The survival, development, and reproduction of Gonipterus platensis (Coleoptera: Curculionidae) on the main Eucalyptus (Myrtaceae) genotypes planted in Brazil
- Published
- Accepted
- Received
- Academic Editor
- Boyd Mori
- Subject Areas
- Agricultural Science, Developmental Biology, Entomology, Zoology, Forestry
- Keywords
- Bioecology, Eucalyptus camaldulensis, Eucalyptus grandis, Eucalypt hybrid, Eucalyptus snout beetle, Eucalyptus urophylla
- Copyright
- © 2022 Oliveira et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. The survival, development, and reproduction of Gonipterus platensis (Coleoptera: Curculionidae) on the main Eucalyptus (Myrtaceae) genotypes planted in Brazil. PeerJ 10:e13698 https://doi.org/10.7717/peerj.13698
Abstract
Background
Gonipterus platensis Marelli (Coleoptera: Curculionidae) is the main defoliating beetle of Eucalyptus L’Hér. (Myrtaceae) plants worldwide. The suitability of Eucalyptus to this pest varies among host plant genotypes. The objective of this study was to evaluate the development, reproduction, and survival of G. platensis on Eucalyptus species and hybrids to assess their suitability to this insect pest in Brazil.
Methods
The survival, development, and reproduction parameters were evaluated with G. platensis feeding leaves of Eucalyptus camaldulensis Dehnh., Eucalyptus grandis W. Hill., Eucalyptus urophylla S.T. Blake and on the hybrids of E. grandis ×E. urophylla ‘H13’ and ‘VR3748’ in the laboratory.
Results
The duration of the larval stage of G. platensis was shorter on E. urophylla. The pupal stage and the period from larva to adult were equally shorter on E. urophylla and E. camaldulensis. The viability of instars of this insect was low on both E. grandis and E. camaldulensis. The complete lifespan, oviposition period and reproduction parameters of G. platensis were greater on E. urophylla, lower on E. camaldulensis and E. grandis, and intermediate on both hybrids tested.
Synthesis
Eucalyptus urophylla is the most suitable host for G. platensis survival, development, and reproduction, while E. grandis and E. camaldulensis are the least suitable.
Introduction
Planted forests cover around 131 million hectares in the world (FAO, 2020). Brazil is one of the biggest producers with 9.55 million hectares in 2021 to produce raw material for bioenergy, firewood, laminate, pulp and paper, timber, and wall panels (Ribeiro, Rodrigues & Ballarin, 2020; IBÁ, 2021). Eucalyptus L’Hér. (Myrtales: Myrtaceae) is the most prominent plant genus in Brazilian forest plantations with around 78% of the area planted (IBÁ, 2021). The rapid growth, easy regeneration and cultivation, adaptation to different geographic location places, multiple uses, among others, contribute to the expansion of Eucalyptus in the world (Tomé et al., 2021). The large areas with forest plantations and increase in the international trade of the wood products (e.g., wood packaging, tree logs, and wood chips), contribute to the introduction and spread of insect pests to uninfested geographic regions with Eucalyptus plantations (Andrade et al., 2016; Almeida et al., 2018; Meurisse et al., 2019; Tomé et al., 2021).
The Eucalyptus snout beetle, Gonipterus platensis Marelli (Coleoptera: Curculionidae), is native to Australia and is the most widely distributed defoliating beetle of Eucalyptus in the world (Hurley et al., 2016). This insect was reported in the Brazilian states of Espírito Santo, Rio Grande do Sul, Santa Catarina, Paraná, and São Paulo with higher damage in plantations of the latter two states (Wilcken et al., 2008; Souza et al., 2016), especially in Eucalyptus grandis W. Hill. × Eucalyptus urophylla S.T. Blake hybrids (Souza et al., 2016).
Gonipterus platensis adults feed on the young and middle-aged leaves and on soft bark of twigs, while its larvae feed exclusively on shoot tips and young leaves (Souza et al., 2016). High infestations of G. platensis cause dieback of shoot tips, which may induce the development of epicormic shoots and severe defoliation of the upper third of tree canopy. Sequential defoliations may result in growth of multiple leader shoots and mortality of branches or even trees (Tooke, 1955). Damage by G. platensis is low in Australia because of the natural resistance of Eucalyptus species and the suppression of this pest by a diversity of natural enemies (Valente et al., 2017; Valente et al., 2019; Afonso et al., 2019). However, this insect causes severe damage on exotic Eucalyptus species in some African, American, and European countries (Reis et al., 2012; Souza et al., 2016; Valente et al., 2018; Schröder et al., 2020), resulting in a constant search for alternatives to control this pest (Nascimento et al., 2017; Damascena et al., 2020; Schröder et al., 2021).
The susceptibility of Eucalyptus genotypes to Gonipterus spp. Schoenherr varies according to the Eucalyptus sections (taxonomic division of subgenus), species, and hybrids. The Symphormitus subgenus includes about 470 species divided into 11 sections according to their taxonomic and molecular characteristics, of which the Exsertaria, Latoangulatae, and Maidenaria sections represent about 90% of the area planted with Eucalyptus in the world (Nicolle & Jones, 2018; Scanavaca Junior & Garcia, 2021). High populations of the G. platensis and extensive defoliation are more common on Eucalyptus species of the Maidenaria section (Garcia et al., 2019; Gonçalves et al., 2019). Gonipterus platensis in its region of origin infests species of the Maidenaria section including Eucalyptus dalrympleana Maiden, Eucalyptus globulus Labill., Eucalyptus nitens (H. Deane & Maiden) Maiden, Eucalyptus ovata Labill., Eucalyptus rubida H. Deane & Maiden, and Eucalyptus viminalis Labill (Mapondera et al., 2012; Garcia et al., 2019). This pest also infests Eucalyptus species of different sections in countries where it has been introduced. In the Iberian Peninsula, species of the Maidenaria section are defoliated into variable levels, but those of the Latoangulatae section, like Eucalyptus saligna Sm., are only lightly defoliated (Reis et al., 2012; Valente et al., 2018; Gonçalves et al., 2019). In Chile, severe defoliation on E. globulus (Maidenaria section) and on species of the Exsertaria section such as Eucalyptus camaldulensis Dehnh. has been reported (Lanfranco & Dungey, 2001). In Brazil, species of the sections Exsertaria, Latoangulatae and Maidenaria are largely planted but Eucalyptus dunnii Maiden, E. globulus and E. viminalis (Maidenaria section) are the most damaged by G. platensis, which also damages other species such as Eucalyptus saligna (var. protusa) (Latoangulatae section) and the hybrids E. grandis × E. urophylla (=HGU) and E. grandis × E. dunnii (Wilcken et al., 2008; Souza et al., 2016).
The losses in wood yield by G. platensis in Brazil varies within plant genetic materials from a mean annual increment (MAI) reduction of 10.0% on E. grandis to 42.8% on E. grandis × E. dunnii hybrids (Souza et al., 2016). In 2003, G. platensis damaged around 50,000 ha of a HGU clonal plantation in Espírito Santo State with its aggressiveness associated with the high susceptibility of this plant material (Wilcken et al., 2008).
Chemical, nutritional and/or morphological differences in leaves between Eucalyptus genotypes, such as secondary compounds, leaf waxes, nitrogen and tannin levels affect the host selection (Gripenberg et al., 2010) and the insect development (Ohmart & Edwards, 1991; Koul , 2008; Behmer, 2009; Gherlenda et al., 2016), which may explain the susceptibility of Eucalyptus genotypes to G. platensis. The selection of host plants by G. platensis is influenced by the emission of volatiles from green leaves and terpenes, such as terpenol 1,8-cineole (Bouwer et al., 2014; Branco et al., 2019), which is more concentrated in Maidenaria species susceptible to G. platensis than Latoangulatae species.
Species of the Latoangulatae section are the most planted Eucalyptus in Brazil and their susceptibility to G. platensis is poorly known, especially in tropical areas, for lack of information on the suitability of these Eucalyptus species to this insect. Field observation, host plant response, and insect pest performance are methods used to evaluate host-plant suitability to phytophagous insects (Donatelli et al., 2017). The field observation method integrates the host plant response and insect pest performance with environmental conditions in natural and uniform outbreaks over experimental areas where uncontrolled environments can lead to experimental errors (Sallé et al., 2017). The host plant response in the field is difficult to evaluate for Gonipterus - Eucalyptus interactions because this insect feeds on trees from one to six years old, with reduced possibilities of being monitored in field conditions. Therefore, the insect pest performance in laboratory, under controlled conditions, allows a more adequate assessment of biological parameters at all stages of the insect than that on field studies.
The objective of this work was to evaluate the development, reproduction and survival of G. platensis fed with leaves of E. camaldulensis, E. grandis, E. urophylla, and of the hybrids HGU ‘H13’ and ‘VR3748’ of Eucalyptus grandis × E. urophylla under controlled conditions, and to determine the susceptibility of these plants to this insect pest. Our hypothesis was that commercial Eucalyptus species/clones cultivated in Brazil affect differently the development, reproduction, and survival of G. platensis. The information can be used to manage this pest, avoiding extensive plantations with susceptible Eucalyptus species, and reducing the risks of population outbreaks in commercial plantations.
Material & Methods
Insect
The rostrum of G. platensis adults is short with an ochraceous brown and often reddish colour with 5.7 to 8.9 mm long for males and 7.5 to 8.9 mm for females (Rosado-Neto & Marques, 1996). Females lay eggs forming capsules covered by a dark secretion mainly composed of excrements. Gonipterus platensis has four larval instars. The colour of the body of the first and second instar larvae is yellow and the others with three dark-green lateral stripes on the dorsum, which distinguish this species from G. pulverulentus, without stripes on the body (Rosado-Neto & Marques, 1996). Gonipterus platensis larvae bury themselves in the soil to pupate in a pupal chamber made of sand and fluids secreted by the larvae.
Insect collection
The experiment was carried out at the Biological Control of Forest Pests Laboratory (LCBPF) at the Department of Plant Protection of the School of Agricultural Sciences (FCA) at the São Paulo State University (UNESP) in Botucatu, São Paulo State, Brazil. Gonipterus platensis adult females and males were manually collected in a field stand of E. grandis × E. urophylla clonal plants in the Espírito Santo State, Brazil, placed in 1 L plastic containers and taken to the LCBPF. At arrival in the laboratory, the insects were kept in rearing cages (40 cm long ×80 cm high ×45 cm wide) at 26 ± 1 °C, 70 ± 10% RH and 14:10 h (L:D) photoperiod receiving fresh HGU shoots as a food source (Wilcken et al., 2008).
Insect rearing
Gonipterus platensis egg capsules were manually collected daily from the rearing cages and individually transferred to glass Petri dishes (9.0 cm diameter) (one capsule/dish), where they were kept in a biochemical oxygen demand (BOD) incubator chamber (model EL202; EletroLab, São Paulo, Brazil) at 26 °C and 14:10 h (L:D) photoperiod to obtain larvae of this insect. One hundred newly-hatched G. platensis larvae were individually placed per transparent, cylindrical plastic container (7 cm high ×4 cm diameter) with a fresh, young leaf of either E. camaldulensis, E. grandis, E. urophylla, or the E. urophylla × E. grandis hybrids ‘VR3748’ and ‘H13’ with each plant genotype representing a treatment. Gonipterus platensis larvae were assessed daily until the pre-pupa stage. The pre-pupae were individualized in plastic containers (7 cm high ×4 cm diameter) on surface of a fine autoclaved sand layer (40 ml of sand and 2.5 cm deep) as a pupation substrate.
The sex of the newly-emerged G. platensis adults was identified according to the external morphology of their fifth abdominal sternite (Rosado-Neto & Marques, 1996). Pairs were formed with healthy and vigorous newly-emerged adults and each pair placed per transparent, conical-shaped plastic container (6 cm high ×10 cm upper opening diameter ×8 cm lower opening diameter) covered with a fine-mesh nylon fabric piece for aeration. The G. platensis couples received shoots with tender leaves of Eucalyptus species or clones as a food source (according to the treatment) and substrate for oviposition. The petiole of these shoots was placed in 2-ml plastic Eppendorf® tubes (Hamburg, Germany) filled with water + gel (Hydroplan-EB–0.25%) to keep them fresh and suitable for insect feeding and oviposition. The shoots consumed were replaced daily by fresh ones. Non-mated G. platensis adults were individualized in 500 mL plastic containers, receiving a Eucalyptus leaf daily according to the treatment, and used to estimate the adult longevity.
Assessed parameters
The periods of larva to adult; complete lifespan (egg + larva + pupa + adult); pre- and oviposition (days) and egg incubation (days) were evaluated; besides viability of larva to adult and pupal stage (%); number, duration (days) and viability of each instar; adult (males and females separately and combined) longevity (days); numbers of egg capsules/female and of larvae hatched/egg capsule; and viability of eggs/egg capsule (%). The number and duration of each instar was daily assessed using a stereomicroscope (Nikon SMZ645) when needed to examine the presence of exuviae and/or head capsules released on the Eucalyptus leaf or containers’ inner surfaces. The pupal life stage period (pre-pupa + pupa) was determined as the period between larva digging in the sand and adult emergence. The pre-pupal and pupal life stage periods were expressed as pupal life stage period because of the impossibility of identifying these stages separately without destructive sampling of pupal chamber.
The reproductive parameters of G. platensis were evaluated with oviposition obtained from the laboratory rearing colony. The leaves with egg capsules were cut from the shoots and each egg capsule placed in a plastic Petri dish (9.0 cm diameter), kept in a BOD incubator chamber (EletroLab model EL202) at 26 °C and 14:10 h (L:D) photoperiod until all viable larvae hatched. The number of G. platensis couples varied between treatments because of differences in larval and pupal viability and in the number of adults obtained. The complete lifespan, obtained by calculating the median duration (i.e., period in which 50% of the individuals completed every stage), was determined per stage and after the death of all individuals.
Experiment design and statistics
The experiment was arranged in a complete randomize design (CRD) with 100 insects (replicates) per Eucalyptus species or hybrid. Egg, larval, pre-pupa, pupa and adult data were collected for each insect. All data were submitted to analysis of normality of residuals and homogeneity of variances (Univariate Procedure; Statistical Analysis System SAS®, 2001). The data with normal distribution of residuals: period of each instar and larva stage (days), pupal period (days), larva to adult period (days), adult longevity (days), pre- and oviposition periods (days), and number of egg capsules/female were subjected to an Analysis of Variance (one way ANOVA), with the means compared by Tukey’s range test (Tukey, 1949), while those that did not follow a normal distribution: viability of larval stage (%), pupal stage (%), and larva to adult (%), and egg viability/egg capsule (%) subjected to the Kruskal-Wallis test (Kruskal & Wallis, 1952), with the means compared by the Nemenyi test (Nemenyi, 1963). The data of viability was previously transformed into arcsine to homogenize the data variance (Haddad & Vendramim, 2000). The survival data were analysed for the larval stage and larva to adult periods (Lifest Procedure; Statistical Analysis System SAS , 2001). The differences per biological parameter of G. platensis among Eucalyptus host genotypes were obtained comparing the means using the Savage test (Savage, 1972). Data of percentage of G. platensis in the larval stage during the time (days) until the transformation into pupa and adult with different Eucalyptus species and hybrids were calculated to verify the uniformity and time to transformation in these stages. The significance level was 0.05.
Results
Instar periods and larval stage viability
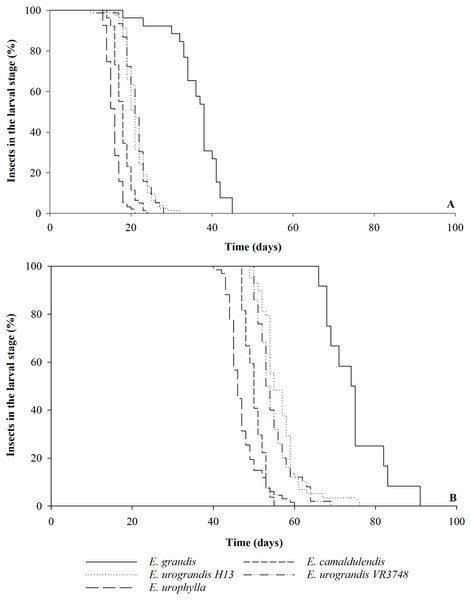
The duration of each instar of G. platensis varied according to the Eucalyptus species or hybrids (Savage test χ2 = 472.96; Pr >χ2 0.0001, Table 1). The durations of first and second instars and of larval stage were shorter on E. camaldulensis and E. urophylla, the third was shorter on E. urophylla and the fourth instar was longer with E. grandis. The duration of the larval stage was shorter on E. urophylla (15.7 ± 0.2 days) than on E. camaldulensis (17.9 ± 0.2 days), HGU ‘H13’ and ‘VR3748’ (20.8 ± 0.4 and 21.3 ± 0.3 days, respectively), and E. grandis (37.6 ± 0.8 days) (Table 1). The last one also showed smaller uniformity and longer time for complete transformation from the larvae population to pupae (Fig. 1A). The larval stage viability was higher on E. urophylla (93.0 ± 2.6%), E. camaldulensis (78.0 ± 4.9%), HGU ‘H13’ (79.0 ± 5.6%), and HGU ‘VR3748’ (76.0 ± 4.9%) than on E. grandis (24.0 ± 4.0%) (Table 1; Fig. 1A).
Pupal period and viability
The pupal stage period of G. platensis was shorter in E. urophylla (31.4 ± 0.4 days) and E. camaldulensis (32.6 ± 0.4 days) than on HGU ‘VR3748’ (33.5 ± 0.5), ‘H13’ (35.3 ± 0.5 days) and E. grandis (37.5 ± 1.7 days). The viability of the pupal stage was higher in the hybrid ‘H13’ (74.7 ± 3.1%), E. urophylla (74.2 ± 6.0%) and the hybrid ‘VR3748’ (65.8 ± 3.3%) than in E. grandis (50.0 ± 3.4%) and E. camaldulensis (33.3 ± 6.5%) (Table 1).
Larva to adult period and viability
The larva to adult period varied between the Eucalyptus genotypes (Savage test χ2 = 189.59; Pr >χ2 0.0001) was shorter in E. urophylla (47.2 ± 0.9 days) and E. camaldulensis (50.5 ± 0.5 days) than in the hybrids ‘VR3748’ (54.8 ± 0.6 days) and ‘H13’ (56.1 ± 0.7 days) and E. grandis (75.1 ± 2.1 days) (Table 1). The last one also showed smaller uniformity and longer time for complete transformation from the larvae population to adult (Fig. 1B). The larva to adult viability was higher in E. urophylla (69.0 ± 3.1%) and the HGU ‘H13’ (59.0 ± 8.1%) and ‘VR3748’ (50.0 ± 4.5%) than in E. camaldulensis (27.0 ± 4.2%) and E. grandis (12.0 ± 3.1%) (Table 1). A total of 50% of the adults emerged within a 46-day period with E. urophylla, compared to a 50, 53, 55, and 74-day periods for E. camaldulensis, the hybrid ‘VR3748’, the hybrid ‘H13’, and E. grandis, respectively (Fig. 1B).
| Parameters | E. camaldulensis | E. grandis | ‘VR3748’ | ‘H13’ | E. urophylla |
|---|---|---|---|---|---|
| I11 | 4.7 ± 0.2c | 9.9 ± 0.5a | 5.8 ± 0.1b | 5.2 ± 0.1bc | 4.5 ± 0.1c |
| I21 | 3.6 ± 0.1d | 9.1 ± 0.3a | 4.9 ± 0.2bc | 5.7 ± 0.2ab | 3.9 ± 0.2cd |
| I31 | 5.0 ± 0.3b | 10.0 ± 0.2a | 5.2 ± 0.2b | 5.0 ± 0.2b | 3.6 ± 0.1c |
| I41 | 4.4 ± 0.3b | 11.5 ± 0.5a | 5.0 ± 0.2b | 5.0 ± 0.2b | 4.3 ± 0.1b |
| LS1 | 17.9 ± 0.2c | 37.6 ± 0.8a | 21.3 ± 0.3b | 20.8 ± 0.4b | 15.7 ± 0.2d |
| V(%)2 | 78.0 ± 4.9a | 24.0 ± 4.0b | 76.0 ± 4.9a | 79.0 ± 5.6a | 93.0 ± 2.6a |
| PS1 | 32.6 ± 0.4bc | 37.5 ± 1.7a | 33.5 ± 0.5ab | 35.3 ± 0.5a | 31.4 ± 0.4c |
| V(%)2 | 33.3 ± 6.5b | 50.0 ± 3.4b | 65.8 ± 3.3a | 74.7 ± 3.1a | 74.2 ± 6.0a |
| LA1 | 50.5 ± 0.5c | 75.1 ± 2.1a | 54.8 ± 0.6b | 56.1 ± 0.7b | 47.2 ± 0.9c |
| A | 47–54 | 66–91 | 50–69 | 49–76 | 43–60 |
| V(%)2 | 27.0 ± 4.2b | 12.0 ± 3.1b | 50.0 ± 4.5a | 59.0 ± 8.1a | 69.0 ± 3.1a |
Notes:
Means followed by the same letter, per row, do not differ by the 1Nemenyi and 2Tukey’s range tests, both at p < 0.05.
Adult complete lifespan and longevity
The adult (females + males) complete lifespan was longer in E. urophylla (223.9 ± 11.2 days) than in the hybrids ‘VR3748’ and ‘H13’ (171.3 ± 14.01 and 146.1 ± 10.6 days, respectively), E. grandis (61.9 ± 16.9 days), and E. camaldulensis (75.2 ± 8.4 days). The longevity of adult females was longer in E. urophylla (283.3 ± 18.3 days) than in E. camaldulensis (103.8 ± 12.6 days) and E. grandis (65.3 ± 27.5 days) and that of males longer in E. urophylla (175.8 ± 10.5 days), ‘VR3748’ (149.8 ± 11.6 days) than in ‘H13’ (127.4 ± 11.0 days), with the second lowest duration on E. camaldulensis (68.1 ± 9.4 days) and the lowest longevity in E. grandis (60.8 ± 21.4 days) (Table 2).
Figure 1: Larval stage and larva to adult period of Gonipterus platensis on Eucalyptus hosts.
Percentage of Gonipterus platensis in the larval stage during the time (days) until the transformation into pupa (A) and adult (B) on different Eucalyptus (E.) species and hybrids at 26 °C and 14:10 h (L:D) photoperiod.Reproduction
The reproductive parameters of adult females of G. platensis varied with Eucalyptus host. The pre-oviposition was longer (55 days) in E. camaldulensis than in E. urophylla (28.7 ± 0.9 days) and the hybrid ‘H13’ (33.2 ± 1.3 days) with intermediate period with the ‘VR3748’ (30.1 ± 1.2 days). The insect laid no eggs on E. grandis and only a single egg capsule on E. camaldulensis (Table 3).
| Eucalyptus hosts | CL1 | Longevity (days) | ||
|---|---|---|---|---|
| Males + Females | Females | Males | ||
| E. camaldulensis | 125.7 ± 8.6d | 75.2 ± 8.4c | 103.8 ± 12.6b | 68.1 ± 9.4cd |
| E. grandis | 137.0 ± 16.9cd | 61.9 ± 16.9c | 65.3 ± 27.5b | 60.8 ± 21.4d |
| ‘VR3748’ | 226.1 ± 14.1b | 171.3 ± 14.01b | 248.0 ± 43.2ab | 149.8 ± 11.6ab |
| ‘H13’ | 202.2 ± 10.7bc | 146.1 ± 10.6b | 195.3 ± 21.2ab | 127.4 ± 11.0bc |
| E. urophylla | 271.1 ± 11.2a | 223.9 ± 11.2a | 283.3 ± 18.3a | 175.8 ± 10.5a |
Notes:
| Eucalyptus hosts | Pre-oviposition1 | Oviposition2 | n |
|---|---|---|---|
| E. camaldulensis | 55.0 | 4.0 | 1 |
| E. grandis | * | * | * |
| ‘VR3748’ | 30.1 ± 1.2ab | 114.6 ± 23.3a | 10 |
| ‘H13’ | 33.2 ± 1.3a | 98.6 ± 31.2a | 12 |
| E. urophylla | 28.7 ± 0.9b | 166.0 ± 19.8a | 18 |
Notes:
Means followed by the same letter, per column, do not differ by the 1Nemenyi and 2Tukey’s range tests, both at p < 0.05.
The G. platensis adult females began oviposition on host shoot tips and leaves up to the following day after copulation. The oviposition period was similar among E. urophylla (166.0 ± 19.8 days) and the hybrids HGU ‘VR3748’ (114.6 ± 23.3 days) and ‘H13’ (98.6 ± 31.2 days) (Table 3).
The number of egg capsules/female was higher in the hybrid ‘VR3748’ (152.3 ± 29.7 egg capsules) and E. urophylla (98.0 ± 14.19 egg capsules) than in ‘H13’ (36.2 ± 6.02 egg capsules). The egg incubation period was similar among Eucalyptus hosts, between 7.1 to 7.3 days. The egg viability/egg capsule was higher in E. urophylla (80.3 ± 3.99%), than on the hybrid ‘H13’ (56.4 ± 6.92%) and intermediate values in ‘VR3748’ (73.2 ± 7.02%). The total number of larvae/egg capsules was higher in ‘VR3748’ (228.30 ± 39.5 larvae) and in E. urophylla (177.7 ± 31.1 larvae) than in ‘H13’ (40.8 ± 9.8 larvae) (Table 4).
| Eucalyptus hosts | ECF1 | EI1 | LH2 | VE/EC (%)2 |
|---|---|---|---|---|
| E. camaldulensis | 1.0 | 7.3 | 4.0 | * |
| E grandis | * | * | * | * |
| ‘VR3748’ | 152.3 ± 29.7a | 7.2 ± 0.08a | 228.3 ± 39.5a | 73.2 ± 7.02ab |
| ‘H13’ | 36.2 ± 6.02b | 7.1 ± 0.10a | 40.8+9.8b | 56.4 ± 6.92b |
| E. urophylla | 98.0 ± 14.19a | 7.2 ± 0.03a | 177.7 ± 31.1a | 80.3 ± 3.99a |
Notes:
Means followed by the same letter, per column, do not differ by the 1Nemenyi and 2Tukey’s range tests, both at p < 0.05.
Discussion
The environmental conditions in the Eucalyptus plantations in South and Southeast Brazil are similar to those of the Gonipterus distribution in Australia, which are a humid subtropical zone with a temperate climate and hot or temperate summers (Crosbie et al., 2012; Mapondera et al., 2012; Alvares et al., 2013). The damage by G. platensis was low in these areas up to 2012 and increased from the end of 2012 (Souza et al., 2016), probably because of the replacement of E. grandis by more productive HGU clones (C.F.W. personal information, 2022). The G. platensis survival, development and reproduction differ among the Eucalyptus species and hybrids with E. urophylla being the most suitable host for this insect, the HGU ‘H13’ and ‘VR3748’ intermediate, and E. grandis and E. camaldulensis the least suitable ones. Higher viability, adult longevity, and fecundity of G. platensis on E. urophylla indicate that species of Latoangulatae section can be suitable hosts for this insect. Nevertheless, the susceptibility of E. saligna, another species of the Latoangulatae, to G. platensis is low (Gonçalves et al., 2019). This suggests different susceptibilities among species within this section. However, E. grandis and E. camaldulensis were poor suitable hosts, and feeding on leaves of these species reduced survival, adult longevity and fecundity of this beetle. Eucalyptus urophylla is native to islands of the Indonesian archipelago and Timor (Hodge & Dvorak, 2015) and is not one of the native hosts of G. platensis in its native region, Tasmania (Australia) (Mapondera et al., 2012). This fact indicates possibilities of G. platensis to adapt to novel hosts as reported for Gonipterus sp. n.2 (Newete, Oberprieler & Byrne, 2011) and other insects. Paropsisterna bimaculata Olivier (Coleoptera: Chrysomelidae) become a pest of E. nitens (Maidenaria section) after this host was introduced in Tasmania. In the past, the insect was thought to be host-specific for species of the subgenus Eucalyptus (de Little & Madden, 1976; Paine, Steinbauer & Lawson, 2011). The oviposition site selection by P. bimaculata females depends on host morphological characteristics because the insect holds the leaf edge while ovipositing and plant kairomones seem to be of low importance (Howlett & Clarke, 2003). On the other hand, Anoplognathus montanus Macleay and A. pallidicollis Blanchard (Coleoptera: Scarabaeidae) that are considered host specific to eucalyptus, can also feed on Schinus molle L. (Anacardiaceae), an exotic plant from South America. This unusual feeding behavior was explained by the presence of similar monoterpenes in both host plants (Steinbauer & Wanjura, 2002).
The shorter larval stage duration on E. urophylla than on other species, possibly, because it has better nutritional value for the larval development of G. platensis. Similarly, E. globulus foliage (Maidenaria section) is considered one the most preferred host to G. platensis in Spain, with a shorter duration of the larval stage (22.1 days) than other species (Cordero-Rivera & Santolamazza-Carbone, 2000; Santolamazza-Carbone, Rodríguez-Illamamola & Cordero Rivera, 2006; Gonçalves et al., 2019). The intermediate larval stage duration of G. platensis on the hybrids HGU ‘H13’ (20.8 ± 0.4 days) and ‘VR3748’ (21.3 ± 0.3 days) is similar to that of this insect on E. globulus (Santolamazza-Carbone, Rodríguez-Illamamola & Cordero Rivera, 2006). The low larval stage viability on E. grandis indicates that this species is inadequate for G. platensis larval development.
The shorter pupal period on E. urophylla indicates the quality of this Eucalyptus species for G. platensis. Higher survival and shorter development period indicate better host quality, as the development duration is extended in inadequate hosts to increase the food intake, especially when the nutrient balance becomes sub-optimal (Chapman, 2013; Bawin et al., 2016). In that case, E. urophylla has both parameters, but a short development period and lower survival of the pupal stage of this insect were found on E. camaldulensis. The higher pupal viability with E. urophylla, HGU ‘H13’ and ‘VR3748’ indicates again the quality of E. urophylla (also presented in the HGUs) for G. platensis development and its mortality in the pupal stage reflecting the conditions to which its larva was exposed (Nestel et al., 2016), with poorer diets increasing pupa mortality (Mohammadzadeh & Izadi, 2018). The lower pupa viability of G. platensis on E. camaldulensis may be due to chemical and/or morphological differences of leaves between Eucalyptus genotypes such as secondary compounds, leaf waxes, nitrogen levels, and tannins (Ohmart & Edwards, 1991; Gherlenda et al., 2016) affecting insect development. Eucalyptus camaldulensis was also inadequate to Gonipterus pulverulentus Lea with lower food conversion efficiency and larval weight among the evaluated hosts, indicating worse larval fitness (Riquelme Virgala et al., 2018).
The shorter larva to adult period and higher survival rates on E. urophylla indicate that this species is a suitable food source to G. platensis (Bawin et al., 2016). Chemical analysis to identify which compounds are responsible for suitable food sources need to be further investigated. In contrast, longer development period in inadequate host plants, as on E. grandis, can increase larval exposure to natural enemies and mortality of G. platensis in the field, according to the slow-growth high-mortality hypothesis (Clancy & Price, 1987; Uesugi, 2015). Variations in the development period of G. platensis among Eucalyptus host species and hybrids suggest an effect of the food quality, as generally reported for insect herbivores (Behmer, 2009). This development period reflects nutritional, morphological, chemical composition, and plant-defence differences between host plants (Paine, Steinbauer & Lawson, 2011; Malishev & Sanson, 2015; Oates et al., 2015; Santadino et al., 2017).
The oviposition period was similar between E. urophylla and HGUs hybrids, the only hosts with oviposition by G. platensis. The higher numbers of egg capsules/female on the ‘VR3748’ and E. urophylla are related to host choice with herbivorous insects choosing those with better conditions for survival and development of their progeny (Gripenberg et al., 2010). The number of egg capsules of Gonipterus sp. n.2 was also high on E. urophylla in South Africa (Newete, Oberprieler & Byrne, 2011). This species laid eggs on E. grandis and E. camaldulensis (Newete, Oberprieler & Byrne, 2011) whereas G. platensis did not, showing different oviposition preferences between these two Gonipterus species. The emission of volatile organic compounds, such as green leaf volatiles and terpenes may influence host plant selection by G. platensis (Bouwer et al., 2014; Branco et al., 2019). The terpenoid 1,8-cineole is probably responsible for the attractiveness and its metabolization by G. platensis results in the production of hydroxylated derivatives that are likely to act as sex pheromones for this beetle (Branco et al., 2020). This terpenoid is highly abundant in the leaf oil composition of Maidenaria species susceptible to G. platensis, like E. dunnii (43.67%), E. globulus (69.10%), E. nitens (47.9%), and E. viminalis (63.73%) and with a low percentage in Latoangulatae species with low damage by G. platensis like E. grandis (0.45%), and E. saligna (0.11%) (Boland, Brophy & House, 1991; Batista-Pereira et al., 2006). The high percentage of 1,8-cineole (53.11%) in the E. urophylla (Latoangulatae section) (Batista-Pereira et al., 2006), makes this species attractive to G. platensis, similar to Maidenaria species.
The unsuitability of E. camaldulensis and E. grandis to G. platensis, compared to the longer pre-oviposition period, lower number of egg capsules/female, and egg viability with the hybrid ‘H13’ indicate the potential of those species for planting as a management strategy for this pest. These plant materials can be used in separate stands on most of the available area or in mixed ones in mosaic or using E. grandis as a barrier in a mosaic landscape (Forrester, Bauhus & Khanna, 2004; Martins et al., 2014).
Conclusions
The shortest egg-to-adult development period, greatest longevity, reproduction, and viability of E. urophylla indicate the suitability of this plant for G. platensis. The intermediate values of the evaluated parameters for G. platensis that fed on the HGU ‘VR3748’ and ‘H13’ indicate that these plants are also appropriate to this pest. The egg-to-adult development period was shorter and the larval stage viability high for G. platensis on E. camaldulensis, but the low larva to adult viability and reproduction impaired the establishment of G. platensis on E. camaldulensis. The longest period and the lowest viability of the larval stage and reproduction of G. platensis on E. grandis indicated this is the least suitable host tested for this insect.
The insect performance method utilized to assess the suitability of Eucalyptus genotypes to G. platensis, allowed to evaluate how host quality affects the survival, development, and reproduction of this insect, and can be replicated for other pest species of economic importance.
The information can be used to manage G. platensis, by avoiding extensive plantations with susceptible species, and reducing the risks of population outbreaks in commercial plantations.