Years of sand fixation with Caragana korshinskii drive the enrichment of its rhizosphere functional microbes by accumulating soil N
- Published
- Accepted
- Received
- Academic Editor
- Muhammad Zia-Ul-Haq
- Subject Areas
- Agricultural Science, Microbiology, Molecular Biology, Plant Science, Soil Science
- Keywords
- Rhizosphere, Microbial community, C. korshinskii, Nitrogen (N), Function prediction, Years of sand fixation
- Copyright
- © 2022 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Years of sand fixation with Caragana korshinskii drive the enrichment of its rhizosphere functional microbes by accumulating soil N. PeerJ 10:e14271 https://doi.org/10.7717/peerj.14271
Abstract
C. korshinskii is one of the most widely-planted sand-fixing legumes in northwest China and exploring its rhizosphere microbiome is of great ecological importance. However, the effect of long-term sand fixation on the composition, diversity, and underlying functions of microbes in the C. korshinskii rhizosphere in dryland ecosystems remain unclear. Here, we performed high-throughput sequencing using a 16S rRNA (absolute quantification) and bacterial functional annotation of prokaryotic taxa (FAPROTAX) analysis and an ITS (relative quantification) and fungal functional guild (FUNGuild) analysis to investigate the C. korshinskii rhizosphere microbiome and metabolic functional groups at different sand-fixing ages (six years, CK6; twelve years, CK12; and eighteen years, CK18) and determined the physicochemical properties of the rhizosphere soil. Results showed that the key bacterial taxa of the rhizosphere were significantly more abundant in CK18 than in CK12 and CK6 at the phylum-class-genus level, and that fungal Glomeromycota was also significantly more abundant in the CK18 rhizosphere compared to CK12 and CK6. Among these bacterial taxa, the enrichment effect of key, functional, genus-level species of bacteria was the most obvious, including Rhizobium, Ensifer, Neorhizobium, Mesorhizobium, Streptomyces, Sphingomonas, and Flavobacterium, which are N-fixing and/or phosphate-solubilizing groups. The significant improvement seen in the physicochemical properties of the CK18 rhizosphere soil, including the higher total nitrogen (TN), available nitrogen (AN), pH, electrical conductivity (EC), higher N:P ratio, and lower C:N ratio, all demonstrated the relationship between the rhizosphere microbes and soil carbon (C) and nitrogen (N) cycling. A redundancy analysis (RDA) of different taxonomic levels indicated a close positive relationship between rhizosphere microbes and AN. In addition, the functional groups of the C. korshinskii rhizosphere bacteria were closely related to soil AN and were mainly composed of chemoheterotrophy and aerobic chemoheterotrophy. A Spearman correlation analysis revealed that these functional groups were mainly identified from bacterial Actinobacteria, Proteobacteria, Verrucomicrobia, Bacteroidetes, and fungal Glomeromycota. Our study provides evidence that the rhizosphere microbes of C. korshinskii are closely related to the accumulation of N in the restoration of desert ecosystems, and that the ecological functional processes they are involved in mainly involve C and N cycles, which play an important role in desertification reversal.
Introduction
In recent decades, an increasing number of studies have reported that revegetation can effectively promote desertification reversal (Lyu et al., 2020; Zhou et al., 2020a), and that the sand-fixing model of combining straw checkerboard with vegetation is one of the most effective measures (Li et al., 2020). In the context of the “Grain for Green” program advocated by the Chinese government, various kinds of drought-tolerant plants have been applied during afforestation (Yu et al., 2020). C. korshinskii, one of the most widely-planted xerophytic legume shrubs in northwest China, is also a pioneer plant for soil and water conservation and sand fixation and has a strong water acquisition strategy in dryland ecosystems (Fang et al., 2008; Wang et al., 2021a; Waseem et al., 2021). One common method of sand restoration is planting N-fixing leguminous shrubs to restore vegetation, so C. korshinskii has been widely-used in long-term restoration projects in dryland ecosystems (Issah et al., 2014; Xu et al., 2019). Studies have reported that C. korshinskii has a unique survival strategy in ecological restoration and a strong resistance to extreme drought (Gao et al., 2018; Zhao et al., 2021), which may be strongly related to its rhizosphere (Hartman et al., 2017). Studying the rhizosphere microorganisms and soil environmental factors of C. korshinskii will likely identify potential microbial functional groups and processes in its rhizosphere that explain this shrub’s ability to grow so well in harsh climates.
Plants depend on the rhizosphere in order to maintain health, absorb nutrients, and resist pathogens. Rhizosphere studies of an annual herbaceous plant showed that soil habitat had a significant effect on rhizosphere community composition and gene expression. The continuous increase of carbohydrate depolymerization genes in the rhizosphere led to the diversity of functional groups, formed obvious niche differentiation, and drove the rhizosphere C cycling process (Nuccio et al., 2020). Importantly, the rhizosphere is part of a dynamic ecological process. Within the rhizosphere, plant roots act as the supplier of nutrients to the soil, and nutrients flow between inorganic and organic substances, which are mediated by the rhizosphere microbes (York et al., 2016). Plant growth is greatly impacted by the availability of soil N and phosphorus (P) in the rhizosphere. The N-fixing ability of legumes helps address the problem of N deficiency in dryland ecosystems (Hartman et al., 2017; Kobayashi, Yamaguchi & Iwasa, 2021). This is because the leguminous rhizosphere depends on powerful rhizobia to form symbiotic nodules and to continuously “fix” atmospheric dinitrogen (N2) into ammonia (NH3) in order to provide the N needed for the host plants (Kobayashi, Yamaguchi & Iwasa, 2021; Yang et al., 2021). Rhizobia in the rhizosphere of legumes can induce plant root nodulation, which significantly improves the soil by N fixation, increasing crop production. There are many species of rhizobia, belonging to 18 genera in the family Rhizobiaceae, of which Rhizobia is the largest genus. Most of a plant’s total N is used in the formation of chloroplasts, so N plays a vital role in plant photosynthesis and production (Lindstrom & Mousavi, 2019); in return, plant photosynthates supply the needs of the rhizosphere microbes, resulting in cooperative relationships between plants and microorganisms, fulfilling the C and N needs of both (Henneron et al., 2020). The rhizosphere N-fixing microbes are numerous and diverse; the main bacteria that come from Proteobacteria include: Alphaproteobacteria (α-rhizobi), Gammaproteobacteria (γ-rhizobi), Betaproteobacteria (β-rhizobia), Deltaproteobacteria, and some Actinomycetes (Vadakattu & Sharma, 2020). Studies have shown that fungal mycorrhizas (e.g., arbuscular mycorrhizal fungi, AMF) attach some bacteria to their mycelia and spores, which play a dual role in P solubilization and N fixation and cover approximately 80% of plants in terrestrial ecosystems (Kiers et al., 2011). AMF mainly originate from the soil fungal phylum Glomeromycota and are responsible for large-scale nutrient migration and C sequestration; furthermore, C availability triggers N utilization through arbuscular mycorrhizal symbiosis, achieving a mutually beneficial trade of C, N and P with AMF hosts (Bucking & Shachar-Hill, 2005; Fellbaum et al., 2012; Kiers et al., 2011). AMF also play a critical role in N and P metabolism (Wang et al., 2021b).
Based on changes in environmental factors, the functional group of rhizosphere microorganisms can trigger the rhizosphere priming effect and promote the functional processes of the rhizosphere soil (Mo et al., 2021; Tkacz & Poole, 2020). Previous studies have shown that key groups of microbes have specialized metabolic functions and are able to maintain the stability of the community in the rhizosphere (Xun et al., 2021). For example, a series of functional traits in legumes are related to symbiotic N fixation, and these traits may determine the successional and functional niches of different legumes (Dovrat et al., 2020; Schulte et al., 2021). Environmental stress (Astorga-Elo et al., 2020; Zhang et al., 2022), degradation of pollutants (Rong et al., 2021), continuous cropping (Alami et al., 2020; Yao et al., 2020), and biological invasion (Gao et al., 2019) could all lead to changes in the microbial functional groups and metabolic processes, specifically, a higher abundance of chemoheterotrophy and aerobic chemoheterotrophy. Chemoheterotrophic bacteria are mainly responsible for the decomposition of organic matter and are the main groups consuming environmental C sources (Kämpfer et al., 1993). The disturbed host, by changing root exudates to recruit functional groups, again regulates soil nutrient cycling, resulting in soil C:N and N:P ratio imbalances (Canarini et al., 2019; Ding, Cong & Lambers, 2021; Mo et al., 2021).
Given the excellent performance of C. korshinskii in the ecological restoration of sandy land over the years (Gao et al., 2018) and also the N fixation characteristics of legumes, there is still a poor knowledge about how the relationship between the composition and functional groups of the leguminous C. korshinskii rhizosphere and soil nutrients changes with years of sand fixation in extremely desertificated ecosystems. Hence, we put forward the following research questions: (1) How do the characteristics of functional microbial composition and soil physicochemical properties in the C. korshinskii rhizosphere change with years? (2) What is the relationship between rhizosphere microbial functional composition and soil properties? According to these research questions, we hypothesized that years of sand fixation with the N-fixing C. korshinskii could effectively enrich the functional rhizosphere microbes by accumulating soil N nutrient. The major aims of our study were to (1) examine the functional microbes and soil properties of the C. korshinskii rhizosphere after 6, 12 and 18 years of sand-fixing, (2) determine and evaluate the relationship between soil properties and microbes of the rhizosphere, and (3) predict the function of the rhizosphere microbes and reveal the relationship between physicochemical properties (especially the soil N nutrient content) and functional groups. Our study will better elucidate the adaptation mechanism of C. korshinskii in extremely arid or sandy habitats from the perspective of rhizosphere microbial ecology, and provide a reference for vegetation restoration.
Materials and Methods
Sampling sites and sampling design
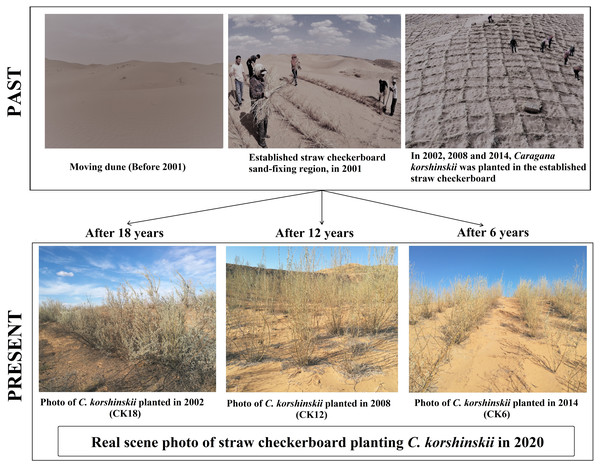
The sampling was carried out in the core area of straw checkerboard-coupled shrub sand fixation in the Baijitan National Nature Reserve (37°58′24″N, 106°24′06″E), located in the southwest margin of the Mu Us Sandy Land, China. The study site is a typical, sandy, ecological recovery area and has an annual precipitation of 230–292 mm, with nearly 70% of the precipitation concentrated in July to September when the summer transition to the autumn months. The site has a semiarid, continental monsoon climate with a mean annual accumulated temperature of approximately 3,334.8 °C, a mean annual temperature of approximately 8.1 °C, and a 157-d frost-free period (Zhou et al., 2020b). This area was a moving dune before 2001, with strong sand storms seriously affecting the local living conditions. In 2001, straw checkerboard sand fixation technology began to be implemented throughout China, including at the study site. Then, starting in 2002, indigenous drought-tolerant seedling shrubs, such as C. korshinskii, Calligonum mongolicum, Corethrodendron scoparium, and C. fruticosum var. mongolicum, were planted yearly on the checkerboard. Among these shrubs, C. korshinskii had the largest planting area. In 2002, 2008 and 2014, straw checkerboards were established, and C. korshinskii was planted successively in the Baijitan Nature Reserve. These three straw checkerboard combined vegetation sand-fixing areas established by C. korshinskii have had significant impacts on sand fixation and desertification reversal, and the moving dunes are now well controlled (Fig. 1). We selected these three areas, established in 2002, 2008, and 2014 as the observation plots in this study, represented by CK18, CK12 and CK6 (Fig. 1), respectively. The study area is in the National Nature Reserve and has not been disturbed by anthropogenic activities.
Figure 1: Sand-fixation process of straw checkerboard-coupled shrubs and performance in the C. korshinskii planting stages.
Soil sampling
In order to keep the samples as consistent as possible, all chosen plots had the same slope and terrain. We chose three plots in each of the three areas (CK6, CK12, and CK18) and two replicate areas (10 m ×10 m, at least 100 m apart), so each area had 6 replicates in total. We collected samples in the summer (on July 26) and fall (on October 17) of 2020.
Before sampling, we excavated the roots of C. korshinskii in advance for preliminary observation and found that the key branches of the C. korshinskii roots, which were interwoven with more root hairs, were concentrated 30–40 cm below the surface of the soil. Studies have confirmed that the root hair region is the region closest to the rhizosphere soil-microbe-host relationship (Koebernick et al., 2017; Ling, Wang & Kuzyakov, 2022). Therefore, we determined that the rhizosphere sampling range of C. korshinskii was 30–40 cm below the soil surface. For each of the two sampling periods, we selected 2–3 healthy C. korshinskii plants of similar size in each replicate area, collecting the top 30 to 40 cm of the rhizosphere soil with a shovel, and mixing it evenly into a duplicate sample. The rhizosphere soil sampling approach involved gently shaking off the bulk soil around the root, brushing the soil tightly attached to the root surface with a sterile brush, and then passing it through a 1-mm soil sieve (we found that using a soil sieve with a 2-mm diameter resulted in too much dead root-bark litter in the final soil samples). After 5 g of rhizosphere soil was collected from each sample plant, the sample soil was put into a numbered tube, placed into a dry ice bucket (McPherson et al., 2018), and then transported to the refrigerator at −80 °C for DNA extraction. Approximately 1 kg of the bulk soil shaken from the plant roots was also collected for a physicochemical properties analysis. At the time of sampling, the wet soil mass was immediately weighed for subsequent determination of soil moisture (SM).
Analysis of soil physicochemical properties
SM was measured by drying to constant mass in an oven at 105 °C. The air-dried rhizosphere soil was then divided into two groups with one group put through a 1-mm soil sieve and the other put through a 0.149 mm soil sieve. The soil pH and electrical conductivity (EC) were measured using a pH meter (PHS-3D, Shanghai Sanxin Instrument, Shanghai, China) and a conductivity meter (DDS-307A, Shanghai Youke Instrument Co. Ltd., Shanghai, China), respectively. Soil organic carbon (SOC), total phosphorus (TP), available phosphorus (AP), total nitrogen (TN), and available nitrogen (AN) were measured using the methods described by Bao (2000). We also calculated the rhizosphere soil stoichiometric ratios (SOC/TN, C:N; SOC/TP, C:P; TN/TP, N:P).
DNA extraction, library preparation, and Illumina MiSeq sequencing
The total microbial genomic DNA was extracted using the FastDNA® SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA) according to the manufacturer’s instructions. In order to understand the changes in the soil microbiome of C. korshinskii after 6, 12 and 18 years, accurate 16S bacterial absolute quantification sequencing was performed (Jiang et al., 2019). The bacterial absolute quantitative sequencing process was as follows: the 16S amplicon library was constructed and sequenced by adding a certain amount of the synthetic “Spike-in Standards” sequences to the sample DNA, and then the standard curve was drawn according to the number of 16S amplicon reads and their absolute copy numbers of spike-in standards. The absolute copy number of 16S rRNA genes was calculated for species within the range of the standard curve in the sample (Yang et al., 2018). Primers 515F/907R (5′- GTGCCAGCMGCCGCGG-3′/5′- CCGTCAATTCMTTTRAGTTT-3′) and ITS1F/ITS2R (5′- CTTGGTCATTTAGAGGAAGTAA-3′/5′- GCTGCGTTCTTCATCGATGC-3′) were used to amplify the bacterial 16S rRNA V4-V5 hypervariable regions (Wang et al., 2019) and the fungal ITS1 gene region (Shi et al., 2021c), respectively. The whole sequencing process was performed using technology from Genesky Biotechnologies Inc., Shanghai, China (201315) (Jiang et al., 2019).
Data analysis
The raw read sequences were processed by QIIME2 (Fung et al., 2021), and the DADA2 plugin was used to identify amplicon sequence variants (ASVs; (Callahan et al., 2016). Taxonomic assignments of ASV representative sequences were performed with a confidence threshold of 0.8 by a naïve Bayes classifier that was trained on the Ribosomal Database Project (RDP) (version 11.5) (http://rdp.cme.msu.edu/). Then, the spike-in sequences were identified, and the total reads were counted. A standard curve for each sample was generated based on read counts and spike-in copy number. The absolute copy number of each ASV in each sample was calculated by using the read counts of the corresponding ASVs, and the spike-in sequence was removed in the subsequent analysis (Jiang et al., 2019).
FAPROTAX (Functional Annotation of Prokaryotic Taxa) was originally used to predict the bacterial metabolic functional groups of aquatic ecosystems (Louca, Parfrey & Doebeli, 2016), but in recent years, it has also been used to predict the C and N metabolic functional groups in terrestrial soil systems (Sansupa et al., 2021). The FUNGuild Database is an annotated database of the functional groups of fungi (Alami et al., 2020). In the present study, we investigated the sand-fixation effect of C. korshinskii, regardless of any association with N fixation and C utilization in the rhizosphere. Therefore, FAPROTAX and FUNGuild analyses were used in this study to predict the functional group characteristics of the C. korshinskii rhizosphere to facilitate subsequent research.
Alpha diversity (Shannon, Simpson and Coverage) and richness (Observed, Chao1 and ACE index) were evaluated using the ASV table by QIIME2 (Fung et al., 2021). Venn diagrams and heatmaps were drawn by the “ggplot2” and “Vegan” R packages (R 4.0.5; https://www.r-project.org/). Changes in the soil microbial community composition, functional groups, and physicochemical properties of the C. korshinskii rhizosphere, as well as their differences between sand fixation years (CK18, CK12 and CK6), and the subsequent correlation analysis (linear and matrix correlation analysis), were measured using one-way ANOVAs with Duncan’s tests by Origin 9.8.0.200 (OriginLab Corporation, Northampton, MA, USA). A redundancy analysis (RDA) was conducted by Canonco 5 to explore the relationship between rhizosphere composition, functional groups, and soil physicochemical properties. The above figures were processed by Adobe Illustrator CS6, and the significance of the difference is indicated by P < 0.05, P < 0.01, and P < 0.001.
Results
Changes in the microbial community composition in the C. korshinskii rhizosphere at different years of sand fixation
The sequences and ASVs of both bacteria and fungi had few differences in the summer and fall samples, so we averaged the summer and fall results from all soil samples for the statistical analysis. We obtained a total of 3,381,990 and 1,060,779 bacterial and fungal community sequences, respectively. The average number of bacterial sequences (211,437) varied from 203,385 to 219,955 per sample, whereas the average number of fungal sequences (66,616) varied from 14,437 to 81,553 per sample (Table S1). A total of 1,862 bacterial ASVs were common among CK18, CK12, and CK6, accounting for 21.3%, 21.8%, and 23.0% of the total bacterial ASVs, respectively. A total of 110 fungal ASVs were common among CK18, CK12, and CK6, accounting for 11.4%, 12.6%, and 14.4% of the total fungal ASVs, respectively (Fig. S1). The rhizosphere microbial community of C. korshinskii was dominated by bacterial groups.
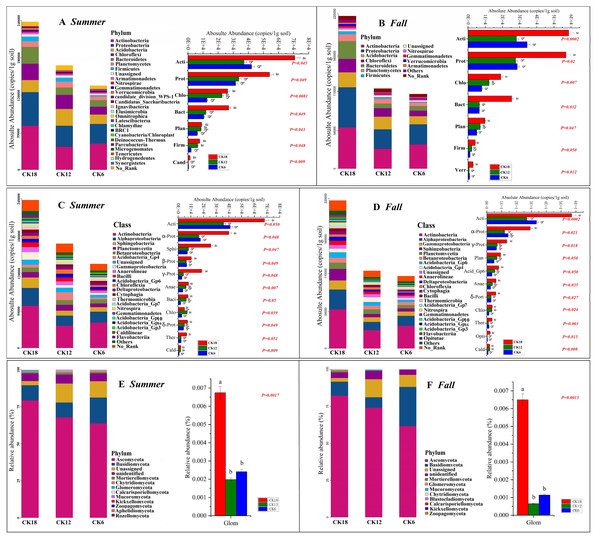
Although there were no significant differences in the alpha diversity of microorganisms between CK18, CK12, and CK6 (Fig. S2), we found that the abundance of some groups of bacteria in the rhizosphere of C. korshinskii increased gradually with time (Fig. 2), with the highest levels found in CK18. These bacterial groups were represented at different taxonomic levels. The dominant phyla that were significantly higher in both summer and fall CK18 samples than in CK12 and CK6 (P < 0.05, 0.01) were: Actinobacteria, Proteobacteria, Chloroflexi, Bacteroidetes, Planctomycetes, and Firmicutes (Figs. 2A–2B). At the class level, Actinobacteria, Alphaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Sphingobacteriia, Betaproteobacteria, Anaerolineae, and Thermomicrobia levels were significantly higher in CK18 (P < 0.05) than in CK12 and CK6 (Figs. 2C–2D). At the fungal phylum level, the relative abundance of Glomeromycota in CK18 was significantly higher (P < 0.01) than in CK12 and CK6 (Figs. 2E–2F).
Figure 2: Abundance of the rhizosphere microbiome (A–B) Composition and differences in bacteria at the phylum level; (C–D) Composition and differences in bacteria at the class level; (E–F) Composition and differences in fungi at the phylum level) of C. korshinskii in summer and fall after 6, 12, and 18 years of sand-fixing restoration.
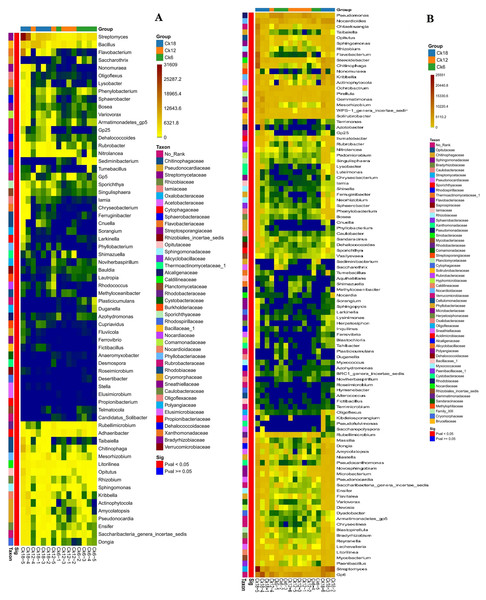
The abundance of genus level groups in the rhizosphere of C. korshinskii in both summer and fall also increased with year, and we found that the bacterial genera were also most abundant in CK18, including Streptomyces, Flavobacterium, Chitinophaga, Kribbella, Mesorhizobium, Opitutus, Actinophytocola, Pseudonocardia, Rhizobium, Amycolatopsis, Sphingomonas, Ensifer, and Neorhizobium, especially Streptomyces (Figs. 3A–3B). Among these genera, Rhizobium, Ensifer, Neorhizobium, Mesorhizobium, Streptomyces, Sphingomonas, and Flavobacterium have typical nitrogen-fixing and/or phosphate-solubilizing characteristics. This result suggests that the enrichment of the rhizosphere microbiome significantly increases with year, with certain functional groups shaping these changes.
Figure 3: Heatmap of the rhizosphere bacterial composition of C. korshinshii at the genus level in both summer (A) and fall (B) after 6, 12, and 18 years of sand-fixing restoration.
Variations in the physicochemical properties of the rhizosphere soil of C. korshinskii and the relationships of these variations with the rhizosphere microbes
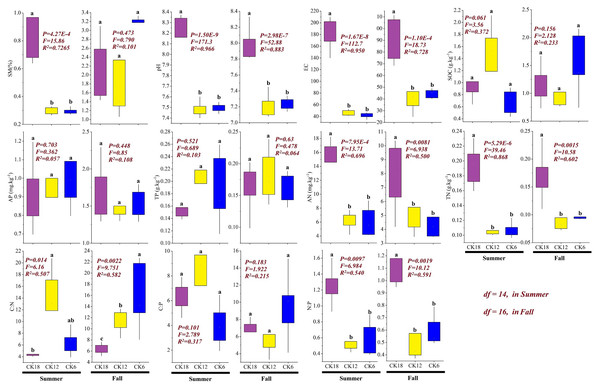
The soil physicochemical properties of the rhizosphere differed among the three stages of C. korshinskii restoration (Fig. 4). In both summer and fall, the soil AN (summer: P < 0.001, F = 13.71, R2 = 0.696; fall: P < 0.01, F = 6.94, R2 = 0.50), TN (summer: P < 0.001, F = 39.46, R2 = 0.868; fall: P < 0.01, F = 10.58, R2 = 0.602), pH (summer: P < 0.001, F = 171.3, R2 = 0.966; fall: P < 0.001, F = 52.88, R2 = 0.883), and EC (summer: P < 0.001, F = 112.7, R2 = 0.95; fall: P < 0.001, F = 18.73, R2 = 0.728) were significantly higher in CK18 than in CK12 and CK6, with no significant difference seen between CK12 and CK6 (P > 0.05). The stoichiometric ratio also changed significantly, and the ratio of N:P in CK18 (summer: P < 0.01, F = 6.98, R2 = 0.54; fall: P < 0.01, F = 10.12, R2 = 0.591) was significantly higher than that in CK12 and CK6, while the C:N ratio (summer: P < 0.05, F = 6.16, R2 = 0.507; fall: P < 0.01, F = 9.75, R2 = 0.582) showed the opposite change, with the C:N ratio significantly lower in CK18 than in CK6 and CK12. These results indicate that over a long period of year, C. korshinskii is able to effectively promote the accumulation of soil N in the rhizosphere.
Figure 4: Changes in the soil physicochemical properties of the C. korshinskii rhizosphere in summer and fall after 6, 12, and 18 years of sand-fixing restoration.
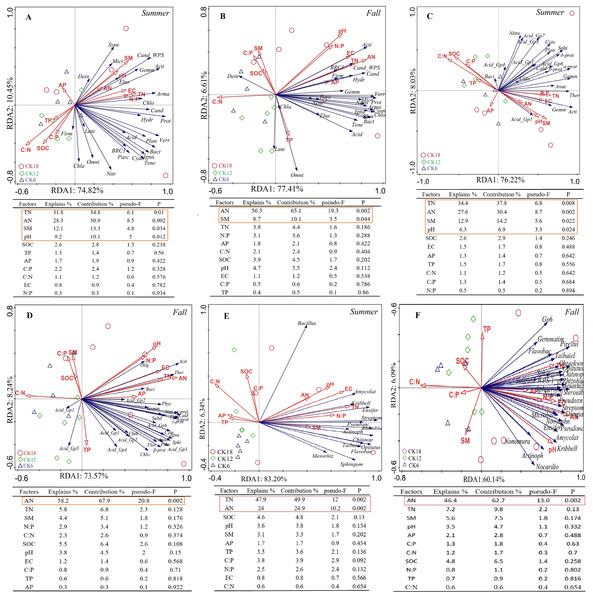
A redundancy analysis (RDA) indicated that the dominant bacterial phyla and classes of the rhizosphere were positively correlated with soil TN, AN, SM, and pH in summer, which explained 85.27% and 84.25% of the total variation at the phylum level (Fig. 5A) and class level (Fig. 5C), respectively. In fall, the dominant bacterial phyla and classes of the rhizosphere were positively correlated with soil AN, which explained 84.02% and 81.81% of the total variation at the phylum level (Fig. 5B) and class level (Fig. 5D), respectively. At the genus level, the dominant genera were positively correlated with soil AN and TN, which explained 89.54% and 66.23% of the total variation in summer (Fig. 5E) and fall (Fig. 5F), respectively. The rhizosphere fungi were related to soil EC and weakly related to soil AN and SM, explaining 84.5% and 46.71% of the total variation in summer (Fig. S3A) and fall (Fig. S3B), respectively. An RDA also showed that the rhizosphere soil characteristics were closely related to the abundance of microorganisms in the rhizosphere of C. korshinskii in both summer and fall, explaining 67.6%, 71.45%, and 73.65% of the total variation at the phylum, class, and genus levels, respectively. These results revealed that the accumulation of N in the rhizosphere was a key factor in the enrichment of the rhizosphere bacterial communities at different taxonomic levels with years.
Figure 5: Ordination plots from a redundancy analysis (RDA) indicate the relationship between the dominant rhizosphere bacterial taxon (A–B) phylum level; (C–D) class level; (E–F) genus level) and soil factors in summer and fall across after 6, 12, and 18 years of C. korshinshii sand-fixing restoration.
Functional prediction and relationship between soil properties and functional groups
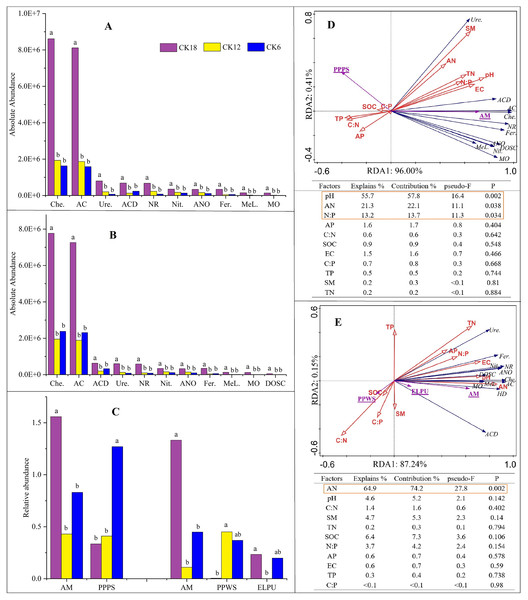
The long-term use of C. korshinskii for sand-fixation afforestation had different effects on the different bacterial functional groups, but little effect on the fungal functional groups (Fig. 6). Bacterial ecological functions, including chemoheterotrophy (Che.), aerobic chemoheterotrophy (AC), ureolysis (Ure.), aromatic compound degradation (ACD), nitrate reduction (NR), nitrification (Nit.), aerobic nitrite oxidation (ANO), fermentation (Fer.), methylotrophy (Met.), and methanol oxidation (MO; Figs. 6A–6B), and the fungal arbuscular mycorrhizal (AM) function (Fig. 6C), were all significantly higher in both seasons of CK18 than in CK12 and CK6 (P ≤ 0.05). In general, the rhizosphere bacteria that were present after 18 years of afforestation with C. korshinskii were dominant in chemoheterotrophy and aerobic chemoheterotrophy.
Figure 6: Functional predictive analysis (FAPROTAX and FUNGuild) of the rhizosphere microbiome and RDA between functional items and soil physicochemical factors among the 18-, 12-, and 6-year-restored dunes in Mu Us Sandy Land.
A-B represent bacteria in summer (A) and fall (B) after 6, 12, and 18 years of sand-fixing restoration.(C) represents fungi in summer (left) and fall (right), while panels (D) and (E) represent RDA of functional groups in summer and fall, respectively. Different uppercase letters in the histogram represent significant differences among different sand-fixing years (P < 0.05). The functional annotation items are abbreviated as follow: chemoheterotrophy (Che.), aerobic chemoheterotrophy (AC), ureolysis (Ure.), aromatic compound degradation (ACD), nitrate reduction (NR), nitrification (Nit.), aerobic nitrite oxidation (ANO), fermentation (Fer.), methylotrophy (MeL.), methanol oxidation (MO), dark oxidation of sulfur compounds (DOSC) in bacteria, are represented in black for RDA; arbuscular mycorrhizal (AM), plant pathogen-plant saprotroph (PPPS), plant pathogen-wood saprotroph (PPWS), endophyte-lichen parasite-plant pathogen-undefined saprotroph (ELPU) in fungi, are represented in underlined purple for RDA.An RDA indicated that most of the ecological functions of the abundant bacteria and fungi were closely related to soil factors (Figs. 6D–6E). In summer, the largest contributor to functional indicators of the soil microbiome was pH, followed by AN and N:P ratio, which accounted for 96.41% of the total variation, combined. In fall, the main soil factor was AN, which explained 87.39% of the total variation. Soil AN was the key factor affecting the microbial functional abundance in the rhizosphere of C. korshinskii. A simple linear regression analysis showed that AN was positively correlated with the ten bacterial ecological functions that were higher in CK18 (Fig. S4). A Spearman’s rank correlation analysis suggested that the functional predictors of these differences were positively correlated with some microbial phyla, including Actinobacteria, Proteobacteria, Chloroflexi, Bacteroidetes, Planctomycetes, Firmicutes, and Verrucomicrobia, and the fungal phylum, Glomeromycota (Fig. S5).
Discussion
Rhizosphere microbes were closely related to soil physicochemical properties
In this study, the rhizosphere microbes of C. korshinskii were well formed and dominated by the key classes of Actinobacteria, α-Proteobacteria, γ-Proteobacteria, δ-Proteobacteria, and β-Proteobacteria, and included Sphingobacteriia (Bacteroidetes), Chloroflexia (Chloroflexi), Thermomicrobia (Chloroflexi), and Caldilineae (Chloroflexi), as well as a small amount of Glomeromycota (Fig. 2). More importantly, the abundance of typical nitrogen-fixing and/or phosphate stabilizing bacterial genera, such as Rhizobium, Ensifer, Neorhizobium, Mesorhizobium, Streptomyces, Sphingomonas, and Flavobacterium, increased significantly in the rhizosphere after 18 years of sand fixation (Fig. 3), which well confirmed our hypothesis. Some of the nitrogen-fixing bacteria formed in the rhizosphere of C. korshinskii in our study were similar to those reported by Rahimlou, Bahram & Tedersoo (2021), who confirmed that some genera of α-Proteobacteria (Rhizobium, Ensifer, Neorhizobium, Allorhizobium, Microvirga, Mesorhizobium, Bradyrhizobium, Azorhizobium, and Methylobacterium) and β-Proteobacteria (Paraburkholderia, Cupriavidus) have potential N-fixing abilities. These nitrogen-fixing groups are mainly derived from the alpha (α), beta (β), delta (δ), and gamma (γ) groups of Proteobacteria as well as Actinomycetes, which form symbionts to facilitate legume growth (Chen et al., 2020a; Vadakattu & Sharma, 2020). This may be because a mutually beneficial symbiotic relationship was established between rhizobia and C. korshinskii, which provided sufficient N nutrients for the rhizosphere of C. korshinskii (Lindstrom & Mousavi, 2019; Wendlandt et al., 2022). Studies have shown that these mutually beneficial relationships promote the accumulation of N in the rhizosphere soil (Welmillage et al., 2021), and that the contribution of symbiotic N fixation in agricultural ecosystems can exceed 80% (O’Hara, 2008), for example, in the rhizosphere of cultivated indica (Zhang et al., 2019a). These results indicate that years of sand fixation using C. korshinskii can effectively increase the abundance of bacterial genera that have functions related to N metabolism.
We found that the abundance of Glomeromycota in the rhizosphere of C. korshinskii also increased significantly after 18 years of sand-fixation restoration (Fig. 2). Arbuscular mycorrhizal fungi (AMF) are mainly derived from the phylum Glomeromycota. Approximately 80% of all plants in terrestrial ecosystems have been colonized by AMF with well-established symbiosis (Smith & Read, 2008). Both plant roots and AM fungal extraradical hyphae can produce enzymes, protons, and carbohydrates, explaining the differences in soil properties between the rhizosphere soil and bulk soil (Johansson, Paul & Finlay, 2004). It has been proven that some bacteria attach to the hyphae and spores of AMF (Scheublin et al., 2010) and stimulate mycelia, spore growth, and germination, as well as mycorrhizal formation (Artursson & Jansson, 2003). These mycorrhizal helper bacteria include gram-negative Proteobacteria (Rhizobium, Azospirillum, Azotobacter, Agrobacterium, Enterobacter, Burkholderia, Klebsiella, Bradyrhizobium, and Pseudomonas), gram-positive Firmicutes (Paenibacillus, Bacillus, and Brevibacillus), and gram-positive Actinomycetes (Streptomyces, Arthrobacter, and Rhodococcus; (Frey-Klett, Garbaye & Tarkka, 2007). Other mycorrhizal helper bacteria, including Sphingomonas, Variovorax, Xenophilus, Hydrocarboniphaga, Brevundimonas, and Microbacterium have recently been identified (Shi et al., 2021a). Rhizobium, Arthrobacter, Sphingomonas, Bacillus, Burkholderia, Pseudomonas, and Flavobacterium all have the dual functions of fixing N and dissolving P (Rodríguez & Fraga, 1999; Shi et al., 2021a; Taktek et al., 2015). It is probable that the mycelia of AMF and these N-fixing bacteria combined to play a role in the rhizosphere of C. korshinskii, resulting in the accumulation of N nutrients in the rhizosphere soil.
Streptomyces was the most abundant bacteria in our study (Fig. 3). It is the largest genus of Actinobacteria and produces high-yield antimicrobial compounds (Hutchings, Truman & Wilkinson, 2019); for example, Arabidopsis thaliana specifically recruits Streptomyces bacteria to its roots to resist pathogenic soil-borne diseases and stimulate the secretion of endogenous hormones in host plants (Worsley et al., 2020). Studies have shown that the enrichment of Streptomyces in the rhizosphere not only improves soil N availability and microbial composition, but also eventually increases the photosynthetic efficiency and yield of legumes (AbdElgawad et al., 2020); therefore, the C. korshinskii rhizosphere in desert systems might also have the same functional processes. In the present study, the long-term establishment of C. korshinskii led to abundant Flavobacterium and Chitinophaga accumulation (Fig. 3). Studies have shown that the combination of the Flavobacterium and Chitinophaga, Bacteroidetes can suppress diseases caused by fungal roots and form functional endophytic bacteria that benefit the host rather than the rhizosphere (Carrión et al., 2019). Recent studies have shown that the phosphatase (PafA) prevalent in Bacteroidetes is mainly synthesized by Flavobacterium, which can rapidly mineralize organophosphorus and release effective phosphate, greatly improving the utilization efficiency of P (Lidbury et al., 2022). These are also the most promising prospects of endophytic bacteria in C. korshinskii roots in desert ecosystems, which we hope to explore in future studies.
Changes seen in the physicochemical properties of the rhizosphere soil further confirmed that the rhizosphere microbiome was closely related to C, N, and P cycling under long-term sand fixation with C. korshinskii, especially the accumulation of N in the rhizosphere (Fig. 4), which fully supported our hypothesis. The higher N content and abundance of associated groups in the rhizosphere of C. korshinskii may be attributed to the release of primary metabolites by root exudates, called the exudation-induced priming effect (EPE), which promotes nutrient mobilization and regulates the stoichiometric ratio of rhizosphere soil (Canarini et al., 2019; Mo et al., 2021; Tian et al., 2019). The acceleration of the N mineralization rate after 18 years of C. korshinskii afforestation resulted in the imbalance of the rhizosphere stoichiometric ratio, such as higher N:P and lower C:N ratios, but no obvious change to the C:P ratio (Fig. 4), indicating that the supply and demand of C and N deviated, with N levels increasing. N:P imbalances have occurred in oligotrophic dune ecosystems, but they mainly occur in invasive woody legumes (Acacia longifolia) (Ulm et al., 2017). In this study, the higher N accumulation in the rhizosphere of C. korshinskii was similar to those seen in invasive plants. Invasive plants generally increase their competitiveness by changing the rhizosphere environment, especially N metabolism (Gibbons et al., 2017). Mikania micrantha, for example, is a typical invasive plant species. It can accelerate the N cycle in the rhizosphere, promoting the accumulation of AN in the rhizosphere to compete with other plants (Yu et al., 2021). The invasive characteristics of Ageratina adenophora include increasing the N content, nitrification rate, ammonification rate, and N fixation rate of the rhizosphere soil, accelerating the process of rhizosphere N cycling (Zhao et al., 2019). C. microphylla is considered an artificially cultivated invasive species (Zhang et al., 2019b), and C. korshinskii, a member of the same genus, may have similar invasive properties, specifically its competitive ability to obtain water (Waseem et al., 2021), survive in dunes (Fang et al., 2011), and create a higher N and N:P in rhizosphere soil, as seen in this study.
A comprehensive RDA found that N enrichment in the rhizosphere was closely related to microbiome involvement (Fig. 5, Fig. S3). N-limitation conditions occur when the microbial demand for N exceeds the supply of N, the excess C is then metabolized and mineralized through the root exudates. When the demand for C exceeds the supply of C, the excess N is mineralized (Schimel & Weintraub, 2003). Root exudates can improve N availability by stimulating microorganisms to accelerate nitrogen cycling (Meier, Finzi & Phillips, 2017). The rhizosphere microbial communities of leguminous shrubs (Hedysarum mongolicum and H. scoparium) in desert ecosystems are mainly influenced by the properties of the soil. The rhizosphere effect recruits and enriches beneficial microbes; in particular, the enrichment of α- and γ-proteobacteria is dependent on organic carbon (Zhou et al., 2020b), resulting in a lower C:N ratio. These results are consistent with the results of this study and indicate that the increase in rhizosphere N nutrients eliminates the N limitation condition, turning it into a P limitation. This promotes the acquisition of photosynthetic C, which affects plant growth and rhizosphere C cycling (Ding, Cong & Lambers, 2021; Liang et al., 2020; Peng et al., 2019; Zhan et al., 2017), further contributing to the higher N:P ratio and the lower C:N ratio in the C. korshinskii rhizosphere during long-term restoration. The N:P ratio of the rhizosphere soil in desert ecosystems has rarely been reported. In subtropical plantations, the N:P ratio in roots is positively correlated with the rhizosphere, and the C:N:P stoichiometry depends on rhizosphere soil properties (Shi et al., 2021b). Most importantly, microbial activation strongly affects the turnover rates of C and N (Mo et al., 2021) or the consumption of C and mineralization and accumulation of N, especially nitrate N (Wang & Tang, 2018). Based on these findings, C. korshinskii is likely able to survive in harsh environments long-term by regulating the ratio of C to N in the rhizosphere (Figs. 4, 5). It is also likely that the rhizosphere’s nitrogen-fixing ability enhances photosynthesis and rhizodeposition to trigger the recruitment of rhizosphere microbes, initiating C and N trading and N cycling, leading to higher aboveground distribution rates and productivity (Henneron et al., 2020). Plants enhance the decomposition of organic matter in the rhizosphere and then change the soil N cycling to control the accumulation of photosynthate C (Henneron et al., 2020; Vance & Heichel, 1991), thus facilitating C and N trading (Kuzyakov & Xu, 2013). This finding was strongly supported by the positive correlation between rhizosphere soil N and the major microbial groups in the RDA ordination plots (Fig. 5, Fig. S3).
Rhizosphere microbial functional groups are shaped by nutrients and promote nutrient cycling
The FAPROTAX analysis in this study showed that the functional groups of the C. korshinskii rhizosphere were divided into two parts: one was responsible for C metabolic groups (Che., AC, ACD, Fer., MeL., MO, and DOSC), and the other was responsible for N metabolic groups (Ure., NR, Nit., ANO), which verified that the potential functions of the rhizosphere microbial community were closely associated with the C and N cycles in the soil (Figs. 6A–6B). Chemoheterotrophic bacteria are usually decomposers and play a role in the in situ remediation and recycling of organic materials in ecosystems (Kämpfer et al., 1993). Che. and AC were the most common metabolic functions of the bacterial communities associated with the C. korshinskii rhizosphere. This is a similar finding to other rhizosphere studies, including a pioneer plant rhizosphere on the Andean Altiplano (Parastrephia quadrangularis; (Zhang et al., 2022), a broad-spectrum herbicide (Clomazone) applied to soil (Rong et al., 2021), the seagrass rhizosphere (Ling et al., 2021), the rhizosphere of Tibetan barley in continuous cropping (Yao et al., 2020), and the rhizosphere of Cistanthe longiscapa in the Atacama desert of Chile (Astorga-Elo et al., 2020). The lower C:N ratio and higher N:P ratio found in the C. korshinskii rhizosphere proves that the rhizosphere functional groups are heterotrophically dependent on root exudates and participate in the C and N cycles, which may be because the extreme drought conditions of sand-fixing systems lead to dramatic changes in the microbial functional groups and in C and N distribution patterns (Schimel, Balser & Wallenstein, 2007). Given the biological needs of microbes, functional processes in the rhizosphere, such as ANO, Nit., and Ure, produce nitrate/ammonium for the host (Gao et al., 2019); in turn, microbes need the host to provide a C source for survival. Chitin is an important C source, and chitinolysis depends on chitinases produced by functional groups of microorganisms (Beier & Bertilsson, 2013), which further contribute to C turnover, such as in Che., AC, ACD, Fer., MeL., MO, and DOSC processes (Rosenberg et al., 2013). This conclusion is supported by the abundance of chemoheterotrophic and nitrogen-fixing microbes observed, which further clarified the C and N trading process in the C. korshinskii rhizosphere.
The abundance of metabolic functions in the rhizosphere was mainly affected by pH, AN, and N:P in the summer and by AN in the fall (Figs. 6D–6E). During both seasons, soil AN was highly correlated with functional groups and most enhanced the AM, ACD, Ure., NR, AC, and Che. functional processes (Fig. S4), suggesting that AN was the main factor promoting microbial metabolic function in the C. korshinskii rhizosphere. The availability of N and P in the soil is known to limit plant growth; when P is chronically limited in nutrient-deficient sand-fixing systems, N-fixing legumes may form nodule symbioses with rhizobia for the transformation and accumulation of N (Ding, Cong & Lambers, 2021; Dovrat et al., 2020; Peng et al., 2019). The intense competition between N and P has been clearly manifested in arid and oligotrophic systems (Cui et al., 2018); legumes use their own N fixation, not only completing the rhizosphere C and N trade under the ground but also dominating the differentiation of the rhizosphere functional groups (Hartman et al., 2017; Henneron et al., 2020; Schulte et al., 2021; Tkacz & Poole, 2020; Yang et al., 2021). The N fixation process requires the activation of nitrogenase in a low-oxygen environment (Gallon, 1981), promoting the coupling of dicarboxylates with rhizobia for nitrogen fixation (Schulte et al., 2021). The rhizosphere microbial functional groups drive N cycling and accumulation (Henneron et al., 2020; Schulte et al., 2021; Wei et al., 2018). The N-fixing environment in the rhizosphere observed in this study may be due to the consumption of rhizosphere oxygen by aerobic chemoheterotrophic groups.
Our study found that the bacterial phyla had a positive effect on the ecological metabolic functions of the C. korshinskii rhizosphere, with the main bacterial phyla being: Actinobacteria, Proteobacteria, Verrucomicrobia, and Bacteroidetes, followed by Firmicutes, Chloroflexi, and Planctomycetes; the fungal phylum Glomeromycota was also present. Most of the bacterial functional groups were involved in N metabolism (e.g., Ure., NR, Nit., ANO), and Glomeromycota was the only fungal phylum closely related to the functional process (Fig. S5), indicating that considerable nutrient exchange occurs in the rhizosphere soil (Sansupa et al., 2021). Louca, Parfrey & Doebeli (2016) confirmed that Proteobacteria (gamma-, alpha-, and beta-), Actinobacteria, Firmicutes (bacilli), and Bacteroidetes (flavobacteria) were most associated with functional groups, which were mostly attributed to environmental changes, but also slightly affected the microbes. This may be because, after several years, the rhizosphere functional components of sand-fixing plants made up for most of the environmental variation, partly explaining the differences in the microbial community composition (Louca, Parfrey & Doebeli, 2016). The large number of symbiotic groups identified from the rhizosphere bacteria in desert environments, such as Streptomyces, Flavobacterium, Rhizobium, Chitinophaga, Kribbella, Mesorhizobium, Opitutus, Actinophytocola, Pseudonocardia, Amycolatopsis, Sphingomonas, and Ensifer, may significantly contribute to the Che., AC, Ure., ACD, NR, and Nit processes (de Vries & Wallenstein, 2017; Hartman et al., 2017; Louca, Parfrey & Doebeli, 2016). The main purpose of microbial functional groups is to serve the host by consuming rhizosphere C and fixing N2 (Hartman et al., 2017; Sansupa et al., 2021; Vadakattu & Sharma, 2020). The microbes were involved in a wide range of ecological functional processes, for example, Sphingomonas (Proteobacteria) contributes to the formation of carbonate through the Ure. process (Stoner et al., 2005) and Nocardioides (Actinobacteria) is involved in the degradation of aromatic compounds (Takagi et al., 2009) and the functional diversity of rhizosphere bacteria in mangroves (Thatoi et al., 2013).
FUNGuild is a functional annotation database containing more than 13,000 fungal species (Chen et al., 2020b), which can provide guidance for saprophytes, pathogens, decomposers or lichen-eating fungi based on their taxonomic characteristics (Nguyen et al., 2016). The enrichment effect of Glomeromycota (Figs. 6C–6E, Fig. S5) in this study further indicated that AMF could enhance the plant uptake of N and P from the soil and participate in nitrification and nutrient decomposition (Bucking & Shachar-Hill, 2005; Fellbaum et al., 2012), promoting the transformation of carbohydrates and ensuring a mutually beneficial nutrient supply (Kiers et al., 2011; Schulte et al., 2021). The extraradical mycelium of AMF accelerates the turnover rate of the C cycle (Staddon et al., 2003). A large number of studies have shown that AMF effectively combines with leguminous rhizobia to promote N fixation, increase the mineralization rate of C and N, and enrich the soil N content (Gan et al., 2021; Wang et al., 2021b; Yin et al., 2021), which are all closely related to the pH and AN around the rhizosphere soil (Gan et al., 2021), consistent with our summer results.
Conclusion
In conclusion, we comprehensively analysed the data from two seasons (summer and fall) and confirmed that, although the microbial diversity of the C. korshinskii rhizosphere did not change over time, the abundance of bacterial phyla (classes), such as Actinobacteria, Proteobacteria (Alphaproteobacteria, Gammaproteobacteria, Betaproteobacteria, Deltaproteobacteria), Chloroflexi (Anaerolineae), Bacteroidetes (Sphingobacteriia), Planctomycetes, Firmicutes, and Thermomicrobia, was significantly higher in CK18 as well as the fungal phylum Glomeromycota. The genera of rhizosphere bacteria, specifically Streptomyces, Flavobacterium, Chitinophaga, Kribbella, Mesorhizobium, Opitutus, Rhizobium, Actinophytocola, Pseudonocardia, Amycolatopsis, and Sphingomonas were significantly higher in CK18. Among these genera, Rhizobium, Ensifer, Neorhizobium, Mesorhizobium, Streptomyces, Sphingomonas, and Flavobacterium are typical nitrogen-fixing and/or phosphate-solubilizing bacteria. The physicochemical properties of the rhizosphere soil showed that sand fixation using C. korshinskii could significantly change the rhizosphere soil properties and increase the soil pH, EC, TN, and AN, with a higher N:P ratio and a lower C:N ratio. The RDA indicated that N content was the main factor affecting the absolute abundance of the C. korshinskii rhizosphere microbiome at different classification levels. Moreover, the FAPROTAX and FUNGuild analyses showed that the ecological functions of the rhizosphere soil were greatly affected by chemoheterotrophy, aerobic chemoheterotrophy, ureolysis, aromatic compound degradation, nitrate reduction, nitrification, aerobic nitrite oxidation, fermentation, methylotrophy, methanol oxidation, dark oxidation of sulfur compounds, and arbuscular mycorrhizal. These functional groups were closely related to soil AN and were mainly identified in Actinobacteria, Proteobacteria, Verrucomicrobia, Bacteroidetes, and fungal Glomeromycota. Our study confirmed that the function of the rhizosphere microbiome of C. korshinskii in desert ecosystems was closely related to the accumulation and transformation of soil N and that the rhizosphere microbiome plays an important role in the cycling of nutrients, providing a reference for future desertification reversal research and efforts.
Supplemental Information
The sequence statistics showing the high quality reads filtered and the corresponding percentage for both bacteria and fungi in summer and fall, for each sample collecting from areas after 6, 12, and 18 years of sand-fixing restoration
Bacterial and fungal abundance in the C. korshinskii rhizosphere based on the number of ASVs in summer and fall after 6, 12, and 18 years of sand-fixing restoration
The percentage in the parenthesis showed the ratio of the common ASVs in each restoration year. CK6, CK12, and CK18 showed 6, 12, and 18 years of sand-fixing restoration with C. korshinskii, respectively.
Comparison of the differences in microbial α-diversity in the C. korshinskii rhizosphere after 6, 12, and 18 years of sand-fixing restoration
CK6, CK12, and CK18 showed 6, 12, and 18 years of sand-fixing restoration with C. korshinskii, respectively.
Ordination plots and the corresponding results of the redundancy analysis (RDA) indicate the relationship between the dominant rhizosphere fungal phyla abundance and soil factors in summer and fall across after 6, 12, and 18 years of sand-fixing restorati
Black arrows represent the dominant fungal groups, while the red arrows represent the soil properties. The dominant fungal groups are abbreviated to the first four initials.