Lyophilized cell-free supernatants of Lactobacillus isolates exhibited antibiofilm, antioxidant, and reduces nitric oxide activity in lipopolysaccharide-stimulated RAW 264.7 cells
- Published
- Accepted
- Received
- Academic Editor
- Mohd Adnan
- Subject Areas
- Biochemistry, Cell Biology, Microbiology
- Keywords
- Lactobacillus, Cell-free supernatants, Antibiofilm, Antioxidant, Anti-inflammatory, Postbiotics
- Copyright
- © 2021 Sornsenee et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Lyophilized cell-free supernatants of Lactobacillus isolates exhibited antibiofilm, antioxidant, and reduces nitric oxide activity in lipopolysaccharide-stimulated RAW 264.7 cells. PeerJ 9:e12586 https://doi.org/10.7717/peerj.12586
Abstract
Background
Probiotics can release bioactive substances known as postbiotics, which can inhibit pathogenic microorganisms, improve immunomodulation, reduce antioxidant production, and modulate the gut microbiota.
Methods
In this study, we evaluated the in vitro antimicrobial effects, antioxidant activity, and anti-inflammatory potential of 10 lyophilized cell-free supernatants (LCFS) of Lactobacillus isolates. LCFS was obtained via centrifugation and subsequent lyophilization of the supernatant collected from the culture medium ofeach isolate. The antibacterial and antibiofilm activities of the LCFS were determined using broth microdilution. The antioxidant potential was evaluated by measuring the total phenolic and flavonoid contents and 2,2-Diphennyl-1-picrylhydrazyl (DPPH) and 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical cation (ABTS+) radical scavenging activities.
Results
All the isolates were able to inhibit the four tested pathogens. The isolates exhibited strong antibiofilm activity and eradicated the biofilms formed by Acinetobacter buamannii and Escherichia coli. All the prepared Lactobacillus LCFS contained phenols and flavonoids and exhibited antioxidant activities in the DPPH and ABTS+ radical scavenging assays. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay revealed that LCFS was not cytotoxic to RAW 264.7 cells. In addition, the ten Lactobacillus LCFS decreased the production of nitric oxide.
Conclusions
All the isolates have beneficial properties. This research sheds light on the role of postbiotics in functional fermented foods and pharmaceutical products. Further research to elucidate the precise molecular mechanisms of action of probiotics is warranted.
Introduction
The term “probiotics” refers to living or dead microorganisms that confer health benefits to a host when administered in adequate amounts (Hotel & Cordoba, 2001). Probiotic microorganisms exert their benefits through two mechanisms: direct effects on living cells and indirect effects involving the production of several metabolites (Vinderola et al., 2007). The most frequently used probiotic microorganisms are lactic acid bacteria (LAB) such as Lactobacillus spp., Lactococcus spp., Carnobacterium spp., Enterococcus spp., Streptococcus spp., Pediococcus spp., and Propionibacterium spp. (Pinto et al., 2020; Sornsenee et al., 2021). Generally, Lactobacillus spp. are the most popular probiotic microbes owing to their “Generally Recognized As Safe (GRAS)” status and their regulation by the US Food and Drug Administration (FDA) for human and animal consumption (FAO/WHO, 2002; Sornsenee et al., 2021). For example, L. acidophilus CL1285, L. casei LBC80R, L. rhamnosus CLR2 (Bio-K Plus International Inc, Laval, Quebec, Canada), L. acidophilus (La-5®), and Bifidobacterium lactis (BB-12®) (Pharma Nord, Nederland), have been used as probiotics in pharmaceutical and diet supplements (Organization, 2002).
The beneficial effects of Lactobacillus as probiotics are not limited to the health of the gastrointestinal tract (GIT) and extend to conditions such as diabetes, obesity, hyperlipidemia, cancer, dementia, Crohn’s disease, and constipation (Plaza-Diaz et al., 2019). Probiotics produce organic acids (acetic acid, propionic acid, and lactic acid), aromatic compounds, diacetyl, hydrogen peroxide, antimicrobial substances, bacteriocins, and other unknown metabolites (Barzegari et al., 2020; Bermudez-Brito et al., 2012; Cremon et al., 2018) that can inhibit several pathogens such as Clostridium difficile (Shahrokhi & Nagalli, 2020), Vibrio parahaemolyticus (Behera, Ray & Zdolec, 2018), carbapenem-resistant Escherichia coli (Chen et al., 2019), Klebsiella pneumoniae (Chen et al., 2019), Listeria monocytogenes (Kariyawasam et al., 2020), Staphylococcus aureus (Melo et al., 2016), Salmonella enteritidis (Sornsenee et al., 2021), and Helicobacter pylori (Ji & Yang, 2021). Probiotics can lower cholesterol levels, boost the immune system, promote the secretion of immunoglobulin IgA, serve as antioxidants, exhibit antidiabetic properties, and suppress inflammation (AlKalbani, Turner & Ayyash, 2019; De Marco et al., 2018; Singhal et al., 2019; Xu et al., 2021). Several studies have shown that Lactobacillus can inhibit biofilm formation by many pathogens (Carvalho et al., 2021; Gómez et al., 2016; Ji & Yang, 2021; Shaaban et al., 2020). Other reports have shown that metabolites produced by probiotics have antivirulence activity (Stefania et al., 2017).
Members of the genus Lactobacillus are gram-positive bacteria, aerotolerant anaerobes or microaerophilic, rod-shaped, and non-spore-forming, with low DNA G+C content (Klaenhammer et al., 2005). This genus comprises 261 species as of March 2020, with extreme diversity at phenotypic, ecological, and genotypic levels (Zheng et al., 2020). We previously identified 10 Lactobacillus isolates from fermented palm sap collected from a local market in the Songkhla Province of Southern Thailand. All Lactobacillus isolates met the established criteria to qualify as potential probiotics, including resistance to gastrointestinal conditions, adherence to human intestinal cells, and susceptibility to transmissible antibiotics. These isolates possessed antimicrobial activity against a wide range of pathogens (Sornsenee et al., 2021). From these data, 10 Lactobacillus isolates are promising potential candidates for use as probiotic applications as functional foods and pharmaceutical products. However, we still lack information about the antibiofilm, antioxidant, and anti-inflammatory activities of Lactobacillus isolates. Thus, the present work aimed (i) to evaluate the antibacterial and antibiofilm activities of lyophilized cell-free supernatants (LCFS) of Lactobacillus against pathogens, (ii) to evaluate the total phenolic and flavonoid contents and free-radical-scavenging activities, and (iii) to evaluate the toxicity of the cell-free supernatants (CFS) and their anti-inflammatory activity using RAW 264.7 cells.
Materials & Methods
Microorganisms and culture conditions
Ten Lactobacillus isolates, including L. paracasei (T0601, T0602, T0603, T0901, T0902, T1301, T1304, and T1901), L. fermentum (T0701), and L. brevis (T0802), were isolated from fermented palm sap collected from a local market in the Songkhla Province of Southern Thailand and characterized as potential probiotics in our previous study. These isolates were used in the present study (Sornsenee et al., 2021). First, they were grown in de Man, Rogosa and Sharpe (MRS) broth (HiMedia, Mumbai, India) at 37 °C for overnight. After that, all isolates were stored at −80 °C in 30% (v/v) glycerol (Sigma, Steinheim, Germany).
Three reference strains, E. coli DMST4212, A. baumannii DMST 2271, and S. aureus DMST 2928, obtained from the Department of Medical Sciences Thailand (DMST), were used in this study. One clinical isolate, methicillin-resistant S. aureus (MRSA), was identified using matrix-assisted laser desorption ionization time-of-flight mass spectrometry/MS mass spectrometry. These strains were cultured on trypticase soy (TSA) agar (HiMedia, Mumbai, India), and the agar plates were incubated at 37 °C for 18 h under aerobic conditions. The colonies were transferred to trypticase soy broth (HiMedia, Mumbai, India) and incubated at 37 °C for 18 h. Each strain was stored at −80 °C in brain heart infusion broth with 30% glycerol until further use.
Preparation of CFS
CFS were prepared according to Melo et al. (2016) with slight modifications. Briefly, each Lactobacillus isolate was cultured in 100 mL of MRS broth and incubated at 37 °C for 18 h under anaerobic conditions. The supernatant was obtained by centrifugation (×6, 000 g, 10 min, 4 °C). The centrifuged supernatant was passed through a sterile 0.22 µm-pore-size filter unit (Sigma, Steinheim, Germany). The filtrate was collected for freeze-drying.
Lyophilization
CFS of each Lactobacillus isolate and MRS medium without Lactobacillus (MRS control) were frozen at −80 °C for 24 h. The samples were lyophilized (Lyophilization Systems, Inc, USA) from −40 ° C to −30 °C, 0.2 mbar. The entire freeze-drying process was performed in 24 h, and the freeze-dried powders were stored at −20 °C. They were then rehydrated with sterile deionized water prior to use.
Determination of minimum inhibitory concentration (MIC) and minimal bactericidal concentration (MBC)
The antibacterial activities of each LCFS against the four pathogenic bacteria were assessed using the method of microdilution in 96-well plates according to the Clinical and Laboratory Standards Institute (CLSI) 2021 guidelines (CLSI, 2021). Serial dilution was performed starting with 100 mg/mL of lyophilized CFS of Lactobacillus in Mueller Hinton broth (MHB) (HiMedia, Mumbai, India). The bacterial suspension (5 × 105 CFU/mL) was inoculated into each well, and the plates were incubated at 37 °C for 18 h. Then, resazurin (Sigma, Steinheim, Germany) was used to determine the MIC values. The MIC was defined as the lowest concentration that completely inhibited the bacterial growth, which presented as a blue color (Hussain et al., 2011). The MBC was determined using the extract that yielded significant MIC values by dropping the culture onto TSA plates. The entire experiment was performed three times with three independent repetitions.
Biofilm inhibition assay
The effects of LCFS of Lactobacillus on biofilm formation of E. coli DMST4212 and A. baumannii DMST 2271 were performed following a method that was modified from published by Yang et al. (2021). Briefly, overnight cultures of E. coli DMST4212 and A. baumannii DMST 2271 were suspended in MHB to a cell density of 5 × 105 CFU/mL and then inoculated into 96-well plates supplemented with 1 × MIC and 2 × MIC of CFS of Lactobacillus. The plates were incubated at 37 °C for 24 h under aerobic conditions. Then, the medium was removed, the biofilms were washed with phosphate-buffered saline (PBS) (pH 7.4) three times, and fixed with 99% (v/v) methanol (200 µL) for 15 min. The biofilm was stained with 0.1% (w/v) crystal violet solution (200 µL) for 10 min. The wells were rinsed four times with distilled water to remove excess dye. The biofilms were dissolved in 95% (v/v) ethanol and absorbance was measured at an optical density (OD) of 570 nm. Each test was performed in triplicate. The percentage of biofilm inhibition was calculated using the following equation:
Biofilm inhibition (%) = [(OD 570 of control well − OD 570 of treated well)/OD 570 of control well] × 100.
Biofilm eradication assays
The effects of LCFS of Lactobacillus on the eradication of biofilms produced by E. coli DMST4212 and A. baumannii DMST 2271 were tested according to reported procedures of Perumal & Mahmud (2013) with slight modifications. Briefly, an overnight culture of each E. coli DMST4212 and A. baumannii DMST 2271 was added to a 96-well microtiter plate and incubated at 37 °C for two days to allow the development of a biofilm. Then, the wells were rinsed with PBS (pH 7.4) to remove non-adherent cells. The biofilms established for two days in each well were subsequently treated with 1 × MIC and 2 × MIC of CFS of Lactobacillus and incubated at 37 °C for 24 h. After incubation, the plates were removed, gently washed with PBS three times, and stained with 0.1% (w/v) crystal violet solution, as described previously, to determine the extent of biofilm inhibition. Each test was performed in triplicate. The percentage of biofilm eradication was calculated using the following equation:
Biofilm eradication (%) = [(OD 570 of control well − OD 570 of treated well)/OD 570 of control well] ×100.
Determination of antioxidant activity
Total phenolic content (TPC) assay
The Folin–Ciocalteu method was used to determine TPC, as described by Chatatikun et al. (2020) with some modifications. Briefly, LCFS of Lactobacillus was diluted in distilled water to a concentration of 50 mg/mL. Subsequently, 100 µL of 0.1 M Na2CO3 solution and 100 µL of 10% Folin–Ciocalteu reagent (Sigma-Aldrich, St. Louis, USA) were mixed in a well of a 96-well plate and incubated for 1 h. The absorbance was measured at 750 nm. A standard curve was plotted using gallic acid with a concentration range of 1.569–200 µg/mL. TPC was determined as gallic acid equivalents (GAE) in mg/g of lyophilized CFS of Lactobacillus.
Total flavonoid content (TFC) assay
The TFC of the LCFS of Lactobacillus was determined using the aluminum chloride colorimetric method (Chatatikun et al., 2020). Briefly, 100 µL CFS of Lactobacillus or quercetin (1.56–100 µg/mL) was incubated with 100 µL of 2% AlCl3 solution in methanol for 30 min at room temperature, and the absorbance was measured at 415 nm. The TFC was calculated from a calibration curve, and the result was expressed as mg quercetin equivalents (QE) per g of lyophilized CFS of Lactobacillus.
2,2-Diphennyl-1-picrylhydrazyl (DPPH) radical scavenging activity
The free-radical-scavenging activities of LCFS of Lactobacillus were measured using the DPPH assay with Trolox (Sigma-Aldrich, St. Louis, MI, USA) as the standard. This assay was performed according to the procedure previously described by Chatatikun et al. (2020) with some modifications. Briefly, 1,000 µg/ml of CFS of Lactobacillus (20 µL) or 1.56 to 100 µg/ml ascorbic acid standard in absolute ethanol was added to 180 µL of DPPH working solution. Then, the mixture was shaken and incubated in the dark for 30 min. The absorbance was read at 517 nm against a blank. The assays were done in triplicate. The DPPH scavenging activity was calculated using the following equation:
% Scavenging activity = 100 × (Abs of control − (Abs of sample − Abs of blank))/Abs of control. IC50, the concentration resulting in 50% inhibition of DPPH, was determined from a graph of free-radical-scavenging activity.
ABTS+ radical scavenging activity
ABTS*+ is generated by oxidation with a strong oxidizing agent (potassium persulfate). The reduction of a blue–green color of ABTS*+ free radical by donating hydrogen antioxidants from LCFS is determined by the decrease of its absorbance. The ABTS+ radical scavenging activity of LCFS of Lactobacillus was evaluated using an ABTS decolorization assay as published by Chatatikun et al. (2020) with modifications. Briefly, ABTS+ was produced by mixing 7 mM ABTS and 2.45 mM potassium sulfate at a ratio of 2:3 (v/v). The ABTS+ was stored in the dark at room temperature for 15 h until it was used. The ABTS+ solution was diluted with methanol to reach an absorbance of 0.70 ± 0.02. Then, 20 µL of CFS of Lactobacillus were mixed with 180 µL of ABTS+ solution and incubated for 45 min. The assays were done in triplicate. The percent inhibition of absorbance at 734 nm was calculated using the following equation:
% Scavenging activity = 100 × (Abs of control − (Abs of sample − Abs of blank))/Abs of control. IC50 was determined as the concentration resulting in 50% inhibition of ABTS+
Determination of anti-inflammatory activity
Cell culture
RAW 264.7 cells, a mouse macrophage cells were kindly provided by Assoc. Prof. Dr. Potchanapond Graidist, Department of Biomedical Sciences and Biomedical Engineering, Faculty of Medicine, Prince of Songkla University, Hatyai, Songkhla, Thailand. RAW 264.7 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Thermo Fisher Scientific, NY, USA) with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin solution (Gibco, Thermo Fisher Scientific) at 37 °C in 5% CO2. The RAW 264.7 cells were subcultured and plated at 80%–90% confluency.
Cell viability assays
MTT assays were performed to assess the effect of LCFS of Lactobacillus on the viability of RAW 264.7 cells with modifications (Khanna et al., 2020). Briefly, RAW 264.7 cells were seeded onto 96-well microplates at 1 × 105 cells/mL and incubated at 37 °C in a 5% CO2 incubator for cytotoxicity assays. The cells were then treated with CFS from Lactobacillus and incubated at 37 °C for 16 h. After incubation, supernatants were discarded and the cells were washed with PBS. A volume of 50 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) solution (Sigma, MO, USA) (0.5 mg/mL in DMEM) was added to each well and incubated for 4 h in the dark after removing the treatment mixture from each well. The formazan crystals were dissolved by adding 100 µL of dimethylsulfoxide (DMSO) solution (Sigma, MO, USA). The OD was measured at 570 nm using a microplate reader. The experiment was repeated three times with triplicate samples. The percentage of cell viability was calculated using the following equation:
% cell viability = (OD of test/OD of untreated control) × 100
Nitric oxide assays
To evaluate their anti-inflammatory activity, the LCFS of Lactobacillus were tested for their ability to reduce lipopolysaccharide (LPS)-induced nitric oxide (NO) generation in RAW 264.7 cells according to the method of Khanna et al. (2020) with slight modifications. Briefly, RAW 264.7 cells were seeded in a 24-well microplate and treated with 96.52 µg/L of LCFS of Lactobacillus with or without 1 µg/ml of LPS (Sigma-Aldrich, St. Louis, USA). RAW 264.7 cells treated with 1 µg/ml of LPS alone were used as the positive control. After 24 h of incubation at 37 °C in 5% CO2, the nitric oxide production was measure by treating the supernatant with an equal volume of Griess reagent (Sigma-Aldrich, St. Louis, USA). The OD was measured at 570 nm using a microplate reader. Each test was performed in triplicate.The concentration of nitric oxide production was calculated using the following equation:
Nitric oxide production = (OD of test/OD of standard) × concentration of standard
Statistical analysis
Data are expressed as mean ± standard error calculated over three independent experiments performed in triplicate. Statistical significance was calculated using One-way ANOVA followed by Tukey’s post-hoc test. p < 0.05 was considered as significant. GraphPad Prism version 9 software was used for all analysis.
Results
Determination of MIC and MBC
The antibacterial activities of the LCFS of Lactobacillus against the four pathogenic bacteria were determined using a broth microdilution assay. As shown in Table 1, the 10 LCFS of Lactobacillus showed strong antibacterial activity and inhibited E. coli DMST4212, A. baumannii DMST 2271, S. aureus DMST 2928, and MRSA with MIC values in the range of 25–50 mg/mL. The MBC values of these LCFS of Lactobacillus were >100 mg/mL. The LCFS of Lactobacillus T0902, T1301, and T1304 did not inhibit S. aureus DMST 2928 or MRSA.
| Isolates | Antimicrobial activity (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | MRSA | E. coli | A. baumannii | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| T0601 | 50 | >100 | 50 | >100 | 25 | >100 | 25 | >100 |
| T0602 | 25 | >100 | 25 | >100 | 25 | >100 | 25 | 100 |
| T0603 | 25 | >100 | 25 | >100 | 25 | >100 | 25 | >100 |
| T0701 | 25 | >100 | 50 | >100 | 50 | >100 | 25 | >100 |
| T0802 | 25 | >100 | 25 | >100 | 50 | >100 | 50 | >100 |
| T0901 | 25 | >100 | 25 | >100 | 25 | >100 | 25 | >100 |
| T0902 | ND | ND | ND | ND | 25 | >100 | 25 | >100 |
| T1301 | ND | ND | ND | ND | 25 | >100 | 25 | >100 |
| T1304 | ND | ND | ND | ND | 25 | >100 | 25 | >100 |
| T1901 | 25 | >100 | 25 | >100 | 50 | >100 | 25 | >100 |
Notes:
This test was performed in triplicate.
- ND
-
Not detectable
- MRSA
-
Methicillin-resistant S. aureus
Reduction of biofilm formation in A. baumannii and E. coli by LCFS of Lactobacillus
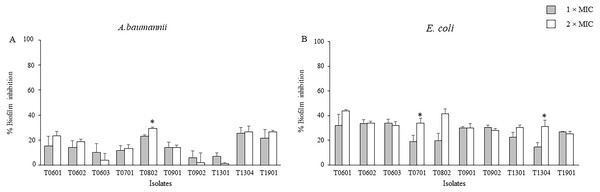
The inhibitory activities of the LCFS of Lactobacillus against biofilm formation by A. baumannii and E. coli were determined using the crystal violet assay. As shown in Fig. 1 and Table S1, the concentration of the CFS tested significantly inhibited biofilm formation by E. coli when compared with the control. At 2 × MIC, the CFS produced by the isolates T0601 and T0802 exhibited the highest inhibition (mean ± standard deviation) of 43.86% ± 1.15% and 41.35% ± 4.19%, respectively, against E. coli biofilm (Table S1). It has been highlighted that at 2 × MIC of the supernatant of the probiotics T0701 and T1304 significantly inhibited E. coli biofilm formation, compared with the concentration at 1 × MIC. The isolate T0802 also exhibited the highest inhibition of 29.33% ± 1.15% against A. baumannii biofilm. A significant difference in inhibition was observed when the bacteria were treated with 2 × MIC of CFS produced by the isolate T0802 when compared with 1 × MIC of the CFS. It has noticed that antibiofilm activity of the supernatant of the probiotics against both S. aureus and MRSA was performed. However, the results demonstrated that the supernatant did not inhibit the biofilms of both the strains.
Figure 1: Effects of the lyophilized cell-free supernatants of Lactobacillus on the inhibition of biofilm formation by A. baumannii (A) and E. coli (B).
The pathogens were grown in a medium supplemented with the cell-free supernatants (CFCs) at different concentrations. CFS-free medium was used as the negative control. The relative percentage of biofilm inhibition was defined as follows: [100 − (mean A570 of treated well/mean A570 of control well) × 100]. The percent inhibition of each datum was compared with its negative control. The data are presented as mean ± standard deviation (* significant difference; P < 0.05).Activity of LCFS on the eradication of the established biofilms of A. buamannii and E. coli
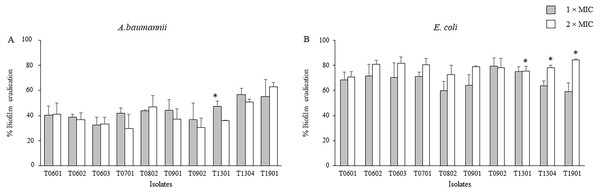
The activity of the LCFS of Lactobacillus on the established biofilms of A. baumannii and E. coli was assessed using the crystal violet assay. As shown in Fig. 2 and Table S2, a significant decrease in the viability of mature two-day-old biofilm-grown cells of both A. baumannii and E. coli was observed after treatment with the LCFS of Lactobacillus at 2 × MIC and 1 × MIC when compared with the negative control (P < 0.05). The CFS from the isolate T1901 resulted in the highest eradication of 62.98% ± 3.54% and 84.34% ± 0.98% of the established biofilm of A. baumannii and E. coli, respectively. A significant difference in the eradication was observed when the bacterial cells were treated with 2 × MIC of CFS produced by the isolate T1901 when compared with 1 × MIC of the CFS.
Figure 2: Effects of the lyophilized cell-free supernatants (LCFS) of Lactobacillus on the inhibition of the established biofilms of A. baumannii (A) and E. coli (B).
The bacteria were grown in a medium supplemented with glucose to produce established biofilms. The established biofilms were treated with LCFS of Lactobacillus at different concentrations. Cell-free supernatant-free medium was used as the negative control. The relative percentage of biofilm eradication was defined as follows: [100 − (mean A570 of treated well/mean A570 of control well) × 100]. The percent inhibition of each datum was compared with its negative control. The data are presented as mean ± standard deviation (* significant difference; P < 0.05).Antioxidant activity of LCFS from Lactobacillus
The antioxidant activities of all isolates were evaluated by measuring the TPC, TFC, DPPH radical scavenging activity, and ABTS+ radical scavenging activity (Figs. 3 and 4).
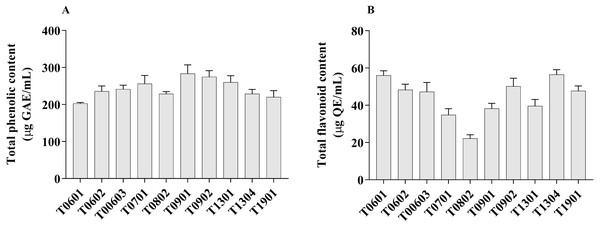
Figure 3: Total phenolic content and total flavonoid content of lyophilized cell-free supernatant of Lactobacillus.
The TPC value of the LCFS of Lactobacillus ranged from 202.7 ± 1.42 µg GAE/g to 283.4 ± 11.91 µg GAE/g (Fig. 3A). LCFS of L. paracasei T0901 showed the highest TPC value (283.4 ± 11.91 µg GAE/g), followed by LCFS of L. paracasei T0902 (274.7 ± 8.34 µg GAE/g) and LCFS of L. paracasei T1302 (260.3 ± 8.69 µg GAE/g).
Values of TFC were determined in mg QE/g of lyophilized CFS of Lactobacillus. The TFC value of the LCFS ranged from 22.26 ± 0.94 µg QE/g to 56.60 ± 1.34 µg QE/g (Fig. 3B). LCFS of L. paracasei T1304 showed the highest TFC value (56.60 ± 1.34 µg QE/g), followed by LCFS of L. paracasei T0601 (56.03 ± 1.23 µg QE/g) and LCFS of L. paracasei T0902 (50.19 ± 2.15 µg QE/g).
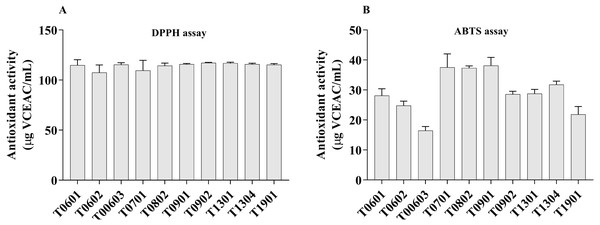
The DPPH radical and ABTS+ radical scavenging activities were used as a tool to investigate the antioxidant properties of the 10 LCFS Lactobacillus isolates (Fig. 4A). The results showed that all the isolates had antioxidant property.
Figure 4: Scavenging activity of lyophilized cell-free supernatant (LCFS) of Lactobacillus isolates, as determined by DPPH assay (A); ABTS radical scavenging activity of LCFS of Lactobacillus isolates (B).
Values are mean ± standard error of the mean of three replicates.The LCFSs of L. paracasei T0902 exhibited strong DPPH radical scavenging activities (117.2 ± 0.26 µg VCEAC/mL), followed by LCFS of L. paracasei T1301 (116.8 ± 0.53 µg VCEAC/mL) and LCFS of L. paracasei T1304 (115.9 ± 0.47 µg VCEAC/mL). This difference was not statistically significant (p > 0.05). The antioxidant activity (ABTS) of all LCFS of L. paracasei isolates ranged from 16.46 ± 0.67 µg VCEAC/mL to 38.1 ±1.37 µg VCEAC/mL. All of these LCFS were significantly different from each other. The LCFS of L. paracasei T0902 displayed the highest ABTS+ radical scavenging activity (38.1 ± 1.37 µg VCEAC/mL), followed by LCFS of L. fermentum T0701 (37.51 ± 2.25 µg VCEAC/mL) and LCFS of L. brevis T0802 (37.32 ± 0.34 µg VCEAC/mL), which were not significantly different from LCFS of L. paracasei T0902.
Cell viability by MTT assay
We evaluated the cytotoxicity of the 10 LCFS of Lactobacillus isolates in RAW 264.7 cells using MTT assays. None of these isolates produced any significant cytotoxicity in the concentration range of 5.00–118.80 mg/mL (Fig. S1). Thus, the LCFS was considered to be safe and was evaluated further.
NO production
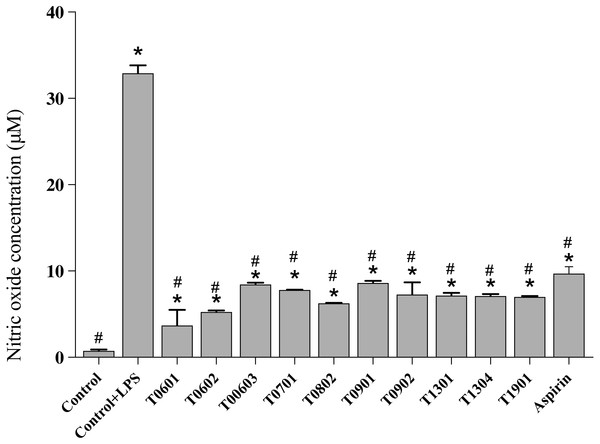
NO is a multifunctional mediator and plays a pivotal role in the immune response to inflammation. Results of the NO assay (Fig. 5) established that the LCFS of Lactobacillus showed a wide range of NO production levels. All of these isolates reduced the NO production to <10 µM (4.17 ± 1.61–8.66 ±0.23 µM) in LPS-stimulated RAW 264.7 cells when compared with untreated LPS-stimulated RAW 264.7 cells (39.89 ± 0.91 µM). Among the isolates, LCFS of L. paracasei T0601 exhibited the lowest NO production (4.17 ± 1.61 µM) in LPS-stimulated RAW 264.7 cells, followed by LCFS of L. paracasei T0602 (5.17 ± 0.05 µM) and LCFS of L. brevis T0802 (6.24 ± 0.04 µM). The NO production of aspirin-treated LPS-stimulated RAW 264.7 cells was 10.06 ± 0.50 µM and was not significantly different from that of the LCFS of Lactobacillus treated LPS-stimulated RAW 264.7 cells.
Figure 5: Inhibition of nitric oxide production in the lipopolysaccharide-stimulated RAW264.7 cells treated with the 10 lyophilized cell-free supernatants of Lactobacillus isolates and aspirin as control.
The results are presented as the mean ± standard deviation of three independent experiments (n = 3). * and # letters shown in the column indicate significant differences (p < 0.05) when compare with control, and control stimulated-LPS, respectivly.Discussion
Probiotics are living microorganisms that confer health benefits to the host when administered in adequate amounts. Moreover, dead bacteria, inactivated bacteria, and bacterial components can also display probiotic properties (Plaza-Diaz et al., 2019). Probiotics are safe, survive in the gastrointestinal tract, produce active molecules that inhibit pathogens, stimulate the immune system, and aid in the improvement of intestinal barrier function and microflora (De Marco et al., 2018; Plaza-Diaz et al., 2019). In our previous report (Sornsenee et al., 2021), 10 lactobacilli isolated from fermented palm sap serve as promising candidates for probiotics since they exhibit potential probiotic properties. Probiotic microorganisms, especially Lactobacillus species, are used as dietary supplements and capsules and in probiotic foods, beverages, and probiotic juices (Saxelin et al., 2005). Commercial Lactobacillu s strains include L. acidophilus NCFM, L. acidophilus La-5, L. casei Shirota, L. casei DN-114 001, L. reuteri DSM 17938, L. rhamnosus GG, L. rhamnosus HN001, L. rhamnosus GR-1, L. paracasei F19, and L. plantarum 299v (Delley et al., 2015; Tremblay et al., 2021). Some Lactobacillus spp. are GRAS by the European Food Safety Authority (EFSA) and FDA (Ogier & Serror, 2008; Plaza-Diaz et al., 2019). The effects of these probiotics on host health have been reported in many studies (Barzegari et al., 2020; Chatatikun et al., 2020; Hotel & Cordoba, 2001; Saxelin et al., 2005; Stefania et al., 2017). Dead bacteria, metabolic by-products, and bacterial molecular components have also been shown to exhibit probiotic effects in various studies (De Marco et al., 2018; Yang et al., 2021). Currently, the term “postbiotic” refers to soluble components with biological activity that could be a safer alternative to the use of whole bacteria (Tsilingiri et al., 2012).
Antimicrobial susceptibility tests showed that all LCFS of Lactobacillus isolates had strong inhibitory effects on the four tested pathogens: E. coli DMST4212, A. baumannii DMST 2271, S. aureus DMST 2928, and MRSA. According to the results of MIC and MBC assays, the MBC/MIC ratio was more than four times that considered to be valuable as a bacteriostatic agent (Levison, 2004). Thus, these LCFS of Lactobacillus isolates are potential antibacterial agents. Our results agree with those of previous studies; for example, Melo et al. (2016) reported that Lactobacillus supernatants inhibited S. aureus. Other reports have shown that the lyophilized cell-free extract of L. casei can inhibit E. coli, Salmonella typhi, Pseudomonas aeruginosa, S. aureus, and MRSA (Saadatzadeh et al., 2013). Lactobacilli can produce various secondary metabolites that exhibit antimicrobial activity, such as organic acids, ethyl alcohol, short-chain fatty acids, bacteriocins, hydrogen peroxide, surfactants, and bacteriocins (Melo et al., 2016; Plaza-Diaz et al., 2019).
Biofilm-related infections are a serious clinical problem and include chronic infections. Since biofilms are not fully available to the human immune system or antibiotics, they are difficult to eradicate and control, which leads to the emergence of antibiotic-resistant strains (Barzegari et al., 2020; Khairy et al., 2020). The present study revealed that all LCFS of Lactobacillus isolates were able to not only inhibit pathogen biofilm formation but also eradicate mature biofilms of E. coli DMST4212 and A. baumannii DMST 2271. Probiotics can interrupt the activity of pathogens and their adhesion to surfaces. Probiotics prevent quorum sensing and biofilm formation, interfere with biofilm integrity, and eradicate biofilms by secreting antagonistic substances (Plaza-Diaz et al., 2019). These data, according to Kim, Kim & Kang (2019), showed that L. brevis DF01 bacteriocin can inhibit the formation of biofilms by E. coli and S. typhimurium. Other study from Rossoni et al. (2018) reported that L. fermentum 20.4, L. paracasei 11.6, L. paracasei 20.3, and L. paracasei 25.4 produce bioactive substances that caused a significant reduction in S. mutans biofilms. Furthermore, the result similar to report from Carvalho et al. (2021), L. plantarum showed promising results against pathogenic biofilms. Some of the bacteriocins eradicate biofilms by inducing the formation of pores on the bacterial cell surface, which leads to ATP efflux, while others exert their biological activity through proteolytic enzymes (Okuda et al., 2013). We consider all LCFS of Lactobacillus isolates to be potentially applicable for reducing the formation of biofilms and for eradicating the established biofilms of E. coli and A. baumannii.
The isolates have desirable properties as potential probiotics. During fermentation, lactobacilli can produce phenolic and flavonoid compounds as end products. The increase in the production of these compounds during the enzymatic hydrolysis of lactobacilli during fermentation leads to an increase in their antioxidant activities (Filannino et al., 2015). In this study, we investigated the total phenolic and flavonoid contents of the LCFS of Lactobacillus isolates. All isolates contained high levels of these compounds. These findings agree with those of Talib et al. (2019) who reported that Lactobacillus spp. showed high antioxidant activities for TPC and TFC. Another study found that L. plantarum can produce high levels of phenolic compounds during fermentation (Xiao et al., 2015). The LCFS of Lactobacillus isolates exhibited strong DPPH and ABT.+ radical scavenging activities. Several probiotics can enhance the activity of antioxidant enzymes or modulate circulatory oxidative stress (Mishra et al., 2015). The CFS of L. acidophilus, L. casei, Lactococcus lactis, L. reuteri, and Saccharomyces boulardii could reduce oxidative damage and free-radical-scavenging rate (De Marco et al., 2018). Liu & Pan, 2010) documented that 12 Lactobacillus strains showed varying capabilities of DPPH radical scavenging. Thus, these results suggest that phenolics and flavonoids are the major compounds responsible for the antioxidant activities.
Inflammation is the mark of many inflammatory disorders such as chronic peptic ulcer, Crohn’s disease, and infections. The intestinal immune system has developed distinct mechanisms to dampen mucosal immunity and to optimize the response against microbiota. NO is a multifunctional mediator and plays an essential role in the immune response to inflammatory activity. Normal NO production in the phagocytes is beneficial for host defense against pathogens and cancer cells (Abdulkhaleq et al., 2018). Proinflammatory cytokines are commonly induced by the LPS cell-wall component of gram-negative bacteria. In this study, the LCFS of Lactobacillus isolates showed low levels of NO production. The supernatant did not exhibit any cytotoxic activity against the RAW 264.7 cells. Recently, there have been a few studies on the anti-inflammatory activity of the CFS of probiotics. Kang et al. (2021) observed that Bifidobacterium bifidum MG731, B. lactis MG741, and L. salivarius MG242 showed low NO production. In another report, the CFS of L. acidophilus and L. rhamnosus GG showed anti-inflammatory properties and modulated the inflammatory response (Maghsood et al., 2018). Thus, reduced NO production by the LCFS of Lactobacillus isolates may be due to the downregulation of inducible NO synthase, the main mediator of various chronic inflammatory diseases (Oh et al., 2012).
Exploiting the LCFS of Lactobacillus isolates in the preparation of probiotic products is an innovative approach and has the potential to replace the living probiotic cells.
Conclusions
The present study revealed that the 10 LCFS of Lactobacillus isolates exhibited antibacterial activity, reduced the formation of biofilms, and eradicated the established biofilm. These supernatants contain phenolic and flavonoid compounds and display antioxidant and anti-inflammatory activities in RAW 264.7 cells. Therefore, they are promising novel postbiotic candidates for use in functional foods and pharmaceuticals. Further research to elucidate the precise molecular mechanisms of action of probiotics is warranted.
Supplemental Information
Toxicity of probiotic on RAW 264.7 cells
The 50% lethal concentration, (LC50) and 5% lethal concentration, (LC5) value of 10 LCFS of Lactobacillus isolates on 264.7 cells.