Wnt signaling related transcripts and their relationship to energy metabolism in C2C12 myoblasts under temperature stress
- Published
- Accepted
- Received
- Academic Editor
- Emiliano Maiani
- Subject Areas
- Agricultural Science, Cell Biology, Genomics, Molecular Biology
- Keywords
- C2C12, Energy metabolism, Glycolysis, Oxidative phosphorylation, Heat/cold stress, Wnt signalling, Transcripts
- Copyright
- © 2021 Risha et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Wnt signaling related transcripts and their relationship to energy metabolism in C2C12 myoblasts under temperature stress. PeerJ 9:e11625 https://doi.org/10.7717/peerj.11625
Abstract
Temperature stress is one of the main environmental stressors affecting the welfare, health and productivity of livestock. Temperature changes can modify cell membrane components, disrupting the crosstalk between the cell and its surroundings by affecting signaling pathways including Wnt signaling pathway, which subsequently disrupts cell energy metabolism. The present study aims to understand the effect of temperature stress on the expression of genes involved in Wnt signaling pathways, and their interaction with energy metabolism in C2C12 myoblasts cells. The C2C12 cells were exposed to cold stress (35 °C), mild heat stress (39 °C) and severe heat stress (41 °C), whereas 37 °C was used as control temperature. Transcript levels of important genes involved in Wnt signaling including Axin2, Tnks2, Sfrp1, Dkk1, Dact1, Cby1, Wnt5a, Wnt7a, Wnt11, Porcn, Ror2, Daam1, and Ppp3ca were significantly altered under severe heat stress (41 °C), whereas eight Wnt signaling-related transcripts (Daam1, Ppp3ca, Fzd7, Wnt5a, Porcn, Tnks2, Lrp6, and Aes) were significantly altered under cold stress (35 °C) compared to control. Under heat stress transcripts of the Wnt/β-catenin inhibitors (Sfrp1, Dkk1, and Cby1) and negative regulators (Dact1 and Axin2) are activated. A positive correlation between oxidative phosphorylation and Wnt-related transcripts was found under high temperatures. Transcripts of the cell membrane receptors, including Lrp6 and Fzd7, and the members of Wnt/Ca+2 signaling pathway, including Ppp3ca and Porcn were downregulated under cold stress. Many Wnt signaling-related transcripts were positively correlated with glycolysis under cold stress. These findings indicate a cross-talk between Wnt signaling and energy metabolism under thermal stress.
Introduction
Temperature fluctuations are among the environmental stresses that cause many changes in living cells and negatively affect animal production in many aspects. Heat stress negatively impacts meat quality (Zaboli et al., 2018; Imik et al., 2012), animal growth (St-Pierre, Cobanov & Schnitkey, 2003), skeletal muscle hypertrophy (Frier & Locke, 2007). It also changes the cellular metabolism (Zhao et al., 2018) and interferes with cellular signaling (Ganesan et al., 2018; Ganesan et al., 2017). On the contrary, mild heat stress can positively impact the healing of injured muscles (Choi et al., 2016). A previous study showed transcriptional changes under thermal stress (Reed et al., 2017). They showed that cold temperature challenge to satellite cells alters the expression of genes involved in cell signalling/signal transduction, whereas high temperature changes the expression of genes related to muscle system development and cell differentiation (Reed et al., 2017).
Temperature fluctuations trigger a response mechanism in the cells, which is believed to start from the membrane (Török et al., 2014). Temperature changes can lead to modifications of membrane components (Ayuyan & Cohen, 2008), particularly in the regions that play an important role in cell signaling (Mollinedo & Gajate, 2015). Wnt signaling is one of these important pathways. The activation of Wnt signaling requires special receptors on the cell membrane, and Wnt ligands (Driehuis & Clevers, 2017).The main element to transmit canonical Wnt signaling is related to LRP6 phosphorylation (Ozhan et al., 2013), and previous studies confirmed that the phosphorylation occurs in the microdomains (Sakane, Yamamoto & Kikuchi, 2010; Sezgin et al., 2017). Evidence pinpoints mediation of Wnt/ß-catenin signal pathway in hyperthermic stress induced cell injuries of human dermal fibroblast (Jiang, Xue & Wang, 2018).
The Wnt signaling pathway is involved in many biological processes during embryonic development. Several Wnt ligands and key components of Wnt signaling in both canonical and non-canonical pathways are also involved in myogenesis (Cisternas et al., 2014). The canonical pathway depends on β-catenin with a regulatory role in gene transcription, whereas the non-canonical pathway is independent of β-catenin (Komiya & Habas, 2008). The non-conical pathway can either be the planar cell polarity Wnt/PCP pathway which is involved in actin remodeling, cell shape and cytoskeleton regulation, or the Wnt/calcium pathway which regulates calcium level in the cells (Komiya & Habas, 2008). Most of the heat-induced genes affecting the WNT pathway typically inhibit WNT signaling was reported (Sun et al., 2015).
Our previous study has shown significant shifts in the energy metabolism of C2C12 myoblasts under heat and cold stress conditions (Sajjanar et al., 2019). Links between thermal stress response especially affecting signaling pathways and cellular energy metabolism is of our interest. Therefore, the aim of our study was to investigate the transcriptional response of Wnt signaling pathway and its interaction with energy metabolism of C2C12 myoblasts under different temperature stress (35 °C, 39 °C and 41 °C compare to 37 °C). The results indicate a possible crosslink between Wnt signaling and energy metabolism under cellular stress response in skeletal muscle cells.
Materials & methods
Cell culture
The mouse skeletal muscle myoblasts cell line C2C12 (ATCC® CRL1772™) was cultivated in 75 cm2 flask with Dulbecco’s Modified Eagle’s medium high glucose (DMEM, Gibco) containing 10% fetal bovine serum (FBS, Biochrom) and 1% penicillin-streptomycin at 37 °C under 5% CO2 till they reached 80% confluence. The passage 9 cells were detached using 0.125% Trypsin-EDTA (Biochrom) and used for the subsequent experiments. For total RNA, 1 × 105 C2C12 cells per well were seeded and cultured in 6 well-plates. For total membranes isolation 5 × 105 C2C12 cells per 75 cm2 flask were seeded and cultured. Other than different temperatures, all cells were incubated at similar conditions. Three independent replicates were performed for each temperature.
Heat/cold stress treatment
Temperature range where mammals can physiological adapt and survive were set between 35 to 41 °C (cold stress 35 °C, control 37 °C, mild heat stress 39 °C, severe heat stress 41 °C). We used the methods as in our previous study (Sajjanar et al., 2019). Briefly, C2C12 cells were seeded and left to attach on the plastic surface for half hour then cultured at four different temperatures (35, 37, 39 and 41 °C) for 72 h in 6-well plates for genomic analysis and 75 cm2 flasks for total membranes protein isolation.
Bioenergetics assay
As described in our previous study (Sajjanar et al., 2019), two different assays were performed to monitor the changes in mitochondrial and glycolytic functions under temperature stress. The results of these two bioenergetics assays under temperature stress have already been reported (Sajjanar et al., 2019). The bioenergetics data were obtained from the same batch and in the same state of the cells as in the RNA experiments. In this study, we assessed the interaction between these two bioenergetics assays with genes related to Wnt signaling pathway. Briefly, cell energy metabolism under thermal stress conditions was examined by measuring mitochondrial function/oxygen consumption rate (OCR) with the Seahorse Cell Mito Stress Test kit. The Cell Mito Stress Test measures key parameters of mitochondrial function by directly measuring the oxygen consumption rate of cells. This test uses modulators of respiration targeting components of the electron transport chain in the mitochondria to reveal key parameters of metabolic function. The compounds (oligomycin, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), and a mix of rotenone and antimycin A) were sequentially added to measure ATP production, maximal respiration, and non-mitochondrial respiration, respectively. The Glycolysis Stress Test is the standard assay for measuring glycolytic function in cells via the direct measurement of the glycolytic activity/extracellular acidification rate (ECAR) with the Seahorse XF Glycolysis kit ECAR in real time. Therefore, glucose, oligomycin, and 2-deoxyglucose were added sequentially to reveal and analyse the key function of glycolytic pathway. Both of these tests revealed how metabolic flux and energy metabolism shift after thermal stress.
Quantitative real-time expression
Genes were selected based on central components of the Wnt signaling pathway including Wnt ligands, receptors and inhibitors of canonical and non-canonical pathway. Total RNA was isolated from C2C12 cells cultured under different thermal conditions using TRI reagent (Sigma-Aldrich) according to the manufacturer’s instructions, then purified by RNeasy MiniKit (Qiagen). DNaseI treatment was done to remove traces of DNA. Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) was used to synthesize cDNA. The BioMark HD Real-time PCR System (Fluidigm, South San Francisco, CA, USA) comprising a 48 × 48 dynamic array with an integrated fluidic circuit (IFC) was used for qPCR analyses. The master mix for the samples consisted of 2.25 µl of the STA and Exo-I –treated sample, 2.5 µl of SsoFast EvaGreen supermix with low ROX (Biorad, Hercules, CA, USA), and 0.25 µl DNA binding dye. The master mix for the assay included 2.5 µL assay loading reagent, 2.25 µL DNA suspension buffer, and 0.25 µL of a 100 µM primer solution (forward and reverse) provided in Table 1. The qPCR cycle program was: initial denaturation at 95 °C for 60 s, followed by 30 cycles of 95 °C for 5 s each (denaturation) and 60 °C for 20 s (annealing). The reference genes Hprt1 and Mrps27 were used as housekeeping genes that showed no significant changes in any of the heat/cold stress treatments. Data analysis was done by 2 − ΔCt method. All experiments were performed three times independently with three technical replicates each.
| Gene symbol | Ref.NCBI | Fwd Primer 5′->3′ | Rev Primer 5′->3′ |
|---|---|---|---|
| Hsf1 | NM_001331214.1 | AACCTGGACAACCTGCAGAC | GGAGGCTCTTGTGGAGACAG |
| Daam1 | NM_001286452.1 | GAACACAAGCATGAGCTGGA | AACACCTCCTCAGAGCCAGA |
| Fzd7 | NM_008057.3 | ATCATCTTCCTGTCGGGTTG | AAGCACCATGAAGAGGATGG |
| Ppp3ca | NM_001293622.1 | ATATCGACGCACCAGTCACA | AAGGCCCACAAATACAGCAC |
| Ror2 | NM_013846.3 | CTTCATTGGGAACCGGACTA | AAGACGAAGTGGCAGAAGGA |
| Dkk1 | NM_010051.3 | AATATGCATGCCCTCTGACC | ACGGAGCCTTCTTGTCCTTT |
| Wnt7a | NM_001363757.1 | GCCCACCTTTCTGAAGATCA | CTGAGGGGCTGTCTTATTGC |
| Wnt11 | NM_001285792.1 | CAGGATCCCAAGCCAATAAA | GACAGGTAGCGGGTCTTGAG |
| Wnt5a | NM_001256224.2 | AAGCAGGCCGTAGGACAGTA | GCCGCGCTATCATACTTCTC |
| Porcn | NM_001308474.1 | GTCCCTGGCATTCATCACTT | GTGTCGTCCACATCGACATC |
| Dvl2 | NM_007888.4 | CAAAGTAACGAGCGTGGTGA | CAGCACTCGTACAGCATCGT |
| Axin2 | NM_015732.4 | AACCTATGCCCGTTTCCTCT | CTGGTCACCCAACAAGGAGT |
| Tnks2 | NM_001163635.1 | TTCAAGTGCAGCAGAAGGTG | GTGGCCCATTTCAACTAGGA |
| Sfrp1 | NM_013834.3 | GCTCAACAAGAACTGCCACA | CTCGGGGAACTTGTCACATT |
| Lrp6 | NM_008514.4 | ACAGAGCCCTGACATCATCC | TGATTTGCGACTGAGTTTGC |
| Csnk1g2 | XM_006513011.1 | GGAGTACGTGCACACCAAGA | GCGATATGGGATGTGCTTCT |
| Aes | NM_001276288.1 | CGCATCAAAGATGAGTTCCA | CTTCACAATTTCCGCCTGTT |
| Cby1 | NM_028634.3 | AAGTTTGAAAACGGCCAGTG | CTGAAAGCATGTCCAGCAGA |
| Dact1 | NM_001190466.1 | GGAATCCATGAAGGAAAGCA | CCTCGCTTTTGAAGTCCAAG |
Total membranes isolations
5 × 105 C2C12 cells were seeded and cultured in T75 Flask and incubated at different temperatures. Three independent replicates were performed for each temperature. C2C12 cells were washed with growth media and scraped off with the homogenization buffer (100 mM HEPES, 250 mM sucrose, 4 mM EDTA), 1mM Dithiothreitol (DTT)) containing protease inhibitor (Mini Protease Inhibitor Cocktail; Merck, Darmstadt, Germany). Subsequently, the samples were disrupted in a Potter homogenizer (10 strokes) and then the cell lysates were centrifuged (800 ×g, 3 min) at 4 °C. The supernatant was centrifuged (105 400 ×g, 2 h) (Optima XPN-100, Beckman Coulter, Brea, USA) at 4 °C to obtain the total membrane fraction. The pellet (total cell membranes) was dissolved in homogenization buffer and stored at −80 °C until further preparation. Total membrane fraction isolations were performed in three independent experiments and three technical replicate at each temperature.
Western blot analysis
The Western blot analyses were performed on total membrane fractions of C2C12 samples and concentrations were measured using Pierce™ BCA Protein Assay Kit (Thermo Scientific, USA). Forty five µg proteins were loaded in each well in the gel (TGX Stain-Free™ FastCast™, BioRad), separated by SDS-PAGE, and then transferred to nitrocellulose membrane with 0.2 µm pore size (catalog no. #1704158, Trans-Blot Turbo, BIO-RAD). The membrane was blocked with 5% milk in Tris buffered saline with Tween-20 (TBST) at room temperature for 45 min. The membrane was then washed with TBST, and then incubated with the primary antibodies against LRP6 (C47E12) antibody (1:1000; catalog no. 3395) and phosphorylated LRP6 (Ser1490) Antibody (1:1000; catalog no. 2568T) at 4 °C for 16 h. The membrane was washed with TBST three times then incubated with 5% milk containing secondary antibodies (Goat Anti-Rabbit IgG H&L (HRP),1:2000, catalog no. ab6721, abcam) for 1 h at room temperature. Last step was by washing the membrane with TBST then incubating with SuperSignal™ West Pico PLUS Chemiluminescent Substrate (catalog no. #34577, Thermo Scientific, USA) Reagent for 1 min. The detection was carried using ChemiDoc Imaging Systems (Bio-rad). We used the total protein amount as a reference to normalize our data. Western blot analysis was performed in three independent replicate at each temperature.
Data analysis
SAS program was used to analyze the normalized data. For the expression data and Western blot, we applied temperature as fixed effect and replicate as random effect in analysis of variance using proc mixed procedure. An adjusting for multiple comparisons across the Type 3 tests for the fixed effect was calculated using the post hoc Tukey-Kramer test. P value < 0.05 was considered statistically significant. Graphs were visualized using GraphPad Prism version 9 (GraphPad Software, La Jolla California USA). Pearson correlation between RNA expression levels of Wnt related genes and energy metabolism values “OCR and ECAR” were calculated and P value <0.05 was considered statistically significant.
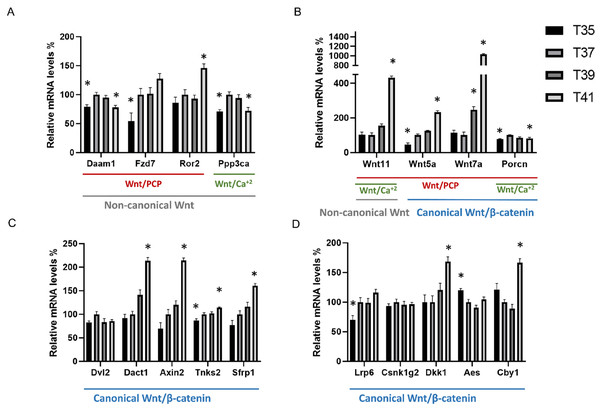
Figure 1: Differentially expressed Wnt-related transcriptions under heat and cold stress.
Wnt signaling pathway including non-canonical pathway (gray line) and canonical pathway (blue line) which divides into the planar cell polarity Wnt/PCP pathway (red line) and Wnt/calcium pathway (green line). (A) Wnt related genes belonging to the non-canonical pathway, (B) Wnt related genes belonging to the non-canonical pathway and canonical pathway, (C and D) Wnt related genes belonging to the canonical pathway. Asterisk (*) denotes significant differences between the control group T37 (37 °C) and the group with temperature stress T35, T39 and T41 (35, 39 and 41 °C) respectively at P < 0.05. A relative mRNA level of control group was adjusted to 100%. Error bars indicate standard error of the mean (SEM).Results
Transcripts related Wnt signaling under cold/heat stress
Considering the important role of Wnt signaling in cell proliferation, we investigated the effect of cold/heat stress on the expression pattern of Wnt pathway (Fig. 1). The first groups (Fig. 1A) of genes included Daam1, Fzd7, Ror2, Wnt11, Wnt5a, Wnt7a and Dvl2, and are known as the components of the non-canonical planar cell polarity pathway (Wnt/PCP). Under thermal stress, the transcript abundance of Fzd7 was significantly decreased in cold stress whereas Ror2 transcripts were significantly increased in severe heat stress. Daam1 was significantly decreased in both cold stress and severe heat stress. Of all Wnt ligand genes, only Wnt5a was significantly decreased in cold stress (35 °C). All Wnt ligands including Wnt5a, Wnt7a and Wnt11 were significantly increased in severe heat stress (41 °C) (Fig. 1B). The transcript abundance of Wnt11 was significantly increased by a factor 4 while Wnt7a was significantly increased by a factor of 10. Interestingly, the transcript abundance of Wnt7a is very low. Especially at cold or control temperature Wnt7a was almost not expressed (ct average = 35) compared to severe heat stress (41 °C) (ct average = 31). No significant change in Dvl2 expression level between temperatures was detected. Besides Ppp3ca, Wnt5a and Wnt11 are also involved in the non-canonical Wnt/Ca+2 pathway. Of these, Ppp3ca was significantly decreased at both cold stress (35 °C) and severe heat stress (41 °C) compared to control. The transcripts involved in the Wnt/β catenin signaling pathway are Wnt5a, Wnt7a, Dvl2, Dact1, Axin2, Tnks2, Sfrp1, Lrp6, Csnk1g2, Aes and Cby1. The transcripts of Axin2, Sfrp1, Dact1 and Cby1 were significantly increased in severe heat stress (Figs. 1C, 1D). Tnks2 was significantly decreased in cold stress and increased in severe heat stress. Lrp6 was significantly decreased at cold stress (35 °C) while Aes was significantly increased. Additionally, we investigated Porcn expression levels which is known to be an important regulator of both canonical and non-canonical Wnt signaling pathway due to its role as a multi-pass transmembrane protein. Transcript abundance of Porcn was significantly decreased in both cold stress (35 °C) and severe heat stress (41 °C) compared to control. There was no significant change in Csnk1g2 RNA expression level between different temperatures. No different RNA expressions of all Wnt ligand genes were found between control (37 °C) and mild heat stress (39 °C) in this study. In addition, under mild heat stress (39 °C), none of the Wnt pathway components were differentially expressed except increased Wnt7a transcript.
Expression pattern of Wnt signaling genes and mitochondrial/ glycolysis functions
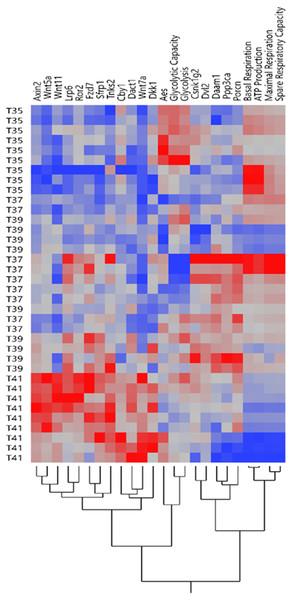
Our previous study showed that temperature stress shifted the OXPHOS and the glycolytic functions in muscle cells line (Sajjanar et al., 2019). Expression pattern of Wnt signaling related genes and mitochondrial/glycolysis functions under heat /cold stress is shown in Fig. 2. The heatmap of expression patterns of Wnt signaling genes and mitochondrial/glycolysis functions can be clearly separated under severe heat and cold stress, whereas the pattern was mixed between control and mild temperature (Fig. 2). Under cold stress, glycolysis is the preferential pathway for energy production by promoting glucose metabolism (Sajjanar et al., 2019). Only expression levels of Aes was significantly increased under cold stress (Fig. 1D) and cluster together with glycolysis and glycolytic capacity (Fig. 2). Other groups of Wnt related transcripts including Axin2, Wnt5a, Wnt11, Lrp6, Ror2, Fzd7, Sfrp1, Tnks2, Cby1, Dact1, Wnt7a and Dkk1 were clustered together and had lower expression under cold temperature and increased expression during severe heat stress. OXPHOS is significantly downregulated during severe heat stress (Sajjanar et al., 2019), while the expression of transcripts in this group was increased, most of which belong to Wnt inhibitors including Axin2, Dkk1, Sfrp1, Dact1 and Cby1. Transcript levels of Csnk1g2, Dvl2, Daam1, Ppp3ca, and Porcn were decreased under severe heat and cold stress and these genes belong to both Wnt/PCP and Wnt/Ca+ pathways.
Figure 2: Expression profile of Wnt signaling genes and mitochondrial/glycolysis functions.
The heatmap of expression patterns of Wnt signaling genes and mitochondrial/glycolysis functions under temperature stress. Hierarchical clustering of the similar profile of expression and energy metabolism at different temperatures.Wnt signaling genes and their relationship to energy metabolism after exposure to Cold/Heat stress
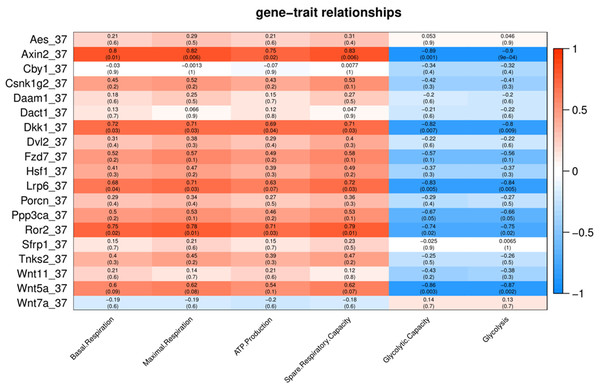
To characterize the association of Wnt pathway-related genes with energy metabolism under temperature stress, we applied correlation analyses between gene expression and energy metabolism under various temperature conditions. At control temperature (37 °C) transcript abundance of five genes (Axin2, Lrp6, Ror2, Dkk1 and Wnt5a) showed a negative correlation (p < 0.05) with glycolysis and glycolytic capacity (Fig. 3). Four genes (Axin2, Lrp6, Dkk1 and Ror2) showed a positive correlation with maximal respiration and spare respiratory capacity (Fig. 3). Three genes (Axin2, Dkk1 and Ror2) showed a positive correlation with ATP production, while four genes (Axin2, Lrp6, Dkk1 and Ror2) showed a positive correlation with basal respiration (Fig. 3).
Figure 3: Correlation matrix of Wnt related genes expression and energy metabolism at 37 °C.
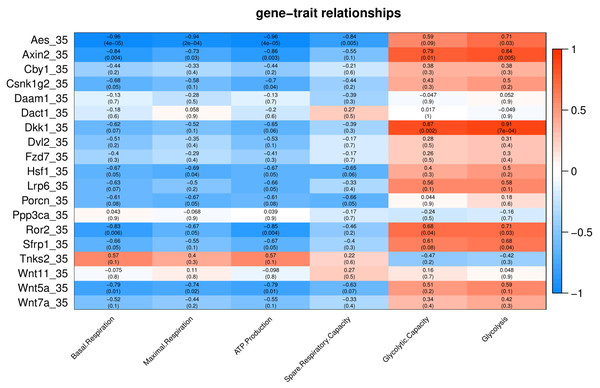
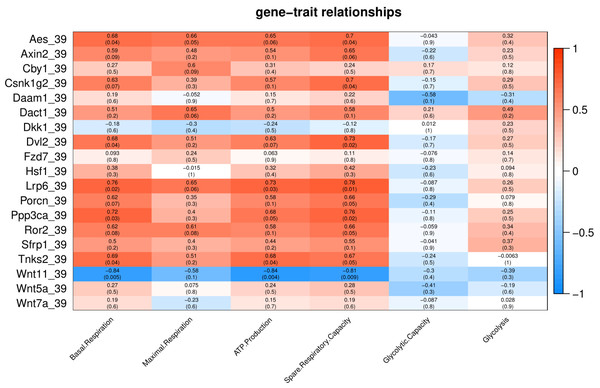
Number in each cell represents the value of correlation coefficient and the corresponding p value. Cell color indicates correlation (red, positive correlation; blue, negative correlation).Under cold stress (35 °C), five genes (Aes, Axin2, Dkk1, Ror2, and Sfrp1) showed a positive correlation (p < 0.05) with Glycolysis (Fig. 4). Different genes showed a negative correlation with basal respiration (Aes, Axin2, Ror2 and Wnt5a), maximal respiration (Aes, Axin2, and Wnt5a), ATP production (Aes, Axin2, Csnk1g2, Ror2 and Wnt5a) and spare respiratory capacity (Aes) (Fig. 4).
Figure 4: Correlation matrix of Wnt related genes expression and energy metabolism at 35 °C.
Number in each cell represents the value of correlation coefficient and the corresponding p value. Cell color indicates correlation (red, positive correlation; blue, negative correlation).Under mild heat stress (39 °C), different genes were positively correlated (p < 0.05) with basal respiration (Aes, Dvl2, Lrp6, Ppp3ca and Tnks2), ATP production (Lrp6, and Tnks2) and spare respiratory capacity (Aes, Csnk1g2, Dvl2, Lrp6 and Ppp3ca) (Fig. 5). Wnt11 showed negative correlation (p < 0.005) with basal respiration, ATP production, and spare respiratory capacity (Fig. 5).
Figure 5: Correlation matrix of Wnt related genes expression and energy metabolism at 39 °C.
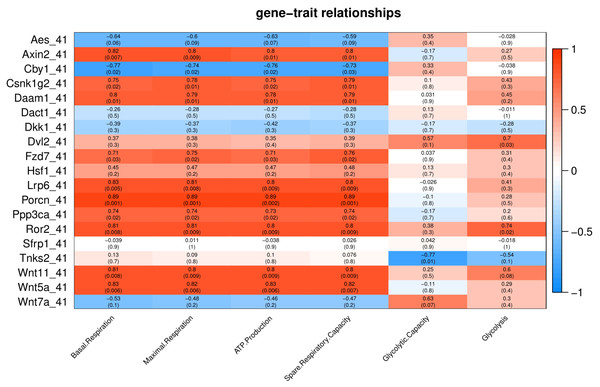
Number in each cell represents the value of correlation coefficient and the corresponding p value. Cell color indicates correlation (red, positive correlation; blue, negative correlation).Under severe heat stress (41 °C), two genes (Dvl2 and Ror2) showed positive correlation (p < 0.05) with Glycolysis, and ten genes (Axin2, Csnk1g2, Daam1, Fzd7, Lrp6, Porcn, Ppp3ca, Ror2, Wnt11 and Wnt5a) showed positive correlation with basal respiration, maximal respiration, ATP production, and spare respiratory capacity (Fig. 6).Tnks2 showed negative correlation with glycolytic capacity (p = 0.01).
Figure 6: Correlation matrix of Wnt related genes expression and energy metabolism at 41 °C.
Number in each cell represents the value of correlation coefficient and the corresponding p value. Cell color indicates correlation (red, positive correlation; blue, negative correlation).Temperature stress induces phosphorylation of LRP6 on cell membranes
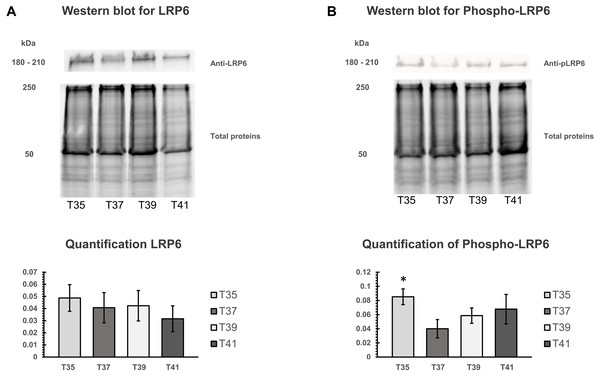
The activation of canonical Wnt signaling pathway requires a specific receptor in cell membranes and Wnt ligands. LRP6 protein is an important receptor in Wnt signaling located on cell membrane. We isolated the cell membranes from cells cultured at different temperatures and measured protein quantity of LRP6 and phospho-LRP6 by Western blot analysis. Total membrane protein was used for normalizing (Figs. 7A, 7B). Three independent experiments were carried out. We found no significant change under heat/cold stress by using LRP6 (Fig. 7A), whereas phosphorylated LRP6 protein was significant higher under cold stress (Fig. 7B). We also found that cells cultured under severe heat stress showed tendency of increased phosphorylated LRP6 protein (Fig. 7B).
Figure 7: Western blot of LRP6 and phosphorylation of LRP6 under temperature stress.
(A) On the upper part, Western blot of total membranes protein of C2C12 using LRP6 antibodies under four different temperatures (T35, T37, T39, and T41) and corresponded of the total membranes proteins, which were used for normalization. The lower part is the quantification of LRP6. (B) On the upper part, Western blot of total membranes protein of C2C12 using phosphorylation LRP6 antibodies under four different temperatures (T35, T37, T39, and T41) and corresponded of the total membranes proteins, which were used for normalization. The lower part is the quantification of phosphor-LRP6. Error bars indicate standard error of the mean (SEM). * indicates p < 0.05 compared to the control group 37 °C.Discussion
Environmental stress including cold/heat stress induces disturbance in the cellular energy metabolism and influences multiple types of signaling molecules including Wnt signaling. The involvement of Wnt signaling during cell proliferation can happen via two pathways, the canonical pathway by mediating the mitosis stages (Kaldis & Pagano, 2009) and the non-canonical pathway which leads to actin polymerization and cytoskeleton regulation (Kim & Davidson, 2011; Dzamba et al., 2009). In this study, selected genes involved in Wnt signaling pathway were evaluated under thermal stress conditions.
Transcripts related Wnt/PCP, Wnt/Ca2+ and Wnt/β-catenin under cold/heat stress
Wnt/PCP pathway involves several proteins. FZD7 favors non-canonical signaling, and its downregulation restrains cell proliferation (Xue et al., 2018). DAAM1 is required for organizing the cytoskeleton (Habas & He, 2001). Downregulation of Daam1 or the knockdown of Wnt5a inhibits migration and invasion of breast cancer cells (Xiong et al., 2018; Prasad et al., 2016). The regulation of Wnt/PCP pathway can also be done via JNK pathway (Yamanaka et al., 2002). Previous studies indicated that Wnt5a and Ror2 activate the noncanonical Wnt signaling pathway, as determined by activation of JNK in cultured cells (Oishi et al., 2003). Here we found overexpression of Ror2 and Wnt5a transcripts together with the down regulation of Daam1 transcript under heat stress. At the same time transcript levels of Wnt11, Wnt5a and Wnt7a were highly increased under heat stress, which may cause an increased intracellular accumulation of Wnt ligands in C2C12 cells.
In this study, two key genes in Wnt/Ca2+ pathway (Ppp3ca and Porcn) were significantly downregulated under both heat/cold stress. Ppp3ca belongs to the Wnt/ca2+ pathway, which can be activated through Wnt11 and Wnt5a (Witze et al., 2013). The Porcn protein has an important role in Wnt ligand trafficking. It modifies Wnt ligands by adding Palmitoleate which is critical for the Wnt secretion. Depleting Porcn will cause intracellular accumulation of Wnt ligands (Barrott et al., 2011).
Wnt/β-catenin is a complicated pathway involving several elements from proteins to transcription factors. During myoblast proliferation, blockade of WNT/β-catenin signaling decreases cell proliferation but does not induce cell death (Suzuki, Pelikan & Iwata, 2015). Real-time determination of cell viability from our previous study using the xCelligence system showed that proliferation of C2C12 was slightly impaired under cold treatment conditions and cell viability was significantly reduced under high thermal stress (Sajjanar et al., 2019). We found lower transcript levels of Lrp6 on the cells under cold stress. Since the LRP6 receptor is located on the cell membrane, we therefore isolated the protein from the cell membrane. No significant increase in LRP6 protein level was found under temperature stress. In contrast, we found that cold stress induces phosphorylation of LRP6 at the cell membrane and possibly triggers further Wnt signaling cascades. Previous studies also confirmed that phosphorylation occurs in microdomains (Sakane, Yamamoto & Kikuchi, 2010; Sezgin et al., 2017). Lypd6 appears to regulate Lrp6 activation, particularly in membrane rafts, which is essential for downstream signaling (Ozhan et al., 2013). The membrane can change its fluidity under the influence of variable temperature (Polezhaev & Volkov, 1979). High temperature tends to increase the distance between membrane phospholipids which will increase the fluidity, while low temperature does the opposite (Fan & Evans, 2015). In our study, a downregulation of Lrp6 and Fzd7 transcripts in C2C12 cell under cold stress was found whereas phosphorylated LRP6 protein was upregulated in the cell membrane. The change of these receptors under cold stress may disturb the activation of the Wnt/β-catenin. Aes (amino-terminal enhancer of split or Tle5) is co-repressor in Tle family which mediates repression of WNT target genes (Brantjes et al., 2001). It is important to note that the temperature influence on the canonical Wnt signal regulation was negative in both heat and cold, but in two different approaches. Under cold stress, the expression of Wnt ligands was reduced while in heat stress we noticed many Wnt inhibitors being overexpressed along with some negative regulators. AXIN2 is considered a negative regulator of the canonical pathway, as it promotes the degradation of β-catenin (Jho et al., 2002; Wu et al., 2012). DACT1 is another negative regulator of the canonical pathway which can mediate dishevelled degradation (Zhang et al., 2006) and inhibit cell proliferation (Zhu et al., 2017). The role of CBY1 in the canonical pathway covers the deactivation of the β-catenin transactivation complex (Takemaru & Moon, 2003). It has been reported that Cby overexpression decreased β-catenin activity (Singh et al., 2007). SFRP1 is an antagonist of Wnt3 where it binds and prevents the activation of the pathway (Yang et al., 2015) and it can promote apoptosis by suppressing proliferation, migration and invasion (Wang et al., 2018). Previous study reported that the transcripts in Wnt signalling pathways including SFRP1 and DACT1 was increased by heat treatment, similar to what we found in this study(Sun et al., 2015). A recent study in IPEC-J2 cells showed downregulation Wnt/β-catenin pathway at 41 °C, and the protein level of its downstream targets AXIN2, GSK3 β, β-catenin, cyclin D1, and c-Myc were also disrupted (Zhou et al., 2020). Our results clearly demonstrate an overexpression of inhibitors (Sfrp1, Dkk1, and Cby1) and negative regulators (Dact1 and Axin2) of Wnt/β-catenin pathway in the high temperature (41 °C). This may indicated that WNT pathways appears to be promoting cell survival during heat stress by suppressing proliferation.
Wnt signaling genes and their relationship to energy metabolism after exposure to Cold/Heat stress
Environmental changes like temperature also play a significant role in energy metabolism, as indicated some previous studies (Sajjanar et al., 2019; Little & Seebacher, 2016; Mitov et al., 2017). We have previously reported that in heat stress, reduced maximal respiration and spare respiratory capacity to promote cell survival, whereas in cold stress, cells prefer glycolysis as a rapid compensatory mechanism to meet the energy demand as an adaptive thermogenic response (Sajjanar et al., 2019). In current study, we found that many Wnt related transcripts under cold stress were positively correlated with glycolysis. Aes was positively correlated with glycolysis under cold stress and cluster with glycolysis. A previous study reported that defects in Wnt signalling may determine a metabolic switch in fuel utilization towards glycolysis (Chafey et al., 2009).
Specific correlation patterns between mitochondrial or glycolytic functions and Wnt signaling pathway-related genes were found in this study. Some transcripts, including Axin2, Ror2 and Wnt5a, were positively correlated with mitochondrial traits in the control and severe heat stress, but negatively in cold stress. A group of genes, including Csnk1g2, Daam1, Fzd7, Porcn, Ppp3ca and Wnt11, correlated positively with mitochondrial traits only in severe heat stress. Wnt signalling is known to promote cell proliferation. To promote cell survival under heat stress, Wnt signaling pathway is activated and there is a change in the expression of Wnt/β-catenin inhibitors, Wnt/PCP, Wnt ligands and Wnt antagonists. We also observed the genes involved in Wnt signaling are positively correlated with energy metabolism. These results indicate that activated Wnt inhibitors or the Wnt antagonists together with reduce metabolic flux promote cell survival under heat stress.
The role of Wnt/β-catenin signaling in mitochondrial biogenesis has been previously reported (An et al., 2010). The regulation of Wnt/β-catenin pathway can be carried out by TNKS1 and TNKS2 (Huang et al., 2009). By controlling AXIN1 and AXIN2 levels and suppressing Wnt/β-catenin signaling, both TNKS1/2 regulate glycolysis (Li et al., 2015). Ror2 overexpression with Wnt5a stimulation and Ror1 knockdown showed that the active non-canonical pathway targets modify other pathways that are involved in cell metabolism like glycolysis and fatty acid metabolism (Bayerlová et al., 2017).
A previous study in Human melanoma cell lines (A2058 and HTB63 showed a positive correlation between Wnt5a and lactic dehydrogenase expression, indicating the role of Wnt5a in glycolytic flux stimulation in cancer cell metabolism (Sherwood et al., 2014). Together, the correlation between Wnt related genes and energy traits was shifted between temperatures, in particular Axin2, Ror2, Lrp6 and Wnt5a had an opposite correlation between the cold stress and the severe heat stress. These findings indicate potential cross-talks between Wnt signaling and energy metabolism.
Conclusion
In summary, we demonstrated a relationship between Wnt related genes and energy metabolism including oxidative phosphorylation and glycolysis under cold/heat stress in C2C12 muscle cell line. Cold stress and severe heat stress lead to transcriptional responses to the regulation of Wnt-related genes in different ways. Thermal stress activates Wnt signaling in C2C12 cells in particular the transcripts of Wnt/β-catenin inhibitors, Wnt/PCP pathway and Wnt ligands are activated under heat stress. Cell membrane receptors Lrp6 and Fzd7 are activated under cold stress. A positive correlation between oxidative phosphorylation and Wnt-related transcripts was found under high temperature while negative correlation was found under cold temperature. More experiments are needed to make definitive conclusions about the relationship between these pathways in vivo under non-diseased/cancerous states.
Supplemental Information
Raw data of the RT-PCR data
Each temperature was performed independently for three replicates, each replicate was performed in three wells, and each sample (well) was performed in two technical replicates.
Original Western blot image for total protein and correspondence blot of LPR6 protein from gel 1, gel 2, and gel 3
All Figure was carried using ChemiDoc Imaging Systems (Bio-rad). We used the total protein amount as a reference to normalize LPR6 protein data. Western blot analysis was performed in three independent experiments.
Raw data of Fig. 2
The detection was carried using ChemiDoc Imaging Systems (Bio-rad). We used the total protein amount as a reference to normalize LPR6 protein data. Western blot analysis was performed in three independent experiments.