Clostridium manihotivorum sp. nov., a novel mesophilic anaerobic bacterium that produces cassava pulp-degrading enzymes
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Biodiversity, Biotechnology, Microbiology, Molecular Biology, Taxonomy
- Keywords
- Cassava pulp-degrading enzymes, Clostridium species, Complete genome sequence, Illumina, Mesophilic anaerobic bacterium, Oxford nanopore technology
- Copyright
- © 2020 Cheawchanlertfa et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Clostridium manihotivorum sp. nov., a novel mesophilic anaerobic bacterium that produces cassava pulp-degrading enzymes. PeerJ 8:e10343 https://doi.org/10.7717/peerj.10343
Abstract
Background
Cassava pulp is a promising starch-based biomasses, which consists of residual starch granules entrapped in plant cell wall containing non-starch polysaccharides, cellulose and hemicellulose. Strain CT4T, a novel mesophilic anaerobic bacterium isolated from soil collected from a cassava pulp landfill, has a strong ability to degrade polysaccharides in cassava pulp. This study explored a rarely described species within the genus Clostridium that possessed a group of cassava pulp-degrading enzymes.
Methods
A novel mesophilic anaerobic bacterium, the strain CT4T, was identified based on phylogenetic, genomic, phenotypic and chemotaxonomic analysis. The complete genome of the strain CT4T was obtained following whole-genome sequencing, assembly and annotation using both Illumina and Oxford Nanopore Technology (ONT) platforms.
Results
Analysis based on the 16S rRNA gene sequence indicated that strain CT4T is a species of genus Clostridium. Analysis of the whole-genome average amino acid identity (AAI) of strain CT4T and the other 665 closely related species of the genus Clostridium revealed a separated strain CT4T from the others. The results revealed that the genome consisted of a 6.3 Mb circular chromosome with 5,664 protein-coding sequences. Genome analysis result of strain CT4T revealed that it contained a set of genes encoding amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic enzymes. A comparative genomic analysis of strain CT4T with closely related species with available genomic information, C. amylolyticum SW408T, showed that strain CT4T contained more genes encoding cassava pulp-degrading enzymes, which comprised a complex mixture of amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic enzymes. This work presents the potential for saccharification of strain CT4T in the utilization of cassava pulp. Based on phylogenetic, genomic, phenotypic and chemotaxonomic data, we propose a novel species for which the name Clostridium manihotivorum sp. nov. is suggested, with the type strain CT4T (= TBRC 11758T = NBRC 114534T).
Introduction
The bio-based economy is an emerging sector with a notable potential for economic growth and with promising business opportunities. It is generally defined as the sustainable exploitation and management of renewable natural resources for producing bio-based products. Recently, biorefineries utilize lignocellulosic and other organic raw materials to generate a spectrum of bio-based products such as biofuels, biochemicals and other high value-added products get attention (FitzPatrick et al., 2010). Biomass feedstocks are grouped into two categories, carbohydrate-rich and oleaginous (Melero, Iglesias & Garcia, 2012). Carbohydrate-rich feedstocks contain starch and non-starch polysaccharides (NSP). Industrial starch-rich by-products such as cassava pulp, wheat bran, rice bran, sago pith residues and brewery-spent grains are available in enormous quantities and vary in terms of starch and NSP, hemicellulose and cellulose components (Cripwell et al., 2015). These materials are potential feedstocks for bio-based production, however, they have first to undergo a pretreatment process for the enhanced production of biofuels, organic acids and other valuable biochemicals (Cripwell et al., 2015; Zhang et al., 2016). The starch granules in the starch-rich by-products are entrapped tightly in the secondary cell wall structure by cellulose, hemicellulose and lignin, thus, the starch cannot be easily released for further conversion (Apiwatanapiwat et al., 2016). Moreover, the costs associated with the pretreatment process, such as the energy, equipment and wastewater treatment costs, have resulted in the slow adoption of the technology.
Thailand is a major cassava producer for the domestic and global markets. Cassava starch factories in Thailand generate approximately 1.5–2.0 million tons of waste cassava pulp annually (Norrapoke et al., 2018). Most of the cassava pulp ends up in landfills, resulting in environmental pollution. The pulp spoils rapidly in the humid, warm tropical environment, and under anaerobic conditions generates methane, thus contributing to global warming and leaching of the soil, entering water sources and creating a nuisance to the air quality near the cassava starch factories that consequently affects human health. The utilization rather than discarding of cassava pulp will, therefore, reduce the negative impact on environmental and human health. On a dry weight basis, cassava pulp is mainly composed of starch (50–60%, w/w) with 15–27% (w/w) cellulose and hemicelluloses contents, pectin (7.0–7.3%, w/w), and lignin (3.4–4.6%, w/w) (Djuma’ali et al., 2012; Vaithanomsat et al., 2013). In general, the saccharification of cassava pulp to fermentable sugars used in the production of high value-added products requires the action of enzymes belonging to glycoside hydrolases (GHs), which hydrolyze the glycosidic bonds of starch, cellulose and hemicellulose contained in lignocellulose (Naumoff, 2011; Linares-Pastén, Andersson & Karlsson, 2014). Thus, the hydrolysis of cassava pulp by enzymatic saccharification requires the interaction of a set of carbohydrate-active enzymes containing amylolytic, cellulolytic and hemicellulolytic activities (Rattanachomsri et al., 2009).
Mesophilic anaerobic Clostridia have been reported to produce enzymes that have a high potential to hydrolyze biomass feedstocks (Doi & Kosugi, 2004). However, there are different dominant groups of enzymes to degrade starch and NSP. Although Clostridium polyendosporum PS-1T (Duda et al., 1987) and C. amylolyticum SW408T (Song & Dong, 2008) have been reported to utilize starch as their carbon source for growth, until now no further studies on the amylolytic enzyme properties of these two microorganisms have been elucidated. So far, the genome of C. amylolyticum SW408T has been sequenced by the Joint Genome Institute (JGI) as part of the Community Science Program in 2016, revealing a total of 27 genes coded for amylolytic-, hemicellulolytic- and cellulolytic-enzymes that mainly consisted of genes encoding for an amylolytic enzyme, with very low hemicellulolytic- and cellulolytic-enzymes, while the C. polyendosporum PS-1T genome is not available and has not been reported in the database. In comparison, mesophilic anaerobic Clostridia, such as C. cellulovorans 743BT (Sleat, Mah & Robinson, 1984; Tamaru et al., 2010), C. cellulolyticum ATCC 35319T (Desvaux, 2005), C. josui JCM 17888T (Sakka et al., 2010), C. acetobutylicum ATCC 824T (Sabathé, Soucaille & Bélaïch, 2002) and C. bornimense M2/40T (Tomazetto et al., 2016) produce highly active cellulolytic enzymes, but provide very low amylolytic- and hemicellulolytic-enzyme activities. In contrast, Clostridium sp. strain MF28 was reported as producing a highly hemicellulolytic enzyme with an efficient capability to degrade hemicelluloses and raw plant biomass, but which expressed a low level of amylolytic- and cellulolytic-enzymes (Li & He, 2016). To date, there has been no report of any mesophilic anaerobic Clostridium capable of producing an array of amylolytic-, hemicellulolytic- and cellulolytic-enzymes which can degrade cassava pulp efficiently. Therefore, only the enzyme systems from microorganism that is infrequently isolated, especially those from mesophilic anaerobic bacteria, possess the ability to degrade starch-based biomass and may, therefore, provide increased opportunities for industrial applications. These bacteria have always predominantly produced a wide range of pH and temperature tolerant enzymes (Himmel et al., 2010). They are very suitable to be used on starch liquefaction and saccharification processes and can save energy, reduce expensive heating steps and reduce adverse chemical reactions at high temperatures. This observation has encouraged us to look for a new mesophilic anaerobic bacterium that can produce an array of amylolytic-, hemicellulolytic- and cellulolytic-enzymes that will function synergistically and cooperatively to degrade cassava pulp, as well as hydrolyze the recalcitrant cell wall structure of the pulp.
The usual parameters used to delineate and describe new bacterial species include 16S ribosomal RNA gene sequence-based identity and phylogeny (Tindall et al., 2010), genomic G + C content diversity and DNA-DNA hybridization (DDH) (Wayne et al., 1987). However, there are some limitations to these parameters, notably because the cutoff values vary dramatically between genera and species. The introduction of high-throughput sequencing techniques has made genomic data available for many bacterial species, and to date, the availability of low-cost, high-performance sequencing continues to expand the diversity of research and applications on a genome-scale. Advances in the next generation of sequencing technologies, e.g., Illumina (Bentley et al., 2008) and Oxford Nanopore Technology (ONT) platforms (Clarke et al., 2009) have been applied to sequencing full-length genetic information of many organisms, by generating short- and long-read sequence data that enables the accurate identification of species-level taxonomy and allows for the de novo assembly of complete genomes. The combination of genomic and phenotypic information will allow a faster and more reliable classification of new isolates of microorganisms (Chun & Rainey, 2014).
In this study, we isolated a novel mesophilic anaerobic bacterium, Clostridium manihotivorum CT4T from the soil of a cassava pulp landfill. The isolated strain demonstrated an efficient degradation of cassava pulp, a by-product of the cassava starch industry. The phenotypic and biochemical characteristics of the isolated strain were reported. To better understand the genetic basis for the cassava pulp degradation by strain CT4T, its genome was entirely sequenced using Illumina and ONT platforms. The genome analysis of strain CT4T identified a set of genes encoding amylolytic-, hemicellulolytic- and cellulolytic-enzymes critical to its ability to degrade cassava pulp, which is rarely found in Clostridium species.
Materials and Methods
Preparation of samples and basal medium
Samples of cassava pulp and soil beneath the waste heap were obtained from a starch factory landfill in Chonburi Province, Thailand. The pulp was ground by an ultra centrifugal mill ZM-100 and sieved through a 0.5 mm mesh screen (Retsch, Haan, Germany). The pulp was washed several times with distilled water to remove the remaining sugar and other dirt, oven-dried at 50 °C until at a constant weight and then stored in plastic bags at 4 °C for further experiments.
The basal medium (BM7; pH 7.0) was composed of (per liter) 1.5 g KH2PO4, 2.9 g K2HPO4, 2.1 g urea, 4.5 g yeast extract, 0.5 g cysteine-HCl, 0.001 g resazurin and 200 µL mineral solution (25.0 g/L MgCl2.6H2O, 37.5 g/L CaCl2.2H2O and 0.3 g/L FeSO4.6H2O). The BM7 was anaerobically prepared in bottles sealed with butyl rubber stoppers, under an atmosphere of high-purity N2, and sterilized by autoclaving at 121 °C for 15 min.
Screening and isolation of cassava pulp-degrading strains
The enrichment and isolation were performed under anaerobic conditions. Approximately 1 g of the soil sample was transferred into Hungate tubes containing 15 mL BM7 (pH 7.0) and 1% (w/v) cassava pulp. After inoculation, each test tube was flushed with N2 and incubated at 37 °C. The culture that showed the highest degradation of pulp, as visually indicated by the remaining cassava pulp (approximately ≤ 50% residue dry weight), was selected and serially diluted into agar-cassava pulp medium that had been preliminarily melted and cooled to 55 °C. The cultures were then subjected to the roll-tube technique for isolating obligate anaerobes (Hungate, 1969), after which solidified samples were incubated at 37 °C. Single colonies were isolated with sterile needles and inoculated into BM7 broth containing cassava pulp. Afterward, the cultures were incubated to study their ability to degrade the cassava pulp. Pure cultures were obtained following repeated sub-culturing (ten times) in BM7 containing cassava pulp.
The composition of cassava pulp and residual cassava pulp digested by C. manihotivorum CT4T, C. polyendosporum PS-1T and C. amylolyticum SW408T were analyzed following the National Renewable Energy Laboratory (NREL) protocol (Sluiter et al., 2008).
16S rRNA gene sequencing
Genomic DNA for 16S rRNA gene sequencing was prepared by phenol-chloroform extraction. The 16S rRNA gene was amplified by PCR using the following primers: 8F (5′–AGAGTTTGATCCTGGCTCAG–3′) and 1492R (5′–GGTTACCTTGTTACGACTT–3′). The PCR reaction conditions were as follows: 94 °C for 3 min, 35 cycles at 94 °C for 30 s, 55 °C for 40 s and 72 °C for 2 min, with a final extension time of 5 min at 72 °C. The amplified fragment was ligated into the pGEM-T Easy vector (Promega, Madison, WI, USA), and the recombinant plasmid was sequenced using T7 and SP6 primers. A sequence similarity search was performed using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi). A phylogenetic tree was generated by the neighbor-joining method with 1,000 bootstrap replications, employing the MEGA version 6.0 (Tamura et al., 2013).
Physiological and biochemical analysis
Gram staining of strain CT4T was conducted using the conventional methodology and confirmed using the KOH test (Powers, 1995). Endospore staining was examined by Schaeffer–Fulton’s method (Schaeffer & Fulton, 1933). Cell morphology was observed by scanning electron microscope (SEM; model SU800 Hitachi, Japan). Substrate utilization was tested by growing the strain in BM7 containing 0.5% (w/v) of the following substrates: D-glucose, D-galactose, D-arabinose, D-rhamnose, D-mannose, D-xylose, D-fructose, D-trehalose, D-raffinose, lactose, sucrose, maltose, cellobiose, mannitol, soluble starch from potato (ACS reagent), birchwood xylan, cellulose powder (Type 20) and Avicel® (PH-101); these chemicals were purchased from Sigma-Aldrich, Saint Louis, MO, USA. Raw cassava starch and cassava pulp were obtained from a starch factory landfill in Chonburi Province, Thailand. After 5 days of incubation, cell growth was assessed by determining the optical density at 600 nm. The fermentation products in the supernatant extracted from the glucose-grown culture were determined by gas chromatography equipped with a flame-ionization detector and a Carbopack B-DA/4% Carbowax 20M column (GC-14A; Shimadzu, Japan). The column, injector and detector temperatures were 170, 230 and 230 °C, respectively. Other biochemical tests were conducted according to the methods of Holdeman, Cato & Moore (1977) and Summanen et al. (1993). The isomers of diaminopimelic acid (DAP) in the cell wall were determined as described by Komagata and Suzuki (Komagata & Suzuki, 1988). Cellular fatty acids were extracted, methylated and analyzed using the standard microbial identification system (MIDI) protocol (Sherlock microbial identification system, version 6.1) while the fatty acids were identified using the TSBA6 database of the microbial identification system (Sasser, 1990). The polar lipids were analyzed from freeze-dried cells by two-dimensional TLC, as described by Minnikin et al. (1984). Appropriate detection reagents were used to visualize the spots: phosphomolybdic acid reagent 5% (w/v) solution in ethanol (Sigma-Aldrich, Saint Louis, MO, USA) was used to detect total polar lipids; ninhydrin reagent (0.2% solution; Sigma-Aldrich) was used to detect amino lipids; Dittmer and Lester reagent (molybdenum blue reagent, 1.3%; Sigma-Aldrich) was used to detect phospholipids and Dragendorff’s reagent (Sigma-Aldrich) was used to detect phosphatidylcholine.
Cultivation and enzyme production
Strain CT4T was anaerobically cultivated in 1,000 mL of BM7 containing 1% (w/v) cassava pulp for 3 days at 37 °C, pH 7.0 under static conditions in an anaerobic chamber (Bactron II, USA). The culture supernatant was collected by centrifugation at 10,000× g for 10 min at 4 °C and subsequently concentrated using a hollow fiber cartridge with a 10-kDa-cutoff membrane (GE Healthcare, USA). The retentate (50-times more concentrated) was then used as the crude enzyme.
Enzyme assays and protein determination
All enzyme assays were performed in 50 mM sodium phosphate buffer (pH 7.0) and incubated at 55 °C for 10 min. The enzymatic activities on 1% (w/v) cassava pulp, soluble starch, pullulan, birchwood xylan, cellulose powder (Type 20) and pectin from citrus peel were assayed by determining the amount of reducing sugars liberated by the Somogyi–Nelson method (Nelson, 1944). One unit (U) of enzyme activity was defined as the amount of enzyme that released 1 µmol of reducing sugar in 1 min under assay conditions. The protein concentration was determined by the Lowry method (Lowry et al., 1951) using bovine serum albumin as a standard.
Library preparation and genome sequencing
The genomic DNA was extracted from the cultures using a blood and cell culture DNA midi kit (Qiagen, Germany) according to the manufacturer’s instructions. Strain CT4T was sequenced using two sequencing platforms: Illumina (Hiseq2500) and ONT (MinION). Illumina sequencing paired-end DNA libraries were prepared following the Illumina DNA manufacturer’s instructions (NEBNext® Ultra™ DNA library prep kit). The size-selected, adaptor-ligated DNA fragments were PCR-amplified using the following protocol: polymerase activation (98 °C for 30 s), followed by 10 cycles (denaturation at 98 °C for 10 s, annealing at 65 °C for 75 s and extension at 65 °C for 75 s) with a final 5 min extension at 65 °C. The DNA libraries were purified by magnetic beads, and their size distribution was checked using Agilent Bioanalyzer DNA high sensitivity chip assay. The DNA fragments were sequenced using the Illumina Hiseq2500 with 2 × 150 bp paired-end protocol (Illumina, Inc., California, USA).
The ONT library preparation and bioinformatics analysis were performed according to Jenjaroenpun et al. (2018). In brief, a total amount of 600 ng genomic DNA was used as input for a rapid sequencing kit (SQK-RAD002) to generate the DNA sequencing library. The library was then loaded onto a flow cell version FLO-MIN106 on a MinION Mk1B (released in 2014 through the MinION Access Program) (Oxford, UK) to perform DNA sequencing for 48 h. Base-calling was performed using the local-based software, Albacore version 1.2.3 (ONT, USA).
Genome assembly and annotation
The high-quality ONT reads (average quality score of >7) were first assembled using combination Minimap2 (Li, 2018) and Miniasm (Li, 2016), resulting in a circular draft genome. The draft genome was polished using Racon (Vaser et al., 2017) and Nanopolish (Loman, Quick & Simpson, 2015), based on the consensus pileup of high-quality ONT reads and additionally using Pilon (Walker et al., 2014), based on short Illumina reads.
Genome annotation was conducted with a Prokka annotation pipeline (Seemann, 2014). The rRNA and tRNA genes were identified with RNAmmer (Lagesen et al., 2007) and Aragorn software (Laslett & Canback, 2004), respectively. Functional classification of protein-coding genes in Clostridium sp. strain CT4T was done by assigning Cluster of Orthologous Group (COG) codes to each gene using eggnog-Mapper (Huerta-Cepas et al., 2017) and eggNOG version 4.5 (Huerta-Cepas et al., 2016).
Average amino acid identity analysis
The first analysis comprised pairwise comparisons of AAIs (Konstantinidis & Tiedje, 2005) of the 665 genomes belonging to the Clostridia class (Cabal et al., 2018). For each pair of genomes, the average AAI was then calculated based on the identities of all conserved reciprocal best matches, a calculation that was not always symmetrical. In such cases, the average of the two AAI values was assigned to each pair of genomes. The AAI tree was built with BIONJ (Gascuel, 1997) to find dissimilarities of AAI values (100% minus AAI).
Comparison of glycoside hydrolase producing genes in strain CT4T with related species
Strain CT4T (GenBank accession number CP025746) was compared with the closely related C. amylolyticum SW408T (NZ_FQZO00000000; NCBI) that had available genomic information in the NCBI database, using OrthoMCL (Chen et al., 2006) to characterize their specific genetic features and identify overlaps among orthologous clusters. The protein sequences were grouped into gene families encoding amylolytic-, hemicellulolytic- and cellulolytic-enzymes, using the criteria: E-value <1E-5 and sequence identity >50%. The genomic information of C. polyendosporum PS-1T was not reported in the NCBI database, therefore, the strain PS-1T was excluded from genome comparison.
Results
Isolation and identification of cassava pulp-degrading bacterium
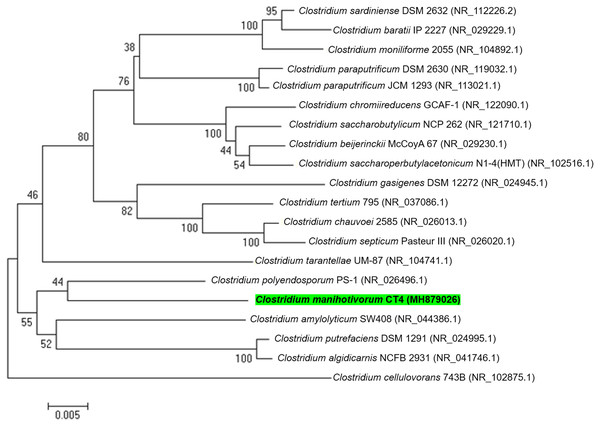
In total, 15 individual colonies were isolated by the roll-tube technique and were subcultured 10 times in BM7 separately, utilizing cassava pulp as carbon sources. Visualization of the roll-tube appearance revealed that isolate CT4T performed best in relation to cassava pulp degradation. Moreover, approximately 60.8% (w/v) removal of dry weight was detected when cultured in BM7 broth. The isolated CT4T thoroughly utilized the starch contains in cassava pulp after 5 days of culturing. The result showed that the isolated CT4T could remove 99.0% starch in cassava pulp, while cellulose and hemicellulose contents were removed by 42.2% and 39.2%, respectively, when compared with the control. Besides, this strain removed starch and non-starch polysaccharide in cassava pulp better than the related species, strain PS-1T and SW408T (Fig. S2). Thus, it was consequently selected for further analysis. Prior to genome sequencing, the 16S rRNA gene sequence of strain CT4T (accession number MH879026) was compared with the nucleotide sequences in NCBI. The analysis revealed that strain CT4T shared 95% sequence identity with Anaerobacter polyendosporus PS-1T, now reclassified as Clostridium polyendosporum comb. nov. (Duda et al., 1987; Stackebrandt et al., 1999) and 94% sequence identity with C. amylolyticum SW408T (Song & Dong, 2008), Clostridium putrefaciens DSM 1291T (Sturges & Drake, 1927), and Clostridium algidicarnis NCFB 2931T (Lawson et al., 1994). Phylogenetic analysis based on 16S rRNA gene sequences and neighbor-joining method indicated that strain CT4T belongs to the genus Clostridium (Fig. 1). Therefore, isolated CT4T was classified as Clostridium sp. CT4T
Figure 1: A phylogenetic tree was constructed from 16S rRNA gene sequences by the neighbor-joining method that showed the relatedness of C. manihotivorum CT4T with other members of the genus Clostridium.
Physiological and biochemical characteristics of strain CT4T
The SEM image revealed that cells of strain CT4T were rod-shaped, and surrounded by a polysaccharide capsule (Fig. 2). Strain CT4T was Gram-positive, single endospore-forming, non-motile and non-flagellate (Table 1). To understand the optimal growth conditions, strain CT4T was cultivated under different pH (pH 4.0–11.0) and temperature (25−50 °C) conditions. Strain CT4T could grow at a wide range of temperatures (25−45 °C) and pH (5.5–7.5) in BM7 medium containing 1% (w/v) cassava pulp. The optimum growth of strain CT4T was found at 37 °C and pH 7.0. Moreover, strain CT4T used a wide range of carbon sources, including D-glucose, D-xylose, D-galactose, D-fructose, D-mannose, D-arabinose, D-rhamnose, D-trehalose, D-raffinose, sucrose, lactose, maltose, mannitol, cellobiose, soluble starch, xylan, cellulose and Avicel®. The main metabolic products of strain CT4T, ranked based on quantity, were acetate, butyrate, ethanol and propionate. Butanol was not observed during growth, whereas it was produced in the closest relative, C. polyendosporum PS-1T (Table 1). In contrast, C. putrefaciens isolated from spoiled ham (Sturges & Drake, 1927), and C. algidicarnis isolated from vacuum-packed refrigerated pork (Lawson et al., 1994) cannot hydrolyze starch despite their relatedness to strain CT4T (Table 1). Strain CT4T presented LL-diaminopimelic acid (LL-DAP) in their cell wall, whereas most members in the genus Clostridium contains meso-diaminopimelic acid. Thus, the strain CT4T was different from the other related strains, except C. putrefaciens that have the same with strain CT4T. The cellular fatty acid profiles of strain CT4T are listed in Table S1. The major fatty acids detected from strain CT4T were C 16:0 (37.4%), C 14:0 (15.0%), anteiso-C 15:0 (5.5%), summed feature 1 (C 13:0-3OH and/or C 15:1isoH; 4.5%), C 19:0cyclo ω8c (4.2%) and C 17:02-OH (4.0%). In terms of their polar lipid profiles, strain CT4T contained phosphatidylethanolamine (PE) and phosphatidylglycerol (PG) as the major polar lipids, while phosphatidylcholine (PC) was found as minor polar lipid. Additionally, three unidentified phospholipids (PL1–PL3) and three unidentified amino lipids (AL1–AL3) were also detected (Fig. S1).
Figure 2: The SEM of C. manihotivorum CT4T grown on basal medium with cassava pulp as the sole carbon source.
| Characteristic | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Isolation source | Decomposed cassava pulp soil | Meadow soil | H2-producing upflow anaerobic sludge blanket reactor | Ham | Vacuum-packed refrigerated pork |
| Cell morphology | Rod | Rod | Rod | Rod | Rod |
| Cell length/width (µm) | 4−8/0.5−1.5 | 4−8/1.5−3.0 | 2.0−7.5/0.5−0.7 | 3−15/0.5−0.7 | 2−5/0.5−1.0 |
| Gram-stain | + | + | + | + | + |
| Endospores formed (amount/cell) | + (1) | + (up to 7) | + (1) | + (1) | + (1) |
| Motility | − | − | + | − | − |
| Flagella | − | NR | + | NR | NR |
| Temperature range/ optimum (°C) | 25−45/37 | 15−45/25−35 | 24−45/37 | 20−25 | 25−30 |
| pH range/optimum | 5.5−7.5/7.0 | 5.5−8.5/6.5 −7.5 | 4.0−9.0/7.0 | 6.0−9.0/8.0 | NR |
| Starch degradation | + | + | + | − | − |
| Fermentation productsa | A, B, E, P, CO2, H2 | A, B, E, L, CO2, H2, b | A, E, CO2, H2 | NR | A, B |
| G + C (mol%) | 32 | 29 | 33 | NR | NR |
| Reference | This study | Duda et al. (1987) | Song & Dong (2008) | Sturges & Drake (1927) | Lawson et al. (1994) |
Notes:
Strains: 1, strain CT4T; 2, Clostridium polyendosporum PS-1T; 3, Clostridium amylolyticum SW408T; 4, Clostridium putrefaciens DSM 1291T; 5, Clostridium algidicarnis NCFB 2931T.
Notes: −, negative; +, positive; NR, no reported.
Based on the 16S rRNA gene sequence similarity, physiological attributes and biochemical properties, strain CT4T was considered to be a novel species of the genus Clostridium. Thus, the strain CT4T was introduced in the namely Clostridium manihotivorum CT4T, which can degrade cassava pulp. The meaning of “manihotivorum” is devouring cassava. This bacterium was deposited as a type strain in the Thailand Bioresource Research Center (TBRC), and NITE Biological Resource Center (NBRC), Japan under accession numbers TBRC 11758T and NBRC 114534T, respectively.
Characterizations of amylolytic-, hemicellulolytic- and cellulolytic-enzymes of C. manihotivorum CT4T
In this study, a C. manihotivorum CT4T was discovered to degrade cassava pulp, which was able to produce the cassava pulp degrading enzymes, including amylolytic-, hemicellulolytic- and cellulolytic-enzymes. In order to characterize the properties of the crude enzyme from strain CT4T, the isolate was cultivated in BM7 medium containing 1% (w/v) cassava pulp at pH 7.0, 37 °C. Afterwards, the culture supernatant was harvested at the early stationary phase (3 days) and concentrated by ultrafiltration technique. The crude enzyme gave the highest activity on cassava pulp (1,901.1 U/g protein), which was 1.56-fold higher than that obtained from soluble starch (1,212.7 U/g protein). In addition, a pullulanase activity of 27.5 U/g protein was detected (Table 2). C. manihotivorum CT4T was also able to produce xylanase (43.5 U/g protein), cellulase (32.0 U/g protein) and pectinase (42.4 U/g protein) as shown in Table 2, which are involved in the degradation of xylan, cellulose and pectin contained in the cell wall structure of cassava pulp, respectively.
The complete genome of C. manihotivorum CT4T and comparative genomics
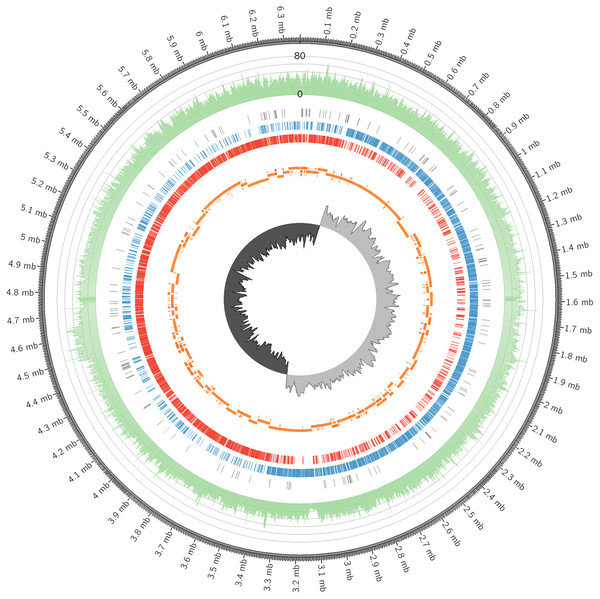
In this study, the complete genome of C. manihotivorum CT4T, deposited in GenBank under the accession number CP025746, was described. A complete, gapless and circular genome assembly was generated, with a total size of 6,364,326 bases and a 40-fold coverage, as shown in Fig. 3 and Table 3. The origin of replication was determined based on GC skew analyses. The average G + C content was approximately 32 mol%, and plasmid was not detected. The DNA G + C content of strain CT4 (32 mol%), was within the range of 23–37% reported for the genus Clostridium (Lawson & Rainey, 2016). Genome annotation was performed using Prokka (Seemann, 2014) and Blast2GO (Conesa et al., 2005). The genome was predicted to have 5,664 protein-coding sequences (CDS), 42 rRNA sequences, 95 tRNA sequences, 1 tmRNA sequence and 153 misc_RNA sequences. Furthermore, NCBI Prokaryotic Genome Annotation Pipeline (PGAP) version 4.11 was also employed to annotate the genome, which provided slightly different result of 5,308 CDSs and 5,654 total genes (Table 3). Hereafter, 5,664 CDSs were used for further analysis, in which the details are available in Data S1. According to the comparison of the genomes between C. manihotivorum CT4T and C . amylolyticum SW408T, the strain CT4T has much larger genome size than the strain SW408T about 2.1 Mb. Moreover, 5,664 CDSs were predicted in C. manihotivorum CT4T whereas only 3,957 CDSs were reported in C. amylolyticum SW408T.
Figure 3: The circular genome map of C. manihotivorum CT4T.
Average amino acid identity and phylogenetic analysis
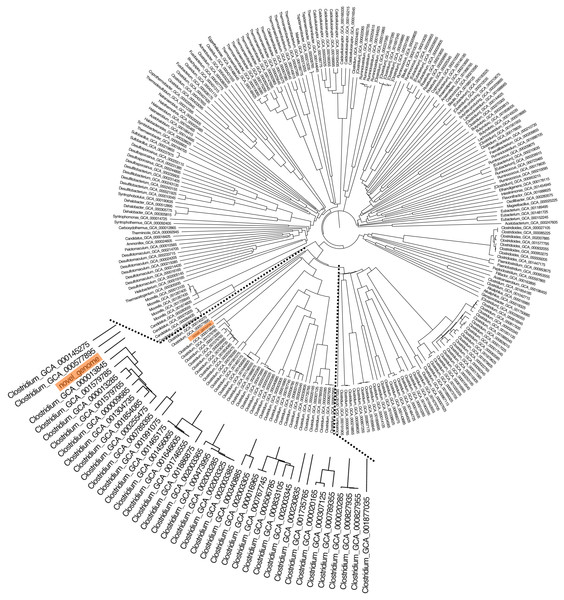
The whole-genome phylogeny of C. manihotivorum CT4T was compared with a unique set of 665 Clostridia class genomes (Cabal et al., 2018), employing the average amino acid identity (AAI) analysis method. AAI has proven to have a better resolution power at the species level than 16S rRNA gene sequence-based comparison (Mahato et al., 2017). The derived phylogenetic tree based on the AAI analysis of all 666 genomes revealed several main clusters (Fig. 4). The strain CT4T was clearly separated from the other Clostridia, in which a single branch was observed. The result aligned with the above physiological and biochemical characteristics that differ from other related type strains.
| Features | Values | |
|---|---|---|
| In-house pipeline | PGAP pipeline | |
| Genome size (bp) | 6,364,326 | 6,364,326 |
| G + C content (mol%) | 32 | 32 |
| Total number of genes | 5,941 | 5,654 |
| Protein-coding sequences | 5,664 | 5,308 |
| rRNA genes | 42 | 42 |
| tRNA genes | 95 | 95 |
| tmRNA | 1 | 1 |
| misc_RNA | 153 | 153 |
Figure 4: The AAI-based tree of C. manihotivorum CT4T and related strains belonging to the 665 Clostridia class.
Functional category of strain CT4T
Approximately, 75% (4,223 out of 5,664) of the protein-coding sequences in C. manihotivorum CT4T were classified into COG functional categories (Table 4): replication, recombinant and repair (L: 548 protein-coding sequences); transcription (K: 381); carbohydrate transport and metabolism (G: 318); amino acid transport and metabolism (E: 276); cell wall/membrane/envelope biogenesis (M: 260) and translation, ribosomal structure and biogenesis (J: 197), based on Tatusov et al. (2000). The COG category (G: 318) comprised mainly protein-coding sequences that were involved in the degradation of starch and polysaccharides contained in lignocellulosic materials, and the transportation of the compounds (Tomazetto et al., 2016). These results suggest that C. manihotivorum CT4T contains genes encoding glycoside hydrolases, related to starch, hemicellulose, cellulose and pectin degrading enzymes (Table 5).
| Code | Functional annotation | Number of genes |
|---|---|---|
| B | Chromatin structure and dynamics | 1 |
| C | Energy production and conversion | 172 |
| D | Cell cycle control, cell division, chromosome partitioning | 38 |
| E | Amino acid transport and metabolism | 276 |
| F | Nucleotide transport and metabolism | 82 |
| G | Carbohydrate transport and metabolism | 318 |
| H | Coenzyme transport and metabolism | 97 |
| I | Lipid transport and metabolism | 71 |
| J | Translation, ribosomal structure and biogenesis | 197 |
| K | Transcription | 381 |
| L | Replication, recombination and repair | 548 |
| M | Cell wall/membrane/envelope biogenesis | 260 |
| N | Cell motility | 56 |
| O | Posttranslational modification, protein turnover and chaperones | 109 |
| P | Inorganic ion transport and metabolism | 30 |
| T | Signal transduction mechanisms | 41 |
| U | Intracellular trafficking, secretion and vesicular transport | 153 |
| R | General function prediction only | 1,134 |
| S | Function unknown | 259 |
| Total | 4,223 |
| Enzymes | EC number | Locus tag | Domains organization |
|---|---|---|---|
| Amylolytic enzymes | |||
| α-Amylase | 3.2.1.1 | CT4_03811, CT4_04618, CT4_04619, CT4_04620, CT4_04873, CT4_05358, CT4_01439 |
CT4_05358: GH13–CBM20 CT4_01439: GH13–CBM53–CBM53 |
| Oligo- α-1,6-glucosidase | 3.2.1.10 | CT4_03811, CT4_04618, CT4_04619, CT4_04620, CT4_04873 | |
| α-Glucosidase | 3.2.1.20 | CT4_03811, CT4_04618, CT4_04619, CT4_04620, CT4_04873, CT4_01877, CT4_03509, CT4_00906, CT4_04500, CT4_04692, CT4_05353, CT4_05689 |
CT4_04500: CBM34–GH13 CT4_04692: CBM34–GH13 |
| Amylo- α-1,6-glucosidase | 3.2.1.33 | CT4_04498 | |
| Pullulanase | 3.2.1.41 | CT4_00906 | |
| Glucan- α-1,6-glucosidase | 3.2.1.70 | CT4_03811, CT4_04618, CT4_04619, CT4_04620, CT4_04873 | |
| Hemicellulolytic enzymes | |||
| Endo-1,4-β-xylanase | 3.2.1.8 | CT4_03195, CT4_04894 | |
| α-Galactosidase | 3.2.1.22 | CT4_04979, CT4_04272 | |
| β-Galactosidase | 3.2.1.23 | CT4_00135, CT4_01004, CT4_01881, CT4_02219, CT4_05037, CT4_05052, CT4_05461, CT4_00379, CT4_01609, CT4_03387 | |
| β-Glucuronidase | 3.2.1.31 | CT4_00135, CT4_01004, CT4_01881, CT4_02219, CT4_05037, CT4_05052, CT4_05461, CT4_04896 | |
| β-Xylosidase | 3.2.1.37 | CT4_03273 | |
| α-L-Arabinofuranosidase | 3.2.1.55 | CT4_03251, CT4_03484, CT4_03690, CT4_02877, CT4_03686 |
CT4_03484: CBM4–GH43 CT4_03690: CBM4–GH43 |
| Endo-β-1,4-mannanase | 3.2.1.78 | CT4_04971, CT4_05469 |
CT4_04971: CBM6–CBM35–GH26 CT4_05469: CBM6–CBM35–GH26 |
| Endo-α-1,5-L-arabinanase | 3.2.1.99 | CT4_01164, CT4_03483, CT4_03685, CT4_05022 | |
| Cellulolytic enzymes | |||
| Endo-β-1,4-glucanase | 3.2.1.4 |
CT4_03367, CT4_01165 CT4_00352, CT4_05071 |
CT4_03367: GH9–CBM3–CBM3 CT4_00352: GH5–CBM46 CT4_05071: GH5–CBM46–CBM3 |
| β-Glucosidase | 3.2.1.21 | CT4_00135, CT4_01004, CT4_01881, CT4_02219, CT4_05037, CT4_05052, CT4_05461, CT4_00940, CT4_01878, CT4_02623, CT4_03388, CT4_03385 | CT4_03385: GH3–CBM6 |
| Endo-β-1,6-glucanase | 3.2.1.75 | CT4_00944 | |
| 6-Phospho-β-glucosidase | 3.2.1.86 | CT4_00135, CT4_01004, CT4_01881, CT4_02219, CT4_05037, CT4_05052, CT4_05461, CT4_05778 | |
| Pectinolytic enzyme | |||
| Pectate lyase | 4.2.2.2 | CT4_00924 | |
Identification of the genes encoding amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic-enzymes in C. manihotivorum CT4T
The genes encoding amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic-enzymes were detected in the genome of C. manihotivorum CT4T (Table 5). Amylolytic enzymes were found in the complete genome of strain CT4T including α-amylase, oligo-α-1,6-glucosidase, α-glucosidase, amylo-α-1,6-glucosidase, pullulanase and glucan-α-1,6-glucosidase that could be classified into endo-acting amylase, exo-acting amylase and debranching amylase. The α-amylase of C. manihotivorum CT4T was predicted to contain starch binding domains (SBDs) of CBM20 (gene locus; CT4_05358) and CBM53 (CT4_01439) while α-glucosidase contained CBM34 (CT4_04500 and CT4_04692), as shown in Table 5. Moreover, the genes encoding hemicellulolytic- and cellulolytic-enzymes, such as endo-1,4-β-xylanase, α-galactosidase, β-galactosidase, β-glucuronidase, β-xylosidase, α-L-arabinofuranosidase, endo-β-1,4-mannanase, endo-α-1,5-L-arabinanase, endo-β-1,4-glucanase, β-glucosidase, endo-β-1,6-glucanase and 6-phospho-β-glucosidase, have been observed to be involved in the hydrolysis of hemicellulose and cellulose. In addition, the genome of C. manihotivorum CT4T also harbors a gene (CT4_00924), which encodes a putative pectate lyase that can be hydrolyzed the internal α-1,4 linked D-galacturonic acid within the pectin.
As illustrated in Fig. 1, phylogenetic analyses showed that strain CT4T forms a cluster with C. polyendosporum PS-1T. The latter cluster forms a sibling group with the C. amylolyticum SW408T, C. putrefaciens DSM 1291T and C. algidicarnis NCFB 2931T branch. However, only the strain SW408T was released as a genome announcement. Thus, the C. amylolyticum SW408T was chosen for comparative genomic exploration. Analysis of genes encoding carbohydrate-active enzymes in the genomes of strains CT4T and SW408T revealed differences in the distribution of genes (Table 6). C. manihotivorum CT4T had a higher number of genes encoding amylolytic enzymes than that of C. amylolyticum SW408T. Moreover, strain CT4T contained more genes encoding hemicellulolytic-, cellulolytic- and pectinolytic-enzymes than C. amylolyticum SW408T, except the debranching enzyme, α-galactosidase.
| Enzymes | EC number | Strains | ||
|---|---|---|---|---|
| CT4 | SW408 | |||
| Amylolytic enzymes | ||||
| α-Amylase | 3.2.1.1 | 7 | 2 | |
| Oligo-α-1,6-glucosidase | 3.2.1.10 | 5 | 1 | |
| α-Glucosidase | 3.2.1.20 | 12 | 8 | |
| Amylo-α-1,6-glucosidase | 3.2.1.33 | 1 | 0 | |
| Pullulanase | 3.2.1.41 | 1 | 0 | |
| Glucan-α-1,6-glucosidase | 3.2.1.70 | 5 | 1 | |
| Total | 31 | 12 | ||
| Hemicellulolytic enzymes | ||||
| Endo-1,4-β-xylanase | 3.2.1.8 | 2 | 0 | |
| α-Galactosidase | 3.2.1.22 | 2 | 5 | |
| β-Galactosidase | 3.2.1.23 | 10 | 4 | |
| β-Glucuronidase | 3.2.1.31 | 8 | 3 | |
| β-Xylosidase | 3.2.1.37 | 1 | 0 | |
| α-L-Arabinofuranosidase | 3.2.1.55 | 5 | 0 | |
| Endo- β-1,4-mannanase | 3.2.1.78 | 2 | 0 | |
| Endo-α-1,5-L-arabinanase | 3.2.1.99 | 4 | 0 | |
| Total | 34 | 12 | ||
| Cellulolytic enzymes | ||||
| Endo-β-1,4-glucanase | 3.2.1.4 | 4 | 0 | |
| β-Glucosidase | 3.2.1.21 | 12 | 3 | |
| Endo-β-1,6-glucanase | 3.2.1.75 | 1 | 0 | |
| 6-Phospho-β-glucosidase | 3.2.1.86 | 8 | 4 | |
| Total | 25 | 7 | ||
| Pectinolytic enzyme | ||||
| Pectate lyase | 4.2.2.2 | 1 | 0 | |
| Total | 1 | 0 | ||
Discussion
As we know, cassava pulp generated in large amounts, as industrial waste during cassava processing is rich in starch and fiber (Norrapoke et al., 2018). Thus, it can be used as a renewable material to produce high value-added products (FitzPatrick et al., 2010). Mostly, bacterial species of the genus Clostridium are known as good degraders of lignocellulosic materials (Doi & Kosugi, 2004). However, not much is known regarding amylase, hemicellulase and cellulase-producing species that are capable of efficient cassava pulp degradation. Among the species isolated from soil samples collected from cassava pulp landfill using the Hungate roll-tube technique, strain CT4T was most effective in degrading cassava pulp. The roll-tube procedure has previously been used to isolate single colonies and pure cultures of bacteria, including Clostridium thermocellum S14 (Tachaapaikoon et al., 2012) and C . amylolyticum SW408T (Song & Dong, 2008). Subsequently, strain CT4T was identified using the 16S rRNA gene sequencing analysis. According to 16S rRNA gene sequence analysis, strain CT4T was phylogenetically related to members of the genus Clostridium (90–95% sequence similarity), with the highest degree of sequence similarity to C. polyendosporum PS-1T (95%) and follow by C . amylolyticum SW408T (94%). These values are at the level suggested to allocate the strain to a novel species of genus Clostridium (Yarza et al., 2008). Moreover, AAI and phylogenetic analysis of the strain CT4T suggested that the newly isolated strain CT4T should be classified as a novel species of the genus Clostridium, known as C. manihotivorum CT4T.
Although C. polyendosporum PS-1T could degrade starch, its activity is not known (Duda et al., 1987). Remarkably, C. polyendosporum PS-1T and C. manihotivorum CT4T have different capacities for endogenous spore formation. While C. polyendosporum PS-1T has the ability to form several endospores in one cell (some cells may produce up to seven), cells of the strain CT4T contained a single endospore (Table 1). Likewise, C. amylolyticum SW408T, a mesophilic anaerobic amylolytic bacterium (and a close relative of strain CT4T), isolated from an H2-producing up-flow anaerobic sludge blanket reactor utilizes several kinds of mono- and di-saccharides and simultaneously hydrolyzes and ferments starch (Song & Dong, 2008). Nonetheless, there are no reports precisely in relation to cassava pulp degradation in this genus. The degradation of cassava pulp by strain CT4T was also compared with that of C. polyendosporum PS-1T and C. amylolyticum SW408T, which are the closest related species. They were inoculated into BM7 containing 1% (w/v) cassava pulp at 37 °C, pH 7.0 for 5 days. C. manihotivorum CT4T grew rapidly, while both strains showed a small amount of growth on cassava pulp. After cultivation, the residue weights of C. manihotivorum CT4T, C. polyendosporum PS-1T and C. amylolyticum SW408T were decreased by 60.8% (w/v), 0.6% (w/v) and 0.4% (w/v), respectively, and compared with the initial dry weight of cassava pulp. Cassava pulp compositions after digested by C. manihotivorum CT4T, C. polyendosporum PS-1T and C. amylolyticum SW408T were analyzed (Fig. S2). C. manihotivorum CT4T showed a high degradation ability for starch, which was 99% starch removal. Moreover, the strain CT4T revealed not only efficient starch degradation but also cellulose, hemicellulose and pectin. By contrast, C. polyendosporum PS-1T and C. amylolyticum SW408T showed ineffective cassava pulp degradation. The starch, cellulose, and hemicellulose contents of the residues were little decreased. The result indicated that C. manihotivorum CT4T might have better cassava pulp degradation ability than C. polyendosporum PS-1T and C. amylolyticum SW408T. The results indicated that the C. manihotivorum CT4T showed greater cassava pulp degradation than the other closely related species. The significantly different degradation of cassava pulp by the crude enzyme from C. manihotivorum CT4T, from the other members of Clostridium, was possibly caused by many factors such as: (1) synergistic interactions among amylolytic-, hemicellulolytic- and cellulolytic-enzymes; (2) the enzymes containing non-catalytic binding domains that linked with catalytic domains known as carbohydrate-binding modules (CBMs); and (3) the weak binding of the enzymes to lignin. Various hemicellulolytic-enzymes including endo-β-1,4-xylanase, β-xylosidase and endo-β-1,4-mannanase were broken down xylan and mannan, the main hemicellulose in cassava pulp that covers the cellulose. Removal of xylan and mannan could help to increase the accessibility of cellulolytic-enzymes (such as endo- β-1,4-glucanase and β-glucosidase) for disruption of cellulose. Synergism between hemicellulolytic- and cellulolytic-enzymes led to enhanced release of the entrapped starch granules from cassava pulp. Consequently, the entrapped starch granules became more available for amylolytic-enzyme which were then effectively hydrolyzed to oligosaccharides and monosaccharides by endo-acting α-amylase, exo-acting α-glucosidase, and debranching enzyme pullulanase. Therefore, the synergism hemicellulolytic- and cellulolytic-enzymes acted cooperatively on decomposition of the hemicellulose-cellulose matrix, leading to increased accessibility of the amylolytic enzymes to the exposed starch granule located within the cassava pulp (Bunterngsook et al., 2017; Poonsrisawat et al., 2017), while CBMs have been reported to assist hydrolysis of insoluble substances by bringing the catalytic domain in close proximity to its substrate (Hervé et al., 2010). Moreover, the cassava pulp degrading enzyme of C. manihotivorum CT4T may be active and low binding to lignin in cassava pulp. Teeravivattanakit et al. (Teeravivattanakit et al., 2017) reported that because the bacterial multifunctional enzyme PcAxy43A from Paenibacillus curdlanolyticus B-6 was a weak lignin-binding enzyme, this enzyme was capable of converting xylan contained in agricultural residues to xylose in one step without chemical pretreatment to remove lignin. Therefore, a weak lignin-binding enzyme is a potential factor for obtaining enzymes suitable for the hydrolysis of lignocellulosic materials (Berlin et al., 2006). Although some Clostridium spp. such as C. amylolyticum SW408T (Song & Dong, 2008), C. thermosulfurigenes H12-1 (Saha, Shen & Zeikus, 1987) and C. butyricum T-7 (Tanaka et al., 1987) have the ability to hydrolyze soluble starch or raw starch by producing α-amylase and β-amylase. However, these three strains do not produce pullulanase, xylanase or cellulase and thus, unlike C. manihotivorum CT4T, lack the properties of cassava pulp degrading enzymes.
To further explore whether C. manihotivorum CT4T could be used to degrade cassava pulp, we analyzed its whole genome for the presence of enzymes involved in cassava pulp degradation. It found that the genome contains various genes encoding amylolytic-, hemicellulolytic- and cellulolytic-enzymes which possess different CBM domains. Those CBM families help in substrate recognition and binding, and thus increase the catalytic activity on insoluble substrates such as CBM20, CBM34 and CBM53 have been reported to act in the degradation of raw starch granules by enabling the enzyme to interact with the starch granules and also disrupt the surface of the starch structure (Machovič & Janeček, 2006; Lombard et al., 2014). Furthermore, hemicellulases and cellulases, including the exo-, endo-types and side-chain acting enzymes, are involved in the hydrolysis of hemicellulose and cellulose contained in lignocellulosic materials (Linares-Pastén, Andersson & Karlsson, 2014). The genome annotation of C. manihotivorum CT4T revealed the presence of gene products of hemicellulases featuring CBMs that have the ability to interact with insoluble substances and support catalytic domains to hydrolyze their substrates (Shallom & Shoham, 2003). For example, the α-L-arabinofuranosidase and endo-β-1,4-mannanase of C. manihotivorum CT4T were predicted to contain CBM4 (gene loci; CT4_03484 and CT4_03690), CBM6 and CBM35 (CT4_04971 and CT4_05469) which have been reported to have a binding function to insoluble xylan (Munir et al., 2014). However, endo-1,4- β-xylanases (gene loci; CT4_03195 and CT4_04894), the main enzymes to attack the xylan backbone of strain CT4T could not find the CBM. To explain how those xylanases were able to degrade xylan in cassava pulp, the enzymes might have other substrate-binding regions which are located at a certain distance from the active site and are called secondary xylan-binding sites (SXS), which function similarly to the CBM (Jommuengbout et al., 2009). Based on the amino acid sequence alignment of endo-1,4-β-xylanase (CT4_04894) with an endo-1,4-β-xylanase in the glycoside hydrolase family 10 (Xyn10) from Penicillium simplicissimum, which is capable of binding to insoluble xylan via the SXS, it was found that the residues E60, N61, K64, H97, W101, N142, E143, Y187, Q218, H220, E250, W283 and W291 of an endo-1,4-β-xylanase from strain CT4T (CT4_04894) were conserved with the SXS of Xyn10 from P. simplicissimum (Schmidt, Gübitz & Kratky, 1999). Besides, cellulolytic enzymes such as endo-β-1,4-glucanase and β-glucosidase in C. manihotivorum CT4T also contained CBM3 (gene locus; CT4_03367), CBM6 (CT4_03385), CBM3 and/or CBM46 (CT4_05071 and CT4_00352), which are known to bind and support catalytic domains to hydrolyze crystalline and amorphous celluloses (Cho et al., 2008; Guillén, Sánchez & Rodríguez-Sanoja, 2010). The results strongly indicated that C. manihotivorum CT4T possesses a set of genes encoding a complete system of amylolytic-, hemicellulolytic- and cellulolytic-enzymes, indicating that C. manihotivorum CT4T is a good candidate for degrading cassava pulp.
Conclusions
In this work, we have highlighted the cassava pulp-degrading enzyme of the isolated strain CT4T. It is a new species of the genus Clostridium that possesses specialized ability to degrade cassava pulp, a property that is occasionally found in this genus. The AAI constructed from C. manihotivorum CT4T revealed differences in the evolutionary relationships among the other Clostridium species. A complete genome sequence studied by Illumina and Oxford Nanopore Technology revealed that C. manihotivorum CT4T possesses a set of genes encoding the enzymes for the decomposition of an industrial starch-rich by-product, cassava pulp. In addition, C. manihotivorum CT4T contained a total of 91 genes encoding amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic-enzymes. Comparative analyses of the C. manihotivorum CT4T with the genome of C. amylolyticum SW408T revealed that strain CT4T had a high proportion and diversity of amylolytic-, hemicellulolytic-, cellulolytic- and pectinolytic-enzymes. The results suggest that C. manihotivorum CT4T is a promising microbe for saccharification of cassava pulp into useful value-added products.
Description of Clostridium manihotivorum sp. nov.
Clostridium manihotivorum sp. nov. (ma.ni.ho.ti.vo’rum. N.L. n. manihot, a botanical genus name (cassava); L. v. voro, to eat, devour; N.L. neut. adj. manihotivorum, devouring cassava).
Cells are Gram-positive, anaerobic, rods, single-endospore forming, non-motile and non-flagellate. Cells are 4.0–8.0 µm long and 0.5–1.5 µm wide. Colonies are 0.5–1.0 mm in diameter after incubation on BM7 agar supplemented with cassava pulp (1%, w/v) at 37 °C for 5 days. Cell growth is observed at 25−45 °C (optimum, 37 °C) and at pH 5.5–7.5 (optimum, 7.0). The strain curdles milk, but is negative for catalase, H2S, and indole. Utilizes D-glucose, D-xylose, D-galactose, D-fructose, D-mannose, D-arabinose, D-rhamnose, D-trehalose, D-raffinose, sucrose, lactose, maltose, mannitol, cellobiose, soluble starch, xylan, cellulose and Avicel®. The end products of glucose fermentation are acetate, butyrate, ethanol, propionate, CO2 and H2. The diagnostic amino acid in their cell wall is LL-diaminopimelic acid (LL-DAP). The major fatty acids are C 16:0, C 14:0, anteiso-C 15:0, summed feature 1 (C 13:0-3OH and/or C 15:1isoH), C 19:0cyclo ω8c and C 17:02-OH. The major polar lipids present are phosphatidylethanolamine (PE) and phosphatidylglycerol (PG). The genome size of the type strain is around 6.3 Mb and the genomic DNA G + C content 32 mol%. The type strain, CT4T (=TBRC 11758T = NBRC 114534T), was isolated from soil collected from a cassava pulp landfill located at Chonburi province, Thailand.
Supplemental Information
The two-dimensional thin-layer chromatograms of the polar lipids from C. manihotivorum CT4T detected with the following reagents:
phosphomolybdic acid (A), Dittmer and Lester reagent (B), ninhydrin reagent (C) and Dragendorff’s reagent (D). PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PC, phosphatidylcholine; AL1-AL3, unidentified aminolipids; PL1-PL3, unidentified phospholipids.
The cellular fatty acid compositions of C. manihotivorum CT4T
The raw data set of enzyme activities produced by C. manihotivorum CT4T
Biodegradation ability for cassava pulp by C. manihotivorum CT4T , C. polyendosporum PS-1T and C. amylolyticum SW408T
The chemical compositions of residual cassava pulp relative to the original weight is shown after cultured with CT4T , PS-1T and SW408T for 5 days.