Settling taxonomic and nomenclatural problems in brine shrimps, Artemia (Crustacea: Branchiopoda: Anostraca), by integrating mitogenomics, marker discordances and nomenclature rules
- Published
- Accepted
- Received
- Academic Editor
- Gabriela Parra Olea
- Subject Areas
- Biodiversity, Evolutionary Studies, Genomics, Taxonomy, Zoology
- Keywords
- Systematics, Phylogeny, New synonymies, Crustacea, Salterns, Salt Lakes, Fossil dating, Mitogenomics
- Copyright
- © 2021 Sainz-Escudero et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Settling taxonomic and nomenclatural problems in brine shrimps, Artemia (Crustacea: Branchiopoda: Anostraca), by integrating mitogenomics, marker discordances and nomenclature rules. PeerJ 9:e10865 https://doi.org/10.7717/peerj.10865
Abstract
High morphological plasticity in populations of brine shrimp subjected to different environmental conditions, mainly salinity, hindered for centuries the identification of the taxonomic entities encompassed within Artemia. In addition, the mismatch between molecular and morphological evolution rates complicates the characterization of evolutionary lineages, generating taxonomic problems. Here, we propose a phylogenetic hypothesis for Artemia based on two new complete mitogenomes, and determine levels of congruence in the definition of evolutionary units using nuclear and mtDNA data. We used a fossil of Artemia to calibrate the molecular clock and discuss divergence times within the genus. The hypothesis proposed herein suggests a more recent time frame for lineage splitting than previously considered. Phylogeographic analyses were performed using GenBank available mitochondrial and nuclear markers. Evidence of gen e flow, identified through discordances between nuclear and mtDNA markers, was used to reconsider the specific status of some taxa. As a result, we consider Artemia to be represented by five evolutionary units: Southern Cone, Mediterranean—South African, New World, Western Asian, and Eastern Asian Lineages. After an exhaustive bibliographical revision, unavailable names for nomenclatural purposes were discarded. The remaining available names have been assigned to their respective evolutionary lineage. The proper names for the evolutionary units in which brine shrimps are structured remain as follows: Artemia persimilis Piccinelli & Prosdocimi, 1968 for the Southern Cone Lineage, Artemia salina (Linnaeus, 1758) for the Mediterranean-SouthAfrican Lineage, Artemia urmiana Günther, 1899 for the Western Asian Lineage, and Artemia sinica Cai, 1989 for the Eastern Asian Lineage. The name Artemia monica Verrill, 1869 has nomenclatural priority over A. franciscana Kellogg, 1906 for naming the New World Lineage. New synonymies are proposed for A. salina (= C. dybowskii Grochowski, 1896 n. syn., and A. tunisiana Bowen & Sterling, 1978 n. syn.), A. monica (= A. franciscana Kellogg, 1906 n. syn., and A. salina var. pacifica Sars, 1904 n. syn.); A. urmiana (= B. milhausenii Fischer de Waldheim, 1834 n. syn., A. koeppeniana Fischer, 1851 n. syn., A. proxima King, 1855 n. syn., A. s. var. biloba Entz, 1886 n. syn., A. s. var. furcata Entz, 1886 n. syn., A. asiatica Walter, 1887 n. syn., A. parthenogenetica Bowen & Sterling, 1978 n. syn., A. ebinurica Qian & Wang, 1992 n. syn., A. murae Naganawa, 2017 n. syn., and A. frameshifta Naganawa & Mura, 2017 n. syn.). Internal deep nuclear structuring within the A. monica and A. salina clades, might suggest the existence of additional evolutionary units within these taxa.
Introduction
Taxonomic practice includes two separated but closely linked activities: the recognition and definition of the biological units resulting from speciation processes and the provision of a universal name for each of those biological units (Wiley, 1981; Minelli, 2003; De Carvalho et al., 2005; Padial et al., 2010). Recognition of biological units follows the classical scientific methodology: observation, hypotheses formulation, data gathering, hypotheses testing, and proposal to the scientific community for further testing, since species are also working hypotheses. Provision of a universal name for each animal is done by strictly applying the rules and recommendations of a code of practice, the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999), provided by the International Commission on Zoological Nomenclature.
Historical confusion between these two activities, identification of biological units and naming them, has rendered taxonomy a sort of obscure, almost mystical, discipline, difficult to accommodate to society or even to be understood by non-taxonomist scientists (Rosen, 1986; Dubois, 2003; Lipscomb, Platnick & Wheeler, 2003; Mace, 2004; Wheeler & Valdecasas, 2005; Garnett & Christidis, 2007; Ebach, Valdecasas & Wheeler, 2011).
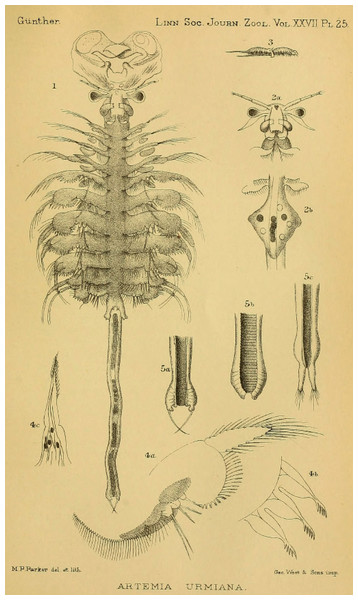
The systematics and nomenclature of the brine shrimp (Artemia Leach, 1819) is a clear example of the problems that nomenclatural practice, when not carefully considered, can generate when studying model organisms. Artemia is a poorly diversified group of small hypersaline water branchiopods (Crustacea, Anostraca), currently conformed by less than a dozen species distributed all over the world, often associated to salt production, and used as a model system for diverse research purposes, as well as a valuable food source in aquaculture (Lenz, 1984; Sorgeloos et al., 1986; Sorgeloos, Dhert & Candreva, 2001; Van Stappen, 1996; Ruebhart, Cock & Shaw, 2008; Amat et al., 2005; Baxevanis, Kappas & Abatzopoulos, 2006). Despite the reduced number of species, the different taxa within Artemia have been referred to, in the scientific literature, with more than 50 names, almost all of them used at the species level (Daday de Deés, 1910; Belk & Brtek, 1995; Rogers, 2013; Asem et al., 2020). Most of the names applied from the end of the eighteen to the mid-twentieth century in Artemia taxonomic characterization were forgotten and not used again by later authors. Some of those names were not accompanied by adequate descriptions or were applied to populations no longer existing or hard to locate, making difficult their subsequent evaluation and application (Fischer, 1851; King, 1855; Liévin, 1856; Verrill, 1869 in part; Grube, 1874; Walter, 1887; Grochowski, 1896). However, that was not the case for some others (Fischer de Waldheim, 1834; Verrill, 1869 in part; Sars, 1904) (Fig. 1). The abandonment of older names brought a new series of species descriptions, sometimes applied to populations already named, generating nomenclatural problems that required direct actions from the International Commission on Zoological Nomenclature (International Commission on Zoological Nomenclature, 1985, 1993). However, these actions from the ICZN, were not enough to stabilize brine shrimp nomenclature, and still today, some names remain problematic. Reasons for this problematic nomenclatural situation are of diverse nature, some of them intrinsic, directly related to the peculiar biological characteristics of Artemia, and some of them extrinsic, related to the human perspective of their study.
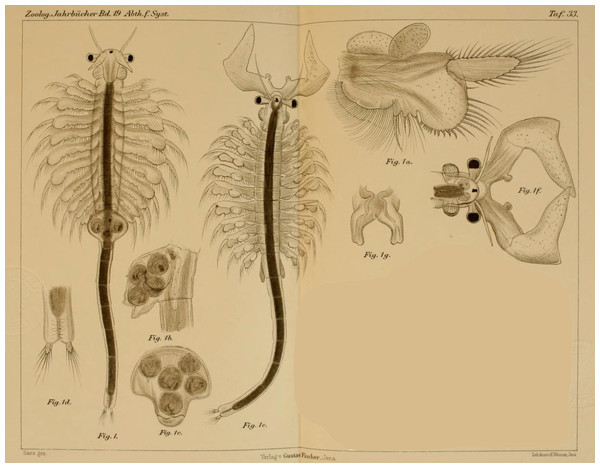
Figure 1: Original illustration of Artemia salina var. pacifica by Sars (1904) from Zoologische Jahrbücher, 19, pl. 33, a high-quality illustration accompanying a precise morphological description of a valid taxon. This is one of the names that, in case molecular data supported their specific ascription, would have nomenclatural priority over Artemia franciscana Kellogg, 1906.
Among the intrinsic factors, we may consider first the extreme morphological and physiological phenotypic plasticity shown by Artemia. Brine shrimps can change dramatically in size, shape or even degree of development of anatomical structures as a function of the salt concentration at which early stages are exposed during their development (Schmankewitsch, 1875, 1876, 1877a, 1877b; Artom, 1907a, 1907b; Asem & Rastegar-Pouyani, 2010; Asem et al., 2010). A second factor involves the diversity of reproductive modes, ranging from the typical bisexual reproduction in Anostraca, to strict parthenogenesis, and from production of resistance eggs (cysts), through almost ovoviviparity (Artom, 1906a, 1906b, 1906c, 1908; Baxevanis, Kappas & Abatzopoulos, 2006; Maccari et al., 2013, 2014). A third source of conflict is the existence of polyploidy, with 3n, 4n and 5n parthenogenetic specimens that can be found either in syntopy with diploid specimens, or forming populations exclusively conformed by diploid or tetraploid parthenogenetic individuals (Artom, 1913, 1921b; Gross, 1932; Barigozzi, 1934, 1980; Barigozzi & Tosi, 1959; Zhang & Lefcort, 1991; Zhang & King, 1993; Sun et al., 1999; Abatzopoulos et al., 2002b, 2003; Maniatsi et al., 2011; Asem, Eimanifar & Sun, 2016). Although it might seem that reproductive attributes could potentially facilitate the taxonomy of Artemia, this diversity was in fact a source of confusion that generated multiple taxonomic descriptions, since names were provided independently for parthenogenetic and bisexual populations.
Taxonomic problems in Artemia are related to changes in taxonomic practice over time. The first period of brine shrimp taxonomy was characterized by a proliferation of new species names, defined on the basis of morphological traits later shown to be plastic, and generally applied to populations of a single saltern or salt-lake (Fischer de Waldheim, 1834; Fischer, 1851; Liévin, 1856; Entz, 1886; Verrill, 1869; Grube, 1874; Walter, 1887; Grochowski, 1896; Günther, 1899; Sayce, 1903; Kellogg, 1906). A second historical period involved definition of species based on reproductive mode and laboratory reproductive isolation, coupled or not with protein or cytogenetic analyses. During this period, previously considered units were redefined yielding a new set of names (Piccinelli & Prosdocimi, 1968; Bowen & Sterling, 1978; Barigozzi, 1980; Cai, 1989a, 1989b; Browne & Bowen, 1991; Pilla & Beardmore, 1994). The third and current period of species delimitation, based mainly on molecular DNA information, generated a few more species names and turned species delimitation based almost exclusively on mitochondrial sequence analyses (Asem, Eimanifar & Sun, 2016; Naganawa & Mura, 2017). In addition to all of these numerous taxonomic proposals, it is necessary to remark a poorly done nomenclatural work, sometimes neglecting basic priority principles, ignoring previous species descriptions, or presenting vague type localities, or even not designating type specimens (Kellogg, 1906; Bowen & Sterling, 1978; Abatzopoulos, Zhang & Sorgeloos, 1998). It is difficult to believe that a proper revision of the nomenclature in accordance to the rules and recommendations of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999) has not been performed yet for one of the most world-wide commercialized invertebrates. Only Asem, Rastegar-Pouyani & De Los Ríos-Escalante (2010) made a clarification attempt, and recently, Asem et al. (2020) reviewed the taxonomic problems of native Asian Artemia. The task has been probably avoided either because the early inclusion of partial genetic data in the definition of taxa blurred the overall picture (Alonso, 1996), or because the early proliferation of names made the selection of valid names for the molecularly defined taxa a complicated task. Worldwide monographs or catalogues of Anostraca included all names under the synonymy of Artemia salina (Linnaeus, 1758) (Linder, 1941; Botnariuc & Orghidan, 1953), or more recently, considered many available names as nomina nuda (Belk & Brtek, 1995; Rogers, 2013).
Recently, different research teams have been trying to disentangle the taxonomic problems derived from the complex biology of brine shrimps (Baxevanis, Kappas & Abatzopoulos, 2006; Muñoz et al., 2008; Kappas et al., 2009; Kappas, Baxevanis & Abatzopoulos, 2011; Maniatsi et al., 2011; Maccari, Amat & Gómez, 2013; Maccari et al., 2014; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016). These researchers have successfully dealt with the origin and relationships of the parthenogenetic strains, and the evolutionary relationships of the polyploid populations. However, the nomenclatural acts necessary to fix the taxonomic situation of the already identified units cannot be undertaken without a full review of the current set of nomenclatural problems. This situation needs to be sorted out, including the identification of truly problematic areas that have direct consequences on species identification, conservation, or economic impact. In this work, we tried to achieve two goals; first, to present an informed hypothesis on how many singular and evolutionary independent taxa can be defined to date within Artemia following the evolutionary species concept (Wiley, 1978), and second, to identify the correct name for each of the biological entities (e.g., species) recovered.
To accomplish this goal, we (1) provide a new mitogenomic robust phylogenetic hypothesis for Artemia, with the inclusion of the first mitogenome of the bisexual A. salina, and of a Mexican population of A. franciscana (= A. monica); (2) propose a documented hypothesis on how many evolutionary independent taxonomic units are recognizable within Artemia by evaluating levels of congruence between already published mitochondrial DNA (mtDNA) and nuclear data, including fast evolving genes; and (3) identify the biological meaning and identity of each of the published names applied to populations of Artemia. In order to accomplish the latter objective, we searched for all the information available in the original bibliographical sources, including original descriptions, reproduction mode, ploidy level, and geographic location of the populations from where names were published.
Materials and Methods
Mitogenome analyses
Adult specimens from Laguna Ojo de Liebre, Guerrero Negro, Baja California Sur (BCS) (Mexico) (Arthropod Collection of Museo Nacional de Ciencias Naturales, MNCN 20.04/12541), and of A. salina from Salobrar de Campos, Es Trenc, Mallorca (Spain) (MNCN 20.04/12092), stored in absolute ethanol, were used for this study. One specimen of each locality was sent to AllGenetics for DNA extraction and high-throughput sequencing. Briefly, total genomic DNA was extracted using the “RealPure MicroSpin kit” (Durviz®) following the protocol described by the manufacturer. Libraries were prepared using the Nextera DNA Library Prep kit (Illumina, San Diego, CA, USA) and sequenced in an Illumina HiSeq 4000 PE100 lane. Raw data were first cleaned using the R package BBmap (sourceforge.net/projects/bbmap). Genome assembly of the Mexican specimen was carried out using as reference a sequence of the complete cox1 gene of a record named as A. franciscana available in Genbank (accession number: NC001620.1), whereas for the sample of A. salina a partial sequence of cox1 was used as seed (accession number: KX925417.1), and to avoid possible bias, checked against EU543451.1 (Muñoz et al., 2008). Finally, annotation was performed using the MITOchondrial genome annotation server 2 (MITOS2) (Bernt et al., 2012), checking manually the start and stop codons of all coding genes. The circular map of the Artemia mitogenome and its constituent genes are represented in Fig. S1. Mitogenomic annotations are specified in Tables S1 and S2. Newly generated mitogenomes were deposited in Genbank with the accession numbers MT495440 and MT495441, respectively.
We gathered all the complete mitogenomes of Anostraca published in the literature and available in GenBank to construct a data set composed of: a mitogenome of A. franciscana from San Francisco Bay (NC001620.1) (Perez et al., 1994; Valverde et al., 1994), a mitogenome of A. sinica (MK069595.1) (Asem et al., 2019), another of A. urmiana, and two mitogenomes from specimens of two populations of Tibet (identified as A. tibetiana, NC021382.1, JQ975177.1, JQ975178.1 respectively) (Zhang et al., 2013). Finally, to include a sample of A. persimilis we merged partial mitogenomic sequences of three genes (cox1, 12S, and 16S) derived from two different Argentinian samples (KX925418, KX925432 (Qian, Yuangao & Liying, GenBank); FJ007810 (Kappas et al., 2009)). Mitogenomes of Streptocephalus sirindhornae Sanoamuang et al., 2000 (KP273593.1 (Liu et al., 2015)) and of Phallocryptus tserensodnomi Alonso & Ventura, 2013 (NC026710 (Fan, Lu & Yang, 2016)) were included as outgroups.
For phylogenetic reconstruction purposes, we considered only protein-coding and ribosomal RNA genes, since tRNA genes are highly conserved and resulted to be non-informative. We first extracted a matrix for each protein-coding gene, then we aligned each gene matrix based on their corresponding amino acid translations according to the invertebrate mitochondrial genetic code using the TranslatorX Web Server (Abascal, Zardoya & Telford, 2010) by selecting the MAFFT algorithm (Katoh et al., 2005). We allowed TranslatorX to determine the most likely reading frame. We cleaned the matrixes by removing poorly aligned sites under the Gblocks protein information criterion (Castresana, 2000). For a less stringent selection of the positions to be discarded we allowed gap positions within the final blocks, and for a more stringent selection we did not allow many contiguous non-conserved positions (Abascal, Zardoya & Telford, 2010). RNA genes were aligned and cleaned through the MAFFT and Gblocks online services (Katoh, Rozewicki & Yamada, 2017; Talavera & Castresana, 2007). PartitionFinder v2 (Lanfear et al., 2017) was used to select the best partition scheme and molecular evolutionary models under the Bayesian Information Criterion (BIC; Schwarz, 1978) (Table S2). Because of previous reports of accelerated nucleotide rates (Crease, 1999; Hebert et al., 2002), we tried to reduce the possible effect of saturation by using a data set including only amino acid sequences of coding mtDNA plus ribosomal genes.
Phylogenetic reconstruction was performed using a Bayesian Inference approach implemented in MrBayes version 3.2.6 (Ronquist et al., 2012), using the amino acid + ribosomal concatenated data. MrBayes analyses consisted of two simultaneous runs of 100 million generations each, sampling trees every 10,000 generations. Mixing and convergence among runs were evaluated by checking the average standard deviation of split frequencies, the EES values and the Potential Scale Reduction Factor (PSRF) for each parameter. A majority consensus tree was reconstructed after discarding the first 2,000 sampled trees as burn-in.
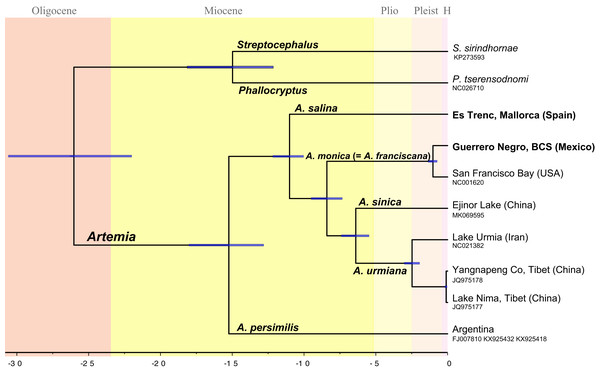
Divergence times across taxa within Artemia (Fig. 2) were estimated using Bayesian relaxed molecular clocks implemented in BEAST version 1.8.2 (Drummond et al., 2012). In order to calibrate the molecular clock, we used information derived from fossil specimens originally identified as A. salina from the Messinian Kalavasos Formation in Cyprus (Manzi et al., 2016). Since the identification of the fossil at the species level is difficult to determine, we considered two alternative scenarios where the fossil might be differently placed. A first scenario (Scheme 1) followed the identification of Manzi et al. (2016) and the fossil was set at the node which clusters all species of Artemia excluding A. persimilis (Fig. 2). Alternatively, the fossil was treated as a member of the Asian clade (Scheme 2) and thus, the calibration point was settled at the node which clusters the Asian species (Fig. S2). These analyses were performed on the concatenated data set partitioned by gene. This matrix was composed of 13 partitions, the first two corresponding to the ribosomal genes, and the remaining corresponding to the protein-coding genes, except NAD4 and NAD4L, and ATP6 and ATP8, which were merged within the same partition. Site models as well as molecular clocks were unlinked across genes. Trees were linked to ensure that all partitions shared the same tree topology. We used uncorrelated lognormal relaxed clocks with an uninformative prior of substitution rates (gamma distribution, initial value = 0.01, shape = 0.01, offset = 0). Manzi et al. (2016) estimated that the age of the sediments where the fossil was found was about 5.55 Ma. This age was used as a minimum age for the node, a prior with a lognormal distribution (offset = 5.55, mean = 5.55, standard deviation = 0.1), in each of the two proposed scenarios above. Birth-Death model was set as tree prior. The analyses were run for 100 million generations, sampling every 10,000; we inspected the trace plots and effective sample sizes in Tracer 1.8.0 (Drummond & Rambaut, 2007). The first 20 million states were discarded as burn-in. We used Bayes Factor comparison as implemented in BEAST to compare the marginal likelihood value of the two alternative scenarios in which the fossil was placed. To perform marginal likelihood estimations using path sampling (PS)/stepping-stone sampling (Baele et al., 2012) we selected the respective option in the MCMC-BEAUti panel following the default settings. We compared the two marginal likelihood values using the likelihood ratio test, 2Ln (H0–H1). We followed the interpretation of Kass & Raftery (1995) according to which values larger than 2 indicate positive support for one model over the other, and values larger than 6 indicate strong positive support.
Figure 2: Chronogram showing lineage divergence times in Artemia obtained using BEAST following the first scenario hypothesis (Scheme 1).
Time indicated in million years (Ma). Dark blue horizontal bars represent 95% HPD (High Posterior Density). A posterior probability value of 1 was obtained for all nodes.Additionally, divergence times across Artemia were estimated by using published nucleotide substitution rates to offer a comparison against the results obtained with fossil evidence. In one case, we used the coxI nucleotide substitution rate that was previously used to date phylogenies of Anostraca (Reniers et al., 2013; Lindholm et al., 2016; Rodríguez-Flores et al., 2020) (Scheme 3, Fig. S3). CoxI nucleotide substitution rate was estimated from the speciation event of two sister species of snapping shrimps (Decapoda: Alpheidae) separated by the closure of the Isthmus of Panama (Knowlton & Weight, 1998). In a second case, we used the average mitochondrial substitution rate for Artemia obtained by Luchetti et al. (2019), who calculated the substitution rates per branch in a time-tree that included representative species of Anostraca, Cladocera and Notostraca (Scheme 4, Fig. S4). For the first case (Scheme 3) we used the concatenated data set partitioned by gene. Site models as well as molecular clocks were unlinked across genes using uncorrelated lognormal relaxed clocks. A lognormal distribution in real space (initial value = 1.0, mean = 0.007, standard deviation = 0.1) was assigned for the ucld.mean parameter of the coxI marker. For the remaining markers, we used uninformative priors (gamma distribution, initial value = 0.01, shape = 0.01, offset = 0). For the second case (Scheme 4), we also used the concatenated dataset with partitions per gene. Site models were unlinked but, differently from the previous case, molecular clocks were linked since the substitution rate was calculated for the entire mitogenome. A lognormal distribution in real space (initial value = 0.0045, mean = 0.0045, standard deviation = 0.18) was assigned for the ucld.mean parameter. The length of the MCMC chain was 100 million generations, sampling every 10,000. Trace plots and effective sample size were inspected in Tracer 1.8.0 (Drummond & Rambaut, 2007). Finally, the first 20 million states were discarded as burn-in.
All analyses were run in the web public resource CIPRES Science Gateway version 3.3 (Miller, Pfeiffer & Schwartz, 2011).
To compare these dating Schemes (1–4) with previous divergence time estimations in Artemia, we replicated our analysis using a nucleotide matrix, instead of the amino acid one already used. This matrix was composed of the same 13 partitions explained above. We relaxed the age limits to avoid fixation of narrow bonds imposed by the date of the fossil. Additionally, we replicated the divergence time estimation of Eimanifar, Van Stappen & Wink (2015) using this nucleotide dataset. To accomplish this, we used the estimated age of the node that separates A. salina from the remaining lineages obtained in Eimanifar, Van Stappen & Wink (2015) (27 Ma, 95% HPD 67.49–10.54 Ma) to calibrate the molecular clock (lognormal distribution, offset = 10.65, mean = 20, standard deviation = 0.6).
Analyses of available nuclear DNA and mtDNA data
A total of 428 nDNA sequences of the ITS region of Artemia (Abatzopoulos et al., 2009; Asem, Eimanifar & Sun, 2016, Asem et al., 2019; Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar et al., 2014; Kappas et al., 2009; Maccari, Amat & Gómez, 2013; Maniatsi et al., 2009; Valsala, Sugathan & Bharathan, 2015; Vikas et al., 2012) and one of Streptocephalus proboscideus (Frauenfeld, 1873) (AY519840) used as outgroup, were downloaded from GenBank and aligned using MAFFT algorithm (Katoh & Toh, 2008). The resulting matrix was cleaned through Gblocks DNA information criterion (Castresana, 2000) excluding several contiguous non-conserved positions and allowing gap positions within the final blocks. A collapsed-haplotype matrix was obtained using the web-based tool ALTER (Glez-Peña et al., 2010) allowing gaps as variable characters. Phylogenetic analyses were performed using a Bayesian Inference approach implemented in MrBayes, using the ITS collapsed data (a total of 226 sequences, including the outgroup). The best substitution model was estimated by setting the command lset nst to mixed. This procedure results in the Markov chain sampling over the space of all possible reversible substitution models, no matter whether they have a name (e.g., HKY, F81) or not. The analysis consisted of a run of 5 million generations, sampling trees every 1,000 generations.
Because of their different effective population sizes, and being differently conditioned by ploidy and inheritance mechanisms, phylogeographic analyses were performed for nuclear and mitochondrial molecular markers separately (Rodríguez-Flores et al., 2017, 2020). Phylogeographic analyses for the New World Lineage, based on cox1 mtDNA data, were performed once all sequences from areas falling outside the assumed native distribution of the New World Lineage (American Continent) were removed (Eimanifar et al., 2014; Eimanifar, Van Stappen & Wink, 2015). A cox1 fragment, extracted from the mitogenome of the specimen from Laguna Ojo de Liebre (Guerrero Negro, Baja California, Mexico) was also included. Sequences were dealt with DNA Sequence Polymorphism version 6.12.01 (Rozas et al., 2017) and collapsed to haplotypes or unique alleles. Gaps in the nuclear marker were treated as variable characters, and consequently a matrix in which each gapped position was considered as a different character was used in the analyses. Networks were constructed through Population Analysis with Reticulate Trees (PopART) 1.7 software (Leigh & Bryant, 2015) applying a TCS algorithm to shape the relationships between alleles. All the information on sequence-haplotype correspondence and their bibliographic sources is shown in Tables 1 and 2.
Note:
Nuclear sequences of the Western Asian Lineage (A. urmiana) used in this study. Names used for populations with available gene sequences are those originally mentioned by their respective authors (Literature referred Taxon). In “GenBank accession number” column, a semi-colon separates sequences by groups according to bibliographic sources, as indicated in “Literature source” column. Symbol “*” indicates that the corresponding sequences were not reported in publications.
Note:
MtDNA sequences of the New World Lineage (A. monica = A. franciscana) used in this study. Names used for populations with available gene sequences are those originally mentioned by their respective authors (Literature referred Taxon). * indicates samples from Mono Lake (California). In “GenBank accession number” column, a semi-colon separates sequences by groups according to bibliographic sources, as indicated in “Literature source” column.
Nomenclature
An exhaustive bibliographical search was undertaken to locate and gather all original publications in which any possible nomenclatural act affecting Artemia was published. The search started with four main sources for synonymies: Daday de Deés (1910), Belk & Brtek (1995), Asem, Rastegar-Pouyani & De Los Ríos-Escalante (2010), Asem et al. (2020), and Rogers (2013). From there, we sought for any additional bibliographic information mentioned in each of the papers consulted. A final search through the Zoological Record database was completed. Each publication was carefully revised in two ways, a first one to obtain data on reproduction mode, ploidy level if available, and precise geographic location of the populations from where names were published; and a second one to evaluate every taxonomic decision made by subsequent authors upon these names in accordance to the rules and recommendations of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999). The second revision included examination of some taxonomic features, including level of detail in the morphological description, designation of type series or holotype, original intention of the author while providing a name (see “Appendix I” for unavailable names), and a subjective evaluation of the methods used to define the evolutionary unit on which the name was applied.
In order to preserve the desired nomenclatural stability, we have tried to assign each of the available names to their respective biological unit. For this task, we used information from type localities (terrae typicae) from which taxa were described, because, even if at the time of the description reproductive mode, ploidy, or mtDNA lineage were not recorded, in some cases they were studied subsequently. Problems arose when type locality was not precise, or when introductions were taking place in the area, rendering impossible to determine if the new data gathered actually corresponded to the originally named population (see nomina dubia in “Appendix II”). There are names that have been applied historically to parthenogenetic populations, but because they are considered to be the same species as their closely related bisexuals (see below), any of the names applied to parthenogenetic populations are also available for naming the species to which they belong (International Commission on Zoological Nomenclature, 1999).
Results
Genome content and organization
The complete mitochondrial genomes of A. salina and A. franciscana are typical circular DNA molecules of 15,436 bp and 15,825 bp, respectively (Tables S1 and S2; Fig. S1). These mitogenomes encoded the typical 37 genes, including 13 protein-coding genes, 22 transfer RNAs and 2 ribosomal RNAs and a putative mtDNA control region. Like many other mitochondrial genomes of arthropods, the major strand (J strand) carried most of the genes (9 PCGs and 13 tRNAs), while the remaining genes were on the minor strand (N strand). Gene order and orientation were the same as indicated in the previously published Artemia mitogenomes (Perez et al., 1994; Valverde et al., 1994; Zhang et al., 2013; Asem et al., 2019).
Phylogeny of Artemia
The topology of the Bayesian phylogram derived from the amino acid + ribosomal concatenated mitochondrial data set was totally congruent with the topology of the ultrametric tree obtained from BEAST (Fig. 2). All nodes are supported with a posterior probability of 1 (PP).
The obtained temporal schemes of diversification in Artemia differ markedly depending on the type of evidence used to calibrate the molecular clock (Fig. 2; Figs. S2, S3 and S4) (Table 3). For example, the earliest diversification event within the genus took place in the Late Miocene according to Scheme 3, or in the Paleocene (Scheme 4). The ages of the speciation events within Artemia under the different schemes are summarized in Table 3.
| Node | Node description |
Scheme 1 Mean/95%HPD Ma* |
Scheme 2 Mean/95%HPD Ma |
Scheme 3 Mean/95%HPD Ma |
Scheme 4 Mean/95%HPD Ma |
Scheme 5 Mean/95%HPD Ma |
|---|---|---|---|---|---|---|
| 1 | First diversification event within Artemia | 15.29/18.15–12.82 | 26.34/32.01–21.44 | 9.73/13.43–6.56 | 60.55/97.36–36.39 | – |
| 2 | Split between A. salina and the Asian + A. monica Clade | 11.02/12.19–10.04 | 19.02/22.12–16.35 | 7.22/9.89–5.18 | 47.42/77.04–25.32 | 27/67.49–10.54 |
| 3 | Split between A. monica and the Asian lineage | 8.42/9.55–7.32 | 14.52/16.55–12.66 | 5.51/7.59–3.97 | 36.71/60.93–18.93 | 34.01/65.42–16.96 |
| 4 | Speciation event that originated A. urmiana and A. sinica | 6.6/7.40–5.47 | 11.03/12.15–9.98 | 3.92/5.37–2.75 | 24.48/43.15–12.34 | 19.99/36.69–9.37 |
Note:
Comparison of different temporal diversification schemes in Artemia. Scheme 1: using the fossil of Artemia described by Manzi et al. (2016) to date the split between A. salina and the Asian + A. monica Clade; Scheme 2: using the Artemia fossil described by Manzi et al. (2016) to date the ancestral node of Asian Artemia; Scheme 3: using the coxI nucleotide substitution rate estimated for Alpheidae (Decapoda) (Knowlton & Weight, 1998); Scheme 4: using a “total mitogenomic” nucleotide substitution rate for Artemia (Luchetti et al., 2019); Scheme 5: using a fossil of Daphnia (Eimanifar, Van Stappen & Wink, 2015) (notice that Eimanifar, Van Stappen & Wink, 2015, tree topology differs from ours in the relative position of A. monica = A. franciscana and A. salina). *Ma stands for Mega anum (1,000,000 years).
Bayes Factor comparison between the model marginal likelihoods of Schemes 1 and 2, favors scheme 1 hypothesis: 2lnBF = 2*((−34245.58) − (−34274.06)) = 58.96, which, according to the scale given in Kass & Raftery (1995), can be interpreted as very strong support in favor of Scheme 1.
All phylogenetic analyses yielded the same tree topology. This topology is described below, incorporating the TMRCAs corresponding to Scheme 1. The sample representing the Southern Cone lineage (A. persimilis) is sister to a clade that includes all the remaining ingroup samples (PP = 1); the splitting event between A. persimilis and the ancestor of all the remaining Artemia took place about 15.3 Ma (95% HPD 18.15–12.8 Ma). This separation event coincides with the split of the outgroup species (P. tserensodnomi and S. sirindhornae). A subsequent speciation event, 11.0 Ma (95% HPD 12.19–10.04 Ma), separated the Mediterranean-South African lineage (A. salina) from the ancestor of all other taxa during the Late Miocene. The clade composed by the North American samples (A. monica, see taxonomic discussion) is sister to the Asian Clade (PP = 1). These two clades started to diverge about 8.4 Ma (95% HPD 9.55–7.32 Ma). The two specimens that conform the North American lineage, Guerrero Negro and San Francisco Bay, diverged in the Pleistocene, 1 Ma (95% HPD 1.37–0.72 Ma). Separation between the Eastern (A. sinica) and Western (A. urmiana) Asian lineages occurred about 6.6 Ma (95% HPD 7.40–5.47 Ma). Mitogenome information suggests that historical isolation within the Western Asian lineage started 2.4 Ma (95% HPD 3.02–1.95 Ma) by the divergence of Tibetan populations from the remaining populations that conform this clade.
The Bayesian analysis of the nuclear marker dataset (ITS region) generated a tree constituted by five well-supported clades (Fig. 3). Main clades show posterior probabilities between 0.90 and 1 (black spots), although relationships among them are not always fully resolved: The Southern Cone Lineage constitutes a well-defined clade and includes bisexual populations from Chile (Pichilemu and Torres del Paine), and Argentina (Buenos Aires) (Baxevanis, Kappas & Abatzopoulos, 2006; Kappas et al., 2009). The New World Lineage is conformed by some well differentiated internal clades, in which specimens from populations from Argentina, Brazil, Canada, Chile, Mexico, Jamaica and USA (Great Salt Lake and San Francisco Bay) are included. Introduced populations from Cape Verde, China, India, Iraq, Iran, Italy, Portugal, South Africa, Sri Lanka and Vietnam (Baxevanis, Kappas & Abatzopoulos, 2006; Kappas et al., 2009; Maniatsi et al., 2009; Vikas et al., 2012; Eimanifar et al., 2014; Valsala, Sugathan & Bharathan, 2015), fall also in this clade. The Asian Lineage is formed by two well defined and separated clades: Western and Eastern Asian clades. The Western clade contains bisexual populations from Iran, Ukraine, Tibet and Kazakhstan, and diploid, triploid, tetraploid and pentaploid parthenogenetic populations from Azerbaijan, China (including Tibetan populations), India, Iraq, Iran, Kazakhstan, Pakistan, Russia, Turkey, Turkmenistan, Ukraine, Uzbekistan; in addition, it includes also parthenogenetic populations form Albania, Egypt, Greece, Italy, Israel, Madagascar and Namibia (Baxevanis, Kappas & Abatzopoulos, 2006; Abatzopoulos et al., 2009; Kappas et al., 2009; Maccari, Amat & Gómez, 2013; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016; Asem et al., 2019). The relationships among populations within the Western Asian Clade remain unresolved. The Eastern Asian clade includes bisexual and parthenogenetic populations from different Chinese locations (Kappas et al., 2009; Maccari, Amat & Gómez, 2013; Eimanifar et al., 2014). Finally, the Mediterranean—South African clade is formed by bisexual populations from Algeria, Cyprus, Egypt, Italy, Libya, Spain, South Africa and Tunisia (Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar et al., 2014).
Figure 3: Bayesian phylogenetic relationships of Artemia based on nuclear ITS1 region sequences (see Materials and Methods for sequence original sources).
Note the position of populations form Tibet and Kazakhstan. Posterior probabilities >0.90 indicated by black dots.Phylogeographic analyses
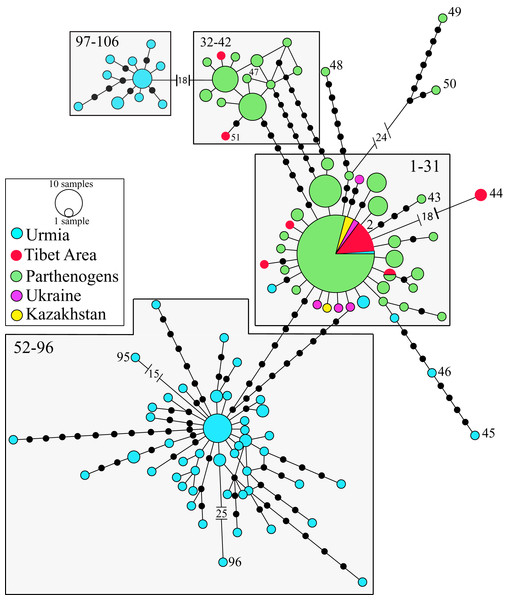
The phylogeographic analysis of the nuclear data set of the Western Asian Clade (A. urmiana) includes 106 different nuclear alleles (Fig. 4). Specimens from almost all parthenogenetic populations and the bisexual populations from Tibet, Kazakhstan, Ukraine and Lake Urmia (Iran) all share a common allele (#2). Divergent alleles (#48, 49 and 50) correspond to parthenogenetic individuals from Greece and Israel (Baxevanis, Kappas & Abatzopoulos, 2006) and #95 and #96 to bisexual individuals from Lake Urmia (Asem et al., 2019). Some specimens from Lake Urmia (#97 to #106) (Eimanifar et al., 2014) are genetically distant from all other samples. Tibetan bisexual specimens from LagKor Co (haplotype #44) (Baxevanis, Kappas & Abatzopoulos, 2006) differ from two other bisexual specimens of the same locality (#2, Maccari, Amat & Gómez, 2013) by the presence of a gap involving 18 positions, probably caused by a single evolutionary event, with no additional substitution events occurring between them. Nuclear data do not show geographic structure, including a widely distributed allele #2, suggesting that introgression or gen flow is occurring across Western Asian mtDNA defined clades (Baxevanis, Kappas & Abatzopoulos, 2006; Maniatsi et al., 2011; Asem, Eimanifar & Sun, 2016). In addition, laboratory crosses demonstrated inter-fertility between bisexual populations from diverse Asian localities, from Lake Urmia (Iran) to Catvis (Kazakhstan) (Pilla & Beardmore, 1994).
Figure 4: Allelic network of the Western Asian Lineage (A. urmiana) based on ITS1 sequence data (see materials and methods for sequence original sources).
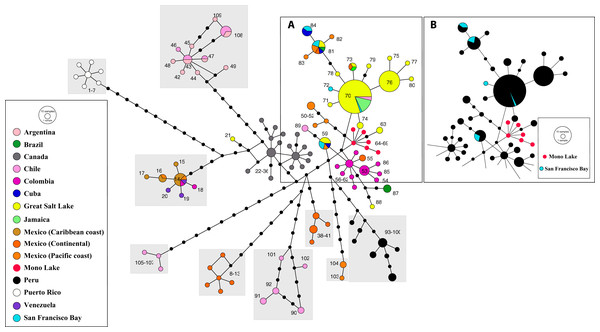
Note that most Tibetan specimens (bisexual populations, in red) share a common allele, or differ by a reduced number of nucleotide substitutions with respect to parthenogenetic populations from all over the continent. Haplotype #44 differs from the widespread haplotype #2 in 18 positions affected by a gap, but otherwise it does not show any nucleotidic change. Size of allele circles is proportional to number of individuals. Numbers indicate allele identification. Black dots separating alleles represent individual nucleotide substitutions. A total of 106 alleles were recorded. Information on sequence-allele correspondence is shown in Table 1.Phylogeographic analysis of the New World Clade included 109 different cox1 haplotypes, published under the names A. franciscana and A. monica (Fig. 5). The haplotype network displays high geographic structure, including multiple cohesive geographic clusters as those from Peru or Puerto Rico, and divergent populations form Mexico, Chile and Argentina. Haplotypes within the core group (Fig. 5A), including those from the Great Salt Lake, Mono Lake and San Francisco Bay (USA), Mexico (Continental and Pacific Coast), Brazil, Cuba, Colombia, Chile, and Jamaica, differ very little with respect to each other. Haplotypes from Mono Lake (type locality of A. monica) are very similar to those from the Great Salt Lake and San Francisco Bay (type locality of A. franciscana) (Fig. 5B).
Figure 5: Haplotype network of cox1 sequence data for the New World Lineage (see Materials and Methods for sequence original sources).
(A) Includes most of the available sequences from western US (Great Salt Lake—yellow, San Francisco Bay—light blue, Mono Lake—red), together with clusters of sequences from México (orange) and Colombia (pink), and presumed introduced populations from Jamaica and Cuba. (B) Is identical to (A), with colors changed to remark visually the close allele proximity between Mono Lake (type locality of A. monica) and San Francisco Bay (type locality of A. franciscana) samples. Nucleotide substitutions between Great Salt Lake and Mono Lake specimens range from 1 to 3. Nucleotide substitutions between Great Salt Lake and San Francisco Bay specimens range from none to 1. According to their position in the network, it is likely that the Great Salt Lake, San Francisco Bay and Mono Lake populations originated from a very recent common ancestor as discussed by Abreu-Grobois & Beardmore (1991). Size of circles proportional to number of individuals sharing haplotype. Numbers identify haplotypes. Black dots separating haplotypes represent individual nucleotide substitutions. A total of 109 haplotypes were included. Information on sequence-haplotype correspondence is shown in Table 2.Discussion
Phylogeny and time of diversification in Artemia
Artemia was recovered as a monophyletic lineage in our mitogenomic phylogeny (Fig. 2), with internal phylogenetic relationships clearly depicting a sister taxon relationship between A. persimilis and the rest of clades, including Old and New World taxa. Previous authors suggested a similar set of relationships based on nuclear and mitochondrial sequences (Baxevanis, Kappas & Abatzopoulos, 2006; Maniatsi et al., 2011; Eimanifar et al., 2014), enabling the rejection of the reciprocal monophyly of the Old Word vs New World taxa.
The dates for the origin of Artemia and of its initial diversification are controversial. Previous authors such as Baxevanis, Kappas & Abatzopoulos (2006) estimated that the origin of Artemia occurred 80–90 Ma, whereas Eimanifar, Van Stappen & Wink (2015) proposed a Late Eocene Origin (34.01 Ma, 95% HPD: 16.96–65.42 Ma). Our estimates provide a much more recent date for the origin of Artemia. Differences between time estimates presented herein and those proposed in previous studies arise from the type of evidence used to calibrate the molecular clock. Geological information is often used to assign a probable age to nodes affected by certain geological event (Hipsley & Müller, 2014; Ho et al., 2015). However, the assumption of divergence as a consequence of specific geological events represents an independent hypothesis that needs to be properly tested and not merely assumed (Kodandaramaiah, 2011; Magallón, 2004). Baxevanis, Kappas & Abatzopoulos (2006) in a pioneer attempt to date the origin of diversification of Artemia, assumed that a series of paleogeographic events were involved in the direct separation of a lineage into a pair of sister taxa, for example, the split of South America from ancient Gondwana in the divergence between the South American A. persimilis and the Eurasian lineages. This approach might produce a considerable overestimation of diversification times, aside of underestimating the cox1 substitution rates in Artemia compared to most arthropods (usually ranging from 1.4 to 2.6% per million year) (Knowlton & Weight, 1998). Eimanifar, Van Stappen & Wink (2015), instead, used an indirect approach to calculate divergence times, estimating the separation between Anostraca and Cladocera using as calibration point a fossil of Daphnia and including samples of Artemia as representatives of Anostraca. However, this approach involved large incomplete sampling, a problem that could affect the estimation of divergence times (Stadler, 2009). Nevertheless, fossils of Artemia were unknown by previous authors and divergence times estimated with the indirect approach of Eimanifar, Van Stappen & Wink (2015) provided a novel overview of the evolutionary history of the family.
Records of fossil specimens provide crucial information on the minimum ages of a clade, although its dating and correct phylogenetic placement is sometimes complex (Thorne, Kishino & Painter, 1998; Magallón, 2004). The identification of Manzi et al. (2016) fossils is problematic since the main character which separates A. salina from other species of Artemia is the absence of a spine at the basis of male penises (Mura & Brecciaroli, 2004), a character that cannot be appreciated in Manzi et al. (2016) fossilized specimens. However, there are some evidences suggesting that the identification of Manzi et al. (2016) fossils as A. salina is probably correct. The location of the fossils, Kalavasos Formation in Cyprus (Manzi et al., 2016), practically rules out the possibility that it corresponds to any of the American lineages. In addition, Muñoz et al. (2008) and Baxevanis et al. (2014) demonstrated that A. salina shows substantial haplotype diversity that appears geographically structured throughout the Mediterranean. This can be considered as an evidence of the continuous presence of A. salina in the Mediterranean area for a very long period of time. Alternatively, the remains could have been part of the Asian Lineage, because they could have been present all over the Eurasian Continent and posteriorly become extinct in the Mediterranean. We have considered this alternative in our dating Scheme 2. The possibility for the fossil to be parthenogenetic, can be ruled out because at least one of the specimens shown by Manzi et al. (2016) is a male. Furthermore, the presumed recent origin of parthenogenesis within Asia (Baxevanis, Kappas & Abatzopoulos, 2006; Maccari, Amat & Gómez, 2013; Maniatsi et al., 2011) would discard such possibility, whereas the fact that the parthenogenetic populations of the Mediterranean share haplotypes with populations from Asia (Maniatsi et al., 2011) is clear signal of their recent arrival to the region.
Considering all available evidences to calibrate the molecular clock and to estimate divergence times within Artemia, it seems quite likely that the times of origin and diversification in Artemia are much more recent than previously considered (Table 3). Although our estimates might be equally probable than previous hypotheses, we consider our Scheme 1 to be a more realistic scenario (Fig. 2) (Scheme 1 is supported vs Scheme 2 in the Bayes Factor comparisons in BEAST). In addition, the fact that the dates estimated according to scheme 1 are closer to those obtained using the general substitution rate for cox1 gene (Scheme 3) (Knowlton & Weight, 1998) makes this hypothesis more likely than those requiring substitutions rates much lower than the general rate (Schemes 4 and 5) (Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar, Van Stappen & Wink, 2015; Luchetti et al., 2019). Luchetti et al. (2019) mutation rate calculated for the entire Artemia mitogenome does not consider different molecular clocks for each individual mitochondrial gene. Therefore, until an exhaustive research about specific molecular substitution rates could be carried out for Artemia, the tempo of diversification within the genus will remain controversial.
Evolutionary units within Artemia and their nomenclature
Based on the phylogenetic and phylogeographic results presented herein, we consider the genus Artemia to be represented by five evolutionary cohesive units (e.g., species), represented by the Southern Cone, Mediterranean—South African, New World, Western Asian, and Eastern Asian Lineages. These units and their nomenclature are discussed in the following paragraphs.
Southern Cone Lineage—Artemia persimilis
The Southern Cone Lineage is a clade geographically restricted to Argentina and Chile. The few populations included in the Southern Cone Lineage are well characterized with respect to the rest of Artemia lineages, by morphological, cytogenetic, allozyme, mtDNA, and nuclear sequence features (Halfer-Cervini, Piccinelli & Prosdocimi, 1967; Halfer-Cervini et al., 1968; Piccinelli & Prosdocimi, 1968; Piccinelli, Prosdocimi & Baratelli Zambruni, 1968; Abreu-Grobois, 1987; Badaracco et al., 1987; Hontoria & Amat, 1992a; Amat et al., 1994; Baratelli & Barigozzi, 1990; Gajardo et al., 1995, 2004; Colihueque & Gajardo, 1996; Rodríguez Gil, Papeschi & Cohen, 1998; Cohen et al., 1999; Cohen, Rodríguez Gil & Vélez, 1999; Zúñiga et al., 1999; De Los Ríos & Zúñiga, 2000). This well-defined evolutionary and taxonomic unit, characterized by a particular chromosome number (2n = 44; while all other bisexual species present 2n = 42) (Abatzopoulos, Kastritsis & Triantaphyllidis, 1986), is sister to all other lineages of Artemia (Fig. 2). The Southern Cone Lineage includes geographically structured nuclear (ITS1) clades (Baxevanis, Kappas & Abatzopoulos, 2006), congruent with mtDNA data (Gajardo et al., 2004) (Fig. 3).
This clade has been referred to, so far, by a unique species name, Artemia persimilis Piccinelli & Prosdocimi, 1968, and except for an unconfirmed report of the species in Italy (Piccinelli & Prosdocimi, 1968; Triantaphyllidis, Abatzopoulos & Sorgeloos, 1998), it has maintained its status as a South American endemic. The synonymic list for Artemia persimilis is as follows:
Artemia persimilis Piccinelli & Prosdocimi, 1968
Artemia persimilis Piccinelli & Prosdocimi, 1968: 116. Terra typica: “Salinas Grandes di Hidalgo, Argentina”. Holotype and the single paratype indicated, held at Museo Civico di Storia Naturale, Verona, Italy (Piccinelli & Prosdocimi, 1968; Belk & Brtek, 1995).
Mediterranean-South African Lineage—Artemia salina
The Mediterranean-South African Lineage comprises two deep geographically structured mitochondrial clades (South African—Mediterranean), with limited separation between them at the nuclear level (Muñoz et al., 2008; Baxevanis et al., 2014), but markedly divergent at the mtDNA level. Mediterranean populations are on turn structured in a Western and an Eastern main nuclear (ITS1 and AFLPs) clades (Triantaphyllidis et al., 1997a; Baxevanis, Kappas & Abatzopoulos, 2006; Baxevanis et al., 2014). The Mediterranean-South African Lineage includes morphologically and genetically diverse populations, with highly modified local morphotypes, but clearly diagnosable from all other lineages (Amat, 1980a; Triantaphyllidis et al., 1997a; Mura & Brecciaroli, 2004). The Mediterranean-South African Lineage is currently known by the name A. salina.
The oldest name for any species of Artemia, Cancer salinus Linnaeus, 1758, was considered problematic (Bowen & Sterling, 1978). Salt extraction at the type locality of Cancer salinus (man-made salterns at Lymington, England) was abandoned, and the brine shrimps disappeared from there, making impossible to collect and study new fresh specimens. We made an inquiry to the Linnean Society (London) to localize any possible material used by Linnaeus (1758) in his description, but the answer was that no material of Cancer salinus was currently preserved at the Institution. However, Mura (1990) and Baxevanis, Kappas & Abatzopoulos (2006) located some material from Lymington at the Natural History Museum (London). Their morphological study confirmed that they represent the traditional and current concept of A. salina (Mura, 1990; Baxevanis, Kappas & Abatzopoulos, 2006).
Once the name Artemia salina (Linnaeus, 1758) is clearly applicable to designate the Mediterranean-South African Lineage (Baxevanis, Kappas & Abatzopoulos, 2006) (neotype designation is however desirable), assignation of additional names to the clade is quite straightforward. Names published for any bisexual taxon in the Mediterranean Region before the introduction of North American specimens a few decades ago, can be undoubtedly assigned to Artemia salina. Mura & Nagorskaya (2005) confirmed the presence of A. salina as the only bisexual species present at that time in Ukraine, an area of potential contact with bisexual populations of the Western Asian Lineage; this information helped us to retain A. arietina Fischer, 1851, under the synonymy of A. salina. Two old names with Mediterranean type localities, and whose reproductive mode was not stated in the original description are treated as nomina dubia, but tentatively included in the synonymic list for A. salina. The resulting synonymic list for the Mediterranean-South African Lineage remains thus as follows:
Artemia salina (Linnaeus, 1758)
Cancer salinus Linnaeus, 1758: 634. Terra typica: “Habitat in Angliae Salinis Limingtonianis.”. Bisexual (Linnaeus, 1758). Baxevanis, Kappas & Abatzopoulos (2006) confirmed the morphological ascription of topotypical specimens. Neotype designation among any of those specimens is desirable.
Gammarus salinus (Linnaeus, 1758): Fabricius, 1775: 419.
Artemisia salina (Linnaeus, 1758): Latreille, 1816: 68. The genus Artemisia was supressed for the purposes of the Principle of Priority and placed on the Official Index of Rejected and Invalid Generic Names in Zoology (International Commission on Zoological Nomenclature, 1985, Opinion 1301).
Eulimene albida Latreille, 1816: 68 (nomen dubium). Terra typica: “…dans la Méditerranée…”. Reproductive mode not indicated (Latreille, 1816). Daday de Deés (1910) included E. albida in the synonymy of A. salina. The name Eulimene Latreille, 1816, does not have nomenclatural precedence over Artemia Leach, 1819 (International Commission on Zoological Nomenclature, 1985, Opinion 1301).
Artemisus salinus (Linnaeus, 1758): Lamarck, 1818: 135. The genus Artemisus was supressed for the purposes of the Principle of Priority (International Commission on Zoological Nomenclature, 1985, Opinion 1301).
Artemia salina (Linnaeus, 1758): Leach, 1819: 543.
Artemia eulimene Leach, 1819: 543 (nomen dubium). Terra typica: “Habite la Méditerranée, près Nice”. Reproductive mode not indicated (Leach, 1819). Desmarest (1823) considered A. eulimene a synonym of Eulimene albida.
Artemis salinus (Linnaeus, 1758): Thompson, 1834: 105.
Artemia arietina Fischer, 1851: 156. Terra typica: “… aus der Umgegend von Odessa stammt”. Bisexual (Fischer, 1851). Daday de Deés (1910) included A. arietina as a variety of A. salina.
Branchipus (Artemia) salinus (Linnaeus, 1758): Grube, 1853: 139.
Branchipus eulimene (Leach, 1819): Grube, 1853: 140.
Branchipus arietinus (Fischer, 1851): Grube, 1853: 140.
Branchipus oudneyi Liévin, 1856: 1. Terra typica: “…die Trona-Seen, und besonders der Bahr-el-Dud… Diesen See bewohnt der berühmte Fezzan-Wurm oder Dud” [Lybia: Fezan: Ubari Trona Lake]. Bisexual (Liévin, 1856). Daday de Deés (1910) included B. oudneyi in the synonymy of A. salina.
Artemia oudneyi (Liévin, 1856): Baird in Liévin, 1856: 1.
Callaonella dybowskii Grochowski, 1896: 100. New synonymy. Terra typica: “im Süsswaser, nämlich im Vrana-See auf der Insel Cherso”. Bisexual populations (Grochowski, 1896). Daday de Deés (1910) commented on the peculiarity of being collected in freshwater at the Croatian Island of Cres (Grochowski, 1896), but treated it as a synonym of Artemia jelskii Grube, 1874. According to the illustration provided by Grochowski (1896), it is morphologically assignable to A. salina.
Artemia dybowski (Grochowski, 1896): Belk & Brtek, 1995: 316.
Artemia tunisiana Bowen & Sterling, 1978: 595. New synonymy. Terra typica: not stated explicitly, but the authors included two populations in the category: “… from Tunis, and from San Bartolomeo, Sardinia”. Type series or type material not designated. Bisexual populations (Bowen & Sterling, 1978).
New World Lineage—Artemia monica (= A. franciscana)
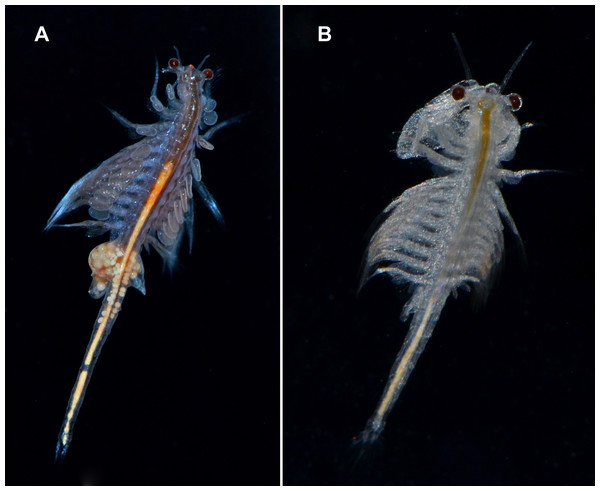
The widely distributed New World Lineage is integrated by multiple geographically structured mitochondrial clades and shows large nuclear sequence variability (Sáez, Escalante & Sastre, 2000; Baxevanis, Kappas & Abatzopoulos, 2006). It has been introduced all over the world (Figs. 6A and 6B) (Eimanifar et al., 2014).
Figure 6: Live specimens of Artemia monica (= A. franciscana).
(A) female; (B) male from an introduced population in Spain (San Fernando, Cádiz). Photographs by M. García-París.Cox1 clades within the New World Lineage are largely divergent and, in most cases reciprocally monophyletic and, once excluding the demonstrated introduced populations (Eimanifar et al., 2014), they are geographically structured (Fig. 5). Some of the well-differentiated mtDNA clades are isolated in the Antilles (Puerto Rico), Mexico, or in different areas of South America, and present high intraspecific FST values based on allozyme data (maximum values of FST = 0.24 to 0.38) (Abreu-Grobois & Beardmore, 1980, 1983; Abreu-Grobois, 1983, 1987; Gajardo & Beardmore, 1993; Pilla & Beardmore, 1994). However, there is no evidence supporting that any of these divergent phylogroups might represent a different species. Specimens from divergent mtDNA lineages (all mentioned under the name A. franciscana) and different geographic origins occur together in introduced populations through Europe and Asia, providing some indication of the lack of genetic isolation among mtDNA phylogroups (Eimanifar et al., 2014). In addition, Abreu-Grobois & Beardmore (1991) studied 22 allozyme loci and recorded intraspecific DNei distances (0.09–0.13) (Nei, 1972) between populations of the Great Salt Lake (Utah), San Francisco Bay (California), and Pekelmeer (Bonaire). Nevertheless, our mitogenomic data show a relatively large divergence between Baja California (Mexico) and San Francisco Bay (USA) populations (separated about 1,300 km), suggesting that divergent phylogroups within the New World Lineage should be studied at the nuclear level before reaching a final conclusion (as already suggested by Bowen et al., 1985).
The only proposal to consider differentiated taxa within the New World Lineage, was prompted by the ecological isolation of Mono Lake (California) population. Brine shrimps from Mono Lake were considered reproductively isolated from nearby populations because of the particular water ionic composition of the Lake (Clark & Bowen, 1976; Bowen et al., 1985), and accordingly treated as a different species under the name A. monica (Conte, Jellison & Starrett, 1988; Dana, Jellison & Melack, 1990, 1995; Dana et al., 1993). The available mtDNA sequences of the endangered A. monica are deeply nested within a western USA clade, which includes samples from nearby populations including the Great Salt Lake and San Francisco Bay (type locality of A. franciscana), as well as some Mexican and Colombian localities (Figs. 5A and 5B). Samples from Mono Lake do not form a monophyletic mtDNA phylogroup, which probably caused that recent authors totally ignored its existence (Abatzopoulos et al., 2002b; Baxevanis, Kappas & Abatzopoulos, 2006; Maniatsi et al., 2011; Eimanifar et al., 2014). Nuclear data based on 22 allozyme loci do not support the isolation of the Mono Lake population either, since it is deeply nested within a western USA nuclear clade including populations from the Great Salt Lake (Utah), San Francisco Bay (California), and Pekelmeer (Bonaire, Antilles) (Abreu-Grobois & Beardmore, 1991; Pilla & Beardmore, 1994). Nei (1972) genetic distance (DNei) between San Francisco Bay (type locality of A. franciscana) and Mono Lake (type locality of A. monica) populations (DNei = 0.05) falls within the usual range for intraspecific populations, and is even lower than that between San Francisco Bay and the Great Salt Lake populations (DNei = 0.09) (Abreu-Grobois & Beardmore, 1991). In addition, Bowen et al. (1985) demonstrated that in laboratory conditions, under highly controlled chloride and carbonate levels, specimens of A. monica (Mono Lake) and of A. franciscana from locations nearby show complete reproductive compatibility and a normal mating behavior. All these data already granted rejection of an independent species status for the Mono Lake population by some authors (Abreu-Grobois & Beardmore, 1983; Triantaphyllidis, Abatzopoulos & Sorgeloos, 1998; De Los Ríos & Zúñiga, 2000). In fact, Abreu-Grobois & Beardmore (1991) proposed an appealing explanation for the maintenance of a relatively high level of gene flow between the Mono Lake population and other extant or extinct populations found in the eastern Sierra Nevada mountains. The existence of many saline—carbonated lakes in the Eastern Sierras likely promoted the presence of populations of Artemia adapted to local chemical conditions more or less similar to those present today in Mono Lake (Bowen et al., 1985; Abreu-Grobois & Beardmore, 1991). Abreu-Grobois & Beardmore (1991) suggested that climatic and hydrological changes during the Holocene caused sequential extinction and recolonization events as a consequence of variations in the ionic levels of these lakes. Under this circumstances gene flow between Mono Lake and those other populations could have been relatively high, possibly favored by avian movements. This scenario could explain the high allelic diversity found today in Mono Lake and their genetic similarity with respect to other Western US populations (Abreu-Grobois & Beardmore, 1991).
Lack of reciprocal monophyly at nuclear and mtDNA levels suggests that gene flow is effectively taking place between the Mono Lake population and neighboring ones (Fig. 5B). Consequently, the ecological differences observed between the population of Mono Lake and other locations in California and Utah (Lenz, 1980, 1984; Dana & Lenz, 1986), suggest that these populations should be considered either as local adaptive ecotypes (Abreu-Grobois, 1983, 1987), as it is the case for extremely adapted populations in other regions (Schmankewitsch, 1875, 1877a, 1877b; Amat, 1980b; Asem & Rastegar-Pouyani, 2010), or the result of an inconclusive speciation process. Consequently, the Mono Lake brine shrimp together with all western North American Artemia are part of a single evolutionary unit.
Belk & Bowen (1990) submitted an application for the conservation of the specific name Artemia franciscana Kellogg, 1906, over some of previously published names with nomenclatural priority over it. The Opinion 1704 of the Commission (International Commission on Zoological Nomenclature, 1993) included the names Artemis guildingi Thompson, 1834, Artemia fertilis Verrill, 1870, and Artemia utahensis Lockington, 1876, in the Official Index of Rejected and Invalid Specific Names in Zoology, and gave precedence to A. franciscana over A. gracilis. However, as a member of the Commission (L.B. Holthius) pointed out, it was premature to deal with the issue before the systematics of Artemia was properly analyzed (International Commission on Zoological Nomenclature, 1993). In fact, populations of Mono Lake (type locality of A. monica) (Verrill, 1869), and from coastal Peru (where the type locality of A. jelskii is located) (Grube, 1874), are likely part of the same taxonomic unit as the populations from San Francisco Bay (type locality of A. franciscana), and both have nomenclatural priority over A. franciscana. In addition, the detailed description of Hawaiian populations (A. salina var. pacifica) (Fig. 1; Sars, 1904) also corresponds to the typical morphology of the New World Lineage of Artemia, and therefore might have also priority over A. franciscana.
The resulting situation is that, besides the Commission’s locked name A. gracilis (a nomen dubium), at least three other names could have precedence over A. franciscana according to the Principle of Priority (A. monica, A. jelskii and A. salina var. pacifica). The Code of Nomenclature indicates that the Principle of Priority may be modified in its operation in the interest of stability and universality. The Code estates that: “23.9.1. prevailing usage must be maintained when the following conditions are both met: 23.9.1.1. the senior synonym or homonym has not been used as a valid name after 1899, and 23.9.1.2. the junior synonym or homonym has been used for a particular taxon, as its presumed valid name, in at least 25 works, published by at least 10 authors in the immediately preceding 50 years and encompassing a span of not less than 10 years”. The first condition is not met by either Verrill’s (1869) A. monica (often used as a valid name after 1899), Grube’s (1874) A. jelskii (a nomen dubium, but used as a valid species name at least by Daday de Deés (1910) as Callaonella jelskii), or Sars’ (1904) A. salina var. pacifica (described after 1899). The oldest name, Artemia monica Verrill, 1869 (published in the same work as A. gracilis and therefore without precedence of one over the other), has been used extensively as a valid species name in the XXth and XXIst centuries (see for example Abreu-Grobois & Beardmore, 1991; Brendonck & Belk, 1997; Asem, Eimanifar & Sun, 2016).
The precedence of A. monica over A. franciscana cannot be reverted under any provision of the Code (International Commission on Zoological Nomenclature, 1999), and thus, Artemia monica Verrill, 1869, becomes the valid name for the New World Lineage of Artemia. Therefore, all populations currently referred to by the name A. franciscana must be referred to as A. monica.
The synonymic list (synonyms and new combinations) for the New World Lineage would remain as follows:
Artemia monica Verrill, 1869
Artemis guildingi Thompson, 1834: plate 1, fig. 11 (unavailable name). Terra typica: “West Indies”. Artemis guildingi Thompson, 1834 was placed in the Official Index of Rejected and Invalid Specific Names in Zoology (International Commission on Zoological Nomenclature, 1993, Opinion 1704).
? Artemia gracilis Verrill, 1869: 248 (nomen dubium). Terra typica: “Near New Haven, in tubs of water from salt marsh”. Verrill (1870) precised: “New Haven, Conn. Charlestown, Mass., on railroad bridge across Charles River in tubs of concentrated sea-water.”. Syntypes (396, 397) at the Peabody Museum of Natural History (New Haven, Connecticut, USA) (Belk & Brtek, 1995).
Artemia monica Verrill, 1869: 249. Terra typica: Not indicated explicitly in the original description, but a few pages earlier, Verrill (1869: 245) stated “… a number of specimens of a new species, A. monica, V., which he collected in Mono Lake, California…”. Syntypes (395) at Peabody Museum of Natural History (New Haven, Connecticut, USA) (Belk & Brtek, 1995). Bisexual (Verrill, 1869).
Artemia fertilis Verrill, 1869: 238 (unavailable name). Terra typica: “Great Salt Lake, Utah,…”. Artemia fertilis Verrill, 1869, was placed in the Official Index of Rejected and Invalid Specific Names in Zoology (International Commission on Zoological Nomenclature, 1993, Opinion 1704).
? Artemia jelskii Grube, 1874: 56 (nomen dubium). Terra typica: “…Callao…”. Types not designated. Bisexual (Grube, 1874). Daday de Deés (1910) treated it as an independent species as Artemia (Callaonella) jelskii Grube, from salterns near Callao, in Perú. The molecular identification of this population is desirable in order to determine its taxonomic placement and make effective this possible synonymy.
Artemia utahensis Lockington, 1876: 137 (unavailable name). Terra typica: “… inhabits the Great Salt Lake of Utah.”. Artemia utahensis Lockington, 1876, was placed in the Official Index of Rejected and Invalid Specific Names in Zoology (International Commission on Zoological Nomenclature, 1993, Opinion 1704).
? Callaonella jelskii (Grube, 1874): Kulczycki, 1885: 591.
Artemia salina var. pacifica Sars, 1904: 630. New synonymy. Terra typica: “… (1) in einem Salzsee, mit 15% Kochsalz, in der Nähe von Honolulu, Hawaiische Inseln, und (2) in einer Lagune, mit 12% Kochsalz, auf der kleinen unbewohnten Koralleninsel Laysan, ungefähr 800 Seemeilen WNW. von Honolulu.”. Bisexual (Sars, 1904; Fig. 1). Although its morphology has been well studied and documented, a molecular identification of this population is desirable.
Artemia franciscana Kellogg, 1906: 596. New synonymy. Terra typica: “… found abundantly in the salterns (evaporating pools), density 1.08 to 1.24, at Redwood City, San Francisco Bay,…”. Types not designated (Belk & Brtek, 1995). Bisexual (Kellogg, 1906).
Western Asian Lineage—Artemia urmiana
The Western Asian Lineage is composed of at least three geographically structured mitochondrial clades, some of them including bisexual and parthenogenetic populations (e.g., Urmia Lake), with apparent gene flow among bisexual populations from all three clades (Maccari, Amat & Gómez, 2013; Asem, Eimanifar & Sun, 2016). Bisexual populations are morphologically diagnosable from all other lineages and they have been introduced in diverse areas, including the Mediterranean region.
Mitochondrial clades within the Western Asian Lineage, usually known as A. tibetiana, A. urmiana, Eurasian Haplotype Complex and A. parthenogenetica (in part), are part of a single nuclear clade (Fig. 4) with alleles widely shared across all mtDNA units (Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016). Some of these mtDNA clades may represent incipient evolutionary units, with relatively low gene flow occurring among them (Kappas, Baxevanis & Abatzopoulos, 2011; Eimanifar et al., 2014) (Fig. 4). However, Zhang et al. (2013) discussed the low divergence found among complete mitogenomes of bisexual populations from Lake Urmia and Tibet, as reported in previous studies (Baxevanis et al., 2005; Baxevanis, Kappas & Abatzopoulos, 2006; Wang et al., 2008).
Mitochondrial clades may, or may not, represent evolutionary units. If time is long enough, coalescence processes, including lineage sorting, generally will end up by depicting concordant clades for mtDNA and nuclear markers (reciprocally monophyletic if gene flow got interrupted, or single clades if gene flow ended up homogenizing the original incipient clades) (Fujita et al., 2012; Sukumaran & Knowles, 2017; but see Albert, Zardoya & García-París, 2007). However, in many groups, particularly in those with little developed prezygotic isolation mechanisms, mtDNA is often well structured across populations that are still linked by gene flow (García-París et al., 2003; Recuero & García-París, 2011). In these cases, and as a consequence of demographic processes such as maternal inheritance, small population or sampling size, lack of recombination, difficulties to move across contact zones, etc… mtDNA clades can appear as reciprocally monophyletic, transmitting the idea that there has been a long period of isolation between populations, whereas analyses of rapidly evolving nuclear markers such as ITS, show evident signs of gene flow across mtDNA breaks (Babik et al., 2005; Rodríguez-Flores et al., 2017). In these cases, discordances between nuclear and mtDNA markers are very useful to determine isolation levels and consequently the evolutionary status of two population groups. In Artemia, which does not show any sign of occurrence of pre-zygotic isolation mechanisms (Pilla & Beardmore, 1994), mitochondrial data have been extensively used to characterize evolutionary units within the Asian Lineages (Eimanifar et al., 2014), or even to describe new taxa (Naganawa & Mura, 2017). However, data from fast evolving nuclear data, mostly ITS1, do not support the recognition of some of those phylogroups or mtDNA clades as independent taxa.
The bisexual population of Lagkor Co in Tibet has been formerly treated as a different species, A. tibetiana (Abatzopoulos, Zhang & Sorgeloos, 1998). The taxon was characterized by having cysts with large diameters (323 µm + 17.2; 330 µm +14.6), the longest known first instar nauplii (667 µm + 32.7), and a large adult size (Abatzopoulos, Zhang & Sorgeloos, 1998). Cox1 mtDNA sequences of A. tibetiana clustered in two non-sister clades, sequentially sister to a clade conformed by populations of A. urmiana plus 2n and 3n parthenogenetic specimens, rendering A. tibetiana a non-monophyletic mtDNA entity (Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016). Genetic divergence based on allozyme analyses and reproductive incompatibility (postzygotic isolation) between the Tibetan and other Asian populations was relatively low (allozymes), or not significantly different (fertility) from that recorded for intraspecific crossings (40–60% fertile specimens according to Abatzopoulos, Zhang & Sorgeloos, 1998). Abatzopoulos et al. (2002b) pointed out that “The likelihood of extensive geographical differentiation cannot be completely ruled out, especially with the limited number of populations investigated here, a fact that can lead to a fallible taxonomy.” Eimanifar et al. (2014) and Asem, Eimanifar & Sun (2016) phylogeographic analyses based on ITS1 sequence data, which included specimens of all Asian taxa, indicated that the nuclear sequences of the ITS1 region from the type locality of A. tibetiana, were almost identical to those of 2n and 3n parthenogenetic specimens and to those of bisexual A. urmiana (Fig. 4). Eimanifar et al. (2014) pointed out that: “The presence of a common haplotype can be simply explained because of the lack of time to generate and sort out new variants among closely related species”, while Kappas, Baxevanis & Abatzopoulos (2011) suggested that the large morphological diversity displayed by A. tibetiana, coupled with a low level of genetic divergence between A. tibetiana and A. urmiana, reflects recent speciation or slow rates of divergence. There are thus several evidences against maintaining the species status of the population named A. tibetiana from an evolutionary species concept perspective (Wiley, 1978): firstly, the absence of a common ancestor for all populations currently included under this name, that is, the different Tibetan populations do not form a monophyletic group, nor are they evolutionarily cohesive (Maccari, Amat & Gómez, 2013; Eimanifar et al., 2014); secondly, they present unclear boundaries in their genetic/morphological differentiation (Kappas, Baxevanis & Abatzopoulos, 2011), especially considering that gene flow has been occurring across Tibetan and non-Tibetan populations until very recently, as inferred from a rapid evolving nuclear marker (Baxevanis, Kappas & Abatzopoulos, 2006; Maccari, Amat & Gómez, 2013) (Fig. 4). Even though the occurrence of partial cross-fertility in F2 and F3 generations cannot be ignored (Triantaphyllidis, Abatzopoulos & Sorgeloos, 1998; Abatzopoulos et al., 2002b), testing the species hypothesis on the basis of this parameter would require analyzing the reproductive compatibility among other populations of the Western Asian Lineage. We agree with Asem et al. (2020) in that the taxonomic status of some Tibetan populations is dubious until more work on their degree of isolation and population speciation trends is performed. However, these considerations do not affect the status of the name A. tibetiana that should be considered as a junior synonym of A. urmiana based on the occurrence of nuclear gene flow between the type locality of A. tibetiana and populations of A. urmiana (Maccari, Amat & Gómez, 2013).
Naganawa & Mura (2017) described recently two Asian Artemia species, A. frameshifta and A. murae. The cox1 fragment used by Naganawa & Mura (2017) to identify the only female studied A. frameshifta (GenBank accession number LC195588) present 11 indels (one to three base pairs long) when aligned with all other sequences of Asian Artemia. Amino acid transcription reveals extensive presence of stop codon positions (TAA and TAG) (Eimanifar, 2014) along the first half of the sequence (for all three possible reading frames). Based on the lack of morphological differentiation of A. frameshifta and on the affinity of the studied sequence (possibly a pseudogene) with sequences of the Western Asian Lineage, we propose the synonymy of A. frameshifta with A. urmiana. With regard to A. murae, Naganawa provided a well-illustrated morphological description and a cox1 sequence fragment (GenBank accession number LC195587). A re-examination of this sequence reveals that, a single position base (Adenine) was introduced at the end of the fragment, generating a displacement of the reading frame involving 28 positions. Its amino acid transcription reveals that this sequence presents a large amount of amino acid changes with respect to other Asian Artemia, which otherwise present highly conservative amino acid sequences. This fact, together with absence of morphological conclusive differences with respect to A. urmiana (as already suggested by Naganawa & Mura, 2017), made us, regretfully, to reconsider the validity of A. murae and include it tentatively in the Western Asian Lineage as a junior synonym of A. urmiana.
A large number of parthenogenetic populations from the Western and the Eastern Asian Lineages studied shared a general common allele of Na+/K+ ATPase (Asem, Eimanifar & Sun, 2016). These data are partially supported by ITS data. However, the diversification described for ITS is very high (Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016; Fig. 2), and inconsistent with general patterns of evolution of ITS markers in Anostraca (Rodríguez-Flores et al., 2017, 2020). These discordances between sets of markers across Asian populations are not reflected at the morphological level, since bisexual populations from Western and Eastern Asian Lineages seem to differ consistently (A. urmiana vs. A. sinica) in agreement with mtDNA clades (Cai, 1989b). While these discordances need to be studied at a deeper level, we prefer to retain as separate evolutionary entities the morphologically (nuclear) and mitochondrially defined Western and Eastern Asian Lineages, in agreement with Cai (1989a, 1989b) and Zheng, Sun & Ma (2004) tests of reproductive incompatibility.
Analyses carried out using mtDNA data (cox1, 12S and 16S), show that all 2n and 3n parthenogenetic specimens cluster together or are nested within a single lineage that also includes some bisexual populations from Lake Urmia, Ukraine, and Tibet (Maniatsi et al., 2011; Asem, Eimanifar & Sun, 2016); mitochondrial differentiation (cox1, 12S and 16S) between 2n and 3n parthenogenetic populations and bisexual populations from Lake Urmia is quite limited (Baxevanis, Kappas & Abatzopoulos, 2006; Maniatsi et al., 2011; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016), suggesting a very recent origin for both parthenogenesis and polyploidy within this clade.
Nuclear data (Fig. 3) suggest that 3n polyploid specimens are occasional evolutionary experiments, likely advocated to extinction, and therefore difficult to be considered as a differentiated taxon (Baxevanis, Kappas & Abatzopoulos, 2006). On the other hand, diploid parthenogenetic populations are well established, and widely distributed, but they will end up as clonal isolated lines, altogether difficult to be considered as a single evolutionary unit or a single taxonomic entity (each female clone is an independent line) (Abatzopoulos et al., 2002a). To complicate matters, the occasional males produced in 2n parthenogenetic populations (MacDonald & Browne, 1987; Mura & Nagorskaya, 2005; Maccari et al., 2013) open a window for the existence of gene flow between males of parthenogenetic origin and bisexual populations when contact is established, which is a relatively frequent situation. This problem requires further analyses, because Baxevanis, Kappas & Abatzopoulos (2006) and Maniatsi et al. (2011) recovered multiple independent origins for parthenogenesis and found large discordances between the evolutionary patterns shown by nuclear and mitochondrial data among parthenogenetic lines. We concur with Baxevanis, Kappas & Abatzopoulos (2006) on considering that 2n and 3n parthenogenetic populations are part of a single Western Asian Lineage and that, at least for the time being, they should not be treated as independent taxa (Fig. 3).
The fact that bisexual, diploid and triploid parthenogenetic populations, are part of a single lineage, highly complicates the nomenclature of the Western Asian Lineage (Asem et al., 2020). There is no information on the level of ploidy or mtDNA data for many of the parthenogenetic populations for which available names have been published, and therefore it is impossible to ascribe those names with certainty to either the Western or the Eastern Asian Lineages. Even having the opportunity to perform molecular analyses of specimens from those localities, the chance that new introductions occurred, would mask original identifications, since 4n (Eastern Asian Lineage) and 2n (Western Asian Lineage) parthenogenetic specimens are currently found together in many areas (Eimanifar et al., 2014). In this sense, some names applied to parthenogenetic populations must remain as nomina dubia until additional information can be obtained. Among these are the two Australian taxa described by Sayce (1903), A. australis and A. westraliensis. Reasons to include them within the Western Asian Lineage are that all parthenogenetic populations so far studied in Australia are reported to be 2n (McMaster et al., 2007; Muñoz & Pacios, 2010).
In order to assure stability, reversion of precedence with respect to A. urmiana Günther, 1899, of names applied to parthenogenetic populations described before 1899 (Branchipus milhausenii Fischer de Waldheim, 1834; Artemia koeppeniana Fischer, 1851, Artemia proxima King, 1855, Artemia salina var. biloba Entz, 1886, Artemia salina var. furcata Entz, 1886, and Artemia asiatica Walter, 1887) is feasible, because as far as we have been able to find, none of them was used as a valid taxon name after 1899 (International Commission on Zoological Nomenclature, 1999). In fact, Daday de Deés (1910) included all of them, except A. asiatica (probably a lapsus, because Daday de Dées mentioned the type locality of A. asiatica as part of the A. salina geographic range), as intraspecific variants or synonyms of A. salina, and were not used again as names for any valid taxon. On the other hand, the name A. urmiana has been used extensively, in at least 25 works, published by at least 10 authors in the immediately preceding 50 years and encompassing a span of not less than 10 years (see for example: Barigozzi et al., 1988; Abreu-Grobois & Beardmore, 1991; Browne & Bowen, 1991; Triantaphyllidis, Abatzopoulos & Sorgeloos, 1998; Abatzopoulos et al., 2006, 2009; Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar, Rezvani & Carapetian, 2006; Eimanifar et al., 2014, 2016; Asem & Rastegar-Pouyani, 2007, 2008, 2010; Asem, Rastegar-Pouyani & Agh, 2007; Asem, Rastegar-Pouyani & De Los Ríos-Escalante, 2010; Asem et al., 2010; Asem, 2008; De Los Ríos & Asem, 2008; Shadrin et al., 2008; Agh et al., 2009; Khomenko & Shadrin, 2009; Ahmadi et al., 2012; Anufriieva & Shadrin, 2012, 2013; Castro Mejía et al., 2013; Eimanifar & Wink, 2013; Zhang et al., 2013; Asem, Eimanifar & Sun, 2016; Naganawa & Mura, 2017; and additional references in Asem & Rogers, 2012). Therefore, and according to the Article 23.9.2. of the International Code of Zoological Nomenclature (International Commission on Zoological Nomenclature, 1999), the name A. urmiana Günther, 1899 can be considered a nomen protectum having thus nomenclatural precedence over the names Branchipus milhausenii Fischer de Waldheim, 1834, Artemia koeppeniana Fischer, 1851, Artemia proxima King, 1855, and Artemia asiatica Walter, 1887 (all of them nomina oblita). Günther (1899) description of A. urmiana is well illustrated and precise (Fig. 7).
Figure 7: Original illustration of Artemia urmiana (nomen protectum) in Günther (1899) from The Journal of the Linnean Society, 27, pl. 25, a high-quality illustration accompanying the original description of A. urmiana.
The synonymic list (synonyms and new combinations) for the Western Asian Lineage remains as follows:
Artemia urmiana Günther, 1899
Branchipus milhausenii Fischer de Waldheim, 1834: 459 (nomen oblitum). New synonymy. Terra typica: Not stated in the original description, but a couple of pages earlier, Fischer de Waldheim (1834: 457) indicated that Milhausen found the species “dans le lac salé Sak en Crimée”. Only female specimens were mentioned or described in the original work.
Artemia mulhausenii (Fischer de Waldheim, 1834): Milne-Edwards, 1840: 370. Many authors considered that Fischer (1851: 155) described a new species of Artemia under the name Artemia muellhausenii Fischer, 1851. However, Fischer (1851) clearly indicated that his description was intended only to improve former descriptions of the same taxon by Fischer de Waldheim (1834 sub Branchipus milhausenii) and Rathke (1836 sub A. salina), both made using materials from Crimea. Fischer (1851) used for Fischer de Waldheim (1834) taxon, the spelling modified by Milne-Edwards (1840), adding an extra-l.
Artemia koeppeniana Fischer, 1851: 157 (nomen oblitum). New synonymy. Terra typica: “… im südlichen Russland gesammelt,”. Only female specimens mentioned (Fischer, 1851).
Branchipus koeppenianus (Fischer, 1851): Grube, 1853: 140.
Artemia proxima King, 1855: 70 (nomen oblitum). New synonymy. Terra typica: “Salt Pans, Newington; Parramatta”. Types not indicated (King, 1855, 1866).
Artemia salina var. biloba Entz, 1886: 105 (nomen oblitum). New synonymy. Terra typica: “Ezen fajta a tömör sótartalmú tavakat, Vizaknan a 20%—os Tökölyit, Tordán a 10%—os Aknafürdőt lakja.” [Romania: Transylvania: Lake Tökölyit in Vizaknán (Ocna Sibiului), Lake Aknafürdőt in Tordán (Turda)]. Type not designated. Only female populations (Entz, 1886). Daday de Deés (1910) established its synonymy with A. salina var. milhausenii.
Artemia salina var. furcata Entz, 1886: 106 (nomen oblitum). New synonymy. Terra typica: “Ezen fajta a hígabb sotartalmú tavakat, Vizaknan a 7.65%—os Vörös-, és Asszonytavat, Tordán a Banyafürdő 4%—os tavait lakja.” [Romania: Transylvania: Lake Vörös and Asszonytavat in Vizaknán (Ocna Sibiului), Lake Banyafürdő in Tordán (Turda)]. Type not designated. Only female populations (Entz, 1886). Daday de Deés (1910) established its synonymy with A. salina var. arietina.
Artemia asiatica Walter, 1887: 926 (nomen oblitum). New synonymy. Terra typica: “In einer Salzquelle zwischen Bend-i-nadyr und dem Brunnen Agamet in der Bergwüste östlich vom Murgab, nahe der Afghanengrenze”. The type series includes only females (Walter, 1887). Walter (1888a, 1888b) indicated that only female specimens were collected in a salt-spring in the hillside of the Afghan border, east of Saryken-Aul at Murgab [Bãlã Morgãb, 35°38′N–63°18′, Afghanistan, see map in Radde & Walter (1889), between Bend-i-nadyr [ca. 35°51′N–63°07′E] and the desert-well Agamet, on 14–26 April 1887, a salty lake with thickened edges by precipitated salt.
Artemia urmiana Günther, 1899: 395 (nomen protectum). Terra typica: “Lake Urmi, in water of specific gravity 1.1138.”. Bisexual (Günther, 1899; Fig. 7). Barigozzi et al. (1988) failed to find bisexual populations at Lake Urmia, and found only parthenogenetic populations.
? Artemia australis Sayce, 1903: 229 (nomen dubium). Terra typica: “Brackish-water, Sandhills, Gleneg, coastal district of South Australia…”. Sayce (1903) stated that over 100 specimens all were females, and that the large number of young forms observed probably were of parthenogenetic origin.
? Artemia westraliensis Sayce, 1903: 230 (nomen dubium). Terra typica: “Lake Aurean, Murchison, West Australia…”. The type series consists of two female specimens (Sayce, 1903).
Artemisia proxima (King, 1855): Dakin, 1914: 294.
Artemisia australis (Sayce, 1903): Dakin, 1914: 294.
Artemisia westraliensis (Sayce, 1903): Dakin, 1914: 296.
Artemia parthenogenetica Bowen & Sterling, 1978: 595. New synonymy. Terra typica: not stated explicitly, but the authors included five parthenogenetic populations in its category: “… Madras and Kutch, India; Port Hedland, Australia; Sète, France; and Yamaguchi-ken, Japan”. Type series or type material not designated, and ploidy not stated (Bowen & Sterling, 1978). It is very possible that materials used in this work included both 2n and 4n parthenogenetic populations, but most of the populations included by Bowen & Sterling (1978) correspond today to the A. urmiana clade (Triantaphyllidis, Abatzopoulos & Sorgeloos, 1998; Muñoz & Pacios, 2010). A lectotype designation (if type specimens exist) or neotype (if they are lost) is necessary to assure the correct synonymization of this name.
? Artemia barkolica Qian & Wang in Qian et al. (1992) (nomen dubium). Terra typica according to Asem et al. (2020): Barkol Lake, Xinjiang, China. Male and female specimens known. Specimens from Barkol Lake were studied at the molecular level by Asem, Eimanifar & Sun (2016); these sequences are nested within the Western Asian Lineage. Asem et al. (2020) already considered this taxon to be composed of several phylogenetic clades (all present in this location), but they did not provide a formal statement on its synonymy.
Artemia urumuqinica Qian & Wang in Qian et al. (1992) (nomen dubium). Terra typica according to Asem et al. (2020): Urumqi Caiwuo Pu Yan Hu, Xinjiang, China. Only female specimens known. Asem et al. (2020) considered it a possible synonym of previously described taxa, but they did not provide any formal statement on its synonymy.
Artemia ebinurica Qian & Wang in Qian et al. (1992). New synonymy. Terra typica according to Asem et al. (2020): Ebinur, Xinjiang, China. Male and female specimens known. Cox1 sequences of specimens from Aibi Lake were studied at the molecular level by Maccari et al. (2013) and Asem, Eimanifar & Sun (2016); these sequences are included within the Western Asian Lineage. Asem et al. (2020) mentioned that they could be considered as synonyms of previously described taxa, but they did not provide any formal statement on its synonymy.
Artemia tibetiana Abatzopoulos, Zhang & Sorgeloos, 1998: 43. New synonymy. Terra typica: “… in Lagkor Co Lake on the high plateaus of Tibet (P.R. China).” “Lagkor Co is a carbonate lake, situated 4,490 m above sea level in the arid-temperate plateau zone of Tibet, at 84° 13′ E and 32° 03′ N… ”. Types not designated. Bisexual (Abatzopoulos, Zhang & Sorgeloos, 1998). The taxon was characterized by presenting large cyst diameter (323 µm + 17.2; 330 µm +14.6), the largest length of first instar nauplii (667 µm + 32.7), and the largest adult size recorded among Artemia species (Abatzopoulos, Zhang & Sorgeloos, 1998). However genetic divergence based on allozyme analyses, and reproductive incompatibility (postzygotic isolation) between this Tibetan populations and other Asian populations studied was relatively low (allozymes), or not significantly different (fertility) than those obtained for intraspecific crossings (Abatzopoulos, Zhang & Sorgeloos, 1998).
Artemia murae Naganawa in Naganawa & Mura (2017): 1684. New synonymy. Terra typica: “Tonkhil nuur (Tonkhil Lake), Tonkhil sum., Gobi-Altai aimag, Mongolia (46°10′10″N 93°55′00″E),…”. Bisexual. This population deserves further molecular analyses (see above).
Artemia frameshifta Naganawa & Mura, 2017: 1688. New synonymy. Terra typica: “Bajan-Onjul, Tov aimag, Mongolia…”. Only female specimens known.
Eastern Asian Lineage—Artemia sinica
The Eastern Asian Lineage is composed of two to three relatively well supported cox1 mtDNA sister clades. The available information on nuclear markers, suggests that either gene flow is still ongoing across them, or that actual isolation across mtDNA clades is so recent that there is no evidence of nuclear isolation (Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016). Again, divergence among mtDNA clades within the Eastern Asian Lineage is low compared to what is found among old mtDNA clades in other species of Artemia, for which the existence of gene flow across mtDNA clades has been demonstrated to occur (Eimanifar et al., 2014). Consequently, all populations structured in cox1 clades within the Eastern Asian Lineage should be treated as a single evolutionary and taxonomic unit.
The Eastern Asian Lineage includes bisexual and 4n–5n polyploid parthenogenetic specimens (Baxevanis, Kappas & Abatzopoulos, 2006; Asem, Eimanifar & Sun, 2016). Bisexual populations are morphologically diagnosable (Cai, 1989a, 1989b), while bisexual and parthenogenetic populations are genetically characterized with respect to all other lineages (Baxevanis, Kappas & Abatzopoulos, 2006), but see Eimanifar et al. (2014) and Asem, Eimanifar & Sun (2016) to get an idea of the large diversity shown by rapidly evolving nuclear data. Mitochondrial DNA variability within either parthenogenetic or bisexual populations is very limited (Naihong et al., 2000). All 4n and 5n parthenogenetic specimens cluster in a single clade (based on cox1, 12S and 16S mtDNA), related but not nested within the bisexual clade from China (Maniatsi et al., 2011; Asem, Eimanifar & Sun, 2016). Cox1 divergence between the 4n and 5n parthenogenetic clade and the bisexual populations is relatively large (Maniatsi et al., 2011; Asem, Eimanifar & Sun, 2016), suggesting that parthenogenesis and polyploidy arose after separation of the two clades. Nuclear data, either ITS1 or slow evolving nuclear genes, such as Na+/K+ ATPase, show that some specimens of 4n populations share alleles with 2n and bisexual populations of the Western Asian Lineage, while all other 4n and 5n display a wide array of alleles some of them related to bisexual populations of the Eastern Asian Lineage (Baxevanis, Kappas & Abatzopoulos, 2006; Asem, Eimanifar & Sun, 2016). However, nuclear data of parthenogenetic polyploid populations are of difficult interpretation since polyploidy generates multiple nuclear copies.
Parthenogenesis and polyploidy are strong speciation factors when sufficient time is provided, sometimes leading to complete isolation and the formation of independent taxa (Chaplin & Hebert, 1997; Mark Welch & Meselson, 2000; Cunha, Doadrio & Coelho, 2008; but see Hurst & Peck, 1996; Schön, Martens & Rossi, 1996). In parthenogenetic Artemia, each polyploidy event could be treated as a speciation event, resulting thus in multiple agamospecies (Mayr, 2001) as indicated by Maniatsi et al. (2011). Despite the time elapsed from their split from the bisexual Eastern Asian Lineage, all 4n specimens studied so far share a common cox1 haplotype, with only one mutation step minor variants (Baxevanis, Kappas & Abatzopoulos, 2006; Maniatsi et al., 2011), consequence of very recent mutation events, or more likely derived from sequence reading problems or PCR noise. This implies that 4n parthenogenetic populations were originated very recently from a bisexual ancestral population sister to the bisexual populations of the Eastern Asian Lineage. Therefore, even if current tetraploid parthenogenetic populations could be isolated (Maniatsi et al., 2011), their hypothetical recent bisexual ancestor is likely not. We again agree with Baxevanis, Kappas & Abatzopoulos (2006), Maniatsi et al. (2011), and Eimanifar, Van Stappen & Wink (2015) in considering that these 4n and 5n parthenogenetic populations are part of an Eastern Asian Lineage, and they should not be treated as an independent taxon from the bisexual populations that originated them.
Only one species name, Artemia sinica Cai, 1989, has been applied with certainty to the Eastern Asian Lineage in addition to A. parthenogenetica (in part). However, it could be possible that some of the nomina dubia included tentatively under A. urmiana, corresponded in fact to the Eastern Asian Lineage (see comments in Asem et al., 2020). Then, some of those names might have priority over A. sinica. The description of A. sinica by Cai (1989a) was published in a short format and latter corrected and completed, with better quality images (Cai, 1989b).
So far, the synonymic list for the Eastern Asian Lineage remains as follows:
Artemia sinica Cai, 1989
Artemia sinica Cai, 1989a: 40. Terra typica: “…from the 150 km2 Xie-chi sulphate salt lake, located east of the city of Yun Chang in the Shan-xi Province in Central China.”. Bisexual (Cai, 1989a, 1989b). The figure presented in Cai (1989a) was published in better quality in Cai (1989b). Cai (1989b) corrected some data of the type locality: Yun Cheng salt lake, Shanxi Province, China; and described the morphologically differential characters. Cai (1989b) indicated that the species presents 42 chromosomes and that it is reproductively isolated from the rest of species in the genus and is morphologically distinguishable from all other bisexual species.
Remarks on the morphology of Artemia
Many authors have studied different aspects of the morphology of Artemia, including qualitative and quantitative traits and its state at different moments of development (Schmankewitsch, 1873, 1877b; Artom, 1907a; Abonyi, 1915; Gilchrist, 1960; Tyson & Sullivan, 1979, 1980; Amat, 1980a, 1980b; Wolfe, 1980; Schrehardt, 1987; Mura, Del Caldo & Fanfani, 1989; Mura, Fanfani & Del Caldo, 1989; Mura, 1990; Hontoria & Amat, 1992a, 1992b; Mura & Del Caldo, 1992; Torrentera & Dodson, 1995; Brendonck & Belk, 1997; Triantaphyllidis et al., 1997a, 1997b; Gajardo et al., 1998; Cohen et al., 1999; Cohen, Rodríguez Gil & Vélez, 1999; Zúñiga et al., 1999; Mayer, 2002; Torrentera & Belk, 2002; Mura & Brecciaroli, 2004; Abatzopoulos et al., 2009; Baxevanis et al., 2005; Asem, Rastegar-Pouyani & Agh, 2007; Asem & Rastegar-Pouyani, 2008; Asem et al., 2010; De Los Ríos & Asem, 2008; Asem & Rastegar-Pouyani, 2010; Vetriselvan & Munuswamy, 2011; Naceur, Jenhani & Romdhane, 2012, 2013; Asem & Sun, 2016). Most of them concluded that inter-populational variability is so high as to impede using the characters studied for species discrimination unless specimens are reared at controlled laboratory conditions (Mura & Brecciaroli, 2004; Abatzopoulos et al., 2009; Asem et al., 2010). Temperature and ionic composition and concentration were mainly responsible for the differences found among populations from close locations or among seasonal cohorts in a single location (Schmankewitsch, 1877b; Abonyi, 1915; Amat, 1980b; Naceur, Jenhani & Romdhane, 2012).
Some quantitative characters, including abdominal length, size and shape of the ovisac, length of the furca, number of setae on furcal branches, and size and shape of head appendages, as eye diameter and length of the antenna, have been shown to enable taxon discrimination when specimens are reared under similar developmental conditions (Hontoria & Amat, 1992a, 1992b). In this situation, Baxevanis et al. (2005) reported that both sexes of bisexual A. urmiana can be differentiated from A. sinica and A. monica (= A. franciscana) based on the display of a very thin and long abdomen, the shape of the ovisac, and the remarkable short furcal branches, which either have few setae or are completely naked. Artemia sinica differs from representatives of the A. monica clade in the relative length/width ratio of the abdomen (Cai, 1989b). In A. persimilis each of the furcal rami of adults bears three to five feathered setae (Cohen et al., 1999), while adults of A. monica bear generally 12 to 15 each (Schrehardt, 1987 sub A. franciscana).
Qualitative characters such as shape, size and ornamentation of the frontal knobs of male antennae, and presence and ornamentation of spine-like projections at the base of penises, are reliable features for the identification of A. persimilis and A. salina, but are less useful for the recognition of other taxa (Triantaphyllidis et al., 1997a; Mura & Brecciaroli, 2004; Abatzopoulos et al., 2009). The frontal knob of the male antenna is sub-spherical, large and poorly ornamented in A. persimilis, it is sub-cylindrical in A. salina, whereas it is sub-spherical but smaller and covered with dense papillae in specimens of A. monica, A. urmiana and A. sinica (Mura & Brecciaroli, 2004). Ornamentation of the basal spines of penis (absent in A. salina) can be used to separate A. persimilis from all other species: A. persimilis presents a few tooth-like protuberances scattered on the surface, while specimens of A. monica, A. urmiana and A. sinica present a dense cover of scale-like projections covering the tip of each penis (Mura & Brecciaroli, 2004).
Adult males of A. salina are characterized by the display of sub-cylindrical frontal knobs, which are sub-spherical in all other species, and by the absence of a basal spine on the penises, (vs. present in all other species). The sub-spherical frontal knob of the male antennae of A. sinica is generally smaller than those of specimens of A. monica (= A. franciscana) and A. urmiana (Cai, 1989a, 1989b).
Identification key to males of Artemia
Note that separation between specimens of A. monica (= A. franciscana), A. sinica and A. urmiana cannot be established with certainty unless specimens are reared under controlled conditions. Morphological characters used in the key were mainly obtained from Cai (1989b), Triantaphyllidis et al. (1997a), Cohen et al. (1999), Mura & Brecciaroli (2004), and Baxevanis et al. (2005).
-
Penises without spine outgrowth on the basal part; antennal frontal knobs sub-cylindricalArtemia salina
Penises with spine outgrowth on the basal part; antennal frontal knobs sub-spherical2
-
Basal spine of the penises without terminal scale-like projections and with a few tooth-like protuberances scattered on surface; frontal knobs large and poorly ornamentedArtemia persimilis
Scale-like, acute, projections covering completely the apical end of the basal spine of the penises; small, densely ornamented frontal knobs with spines and setae3
-
Abdomen proportionally long, furcal branches remarkably short, rami with few to none plumose setaeArtemia urmiana
Abdomen proportionally short, furcal branches each with less than 15 plumose setae4
-
Frontal knobs large, with large basis; abdominal segments proportionally broadArtemia monica (= A. franciscana)
Frontal knobs small, with small basis; abdominal segments proportionally slenderArtemia sinica
Conclusions
The proper names for the evolutionary units in which brine shrimps are structured remain as follows: Artemia persimilis Piccinelli & Prosdocimi, 1968 for the Southern Cone Lineage; Artemia salina (Linnaeus, 1758) for the Mediterranean-South African Lineage; Artemia monica Verrill, 1869 (= A. franciscana Kellogg, 1906) for the New World Lineage Artemia urmiana Günther, 1899 for the Western Asian Lineage; and Artemia sinica Cai, 1989 for the Eastern Asian Lineage.
Future research to identify species-level lineages in Artemia is still required in different geographic areas. The Mediterranean and South African populations of A. salina are so distant geographic and genetically that they could represent two independent taxonomic units (Muñoz et al., 2008; but see Baxevanis et al., 2014). The mtDNA phylogeographic structure within A. monica (= A. franciscana), depicts a series of relatively isolated units (Puerto Rico, México among others, see Fig. 5) so separated from the remaining ones, that deserve a detailed nuclear study to set the level of gene flow among them. The extent of gene flow occurring among the different Tibetan populations and also with respect to other Asian populations needs to be revised. Since the population of A. tibetiana from the type locality shows a relatively high-level of gene exchange with Asian populations of A. urmiana (Baxevanis, Kappas & Abatzopoulos, 2006; Eimanifar et al., 2014; Asem, Eimanifar & Sun, 2016), its synonymy seems to be justified. But, other Tibetan populations might not be subjected to equal amounts of gene flow, and could represent undescribed taxa (Kappas, Baxevanis & Abatzopoulos, 2011; Eimanifar et al., 2014). Finally, bisexual Hawaiian (Sars, 1904) and coastal Peruvian (Grube, 1874) populations, bisexual and parthenogenetic Chinese populations (Qian et al., 1992), and parthenogenetic populations from Australia (Sayce, 1903), all require of molecular data to guarantee a precise identification to confirm their synonymy with other published names.
Appendix I
Nomina nuda and other unavailable names in Artemia
A nomen nudum is “a name that, if published before 1931, fails to conform to Article 12; or, if published after 1930, fails to conform to Article 13. A nomen nudum is not an available name, and therefore the same name may be made available later for the same or a different concept; in such a case it would take authorship and date [Arts. 50, 21] from that act of establishment, not from any earlier publication as a nomen nudum.” (International Commission on Zoological Nomenclature, 1999). Article 12 explicitly indicates that: “To be available, every new name published before 1931 must satisfy the provisions of Article 11 and must be accompanied by a description or a definition of the taxon that it denotes, or by an indication”. Article 13 explicitly indicates: “T. be available, every new name published after 1930 must satisfy the provisions of Article 11 and must 13.1.1. be accompanied by a description or definition that states in words characters that are purported to differentiate the taxon, or 13.1.2. be accompanied by a bibliographic reference to such a published statement, even if the statement is contained in a work published before 1758, or in one that is not consistently binominal, or in one that has been suppressed by the Commission (unless the Commission has ruled that the work is to be treated as not having been published [Art. 8.7]), or 13.1.3. be proposed expressly as a new replacement name (nomen novum) for an available name, whether required by any provision of the Code or not.”
According to the International Commission on Zoological Nomenclature (1999) criteria for a name to be considered a nomen nudum (Articles 12 and 13 of the Code), none of the following Artemia names are nomina nuda: Eulimene albida Latreille, 1816; Branchipus milhausenii Fischer de Waldheim, 1834; Artemia salina f. arietina Fischer, 1851; Artemisia proxima King, 1855; Branchipus oudneyi Liévin, 1856; Artemia jelskii Grube, 1874; Artemia salina var. biloba Entz, 1886; Artemia salina var. furcata Entz, 1886; Callaonella dybowski Grochowski, 1896; Artemia westraliensis Sayce, 1903; and Artemia salina var. pacifica Sars, 1904. They are all available names.
However, “Artemia elegans Seale, 1933”, “Artemia americana Barigozzi, 1974”, “Artemia odessensis Barigozzi, 1980”, “Artemia sinica aibihuensis Yin, Zhang & You, 2013”, “Artemia sinica gahaiensis Yin, Zhang & You, 2013”, “Artemia sinica jingyuhuensis Yin, Zhang & You, 2013”, and “Artemia sinica xiaochaidanensis Yin, Zhang & You, 2013”, meet the requirements to be considered nomina nuda and therefore are unavailable (Asem et al., 2020; see also Article 11.5).
All other names from Simon (1886), Samter & Heymons (1902), Artom (1906a, 1906b, 1906c, 1912, 1921b), and Perrier (1929), included by Rogers (2013) as nomina nuda, and those included by Vikas et al. (2012) (Asem et al., 2020) rather correspond to denominations that the authors never intended to become taxonomic nomenclatural acts, or to names that actually were never used by them. Artom (1905, 1906a, 1906b, 1906c, 1907a, 1907b, 1908, 1911, 1912, 1913, 1921a, 1921b, 1922, 1924, 1926, 1931) performed a series of meticulous experiments demonstrating the existence of an ovoviviparous reproductive mode, and the presence of parthenogenetic and also tetraploid populations of Artemia, which he considered to be differentiated species. However, Artom (op. cit.) never intended to provide new names for these species or describing them (Bond, 1934); contrary to the opinion of Barigozzi (1974, 1980). Artom (op. cit.) used names as biological terms, in Italian, referring to the biological traits of the populations he was studying: “Artemie sessuate”, “Artemie partenogenetiche”, “Artemia di Cagliari”, “Artemia partenogenetica di Capodistria”, “varietà sessuata”, “forma sessuata”, “Artemia sessuata”, “varietà partenogenetica”, “Artemie partenogenetiche di Marsiglia e di Capodistria”, “Artemia a partenogenesi indefinita”, “Artemia sessuata di Cagliari”, “Artemia sessuata di Cagliari”, “Artemia salina sessuata di Cagliari”, “Artemia partenogenetica di Capodistria”, “Artemia salina partenogenetica di Capo d’Istria”, “Artemia salina di Capodistria”, “Artemia micropirenica”, “Artemia macropirenica”, “Artemie micropireniche”, “Artemie macropireniche”, “Artemia salina partenogenetica di Odessa”, “Artemia partenogenetica di Odessa”, “Artemie (univalens di Cagliari e bivalens di Capo d’Istria)”, “Artemia bivalens”, “Artemia univalens”, “specie univalens sessuata (Cagliari)”, specie “bivalens partenogenetica (Capodistria e Odessa)”, “Artemia salina univalens”, “Artemia salina bivalens”, “Artemia salina bivalens di Capo d’Istria”, “Artemia salina di Cagliari (univalens)”, “Artemia salina di Capo d’Istria (bivalens)”, “Artemia salina bivalens di Odessa” (Artom, op. cit.; italics as in the original). These adjectives, used in different forms in the same page, are not taxonomic actions and are not available for nomenclatural purposes (Art. 1.3.5). Therefore, they cannot be included in the synonymy of any species of Artemia (Belk & Brtek, 1995), nor treated as nomina nuda (Rogers, 2013). A similar situation occurs with Samter & Heymons (1902) diverse expresions; however, Samter & Heymons (1902) never used a name such as “Artemia cagliaritana”, wrongly attributed to them in various synonymic lists (nomen dubium according to Belk & Brtek (1995), and nomen nudum according to Rogers (2013 sub “Artemia cagliartiana”), or to Barigozzi (1980: 150 sub “Artemia calaritana”)). However, Artom (1905: 286) did use such a name to indicate that there is no justification to legitimate the creation of a new species: “… non sono tali da legittimare la creazione di una nuova specie di Artemia Cagliaritana”, obviously referring to the geographic location of his samples, as stated a few lines below: “…di una especie Cagliaritana”. These words cannot be considered a nomenclatural act. In a similar manner, Simon (1886) subdivision of morphotypes in A. salina: “Forma principalis”, “Forma intermedia”, “Forma Milhauseni”, and “Forma Köppeniana”, were not intended to be used as taxonomic entities. However, Daday de Deés (1910) made the name A. salina var. principalis available. Barigozzi (1974, 1980) with an evident disregard for taxonomic nomenclature, complicated matters by proposing infrasubspecific and new (unavailable) names and by considering Artom’s (op. cit.) descriptions, nomenclatural acts.
In summary, the following names mentioned by previous authors (Belk & Brtek, 1995; Rogers, 2013) are unavailable for taxonomic nomenclature purposes and consequently should be stated as such, or simply should not to be placed in the synonymic list of any species of Artemia: “Artemia salina f. intermedia Simon, 1886”; “Artemia salina f. principalis Simon, 1886”; “Artemia cagliaritana Samter & Heymons, 1902”; “Artemia salina partenogenetica Artom, 1906”; “Artemia salina sessuata Artom, 1912”; “Artemia salina univalens Artom, 1912”; “Artemia salina bivalens Artom, 1912”; “Artemia bivalens partenogenetica Artom, 1912”; “Artemia micropirenica Artom, 1921”; “Artemia salina f. typica Perrier, 1929”; “Artemia elegans Seale, 1933; Artemia americana Barigozzi, 1974”; “Artemia odessensis Barigozzi, 1980”; “Artemia kazakhastan Vikas et al., 2012”; and “Artemia china Vikas et al., 2012”. See Asem et al. (2020) for details with respect for these two last names.
Some additional names, “Artemia salina var. arietina f. brachycerca Daday de Deés, 1910”; “Artemia salina var. arietina f. dolichocerca Daday de Deés, 1910”; “Artemia salina var. arietina f. eurycerca Daday de Deés, 1910”; “Artemia salina var. arietina f. oligotricha Daday de Deés, 1910”; “Artemia salina var. arietina f. polytricha Daday de Deés, 1910”; and “Artemia salina var. principalis Daday de Deés, 1910”, were used to describe intrapopulational variation and infrasubspecific taxa and therefore, are also unavailable according to the International Commission on Zoological Nomenclature (1999).
Once all these aforementioned names are removed from consideration, there are still 31 available names that could be applied to taxa within Artemia (see main text and “Appendix II”).
Appendix II
Nomina dubia in Artemia
A nomen dubium is “a name of unknown or doubtful application”, but nonetheless, an available name (International Commission on Zoological Nomenclature, 1999). There are many names of doubtful application in Artemia (Belk & Brtek, 1995). The incorporation of reproductive biology and molecular data to the current species concepts, makes very difficult the assignation of the older and some recent names to the taxon they belong to.
Names falling within the category of nomina dubia are: Eulimene albida Latreille, 1816, “…dans la Méditerranée…”, type locality not precise and reproductive mode not indicated (Latreille, 1816). Artemia eulimene Leach, 1819, “Habite la Méditerranée, près Nice”, type locality precise, but reproductive mode not indicated (Leach, 1819). Artemia proxima King, 1855, “Salt Pans, Newington; Parramatta”, type locality impossible to locate (according to Sayce (1903)), but currently Newington and Parramatta are suburbs of Sydney (New South Wales); Newington produced large quantities of salt along the 19th century; in case the population still exists, it requires DNA data. Artemia gracilis Verrill, 1869, “Near New Haven, in tubs of water from salt marsh”, type locality precise, but not found again; if the population still exists requires DNA data. Artemia jelskii Grube, 1874, “…Callao…”, it is possible that the specimens studied were originated from any saltern along the coast of Perú, but shipped to Europe from Callao, the main commercial port at the time in the area; if the population still exists it would be necessary to study its genetic identity. Artemia australis Sayce, 1903, “Brackish-water, Sandhills, Glenelg, coastal district of South Australia…”, probably parthenogenetic based on the material studied originally (Sayce, 1903), requires DNA data. Artemia westraliensis Sayce, 1903, “Lake Aurean, Murchison, West Australia…”, probably parthenogenetic based on the type series (Sayce, 1903); if the population still exists requires DNA data. Artemia barkolica Qian & Wang, 1992, sequences are nested within the Western Asian Lineage, but there are contradictory data on the reproductive mode of this population (Asem et al., 2020). Artemia urumuqinica Qian & Wang, 1992, is likely a synonym of A. urmiana, but more clarifying data are necessary. For these names, we can only make a tentative attempt of species allocation.
Supplemental Information
Circular map of the Artemia mitogenome.
The abbreviations for the genes are as follows: cox1, cox2, and cox3 refer to the cytochrome C oxidase subunits; CytB refers to cytochrome B; and nad1–6 refers to NADH dehydrogenase subunits; atp6 and atp8 refer to subunits 6 and 8 of F0 ATPase; rrnL and rnnS refer to the 16s and 12S rRNA genes. The major non-coding region, D-loop and associated promoters (DLP), is shown in grey. Arrows indicate the direction of transcription. Protein coding genes are depicted in green, tRNAs in pink and ribosomal RNA in red.
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the second scenario hypothesis (Scheme 2).
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the second scenario hypothesis (Scheme 2). Time indicated in million years (Ma). Dark blue horizontal bars represent 95% HPD (High Posterior Density). A posterior probability value of 1 was obtained for all nodes.
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the third scenario hypothesis (Scheme 3).
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the third scenario hypothesis (Scheme 3). Time indicated in million years (Ma). Dark blue horizontal bars represent 95% HPD (High Posterior Density). A posterior probability value of 1 was obtained for all nodes.
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the fourth scenario hypothesis (Scheme 4).
Chronogram showing lineage divergence times in Artemia obtained using BEAST following the fourth scenario hypothesis (Scheme 4). Time indicated in million years (Ma). Dark blue horizontal bars represent 95% HPD (High Posterior Density). A posterior probability value of 1 was obtained for all nodes.
Annotations of the mitogenome content of A. salina..
Mitochondrial DNA contains 13 protein genes, two rRNA genes and 22 tRNA genes. Length, size, positions, start/stops codons (protein genes) and anticodons (tRNA) are specified per gene. DLP stands for D-loop and associated promoters. *TAA stop codon is completed by the addition of 3’ A residues to the mRNA.
Annotations of the mitogenome content of Artemia franciscana.
Annotations of the mitogenome content of Artemia franciscana. Mitochondrial DNA contains 13 protein-coding genes, two rRNA genes and 22 tRNA genes. Length, size, positions, start/stops codons (protein genes) and anticodons (tRNA) are specified per gene. DLP stands for D-loop and associated promoters. *TAA stop codon is completed by the addition of 3’ A residues to the mRNA.