Genome-wide analysis and expression profiling of the heat shock transcription factor gene family in Physic Nut (Jatropha curcas L.)
- Published
- Accepted
- Received
- Academic Editor
- Kenta Nakai
- Subject Areas
- Bioinformatics, Molecular Biology, Plant Science
- Keywords
- Jatropha, Heat shock transcription factor, Gene family, Abiotic stress, Gene expression
- Copyright
- © 2020 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Genome-wide analysis and expression profiling of the heat shock transcription factor gene family in Physic Nut (Jatropha curcas L.) PeerJ 8:e8467 https://doi.org/10.7717/peerj.8467
Abstract
The heat shock transcription factor (Hsf) family, identified as one of the important gene families, participates in plant development process and some stress response. So far, there have been no reports on the research of the Hsf transcription factors in physic nut. In this study, seventeen putative Hsf genes identified from physic nut genome. Phylogenetic analysis manifested these genes classified into three groups: A, B and C. Chromosomal location showed that they distributed eight out of eleven linkage groups. Expression profiling indicated that fourteen JcHsf genes highly expressed in different tissues except JcHsf1, JcHsf6 and JcHsf13. In addition, induction of six and twelve JcHsf genes noted against salt stress and drought stress, respectively, which demonstrated that the JcHsf genes are involved in abiotic stress responses. Our results contribute to a better understanding of the JcHsf gene family and further study of its function.
Introduction
The heat shock transcription factor (Hsf) as the direct transcriptional activator of genes plays an important role in regulating plant growth, development and in response to abiotic stresses, such as heat, drought and salt stress (Von Koskull-Döring, Scharf & Nover, 2007). Under heat stress condition, Hsfs functioned as the molecular chaperone protects cells structure in protein folding and assembly process (Hu, Hu & Han, 2009).
Hsfs well-known as a group of DNA-binding proteins, specially recognize the binding motifs AGAAnnTTCT which conserved in the promoter regions of Hsf-inducible genes (Guo et al., 2016). Despite a considerable variety in size and sequence, the main domains of Hsf in eukaryotes are conservative. A typical Hsf was composed of the DNA binding domain (DBD), the oligomerization domain (OD), nuclear localization signal (NLS), nuclear export signal (NES), repressor domain (RD) and C-terminal activator peptide motif (AHA) (Nover et al., 2001; Guo et al., 2016). Since the first Hsf gene was cloned and characterized in the yeast (Wiederrecht, Seto & Parker, 1988), more and more Hsf proteins had been cloned and characterized in plants. The plants Hsfs were separated into three groups (A, B and C), and further divided into 16 subgroups (A group: A1-A9; B group: B1-B5; C group: C1-C2) (Nover et al., 2001; Hu, Hu & Han, 2009; Scharf et al., 2012). Both AHA and adjacent NES motifs in the C-terminal are specific of Hsf A, but absent in Hsf B and Hsf C (Nover et al., 2001). Increasing research indicated that HSF is involved in plant development, biotic and abiotic stress. For example, overexpression of HsfA1a, HsfA1b, HsfA2 or Hsf3 in Arabidopsis promoted its stress tolerance (Qian et al., 2014; Albihlal et al., 2018; Ogawa, Yamaguchi & Nishiuchi, 2007; Prandl et al., 1998). Similar stress tolerance phenotypes were also found in tobacco (Personat et al., 2014). In contrast, the hsfa1a/b/d/e quadruple mutant exhibited a severe growth retardation phenotype (Yoshida et al., 2011). Additionally, AtHsfA6a-overexpressing Arabidopsis demonstrate a significantly decreased germination under ABA treatment (Hwang et al., 2014). It is worth noting that HsfA2, which shared similar structure with HsfA1 but with a different expression profile, was strongly induced under long-term or cycled heat stress conditions in Arabidopsis (Charng et al., 2007; Nishizawa-Yokoi et al., 2009). This indicated that even the orthologous genes play a divergent role in different plants.
Based on released genome sequences, genome-wide analyses of Hsf family performed in various plant species, such as Arabidopsis (Guo et al., 2008), rice (Guo et al., 2008; Mittal et al., 2009; Hu, Hu & Han, 2009), pepper (Guo et al., 2015), Chinese cabbage (Huang et al., 2015), maize (Lin et al., 2011), Triticum aestivum (Xue et al., 2014), tea plant (Camellia sinensis) (Liu et al., 2016), soybean (Glycine max) (Chung, Kim & Lee, 2013), Brassica oleracea (Lohani et al., 2019), Salix suchowensis (Zhang et al., 2015a) and sesame (Dossa, Diouf & Cisse, 2016). These investigations are of great significance for the function analysis of Hsfs refers to plant development and in response to abiotic stresses.
Physic nut (Jatropha curcas L.), which is well-known as a renewable resource for biodiesel production, has a high tolerance to drought and salt stress (King et al., 2009; Divakara et al., 2010). To date, the genome sequence and expression profile of physic nut under several abiotic stresses were available (Wu et al., 2015; Zhang et al., 2014; Zhang et al., 2015b). Therefore, the Hsf family in physic nut could be characterized at the molecular level. However, no detailed study of JcHsf family genes performed. In this study, a total of 17 putative JcHsf genes were identified. The classification, phylogenetic reconstruction, chromosome distribution, gene structure and conserved motifs of the JcHsfs were predicted and analyzed. In addition, the expression profile of JcHsf genes was analyzed under normal condition and in response to salt and drought stress. Taken together, these results these results provide important information for further study of functional genes in the physic nut Hsf family and will provide insights into the functional analysis of other plants Hsf genes.
Materials & Methods
Plant materials and treatment
The inbred physic nut cultivar GZQX0401 were used in this study. The seed germination, planting conditions, stress treatment and material collection were performed as described previously (Zhang et al., 2014; Zhang et al., 2015b).
RNA isolation and gene expression analysis
Total RNA was extracted from ∼100 mg leave samples using the modified CTAB method (Zhang et al., 2014). The RNA was then reverse transcript into cDNA by M-MLV reverse transcriptase (Promega). For qRT-PCR analysis, primers used in this study were designed by primer 6.0 (Table S1). Each reaction was performed using TaKaRa Ex Taq HS Kit according to the instructions of the manufactor. The expression levels were calculated using the 2−ΔΔCTmethod. Each PCR assay was run in duplicate for three independent biological replicates. For gene expression profiles analysis, the number of expressed tags was calculated and then normalized to TPM (number of transcripts per million tags). Heatmap of Hsf family expression in different tissues was constructed based on the number of TPMs. While the heatmap of Hsf family expression under salt and drought stress was constructed by log2 conversion of TPM values.
Identification and characteristics of Hsf genes in physic nut
To identify the Hsf family genes of physic nut, the HSF protein sequences of in Arabidopsis and rice was used as query sequences to execute BLASTP search against the physic nut genome. Target gene sequences were selected with e-value cut-off less than 1e−10. The Hsf protein sequences of Arabidopsis and rice were downloaded from TAIR (http://www.arabidopsis.org/) and the website (http://plntfdb.bio.uni-potsdam.de/v3.0/), respectively. In addition, the HMM file built based on Hsf domain (PF00447) was used to perform HMM searches against the local protein databases of the physic nut by HMMER3 (Eddy & Pearson, 2011). In order to confirm the accuracy of identified genes, the SMART program was used to detect DBD domains and coiled-coil structures (SMART: http://smart.embl-heidelberg.de/). Those protein sequences lacking the DBD domain or a coiled-coil structure were removed. Next, Clustal X was aligned to remove redundant sequences of the confirmed JcHsf sequences (Larkin et al., 2007). Finally, the online ExPasy program (http://www.expasy.org/tools/) was applied to analysis the length, molecular weight and isoelectric point parameters of each JcHsf protein.
Structure and motif analysis of JcHsf genes
The exon/intron structures of JcHsf genes were elucidated by Gene Structure Display Server (GSDS, http://gsds.cbi.pku.edu.cn/) (Guo et al., 2007). Conserved motifs were analyzed using the MEME Suite version 5.0.5 (http://meme-suite.org/tools/meme; Bailey et al., 2009). The parameters were set as the default value except the numbers of different motifs, which was set to 30.
Chromosomal locations, multiple sequence alignment and phylogenetic analysis of JcHsf genes
All identified JcHsf genes were mapped on the eight out of eleven linkage group base on physic nut genome database. The maximum likelihood mapping algorithm and Kosambi mapping function are used to calculate the map distance within cM (Wu et al., 2015). The linkage map of JcHsf genes was mapped using the Map-Chart software package. Amino acid sequences of Hsf protein were aligned by Clustal X (version 1.83). GeneDoc was then used to manually edit the results. The phylogenetic tree was constructed among Arabidopsis thaliana, Oryza sativa, vitis vinifera and Jatropha curcas L. using the maximum likelihood method in MEGA5 with 1,000 replicates (Tamura et al., 2011). The HSF proteins from four plant species were listed in Table S2.
Results
Identification and chromosomal localization of JcHsf gene family
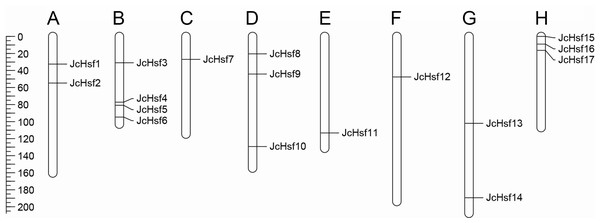
Seventeen (17) putative genes of JcHsf family were identified in the present study, and they were named JcHsf1-17 from top to bottom according to their position on the physic nut linkage groups (LGs) 1 to 11. The accession numbers in GenBank and detailed information of JcHsf gene family were listed in Table 1. The JcHsf protein lengths ranged from 214 aa (JcHsf 7) to 560 aa (JcHsf 11), The molecular masses of the JcHsf proteins were predicted between 28.3 to 62.3 KDa, and the theoretical pIs were ranged from 4.67 to 9.05 (Table 1). Chromosomal location indicated that no JcHsf was mapped on LGs 2, 4 and 10, and 17 JcHsf genes were not randomly distributed in the other 8 LGs (Wu et al., 2015) (Fig. 1). Large difference in number of JcHsf genes located in each LG. The result showed four JcHsf genes on LG3, three on LG6 and 11, two on LG1 and 9, and one on LG5, 7 and 8 (Fig. 1).
| Genes | Gene ID | Protein length (aa) | pI | MW (kDa) | Location |
|---|---|---|---|---|---|
| JcHSF1 | JCGZ_02229 | 359 | 5.42 | 41.4 | LG1 |
| JcHSF2 | JCGZ_01081 | 495 | 5.93 | 55.2 | LG1 |
| JcHSF3 | JCGZ_17108 | 517 | 4.81 | 56.3 | LG3 |
| JcHSF4 | JCGZ_04789 | 358 | 4.74 | 41.1 | LG3 |
| JcHSF5 | JCGZ_21617 | 406 | 5.04 | 46.2 | LG3 |
| JcHSF6 | JCGZ_21433 | 214 | 9.05 | 24.8 | LG3 |
| JcHSF7 | JCGZ_26137 | 311 | 5.44 | 34.4 | LG5 |
| JcHSF8 | JCGZ_21103 | 242 | 7.59 | 28.3 | LG6 |
| JcHSF9 | JCGZ_06744 | 560 | 4.77 | 62.3 | LG6 |
| JcHSF10 | JCGZ_02539 | 497 | 5.19 | 55.2 | LG6 |
| JcHSF11 | JCGZ_12391 | 288 | 5.54 | 31.2 | LG7 |
| JcHSF12 | JCGZ_21870 | 357 | 5.15 | 40.8 | LG8 |
| JcHSF13 | JCGZ_03936 | 425 | 4.91 | 48.5 | LG9 |
| JcHSF14 | JCGZ_20639 | 333 | 5.45 | 36.6 | LG9 |
| JcHSF15 | JCGZ_07238 | 320 | 5.94 | 35.7 | LG11 |
| JcHSF16 | JCGZ_07430 | 473 | 4.85 | 52.5 | LG11 |
| JcHSF17 | JCGZ_07655 | 380 | 4.67 | 42.7 | LG11 |
Figure 1: Distribution of JcHsf genes on physic nut chromosomes according to the linkage map.
(A) Linkage group 1. (B) Linkage group 3. (C) Linkage group 5. (D) Linkage group 6. (E) Linkage group 7. (F) Linkage group 8. (G) Linkage group 9. (H) Linkage group 11. In total, 17 JcHsf genes were mapped to eight linkage groups (LGs). The scale is in centiMorgans (cM).Phylogenetic and structures analysis of JcHsf gene family
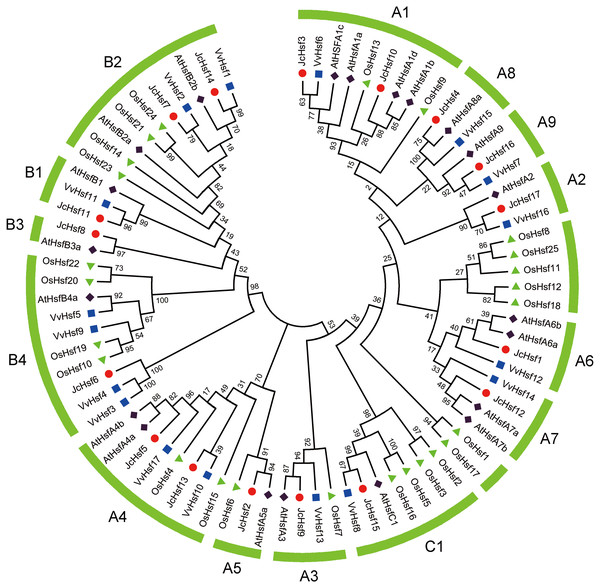
To survey the evolutionary relationships of JcHsf gene family, protein sequences from other three well-studied and representative species, including a dicot Arabidopsis, a monocot rice and grape, were selected to construct a phylogenetic tree (Fig. 2). Similar to the Hsfs from other three plant species, JcHsfs were divided into three groups (Group A, B and C) according to previous study on AtHsfs (Guo et al., 2008). The largest group A was then classified into nine subgroups (A1-A9), including 11 members (JcHsf1, JcHsf2, JcHsf3, JcHsf4, JcHsf5, JcHsf9, JcHsf10, JcHsf12, JcHsf13, JcHsf16 and JcHsf17), accounted for 64.7% of total JcHsfs. The next group B was classified into four subgroups (B1-B4), with five members (JcHsf6, JcHsf7, JcHsf8, JcHsf11 and JcHsf14), accounted for 29.4%. Only JcHsf15 was classified into the smallest group C, which represented 5.9%. Results showed a close evolutionary relationship with dicotyledons plants.
Figure 2: Phylogenetic relationships of Arabidopsis, rice, grape and physic nut Hsf proteins.
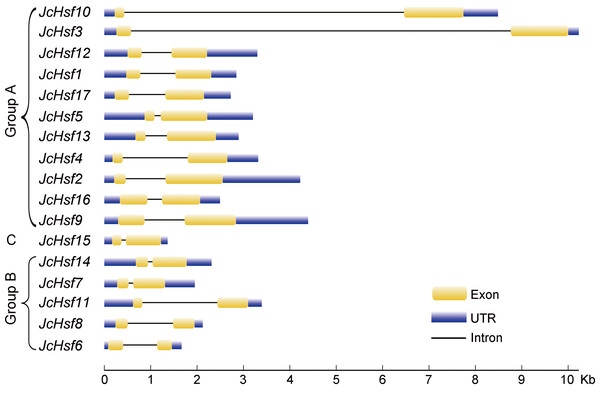
The unrooted tree was constructed, using the MEGA5.0 program, by the neighbor-joining method. Bootstrap values were calculated for 1,000 replicates.During the evolution process, the exon/intron structural within a gene family appears divergence, which of great significance to study the evolution of gene families. So the exon/intron structures of JcHsf genes were analyzed in the present study. It was shown that all JcHsfs had only one intron, illustrating a very highly conserved exon/intron splicing arrangement exists in physic nut (Fig. 3). Although physic nut Hsf genes shared same intron number, the length of intron was different in the groups. For instance, in the subgroup A1, the intron length of JcHsf3 was much longer than that of JcHsf10.
Figure 3: Intron-exon structure of JcHsf genes.
Yellow boxes indicate the exon regions, black lines indicate introns and blue boxes indicate upstream and downstream UTR regions. The lengths of the boxes and lines were scaled according to the length of the genes.Conserved domains and motifs of JcHSF proteins
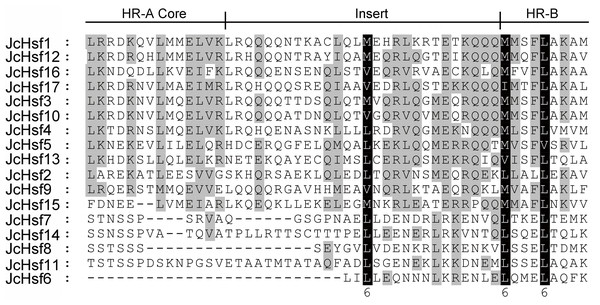
To well investigate the gene structure in the aspects of functional divergence, the conserved motifs/domains of the JcHsfs were analyzed based on the reported information about AtHsfs and OsHsfs (Guo et al., 2008), which contribute to discern a serious about putative functional domains for all JcHsfs. DBD, HR-A/B, NLS, NES, AHA, and RD conserved domains were confirmed in JcHsf proteins (Table 2). Multiple alignment analyses clearly showed that the DBD, most conserved section of JcHsfs, was located in the N-terminal region (Fig. 4). Based on the differences between the HR-A and HR-B regions, three types of Hsfs were identified in the physic nut (Fig. 5).
| Gene name | Group | DBD | NLS | NES | AHA | RD |
|---|---|---|---|---|---|---|
| JcHsf1 | A6 | 41-134 | (226)KDKMK7KKRRR | (347)LVEQLGYL | (319)EGFWDDLLNE | |
| JcHsf2 | A5 | 23-116 | (216)KKRR | (361)LNLTL | (443)DVFWEQFLTE | |
| JcHsf3 | A1 | 44-137 | (242)NRR5KKRRLK | (503)LTEKMGLL | ||
| JcHsf4 | A8 | 12-106 | (205)KENNWR | (298)DSAWEQFLL | ||
| JcHsf5 | A4 | 11-104 | (206)RKRR | (162)MQALKE | (342)DVFWEQFL | |
| JcHsf9 | A3 | 130-223 | (324) RMNRKFVK | |||
| JcHsf10 | A1 | 9-102 | (204)KR6KKRR | (483)LTEQMELL | (432)DVFWEQFL | |
| JcHsf12 | A7 | 41-134 | (227)KDKRK7KKRRR | (345)LAERLGYL | (322)DGFWDELLSE | |
| JcHsf13 | A4 | 12-105 | (203)NKKRR | (161)IMSLCE | (365)DHFWEYFLTE | |
| JcHsf16 | A9 | 138-231 | (320)KRKRQR8KKRR | (447)LELEDL | ||
| JcHsf17 | A2 | 42-135 | (230)KK9KRR | (369)LVDQMGYL | (282)ETLFSAALDD | |
| JcHsf7 | B2 | 21-114 | (257)KRGR | (249)LFGV | ||
| JcHsf8 | B3 | 27-120 | (179)KRKCK | (207)PKLFGVRL | (209) LFGV | |
| JcHsf11 | B1 | 7-100 | (264)KTKKRGR | (254)FKLFGVLL | (256) LFGV | |
| JcHsf14 | B2 | 27-120 | (282)KRMRK | (274) LFGV | ||
| JcHsf6 | B4 | 34-140 | (208)NKIRRL | |||
| JcHsf15 | C1 | 7-100 | (184)KKRR |
Figure 4: Multiple sequence alignment of the DBD domains of 17 members of the JcHsf protein family.
Multiple alignment results clearly revealed highly conserved DBD domains among physic nut Hsf genes.Figure 5: Multiple sequence alignment of the HR-A/B regions of the Hsf protein family in physic nut.
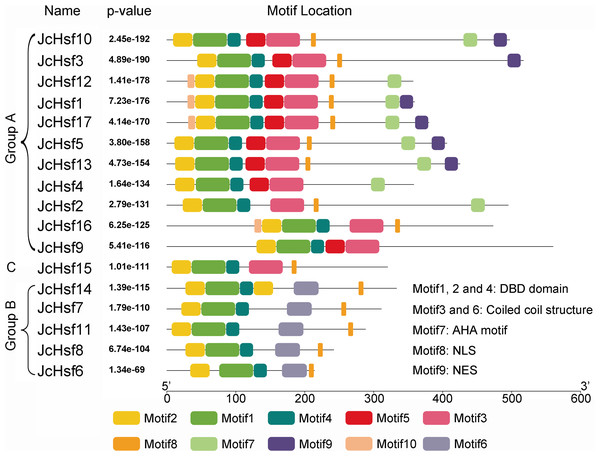
The scheme at the top depicts the locations and boundaries of the HR-A core, insert, and HR-B regions within the HRA/B regions.The motif of JcHsf protein was also analysis using the MEME motif search tool (Fig. 6). Among the ten detected motifs, motifs 1, 2, and 4 including the highly conserved DBD existed in all the JcHsfs (Fig. 7). The motif 6 representing the HR-A/B region was found in all class B JcHsfs, while which was replaced by motif 3 in classes A and C. Most JcHsf proteins contain Motifs 8 representing NLS except JcHsf4 and JcHsf9. Six proteins in the subgroup A1, A2, A4 and A6 had motif nine which consisted of NES. In additionally, motifs 10 containing AHA were discovered in the C-terminus of most groups A JcHsfs. Moreover, some unknown motifs were also detected in this study.
Figure 6: Distribution of conserved motifs in the JcHsf family members.
The names of all members of the gene clusters defined and the combined P values are shown on the left side of the figure, while motif sizes are indicated at the bottom of the figure. Different motifs are indicated by different color numbered 1–10. For details of motifs, refer to Table S3.Figure 7: Motif logo, the amino acid composition of each conserved motif.
Amino acid sequences of each motif identified by MEME tools. The font size indicates the frequency of the corresponding amino acid. Motif location and combined E-value are shown.Expression profiles of JcHsf genes in different tissues
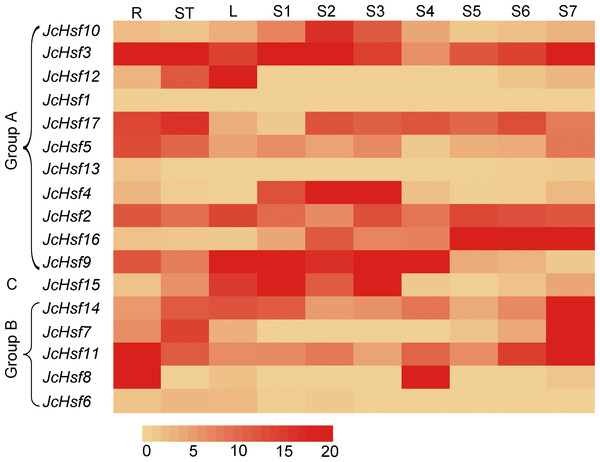
To gain insight into the function of JcHsf genes, their expression patterns in different tissues were analyzed. Results showed that JcHsf1 was not expressed in all the tissues, JcHsf6 and JcHsf13 with low expression levels were shown in different tissues and developing seeds. Of the other fourteen JcHsf genes, four (JcHsf3, 5, 11 and 8), two (JcHsf7, 17) and three (JcHsf2, 9 and 12) showed the highest expression level in the root, stems and leaves, respectively. Four (JcHsf4, 9, 10 and 15) showed a much higher expression level in the seed early developing stage (S1–S4). Six (JcHsf2, 11, 14 and 16) showed much higher expression level in the seed late developing stage (S5–S7). Two (JcHsf3 and 17) showed sustained high expression throughout the seed development stages (Fig. 8).
Figure 8: Expression analysis of the JcHsf genes in different tissues.
R: roots, St: stem cortex, L: leaves S1–S7 correspond to seven different developing physic nut seeds: on 14, 19, 25, 29, 35, 41, and 45 days after pollination. The color scale represents signal values.Expression profiles of JcHsf genes under drought and salt stress
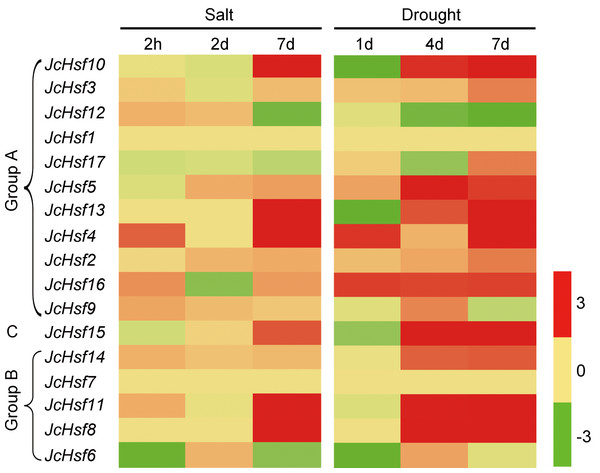
In order to identify the expression pattern of JcHsf genes in response to salt and drought stress, the reported expression profile data of these genes in leaves 2 h, 2d and 7d under salt stress (Zhang et al., 2014) and 1d, 4d and 7d under drought stress (Zhang et al., 2015b) were analyzed (Fig. 9). The results showed that six genes (JcHsf4, 8, 10, 11, 13 and 15) were significantly up-regulated and two genes (JcHsf 6 and 12) were down regulated in both salt and drought stress condition. In addition, the other six genes (JcHsf2, 3, 5, 14, 16, 17) highly up-regulated in response to drought were also identified.
Figure 9: Expression analysis of JcHsf genes in leaves under salt and drought stress.
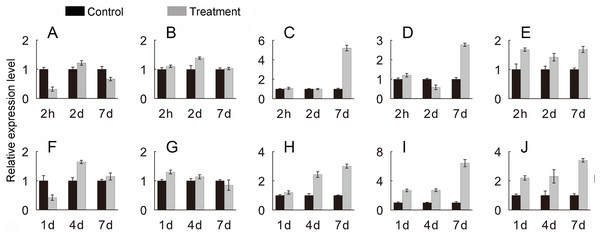
Log2 based values (signal values for treatment/signal value for control) were used to create the heat map based on transcriptomic data for Hsfs. The color scale represents signal values.To verify the JcHsf genes expression pattern from the RNA-seq data, the qRT-PCR were performed to detect the transcriptional level of group B JcHsf (6, 7, 8, 11 and 14) in leaves under salt and drought stress (Fig. 10). The results were basically consistent with the changes in the expression of RNA-seq, indicating that the data of RNA-seq were generally accurate.
Figure 10: Expression level of selected JcHsf genes.
qRT-PCR analysis of selected genes in leaves after salt and drought treatments at different time points. (A) JcHsf6 under salt stress. (B) JcHsf7 under salt stress. (C) JcHsf8 under salt stress. (D) JcHsf11 under salt stress. (E) JcHsf14 under salt stress. (F) JcHsf6 under drought stress. (G) JcHsf7 under drought stress. (H) JcHsf8 under drought stress. (I) JcHsf11 under drought stress. (J) JcHsf14 under drought stress. Relative expression was normalized with JcActin as the reference gene. Bars show standard deviations of the repeats. The experiment involved three biological replicates, each with two technological replicates.Discussion
Physic nut has been emerged as a renewable resource for biodiesel production, which can be grown on the barren soil (King et al., 2009; Divakara et al., 2010). Until now, little is known about the response mechanism to abiotic stress in physic nut. More and more evidence suggests that Hsfs play central roles in plant developmental and response to abiotic stresses (Lin et al., 2018; Lohani et al., 2019). However, there have been no reports of any studies on the Hsf genes in physic nut, so it is necessary to investigate the new Hsf genes in physic nut. In this study, 17 Hsf genes were found in physic nut genome (Table 1) (Wu et al., 2015).
Phylogenetic tree suggested that, JcHsf proteins could be divided into three groups (Fig. 2), similar to Arabidopsis and other plants (Guo et al., 2008; Lin et al., 2011; Liu et al., 2016). The Hsf gene numbers of some specific subgroups in physic nut were different from Arabidopsis. For example, the number of subgroup A1, A6 and A7 in physic nut were less than that in Arabidopsis. One possible reason is that during the early stages of evolution, JcHsf genes have not yet experienced chromosome fragment replicate events (Wu et al., 2015). Another possible reason is that the Arabidopsis may acquire the Hsf genes while the physic nut lost it from their common ancestor (Wu et al., 2015).
Gene’s structure analyses indicated that all Hsf genes were found to contain only one intron (Fig. 3). Although physic nut Hsf genes shared same intron number, the intron length differed across the groups, which revealed the very highly conserved exon/intron splicing arrangement. Notably, JcHsf4 and JcHsf7 in the subgroup A1 has a long intron which made them different from other JcHsf gene members because of their larger size (9,741 bp and 7,519 bp respectively). In general, the gene structure of members in the same class has the similar domain or motif. In this study, although motif 3 and 6 were included in coiled coil structure, but motif 3 was only presented in group A and C, while motif 6 was just detected in group B (Fig. 6). These motifs were specific to some group, which are awaited for participating in group-specific function. Additionally, there are no AHA motifs required for transcriptional activity in the three members of group A (JcHsf3, JcHsf9 and JcHsf16). Previous studies showed that these proteins may gain function through binding to other menbers of group A to form hetero-oligomers (Guo et al., 2008).
Gene’s expression profiles are often related to their functions (Guo et al., 2008). In the present study, the expression profiles of each JcHsf gene in roots, stems, leaves and developing seeds were investigated (Jiang et al., 2012). Most JcHsfs were detected highly expressed in different tissues and developing seeds. It illustrated that Hsf genes are critical regulators involved in plant growth and development (Fig. 8). In addition, JcHsf10 expression was highest in S2 stage of developing seeds (Fig. 8); its homolog AtHsfA1b was reported to regulate multiple developmental genes under heat stress in Arabidopsis (Albihlal et al., 2018). Therefore, it can be inferred that, at the early stage of developing seed; high level of JcHsf10 may take part in regulating seed development of physic nuts under heat stress.
Studies on genome-wide expression analysis of different plants showed that the expression level of some Hsf genes changed in response to different abiotic stresses (Mittal et al., 2009; Guo et al., 2015; Dossa, Diouf & Cisse, 2016; Liu et al., 2016; Lohani et al., 2019). Consistent with this, 14 JcHsf genes out of 17 were up- or down-regulated under at least one stress condition (Fig. 9). Among these 14 genes, 11 genes showed at least 2 fold up- or down-regulated in at least one-time point in response to both salt and drought stimuli (Fig. 9), which is consisted with previous studies on Hsfs in various plant species (Chauhan et al., 2011; Li et al., 2014; Dossa, Diouf & Cisse, 2016). Overexpression Arabidopsis HsfA1a had a positive effect on tolerance to various stressors by acting the inducible heat shock protein expression (Qian et al., 2014). Its physic nut homolog JcHsf3 was highly expressed in all tested tissues (Fig. 8) and up-regulated significantly at 7d in leaves after drought stress (Fig. 9), suggesting a role response to drought stress. AtHsfA2 is essential for acquiring thermotolerance in Arabidopsis (Charng et al., 2007), and its homolog JcHsf17 presented high level in roots, stems and after S2 stage of developing seeds (Fig. 8), and also up-regulated significantly at 7d in leaves after drought stress (Fig. 9). The result suggests that JcHsf17 may play roles in both thermo and drought tolerance in physic nut. In addition, AtHsfB1 and AtHsfB2b, took part in regulating the expression of defensin gene Pdf1.2, plays an important role in pathogen resistance (Kumar et al., 2009), and their homolog in physic nut were JcHsf11 and JcHsf14, respectively. JcHsf11 and JcHsf14, both classified into group B, presented similar expression pattern under normal and abiotic condition (Figs. 8 and 9). It indicated that JcHsf11 and JcHsf14 may involve in pathogen resistance in physic nut. In brief, we speculate that JcHsf genes may participate in many aspects of the developmental process in physic nut, and their roles deserve further study.
Conclusions
In summary, 17 JcHsf genes were identified from the physic nut genome, and gene information provided, including chromosomal localization, phylogenetic and structure analysis. Most JcHsf genes showed a differential expression pattern, indicating the great significance of JcHsf in plant development and stress response. Although the exact function of the JcHsf genes cannot still be indicated, this study lays a foundation for further study on its potential role in regulating developmental process and stress response in physic nut.