dendrogram constructed using UPGMA based on Jaccard’s similarity coefficients dependent on genetic similarity, inter and intra-species diversity ranged from 0.05 to 0.70 (Fig. 10), the Aspergillus isolates were grouped into two different clusters with about 65% genetic similarity. The first cluster contained only Aspergillus TU-1 and TU-20. While, the second cluster contained most Aspergillus isolates. The second cluster contained two sub-cluster, the first one contained Aspergillus TU-3 and TU-25 in the same group, Aspergillus TU-4, TU-13, TU-8, TU-9, TU-14, TU-15, TU-16, TU-2, TU-19, TU-5, TU-6, TU-23 and TU-24 were found in the second group. Moreover, the second sub-cluster contained Aspergillus TU-31, TU-32, TU-33, and TU-35 and TU-36. The dendrogram showed the highest genetic similarity between Aspergillus TU-5 and TU-6, while the lowest relationship was between Aspergillus TU-1 and TU-36. The dendrogram constructed using UPGMA based on Jaccard’s similarity coefficients (Fig. 6) indicated that Aspergillus TU-2 and TU-16 were in the same sub-cluster and appeared more similar to each other than Aspergillus TU-19.
Detection of aflatoxin and ochratoxin-producing genes
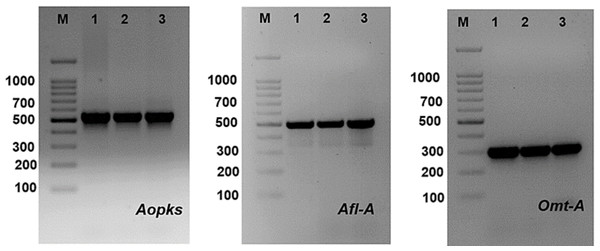
PCR technique using four sets of primers was used to discover different genes involved in the biosynthetic pathways of mycotoxin (aflatoxin and ochratoxin). Bands of the fragments of afl-A, omt-A and Aopks genes can be visualized at 497, 300 and 549 bp, respectively (Table 7, Fig. 11 and Fig. S3). Aflatoxigenic strains (Aspergillus ochraceus TU-2, Aspergillus neoflavipes TU-3, Aspergillus flavipes TU-9, Aspergillus caespitosus TU-13, Aspergillus caespitosus TU-14, Aspergillus flavipes TU-15, Aspergillus ustus TU-19, Aspergillus aculeatus TU-20, Aspergillus oryzae TU-23, Aspergillus flavus TU-24, Aspergillus neoflavipes TU-25, Aspergillus arcoverdensis TU-31, Aspergillus europaeus TU-32, Aspergillus arcoverdensis TU-33, and Aspergillus europaeus TU-36) showed 497 bp DNA fragments with afl-A primer. Moreover, Aflatoxigenic strains (Aspergillus oryzae TU-23, Aspergillus flavus TU-24, Aspergillus europaeus TU-32 and Aspergillus europaeus TU-36) showed 300 bp DNA fragments that corresponded to the complete aflatoxin B1 producing gene. In addition, Aspergillus arcoverdensis TU-31, Aspergillus arcoverdensis TU-33 and Aspergillus niger TU-35 showed 549 bp DNA fragments with Aopks primer. On the other hand, some strains have the phosphate solubilization gene, glucose dehydrogenase (Gdh), such as Aspergillus flavipes TU-15, Aspergillus oryzae TU-23, Aspergillus flavus TU-24, Aspergillus neoflavipes TU-25 and Aspergillus europaeus TU-32.
| Primers | Total bands | Monomorphic bands |
Polymorphic bands |
Monomorphism (%) | Polymorphism (%) |
|---|---|---|---|---|---|
| ISSR-4 | 21 | 5 | 16 | 23.80 | 76.20 |
| ISSR-5 | 22 | 7 | 15 | 31.80 | 68.20 |
| ISSR-8 | 14 | 6 | 8 | 42.85 | 58.15 |
| ISSR-18 | 18 | 7 | 11 | 38.89 | 61.11 |
| ISSR-28 | 17 | 7 | 10 | 41.18 | 57.82 |
| Total | 92 | 32 | 60 |
Figure 9: ISSR-PCR profile of the 22 Aspergillus isolates generated with ISSR primers.
M is 100 bp DNA ladder.Figure 10: Dendrogram of genetic similarity and inter and intra-species diversity of the Aspergillus isolates.
| Isolates | Species | Gdh gene |
AflA gene |
OmtA gene |
Aopks gene |
|---|---|---|---|---|---|
| TU-1 | Aspergillus calidoustus | – | – | – | – |
| TU-2 | Aspergillus ochraceus | – | + | – | – |
| TU-3 | Aspergillus neoflavipes | – | + | – | – |
| TU-4 | Aspergillus venezuelensis | – | – | – | – |
| TU-5 | Aspergillus caespitosus | – | – | – | – |
| TU-6 | Aspergillus caespitosus | – | – | – | – |
| TU-8 | Aspergillus venezuelensis | – | – | – | – |
| TU-9 | Aspergillus flavipes | – | + | – | – |
| TU-13 | Aspergillus caespitosus | – | + | – | – |
| TU-14 | Aspergillus caespitosus | – | + | – | – |
| TU-15 | Aspergillus flavipes | + | + | – | – |
| TU-16 | Aspergillus europaeus | – | – | – | – |
| TU-19 | Aspergillus ustus | – | + | – | – |
| TU-20 | Aspergillus aculeatus | – | + | – | – |
| TU-23 | Aspergillus oryzae | + | + | + | – |
| TU-24 | Aspergillus flavus | + | + | + | – |
| TU-25 | Aspergillus neoflavipes | + | + | – | – |
| TU-31 | Aspergillus arcoverdensis | – | + | – | + |
| TU-32 | Aspergillus europaeus | + | + | + | – |
| TU-33 | Aspergillus arcoverdensis | – | + | – | + |
| TU-35 | Aspergillus niger | – | – | – | + |
| TU-36 | Aspergillus europaeus | – | + | + | – |
Figure 11: PCR amplification of some mycotoxin genes in studied Aspergillus species, where Aopks, Afl-A and Omt-A genes molecular weight are (549, 497 and 300 bp), respectively.
Discussion
The long-term existence of species depends on genetic diversity, which is also crucial to their protection (Frankham, 2012). In addition, genetic diversity is crucial for how populations react to environmental variables (Hoelzel, Bruford & Fleischer, 2019). Consequently, understanding the genetic diversity of populations is crucial for the preservation and appropriate use of genetic resources, as well as their underlying individual and subpopulation components (Pazouki et al., 2015). Species of the genus Aspergillus are widely dispersed everywhere in the environment (Krishnan, Manavathu & Chandrasekar, 2009; Mazrou et al., 2020). The genus is divided into seven subgenera, subdivided into a number of sections, each containing a few to several closely related species (Nouripour-Sisakht et al., 2017). The morphological characteristics of the isolated Aspergillus species were investigated. The colony of A. ochraceus TU2 was yellow-gold on Czapek Agar (CZA) medium. Spherical small conidia and smooth finely roughened (Raper & Fennell, 1965; Nyongesa, Okoth & Ayugi, 2015). While, A. europaeus was yellowish grey, velutinous to floccose and Sclerotia and Hülle cells were absent (Nguyen et al., 2020). The colony of A. flavus was granular, floccose, yellowish green and cleistothecia was present (Raper & Fennell, 1965; Jernejc & Cimerman, 2001; Gautam & Bhadauria, 2012; Zulkifli & Zakaria, 2017; Dewi, 2018). Colonies of A. flavipes and A. neoflavipes were floccose to cottony. Hülle cells were absent and, ascomata (Hubka et al., 2015). On the other hand, the colony of Aspergillus arcoverdensis was white to orange, white, floccose, and cleistothecia was not present (Samson et al., 2014; Matsuzawa et al., 2015). Conidia of A. caespitosus and A. venezuelensis were globose, globose to subglobose. Ascomata, ascospores, globose and Hülle cells were present (Samson et al., 2014; Chen et al., 2016). On the other side, colonies of Aspergillus aculeatus and A. niger were dark brown/gray tones, dark brown to black, velvety, granular, and pale to yellow. Fruiting bodies were absent (Raper & Fennell, 1965; Jernejc & Cimerman, 2001; Silva et al., 2011; Gautam & Bhadauria, 2012; Samson et al., 2014; Nyongesa, Okoth & Ayugi, 2015; Dewi, 2018). While, colonies of A. calidoustus and A. ustus were floccose, blond/greyish yellow, and greyish brown to dark brown. Conidiophore was brown and smooth (Raper & Fennell, 1965; Houbraken et al., 2007).
Aspergillus species’ taxonomy and evolutionary relationships are based on the morphological characteristics, extrapolated data and partial DNA sequences of different genetic targets (Varga, Frisvad & Samson, 2011). However, lack of pigment or poor sporulation, inter-specific similarities and intra-specific variability, and variation in growth requirements for some isolates may influence the outcome of morphological identification (Rodrigues et al., 2009). Therefore, molecular methods are necessary for distinguishing and/or (re-) classifying similar and complex Aspergillus taxa, as well as for the discovery of new species. An international Aspergillus working group has recommended the use of molecular identification based on the ITS region for subgenus/section-level identification (Hu et al., 2021). However, comparative sequence-based identification using the ITS1 region does not always enable discrimination between closely related species (Samson et al., 2014), which might be due to inadequate sequence variability or issues with the reliability of the ITS sequences deposited in reference databases (Nilsson et al., 2006). Some housekeeping genes, such as BT2, calmodulin and rodlet A, have been confirmed as good genetic indicators for identifying species within different sections, such as Fumigati, Usti, Nigri and Terrei (Houbraken et al., 2020), but they are not able to differentiate, for example, species that are phylogenetically close to A. parasiticus (Vesth et al., 2018), and therefore sequence analyses of other loci are required for accurate identification.
Owing to polymorphism and DNA sequence length differences in introns, intron-rich protein-coding genes such as β-tubulin, calmodulin, actin and TEF-1 α are recommended for the discrimination of fungal species (Sklenář et al., 2020). Hence, the identical size (700 nt) and high homology (92.6%) observed among all the strains reflected the lack of introns in the TEF-1a gene region studied here. Phylogenetic analysis of the TEF-1 α genes showed a clade consisting of A. flavus, A. oryzae, A. ochraceus and A. tamari (Flavi section), supported by a bootstrap value of 97%, next to the members of A. avenaceus and A. alliaceus, as a separate group with a bootstrap value of 100% REFF. Pairwise comparison of the DNA sequences of both the ITS and TEF-1 α genes in this study showed a single SNP difference between A. flavus and A. oryzae. Regarding the evolutionary origins of A. oryzae and A. flavus, based on the region neighboring the cyclopiazonic acid biosynthesis gene cluster, Chen et al. (2016) suggested that A. oryzae most likely descended from an ancestor that was the ancestor of A. minisclerotigenes or A. parvisclerotigenus, producing both B- and G-type aflatoxins, while A. flavus descended from an ancestor of A. parasiticus. Although several lines of evidence show that A. oryzae is a morphological variant of A. flavus, it was suggested that these taxa should be retained as separate species because of the regular confusion that conspecificity might generate in the food industry (Varga, Frisvad & Samson, 2011; Rodrigues et al., 2009).
Differentiation of some species of black aspergilli, such as A. niger and A. tubingensis, which are common in both clinical and environmental settings, remains difficult (Criseo, Bagnara & Bisignano, 2001). The taxa can be differentiated by DNA sequences reflecting the cytochrome b (Schmidt-Heydt et al., 2009), ITS (Abdel-Hadi, Carter & Magan, 2011) and β-tubulin (Mutegi et al., 2012) genes. In this study, a sequence difference count matrix based on nt pairwise comparison of the TEF-1 α gene with a similarity of 81.8% provided evidence that this locus is more valuable than ITS (97.9% similarity) for species discrimination of these two closely related species.
The phylogenetic tree of the TEF-1 α genes revealed a cluster consisting of Fumigati and Clavati sections. Closely related species, A. fumigatus, A. fischeri and A. quadricinecta (section Fumigati), formed well-supported clades in the TEF-1 α and BenA gene analyses, with bootstrap values of 98 and 100%, respectively. The phylogenetic tree derived from TEF-1a indicated that A. clavatus, A. clavatonanicus and A. nutans (section Clavati) form a sister group with section Fumigati, with a bootstrap value of 76%, and the phylogenetic analysis of partial DNA sequences of BT2 genes provided a bootstrap value of 100%.
Many researchers have investigated the function of fungi in the contamination and generation of aflatoxin in different crops (El-Shanshoury et al., 2014). Studying mycotoxigenic fungus’s molecular genetics and metabolism is an intriguing approach to reducing crop contamination with mycotoxin (El-Kady & Youssef, 1993). The study of the genetic variations between both aflatoxigenic and non-aflatoxigenic strains is of interest. It is estimated that between 300 and 400 different mycotoxins exist, but the ones produced by Aspergillus have received the most attention due to their potential impact on human, animal, and plant health. Aspergillus species are responsible for the production of numerous severe and fatal biotoxins. These include aflatoxins (AFTs), patulin (PAT), ochratoxins (OTA), aflatrem (AT), citrinin (CIT), cyclopiazonic acid (CPA), secalonic acids (SA), sterigmatocystin (ST), terrein (TR), and gliotoxin (GT) (Ráduly et al., 2020). This knowledge has been utilized for controlling the production of aflatoxins. This study utilized traditional PCR techniques to explore the presence of mycotoxin genes in different Aspergillus isolates which should be useful to understand mycotoxins contamination in the soil.
Conclusion
Herein, various genera of fungi were isolated from soil invertebrates (millipedes, Armadillidium vulgare and Porcellio laevis) in Taif Governorate, Saudi Arabia. The isolates were identified based on the morphological characteristics and molecular analysis of ITS and TEF DNA sequences. Aspergillus spp. were found to be the most common in the samples. ISSR-PCR markers were used to study the genetic diversity of Aspergillus isolates. Detection and identification of mycotoxin genes in Aspergillus isolates were revealed.