Consequences of removal of exotic species (eucalyptus) on carbon and nitrogen cycles in the soil-plant system in a secondary tropical Atlantic forest in Brazil with a dual-isotope approach
- Published
- Accepted
- Received
- Academic Editor
- Xavier Le Roux
- Subject Areas
- Biodiversity, Conservation Biology, Ecology, Forestry
- Keywords
- Superficial soil layer, Litter, Soil organic matter, Stable isotopes, Eucalyptus removal, Nutrient cycles
- Copyright
- © 2020 Teixeira et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Consequences of removal of exotic species (eucalyptus) on carbon and nitrogen cycles in the soil-plant system in a secondary tropical Atlantic forest in Brazil with a dual-isotope approach. PeerJ 8:e9222 https://doi.org/10.7717/peerj.9222
Abstract
The impact of exotic species on heterogeneous native tropical forest requires the understanding on which temporal and spatial scales these processes take place. Functional tracers such as carbon (δ13C) and nitrogen (δ15N) isotopic composition in the soil-plant system might help track the alterations induced by the exotic species. Thus, we assess the effects from the removal of the exotic species eucalyptus (Corymbia cytriodora) in an Atlantic forest Reserve, and eucalyptus removal on the alteration of the nutrient dynamics (carbon and nitrogen). The hypotheses were: (1) the eucalyptus permanence time altered δ13C and δ15N in leaves, soils and litter fractions (leaves, wood, flowers + fruits, and rest); and (2) eucalyptus removal furthered decomposition process of the soil organic matter. Hence, we determined the soil granulometry, the δ13C and δ15N in leaves, in the superficial soil layer, and litter in three sites: a secondary forest in the Atlantic forest, and other two sites where eucalyptus had been removed in different times: 12 and 3 months ago (M12 and M3, respectively). Litter samples presented intermediate δ13C and δ15N values in comparison with leaves and soil. In the M3, the greater δ13C values in both litter rest fraction and soil indicate the presence, cycling and soil incorporation of C, coming from the C4 photosynthesis of grassy species (Poaceae). In the secondary forest, the soil δ15N values were twice higher, compared with the eucalyptus removal sites, revealing the negative influence from these exotic species upon the ecosystem N dynamics. In the M12, the leaves presented higher δ13C mean value and lower δ15N values, compared with those from the other sites. The difference of δ13C values in the litter fractions regarding the soil led to a greater fractioning of 13C in all sites, except the flower + fruit fractions in the secondary forest, and the rest fraction in the M3 site. We conclude that the permanence of this exotic species and the eucalyptus removal have altered the C and N isotopic and elemental compositions in the soil-plant system. Our results suggest there was organic matter decomposition in all litter fractions and in all sites. However, a greater organic matter decomposition process was observed in the M3 soil, possibly because of a more intense recent input of vegetal material, as well as the presence of grassy, easily-decomposing herbaceous species, only in this site. Therefore, the dual-isotope approach generated a more integrated picture of the impact on the ecosystem after removing eucalyptus in this secondary Atlantic forest, and could be regarded as an option for future eucalyptus removal studies.
Introduction
Tropical forests have been reduced to fragments of their original extensions on account of activities such as urbanization, agriculture, and the replacement of native vegetation by exotic tree species for commercial purposes, as eucalyptus and pine (Morellato & Haddad, 2000; Baptista & Rudel, 2006; Wright & Muller-Landau, 2006; Brockerhoff et al., 2008; Vitória, Alves & Santiago, 2019). Historically in Brazil, the eucalyptus plantation has been associated with the suppression of native species, mainly in the Atlantic forest southeast region (Brockerhoff et al., 2008). In biome restoration-aimed efforts, this fast-growing exotic species has been used as a renewable alternative in order to connect fragments from the installation of ecological corridors, or the mixed plantation together with native species (Brockerhoff et al., 2008). However, the presence of eucalyptus close or consorted to secondary forests changes the natural dynamics of these forests, since this species offers fewer resources for the pollinating and dispersing fauna and which may interfere in both C and N cycles (Guo & Sims, 1999; Dutta & Agrawal, 2001). The eucalyptus litter decomposes slowly, has low nutritional attributes, and presents a higher C/N ratio in comparison to the majority of other native species from tropical forests (Rezende, Garcia & Scotti, 2001; Villela et al., 2001; Gama-Rodrigues et al., 2008).
In the sites destined exclusively to biodiversity protection, the eucalyptus has been suppressed from vegetation via removal, mixed plantations, and deforestation, which may cause variations in C and N cycles within the forest ecosystem compartments (Costa, Gama-Rodrigues & Cunha, 2005; Gama-Rodrigues et al., 2005). The N soil supplies are specially controlled over the climate conditions and by the vegetation (Vitousek & Matson, 1984). The forests stock C, which is accumulated between the photosynthesis and breathing balance in this ecosystem (Pregitzer & Euskirchen, 2004). Nonetheless, the C cycle and supply throughout the forest depend on its own age, from the period of natural or anthropic disturbances, and from practices of landscape usage such as deforestation and degradation (Houghton, 2005). Hence, the vegetation removal and/or replacement is likely to transform a C-storage ecosystem into a C-source one to the atmosphere (Bayer et al., 2004; Diekow et al., 2005).
The C and N cycles are fundamental ecological processes in the soil-plant system dynamics, thus influencing growth strategies, the subsistence of vegetal species, and the efficient applications of these elements by the community (Vitousek & Sanford, 1986). Within this system, the litter works as a reservoir for these and other elements. The soil organic matter decomposition rate and, consequently, the C and N release to the soil are related to biotic factors, namely the soil microbial activity, N concentration in plants, the chemical composition of the litter material, as well as abiotic variables, as the soil humidity, temperature, texture and depth (Stemmer, Gerzabek & Kandeler, 1998; Bustamante et al., 2004; Martinelli et al., 2009; Trumbore & Camargo, 2009). The soil texture influences the dynamics of C and N, whence more clayey and humid soils present higher retention capacity of organic matter (Stemmer, Gerzabek & Kandeler, 1998; Telles et al., 2003; Gama-Rodrigues et al., 2005). In more sandy soils, with low retention capacity of organic matter, few differences are observed between C and N values in the soil and those of the organic litter (Rosell, Galantini & Iglesias, 1996). The C isotopic composition (δ13C) from the litter and the soil organic matter depicts the combination of past and current vegetations (Freitas et al., 2001; Mardegan et al., 2009). In the leaves, the stomas control CO2 entry into the substomatal cavity, which has a direct impact on δ13C present in vegetal tissues. At first, any factor that alters the atmospheric CO2 isotopic ratio and/or the ci/ca (internal C/atmospheric C) relationship, will then modify δ13C (Farquhar, O’Leary & Berry, 1982; Mardegan et al., 2009; Vitória et al., 2016, 2018; Teixeira et al., 2018). Hence, in the leaves, the δ13C is determined by processes which interfere and modulate photosynthesis, for example the photosynthetic syndrome, the environmental conditions (for instance irradiance, temperature, humidity), and the availability of water and nutrients (Farquhar, Ehleringer & Hubick, 1989; Teixeira et al., 2015, 2018; Vitória et al., 2016, 2018; Vitória, Alves & Santiago, 2019).
Several biogeochemical processes contribute toward the N isotopic composition (δ15N) in leaves, that include nitrification, mineralization, lixiviation, denitrification, volatilization, abiotic factors (temperature and humidity), bacterial colonization, time and space variations in terms of N availability in the soil, the type of N source (organic or inorganic), soil texture, among others (Dawson et al., 2002; Ometto et al., 2006; Vallano & Sparks, 2013). Tropical forests are dynamic environments for the N-cycle-related processes, thus resulting in N fractionation and enrichment of 15N in the soil-plant system (Martinelli et al., 1999; Nardoto et al., 2008; Craine et al., 2009; Vitória et al., 2018). Leaf C/N ratio and soil N mineralization rates influence soil organic matter dynamics and consequently, the δ15N in leaves (Craine et al., 2009). Soil N transformation rates and N losses to the atmosphere will, ultimately, regulate N availability in an ecosystem and consequently, the δ15N in the soil-plant system (Craine et al., 2009). Since N availability is higher in tropical forests than in temperate forests (Vitousek & Sanford, 1986; Martinelli et al., 1999; Boeger, Wisniewski & Reissmann, 2005), these tropical forests will have, as a consequence, higher δ15N in the soil-plant system compared to the temperate forests under lower N availability (Craine et al., 2009). The high amount of precipitation and almost no seasonality in temperature and humidity in those tropical forests tend to provide favorable conditions to decomposition activity by soil organisms, and consequently high decomposition rates most of the year (Vitousek & Matson, 1984; Davidson et al., 2007; Nardoto et al., 2008; Viani, 2010; Gragnani, 2014). Therefore, both litter decomposition and nutrient mineralization promote nutrient availability in soil, mainly N and P, thus triggering variations in δ15N (Dawson et al., 2002; Bustamante et al., 2004; Ometto et al., 2006). Tropical and humid rainforests have higher concentrations of C and N in the soil during rainy season (Mazurec, 2003; Gama-Rodrigues et al., 2008). The decomposition acceleration in rainy season is attributed to an increment in decomposers and macro-fauna, and, in some forests, to a more significant growth of fine roots, caused by the availability of water and nutrients (Stemmer, Gerzabek & Kandeler, 1998; Bustamante et al., 2004; Martinelli et al., 2009).
In the União Biological Reserve (Rebio União), a fragment of the Atlantic forest with integral nature protection located in southwest Brazil presents specific forestry management which consists on the removal of eucalyptus trees (Corymbia citriodora) until 2015 aiming to restore the native flora. With management, the irradiance patterns, humidity, and site temperature were altered, as well as the δ13C from the main regenerating species from the understory (Teixeira et al., 2015, 2018; Vitória et al., 2016). Other ecological processes might as well have been affected by management, like the C and N dynamics in the soil-plant system.
The objective of this work was to study the forestry management effect of eucalyptus removal in the Rebio União by determining δ13C and δ15N in the soil-plant system, as well as the soil granulometry in three sites: a secondary forest in the Atlantic forest, and two other sites where eucalyptus had been removed at different times: 12 months and 3 months before sampling (M12 and M3, respectively). These study hypotheses were: (1) the eucalyptus permanence time altered the δ13C and δ15N in leaves, soil, and organic litter fractions (leaves, wood, flowers + fruits and rest) and; (2) eucalyptus removal furthered decomposition process of the soil organic matter.
Materials and Methods
Site of study, species and sampling period
This study was held in the União Biological Reserve (Rebio União), in Rio de Janeiro State, Brazil (22°27′S; 42°02′W). Field experiments were approved by SISBIO/ICMBio/MMA, to develop the project “Capacidade aclimatativa de espécies da Mata Atlântica após manejo de eucalipto na Reserva Biológica da União” at the Rebio União, Rio de Janeiro, Brazil, under the licence number 37996-3.
The climate in the region is humid tropical Aw (Alvares et al., 2013), with a yearly average temperature of 25 °C and an annual rainfall of 1,920 mm (85% being between October and April). This Reserve comprises around 7,800 ha of ombrophilous dense Atlantic forest, of which around 220 ha had the native tree vegetation suppressed and replaced by eucalyptus crops (Corymbia citriodora) (Hook.) K.D. Hill & L.A.S. Johnson. The eucalyptus tree plantations ceased to receive silvicultural treatments related to understory removal in 1996. The eucalyptus was clear-cut between 2009 and 2015.
We realized this research in three latosolic dystrophic red–yellow argisoil sites, classified according to Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) (1999), Miranda, Canellas & Nascimento (2007) and Dos Santos et al. (2018). One site is a well-preserved secondary forest; whereas the other two are eucalyptus-harvested sites, employing clear-cutting, with a single difference related to after-handling time: the M12 (where eucalyptus had been removed 12 months before our evaluation); and the M3 (where eucalyptus removal had happened three months before assessment) (Fig. 1; Table 1; Data S1). The post eucalyptus removal times were chosen according to the management activities in the Rebio União, and is sufficient to detect processes related to the decomposition of organic matter. For each of these sites, three ecosystem compartments were studied: photosynthetically active leaves, organic litter fractions, and the soil, due to its relation with organic matter decomposition processes.
Figure 1: Geographic map indicating the secondary forest and other two sites where eucalyptus had been removed at different times 12 and 3 months ago (M12 and M3, respectively), União Biological Reserve, Brazil.
| Secondary forest | M12 | M3 | |
|---|---|---|---|
| Understory of native species | Dense vegetation | Well-developed vegetation | Sparce vegetation |
| Mean irradiance (μmol m−2 s−1)a | 9 | 1.480 | 1.100 |
| Total site (ha) | – | 5.02 | 9.59 |
| Secondary forest distance (km) | – | 1.3 | 0.35 |
| Eucalyptus trees spaced (m) | – | 1.5 × 3b | 1.5 × 3b |
| Eucalyptus removal management | No management | Yes | Yes |
| Management time (months) | – | 12 | 3 |
| Presence of grasses | No | No | Yes |
Notes:
For the elemental and isotopic analyses of C and N, we considered the most abundant tree species within the eucalyptus removal sites M12 and M3 (Evaristo, Braga & Nascimento, 2011), being all of those from the initial succession stratum (Lorenzi, 2000): Siparuna guianensis Aubl. (Siparunaceae), Xylopia sericea A. St.-Hill (Annonaceae), and Byrsonima sericea D.C. (Malpighiaceae). We collected the leaves from the eucalyptus removal sites with individuals from 2 to 5 m high, and diameter at breast height (DBH) >5 cm. For a better diversity-related sampling within the secondary forest site, we collected samples from 20 species (Data S2) according to their importance value index (IVI), all of those ranging between 10 and 15 m tall, and with a DBH >10 cm. The leaves, plant litter, and soils were collected in December 2013 during the rainy season.
Determination of the elemental and isotopic composition of C and N within the leaves, litter and soil compartments and sample number
We standardized collected samples of the third sun-exposed photosynthetically active pair of leaves, once photosynthetic activity and irradiance variation are important factors to determine δ13C (Vitória et al., 2016). Young leaves are not photosynthetically active and enriched in 13C (Cernusak et al., 2009), similar to shadow leaves (Vitória et al., 2016). Besides, great variation can be found in δ13C within a single tree crown on account of the variations in environmental conditions (Le Roux et al., 2001; McDowell et al., 2011). Thus, sun-exposed branches of individuals of the secondary forest were collected from the canopy by using high pruning shears. Sun-leaves from eucalyptus removal areas (M3 and M12 sites) were manually collected. For each species, 5–7 leaves per individual were sampled and then dried in an oven at 50 °C for 48 h (Vitória et al., 2016). Afterward the leaves were macerated in liquid N and homogenized per individual. Regarding the eucalyptus removal sites, the samples collected comprise 5–15 individuals (n = 5–15) of the three species from the regenerating understory in both the M12 and the M3 sites. For a better diversity-related sampling within the secondary forest site, we collected samples from 20 species (Data S2). For all of these 20 species, 5 adult individuals had their leaves sampled, except for the following species: Ficus gomelleira (n = 4), Cupania racemosa (n = 4), Brosimum glazioui (n = 4), Virola gardneri (n = 2), Micropholis guianensis (n = 2), Ocotea diospyrifolia (n = 2). Thus, for the secondary forest, we collected samples from 2 to 5 individuals (n = 2–5).
The litter layer on the soil was collected via a 20 × 20 cm metal frame randomly arranged 25 times in each of the three study sites, totaling 75 samples with 400 cm2 of litter. The collect points were chosen at random. The collected material was dried at constant weight in an oven at 50 °C for 48 h (Mayor et al., 2014; Camara et al., 2018). These samples were screen-sorted by the following fractions: leaves (leaves and leaflets, whole or fragmented); wood (branch or bark pieces with a diameter smaller than 2 cm, and fragments), flower + fruits (flowers, inflorescence or organ, and fragments recognized as such, fruits and seeds, whole or fragmented), and the rest (residues smaller than 2 mm, animal-origin and unknown matter) (Proctor et al., 1983). The 25 dried litter fractions were macerated and grouped in 5 composed samples for each soil-litter fraction per site (n = 5).
The superficial soil (0–10 cm) was collected from below the litter layer withdrawn, totalizing 75 samples with 400 cm2 of soil. The 75 soil samples were dried at constant weight in an oven at 60 °C for 48 h (Mayor et al., 2014; Severo et al., 2017, Camara et al., 2018). No decarbonation was performed once previous tests verified the absence of inorganic carbon in the soil (Camara et al., 2018). A soil sub-sample was used to determine granulometric fractions (item 3), and another sub-sample was sieved in a 2 mm mesh and homogenized for isotopic and elemental determination of C and N (n = 25) (Telles et al., 2003).
Leaves, organic litter fractions, and soils were dried, ground, weighed (1 mg approximately), and inserted in tin capsules for C and N elemental and isotopic determination. All samples were combusted in an elemental analyzer (Flash 2000 Organic Elemental Analyser) which measured elemental concentrations of C and N; coupled to a stable isotope ratio mass spectrometer (IR-MS Delta V Advantage; Thermo Scientific, Waltham, MA, USA); and combined with a ConFloIV Interface (Thermo Scientific, Waltham, MA, USA), which measured the isotopic composition of C and N. Pee Dee Belemnite (PDB) and atmospheric N were used as standard values for C and N analyses, respectively. The values of PDB δ13C and atmospheric δ15N are respectively, 0.0111802‰ and 0.0036‰. The analytical precision was ±0.1 for δ13C and ±0.2 for δ15N, and the accuracy for elemental and isotopic compositions were determined by certified Organic Analytical Standard (OAS): Wheat Flour Standard OAS/Isotope Cert. 114858 and Low Organic Content Soil Standard OAS/Isotope Cert. 201613. The values of OAS 114858 were 39.38 ± 0.19% for C; 1.36 ± 0.23% for N; −27.21 ± 0.13‰ for δ13C; 2.85 ± 0.17‰ for δ15N. The values of OAS 201613 were 1.52 ± 0.02% for C; 0.13 ± 0.02% for N; −27.46 ± 0.11‰ for δ13C; 6.70 ± 0.15‰ for δ15N.
Carbon and nitrogen stable isotope values are reported in ‘‘delta” notation as δ values in parts per thousand (‰).
Determination of the soil granulometric fractions
After soil preparation procedures, as aforementioned, sub-samples were sieved, separating >2 mm fractions by sifting in successive intervals. Soil texture (granulometry) was determined in the soil fraction <2 mm. The methodology used here followed Wentworth (1922) by using a laser diffraction particle analyzer (SALD-3101; Shimadzu, Kyoto, Japan).
For precision analytical control, we measured the analytical variation among the analytical triplicates in every 20 samples, with an acceptable variation coefficient inferior to 10%. The accuracy surpassed 90%. Three certified samples supplied by the manufacturer of the equipment (Shimadzu, Kyoto, Japan) with differentiated particle size range (JIS class 11, Licopodium and glass beads, Data S3) were used to evaluate the accuracy of the soil granulometric fractions. The limit of detection herein was 0.1%.
The granulometric distribution was compiled by the software SysGran (3.0), and the data was grouped in sand, silt, and clay. The data are expressed in mean and standard error values.
Statistical analysis
In order to assess the normality in the data, the Shapiro Wilk (P ≤ 5%) test was conducted. After assessing the normality and homogeneity, the data underwent parametrical analyses. We analyzed differences between means via ANOVA (two-way), followed by Tukey test (P ≤ 5%). The factors considered herein were the compartments (leaves, litter fractions, and soil), sites, and granulometric fractions; it all aims to understand the interference of eucalyptus permanence time in the δ13C and δ15N in leaves, soil and organic litter fractions. The variance analysis was realized in the software Statistica 7.0. We calculated the sand regression coefficient correlations with Sigma Plot 11.0 software package Statistical Package for the Social Sciences (SPSS).
The C isotopic fractionation during organic matter decomposition was calculated from the difference between the δ13C in the soil and the litter fractions. The same was employed to N, by using δ15N. We inferred the soil organic matter decomposition based on these calculations.
Results
The relationship between C and N in the soil-plant system
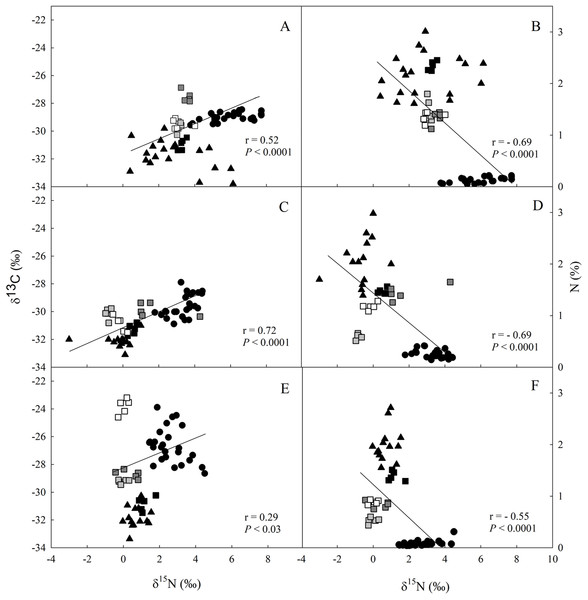
The values of δ13C and δ15N were higher in the soil > litter > leaves for all studied sites (Fig. 2; Data S4–S6). We observed a direct relationship between δ13C and δ15N (Figs. 2A, 2C and 2E), and an inverse one between δ15N and N in all three sites (Figs. 2B, 2D and 2F).
Figure 2: Linear regression between nitrogen (δ15N) and carbon (δ13C) isotopic composition and between δ13C and the elemental concentration of N (%) in different ecosystem compartiments for studied sites at the União.
Linear regression between nitrogen (δ15N) and carbon (δ13C) isotopic composition (A, C and E), and between δ13C and the elemental concentration of N (%) (B, D and F) for leaves (closed circle), litter fractions (squares: white—rest fraction; dark grey—fruits + flowers fraction; light grey—wood fraction; black—leaf fraction) and soil (closed triangle) in the secondary forest (A and B); site managed 12 months ago (M12) (C and D); and site managed 3 months ago (M3) (E and F) in União Biological Reserve (Rebio União), Brazil. December, 2013.Regardless of the site, the elemental concentration values for C and for the C/N ratio were respectively distributed: litter fractions > leaves > soil (Table 2). For N, leaves had higher values than litter, being its concentration thus distributed: leaves > litter fractions > soil (Table 2). Significant higher concentrations of C and N in the soil were found in the M12 site, when compared with the secondary forest and to the M3 site (Table 2). The N concentrations of all plant litter fractions were significantly smaller in the two eucalyptus removal sites (M12 and M3, Table 2). The C/N ratio in the leaves was higher in the M12 site. Higher C/N ratio values in wood fractions were obtained in the eucalyptus removal sites, in comparison with those of the secondary forest (Table 2).
| Site | Fraction | C (%) | N (%) | C/N |
|---|---|---|---|---|
| Secondary forest | Leave | 42.4 ± 0.4 Bb | 2.1 ± 0.1 Aa | 21.1 ± 0.5 Bb |
| Leaves litter | 46.9 ± 0.7 Ba | 2.3 ± 0.1 Aa | 23.4 ± 0.5 Cb | |
| Residue litter | 50.1 ± 0.6 Aa | 1.4 ± 0.1 Ab | 43.4 ± 1.3 Ba | |
| Flower + fruit litter | 47.7 ± 0.1 Aa | 1.3 ± 0.1 Ab | 42.0 ± 1.9 Ba | |
| Wood litter | 46.5 ± 1.9 Aa | 1.5 ± 0.1 Ab | 36.9 ± 1.4 Ba | |
| Soil | 1.5 ± 0.1 Bc | 0.1 ± 0.1 Bc | 16.5 ± 2.8 Ac | |
| M12 | Leave | 44.8 ± 0.7 Ab | 2.6 ± 0.2 Aa | 44.8 ± 2.4 Ab |
| Leave litter | 48.6 ± 0.4 Ba | 1.5 ± 0.1 Ba | 38.6 ± 0.5 Bc | |
| Residue litter | 48.4 ± 0.6 Aa | 1.2 ± 0.1 Bb | 48.0 ± 0.8 Bb | |
| Flower + fruit litter | 49.8 ± 0.3 Aa | 1.5 ± 0.1 Aa | 40.5 ± 1.6 Bb | |
| Wood litter | 48.4 ± 0.8 Aa | 0.6 ± 0.1 Bc | 96.3 ± 4.5 Aa | |
| Soil | 3.3 ± 0.1 Ac | 0.3 ± 0.1 Ad | 15.8 ± 0.8 Ad | |
| M3 | Leave | 45.2 ± 0.5 Aa | 2.0 ± 0.2 Ba | 23.4 ± 0.9 Bc |
| Leaves litter | 51.4 ± 1.1 Aa | 1.4 ± 0.1 Ba | 43.3 ± 0.9 Ab | |
| Residue litter | 45.1 ± 0.4 Ba | 0.9 ± 0.1 Cb | 59.6 ± 1.4 Aa | |
| Flower + fruit litter | 46.3 ± 0.6 Aa | 0.8 ± 0.1 Bb | 65.1 ± 2.7 Aa | |
| Wood litter | 47.5 ± 0.6 Aa | 0.5 ± 0.1 Bb | 106.2 ± 5.9 Aa | |
| Soil | 1.5 ± 0.1 Bb | 0.2 ± 0.1 Bc | 21.2 ± 0.7 Ac |
Note:
Mean and standard error (SE) of the total concentration for C, N, and the C/N ratio in leaves, litter fractions (leaves, residue, flower + fruits, and wood) and soil in the secondary forest, sites managed 12 months ago (M12), and sites managed 3 months ago (M3) at the União Biological Reserve (Rebio União), Brazil. December 2013. Uppercase letters compare the sites and the lowercase letters compare the compartments.
Decomposition of organic matter in the litter fractions
There was an enrichment in the 13C between litter fractions and soil in all sites (except for flower and fruit fractions from the secondary forest and the fraction of the rest in the M3 site) (Table 3). On the other hand, there was no enrichment in 15N in any of the studied sites, being observed only negative values at the time of calculating differences between litter fractions to the soil, for each site (Table 3).
| Site | Decomposition | δ13C (‰) | δ15N (‰) |
|---|---|---|---|
| Secondary forest | Leave litter | 2.0 | −2.7 |
| Residue litter | 0.5 | −2.9 | |
| Flower + fruit litter | −1.5 | −2.4 | |
| Wood litter | 0.7 | −2.9 | |
| M12 | Leave litter | 1.7 | −2.8 |
| Residue litter | 1.3 | −3.3 | |
| Flower + fruit litter | 0.3 | −1.6 | |
| Wood litter | 0.6 | −2.5 | |
| M3 | Leave litter | 4.2 | −1.4 |
| Residue litter | −2.8 | −2.6 | |
| Flower + fruit litter | 2.0 | −2.2 | |
| Wood litter | 2.6 | −2.6 |
Note:
Organic matter decomposition from the difference between δ13C and δ15N values of the soil and the different litter fractions (leaves, residue, flower + fruits, and wood) at the União Biological Reserve (Rebio União), Brazil in secondary forest, site managed 12 months ago (M12) and site managed 3 months ago (M3).
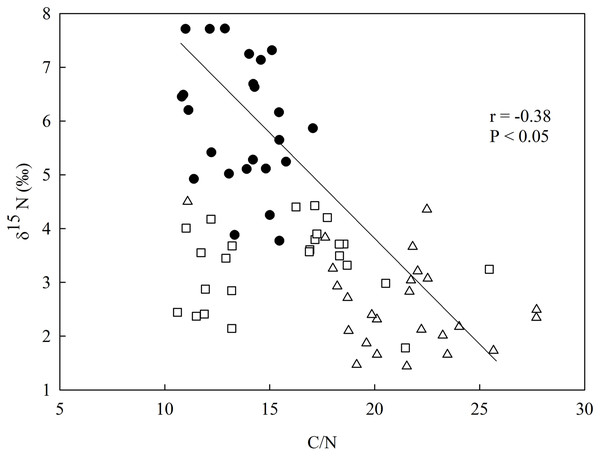
There was a negative relationship between δ15N and the C/N ratio in the soil (Fig. 3), having the secondary forest the highest value for δ15N in comparison to the eucalyptus removal sites, which did not vary between one another. The soil C/N ratio was higher in the most recent eucalyptus removal site (M3) than in the M12 site and the secondary forest (Fig. 3).
Figure 3: Linear regression between nitrogen isotopic composition (δ15N) and the in-soil C/N ratio in the studied sites at the União Biological Reserve (Rebio União), Brazil.
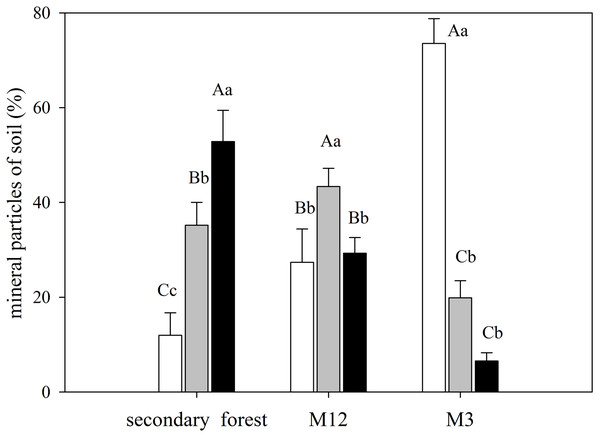
Linear regression between nitrogen isotopic composition (δ15N) and the in-soil C/N ratio in the secondary forest (black circles), the site managed 12 months ago (M12, white square), and the site managed 3 months ago (M3, grey triangle) at the União Biological Reserve (Rebio União), Brazil. December, 2013.The secondary forest soil, where greater values of δ15N were found, presented higher clay content, whereas in the M3 site, which presented a higher C/N ratio, had also featured a more significant amount of sand (Fig. 4; Data S7).
Figure 4: Mean and standard error (SE) of sand, silt, and clay percentage for the studied sites at the União Biological Reserve.
Mean and standard error (SE) of sand percentage (2 mm–63 µm) in white; silt (63 µm–4 µm) in light grey; and clay (<4 µm) in black in the superficial soil layer in secondary forest; site managed 12 months ago (M12); and site managed 3 months ago (M3) in União Biological Reserve (Rebio União), Brazil. December, 2013. Uppercase letters compare between sites, and lowercase letters compare between fractions.Comparison of δ13C and δ15N between the compartments and sites
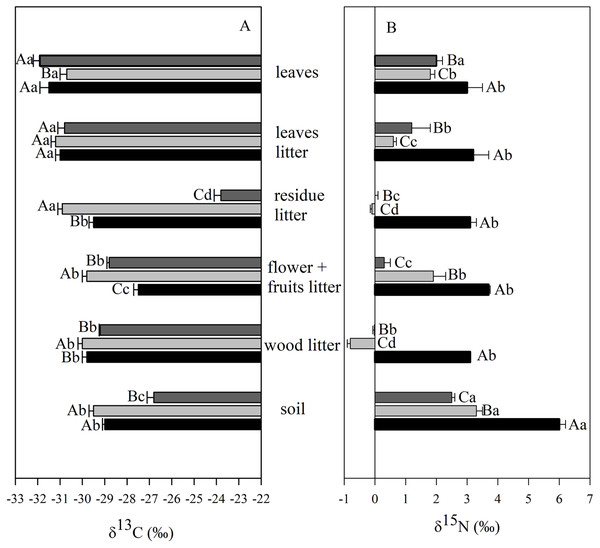
The δ13C values of soil varied from −26.7‰ and −29.6‰ and were consistently higher than those found in leaves and leaf litter fractions (Fig. 5A). The mean soil δ13C in the M3 site was higher than those from both the secondary forest and the M12 site. Significant differences were observed in δ13C values of leaves for the M12 site, in comparison to the other two sites; and also with foliar δ13C values ranging from −31.6‰ (secondary forest) to −32.2‰ (M3 site). The δ13C value of the litter rest fraction in the M3 site was higher than those from the secondary forest and the M12 site (Fig. 5A). In the organic litter, we found that the rest fraction presented the highest δ13C value (−23.8‰, M3 site), whereas the lowest value was found in the leaf fraction (−31.3‰, M12 site).
Figure 5: Mean and SE (standard error) of carbon (δ13C) and nitrogen (δ15N) isotopic composition in different ecosystem compartments at the União Biological Reserve.
Mean and SE (standard error) of carbon (δ13C) (A) and nitrogen (δ15N) (B) isotopic composition in leaves, litter fractions (leaves, residue, flower + fruits and wood) and soil from secondary forest (black); site managed 12 months ago (M12) (light grey); and site managed 3 months ago (M3) (dark grey) in União Biological Reserve (ReBio União), Brazil. December, 2013. Uppercase letters compare between sites, and lowercase letters compare between compartments.The δ15N values have significantly been higher in the secondary forest samples, when compared with those from the eucalyptus removal sites, regardless of the compartment under analysis (Fig. 5B). Concerning the soil, only positive values were verified, which varied from 2.6‰ to 6.0‰. Positive values were also observed for leaves, going from 1.6‰ to 2.9‰. Negative values of δ15N were registered only in a few litter fractions (rest and wood) from the M12 site.
Discussion
Variation of carbon isotopic composition (δ13C) in all compartments in the eucalyptus removal sites
The different δ13C variations of compartments between the eucalyptus removal sites and the secondary forest are mainly caused by the difference in the floristic composition, the organic litter input, and the irradiance, which consequently promotes variations in temperature and humidity, thus affecting processes such as assimilation of C and decomposition. The eucalyptus removal has altered the δ13C in every compartment of the managed sites, in comparison with the secondary forest. The higher δ13C values of the residue litter fraction and the soil in the M3 site reflect the presence, and carbon soil incorporation originated from the photosynthesis of Poaceae species (grassy), with photosynthetic C4 syndrome (Farquhar, 1983). The regenerating understory of tree species in the Atlantic forest in the M3 site was less developed than in the M12 site, possibly on account of the less time for native vegetation to grow and the competition with grassy that is abundant in the M3 site. C4 species present a greater δ13C (ranging from −15‰ to −9‰) than that of the tree species, which feature photosynthetic C3 syndrome (whose δ13C values may vary from −35‰ to −23‰) (Farquhar, Ehleringer & Hubick, 1989; Martinelli et al., 2009). The grassy species contributed to the organic matter onto the soil, raising the δ13C of soil from the M3 site. In the secondary forest, δ13C values were higher in the sequence soil > litter fractions > leaves. A similar pattern was observed in the eucalyptus removal sites, except for the rest litter fraction in the M3 site given the reason aforementioned. The highest soil δ13C value, in comparison with the remaining analyzed compartments, may be explained by the fast mineralization of the components with lower δ13C values (Gerschlauer et al., 2018). Differences between the δ13C from the distinct litter fractions and the δ13C from the soil are interpreted as consequences of the litter organic matter decomposition (Gerschlauer et al., 2018). During decomposition of litter, fractionation occurs due to the decomposers that preferentially use 12C resulting in 13C-enrichment in the remaining soil organic matter (Lichtfouse et al., 1995). The difference in δ13C value (δ13C litter − δ13C soil) and the low C/N ratio in the soil, observed in the M3 site, agree with the idea about altering the dynamics of this soil organic matter.
Our results suggest there was organic matter decomposition in every litter fraction and in every site. However, a higher decomposition of the organic matter determined from the δ13C in the M3 site was also observed. This result possibly comes from the more significant recent input of vegetal matter into the M3 site soil, as well as the presence of grassy (herbaceous species of rapid decomposition) exclusively in this site. Despite the soil humidity reduction caused by microclimatic alterations from recent handling in the M3 site (such as less humidity, higher irradiance, temperature, and winds), the input increase was enough to evidence more significant decomposition in this particular site.
The highest δ13C values found in the M12 site leaves, compared to both secondary forest and the M3 sites, are explained by the fact that this vegetation was formed under greater irradiance, for 1 year after handling. Sites exposed to more intense irradiance provide the environmental conditions which increase the δ13C values within the photosynthesizing tissues (Ehleringer et al., 1986; Domingues et al., 2005; Van der Sleen et al., 2014; Teixeira et al., 2015; Vitória et al., 2016).
In all sites, the δ13C values from the litter leaf fractions were a reflex of the δ13C living leaves. However, despite the previous discussion, the influence of the irradiance variation upon δ13C values in leaves was not the single cause for δ13C values in the litter rest fraction, mainly in the M3 site, where grassy (C4 species) enrich the soil with 13C. Compared with litter leaves, the remaining fractions (“flowers + fruits” and wood) presented higher δ13C values. Variations between the δ13C from the source and sink organs have been widely reported, is the enrichment extension variation of the sink organs δ13C dependent on the tissue, species, and environmental conditions (Cernusak et al., 2009; Vitória et al., 2016).
Among the causes of isotopic variation between source and sink is the variability in the biochemical tissue composition (Cernusak et al., 2009). The leaves present higher cellulose concentrations than do stems and roots which possess more lignin. After eucalyptus removal in the M3 and the M12 sites, the cellulose from leaves decomposed faster than did the lignin from stems, thus changing the soil δ13C. Cellulose is more enriched with 13C than lignin and lipids (Badeck et al., 2005; Cernusak et al., 2009). Another isotopic difference-contributing factor between source and sink is the isotopic fractionation during night breathing. The CO2 respired in the dark by the aerial part is significantly enriched with 13C when compared with the respiratory substrate pool, whereas the CO2 aspired by the roots does not present enrichment (Klumpp et al., 2005). Moreover, stems grow preferentially at night (Steppe et al., 2005; Saveyn, Steppe & Lemeur, 2007). This is when the exported carbohydrates present higher δ13C values. As a consequence, the wood features enrichment of 13C when compared with the leaves. Also, carbohydrates exported during the day present lower δ13C values, and supply preferentially for the leaf growth, which occurs faster during the day (Walter & Schurr, 2005).
However, it is essential to consider that the nature of the site sampling, which occurred under a unique field experimental condition, prevents the replication so as to increase the robustness of this data. In this way, although the data indicate that the presence of grassy and the higher irradiance was determining factors for the alteration of δ13C in the analyzed compartments, these data should be regarded cautiously.
Differences in nitrogen isotopic composition (δ15N) in the soil-plant system related to eucalyptus removal
The higher δ15N values within the soil-plant system in the secondary forest, compared to the eucalyptus removal sites, can relate to differences in the composition of vegetal species, and in the strategies employed by them regarding the use of the soil nutrients (Viani, 2010). Depending on the variation in the functional diversity among coexisting species, one expects a more efficient use of resources and, in parallel, a reduction in the interspecific competition which, consequently, may increase the ecosystem productivity (Naeem et al., 2009). Some species may also disproportionately contribute to the ecosystem-related processes on account of a high specialization level, as the symbiotic nitrogen fixation (Reed, Cleveland & Townsend, 2008). Although tropical forests have an immense nutrient stock, forest sites under management, in general, may lack nutrients, thus presenting a decrease in these stocks (Bellote, Dedecek & Silva, 2008). Besides, other factors such as the dominant species, the management period and activity type, the rain cycles and intensity, and the soil properties interfere in the dynamics of nutrients (Bellote, Dedecek & Silva, 2008).
Our data shows the influence of eucalyptus removal on the superficial soil layer, being the eucalyptus removal sites (M3 and M12), the ones to present lower δ15N in comparison with the secondary forest. Similar δ15N values, to those from the secondary forest, were also found in: other Atlantic forest soils (between −1.2‰ and 6.0‰) (Viani, 2010; Gragnani, 2014); in the litter (between −1.8‰ and 1.8‰) (Owen, 2013); and in tree leaves from tropical forests (between −3.0‰ and 12.0‰) (Davidson et al., 2007; Nardoto et al., 2008, Vitória et al., 2018).
Other aspects regarding the retention of N and the difference in δ15N values in the soil-plant system between the sites should be taken into account. First, there is the N mobility before the foliar senescence, being its concentration thus distributed: leaves > litter fractions > soil in all the three sites. Second, the differences in soil textures, where more clayey soil was found in the secondary forest than in the eucalyptus removal sites. Clayey soils have greater retaining capacity when compared with sandy ones, especially the N ammoniacal form (Trumbore & Camargo, 2009). Clay soils present more intense processes of mineralization, nitrification, and denitrification than do sandy soils in the same region, and that influences the forest productivity and nitrogen availability (Keller et al., 2005). This suggests that N and δ15N are relatively higher in sites with greater clay concentration (Martinelli et al., 1999; Davidson et al., 2007; Houlton et al., 2007; Craine et al., 2009). Nonetheless, greater water availability also tends to increase N concentration and δ15N values in leaves and soils (Austin & Vitousek, 1998; Handley et al., 1999; Amundson et al., 2003; Houlton et al., 2007; Nardoto et al., 2008).
Even though the relationship between clay and soil δ15N might be promote higher N fractionation (via nitrification, denitrification, and volatilization), in clay-rich soils the relative proportion of N fractionation might not be directly influenced by clays, but by the stability of clay particles containing 15N-enriched organic matter. As a result of the higher organic matter decomposition and/or higher clay concentrations, it is possible for soils from hotter and/or drier ecosystems to have a greater proportion of N in the organic matter associated with minerals, as opposed to having a higher proportion of N that is lost by fractionation processes (Craine et al., 2015).
The lower C/N litter ratio limits the N in the soil, thus raising the organic matter decomposition rates by the biota (Berhe, 2012). The C/N ratio in the leaves was greater in the M12 site. Higher C/N ratio values in wood fractions were obtained in the eucalyptus removal sites, in comparison with those of the secondary forest. That demonstrates the slow eucalyptus branch wood decomposition compared to that of the native species. Therefore, the secondary forest site, with less C/N ratio in the litter, has its N-rich organic compounds consumed rapidly; while the excess of N is mineralized, increasing hence the δ15N value in the soil. That explains the negative relationship between δ15N and the C/N ratio, which is also reported by big-scale evaluations (Amundson et al., 2003; Craine et al., 2015).
Whenever both soil and plant contain high δ15N values, one may infer the presence of organic matter and decomposition activity in the soil, aside from variables such as temperature and gas flow into the atmosphere, which also interfere in the system (Craine et al., 2015). More humid soils (clayey) usually have more nitrogen gases emissions than those sandy soils (Davidson et al., 2000; Keller et al., 2005). Soils with larger quantities of silt and clay present a lower C/N ratio (Stemmer, Gerzabek & Kandeler, 1998; Christensen, 2001), as observed in the study herein.
The 15N-rich organic matter decomposition, in general, has smaller δ15N values in mountainous ecosystems from tropical forests than do a wide range of tropical forests in low lands worldwide with average values ranging from 3‰ to 14‰ (Martinelli et al., 1999; Nardoto et al., 2014). Such low δ15N values were reported in hilly ecosystems and were related to both high concentration of NO3− in soil solution and significant N lixiviation rates (10–15 kg N ha−1 year−1) (Gütlein et al., 2016).
A significant increase in soil δ15N has also been observed in forests where recent fire events took place (Hemp, 2005; Zech et al., 2011). Vegetation burning may cause loss of gaseous NO2 resulting in depleted values of δ15N (Zech et al., 2011). Some disturbances in the ecosystem (forest fire, selective cutting, and others) trigger changes in the vegetal coat which affects the organic matter dynamics, and which may be regarded as variations in the C/N ratio in the soil (Saiz et al., 2016). Hence, this work suggests that the high δ15N values in leaves and soil from the secondary forest are a consequence of relatively clay-rich soil combined with a moderate water deficit (Ometto et al., 2006; Nardoto et al., 2008; Quesada et al., 2012) which, consequently, would then lead to greater availability and loss of N (Ometto et al., 2006; Nardoto et al., 2008).
The alteration in vegetation after eucalyptus removal modified the soil properties and furthered the decomposition processes of soil organic matter
The higher concentration of C and N in the soils of the M12 site is associated with two factors mainly: (1) greater eucalyptus litter entry in the system regarding eucalyptus logging, once only eucalyptus tree trunks were removed from the site in order to be commercialized; and (2) longer period since eucalyptus removal in this site, in comparison with the M3 site, which enabled the decomposition and incorporation of the M12 site litter-origin nutrients by the soil. However, the soil C concentration (3.3%) in the M12 site is among the lowest values reported for tropical forest soils, which vary from 3.0% (Mazurec, 2003) to 9.0% (Clevelario Júnior, 1996). A similar concentration of elemental N in the soil, found in the M12 site (0.3%) had been reported by Villela et al. (2001) in superficial soils of eucalyptus crops (0.31%) in the same studied site (Rebio União). The concentration of elemental N is greater in the leaves than it is in the soil and the litter fractions, which may be explained by the translocation of this mobile element in the plant before the foliar senescence (Aerts & Chapin, 2000; Satti et al., 2003; Kuang et al., 2010). The great necessity of N by this organ is due to the photosynthetic process in which many enzymes are entailed, as protein molecules with a catalytic function that contain N in their structures (Paul & Pellny, 2003).
The biotic and abiotic conditions fostered the decomposition of 13C-enriched compounds in the M3 site. In more sandy-textured soils (M3 site), the organic matter tends to decompose more rapidly, which has less capacity to retain organic matter (Rosell, Galantini & Iglesias, 1996). The more intense presence of sand also favours the lixiviation of more soluble organic compounds, which might dissipate after the litter deposition. Moreover, the greater presence of grassy (C4 plants) in the M3 site soil, compared with that of the M12 site, presented a higher value for δ13C in the soil because the C4 species showed less isotopic discrimination than did C3 species. From that, the M3 site soil had a higher δ13C value (Farquhar, 1983). Studies on the Atlantic forest soils (Vitorello et al., 1989; Bonde, Christensen & Cerri, 1992) and the Amazon forest soils (Desjardins et al., 1996) stated that soils with greater clay quantity had higher values for δ13C. Even so, it was not confirmed by this research, where the δ13C value was higher in the M3 site soil, which had a more considerable amount of sand. Certainly, the presence of both grassy species and sandy soil in the M3 site was an influencing factor upon δ13C values in this soil.
The relationship between the C/N ratio and the δ15N may be used as a tool for verifying degradation and stabilization of the soil organic matter in disturbed systems, in comparison with the secondary forest, which has already been observed (Conen et al., 2008). In the present study, the soil δ15N increased along with the decrease in the soil C/N ratio soil. Craine et al. (2015), studying 6,000 soil samples from 910 sites, had also noted the increase in soil δ15N associated with a decrease in the soil C/N ratio, and as a consequence of greater stabilization of the soil organic matter. Soils with high clay content, as it is the case of the secondary forest, also have high soil δ15N. The clay and silt fractions are frequently associated with this soil organic matter, thus hampering the decomposition process (Liao, Boutton & Jastrow, 2006).
The organic matter with a longer incorporation time into the soil should be a better indicator of the isotopic signature of the decomposer than of the initial input from the fall of branches and plant leaves (Craine et al., 2015). For this reason, the soil organic matter fractions generally show higher δ15N values in humidified soils (Liao, Boutton & Jastrow, 2006; Marin-Spiotta et al., 2009). Liao, Boutton & Jastrow (2006) quantified C and N stable isotopes in the soil, as well as their granulometry, in sites where tree species and bushes (C3 plants) were replaced by pastures (C4 plants). According to these authors, the soils with higher silt and clay fractions were those with higher δ15N values, thus suggesting organic matter stabilization (Marin-Spiotta et al., 2009).
The data presented herein indicate the alteration of C and N dynamics in the ecosystem considering land use (presence and subsequent removal of the exotic eucalyptus species from the Atlantic forest fragment). It is important to emphasize the need for caution in the interpretation of these data since there are limitations around this type of experimental design. In studies that make use of real situations in the field, true replicates (sites) are difficult to work. In addition, this study addressed isotopic and soil granulometry analysis. More robust information can be obtained when other indicators and characteristics of vegetation and soil are also analyzed, including functionality attributes, photosynthetic performance, details of soil organic matter composition, among others. However, it is of utmost importance to understand how this exotic species affects the functioning of the ecosystem since eucalyptus has been planted all over the world for commercial or forest restoration purposes, either as monoculture or in symbiotic consortia with native tropical forests (Guariguata, Rheingans & Montagnini, 1995; Parrotta, Turnbull & Jones, 1997; Feyera, Beck & Lüttge, 2002; Baptista & Rudel, 2006). The substitution of native forest cover by the exotic eucalyptus species has been widely used in order to give financial returns to the landowners (Baptista & Rudel, 2006). In some cases, the economic and environmental purposes are enhanced simultaneously, being the eucalyptus used for its rapid growth in deforested areas (Feyera, Beck & Lüttge, 2002), facilitating later forest restoration, as seen in plantations of this exotic species in Central America and the Caribbean (Guariguata, Rheingans & Montagnini, 1995; Parrotta, Turnbull & Jones, 1997). The fact that tropical forests are being the focus of recovery efforts after disturbances and degradation is mainly due to the urgent need to mitigate the consequences of climate change. In this sense, the data presented here are important to improve the understanding of the beneficial effect of the eucalyptus removal in the ecosystem.
Conclusions
The removal of the exotic species altered temporally and differently the isotopic and elemental ratios of C and N in the different ecosystem compartments due to the floristic composition (appearance and abundant presence of grassy in the M3 site), the alteration in the environmental conditions (irradiance, water availability, and temperature), variations in mineralization and nitrification rates, and of the decomposition and input from the organic litter.
The soil δ13C was higher in the site where the eucalyptus was harvested due to the facilitation of the appearance of grasses with photosynthetic C4 syndrome that were decomposed and incorporated in the soil. Under the environmental conditions studied, such decomposition and incorporation occurred in a short period of time, as the highest values of δ13C were observed in the area where the eucalyptus was removed 3 months ago (M3 site), but no later than 12 months (M12 site). Another aspect that contributed to the increase of soil δ13C where the eucalyptus was harvested was the higher irradiance input, which increased the values of δ13C in the photosynthesizing leaves that will be decomposed afterward.
The soil δ15N also increased according to the increase in the time of eucalyptus harvesting (higher in the M12 site than in the M3 site), altering the dynamics of the organic matter in this compartment.
Therefore, the dual-isotope approach was able to generate a more integrated picture of the impact in the ecosystem after the eucalyptus removal in this secondary Atlantic forest. Finally, this tool should be considered as an option for future eucalyptus removal studies, taking into account the particularity of the data generated given the absence of sample replicas. Nevertheless, our data were able to detect the influence of this exotic species on the isotopic changes of C and N in all compartments of the ecosystem.
Supplemental Information
Images of União Biological Reserve (Rebio União), Brazil. (A) secondary forest; (B) site managed 12 months ago (M12); (C) site managed 3 months ago (M3).
Family and species sampled in the secondary forest of União Biological Reserve (Rebio União), Brazil.
Family and species sampled in the secondary forest of União Biological Reserve (Rebio União), Brazil.