Isotopic biomonitoring of anthropic carbon emissions in a megalopolis
- Published
- Accepted
- Received
- Academic Editor
- Nicola Carslaw
- Subject Areas
- Ecology, Plant Science, Atmospheric Chemistry, Environmental Contamination and Remediation, Environmental Impacts
- Keywords
- Biomonitoring, Environmental pollution, Global ecology, Stable isotopes, Tillandsia recurvata, Mexico, δ13C
- Copyright
- © 2020 Díaz-Álvarez and de la Barrera
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Isotopic biomonitoring of anthropic carbon emissions in a megalopolis. PeerJ 8:e9283 https://doi.org/10.7717/peerj.9283
Abstract
Atmospheric pollution has become a serious threat for human health and the environment. However, the deployment, operation and maintenance of monitoring networks can represent a high cost for local governments. In certain locations, the use of naturally occurring plants for monitoring pollution can be a useful supplement of existing monitoring networks, and even provide information when other types of monitoring are lacking. In this work, we (i) determined the tissue carbon content and the δ13C values for the epiphytic CAM bromeliad Tillandsia recurvata and the relationship of both parameters with the existing CO concentrations in the Valley of Mexico basin and (ii) mapped the spatial distribution of such elemental and isotopic composition for this plant within the basin, in order to assess its potential as an atmospheric biomonitor of carbon monoxide, a pollutant with important repercussions on public health. The CO concentrations in the basin ranged from 0.41 ppm at rural locations to 0.81 ppm at urban sites. The carbon content of T. recurvata, which averaged 42.9 ± 0.34% (dry weight), was not influenced by the surrounding CO concentration. In contrast, the δ13C depended on the sites where the plants were collected. For example, the values were −13.2‰ in rural areas and as low as –17.5‰ in an urban site. Indeed, the isotopic values had a positive linear relationship with the atmospheric CO concentrations. Given the close relationship observed between the isotopic composition of T. recurvata with the CO concentrations in the Valley of Mexico, the δ13C values can be useful for the detection of atmospheric carbonaceous emissions.
Introduction
Atmospheric pollution has become a serious threat for human health and the environment. This is especially worrying for populous cities, which is a frequent case throughout Latin America (Kampa & Castanas, 2008; Rioja-Rodríguez et al., 2016). For example, Mexico City, with vigorous industrial and household activities, as well as numerous motor vehicles of all classes, has seen an increase of emissions of different pollutants to the atmosphere. This has resulted in a higher incidence of respiratory and cardiovascular diseases, which already cause at least 9,600 premature deaths annually just in this megalopolis and 20,000 in the whole country (Stevens et al., 2008; Instituto Nacional de Estadística y Geografía (INEGI), 2011). In addition, atmospheric pollution is one of the leading causes of biodiversity loss and ecosystem change both, from nitrogen deposition and the release of greenhouse gas emissions (Sala et al., 2000; Rockström et al., 2009; Hooper et al., 2012).
Carbon monoxide (CO) is among the main atmospheric pollutants with public health repercussions. Not only can acute exposure to high concentrations of CO lead to death, but the chronic exposure to low concentrations of this gas has been associated with cardiovascular and neurological damage (World Health Organization WHO, 1999; Townsend & Maynard, 2002; Prockop, 2005; Chen et al., 2007). CO results from the combustion of carbonaceous fuels, which also produces carbon dioxide. The proportion in which they are emitted depends on the quality of the combustion. For example, if a motor works in optimal conditions, that is, when the mixture of air, fuel and temperature inside an automobile engine is ideal, a complete combustion of the fuel is achieved, resulting in a complete oxidation of carbon and the subsequent emission of CO2, predominantly. For example, 3.8 L of gasoline, whose weight is 2.7 kg, can emit 9 kg of CO2 and ideally very low or insignificant amounts of CO (Salameh, 2014). Nevertheless, when the engine conditions are not optimal, an incomplete combustion/oxidation generates higher emissions of CO; this generally occurs in the engines of old cars and during heavy traffic congestions, which are frequent in the Valley of Mexico, where the vehicular fleet commonly exceeds 20 years of operation (Williams, 1990; Turnbull et al., 2011a; Silva, Arellano & Worden, 2013; Salameh, 2014; SEDEMA Secretaría del Medio Ambiente de la Ciudad de México, 2016a).
A characterization of air quality, by means of monitoring the concentration and distribution of atmospheric pollution, is thus imperative, including the monitoring of carbon emissions. However, the deployment, operation and maintenance of monitoring networks can represent high costs that in some cases exceed the budget and priorities of the local governments (Díaz-Álvarez et al., 2019). For example, only 77 cities from 17 countries in Latin America and the Caribbean have public information about atmospheric pollution, while 16 countries in the region do not release data at all (Rioja-Rodríguez et al., 2016). In Mexico, federal environmental and health regulations mandate the deployment of air quality monitoring networks for cities whose population exceeds half a million and for settlements with emissions surpassing 20,000 tons of regulated pollutants per year (SEMARNAT, 2012). However, as it occurs all over Latin America and the Caribbean, the monitoring of air quality is not evaluated in many localities as required (Rioja-Rodríguez et al., 2016; Instituto Nacional de Ecología y Cambio Climático (INECC), 2017).
The use of naturally occurring plants as biomonitors can supplement existing air quality monitoring systems and in some cases, when a monitoring system is lacking, be utilized for the early detection of increasing atmospheric pollution, considering that the elemental and isotopic composition of plant tissues can respond to the concentration of some pollutants (Díaz-Álvarez, Lindig-Cisneros & de la Barrera, 2018; Díaz-Álvarez et al., 2020). Once the origin and concentration of the pollution is determined, reduction and mitigation actions can be implemented. A particularly suitable group of plants for biomonitoring are those that depend exclusively from atmospheric sources for their mineral nutrition, commonly named “atmospheric plants” (Markert, Breure & Zechmeister, 2003; Vianna et al., 2011; Pellegrini et al., 2014; Díaz-Álvarez & de la Barrera, 2018; Díaz-Álvarez, Lindig-Cisneros & de la Barrera, 2018). One of such species is T. recurvata (L.) L (Schmitt, Martin & Luttge, 1989). This CAM bromeliad is distributed from the southern United States to Argentina and Chile, and it is commonly found growing on different built structures in cities (Schrimpff, 1984; Díaz-Álvarez & de la Barrera, 2018). Additionally, the plant can remain physiologically active year-round and, thanks to its absorptive trichomes, carry out bioaccumulation of different atmospheric pollutants including heavy metals, polycyclic aromatic hydrocarbons, nitrogen, sulfur and carbon (Schrimpff, 1984; Zambrano et al., 2009; Castañeda Miranda et al., 2016; Díaz-Álvarez & de la Barrera, 2018: Piazzetta, Ramsdorf & Maranho, 2018). Although, the carbon isotopic composition of this plant has been reported for polluted and non-polluted sites, this is the first time that a specific relationship between carbon emissions and the plant’s responses is determined.
By means of an extensive sampling throughout the Valley of Mexico, we (i) determined the carbon content and the δ13C values for the epiphytic CAM bromeliad T. recurvata and their relationship with the prevailing CO concentrations, and (ii) mapped the spatial distribution of such elemental and isotopic composition of this plant in the basin, in order to assess the potential that this plant has as an atmospheric biomonitor of CO.
Materials and Methods
Study region
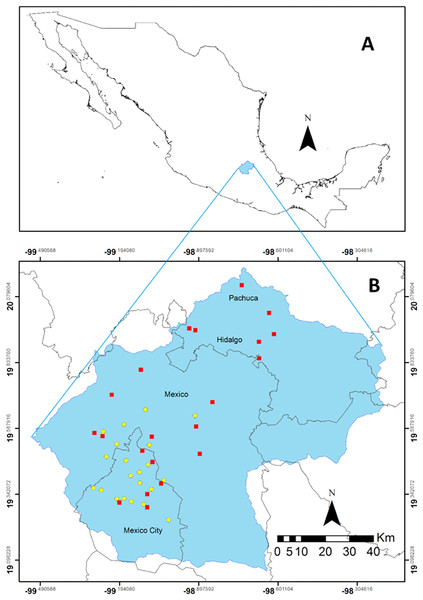
The study was conducted in the Valley of Mexico basin which covers an area of 7,500 km2, with a mean elevation of 2,240 m, and a mean annual precipitation of 600 mm that can reach up to 1,300 mm in the surrounding mountains at 5,400 m (Fig. 1; Calderón & Rzedowski, 2005; Servicio Meteorológico Nacional (SMN), 2016). The basin includes portions of the states of Hidalgo, Mexico, and Mexico City. Pachuca, the capital of Hidalgo, at the north has a population of 3 million. At the southern portion of the Valley sits Mexico City, whose population reaches 20 million. Additionally, various small towns and settlements with industrial or agricultural activities contribute to the 30 million inhabitants of the basin (Instituto Nacional de Estadística y Geografía (INEGI), 2011; Díaz-Álvarez & de la Barrera, 2018).
Figure 1: Region in central Mexico where the study was conducted.
Location of the Valley of Mexico (blue polygon) in Mexico (A). Spatial distribution of the collecting sites throughout the Valley represented by the red squares. The stations belonging to the automatic monitoring network are indicated by yellow dots (B). This network is located between Mexico City and the State of Mexico in the most populated zone of the region.Atmospheric CO concentration and biomonitoring
The Mexico City environmental authority has deployed an Automatic Atmospheric Monitoring Network (http://www.aire.cdmx.gob.mx/default.php) comprised of 33 stations, which monitor different parameters, including wet N deposition, O3, NOx, NO2, NO, PM10 PM2.5 and CO (Fig. 1). We calculated the mean concentration of CO (ppm) between January and November 2014 for each one of the 21 stations that recorded this parameter.
We determined the relationship between CO concentrations in the Valley of Mexico and the carbon content and the δ13C values was determined for the epiphytic CAM bromeliad T. recurvata, which has an ample distribution in the Americas. This plant has been utilized as a biomonitor of atmospheric pollution and can be easily found in the Valley of Mexico (Zambrano et al., 2009; Díaz-Álvarez, Lindig-Cisneros & de la Barrera, 2018; Díaz-Álvarez & de la Barrera, 2018; Piazzetta, Ramsdorf & Maranho, 2018). Sampling sites were determined in two steps. First, potential sites for the occurrence of T. recurvata were identified utilizing Google Earth’s satellite scenes, followed by a corroboration by means of the StreetView function where available. In particular, we identified the presence of natural protected areas, vegetation stands in rural/agricultural areas, or parks and other vegetated features in urban areas, where trees and shrubs that could act as phorophytes for T. recurvata were be present. A total of 73 sites were identified within the basin. Second, a stratified sampling (within the identified vegetated sites; Wang et al., 2012) was conducted on 3–15 November 2014, which occurred towards the end of the rainy season, late in the growing period for T. recurvata. After discarding those sites where T. recurvata was not found and those where access was not possible, plant samples were collected for elemental and isotopic analyses from 22 sites (Fig. 1), including urban parks (7 sites), built urban structures (4 sites), agricultural sites (6 sites), and natural protected areas (5 sites).
At each site, newly formed, fully developed leaves, which can be visually differentiated from those that grew in previous years, were collected (Permit SGPA/DGGFS/712/2767/14, Secretaría de Medio Ambiente y Recursos Naturales, Mexico) from 5 mature individuals growing at least 5 m apart (Harmens et al., 2008; Díaz-Álvarez et al., 2019). The samples were dried at 60 °C in a gravity convection oven until reaching constant weight. Sample preparation for stable isotope analyses was conducted following Díaz-Álvarez & de la Barrera (2018). The carbon isotope ratios, reported in parts per thousand were calculated relative to Vienna–Pee Dee Belemnite (VPDB). The analytical precision for the δ13C was 0.2 ± 0.07‰ (SD). The natural abundances of 13C were calculated as: where, R is the ratio of 13C/12C for carbon isotope abundance for a given sample (Ehleringer & Osmond, 1989; Evans, 2001).
Statistical and spatial analyses
Data were analyzed following Díaz-Álvarez & de la Barrera (2018). In particular, linear regressions were calculated to determine the relationship between CO concentrations and carbon content (% dry weight), as well as the isotopic composition (δ13C values) for T. recurvata in the Valley of Mexico. The differences between sites for the carbon content and the δ13C values were determined by means of the Kruskal–Wallis one-way analysis of variance by ranks, followed by a Nemenyi’s post-hoc tests for pairwise multiple comparisons (p ≤ 0.05). The analyses were conducted using the Pairwise multiple comparison of mean ranks package (PMCMR) in R (version 3.5.3, R Core Team, R foundation for Statistical Computing, Vienna, Austria; Pohlert, 2014).
Data interpolation for CO concentration within Mexico City and for T. recurvata tissue carbon content and δ13C values throughout the Valley of Mexico were conducted with the Ordinary Kriging model contained in ArcGIS 10.5 (ESRI, Redlands, CA, USA), which was also utilized to create the maps. This interpolation method is based on the assumption that data are spatially autocorrelated (Cressie, 1988; Wang et al., 2002; Wong, Yuan & Perlin, 2004), which generally is the case for atmospheric pollutants, as their concentration is higher with proximity to the source, thus influencing the ensuing plant responses (Stevens et al., 2004; Díaz-Álvarez, Lindig-Cisneros & de la Barrera, 2018). This allows the estimation of parameters of interest in relatively large geographical regions (Liao, Li & Zhang, 2017), where only a sparse sampling is available (Oliver & Webster, 2014). While interpolation protocols have been developed that improve on Ordinary Kriging, this model is among the most utilized methods in environmental studies, including those considering atmospheric pollution (Liao, Li & Zhang, 2017; Gupta et al., 2018; Gómez-Losada et al., 2019; Huang et al., 2020).
Results
Spatial distribution of CO concentrations
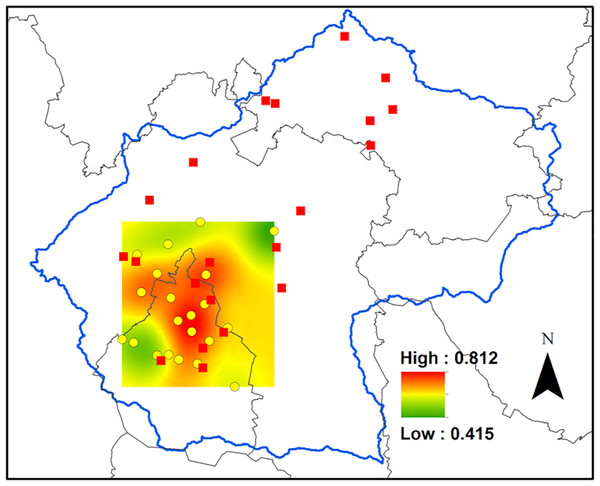
The CO concentration, averaged 0.67 ± 0.03 ppm in Mexico City, where the monitoring network is deployed (Fig. 2). The central and northern portions had the highest concentrations of CO, reaching a maximum of 0.81 ppm, and the lowest concentration was found at the northeast end of the distribution of the monitoring network, where it reached a mean value of 0.41 ppm (Fig. 2).
Figure 2: Carbon monoxide concentrations in Mexico City.
Ordinary kriging for the carbon monoxide concentrations, in parts per million, inside the area covered by the air quality network in the Valley. The data consisted of mean concentration of CO during the period that comprises January to November 2014. The data utilized for this analysis are available at http://www.aire.cdmx.gob.mx.Carbon content and isotopic composition for Tillandsia recurvata
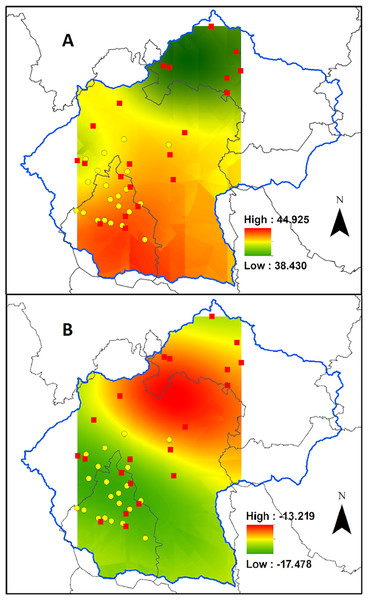
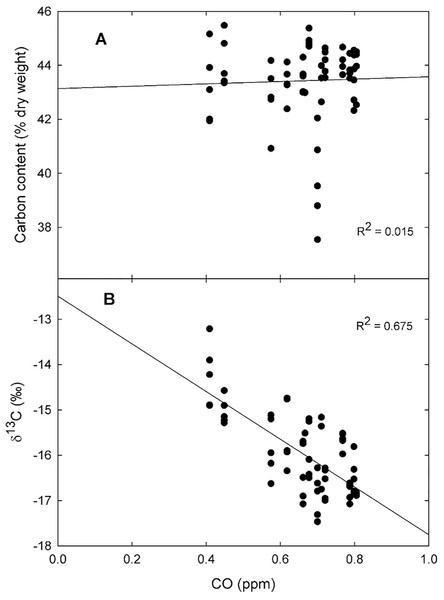
On average, the tissue carbon content was 42.9 ± 0.3% (dry weight) ranging from 38.4% for plants collected in the urban area of a small town at the north-west portion of the Valley, to 44.9% at the southern part of the Valley, in the middle of the Mexico City (Fig. 3A). However, the carbon content was not affected by the CO concentration recorded during 2014 by the Mexico City monitoring network (R2 = 0.015; Fig. 4A).
Figure 3: Biomonitor carbon status in the Valley of Mexico.
Ordinary kriging for the carbon content (A) and δ13C values (B) for Tillandsia recurvata in the Valley of Mexico.Figure 4: Biomonitor responses to carbon monoxide.
Linear regression for the relationship between CO concentration in ppm during 2014 and the carbon content (A), and the δ13C values (B) for Tillandsia recurvata at the Valley of Mexico (n = 5).Contrasting with what occurred for the carbon content, the δ13C values responded positively to the atmospheric CO concentration, being lower in sites with higher concentrations than in those with lower CO concentrations (Fig. 4B; R2 = 0.675). For example, the δ13C values reached –16.2‰ in Pachuca, a populous city at the north of the Valley, which is lower than in some rural sites further south (Fig. 3B). The δ13C values were as negative as –17.5‰ for plants collected in an urban site where the mean CO concentration averaged 0.70 ppm during 2014 (Fig. 3B). In contrast, the highest δ13C values of −13.2‰ were found for plants growing at the archeological site of Teotihuacan, whose CO concentration was the lowest recorded inside the monitored area (p < 0.05; Fig. 3B).
Discussion
The atmospheric concentration of CO in the area covered by the monitoring network tended to be higher in the vicinity of the numerous important and busy motorways that have been built in this region and are utilized by the 5.2 million vehicles registered in Mexico City and its metropolitan area, in addition to various thousands of visiting vehicles from other cities and states (SEDEMA Secretaría del Medio Ambiente de la Ciudad de México, 2016a). Indeed, motor vehicles are the main source of CO for the region, contributing with 96% of the total emissions, which amount to nearly 700 million tons just in Mexico City; the remaining 4% originated from industrial and domestic sources (SEDEMA, Secretaría del Medio Ambiente de la Ciudad de México, 2016b). However, it is worth mentioning that the CO concentrations of 3.8 ppm measured in Mexico City and its metropolitan area during this study did not exceed the Mexican standard (exposure to 11 ppm for 8 h) nor the World Health Organization’s (9 ppm) and the United States Environmental Protection Agency’s criteria for this pollutant (9 ppm; World Health Organization WHO, 1999; SEMARNAT, 2012; SEDEMA Secretaría del Medio Ambiente de la Ciudad de México, 2016a).
While the carbon content of T. recurvata was insensitive to the CO concentration, the δ13C of newly formed leaves adequately biomonitored the prevailing atmospheric pollution, with values that were substantially more negative in polluted sites than in “clean” sites, a pattern that has been previously documented with atmospheric biomonitors (Martin, 1994; Lichtfouse, Lichtfouse & Jaffrézic, 2003; Zambrano et al., 2009; Cobley & Pataki, 2019). When interpreting the isotopic signature of carbon biomonitors it is important to consider that while CO is but a small fraction of the urban carbonaceous emissions, it is produced from combustion simultaneously with CO2, a gas that is usually not included in urban air quality monitoring protocols (SEMARNAT, 2012; SEDEMA, Secretaría del Medio Ambiente de la Ciudad de México, 2016b). Thus, the plants are in fact mostly recording the isotopic signal of CO2 assimilated by photosynthesis. However, the relationship between atmospheric CO and CO2 concentrations is usually linear, so that the “calibration” conducted in the present study utilizing CO (the only carbon gas that is monitored) as an integrative proxy for urban carbon emissions could be utilized in other unmonitored cities (Turnbull et al., 2011b; Silva, Arellano & Worden, 2013; Gromov, Nrenninkmeije & Jöckel, 2017).
The carbon content in plant tissues is commonly ca. 50% on a dry mass basis, although it varies depending on different factors, such as the developmental stage, organ, species, latitude, water availability, nutrient availability and the CO2 concentration prevalent during organ development (Díaz-Álvarez, Lindig-Cisneros & de la Barrera, 2015; Ma et al., 2018). High CO2 concentrations, such as those found in urban environments, like Mexico City, are among the factors leading to higher photosynthetic rates in CAM plants (Drennan & Nobel, 2000; Andrade et al., 2007; Smith et al., 2009; Zotz et al., 2010; SEDEMA Secretaría del Medio Ambiente de la Ciudad de México, 2016a). However, when plants are exposed to different concentrations of hazardous gases such as NOx, SO2 and CO, the net photosynthetic rate and the photosynthetic pigment content can decrease, because the resulting oxidative stress alters the carboxylation process, leading to a net reduction of the carbon content (Bytnerowicz et al., 2001; Mittler, 2002; Muneer et al., 2014). Such gases are monitored and have actually been detected in areas of Mexico City and Pachuca, although it appears that their concentration is not high enough to cause a tissue carbon reduction for T. recurvata (Díaz-Álvarez & de la Barrera, 2018; SEDEMA Secretaría del Medio Ambiente de la Ciudad de México, 2016a). In this respect, this species displays a physiological resistance to O3 and SO2, which may contribute to the results observed (Benzing et al., 1992).
Three factors can help explain the general carbon isotopic pattern observed for T. recurvata throughout the study. The first factor is a large difference in the isotopic signature for CO2 from different sources. In particular, the δ13C values of the air from natural environments can reach –8‰ (Pichlmayer et al., 1998; Widory & Javoy, 2003). In contrast, the carbon in the air is depleted of 13C in sites where motor vehicles and the industrial activities are common. In this regard, the δ13C values for coal burning, gasoline, diesel and natural gas range from –25 to –42‰ (Pichlmayer et al., 1998; Röckmann et al., 2002; Pataki, Bowling & Ehleringer, 2003; Widory & Javoy, 2003; Semmens et al., 2014; Naus, Röckmann & Popa, 2018).
A second factor is an increasing isotopic discrimination against 13C that occurs for CAM plants exposed to high CO2 concentrations (Zhu, Goldstein & Bartholomew, 1999). In this case, given that increasing concentrations of CO2 can inhibit the activity of phosphoenolpyruvate carboxylase (PEPc), which is already near saturation at natural concentrations of CO2 (Ting, 1994). PEPc has an isotopic discrimination that ranges between 2 and 10‰, which explains the common δ13C values for CAM plants. However, if PEPc is inhibited, the ribulose bisphosphate carboxylase/oxygenase, an enzyme with a higher discrimination of 22–27‰, will conduct most of the carboxylation, resulting in the observed δ13C values, which were more negative in polluted sites (Ehleringer & Osmond, 1989; Farquhar, Ehleringer & Hubick, 1989; Ting, 1994; McNevin et al., 2007; Smith et al., 2009; Cernusak et al., 2013).
A third potential factor influencing the observed pattern is air temperature in cities. Warmer than natural nocturnal air temperatures, such as those resulting from the urban heat island in Mexico city, drive changes in the carbon fixation cycle of CAM plants, which in turn leads to an increased isotopic discrimination against 13C (Troughton & Card, 1975; Farquhar, Ehleringer & Hubick, 1989; Jauregui, 1997; Zhu, Goldstein & Bartholomew, 1999; Cui & De Foy, 2012; Cernusak et al., 2013).
Conclusions
Owing to the close relationship observed between the isotopic composition of T. recurvata and the atmospheric CO concentration in the Valley of Mexico, the δ13C values can be useful for characterizing carbonaceous pollution. In addition, given that the emissions of CO and CO2 are accompanied by the emission of other pollutants, such as NOx, SO2, and heavy metals, which results in the formation of secondary pollutants such as O3 and particulate matter, this plant can be deemed as an ideal candidate for implementing broader monitoring studies in regions where automatic monitoring networks are not available and the bromeliad is abundant.
Supplemental Information
Raw data.
Geographic coordinates of (A) Mexico City’s Air Quality Network Monitoring Stations, (B) Carbon monoxide concentrations during 2014, and (C) Tissue carbon content (% dry mass) and δ13C of Tillandsia usneoides sampled during 2014 in the Mexico Valley.