Modifying effects of leaf litter extracts from invasive versus native tree species on copper-induced responses in Lemna minor
- Published
- Accepted
- Received
- Academic Editor
- Jörg Oehlmann
- Subject Areas
- Plant Science, Soil Science, Ecotoxicology
- Keywords
- Copper toxicity, Invasive species, Leaf litter extracts, Lemna, Lipid peroxidation, Oxidative stress enzymes, DOM
- Copyright
- © 2020 Karitonas et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Modifying effects of leaf litter extracts from invasive versus native tree species on copper-induced responses in Lemna minor. PeerJ 8:e9444 https://doi.org/10.7717/peerj.9444
Abstract
Invasive plant species tend to migrate from their native habitats under favourable climatic conditions; therefore, trophic and other relationships in ecosystems are changing. To investigate the effect of natural organic matter derived from native Alnus glutinosa tree species and from invasive in Lithuania Acer negundo tree species on copper toxicity in Lemna minor, we analysed the dynamics of Cu binding in aqueous leaf litter extracts (LLE) and plant accumulation, morphophysiological parameters, and antioxidative response. The results revealed that A. glutinosa LLE contained polyphenols (49 mg pyrogallol acid equivalent (PAE)/g DM) and tannins (7.5 mg PAE/g DM), while A. negundo LLE contained only polyphenols (23 mg PAE/g DM). The ability of LLE to bind Cu increased rapidly over 1.5–3 h to 61% and 49% of the total Cu concentration (6.0 ± 0.9 mg/L), respectively for A. glutinosa (AG) and A. negundo (AN), then remained relatively stable until 48 h. At the same time, L. minor accumulated 384, 241 or 188 µg Cu/g FW when plants were exposed to Cu (100 µM CuSO4), Cu with 100 mg/L dissolved organic carbon (DOC) from either AG LLE or AN LLE, accordingly. Catalase (CAT) and guaiacol peroxidase (POD) played a dominant role in hydrogen peroxide scavenging when plants were exposed to Cu and 10 or 100 mg/L DOCAG mixtures in both the first (up to 6h) and the second (6–48 h) response phases. Due to functioning of oxidative stress enzymes, the levels of the lipid peroxidation product malondialdehyde (MDA) reduced in concentration-dependent manner, compared to Cu treatment. When combining Cu and DOCAN treatments, the most sensitive enzymes were POD, ascorbate peroxidase and glutathione reductase. Their activities collectively with CAT were sufficient to reduce MDA levels to Cu-induced in the initial, but not the second response phase. These data suggest that leaf litter extracts of different phenolic compositions elicited different antioxidant response profiles resulting in different reductions of Cu stress, thus effecting L. minor frond and root development observed after seven days. The complex data from this study may be useful in modelling the response of the aquatic ecosystem to a changing environment.
Introduction
Copper is needed for normal plant growth and development and is a cofactor for physiological processes such as photosynthesis, mitochondrial respiration, superoxide scavenging, ethylene sensing and lignification (Maksymiec, 1997). However, copper released into the environment in surplus concentrations is toxic to plants (Naumann, Eberius & Appenroth, 2007). Transition metals, including Cu, stimulate the formation of hydroxyl radicals (⋅OH) from the non-enzymatic chemical reaction between superoxide (O2−) and H2O2 (Haber-Weiss reaction). Excess Cu can induce negative effects including the production of reactive oxygen species (ROS) via Fenton reaction (Halliwell & Gutteridge, 1984). ROS, in turn, can oxidize lipids (De Vos et al., 1991), disrupt protein functions due to binding to sulphydryl groups (Weckx & Clijsters, 1996) and inhibit photosynthesis and electron transport (Thomas et al., 2013; Xia & Tian, 2009). Plant growth can be inhibited as a result.
The presence of oxygen in intracellular environments due to aerobic metabolism poses a constant oxidative threat to cellular structures and processes. ROS affect the metabolism, growth and development of plant cells. ROS formation and consumption are tiny balanced and coherent in cells. At any condition in which cellular redox homeostasis is disrupted, ROS production becomes far greater than the capacity of the tissues to scavenge them, thus can be defined as oxidative stress (Alscher, Donahue & Cramer, 1997). The environmental stressors can increase the synthesis of non-enzymatic antioxidants such as thiol tripeptides, glutathione and ascorbate and α-tocopherol, as well as the modification of the activity of antioxidant enzymes including superoxide dismutase, catalase, glutathione peroxidase, ascorbate peroxidase and glutathione reductase (Foyer et al., 1997; Schützendübel & Polle, 2002). Accordingly, excess of Cu in plants can cause oxidative stress, and therefore change antioxidative pathways (Babu et al., 2003; De Vos et al., 1992; Gupta et al., 1999; Teisseire & Guy, 2000; Wang et al., 2004).
The solubility, adsorption, transport and toxicity of metals in natural surface waters are strongly influenced by complexation with dissolved organic matter (DOM) (Kim et al., 1999; Koukal et al., 2003; Manceau & Matynia, 2010; Marx & Heumann, 1999). Natural waters contain different concentrations of DOM, which, depending on the geographical area are ranging between < 1 and 100 mgC/L (Wetzel, 2001). Higher DOM concentrations (up to 300 mgC/L) have also been reported in some Canadian wetlands (Blodau, Basiliko & Moore, 2004). DOM is a complex and polymorphous mixture, which includes proteins, carbohydrates, polyphenols, and other vital compounds that originate chiefly from the degradation of plant and animal matter (Stevenson, 1994). A major fraction of DOM in waters comprises humic substances representing more than 60 −80% of the total dissolved organic carbon (DOC), which consists mainly of humic and fulvic acids (Steinberg, 2003).
Leaf litter is a readily available allochthonous source of DOM and plays an important role in freshwater ecosystems serving as a key source of nutrients (Tank et al., 2010). The enrichment with DOM relates to litter quality, which, in turn, is leaf species-dependent and furthermore can depend whether they are of native or non-native origin (Casas et al., 2013). In addition, leaf litter emits phenolic compounds including tannins, a significant component of plant secondary metabolites (Lachman et al., 2011; Lin et al., 2006). Tannins may provide a nutrient conservation mechanism by reducing decomposition rates of litter and decreasing nitrogen leaching potential (Lin et al., 2010). It has been found that DOM might cause oxidative stress in freshwater organisms (Nimptsch & Pflugmacher, 2008; Steinberg et al., 2003), and leaf litter leachates obtained from various tree species such as white pine Pinus strobus and red oak Quercus robur (Earl, Cohagen & Semlitsch, 2012) can be toxic to aquatic organisms. Moreover, it has been suggested that black alder Alnus glutinosa, native in Lithuania species, and boxelder maple Acer negundo, invasive in Lithuania species, impacted the same aquatic organisms in different ways (Krevš et al., 2013; Manusadžianas et al., 2014). A. negundo became widespread in Lithuania after its escape from cultivation in the mid-twentieth century (Gudžinskas, 1998). It colonized coastal zones of lakes and rivers that are dominated by autochthonous A. glutinosa (Prieditis, 1997). In this context, it might be interesting to reveal the potential of the DOM obtained from diverse species to modify metal effects on aquatic plants.
Alongside the natural sources, the increase of DOM in water bodies depends on rural and municipal activities. Similar anthropogenic sources of copper appearance were emphasized, i.e., agriculture and industrial wastes (Hou et al., 2007; Panagos et al., 2018), and combating massive growth of cyanobacteria (Huh & Ahn, 2017). To investigate possible phytotoxicity effects, we used duckweed Lemna minor, a well-known bioindicator of eutrophic water bodies (Environment Canada, 2007; US Environmental Protection Agency, 2012). This plant is considered to be a suitable model for physiological and ecotoxicological studies due to its small size, fast growth rate, vegetative reproduction, ease of culture and sensitivity to numerous pollutants. Lemna has been used for antioxidative response studies (Forni et al., 2012; Radić & Pevalek-Kozlina, 2010; Razinger et al., 2007; Teisseire, Couderchet & Vernet, 1998; Teisseire & Guy, 2000; Zezulka et al., 2013). However, information on the involvement of oxidative stress under the combined treatment of Cu and DOM obtained from leaf litter extracts is lacking in L. minor.
The main objective of this study was to investigate the effect of natural organic matter derived from native Alnus glutinosa tree species and from invasive in Lithuania Acer negundo tree species on copper toxicity in L. minor. We focused on time-dependent alterations of (1) Cu binding to DOM in media and accumulation in the plant, (2) morphophysiological parameters (frond area and root length), and (3) oxidative stress characteristics such as lipid peroxidation, hydrogen peroxide content and the activities of antioxidant enzymes, i.e., catalase, guaiacol and ascorbate peroxidases, and glutathione reductase. We limited our observations of oxidative stress characteristics to 48 h; thus, relatively high concentrations of 100 µM CuSO4 and up to 100 mg/L DOC were used.
Material and Methods
Plant material
We have been collecting duckweed (Lemna minor L.) plants from a local freshwater pond (54°75′N, 25°29′E; Verkiai Regional Park, Vilnius) and cultured fronds under controlled conditions for several years. Species specificity of experimental clone morphologically similar to L. minor was proved by sequencing the chloroplast DNA fragment (611 bp) at the Laboratory of Molecular Ecology of the Nature Research Centre (Vilnius). The highest chloroplast DNA sequence identity (99%) to L. minor strain RDSC 7210 (Sequence ID KX212888.1), which included ATPase subunit I gene, partial cds; atpF-atpH intergenic spacer, complete sequence; and ATPase subunit II gene, partial cds, indicated that plant clones used for the experiments in the current study should be attributed to L. minor species.
A stock culture was cultivated in 500-mL plastic containers 60 × 85 × 100 (mm, WxLxH) in a modified Steinberg growth medium containing 3.46 mM KNO3, 1.25 mM Ca(NO3)2, 0.66 mM KH2PO4, 0.072 mM K2HPO4, 0.41 mM MgSO4, 0.63 µM ZnSO4, 1.94 µM H3BO3∗, 0.18 µM Na2MoO∗, 0.91 µM MnCl2∗, 2.81 µM FeCl3∗ and 4.03 µM Na2EDTA∗∗ (chemicals were from Roth, Acros Organics∗ and Fisher∗∗). The pH was adjusted to 6.0 with 1 M NaOH.
Stock cultures and treated plants were kept in growth chambers at 24 ±2 °C under constant illumination with cool white fluorescent light at a photosynthetic photon flux density of 160µmol m−2 s−1.
Preparation of aqueous extracts
Fallen leaves of A. negundo and A. glutinosa were collected in Rudnia (54°05′N, 24°40′E; Varena district, Lithuania) in autumn. After collection, tree leaves were dried for 10 days in the shade at room temperature. The dried materials (without petiole and central vein) were mechanically ground to obtain a homogenous powder. One gram powder was extracted in 100 mL of deionised water (dH2O) for 3 h at 65 °C followed by rapid filtration through a Whatman #3 disk in order to obtain a clear crude extract solution, and then re-filtered through a nitro-cellulose paper filter (0.2 µm) to reduce the risk of interference by microorganisms.
Experimental design
The experimental scheme comprised control (growth medium) and four treatments (growth medium supplemented with Cu (100µM CuSO4 or 6.4 mg Cu/L); 100 mg/L DOC; Cu + 10 mg/L DOC and Cu + 100 mg/L DOC. Plants were incubated in 500-mL plastic containers (60 × 85 × 100) having 200 mL of the corresponding medium. Media were adjusted to pH 6.
For growth experiments, healthy colonies with 2–3 fronds from stock cultures were transferred to the containers with the corresponding exposure medium. The frond area (zones without the signs of chlorosis) and root length were measured at 0-day and after seven days by using image control system (Software MOTIC 2.0). The growth rate per day was calculated with the following equation r = (lnxt2-lnxt1)/t2-t1, where xt1 and xt2are the values of observation parameter at t1 and t2 day, respectively. Two independent experiments in quadruplicate were conducted.
To study H2O2 kinetics, lipid peroxidation and antioxidant enzyme activities, the cultures were started by transferring healthy colonies with 3–4 fronds from stock cultures into four containers (0.8–0.9 g in each container) for each corresponding exposure medium. Plant samples were collected 0.75, 1.5, 3, 6, 12, 24 and 48 h after the onset of the exposure. For each exposure time, we prepared new exposure medium. Two independent experiments in quadruplicate for each parameter at each exposure time were conducted. Then samples of L. minor (0.2 g each) were processed after Hildebrandt et al. (1986). Cold potassium phosphate buffer (0.1 M, pH 7.0) containing 1% (w:v) polyvinylpyrrolidone and 1% (v:v) Triton X-100 was added to chilled (4 °C) mortar and pestle containing the sample. Each sample was macerated with one mL of extracting buffer and was further grounded with another one mL of the buffer. A 1.5 mL aliquot of homogenate was centrifuged at 15,000 g for 15 min at 4 °C (Hou et al., 2007). The supernatant was frozen immediately for future total protein content and enzyme assays. Total protein content was determined using bovine serum albumin (BSA) as standard (Bradford, 1976). Spectrophotometric measurements were carried out on a Libra S32 PC UV–VIS (Biochrom, UK).
Chemicals used for the determination of the total protein content and oxidative enzyme activities were purchased from Sigma-Aldrich (ascorbic acid, BSA, Na2EDTA, Folin-Ciocalteau’s phenol reagent, hide powder, H2O2, pyrogallol, polyvinylpyrrolidone, sodium carbonate decahydrate, Triton X-100, thiobarbituric acid (TBA), trichloroacetic acid (TCA)) and from Roth (GSSG, KH2PO4, K2HPO4, NADPH, TRIS).
Determination of Cu
Before determining the metal content, the plants from control and Cu, Cu + 10 mg/L DOC and Cu + 100 mg/L DOC treatments (one experiment with four replicates at each exposure time) were washed triple with deionised water. All liquids on the surface of plant materials were blotted with paper towels. 0.2 g of fresh weight of plant materials was transferred to a ceramic crucible (Haldenwanger, Waldkraiburg, Germany) to destroy the combustible (organic) portion of the sample by thermal decomposition in a muffle furnace (SNOL-1,6.2,5, Borispol, Ukraine) at 450–550 °C for 2–3 h. After the sample was digested in 0.5 mL pure HNO3 (Roth, Karlsruhe, Germany) and heated until the acid evaporates up to a half volume and made up to final volume of five mL with dH2O.
Cu fractionation in treatment solutions (without plants) followed Adam et al. (2014) procedure. The fraction with dissolved Cu was obtained after ultrafiltration for 1 h in Microsep™Advance Centrifugal Devices (Pall Corporation, Ann Arbor, MI, USA) containing polyethersulfone membranes with a cut-off of 1 kDa, at 5,000 g (5430R, Eppendorf, Hamburg, Germany). Cu content was determined in duplicate at 0.75, 1.5, 3, 6, 12, 24 and 48 h before and after ultrafiltration by Perkin Elmer Optima 7000 Dual View ICP Optical Emission Spectrometer (Waltham, MA, USA) with standard method and calculated according to the standard curve. Cu concentration was expressed as mg/L or µg/g fresh weight (FW).
CuSO4 5H2O was from Sigma-Aldrich (purum p.a.).
Catalase (CAT) EC1.11.1.6
CAT was determined according to Aebi (1984). The assay medium contained 50 mM potassium phosphate buffer (pH 7.4, 25 °C), 12.5 mM H2O2, 50 µl supernatant containing enzyme extract and dH2O to make up the volume to three mL. The reaction was initiated by adding H2O2. The decrease in absorbance of H2O2 was recorded at 240 nm for 60 s with 50 mM potassium phosphate buffer used as the blank. The enzyme activity was calculated from the initial rate of the reaction using extinction coefficient ε = 0.04 mM−1 cm−1 for H2O2.
Ascorbate peroxidase (APX) EC 1.11.1.11
APX activity was measured according to the method described by Nakano & Asada (1981). The three mL reaction medium was composed of 50 mM potassium phosphate buffer (pH 7.0, 25 °C), 0.5 mM ascorbic acid, 0.1 mM Na2EDTA, 0.1 mM H2O2 and 100 µL supernatant containing enzyme extract. The reaction was initiated by adding H2O2. The decrease in the optical density to ascorbic acid was recorded at 290 nm for 30 s. The enzyme activity was calculated from the initial rate of the reaction using ε = 2.8 mM −1 cm−1for ascorbate.
Guaiacol peroxidase (POD) EC 1.11.1.7
POD activity was determined according to Upadhyaya et al. (1985). The assay medium contained 2.5 mL of 50 mM potassium phosphate buffer (pH 6.1, 25 °C), one mL 1% H2O2, one mL 1% guaiacol and 50 µL supernatant containing enzyme extract. The reaction was initiated by adding supernatant containing enzyme extract. The change in the optical density was recorded at 420 nm for 1 min. The enzyme activity was calculated using ε = 26.6 mM−1 cm−1 for oxidized tetraguaiacol polymer.
Glutathione reductase (GR) EC 1.6.4.2
GR activity was determined according to Mannervik (1999). The assay medium contained 0.2 M potassium phosphate buffer (pH 7.0)/2 mM Na2EDTA, 20 mM GSSG, 2 mM NADPH and 100 µL supernatant containing enzyme extract and dH2O to make up the volume to one mL. The reaction mixture was equilibrated at 30 °C. The decrease in the NADPH concentration was recorded at 340 nm for 1 min against the assay solution. Corrections were made for the non-enzymatic oxidation of NADPH by recording the decrease at 340 nm without adding GSSG to the assay medium. The enzyme activity was calculated from the initial rate of the reaction after subtracting the non-enzymatic oxidation using ε = 6.2 mM−1 cm−1 for NADPH.
Determination of hydrogen peroxide
The level of H2O2 in plant was determined according to Jana & Choudhuri (1981) with slight modification (Chen, Lin & Kao, 2000). H2O2 was extracted by homogenizing 0.2 g plant with 2 ml of phosphate buffer (50 mM, pH 7.0). The homogenate was centrifuged at 6,000 g for 25 min at 4 °C. 900 µL of the supernatant was mixed with 300 µL of 0.1% titanium chloride in 20% (v/v) H2SO4 and mixture was then centrifuged at 6,000 g for 15 min. The intensity of yellow colour of the supernatant was measured at 410 nm. H2O2 level was calculated using the extinction coefficient 0.28 µmol−1cm−1.
Determination of lipid peroxidation
The level of lipid peroxidation in plant was assessed by thiobarbituric acid (TBA) reactive metabolites chiefly malondialdehyde (MDA) as described by Heath & Packer (1968). Plant tissues (0.2 g) were extracted in two mL of 0.25% TBA made in 10% TCA. Extract was heated at 95 °C for 30 min and then quickly cooled on ice. After centrifugation at 10 000 g for 10 min, the absorbance of the supernatant was measured at 532 nm. Correction of non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The level of lipid peroxidation was expressed as MDA concentration formed using ε = 155 mM−1 cm−1.
Total phenol and tannin contents
The total phenol content of the dry leaves was measured spectrophotometrically after reaction with Folin-Ciocalteau phenol reagent, according to the manual method described by Singleton, Orthofer & Lamuela-Raventós (1998) with little modifications (Amorim et al., 2008; Atanassova, Georgieva & Ivancheva, 2011). A one mL aliquot of extracts or a standard solution of pyrogallol acid was added to a 25 mL volumetric flask containing nine mL of dH2O. One mL of the Folin-Ciocalteau phenol reagent was added to the mixture and shaken. After 5 min, 10 mL of 7% Na2CO3 solution was added to the mixture. The solution was diluted to 25 mL with dH2O and incubated for 30 min at room temperature. Absorbance was measured at 760 nm against a blank prepared with dH2O.
For determination of tannin content, aqueous extracts of leaves were shaken with hide powder for 60 min in ultrasonic bath. The non-tannin phenolics in the clear supernatant were determined in the way similar to this of total phenol content. Tannin content was calculated as a difference between total phenolic and non-tannin phenolic contents in the extract. Pyrogallol acid in deionised water was used for making a standard curve. Total phenol and tannin values are expressed as pyrogallol acid equivalents (PAE) in mg/g DM.
Dissolved organic carbon
DOC concentration in DOM extracts was determined according to ISO 8245:1999 in a certified analytical laboratory (JSC Water Investigations, Vilnius, Lithuania).
Statistical analysis
The statistical analysis was carried out using the software PASW Statistics 18.0 (Predictive Analytics Software, IBM).
To validate an aggregation of the replicates from two experiments on frond and root growth rates, two-way MANOVA was used. The factors in the analysis were a two-level experiment factor (experiment 1 and experiment 2) and a seven-level treatment factor. To validate an aggregation of the replicates from two experiments on specific enzyme activity, MDA or H2O2 concentrations, two-way ANOVA was used. The factors in the analysis were a two-level experiment factor (experiment 1 and experiment 2) and a five-level treatment factor. In both analyses, there was no significant difference between two levels of experiment factor and interaction between the factors (p > 0.05), therefore, the replicates were pooled to yield n = 8.
After checking for normality (Shapiro–Wilk test) and homogeneity of variances (the Levene test) the differences of treatments from control within each leaf species were analysed by one-way ANOVA and the Dunnet test (α = 0.05). Additionally, Tukey post-hoc test was used for differences among treatments (α = 0.05).
Results
Frond and root growth rate
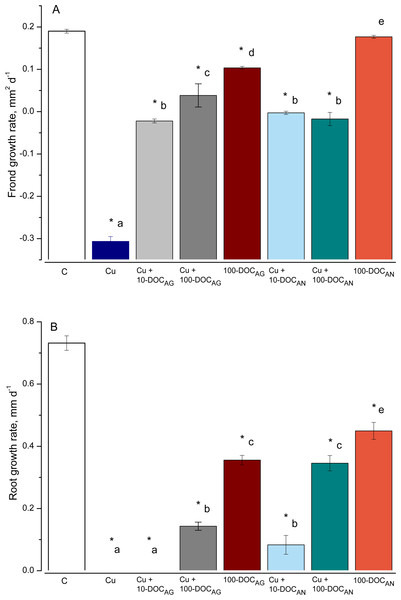
The duckweed frond and root growth rates (FGR and RGR, respectively) were significantly affected by all treatments, with an exception of FGR in the treatment with 100 mg/L DOC derived from A. negundo (100-DOCAN) (Fig. 1). Irrespective of the kind of leaf litter extract (LLE) and the type of treatment, the reduction in root length was higher than in the frond area.
Figure 1: Frond and root growth of L. minor exposed to copper, leaf litter extracts (LLE) of Alnus glutinosa or Acer negundo, and combinations of Cu and LLE.
Frond (A) and root (B) growth rates of L. minor in control (C) and exposed for seven days with 100 µM CuSO4 (Cu), mixtures of Cu and A. glutinosa leaf litter extracts (LLE) of 10 and 100 mg/L DOC (Cu+10-DOC_AG and Cu+100-DOC_AG), mixtures of Cu and A. negundo LLE of 10 and 100 mg/L DOC (Cu+10-DOC_AN and Cu+100-DOC_AN) as well as with 100 mg/L DOC of A.glutinosa (100-DOC_AG) and A. negundo (100-DOC_AN) LLE. Data represent mean ± SD (n = 8). Asterisks indicate significant difference from the control, different letters indicate significant difference among the means (α = 0.05).As for the effect on fronds, Cu (100 µM or 6.4 mg/L) slowed the development of frond area by 97% after seven-day exposure (Fig. 1A). Due to the development of chlorosis and thus the decrease of a photosynthetically active square, the FGR was negative (−0.3 mm2 d−1). The 100-DOCAN diminished insignificantly the development of the frond area. Due to chlorosis, the FGR was −0.02 mm2 d−1 in Cu + 10-DOCAG treatment, and 5.0 times lower than the controls in Cu + 100-DOCAG. The mixtures of Cu with A. negundo LLE of 10 and 100 mg/L DOC both stopped the growth of fronds.
As for the effect on roots, the growth was not observed over seven days in L. minor treated by Cu (Fig. 1B). 100 mg/L DOC derived from A. glutinosa (100-DOCAG) significantly diminished the development of roots (root growth rate (RGR) decreased 2.1 times compared to controls). The 100-DOCAN significantly decreased the RGR by 1.6 times. The mixture of Cu + 10-DOCAG inhibited growth of the roots totally, while the effect of Cu + 100-DOCAG was lower (RGR decreased 5.1 times compared to controls). The mixtures of Cu with A. negundo LLE of 10 and 100 mg/L DOC both diminished RGR by 8.8 and 2.1 times compared to controls, respectively.
Total phenols and tannins
Polyphenol and tannin concentrations in the DOM of A. glutinosa leaf litter were quite high (49 and 7.5 mg PAE/g DM, respectively), meanwhile DOM of A. negundo leaf litter had as much as twice lower polyphenol concentration (23 mg PAE/g DM) and no tannins.
Cu accumulation
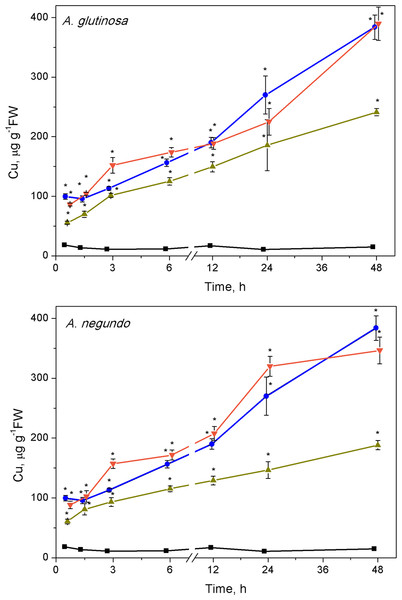
The ability of LLE to bind Cu increased rapidly. Lower concentrations of dissolved Cu were measured (after ultrafiltration) in the mixtures of Cu + 100-DOCAG and Cu + 100-DOCAN already after 45 min, making up 61.9% and 63.8% of the total Cu (6.0 ± 0.9 mg/L, n = 26), respectively. The percent of dissolved Cu decreased further until 1.5 h in the case of 100-DOCAG and then remained relatively stable (39.3% of the total Cu) up to the end of exposure at 48 h, while it diminished until 3 h in the case of 100-DOCAN and then remained relatively stable up to the end of exposure (51.4% of the total Cu).
The Cu concentration in L. minor increased with time in all treatments (Fig. 2). The plant accumulated 99.6 ± 4.6 and 384 ± 20.4 µg Cu/g FW in the treatment with Cu, respectively at 45 min and 48 h. Similar Cu uptake was found in plants treated with Cu + 10-DOCAG or Cu + 10-DOCAN. However, 100 mg/L DOC obtained from each of the leaf extracts diminished Cu uptake compared to Cu-treatment throughout all the exposure periods (Fig. 2). Accumulated Cu content was 55.6 ± 2.7 and 241 ± 6.1 µg Cu/g FW, respectively at 45 min and 48 h for A. glutinosa, and 60.8 ± 4.0 and 188 ± 7.8 µg Cu/g FW, respectively at 45 min and 48 h for A. negundo treatments.
Figure 2: Cu accumulation in L. minor treated by Cu, and combinations of Cu and leaf litter extracts from A. glutinosa or A. negundo.
Cu concentration in L. minor treated by Cu and Cu+DOC of leaf litter extracts (µg g−1FW). Plants were incubated in control medium (■), 100 µM Cu (●), 100 µM Cu+10 mg/L DOC (▾), 100 µM Cu + 100 mg/L DOC (▴) from A. glutinosa or A. negundo. Each value represents mean ±SD (n = 4). Asterisks indicate significant difference from the control at α = 0.05.Hydrogen peroxide
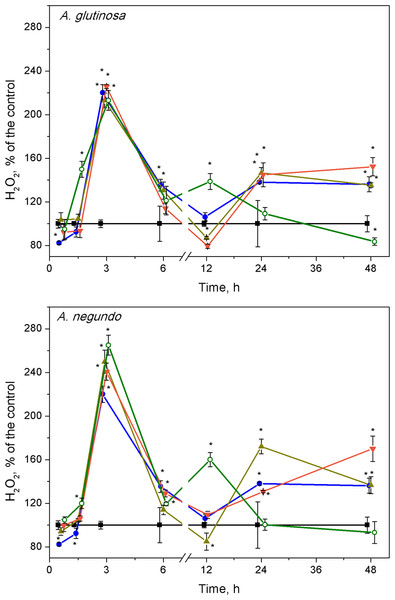
The highest changes in hydrogen peroxide levels in plants were induced at early stages of the treatments. The H2O2 content increased significantly after 3 h of exposure to Cu, its combinations with either A. glutinosa or A. negundo extracts, and to 100-DOCAG or 100-DOCAN alone (Fig. 3). At this exposure time, it was approximately by 2.2 times higher in Cu, Cu+10-DOCAG, Cu+100-DOCAG and 100-DOCAG treatments than in control (0.27 ± 0.10 mM, n = 56), and there were no significant differences among these treatments. However, treatments that included A. negundo extracts induced DOC–dependent H2O2 levels in L. minor. The H2O2 levels in Cu+10-DOCAN and Cu+100-DOCAN treatments exceeded that of Cu treatment by 21% and 30%, respectively, and reached maximum 40% value in the treatment of 100 mg/L DOCAN.
Figure 3: Concentration of hydrogen peroxide in L. minor treated by Cu, leaf litter extracts and Cu + leaf litter extracts.
Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC from A. glutinosa or A.negundo (o), and Cu+10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ± SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05.At 12 h exposure, irrespective to the kind of leaf extract, the level of hydrogen peroxide in the treatments of Cu and its combinations with the extracts did not exceed that of control, but it was by 60% higher in the treatments of the leaf extracts alone (Fig. 3). Subsequently, within the 24–48 h exposure period, the level of H2O2 in L. minor treated with 100-DOCAG or 100-DOCAN decreased to that of control, whereas Cu and its combinations with leaf extracts induced augmentation of hydrogen peroxide up to 1.5–1.7 times.
Lipid peroxidation
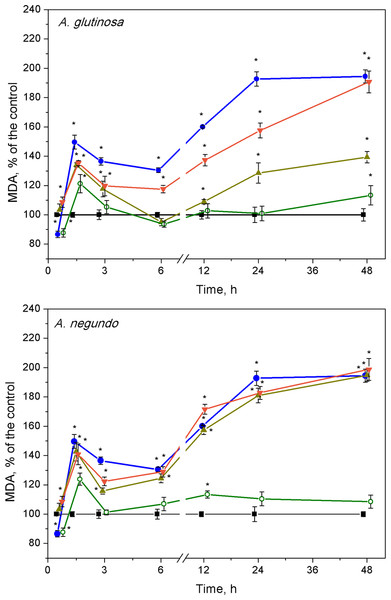
After 1.5 h of exposure, a significant increase of MDA concentration in L. minor over the control levels (Fig. 4, 10.1 ± 2.13 nM/g FW and 9.58 ± 2.22 nM/g FW, left and right graphs, respectively) was observed in all treatments, and irrespective to the leaf species (Fig. 4). This 20–45% increase in MDA content was led by its decrease that continued up to 3–6 h. Then, MDA augmentation was observed up to 48 h in the treatments with Cu and the mixtures of Cu and the extracts of both leaf species, however in different strength. Specifically, in the case of A. glutinosa, the addition of 100 mg/L DOC suppressed Cu-induced augmentation of MDA more strongly than the addition of 10 mg/L DOC (Fig. 4). At 48 h, no significant difference in MDA was observed between Cu and the combined treatment of Cu + 10-DOCAG. Contrary, in the case of A. negundo, the addition of neither 10 nor 100 mg/L DOC were able to change significantly the course of Cu-induced augmentation of MDA (Fig. 4), the level of which, after 48 h, reached as almost twice higher as the control level.
Figure 4: Lipid peroxidation expressed as MDA concentration in L. minor treated by Cu, leaf litter extract of A. glutinosa or A. negundo, and combinations of Cu and corresponding extracts.
Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC (o), and Cu + 10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ± SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05.After the initial approximately 20%-peak in MDA content at 1.5 h, the effect of 100 mg/L DOC extracts obtained from the NOM of both leaf species was weak, especially in the case of A. glutinosa, and showed no more than 15% deviation from the control level of MDA in exposures longer than six hours (Fig. 4).
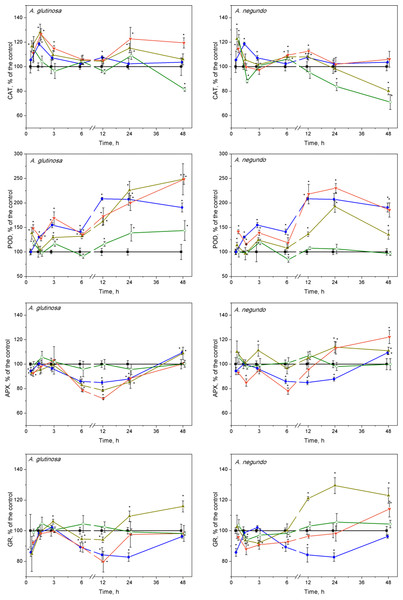
CAT activity
After 45 min exposure of L. minor plants, significant increases of CAT activities were observed in the treatments of Cu + 100-DOCAG, Cu + 100-DOCAN and 100-DOCAN (Fig. 5). After 1.5 h, the plants responded by significant increases of CAT activities to Cu, Cu + 10-DOCAG and Cu + 100-DOCAG treatments in case of A. glutinosa, while the reaction in case of A. negundo was weaker. Then, after the initial increases in CAT activity, the reaction, in general, slowed down towards the control level (682 ± 188 nkat/mg protein and 800 ± 121 nkat/mg protein for treatments of A. glutinosa and A. negundo, respectively; mean ± SD, n = 56) from 3rd hour and up to the end of exposure at 48 h, with irregular deviations. The exceptions comprised the decreasing tendency in CAT activities in the treatments of 100 mg/L DOC of both leaf species and with Cu + 100-DOCAN reaching significantly lower levels from those of controls by 20–30% at the end of exposure as well as the increasing tendency in CAT activities in the treatment of Cu + 10-DOCAG reaching significantly higher level from the controls by 20% at the end of exposure (Fig. 5).
Figure 5: Oxidative stress enzyme activities in L. minor treated by Cu, leaf litter extract from A. glutinosa or A. negundo, and combinations of Cu and respective extracts.
Activities of catalase (CAT), guaiacol peroxidase (POD), ascorbate peroxidase (APX) and glutathione reductase (GR) in L. minor treated by Cu, DOC of leaf litter extracts from A. glutinosa or A. negundo, and combinations of Cu and the DOC of respective extract. Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC (o), Cu+10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ±SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05.POD activity
Guaiacol peroxidase activities were significantly enhanced after treatments of Cu and its mixture with 10 and 100 mg/L DOC from A. glutinosa, but not with the 100-DOCAG, during the 45 min–48 h period reaching a 2–2.5-fold increase at 24 and 48 h (Fig. 5). In case of A. negundo, the increases of POD activity over control (316 ± 122 nkat/mg protein and 406 ± 112 nkat/mg protein for treatments of A. glutinosa and A. negundo, respectively; mean ± SD, n = 56) were significant later, throughout the 12–48 h period, in the same treatments as above, and again with the exception of 100-DOCAN (Fig. 5).
APX activity
No ascorbate peroxidase activity alterations were observed at 45th min, meanwhile, at 1.5 h, the 15–20% decreases were evident in the treatments of Cu + 10-DOCAG and Cu + 100-DOCAG, and Cu + 10-DOCAN (Fig. 5). Then, at the 6–24 h period, the significant suppression of APX activities was observed in the treatments of Cu, Cu + 10-DOCAG and Cu + 100-DOCAG, and at 6th hour, in the treatment of Cu + 10-DOCAN (Fig. 5). At the end of exposure at 48-h, the activities of all treatments did not differ from those of controls (32.6 ± 6.99 nkat/mg protein and 15.2 ± 2.47 nkat/mg protein for treatments of A. glutinosa and A. negundo, respectively; mean ± SD, n = 56).
GR activity
The glutathione reductase activity changes in L. minor were irregular during the 12 h of exposure in all treatments with the DOM of A. glutinosa and Cu alone, then GR activity decreased by approximately 20% below control level (409 ± 46.6 nkat/mg protein and 430 ± 72.6 nkat/mg protein for treatments of A. glutinosa and A. negundo, respectively; mean ± SD, n = 56) in the treatments of Cu and Cu + 10-DOCAG (Fig. 5). At 24 and 48 h, GR activity increased up to approximately 20% in the treatment of Cu + 100-DOCAG. In the case of A. negundo, the activity of GR activity was slightly, yet significantly, suppressed in the treatments of Cu + 10-DOCAN and Cu + 100-DOCAN, during the 1.5–3-h period. Then GR activity tended to recover to the control level at 24 h and to exceed it at the end of 48 h exposure period, in the treatment of Cu + 10-DOCAN, while a significant increase in 20–30% above control level was seen in the treatment of Cu + 100-DOCAN during the period of 12–48 h (Fig. 5). No significant alterations of GR activities were found in the LLE of 100 mg/L DOC of either species.
Discussion
Our results showed a high accumulation of copper in L. minor, which increased with time over 48 h. Similar accumulation properties of duckweed for this metal have already been documented for various exposure durations (Drost, Matzke & Backhaus, 2007; Kanoun-Boulé et al., 2009; Razinger et al., 2007). The accumulation of metals in aquatic plants is often accompanied by a variety of morphological and physiological changes, some of which directly contribute to the tolerance capacity of plants (Prasad et al., 2001; Xing, Huang & Liu, 2010). L. minor exposed to Cu, the extracts from A. glutinosa or A. negundo leaf litter, and to the combination of Cu with the extracts indicated inhibition of the growth parameters, enhanced levels of the lipid peroxidation and altered enzyme activities. To explore oxidative damage, we applied certainly toxic concentration of Cu (100 µM) within up to 48-hour exposure durations as compared to growth inhibition values of 7-d EC50, i.e., 2.7 and 9.7 µM reported by Nauman et al. (2007) and Drost et al. (2007), respectively. The time span longer than about one day can already be considered as long-term since the amount of L. minor biomass doubles every two days (Environment Canada, 2007) and the doubling time for fronds ranges from 1.3 to 2.8 days (Wang, 1990) under optimal nutrient, light and temperature conditions. According to our findings, the reduction in root growth rate was higher than in the frond growth rate, irrespective of the kind of treatment. It is known that root length inhibition is a more sensitive endpoint than that of the frond area (Gopalapillai, Vigneault & Hale, 2014). However, the concentration-dependent antagonistic action of Cu and A. glutinosa extract mixture was evident on fronds and roots, while in the case of Cu and A. negundo extract mixture this was evident on roots only, suggesting that the origins of the diminishing influence of A. glutinosa and A. negundo extracts on Cu-induced toxicity effects should not be the same.
The complex interactions between physical-chemical and biological factors in the aquatic medium may change metal bioavailability (Cuss & Guéguen, 2012; Koukal et al., 2003). The data obtained in Cu measurements in L. minor within 45 min–48 h period revealed that the accumulation rate in the combined treatments of Cu and A. glutinosa or A. negundo leaf extracts (100 mg/L DOC) was respectively two or three times lower than that in the CuSO4 treatment (∼6 µg g−1 FW h−1). The Cu accumulation dynamics at a constant rate did not correspond with the free Cu ions concentration dynamics in media, wherein it reached relatively constant level after 1.5 h or 3 h, respectively for mixtures of Cu and A. glutinosa or A. negundo leaf extracts, and the Cu2+ levels were respectively 39% and 51% of that of nominal Cu concentration. Fact that Cu2+ concentration in the medium with NOM from A. negundo leaves remained higher than that from A. glutinosa, but a higher amount of Cu accumulated in L. minor in the case of NOM from A. glutinosa leaves suggests that part of Cu could enter the cell being bound on NOM or could be bound in the cell wall, and this part was higher in the case of A. glutinosa leaf extract. This assumption is supported by the comparative analysis of effects on L. minor root growth rate (RGR). As large as twice the increase of inhibition of RGR (= (RGRCu+100−DOC - RGR100−DOC)/RGR100−DOC) was found for the mixture of Cu and A. glutinosa leaf extract as for Cu and A. negundo (Fig. 1). Otherwise, a stronger effect on L. minor exposed to the mixture of Cu and A. negundo leaf extract should be observed; at least, due to a higher concentration of Cu ions measured in the medium. The mechanisms of copper ions translocation from the medium into plant cell comprise, at least, transition metal transporters (González-Guerrero et al., 2016; Palmgren & Nissen, 2011) including low-molecular-weight organic molecules (Sinclair & Krämer, 2012) and binding in the cell wall. It was estimated that 74% of Cu accumulated in the cell wall of macrophytic alga Nitellopsis obtusa, when its internodal cells were treated with 50 µM of CuSO4 (Manusadžianas et al., 2017).
Plants exposed to long-term stress pass through different physiological states, from resistant to exhaustive (Lichtenthaler, 1996); thus, different kinetics of primary and successive plant responses might be expected. We found two phases of responses: namely, the first (up to 6 h) and the second one (within 6–48 h) could be identified for lipid peroxidation and hydrogen peroxide, and also, in part, enzyme activity kinetics. In addition, frond abscission/disintegration delimitated these response phases at the physiological level. Disintegration with successive chlorosis/necrosis is a recognized symptom of toxicity in fronds of Lemna exposed to pollutants (Khellaf & Zerdaoui, 2009). Release of daughter fronds from the metal stressed mother frond would increase the chance of the daughter fronds survival (Li & Xiong, 2004).
The high content of Cu (100 µgCu/g FW) accumulated in L. minor over initial 45 minutes caused a half-fold increase in the amount of MDA at 1.5 h and temporal increase of H2O2 with the peak at 3 h. It is widely accepted that malondialdehyde, the end product of lipid peroxidation, can be used as an indicator of membrane damage (Heath & Packer, 1968) and oxidative stress (Zezulka et al., 2013). At the end of the first response phase (plant resistance state), we observed the frond abscission. The oxidative stress progressed during the second response phase (plant exhaustive state) in terms of up to a 2-fold increase in MDA content and elevated H2O2 over control level at 48 h. A similar picture, in general, could be seen when plants were exposed to Cu and its combinations with leaf litter extracts; however, in the case of Cu and A. glutinosa leaf extract mixture, MDA content in L. minor was inversely dependent on DOC concentration in the extract (Fig. 4).
The response patterns of the plants exposed to each of the leaf litter extracts singly were mainly the same as exposed to Cu or Cu combined with the extracts, during the first response phase (up to 6 h). However, the levels of H2O2 and MDA relaxed to that of control during the second response phase. Therefore, it can be suggested that plants treated with extracts alone underwent oxidative stress in terms of H2O2 production and MDA increase, however, due to enzymatic activity they were able to resist this initial stress and consequently avoid detrimental effects in terms of growth and development at the final 7-day exposure, yet partially slowed down (Figs. 1 and 3 and 4).
In the first response phase, alterations of oxidative stress enzymes in L. minor were observed as early as after 45-min, the shortest exposure period. Specifically, Cu or its combinations with A. glutinosa extracts induced CAT activity increases that were not led by significant overrun of the control levels of H2O2 and MDA. This should show that the initial response of the enzymes prevented membrane damage due to ROS generation. Later on, however, concentrations of H2O2 and MDA significantly increased over the control reaching their peak values within 1.5–3 h, and this coincided with the maximum CAT activity. Bayliak, Semchyshyn & Lushchak (2006) and Martins & English (2014) have demonstrated that increasing H2O2 levels (up to 1mM) in yeast cells stimulate CAT. We measured H2O2 concentrations of 0.6–0.7 mM to be the highest ones in treated L. minor. Similar fast mobilization of CAT was considered as a cellular adaptation in primary leaves of Phaseolus vulgaris to cope with H2O2 overproduction generated by Cu2+ (Weckx & Clijsters, 1996). Interestingly, the increase in CAT activity during the initial 1.5 h was followed by attenuation of APX activity (Fig. 5). This might be linked with the decreased GR activity since GR converts GSSH into GSH by consuming NADPH and so maintains AsA level. One of the characteristic properties of APX that distinguishes it from POD, cytochrome c peroxidase and GR, is the rapid inactivation of the enzyme under conditions where an electron donor is absent (Miyake & Asada, 1996). The lower reduction state of AsA could be due to both the insufficient supply of electrons from GSH via direct interaction between GSH and AsA, and inadequate activities of MDHAR and DHAR (Asada & Takahashi, 1987; Foyer & Halliwell, 1976). In our experiments, GR activity began to increase after the initial decrease at 45 min, restoring the control level after three hours of exposure to Cu and Cu+DOCAG (Fig. 5). This increase of GR activity created, indirectly, favourable conditions for augmentation of ascorbic acid content, which allowed the APX activity to increase toward the control level during 1.5–3 h. Results of Xiang & Oliver (1998) have shown that the liquid culture of Arabidopsis tissues exposed to 100 µM Cu2+ respond fairly rapidly by increasing the transcript levels of the genes encoding the GSH-synthesizing enzymes (GSH1, GSH2) and the GSSG-reducing enzyme (GR1). Elevated transcript levels were evident one hour after the exposure to Cu2+, plateaued at six hours, and remained high for 18 h, and the high levels of transcripts continued for a few days under this condition. Another factor that prevented H2O2 accumulation in L. minor treated by Cu and Cu+DOCAG could be elevated POD activity within a 3–6-h period, which compensated gradual decrease of CAT activity toward the control level (Fig. 5). Similar concomitant activation of POD and inactivation of CAT has been found in various plants as an oxidative stress response to pathogen within 30 h (Madhusudhan et al., 2009).
The observed alterations in enzyme activities are likely related to oxidative reactions due to increased H2O2 level, which may eventually yield increased lipid peroxidation. Indeed, at the end of the first response phase, the highest MDA content in L. minor was found in the treatment with Cu (Fig. 2), which, however, was diminished by the DOCAG, but not by the DOCAN, in a concentration-dependent manner. The latter could be due to higher contents of polyphenols in the DOC of A. glutinosa than that of A. negundo, respectively 49 and 23 mg PAE/g DM and, in addition, tannins (7.5 mg PAE/g DM) that were lacking in the DOC of A. negundo. It has been found that tannins exhibit strong antioxidant properties in comparison to low molecular weight phenolic compounds. The presence of catechol or galloyl groups in tannin structure are essential to complex formation with transition metals (Andjelkovic et al., 2006) including copper (Brown & Kelly, 2007; Miller et al., 1996). Phenolic compounds with additional hydroxy groups on aromatic ring bind Cu2+ more efficiently (Brown & Kelly, 2007). The lack of tannins and low polyphenol content, and thus weak Cu binding ability in A. negundo LLE may explain, at least in part, negligible impact of A. negundo-derived DOM on the moderation of Cu-induced MDA levels. However, it is also known that phenolics can display prooxidant activities in the presence of metal ions in plants (Decker, 1997; Azam et al., 2004).
In the second response phase, Cu and its combination with A. glutinosa extracts induced a continuous increase of MDA concentration up to the end of 48-h exposure. This could be related to inactivation of APX, despite that the CAT and POD activity remained above the control level. It is known that inhibition of APX results in an increased level of H2O2 that contributes to defence gene activation and acts as a substrate for POD involved in defence responses such as lignification and crosslinking of cell wall proteins (Bradley, Kjellbom & Lamb, 1992). DOC of A. glutinosa is characterized by higher contents of polyphenols and tannins that are known to support the primary detoxification system (Yamasaki, Sakihama & Ikehara, 1997). Our results also showed that A. glutinosa leaf extract stimulated POD when it acted individually. In the Cu+DOCAG treatments, exogenous polyphenols and tannins and continuous increase of POD activity, exceeding that of Cu-induced at the end of the 48-h period, suggests that L. minor avoids H2O2 overproduction through a phenolic-dependent resistance mechanism. This possibility is further supported by the observation that MDA content was diminished by the DOCAG in a concentration-dependent manner, which was also true for the first response phase.
Combined treatments of Cu and A. negundo extracts, within initial 3 h, induced different responses of certain oxidative stress enzymes in relation to those of Cu and A. glutinosa extracts. Contrary to the case of A. glutinosa, when CAT enzyme activity decreased monotonically toward controls (the tendency observed up to 6 h), a rapid decrease of CAT activity to control level at 1.5 h indicated that the ability to scavenge H2O2 in plants was weakened. Indeed, A. negundo extract (100 mg DOC/L) even acting individually induces fast inactivation of CAT. During 3–6 h period, the relatively higher activity of POD in the relation of CAT, APX and GR activities in the treatments of Cu and A. negundo extracts indicated that actual level of lipid peroxidation could be mainly associated with the POD scavenging of H2O2. Inactivation of both CAT and the AsA-GSH pathway may prevent the cell from depleting NAD(P)H reserves (Heineke et al., 1991). Overall, within three hours of exposure, the treatments of Cu in combination with DOC from either A. negundo or A. glutinosa extract yielded lower MDA levels than in the treatment of Cu, and this was supported by various responses of ROS enzymes. However, after six hours of exposure, plants were unable to cope with H2O2 excess in the treatments of Cu+DOCAN when lipid peroxidation augmented up to the level observed in Cu-treatment, in contrast to Cu+DOCAG when lipid peroxidation was lower (Fig. 4). This distinction between the influences of various leaf species extracts at the background of Cu action could be caused by the differences in contents of polyphenolic compounds (see above).
Extended exposure of L. minor for 6–48 h in the mixture of Cu+100-DOCAN induced higher activities of APX and GR. This finding indicates that consumption of H2O2 can be associated not only with a POD (see below), but with the enzymatic activities in the AsA-GSH cycle, as well. Alteration of these enzymes was led by the inactivation of CAT. The first reason for the latter could be an excess level of AsA in the presence of Cu. Davison, Kettle & Fatur (1986) have shown that AsA alone is not very damaging and that AsA’s inhibitory action can be released by O2, H2O2 or Cu2+, i.e., when AsA is oxidized to semidehydroascorbate (or ascorbyl radical). Ascorbate toxicity depends on the presence of copper (or iron) and oxygen, but oxygen is not required in the presence of H2O2 (Samuni et al., 1983). Another reason for progressive inactivation of CAT observed in our study could be the presence of phenolic compounds in leaf litter extracts. Although the quantity and composition of the extracts differed, the effect on CAT activity decrease was evident in 100 mg/L DOC of either extract, especially at the end of 48-h exposure. The similar inhibitive action of certain phenolic compounds on CAT within 24-h exposure has been found for thermophilic fungi (Yüzügüllü et al., 2011).
POD activity did not differ between the treatments of Cu and either Cu+DOCAN or Cu+DOCAG at 24th hour of exposure. However, the extract type probably determined the opposing POD response at 48th hour of exposure. At this time point, the highest POD activity (exceeding that of the Cu-induced) was observed in the case of Cu+DOCAG. The lack of the differences in MDA levels in the second phase of L. minor response to Cu or its combinations with DOCAN suggests that, under the influence of A. negundo extracts, CAT, POD and AsA-GSH cycle enzymes were unable to minimize the oxidative damage induced by Cu. This is the opposite to the action of A. glutinosa extracts.
Invasive plant species tend to migrate from their native habitats under favourable climatic conditions; therefore, trophic and other relationships in ecosystems are changing. It has been suggested that black alder Alnus glutinosa, native species in Lithuania, and boxelder maple Acer negundo, invasive species in Lithuania, impact the same aquatic organisms in different ways (Krevš et al., 2013; Manusadžianas et al., 2014). In this context, we revealed the potential of diverse species DOM to modify Cu toxicity effects on Lemna minor. Both types of leaf litter extracts protected L. minor from deleterious effects of lipid peroxidation products during the first response phase (up to 6 h) when plants activated stress-coping mechanisms. Throughout the second response phase (6–48 h), cellular defence mechanisms were impaired and the vitality of L. minor steadily decreased. Overall, the analyses of Cu accumulation in L. minor and binding on DOM, and the dynamics of MDA content that represents integrative biochemical response, suggest that the reason of beneficial action of A. glutinosa extracts compared to that of A. negundo is based on the higher contents of polyphenols and tannins.
Conclusions
We revealed that leaf litter extracts of black alder Alnus glutinosa, native species in Lithuania, and boxelder maple Acer negundo, invasive species in Lithuania have various potential to modify Cu toxicity effects on Lemna minor. Analyses of duckweed responses, dynamics of Cu accumulation in the plant and its binding on the DOM in media allowed to conclude that both types of leaf litter extracts protected L. minor from deleterious effects of lipid peroxidation products during the first response phase within 6 h, however, cellular defence mechanisms were impaired during the prolonged exposure within 6–48 h. The differences in antioxidant enzyme activity profiles ascertained in L. minor treated by mixtures of Cu and various leaf extracts over two days were considered to condition dissimilar effects on the development of plant fronds and roots observed after seven days. The complex data obtained in the current study could be useful for modelling of aquatic ecosystem responses to the changing environment.
Supplemental Information
Frond and root growth rates of L. minor treated by Cu, tree leaf litter extracts or combinations of Cu and the extracts
Frond (A) and root (B) growth rates of L. minor in control (C) and exposed for seven days with 100 µM CuSO4 (Cu), mixtures of Cu and A. glutinosa leaf litter extracts (LLE) of 10 and 100 mg/L DOC (Cu+10-DOC_AG and Cu+100-DOC_AG), mixtures of Cu and A. negundo LLE of 10 and 100 mg/L DOC (Cu+10-DOC_AN and Cu+100-DOC_AN) as well as with 100 mg/L DOC of A. glutinosa (100-DOC_AG) and A.negundo (100-DOC_AN) LLE. Data represent mean± SD (n = 8). Asterisks indicate significant difference from the control, different letters indicate significant difference among the means (α = 0.05).
Accumulation of Cu in Lemna minor treated with Cu or combinations of Cu and tree leaf litter extracts
Cu concentration in L.minor treated by Cu and Cu+DOC of leaf litter extracts (µg g−1 FW). Plants were incubated in control medium (■), 100 µM Cu (●), 100 µM Cu+10 mg/L DOC (▾), 100µM Cu + 100 mg/L DOC (▴) from A. glutinosa or A.negundo. Each value represents mean ± SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05. t–¿from the control at α = 0.05.
Hydrogen peroxide concentrations in Lemna minor treated with Cu, leaf litter extract from A. glutinosa or A. negundo, and combinations of Cu and corresponding extracts
Concentration of H2O2 in L.minor treated by Cu, DOC or combinations of Cu and DOC of corresponding leaf litter extracts. Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC from A. glutinosa or A. negundo (o), and Cu+10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ± SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05. from the control at α = 0.05.
Lipid peroxidation as MDA concentration in L.minor treated with Cu, tree leaf litter extracts or combinations of Cu and the extracts
Lipid peroxidation expressed as MDA concentration in L.minor treated by Cu, DOC or combinations of Cu and DOC from A. glutinosa or A.negundo leaf litter extracts. Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC (o), and Cu+10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ± SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05. t difference from the control at α = 0.05.
Activities of catalase, guiacol peroxidase, ascorbate peroxidase and glutathione reductase in Lemna minor treated with Cu, tree leaf litter extracts or combinations of Cu and the extracts
Activities of catalase (CAT), guaiacol peroxidase (POD), ascorbate peroxidase (APX) and glutathione reductase (GR) in L. minor treated by Cu, DOC of leaf litter extracts from A. glutinosa or A. negundo, and combinations of Cuandthe DOC of respective extracts. Plants were incubated in control medium (■), 100 µM Cu (●), 100 mg/L DOC (o), Cu+10 mg/L DOC (▾) or Cu + 100 mg/L DOC (▴). Each value represents mean ±SD (n = 8). Asterisks indicate significant difference from the control at α = 0.05.