First record of Trichinella in Leopardus guigna (Carnivora, Felidae) and Galictis cuja (Carnivora, Mustelidae): new hosts in Chile
- Published
- Accepted
- Received
- Academic Editor
- Whitney Kistler
- Subject Areas
- Parasitology, Veterinary Medicine, Zoology
- Keywords
- Trichinella, Wildlife, Neotropics, Reservoir, Felidae, Mustelidae, Chile, Zoonoses, Mammals
- Copyright
- © 2021 Echeverry et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. First record of Trichinella in Leopardus guigna (Carnivora, Felidae) and Galictis cuja (Carnivora, Mustelidae): new hosts in Chile. PeerJ 9:e11601 https://doi.org/10.7717/peerj.11601
Abstract
Background
Trichinellosis is a zoonotic disease with a worldwide distribution. It is caused by several species of nematodes in the genus Trichinella. Trichinella spp. are transmitted through predation or carrion consumption and occur in domestic and sylvatic cycles. In humans trichinellosis occurs due to the consumption of raw or undercooked, infected meat and is mainly associated with the household slaughter of pigs or the consumption of game animals without veterinary inspection, a cultural practice that is difficult to resolve. Therefore, knowledge of this parasite’s reservoir is relevant for better implementing public health strategies. The aim of this study was to assess the presence of Trichinella sp. in several carnivore and omnivore vertebrates in central-southern Chile.
Methods
We collected muscle tissue from a total of 53 animals from 15 species and were digested to detect Trichinella larvae which were further identified to species level using molecular techniques.
Results
We detected Trichinella larvae in Leopardus guigna (Felidae) and Galictis cuja (Mustelidae). We identified the larvae collected from L. guigna as Trichinella spiralis, but we were unable to molecularly characterize the larvae from G. cuja. This is the first record of Trichinella in a native mustelid of South America and the first record of T. spiralis in L. guigna. This study identified two novel hosts; however, further work is needed to identify the role that these and other hosts play in the cycle of Trichinella in Chile.
Introduction
Trichinellosis is a disease that is distributed worldwide and is caused by nematodes in the genus Trichinella (Korhonen et al., 2016). It is considered neglected and emerging in some regions (Dupouy-Camet, 1999; Murrell & Pozio, 2000; Bruschi, 2012; Boutsini et al., 2014). Trichinella nematodes are transmitted from animals to humans by the ingestion of raw or undercooked infected meat.
Trichinella is transmitted among non-human animals via predation and carrion consumption; therefore, it circulates among carnivorous and omnivorous vertebrates. Two cycles have been described: the domestic (encompassing mainly pigs, rats, dogs, and cats) and the sylvatic (encompassing free-range vertebrates) cycles (Pozio, 2000; Pozio, 2007; Loutfy et al., 1999). These cycles can be connected and fed back by invasive rats and other synanthropic animals (Pozio, 2000). The domestic cycle was the primary cause of human infections; however, improvements in pork production have reduced outbreaks globally (Devleesschauwer et al., 2015; Murrell, 2016). The improvements to pork production changed the epidemiology of trichinellosis in human populations. Trichinella infections now primarily occur during the consumption of meat from unregulated sources, mainly backyard pork production and the consumption of game animals (Pozio, 2014; Tryland et al., 2014; Fichi et al., 2015; Kärssin et al., 2017).
At present, there are 10 recognized species of Trichinella around the world and three additional genotypes that have not yet been identified as distinct species (Korhonen et al., 2016; Sharma et al., 2020). Most species infect only mammals (Klun et al., 2019; Bilska-Zając et al., 2020), including marine mammals (Tryland et al., 2014; Pasqualetti et al., 2018). However, Trichinella pseudospiralis Garkavi, 1972 also infects birds, and Trichinella zimbabwensis Pozio et al., 2002 and Trichinella papuae Pozio et al., 1999 infect reptile hosts (Korhonen et al., 2016). Thus, obtaining ecological and epidemiological knowledge of the transmission cycle is relevant for reducing the incidence of this parasite.
In South America, Trichinella spp. infections have been detected in Argentina, Bolivia, Chile (larvae isolation), Brazil, and Ecuador (antibody detection) with most studies focusing on the domestic cycle (Bjorland et al., 1993; Ribicich et al., 2020). Four species have been reported: Trichinella spiralis Owen, 1835, Trichinella patagoniensis Krivokapich et al., 2012, Trichinella britovi Pozio et al., 1992, and T. pseudospiralis (Krivokapich et al., 2006; Krivokapich et al., 2012; Krivokapich et al., 2015; Krivokapich et al., 2019). Additionally, Trichinella infections have been documented from eight wild species: cougar (Puma concolor Linnaeus, 1771), wild boar (Sus scrofa Linnaeus, 1758), fox (Lycalopex gymnocercus gracilis Fischer, 1814), opossum (Didelphis albiventris Lund, 1840), sea lion (Otaria flavescens Shaw, 1800), pecarí (Tayassu tajacu Palmer, 1897), armadillo (Chaetophractus villosus Desmerest, 1804), and pericote (Graomys centralis Thomas, 1902) (Minoprio, Abdon & Abdon, 1967; Ribicich et al., 2020; Soria et al., 2010).
In Chile, the domestic cycle is fairly well-studied (Alcaíno & Arenas, 1981; Schenone et al., 2002; Landaeta-Aqueveque et al., 2021), but the sylvatic cycle is largely unknown. Trichinella spiralis is the sole species that has been reported in Chile (Schenone et al., 2002; Landaeta-Aqueveque et al., 2015; Hidalgo et al., 2019; Echeverry et al., 2021; Espinoza-Rojas et al., 2021). Among non-domestic animals, cougars, American minks (Neovison vison Schreber, 1777) and wild boar are the only wild/feral hosts with documented infections (Landaeta-Aqueveque et al., 2015; Hidalgo et al., 2019; Echeverry et al., 2021; Espinoza-Rojas et al., 2021). In addition to those reports, other studies have not found infected animals (Alvarez et al., 1970; González-Acuña et al., 2010; Ramirez-Pizarro et al., 2019). Therefore, the objective of this study was to assess the presence of Trichinella sp. in carnivorous and omnivorous wild vertebrates from south-central Chile.
Materials & Methods
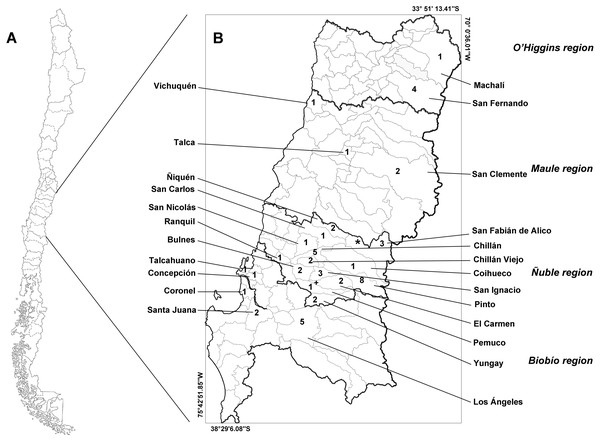
The study area includes four administrative regions of Chile: the O’Higgins, Maule, Ñuble, and Biobío regions (Fig. 1). These regions feature a transitional climate that falls somewhere between the classifications of warm Mediterranean (Csb, after Köpen classification) and wet temperate oceanic (Cfb, after Köpen classification). These regions lie within the limits between central and southern Chile.
Figure 1: Map of Chile (A) and the studied administrative regions (B).
The italicized text indicates the name of the regions, and the Roman text indicates the name of the communes. Infected animals are presented with the symbols “+” (Leopardus guigna) and “*” (Galictis cuja). The numbers indicate the number of animals examined in each commune. Thick lines indicate the regional limits, while thin lines indicate the limits of the communes.This study considered animals that were found dead, mainly run over by a vehicle, or that died in wild animal rescue/rehabilitation centers (Fauna Rehabilitation Center of the Universidad de Concepción; Wild Fauna Rehabilitation Center of the Universidad San Sebastián) from 2013 to 2020. We examined at least 1 g of muscle (10 g, when possible) of these animals to determine the presence of Trichinella spp. larvae. We then selected the following muscles for parasitological examination: the diaphragm, masseter, tongue, quadriceps (in mammals), pectoral (in birds), and intercostals (in all animals).
We performed artificial digestion of the muscles following the method described by Gajadhar et al. (2019) and preserved the larvae in 96% ethanol. For molecular identification, we extracted DNA from a pool of 10 Trichinella larvae isolated from each positive animal using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) and used 10 ng of DNA for identification at the species level by nested polymerase chain reaction (PCR), following a modification of the protocol of Zarlenga et al. (1999). We performed the reactions at a final volume of 25 µL. We used the following primers: Ne forward (5′-TCTTGGTGGTAGTAGC-3′) and reverse (5′-GCGATTGAGTTGAACGC-3′) in the first PCR (0.5 µM of each primer), and 12.5 µL of GoTaq Green Master Mix (Promega Corporation, Madison, WI, USA). We amplified the DNA in a thermocycler (MultiGene™ OptiMax Thermal Cycler; Labnet International, Inc., Edison, NJ, USA) under the following cycling conditions: 95 °C ×1 min for initial denaturation, followed by 40 cycles of 95 °C ×30 s; 56 °C ×1 min, and 72 °C ×1 min; and a final extension of 72 °C ×2 min. Then, we used 0.5 µM of each Primers I forward (5′-GTTCCATGTGAACAGCAG-3′) and reverse (5′- CGAAAACATACGACAACTGC-3′) in a second PCR under same conditions with an annealing temperature of 55 °C. The PCR products were subjected to electrophoresis in 2% agarose gel. We used master mix without the DNA as the negative control, and T. spiralis larvae obtained from a previous study (Landaeta-Aqueveque et al., 2015) as a positive control of the PCR.
Bioethical considerations: this study met the International Guiding Principles for Biomedical Research Involving Animals. The Comité de Ética of the Facultad de Ciencias Veterinarias of the Universidad de Concepción approved the study (CBE-47-2017).
Results
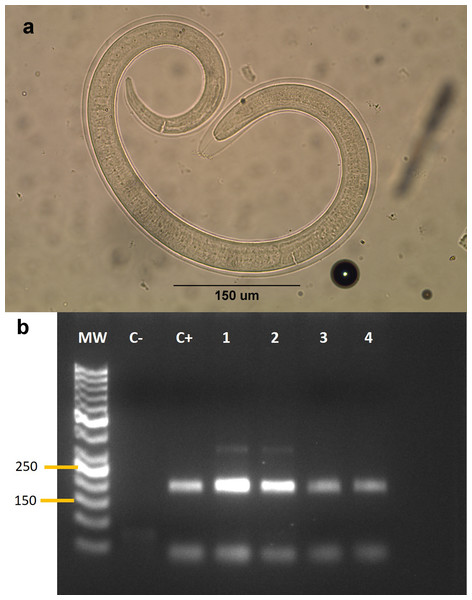
We collected samples from 53 animals. The sample was composed of 28 mammals, 24 birds and one reptile (Table 1). The weight of the examined muscle samples were at least 10 g with the exception of D. bozinovici and P. chamissonis with samples sizes of 3 g and 1 g, respectively. Trichinella larvae were isolated only from one Leopardus guigna Molina, 1782 (güiña; 52 larvae per gram of muscle) and one Galictis cuja Molina, 1782 (lesser grison; 0.3 larvae per gram of muscle), both from the Ñuble region (Fig. 1). We were unable to amplify DNA from the larvae isolated from the grison. However, we were able to amplify a PCR product of 173 bp from the güiña which is consistent with our T. spiralis positive control (Fig. 2) and the size described for this species (Pozio & Zarlenga, 2019).
| Species | Infected/Analyzed (%) | Class |
|---|---|---|
| Glaucidium nana King, 1828 (Austral pygmy owl) | 0/1 (0) | Aves |
| Bubo magellanicus Gmelin, 1788 (Magellanic horned owl) | 0/2 (0) | Aves |
| Tyto furcata Temminck, 1827 (American barn owl) | 0/5 (0) | Aves |
| Strix rufipes King, 1828 (Rufous-legged owl) | 0/2 (0) | Aves |
| Parabuteo unicinctus Temminck, 1824 (Harris’ hawk) | 0/11 (0) | Aves |
| Coragyps atratus Bechstein, 1793 (Black vulture) | 0/1 (0) | Aves |
| Cathartes aura Linnaeus, 1758 (Turkey vulture) | 0/1 (0) | Aves |
| Pelecanus thagus Molina, 1782 (Peruvian pelican) | 0/1 (0) | Aves |
| Grampus griseus Cuvier, 1812 (Risso’s Dolphin) | 0/1 (0) | Mammalia |
| Otaria flavescens Shaw, 1800 (South American sealion) | 0/1 (0) | Mammalia |
| Leopardus guigna Molina, 1782 (Güiña) | 1/6 (16.67) | Mammalia |
| Lycalopex culpaeus Molina, 1782 (Culpeo fox) | 0/2 (0) | Mammalia |
| Galictis cuja Molina, 1782 (Lesser grison) | 1/17 (5.88) | Mammalia |
| Dromiciops bozinovici D’Elía, Hurtado and D’Anatro, 2016 (‘Monito del monte’) | 0/1 (0) | Mammalia |
| Philodryas chamissonis Wiegmann, 1834 (Long-tailed snake) | 0/1 (0) | Reptilia |
Figure 2: (A) Larva of Trichinella sp. isolated from a Galictis cuja. (B) Gel electrophoresis of PCR products.
(B) MW: Marker of 50 bp. C-: negative control. C+: Trichinella spiralis positive control. Lanes 1–4: isolates from Leopardus guigna.Discussion
Detecting Trichinella infection is a challenge in wild fauna of Chile because most carnivore vertebrates are protected by law (SAG, 2012). This protection is due to conservation concerns or because these animals aid in pest control. Therefore, only invasive animals can be hunted to assess Trichinella infection (Hidalgo et al., 2019; Ramirez-Pizarro et al., 2019; Espinoza-Rojas et al., 2021). This has resulted in few studies that have assessed the presence of Trichinella infection in native wildlife in Chile (Alvarez et al., 1970; González-Acuña et al., 2010; Hidalgo et al., 2013; Landaeta-Aqueveque et al., 2015; Echeverry et al., 2021). Although one of these studies sampled a broad range of mammalian species including güiñas and lesser grisons, it did not detect Trichinella spp. (Alvarez et al., 1970).
Studies in Argentina examined another wild felid, the Geoffroy’s cat (Leopardus geoffroyi D’ Orbigny and Gervais, 1844), and the lesser grison with negative results (Ribicich et al., 2010; Winter et al., 2018). Thus, this is the first record of Trichinella spp. larvae in a native mustelid in South America, and the first record of T. spiralis in the güiña. The güiña is the second reported South American felid host for this species.
Previously, other mustelids have been reported to host Trichinella infections: American mink infected with T. spiralis in Chile (Espinoza-Rojas et al., 2021) and with T. spiralis, T. britovi, and T. pseudospiralis in Poland (Hurníková et al., 2016) and the European badger (Meles meles Linnaeus, 1758) infected with T. britovi in Romania (Boros et al., 2020). Similarly, other felids have reportedly harbored Trichinella larvae. Trichinella infections have been reported in cougars across most of their range including with T. spiralis in Chile (Landaeta-Aqueveque et al., 2015; Echeverry et al., 2021), T. patagoniensis in Argentina (Krivokapich et al., 2012), T. spiralis and T. pseudospiralis in the United States (Reichard et al., 2015), Trichinella nativa Britov and Boev, 1972, T. pseudospiralis, Trichinella murrelli Pozio and La Rosa, 2000, and Trichinella T6 in Canada (Gajadhar & Forbes, 2010). Additionally, infections have been reported in Canadian lynx (Lynx canadensis Kerr, 1792) with Trichinella T6 in Canada (Gajadhar & Forbes, 2010), Eurasian lynx (Lynx lynx Schreber, 1777) with T. britovi, and the European wildcat (Felis silvestris Schreber, 1777) with T. britovi and T. spiralis (Pozio et al., 2009).
The güiña is one of the smallest felids in the world. It is distributed across Chile and Argentina between latitudes of 33°S and 48°S (Napolitano et al., 2014). This felid consumes micromammals such as rodents as primary prey (Delibes-Mateos et al., 2014; Figueroa, Corales & Rau, 2018); consequently, rodents could be the source of infection. Rodents have been recognized as hosts of T. spiralis, mainly in the domestic environment in Chile (Schenone et al., 1967; Schenone et al., 2002). This record is in accordance with the fact that güiñas have been frequently infected by pathogens from free-roaming domestic animals (Ortega et al., 2020; Sacristán et al., 2020); although T. spiralis is not an important pathogen for the health of non-human animals, its presence in the güiña highlights the need for pathogen surveillance in the rural–sylvatic interphase.
The lesser grison is a neotropical mustelid that inhabits an area spanning southern Peru, Uruguay, and Paraguay to southern Chile and Argentina, encompassing several environments (Prevosti & Travaini, 2005). It is a generalist predator and rodents comprise an important part of its diet (Ebensperger, Mella & Simonetti, 1991; Zapata et al., 2005). Given that, and considering how other pathogens have spilled from domestic animals (Megid et al., 2013; Pedrassani et al., 2018), this species might most likely be infected in domestic environments. However, identification of the Trichinella species harbored by the lesser grison helps to better understand the source of infection, given that not all Trichinella species identified in South America have been reported in the domestic cycle. For instance, T. patagoniensis has been reported only in cougars (Krivokapich et al., 2008; Krivokapich et al., 2012).
To the best of our knowledge, there are no reports of the güiña as prey of larger predators, whereas the lesser horned owl (Bubo magellanicus) is the sole predator to be reported for the lesser grison (Prevosti & Travaini, 2005). In that respect, T. pseudospiralis, also zoonotic, is the only species of the genus that has reportedly infected birds, and this may be the only species of Trichinella that could be transmitted from the grison to the owl. However, this species has not been reported in Chile and one record of a single pig from Argentina represents the only report in South America (Krivokapich et al., 2015). Therefore, it is unlikely that this owl could play a role in the sylvatic cycle of Trichinella in Chile. Hence, whether güiña and lesser grison participate in the reservoir or constitute dead-end hosts is unknown, and the most likely way for Trichinella larvae to be transmitted from these hosts seems to be their consumption by carrion-consuming mammals. Furthermore, human trichinellosis resulting from the direct consumption of a wild mammal has also been reported worldwide (García et al., 2005; Fichi et al., 2015); however, neither güiñas nor grisons are typical prey for hunters to eat, nor is their hunting permitted by law in Chile (SAG, 2012). However, further studies are needed to evaluate these hypotheses.
It is worth noting that the two types of mammal host species reported herein had the largest sample sizes, suggesting that larger samples of other mammals could represent new hosts for Trichinella. In contrast, the lack of findings identified by Alvarez et al. (1970) may have been due to the real absence of larvae in their samples, as well as to the parasitological technique (trichinoscopy) used, which is of lower sensitivity (Forbes, Parker & Scandrett, 2003).
Conclusions
This is the first record of Trichinella larvae in a native mustelid, G. cuja, in South America, as well as the first record of T. spiralis in L. guigna. Thus, this study increased the number of mammals infected with Trichinella larvae in the neotropics, enhancing the need to identify the role played by neotropical animals in the reservoir for humans. This underlies how studying the rural–sylvatic interphase is of utmost importance.