Rediscovery of Osteocephalus vilarsi (Anura: Hylidae): an overlooked but widespread Amazonian spiny-backed treefrog
- Published
- Accepted
- Received
- Academic Editor
- Tatiana Tatarinova
- Subject Areas
- Ecology, Taxonomy, Zoology
- Keywords
- Amphibia, Amazonia, Brazilian biodiversity, Integrative taxonomy, Advertisement call, Tadpoles, White-sand ecosystems
- Copyright
- © 2019 Ferrão et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Rediscovery of Osteocephalus vilarsi (Anura: Hylidae): an overlooked but widespread Amazonian spiny-backed treefrog. PeerJ 7:e8160 https://doi.org/10.7717/peerj.8160
Abstract
Osteocephalus vilarsi (Melin, 1941) is an Amazonian treefrog species known for over 75 years from its holotype only. Due to a lack of published data on its morphological diagnostic characters and their variations, as well as the absence of molecular, acoustic and ecological data supporting its identity, a highly dynamic taxonomic history has led this species to be confused and even synonymised with other Osteocephalus species from distinct species groups. The molecular phylogenetic relationships of O. vilarsi were investigated based on recently collected specimens from eight Northwestern Brazilian localities in the state of Amazonas, leading to its removal from the Osteocephalus taurinus species group and placement in the Osteocephalus planiceps species group. Furthermore, detailed data on morphology and colour variation are provided, as well as advertisement call and tadpole descriptions. Finally, the currently known geographic range of O. vilarsi is considerably extended, first data on the natural history of the species are provided, and the possible ecological preference of O. vilarsi for Amazonian white-sand forests is discussed.
Introduction
The spiny-backed treefrogs belonging to the genus Osteocephalus Steindachner, 1862 are medium to large-sized arboreal frogs inhabiting primary and secondary forests in the vast area of the Orinoco and Amazonian basins (Frost, 2019). They are distributed from Colombia, Venezuela and the Guyanas to Bolivia and Brazil (Jungfer et al., 2013) and their altitudinal range varies from sea level in Venezuela, the Guianas, Brazil (e.g. O. taurinus Steindachner, 1862) to up to 2,200 m in Ecuador (e.g. O. festae Peracca, 1904). With 24 currently recognised species, the genus Osteocephalus represents the most specious genus of the subfamily Lophyohylinae Miranda-Ribeiro, 1926 (Frost, 2019). The increasing attention paid to spiny-backed treefrog systematics and biogeography in the last decade (Moravec et al., 2009; Jungfer, 2010; Ron et al., 2010, 2012; Hoogmoed, 2013; Jungfer et al., 2013, 2016; Moravec et al., 2016) has significantly improved our knowledge on Osteocephalus phylogeny, resulting in nomenclatural and biogeographical corrections, descriptions of new taxa and the definition of new candidate species. Among the presently accepted species, O. vilarsi (Melin, 1941) is known only from its holotype and represents the most enigmatic species of Osteocephalus (Jungfer et al., 2013; Frost, 2019).
Osteocephalus vilarsi was originally described as Hyla (Trachycephalus) vilarsi, based on a gravid female collected by indigenous people at Missão Taracuá, in 1924. This mission is located on the right bank of the upper Uaupés River (in the state of Amazonas, Brazil, 00°07′56′N, 68°33′03″W, ca 100 m a.s.l.), a tributary of the Negro River (Caldwell, Lima & Keller, 2002). Melin (1941) highlighted the similarity between O. vilarsi and O. taurinus, but, nevertheless, differentiated the new species only from O. planiceps Cope (1874). O. vilarsi has experienced taxonomic problems since its description. First, the species was synonymised with O. taurinus by Bokermann (1966), without further justification. A few years later, Cochran & Goin (1970) directly compared two specimens of O. leprieurii (Duméril & Bibron, 1841; MCZ 28042, CNHM 69716) from Colombia to the holotype of O. vilarsi and considered the latter name a junior synonym of O. leprieurii. Trueb & Duellman (1971) disagreed with the allocation of O. vilarsi in the synonymy of O. leprieurii and argued that O. vilarsi was a junior synonym of O. taurinus based on the fact that both species share moderately exostosed dermal roofing bones, distinctly elevated lateral frontoparietal edges and spots on the throat, chest and flanks. O. vilarsi was considered the junior synonym of O. taurinus for 30 years, until its resurrection as a valid species by Jungfer (2010). In his study, Jungfer redescribed in detail the holotype of O. vilarsi and compared it to other Osteocephalus species, mainly O. taurinus, O. planiceps and O. leprieurii. In addition, Jungfer (2010) highlighted that specimens reported as O. planiceps by Gordo & Neckel-Oliveira (2004) from the Jaú National Park on the lower Negro River (the only Brazilian record for O. planiceps) may represent O. vilarsi instead of O. planiceps.
In a taxonomic and systematic revision based on molecular and morphological data, Jungfer et al. (2013) proposed and defined five Osteocephalus species groups. Due to external morphology similarities (genetic data were not available for O. vilarsi) these authors tentatively associated O. vilarsi to the O. taurinus species group. In the same study, Jungfer et al. (2013) revealed nine candidate species for the Osteocephalus genus, including O. planiceps (Ca1_Neblina411). This candidate species is known from one specimen collected in Venezuela, close to the Brazilian border (Neblina Base Camp, Rio Mawarinuma, Amazonas; ca 140 m a.s.l.). Neblina Base Camp is located ca. 275 km east of Missão Taracuá, the type locality of O. vilarsi. The vegetation of both localities is characterised as a white-sand forest (Adeney et al., 2016) and both the holotype of O. vilarsi and the specimen of O. planiceps (Ca1_Neblina411) (sensu Jungfer et al., 2013) were collected at a similar elevation. With regard to this, it is interesting that specimens of O. planiceps reported from Jaú National Park (Gordo & Neckel-Oliveira, 2004), supposed to be O. vilarsi (Jungfer, 2010), were also collected in a white-sand forest.
In the frame of herpetological surveys of white-sand forest areas in the vicinity of the municipality of Novo Airão (west bank of Negro River, in the state of Amazonas, Brazil) carried out from 2015 to 2018, adults, juveniles and tadpoles belonging to a species of Osteocephalus morphologically corresponding to the description of O. vilarsi and genetically clustering with O. planiceps CA1 (sensu Jungfer et al., 2013) were collected. After morphological comparison of these specimens to the holotypes of O. planiceps and O. vilarsi, as well as to museum specimens of other Osteocephalus species, we attributed these individuals to O. vilarsi. Subsequently, we confirmed our determination by comparison of mitochondrial DNA sequences from these specimens with the DNA of individuals collected directly at the type locality of O. vilarsi.
Further revision of available Osteocephalus collections revealed that the specimens of Osteocephalus reported from Jaú National Park by Gordo & Neckel-Oliveira (2004) actually represent O. vilarsi, and that additional voucher specimens of this species from the Park territory are available. Moreover, specimens have already been sampled at several other localities in the west and north regions of the Negro River (Amazonas, Brazil; see Material and Methods and Fig. 1). Recently, the name O. vilarsi was used for specimens collected in Santa Isabel do Rio Negro area (Amazonas, Brazil) by Menin et al. (2017), but without providing any data on the morphology of these frogs or any explanation concerning the determination method. Examination of the respective voucher specimens (see Appendix 1 in Menin et al., 2017) revealed that all seven specimens listed as O. vilarsi represent a species belonging to the O. taurinus group. Interestingly, only one actual specimen of O. vilarsi (CZPB-AA 239) from Santa Isabel do Rio Negro material exists, but it was wrongly determined as O. planiceps (in addition, the second specimen listed by the above authors as O. planiceps, CZPB-AA 240, was determined incorrectly and represents a species belonging to the O. taurinus group).
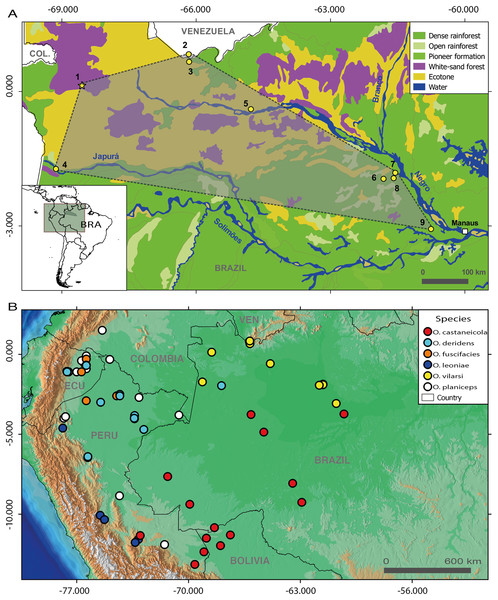
Figure 1: Geographic distribution of members of the Osteocephalus planiceps species group.
(A) Distribution of O. vilarsi in north-western Brazil and southern Venezuela on a vegetation background map. (B) Distribution of all species of O. planiceps species group on an altitudinal background map according to Jungfer et al. (2013), GBIF (www.gbif.org) and own unpublished records. Localities in map A (all in the state of Amazonas, Brazil, except for locality 2 in the Amazonas Department, Venezuela): 1, Missão Taracuá; 2, Venezuelan slope of Pico da Neblina; 3, Brazilian foothills of Pico da Neblina; 4, left bank of Japurá River; 5, Ayuanã River, Santa Isabel do Rio Negro; 6, Miratucu Lake, Jaú National Park; 7, Seringalzinho Village, Jaú National Park; 8, Jaú National Park; 9, Rio Negro Sustainable development reserve. A star denotes the type locality of O. vilarsi (Missão Taracuá). Vegetation types according to Instituto Brasileiro de Geografia e Estatística (1992).In this study, we provide a detailed phylogeny of Osteocephalus and report detailed data on morphological variation and colouration of O. vilarsi, also describing its advertisement call and tadpoles. Finally, we extend the known range of O. vilarsi, provide first data on its natural history, and discuss the possible association of this species with Amazonian white-sand forests.
Materials and Methods
Examined material
The examined material attributed to O. vilarsi consists of 29 adults, two juvenile specimens and 10 tadpoles collected by different researchers from eight Brazilian localities in the state of Amazonas (for details see Fig. 1A; Table S1). This material includes three adult females from the type locality of O. vilarsi (Missão Taracuá) (Appendix I). In addition, we analysed DNA sequences of the only known Venezuelan specimen (AMNH 131254) collected in the Department of Amazonas close to the Brazilian border (Fig. 1A; Table S2). This individual was first reported as O. leprieurii by Faivovich et al. (2004) and, later, as new candidate species O. planiceps (Ca1_Neblina411) by Jungfer et al. (2013).
Specimens of O. vilarsi collected by us in the Brazilian localities Novo Airão and São Gabriel da Cachoeira were recorded at night through visual encounters. Tadpoles were collected in a temporary shallow pond on white-sand soil at Rio Negro Sustainable Development Reserve (RDS Rio Negro). Adults were anesthetised and killed with a topic 2% Benzocaine solution, fixed in 10% formalin and stored in 70% alcohol. Tadpoles were killed with a 5% lidocaine solution and preserved in 5% formalin. Before fixation of recently collected specimens (RDS Rio Negro and Pico da Neblina), tissue samples from adults and one tadpole were extracted, conserved in pure alcohol and stored at the Laboratory of Albertina Lima and Collection of Genetic Resources at Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Brazil. Adults and tadpoles were deposited at INPA’s zoological collection herpetological section (INPA-H). See Menin et al. (2017) for sampling methods in the municipality of Santa Isabel do Rio Negro.
Specimens were collected under ICMBio/RAN permit (Reg. 659755 Nos. 13777 and 52206-1). ICMBio and RAN are institutions of the Ministry of Environment, Government of Brazil. These permits were subject to approval of all procedures for collecting and euthanizing frogs.
Collections acronyms are as follows: AMNH, American Museum of Natural History, New York, USA; APL, Albertina P. Lima field numbers, INPA, Manaus, Brazil; AJC, Andrew J. Crawford field numbers; CBF, Colección Boliviana de Fauna, La Paz, Bolivia; CFBH, Célio F.B. Haddad field numbers, UNESP, Rio Claro, Brazil; CORBIDI, Centro de Ornitología y Biodiversidad, Lima, Peru; CZPB-AA, herpetological section of the Coleção Zoológica Paulo Bührnhein, Manaus, Brazil; EPN, Escuela Politecnica Nacional, Quito, Ecuador; GGU, Giussepe Gagliardi-Urrutia field numbers at UNAP, Iquitos, Peru; GNM, Göteborg Natural History Museum; INPA-H, herpetological section of the zoological collection of INPA; IRSNB, Royal Belgian Institute of Natural Sciences, Brussels, Belgium; KHJ-F, Karl-Heinz Jungfer field numbers; MAR, Marco Rada field numbers; MCZ-A, Harvard Museum of Comparative Zoology, Cambridge, USA; MG, Marcelo Gordo field number; MHNLS, Museo de Historia Natural La Salle, Caracas, Venezuela; MHNC, Museo de Historia Natural, Universidad Nacional de San Antonio Abad del Cusco, Cusco, Peru; MUSM, Museo de Historia Natural de la Universidad de San Marcos, Lima, Peru; MSH, Marinus S. Hoogmoed field numbers; MTR, Miguel T. Rodrigues field numbers; MZUSP, Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil; NMP-P6V, National Museum, Prague, Czech Republic; QCAZ, Museo de Zoología, Pontifica Universidad Católica del Ecuador, Quito, Ecuador; SMNS, Staatliches Museum für Naturkunde, Stuttgart, Germany; SMS, Sergio Marques de Souza field numbers; TG, Taran Grant field numbers; UA, Universidad de los Andes, Bogotá, Colombia.
Species identification
The collected specimens were attributed to O. vilarsi on the basis of morphological comparison with the female holotype of O. vilarsi (NHMG 488) through photographs and data presented in its redescription available in Jungfer (2010). Apart from that, only two species of Osteocephalus occurring in Missão Taracuá (the type locality of O. vilarsi) possess frontoparietal ridges on the head: O. vilarsi and the morphologically clearly different O. taurinus. In our phylogeny, individuals of O. vilarsi from Missão Taracuá clustered with all individuals attributed to O. vilarsi from other localities. The intraspecific genetic distance among them was very short even between specimens from geographically distant localities.
DNA extraction and phylogenetic analyses
Genomic DNA was extracted from 11 samples of O. vilarsi: three adults from the type locality (Missão Taracuá), three adults and one juvenile from the Brazilian foothills of the Neblina mountain range, three adults and one tadpole from RDS Rio Negro. Additionally, we extracted genomic DNA from one specimen of O. taurinus from Missão Taracuá and one tadpole of O. taurinus from northern Purus-Madeira Interfluve (Amazonas, Brazil). Extractions were obtained through the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA), following manufacturer protocols. Primers 16Sar and 16Sbr (Palumbi et al., 1991) were used to amplify a ~553 (476–588) bp-long fragment of the 16S rRNA mitochondrial gene. Polymerase chain reaction (PCR) protocols included a reaction mix with final volume of 15 μL, containing 2.0 μL ddH2O, 1.5 μL 25 mM MgCl2, 1.5 μL 10 mM dNTPs (2.5 mM of each dNTP), 3 μL 5X amplification buffer (75 mM Tris HCl, 50 mM KCl, 20 mM (NH4) 2SO4), 1.5 μL 2 μM solution of each primer, 0.3 μL Taq DNA polymerase 5 U/μL (Biotools, Madrid, Spain) and 1.5 μL of genomic DNA (about 30 ng/μL). Fragment amplification involved a pre-heating step at 73 °C for 60 s, followed by 35 denaturation cycles at 94 °C for 10 s, primer annealing at 50 °C for 35 s and an extension at 72 °C for 90 s, followed by a final extension step at 72 °C for 10 min. Sequencing reactions were carried out after PCR purification using exonuclease and thermosensitive alkaline phosphatase, following the manufacturer’s recommendations (Thermo Fisher Scientific, Waltham, MA, USA) and followed by the use of aABI BigDye Terminator Cycle Sequencing Kit following the manufacturer’s instructions. Forward and reverse primers were used in the sequencing reactions (annealing temperature of 50 °C) and resolved on an ABI 3130xl automatic sequencer. Sequences were manually verified at Geneious (Kearse et al., 2012).
In order to infer the phylogenetic relationships of O. vilarsi, we selected 80 sequences of 16S, 81 sequences of 12S rRNA (12S), 43 sequences of NADH dehydrogenase subunit 1 (ND1), 30 sequences of cytochrome oxidase I (COI) and 27 sequences of cytochrome b (CYTB) from GenBank corresponding to 81 specimens of Osteocephalus and four specimens of Dryaderces. The final matrix was composed by 98 terminals representing all nominal species of Osteceophalus (except O. duellmani) and two candidate species revealed by Jungfer et al. (2013) plus two specimens of Dryaderces pearsoni (Gaige, 1929) and two specimens of Dryaderces sp. CA1 used as outgroups (Table S2). Nuclear markers were not included in the dataset due to the absence of available sequences for approximately 90% of the analysed samples.
Sequences of each marker were aligned separately in Bioedit (Hall, 1999) using the ClustalW algorithm (Thompson, Higgins & Gibson, 1994) and manually checked. Alignments were concatenated in MESQUITE 3.5 (Maddison & Maddison, 2018) and the final matrix was constituted of 4382 bp. PartitionFinder 2.1.1 (Lanfear et al., 2017) was used to infer the best nucleotide evolution model and partitions schemes through PhyML (Guindon et al., 2010) and Bayesian Information Criterion. The best-fit partition scheme and model evolution are displayed in Table 1.
| Schemes | Best model | Subset partitions | Subset alignment |
|---|---|---|---|
| 1 | GTR+I+G | 12S, 16S, ND1\1, CYTB\2 | 1–950, 951–2383, 3037–3997, 3999–4382 |
| 2 | K80+I+G | COI\1 | 2384–3036 |
| 3 | HKY+I | COI\2, ND1\2, CYTB\3 | 2385–3036, 3038–3997, 4000–4382 |
| 4 | GTR+I+G | COI\3, ND1\3, CYTB\1 | 2386–3036, 3039–3997, 3998–4382 |
Note:
Numbers after slashes represent codons of protein coding markers.
The phylogenetic relationship was reconstructed using Bayesian Inference (BI). The BI tree was inferred in MrBayes 3.2.6 (Ronquist et al., 2012) using four runs of 10 million generations with a Metropolis-coupled Markov chain Monte Carlo algorithm. Each run comprised four Markov chains, with probabilities sampled every 1,000 generations. Convergence among runs and model stationarity parameters were verified using Tracer 1.7 (Rambaut et al., 2018). Tree files were combined in LogCombiner 1.8.4 (Drummond et al., 2012) after discarding 25% of the trees. The majority rule consensus tree was built using TreeAnnotator 1.8.4 (Drummond et al., 2012). The average Kimura-2-parameter (K2P) (Kimura, 1980) and uncorrected pairwise genetic distances between O. vilarsi and the remaining dataset of Osteocephalus were estimated using MEGA 6.0 (Tamura et al., 2013).
Adult morphology
Sex and maturity were inferred through the presence or absence of secondary sexual characters (e.g. vocal sac, vocal slits, skin texture, nuptial excrescences on prepollex, presence of eggs). Measurements are given in mm and were taken to the nearest 0.1 mm using a dissecting microscope and a digital calliper. Nine measurements were taken according to Duellman (1970) as follows: snout–vent length (SVL), head length (HL: distance from the posterior edge of the jaw articulation to the tip of the snout), head width (HW at jaw angle), horizontal eye diameter (ED), tibia length (TL), horizontal tympanum diameter (TD), minimal interorbital distance (IOD), upper eyelid width (UEW), eye-nostril distance (EN). One measurement was taken following Heyer et al. (1990): thigh length (THL). In addition, disc width on Finger III (3FD) and Toe IV (4TD) were also measured.
Some Osteocephalus descriptions and redescriptions (Ron & Pramuk, 1999; Jungfer & Lehr, 2001; Jungfer, 2010; Jungfer et al., 2016) have stated that foot length followed Duellman (1970), who stated FL as ‘the distance from the proximal edge of the inner metatarsal tubercle (the large tubercle at the base of the first toe) to the tip of the longest (fourth) toe (including the disc)’. However, the FL is always longer than TL in those studies, which is not consistent with Duellman’s method. In our study, FL was taken from the tip of Toe IV to the heel, in order to make this measurement comparable to those provided in recent Osteocephalus studies. Webbing formulae follow the standards of Savage & Heyer (1967) as modified by Myers & Duellman (1982). Colour in life was described based on field notes and on digital photographs.
Morphological statistics
Although O. vilarsi and O. planiceps are not sister species according our phylogenetic results and the phylogeny published by Jungfer et al. (2013), these species are morphologically similar. Due to that, we performed a Principal Component Analyses (PCA) with morphometric data in order to investigate if the morphometric multivariate space occupied by these two species overlap. The PCA was performed with 12 morphometric ratios (HL/SVL, HW/SVL, IOD/SVL, EN/SVL, ED/SVL, UEW/SVL, TD/SVL, 3FD/SVL, 4TD/SVL, THL/SVL, TL/SVL, FL/SVL). As the SVL seems an important morphological character to differentiate species of Osteocephalus, we also included the SVL in the PCA. Analyses were conducted separately for adult males and females to avoid biases attributed to sexual dimorphism. PCAs were implemented in R environment (R Core Team, 2018) through the function ‘prcomp’ of the package stats. We set the parameters ‘scale’. and ‘center’ as ‘TRUE’ to scale and centre morphometric variables, respectively. The numbers of retrieved Principal Components (PCs) were determined through Broken Stick Model. PCA graphs were obtained trough the function ‘autoplot’ of the package ggplot2 (Wickham, 2016). Adult specimens of O. planiceps from Peru and Ecuador housed in the MCZ-A and NMP-P6V collections were used in the PCA and measured as described for O. vilarsi. Morphometric measurements of both species are available in Table S3. Results are presented in the subsection Comparaisons.
Tadpoles
The developmental stages of 10 tadpoles were determined according to Gosner (1960). Eight morphometric measurements were taken according to Altig & McDiarmid (1999) as follow: total length (TL); body length (BL); tail length (TAL); maximum tail height (MTH); tail muscle height (TMH); tail muscle width (TMW); internarial distance (IND); interorbital distance (IOD). Three measurements were taken following Randrianiaina et al. (2011): maximum body width (BW); maximum oral disc width (ODW) and size of the dorsal gap of marginal papillae (DG). Morphometric measurements were taken to the nearest 0.1 mm with a micrometer coupled to a dissecting microscope. Tadpole descriptions follow Schulze, Jansen & Köhler (2015) and were based on five tadpoles in Gosner stage 36. Colour was described based on one tadpole raised in laboratory until metamorphosis.
Bioacoustics
Advertisement calls of O. vilarsi were recorded at two localities in the state of Amazonas, Brazil: (1) RDS Rio Negro, located in the municipality of Novo Airão: calls of one male were recorded using an Olympus LS-14 Linear PCM Recorder on 24 and 25 October 2017 (air temperature 25–26 °C); (2) Seringalzinho Village, Jaú National Park, located in the municipality of Novo Airão: calls of two males were recorded with a Sennheiser ME66 external microphone in combination with a Marantz PMD 670 recorder on 25 April 2000. Air temperature at the moment of recording was not determined. Both recordings were made at a sampling rate of 44.1 kHz, sample size of 16 bits and stored in wave format. Calls of O. vilarsi were housed at the SAPOTECA, an advertisement call repository of the Research Programme on Biodiversity (PPBIO/INPA), Manaus, Brazil. Calls can be requested at https://ppbio.inpa.gov.br/en/sapoteca/contact.
Temporal and spectral parameters of 23 calls were measured, seven from the male recorded at RDS Rio Negro (not collected) and 16 of the two males recorded at Seringalzinho Village (INPA-H 40455 = 11 calls; INPA-H 40467 = five calls). Calls were analysed using Raven 1.5 (Bioacoustics Research Programme 2015) through a Blackman window, 3 dB Filter Bandwidth of 80 Hz, overlap of 80%, hop size of 4.1 ms, and DFT size of 2,048 samples. Oscillograms were used to measure the call, note and pulse duration and the inter-note and inter-pulse interval. The number of notes, pulses per notes and harmonics were counted through spectrogram visualisations. The dominant frequency of pulses in each note was determined from power spectrum graphs through the function Peak Frequency. The Seewave package version 2.0.5 (Sueur, Aubin & Simonis, 2008) was used to generate advertisement call figures. Seewave was set up as follows: Hanning window and 512 resolution points (FFT). The note-centered approach (Köhler et al., 2017) was used to describe the advertisement call. Call terminology follows Köhler et al. (2017).
Although the advertisement call of O. planiceps has been described by Ron & Pramuk (1999), we analysed 19 advertisement call from three males of this species from Ecuador in order to obtain the same acoustic parameters measured for O. vilarsi in the present study. Advertisment calls of O. planiceps were downloaded from Anfibios del Ecuador (https://bioweb.bio/faunaweb/amphibiaweb/: Ron, Merino-Viteri & Ortiz, 2019). Recordings were made by Morley Read in: (1) Reserva Biológica Jatun Sacha, Provincia Napo; (2) Parque Nacional Yasuní and (3) Pompeya Sur, cerca del río Napo, both in Provincia Orellana.
Results
Molecular phylogenetic analyses
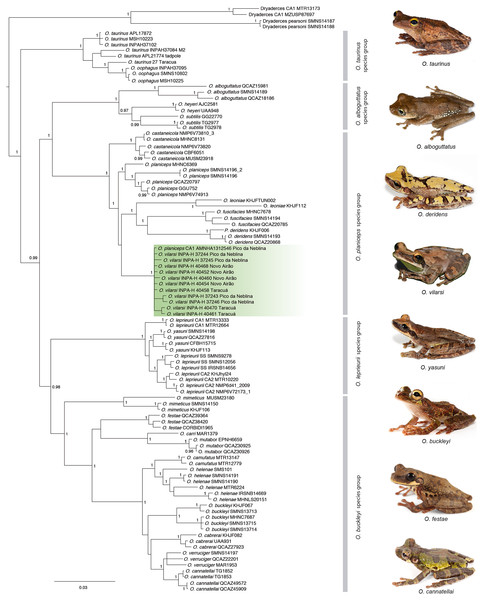
The Bayesian phylogenetic reconstruction based on five mitochondrial markers recovered the genus Osteocephalus as monophyletic (Fig. 2). The five species groups previously determined within Osteocephalus were strongly supported, as well as their evolutionary relationships. The O. taurinus species group is the sister clade to a large clade containing the remaining species groups. Within this large clade, the O. alboguttatus species group is the sister clade to the O. planiceps species group. The O. alboguttatus + O. planiceps species group is sister to the clade containing the O. leprieurii and O. buckleyi species groups (Fig. 2). With few exceptions, the inter-species relationships were highly supported (Fig. 2).
Figure 2: Phylogeny of the genus Osteocephalus based on five mitochondrial markers (12S, 16S, COI, CYTB, ND1; 4382 bp).
Posterior probability values are depicted close to nodes (only values ≥0.95 are shown) and vertical bars denote the distinct species groups within Osteocephalus. Samples corresponding to O. vilarsi are highlighted. Photographs: Santiago Ron, www.bioweb.bio (O. taurinus, O. alboguttatus, O. deridens, O. yasuni, O. buckleyi, O. festae, O. cannatellai), Vinicius Tadeu de Carvalho (O. vilarsi).Our phylogenetic analyses clearly confirmed the status of O. vilarsi as a valid species. All samples related to this species, including those from the type locality, form a strongly supported and monophyletic clade (Fig. 2). In addition, the individual from the Venezuelan slope of Pico da Neblina, previously considered a candidate species related to O. planiceps (Ca1_Neblina411 sensu Jungfer et al., 2013), nested within the same clade as O. vilarsi. Despite the extensive geographic distances between the known localities of O. vilarsi, the average intraspecific K2P distance within this clade was surprisingly low (ca. 0.4%; Table 2).
| Species | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | O. vilarsi | 0.4 | 2.6 | 3.6 | 4.9 | 4.4 | 4.3 | 7.4 | 6.4 | 6.3 | 6.0 | 6.1 | 5.7 |
| 2 | O. planiceps | 2.6 | 1.0 | 2.6 | 4.0 | 4.1 | 4.6 | 6.9 | 5.6 | 6.7 | 6.7 | 6.8 | 6.5 |
| 3 | O. castaneicola | 3.7 | 2.7 | 0.6 | 4.3 | 4.7 | 5.4 | 7.3 | 6.0 | 7.5 | 7.5 | 7.6 | 7.4 |
| 4 | O. leoniae | 5.1 | 4.2 | 4.4 | 3.2 | 5.3 | 6.0 | 9.0 | 7.9 | 8.7 | 8.8 | 8.7 | 8.5 |
| 5 | O. fuscifacies | 4.6 | 4.3 | 4.9 | 5.6 | 1.0 | 5.2 | 8.5 | 7.2 | 7.9 | 7.6 | 8.0 | 7.6 |
| 6 | O. deridens | 4.4 | 4.8 | 5.7 | 6.3 | 5.4 | 0.7 | 8.7 | 7.5 | 9.1 | 9.1 | 9.2 | 9.1 |
| 7 | O. oophagus | 7.9 | 7.3 | 7.8 | 9.7 | 9.1 | 9.5 | 0.1 | 2.1 | 6.7 | 7.1 | 7.0 | 6.6 |
| 8 | O. taurinus | 6.8 | 5.9 | 6.4 | 8.4 | 7.6 | 8.0 | 2.1 | 1.3 | 5.3 | 5.6 | 5.6 | 5.1 |
| 9 | O. leprieurii SS | 6.6 | 7.0 | 7.9 | 9.3 | 8.4 | 9.8 | 7.1 | 5.5 | 0.5 | 1.9 | 1.2 | 1.5 |
| 10 | O. leprieurii CA1 | 6.4 | 7.0 | 8.0 | 9.5 | 8.1 | 9.8 | 7.5 | 5.8 | 1.9 | 0.0 | 1.8 | 1.2 |
| 11 | O. leprieurii CA2 | 6.4 | 7.3 | 8.1 | 9.4 | 8.5 | 10 | 7.4 | 5.9 | 1.3 | 1.8 | 0.5 | 1.4 |
| 12 | O. yasuni | 5.9 | 6.9 | 7.9 | 9.2 | 8.1 | 9.8 | 7.0 | 5.4 | 1.6 | 1.2 | 1.4 | 0.6 |
Contrary to previous hypotheses based on morphology, O. vilarsi is not close related to members of the O. taurinus species group. Instead, the species appears nested within the O. planiceps species group, where it occupies (with low support) a sister position to a strongly supported clade comprising Osteocephalus leoniae Jungfer & Lehr, 2001, Osteocephalus fuscifacies Jungfer, Ron, Seipp & Almendariz, 2000 and Osteocephalus deridens Jungfer, Ron, Seipp & Almendariz, 2000 (Fig. 2). Regarding the interspecific K2P genetic distances within analysed species of the O. planiceps species group (based on 16S gene), O. vilarsi presented the lowest values when compared to O. planiceps (2.6%), and the highest values when compared to O. leoniae (5.1%).
Compared to other Osteocephalus species sympatric with O. vilarsi, the average K2P genetic distances between O. vilarsi and members of the O. planiceps species group were lower than those observed between O. vilarsi and members of the O. leprieurii and O. taurinus groups. The average K2P genetic distance between O. vilarsi and members of the O. leprieurii group ranged from 5.9% to 6.6%, while the distance between O. vilarsi and members belonging to the O. taurinus species group were 6.8% and 7.9% (Table 2). For complete genetic distance values, see Table 2.
Osteocephalus vilarsi (Melin, 1941)
Hyla (Trachycephalus) vilarsi Melin, 1941: p. 40, Fig. 21.
Osteocephalus taurinus: Bokermann (1966), Trueb & Duellman (1971).
Osteocephalus leprieurii: Cochran & Goin (1970); Faivovich et al. (2004); Faivovich et al. (2005); Wiens et al. (2006); Jowers, Downie & Cohen (2008); Lampo, Chacón & Nava (2009); Wiens et al. (2010); Salerno et al. (2012).
Osteocephalus leprieurii B: Fouquet et al. (2007).
Osteocephalus ‘leprieurii’: Moravec et al. (2009); Ron et al. (2012).
O. vilarsi: Jungfer (2010); Jungfer et al. (2013); Frost (2019).
Osteocephalus planiceps: Gordo & Neckel-Oliveira (2004); Menin et al. (2017).
O. planiceps (Ca1_Neblina411): Jungfer et al. (2013).
Holotype
NHMG 488, adult female from Missão Taracuá, right bank of Uaupés River (an upper Negro River tributary), elevation ca. 100 m, Amazonas, Brazil, collected on 7 April 1924 (Melin, 1941; Caldwell, Lima & Keller, 2002). The holotype (as GNM 488) was redescribed and figured by Jungfer (2010).
Amended diagnosis
Osteocephalus vilarsi is a medium-sized species (as defined by Jungfer, 2010) of the O. planiceps species group according to the phylogeny presented in Jungfer et al. (2013) and in the present study. The species can be diagnosed by the combination of the following characters: (1) SVL 47.5–58.4 mm in adult males and 54.6–65.3 mm in adult females; (2) frontoparietal ridges on the head; (3) truncate snout in dorsal view and rounded in lateral view; (4) skin on dorsum of adult males conspicuously tuberculated; (5) vocal slits in males; (6) vocal sac distinct, subgular, moderately expanded laterally to the area between the tympanum and forearm insertion; (7) absence of subdigital nuptial excrescences in breeding males; (8) distal subarticular tubercle on Finger IV bifid; (9) toe length I < II < III < V < IV; (10) adults exhibit white tibiofibular bones, bicoloured iris (upper part bright golden with dark brown veins and fine incomplete dark brown radiation, lower part silver to bronze with dense bold dark brown veins and/or incomplete radiation) and light subocular spot; (11) metamorphs present iris entirely bright red without black reticulation, dorsum and flanks grey with dark grey blotches and spots and absence of reddish orange blotches on hand, elbow, knees, discs, and heels; (12) tadpoles at Gosner stage 36 TL = 33.0–34.5 mm, rounded snout in dorsal view, and LKRF = 2(2)/5–6(1); (13) advertisement call composed by two (169 ± 9 ms (144–180 ms)) or three notes (276 ± 48 ms (162–337 ms)), first note always formed by two pulses, and note duration of single notes lasts 51 ± 9 ms (36–65 ms).
Comparisons
Based on the phylogenetic relationship among O. vilarsi and other Osteocephalus, detailed interspecific comparisons were made with other members of the O. planiceps species group (O. castaneicola Moravec, Aparicio, Guerrero-Reinhard, Calderón, Jungfer & Gvoždík, 2009; O. deridens; O. fuscifacies; O. leoniae; O. planiceps). Due to historical misidentification of O. vilarsi with members of the O. taurinus (O. taurinus; O. oophagus Jungfer & Schiesari, 1995) and O. leprieurii (O. leprieurii; O. yasuni Ron & Pramuk, 1999) species groups, comparisons between O. vilarsi and these species were also provided. Characteristics of the compared species are presented in parentheses.
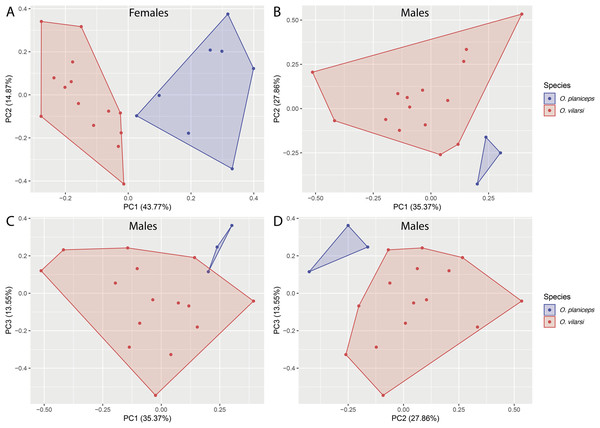
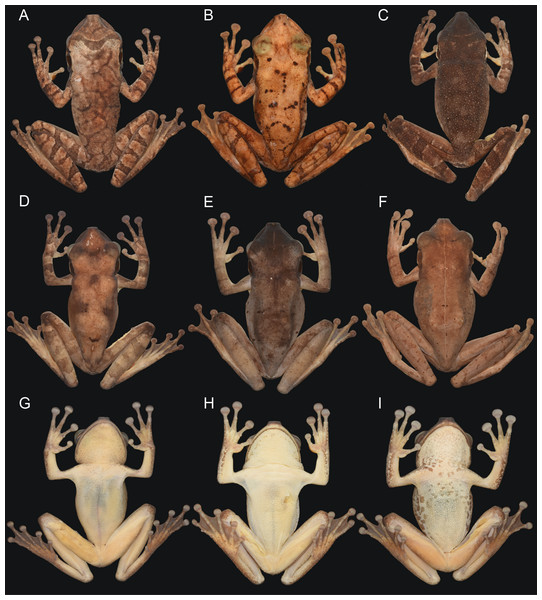
Osteocephalus vilarsi differs from members of the O. planiceps species group, as follows: from O. castaneicola by a truncate snout in dorsal view, the presence of vocal slits in males, vocal sac distinct and subgular, bifid distal subarticular tubercle under Finger IV (rounded snout in dorsal view, absence of vocal slits, vocal sac indistinct, single distal subarticular tubercle under Finger IV); from O. deridens by SVL up to 58.4 mm in adult males and 65.3 mm in adult females, conspicuously tuberculated dorsum in adult males and by white tibiofibular bones (SVL up to 34.9 mm in males and 43.7–50.6 mm in females, dorsal skin of adult males smooth, green tibiofibular bones; Jungfer et al., 2000); from O. fuscifacies by SVL up to 58.4 mm in adult males and 65.3 mm in adult females, a conspicuously tuberculated dorsum in adult males, the presence of a light subocular spot and by white tibiofibular bones (SVL 38.3–45.6 mm in males, adult male dorsum smooth, subocular light spot absent, green tibiofibular bones; Jungfer et al., 2000); from O. leoniae by the relative toe length I < II < III < V < IV, upper part of the iris bright golden with dark brown veins and fine incomplete dark brown radiation and by white tibiofibular bones (relative toe length I < II < III = V < IV, upper part of the iris yellow, green tibiofibular bones; Jungfer & Lehr, 2001); from O. planiceps (Fig. 3) by SVL up to 58.4 mm in adult males and 65.3 mm in adult females (SVL up to 65.4 mm in examined males and 83.2 mm in examined females: Table 3), white tibiofibular bones in life (green tibiofibular bones in life (Figs. 3C–3G) in 42 examined specimens of O. planiceps from Ecuador and 10 from Peru, against only one specimen with white tibiofibular bones in Ecuador). The metamorph of O. vilarsi (Fig. 3D) has an entirely bright red iris without black reticulation (black-reticulated iris, upper portion with red pigmentation on yellow ground and lower iris tan with red pigmentation near the pupil in metamorph of O. planiceps; Fig. 3H). The PCAs showed that the multivariate morphometric space occupied by females of O. vilarsi and O. planieps does not overlap and poorly overlaps in males (Fig. 4). The first two PCs retrieved by the Broken Stick Model in the PCA with females explained approximately 59% of the morphometric variation, while the first three PCs in the male analyses explained 63% of the variation (Fig. 4). The most important morphometric variables contributing to PC1 in female PCA were SVL, UEW, HW and ED. In the male PCA, the most important morphometric variables to PC1 were HW, T4W, TD and FL. See Table 4 for the contribution of all morphometric variables for other PCs. Additionally, the advertisement call of O. vilarsi is different from that of O. planiceps in structural and temporal parameters. The call duration of two-note (169 ± 9 ms (144–180 ms)) and three-note calls (276 ± 48 ms (162–337 ms)) of O. vilarsi is shorter (two-note calls = 229 ± 28 ms (198–259 ms), three-note calls = 402 ± 31 ms (360–465 ms) in O. planiceps); the first note of the advertisement call of O. vilarsi is always composed by two pulses (first note is mostly single (n = 18 or 95%) in O. planiceps). Single-note duration in O. vilarsi lasts 51 ± 9 ms (36–65 ms; n = 16) and is usually shorter than in O. planiceps (80 ± 13 ms (60–108 ms; n = 50)).
Figure 3: Adults and metamorphs of Osteocephalus vilarsi (A–D) and Osteocephalus planiceps (E–H).
(A) Female, INPA-H 40461, from Missão Taracuá, Amazonas, Brazil. (B) Uncollected male, from RDS Rio Negro, Amazonas, Brazil. (C) Male, INPA-H 40459, from RDS Rio Negro, Amazonas, Brazil. (D) Juvenile, from RDS Rio Negro, Amazonas, Brazil. (E) Female, NMP-P6V 71264/2, from Anguilla, Loreto, Peru. (F) Male, QCAZA 148944, from Yasuní National Park, Orellana, Ecuador. (G) Female, QCAZA 44420, from Chiru Isla, North bank Napo River, Orellana, Ecuador. (H) Juvenile, QCAZA 52442, from Cuyabeno Wildlife Reserve, Sucumbíos, Ecuador. Photographs: Vinicius Tadeu de Carvalho (A), Jiří Moravec (B–C, E), Albertina Pimentel Lima (D), Santiago Ron, www.bioweb.bio (F–H).Figure 4: Multivariate morphometric space occupied by Osteocephalus vilarsi and O. planiceps.
Principal Components Analysis (PCA) conducted with the SVL and 12 morphometric ratios of 22 females (A) and 18 males (B–D). The number of plotted PCs for each sex was determined by Broken Stick Model.| Variables | Osteocephalus vilarsi | Osteocephalus planiceps | |||
|---|---|---|---|---|---|
| Males (n = 15) | Females (n = 14) | Holotype | Males (n = 3) | Females (n = 8) | |
| SVL | 54.4 ± 2.7 (47.5–58.4) | 60.1 ± 3.2 (54.6–65.3) | 62.2 | 64.1 ± 1.2 (63.1–65.4) | 72.9 ± 4.9 (66.2–83.2) |
| HL | 18.8 ± 0.9 (17.2–20.2) | 20.7 ± 1.3 (19.0–23.0) | 20.1 | 21.3 ± 0.4 (20.9–21.6) | 24.0 ± 1.8 (21.9–27.8) |
| HW | 18.3 ± 0.9 (16.9–19.9) | 20.0 ± 1.1 (18.5–21.6) | 19.6 | 20.6 ± 0.6 (20.1–21.2) | 23.4 ± 1.5 (22.1–26.8) |
| IOD | 5.0 ± 0.5 (4.1–5.6) | 5.8 ± 0.7 (4.9–6.8) | – | 7.0 ± 0.2 (6.9–7.3) | 8.3 ± 0.8 (7.3–9.3) |
| EN | 5.6 ± 0.5 (4.8–6.5) | 6.6 ± 0.6 (5.6–7.7) | 6.2 | 7.0 ± 0.3 (6.8–7.3) | 8.5 ± 0.8 (7.3–10.0) |
| ED | 5.7 ± 0.3 (5.2–6.2) | 6.3 ± 0.4 (5.9–7.2) | 6.2 | 6.8 ± 0.1 (6.7–6.9) | 6.8 ± 1.0 (5.7–8.5) |
| UEW | 5.5 ± 0.4 (4.7–5.9) | 5.9 ± 0.4 (5.3–6.4) | – | 5.9 ± 0.2 (5.7–6.0) | 6.3 ± 0.8 (5.2–7.6) |
| TD | 4.3 ± 0.4 (3.6–4.9) | 4.8 ± 0.4 (4.2–5.7) | 4.8 | 4.9 ± 0.1 (4.8–5.0) | 5.5 ± 0.3 (5.1–6.1) |
| 3FD | 3.0 ± 0.3 (2.4–3.3) | 3.4 ± 0.3 (3.1–3.9) | 2.3 | 3.7 ± 0.4 (3.4–4.1) | 4.4 ± 0.6 (3.7–5.7) |
| THL | 28.9 ± 1.7 (25.5–32.0) | 32.1 ± 1.9 (28.8–34.8) | 31.6 | 33.9 ± 1.6 (32.3–35.5) | 37.4 ± 3.1 (32.4–42.3) |
| TL | 30.6 ± 1.8 (26.5–33.5) | 34.3 ± 2.2 (30.4–37.9) | 34.5 | 36.3 ± 2.2 (34.4–38.7) | 41.0 ± 2.9 (36.8–46.5) |
| FL | 37.6 ± 2.2 (33.4–41.0) | 42.4 ± 2.7 (38.2–46.2) | 42.0 | 43.8 ± 1.2 (43.0–45.2) | 49.5 ± 3.3 (44.5–55.6) |
| 4TD | 2.6 ± 0.2 (2.2–3.0) | 3.0 ± 0.2 (2.7–3.4) | – | 3.0 ± 0.1 (2.9–3.1) | 3.9 ± 0.5 (3.1–5.0) |
| Variables | Males (n = 18) | Females (n = 22) | |||
|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC1 | PC2 | |
| SVL | 0.245 | −0.325 | 0.339 | 0.355 | 0.033 |
| HL/SVL | −0.321 | 0.294 | 0.191 | −0.306 | −0.121 |
| HW/SVL | −0.392 | 0.226 | 0.011 | −0.308 | −0.112 |
| IOD/SVL | 0.238 | −0.224 | 0.502 | 0.307 | 0.333 |
| EN/SVL | −0.249 | −0.254 | 0.382 | 0.150 | 0.312 |
| ED/SVL | 0.058 | 0.358 | 0.239 | −0.307 | −0.373 |
| UEW/SVL | −0.271 | 0.290 | 0.107 | −0.342 | −0.205 |
| TD/SVL | −0.343 | 0.032 | 0.291 | −0.237 | 0.128 |
| F3W/SVL | −0.180 | −0.342 | 0.238 | 0.257 | −0.311 |
| THL/SVL | −0.284 | −0.251 | −0.306 | −0.269 | 0.176 |
| TL/SVL | −0.129 | −0.385 | −0.265 | −0.164 | 0.364 |
| FL/SVL | −0.335 | −0.301 | −0.195 | −0.231 | 0.431 |
| T4W/SVL | −0.358 | −0.107 | 0.197 | 0.288 | −0.347 |
| Variance (%) | 35.37 | 27.86 | 13.55 | 43.77 | 14.87 |
| Cumulative (%) | 35.37 | 63.23 | 76.78 | 43.77 | 58.65 |
Furthermore, O. vilarsi tadpoles in Gosner stage ≥45 differ from tadpoles of other members of O. planiceps species groups by the presence of dark grey blotches and spots on the dorsum and flanks (dark blotches and spots absent; Moravec et al., 2009; Jungfer et al., 2013).
Osteocephalus vilarsi can be differentiated from all members of the O. taurinus species group by a bicoloured iris, with the upper part bright golden with dark brown veins and fine incomplete dark brown radiation and lower part silver to bronze with dense dark brown veins and/or incomplete radiation and by white tibiofibular bones (iris greenish gold with bold dark brown regular radiation, green tibiofibular bones in O. taurinus and O. oophagus at their type localities; Jungfer & Schiesari, 1995; Lima et al., 2006; Jungfer, 2010). Additionally, males of O. vilarsi differ from those of O. taurinus by having vocal sac distinct, subgular and moderately distensible (vocal sac subgular, paired and strongly distensible). Tadpoles of O. vilarsi at Gosner stage 36 differ from those of O. oophagus at same stage by having TL = 33.0–34.5 mm, rounded snout in dorsal view, and LKRF = 2(2)/5–6(1) (TL = 28.9 mm, nearly truncate snout in dorsal view and LKRF = 2(2)/3 in O. oophagus at its type locality; Jungfer & Schiesari, 1995). Moreover, recently metamorphosed O. vilarsi individuals differ from members of the O. taurinus species group by the absence of reddish orange blotches on hand, elbow, knees, discs and heels (reddish orange blotches present; Lima et al., 2006; Jungfer et al., 2013).
Osteocephalus vilarsi differs from all members of the O. leprieurii species group by the presence of frontoparietal ridges on the head and by the absence of subdigital nuptial excrescences in adult males (frontoparietal ridges absent, subdigital excrescences present in breeding males; Ron & Pramuk, 1999; Jungfer & Hödl, 2002). Moreover, males of O. vilarsi differ from those of O. leprieurii and O. yasuni by having vocal sac distinct, subgular and moderately distensible (vocal sac subgular, paired and distensible ventrolaterally to laterally in O. leprieurii and laterally in O. yasuni; Jungfer & Hödl, 2002; Ron & Pramuk, 1999). Additionally, recently metamorphosed O. vilarsi individuals diffes from those from the O. leprieurii species group by a grey dorsum with dark grey blotches and bright orange iris (brown dorsum with dark brown blotches and greyish iris; Jungfer & Hödl, 2002; Jungfer et al., 2013).
Morphological description of additional adult specimens
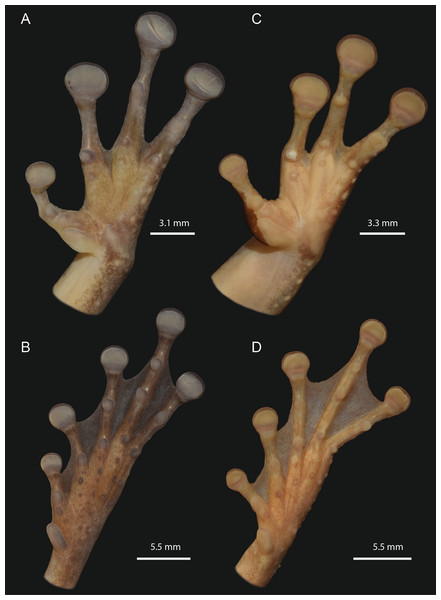
The morphological characteristics of 29 adult specimens of O. vilarsi from eight Brazilian localities are summarised as follows (adult specimen measurements are given in Table 3): medium size, SVL 47.5–58.4 mm in adult males, 54.6–65.3 mm in adult females; skin of dorsal surface with numerous minute low tubercles in females and dense protuberant tubercles bearing keratinised spinous tips in breeding males (Fig. 5); rounded snout in lateral view, truncate in dorsal view; elevated canthus rostralis, sharply angular, medially curved; deeply concave loreal region; low well-marked frontoparietal ridges in adults; large, round to oval tympanum, about 64–76% of the eye diameter, distinct tympanic annulus; markedly developed supratympanic fold; vocal slits present; vocal sac distinct, subgular, moderately expanded laterally to the area between the tympanum and forearm insertion; large, prominent, angular vomerine odontophores, narrowly separated medially, between oblique choanae, bearing 8–16 vomerine teeth each; low ulnar and tarsal tubercles present; axillary membrane present; basal webbing on hand (webbing formula II (2–2+)—3+ III (3––3)—(3––3) IV) (Figs. 6A and 6C); toes about three fourths webbed (webbing formula I (1+–11/2)—( 11/2–2+) II (1–1+)—(11/2–2+) III (1–1+)—(2−–21/2) IV (11/2–2−)—(1–1+) V) (Figs. 4B and 4D); bifid distal subarticular tubercle under the fourth finger (except in two females from the type locality); dark keratinous nuptial excrescences covering the inner prepollex surface and extending laterally up to the thumb disc in males (Fig. 6C).
Figure 5: Dorsal and ventral views of adult females and males of Osteocephalus vilarsi.
(A) INPA-H 37246, male, SVL = 55.5 mm, from the foothills of the Neblina mountain range. (B) INPA-H 40472, male, SVL = 57.2 mm, from Seringalzinho Village, Jaú National Park. (C) CZPB-AA 239, male, SVL = 58.4 mm, from the east bank of Ayuanã River, in the municipality of Santa Isabel do Rio Negro. (D) INPA-H 40461, female, SVL = 61.2 mm, from Missão Taracuá. (E) CZPB-AA 1421, female, SVL = 63.8 mm, from the northern bank of Japurá River. (F) INPA-H 40453, female, SVL = 54.6 mm, from Miratucu Lake, Jaú National Park. (G) INPA-H 40461, female. (H) INPA-H 40468, male, SVL = 55.9 mm, from RDS Rio Negro. (I) CZPB-AA 1421, female. All localities in the state of Amazonas, Brazil. Photographs and plate: Marcelo Gordo & Miquéias Ferrão.Figure 6: Detailed ventral views of the left hand and foot of Osteocephalus vilarsi.
(A and B) INPA-H 40461, adult female, SVL = 61.2 mm, from Missão Taracuá (type locality). (C and D) INPA-H 40455, adult male, SVL = 54.5 mm, from Seringalzinho Village, Jaú National Park. All localities in the state of Amazonas state, Brazil. Photographs and plate: Marcelo Gordo & Miquéias Ferrão.In life (Figs. 7 and 8), adult specimens pale brown, dorsally yellowish brown to reddish brown, with or without a pattern of dark brown to black irregular markings; interorbital stripe and dark brown canthal stripe present (interorbital stripe narrower than the diameter of the eye in all specimens except for female INPA-H 40462, in which a triangular spot is formed); a narrow pale supralabial line expanding in a subocular spot; creamy to yellowish white flanks, with or without irregular brown markings or small spots; light brown hidden thigh surfaces; creamy or yellowish white throat and belly with or without diffuse small pale brown markings or spots; a narrow dark line present along the mandible; fleshy pink to slightly orange ventral thigh surfaces; bicoloured iris with a dark brown horizontal stripe, bright golden above with dark brown veins and fine incomplete dark brown radiation, silver to bronze below with dense bold dark brown veins and/or incomplete radiation (Figs. 7 and 8); white tibiofibular bones (Figs. 7E and 8F).
Figure 7: Colouration in life of females of Osteocephalus vilarsi.
(A) INPA-H 40461, SVL = 61.2 mm, from Missão Taracuá. (B) INPA-H 40470, SVL = 65.3 mm, from Missão Taracuá. (C) Uncollected specimen from the Jaú National Park. (D) Uncollected specimen from RDS Rio Negro. (E) INPA-H 40470. (F) Uncollected specimen from RDS Rio Negro. All localities in the state of Amazonas state, Brazil. Photographs: Vinicius Tadeu de Carvalho (A, B, E), Marcelo Gordo (C), Jiří Moravec (D, F).Figure 8: Colouration in life of males of Osteocephalus vilarsi.
(A) INPA-H 40465, SVL = 55.5 mm, from RDS Rio Negro. (B) Uncollected, from RDS Rio Negro. (C) INPA-H 40459, SVL = 51.1 mm, from RDS Rio Negro. (D) INPA-H 40455, SVL = 54.5 mm, from the Jaú National Park, displaying the lateral projection of the vocal sac between the tympanum and arm. (E) Lateral view of a partially expanded vocal sac in male INPA-H 40465. (F) INPA-H 40459, from RDS Rio Negro. All localities in the state of Amazonas, Brazil. Photographs: Jiří Moravec (A–C, E, F), Marcelo Gordo (D).Sexual dimorphism
In addition to the presence of vocal slits, vocal sac and dark keratinous nuptial excrescences in males, O. vilarsi also exhibits sexual dimorphism of body size and dorsal skin texture (Figs. 5–8). Adult males possess numerous protuberant spinous tubercles distributed on the dorsal surfaces of the head, body and limbs (Figs. 8A–8F). On the other hand, tubercles in adult females are minute and flat (Figs. 7A–7D).
Advertisement call
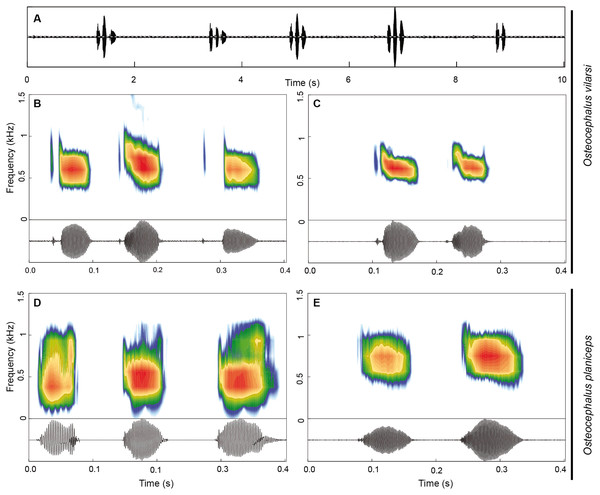
The advertisement call of O. vilarsi (Figs. 9A–9C) exhibits a call duration of 211 ± 61 ms (144–337; n = 23) and consists of notes emitted in pairs (n = 14; common in less motivated males) or trios (n = 9; common in motivated males). The first note of calls is always formed by a pair of pulses (n = 23). The second and third notes consist of one (n = 16) or two pulses (n = 16). Note duration of pulsed and unpulsed notes ranges from 36 to 116 ms (59 ± 13; n = 55), while the note duration of pulsed notes is 45–116 ms (63 ± 13; n = 39) and of the single note is 36–65 ms (51 ± 9; n = 16). The inter-note interval lasts 39–73 ms (54 ± 10; n = 32). The pulse duration of the first pulse in pulsed notes is shorter 3–10 ms (6 ± 1; n = 39) than that of the second pulse 18–75 ms (47 ± 12; n = 39). Pulses are interrupted by a short inter-pulse interval lasting 3–37 ms (9 ± 7; n = 39). The first pulse is tonal while the second pulse contains 1–4 (2 ± 1) harmonics. The dominant frequency is located in the fundamental harmonic. The overall dominant frequency is 474–948 Hz (624 ± 71; n = 91). The first pulse is characterised by a dominant frequency of 560–948 Hz (685 ± 59; n = 36), whereas the dominant frequency of the second pulse is slightly lower, at 474–689 Hz (584 ± 44; n = 55). Bioacoustic parameters of the advertisement call of O. vilarsi and O. planiceps (Figs. 9D–9E) are presented in Table 5.
Figure 9: Advertisement call of Osteocephalus vilarsi and O. planiceps.
(A) Oscillogram of a series of five calls of O. vilarsi from the Jaú National Park, Amazonas, Brazil (INPA-H 40455, SVL = 54.5 mm). Spectrograms (upper graphs) and oscillograms (lower graphs) depicting calls composed by three (B) and two (C) pulsed notes of O. vilarsi from the Jaú National Park (B) INPA-H 40455 and at RDS Rio Negro (C) unvouchered, Novo Airão, Amazonas, Brazil. Spectrograms (upper graphs) and oscillograms (lower graphs) depicting calls of O. planiceps composed by three (D) and two (E) single notes recorded at Pompeya Sur (D), Orellana, Ecuador and at Reserva Biológica Jatun Sacha (E), Napo, Ecuador.| Traits | O. vilarsi (n = 3) | O. planiceps (n = 3) |
|---|---|---|
| Overall call duration (ms) | 211 ± 61 (144–337; n = 23) | 369 ± 134 (108–608; n = 19) |
| Number of notes | 2.4 ± 0.5 (2–3; n = 23) | 2.8 ± 0.8 (1–4; n = 19) |
| Two-note call duration (ms) | 169 ± 9 (144–180; n = 14) | 229 ± 28 (198–259; n = 5) |
| Three-note call duration (ms) | 276 ± 48 (162–337; n = 9) | 402 ± 31 (360–465; n = 10) |
| Four-note call duration (ms) | – | 580 ± 33 (544–608; n = 3) |
| Overall note duration (ms) | 59 ± 13 (36–116; n = 55) | 79 ± 14 (58–108; n = 53) |
| Single note duration (ms) | 51 ± 9 (36–65; n = 16) | 80 ± 13 (60–108; n = 50) |
| Pulsed note duration (ms) | 63 ± 13 (45–116; n = 39) | 93 ± 6 (87–97; n = 39) |
| Inter-note interval (ms) | 54 ± 10 (39–73; n = 32) | 80 ± 20 (34–155; n = 34) |
| First-pulse duration (ms) | 6 ± 1 (3–10; n = 39) | 15 ± 4 (12–20; n = 3) |
| Second-pulse duration (ms) | 47 ± 12 (18–75; n = 39) | 61 ± 4 (58–65; n = 3) |
| inter-pulse Interval (ms) | 9 ± 7 (3–37; n = 39) | 17 ± 2 (15–19; n = 3) |
| Dominant Frequency (Hz) | 624 ± 71 (474–948; n = 9) | 639 ± 198 (323–1162; n = 53) |
Note:
Values are presented as mean ± standard deviation (minimum–maximum). Abbreviation: Hz, hertz; ms, millisecond; n, sample size.
Tadpoles
The following description is based on five tadpoles at Gosner stage 36 (INPA-H 40471) collected at RDS Rio Negro, Novo Airão, Amazonas, Brazil. Total length 34.0 ± 0.6 mm (33.0–34.5 mm). Body compressed in lateral view, ovoid in dorsal view; maximum body height lower than the maximum tail height; body length 44–50% of the tail length. Rounded snout in both dorsal and lateral views (Figs. 10A and 10B). Nostrils located dorsolaterally and directed anterolaterally; circular opening. Internarial distance narrower than the interorbital distance (IND/IOD = 0.69–0.74). Eyes located and directed dorsolaterally. Oral disc (Figs. 10D and 10E) located and directed anteroventrally, large (ODW/BW = 0.43–0.47), with protuberant labia; presence of marginal papillae gap in the medial region of the upper labium present (32–35% of oral disc width); cylindrical and long papillae; one to three rows of papillae on the central and posterior portions of the upper labium; two rows of papillae on the entire lower labium, except for the corner (three rows). Slender upper jaw sheath, finely serrated and arc-shaped; lower jaw sheath as slender and finely serrated as the upper one, V-shaped. Labial keratodont row formula (LKRF) 2(2)/5–6(1); A-2 gap small but larger than the P-1 gap. Single and sinistral spiracle; inner wall free from the body; in lateral view, the spiracle surpasses the outline of the lower margin of the tail muscle in the distal portion. Dextral vent tube, attached to the ventral fin, right wall displaced dorsally. Dorsal and ventral fin similar in height; the ventral fin does not surpass the lower margin of body; flagellum absent.
Figure 10: Preserved tadpole of Osteocephalus vilarsi at developmental Gosner stage 36 (INPA-H 40471).
Specimen collected at RDS Rio Negro, municipality of Novo Airão, Amazonas state, Brazil. Dorsal (A), lateral (B) and ventral (C) views. Detailed views of the oral disc (D) and body (E). Photographs: Jeni Lima Magnusson.Measurements (in mm) are as follows, expressed as means ± standard deviation (range): TL, 34.0 ± 0.6 (33.0–34.5); BL, 10.8 ± 0.2 (10.5–11.0); BW, 7.3 ± 0.2 (7.0–7.6); TAL, 23.2 ± 0.7 (22.0–24.0); TMH, 2.4 ± 0.1 (2.3–2.5); MTH, 6.5 ± 0.1 (6.3–6.7); IOD, 4.4 ± 0.2 (4.0–4.5); IND, 3.2 ± 0.1 (2.8–3.4); TMW, 3.2 ± 0.1 (3.0–3.3); ODW, 3.3 ± 0.1 (3.1–3.4); ODG, 1.0 ± 0.0 (1.0–1.0).
Tadpole and metamorph colouration
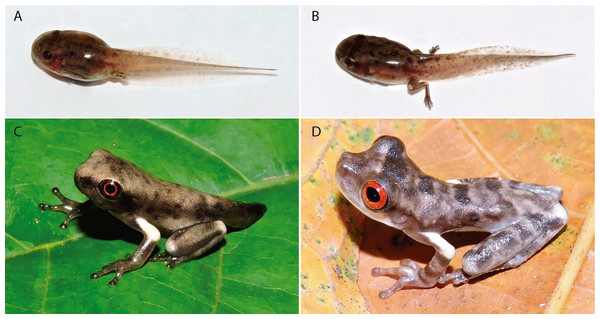
In life, tadpoles at Gosner stage 36 display a light brown dorsum with irregularly distributed dark brown blotches (Fig. 11A); a dark brown band from the nostril to the anterior portion of the orbits; a black iris with a red ring around pupil. Translucent venter. Dorsal portion of the tail muscle bronze; ventral portion pinkish cream; fins translucent with light brow blotches. Tadpoles at Gosner stage 39–40 are similar in colour pattern (Fig. 11B), except for the dorsum colour and fin blotches (that become darker) and by the presence of a large cream blotch on the knee and heel. Tadpoles at Gosner stage 45 display a silvery cream dorsum with dark and light grey blotches and spots (Fig. 11C); a dark grey band covering the lateral of head, from the snout to the posterior portion of the tympanum and on the upper lips; black iris with a red ring around pupil; grey fingers, hands, forearms, toes and feet; white upper arm; white blotch on the knee and heel; light grey groyne; dark grey tail; white venter.
Figure 11: Ontogenetic colouration changes of tadpoles and metamorphs of Osteocephalus vilarsi.
Specimens collected at RDS Rio Negro, municipality of Novo Airão, Amazonas, Brazil. (A) Tadpole at Gosner stage 36. (B) Tadpole between Gosner stage 39 and 40. (C) Metamorph at Gosner stage ~45. (D) Recently metamorphosed juvenile. Photographs: Jiří Moravec (A–C), Albertina Pimentel Lima (D).In life, metamorphs present a grey dorsum with dark grey blotches (Fig. 11D); a large dark grey band on the interorbital region. White subocular region, extending to the anterior infratympanic area; a dark stripe covering supratympanic region, from the posterior corner of the eyes to the arm insertion to the body. Bright red iris and black oval pupil. Limbs grey, except for the upper arm (white); four transversal dark grey stripes on the thigh; three horizontal dark grey stripes on the forearm and tarsus; light grey blotch on knee; white blotch on the heel. Venter white.
Reproductive behaviour
Two O. vilarsi calling males were observed at RDS Rio Negro (municipality of Novo Airão, Negro–Solimões interfluve) during 24–26 October 2017. The first male (not collected) called from the same place for three consecutive nights. It occupied a calling place on a horizontal leaf of herbaceous plant ca. 60 cm above the ground. The plant was growing close to a small phytotelma formed by trunk ridges of a large fallen tree (Fig. 12B). The phytotelma was approximately at the same height above ground as the calling place. The second male was found calling on vegetation ca. 40 cm above the ground at a small temporary forest puddle. Both breeding places were also occupied by calling males of Rhinella sp. (Rhinella margaritifera species group). Tadpoles from RDS Rio Negro were found in a shallow puddle not connected to a stream in a secondary white-sand forest. Specimens from the Jaú National Park were collected and observed in reproduction (May 2000) on the ground or up to 1 m high in shrub branches in open white-sand forests known as campina (canopy below 10 m), near or above temporary puddles. Some calling males presented territorial behaviour by altering their vocalisation in response to a playback (not recorded). Based on those notes, it appears that O. vilarsi can breed in small water body sources in closed forests (e.g. small temporary puddles, phytotelmata), as well as in open forests.
Figure 12: Habitat of Osteocephalus vilarsi at RDS Rio Negro, municipality of Novo Airão, Amazonas, Brazil.
(A) A typical O. vilarsi habitat in an ecotone between a white-sand forest and dense rainforest in September 2018. (B) An O. vilarsi breeding place in a disturbed forest in October 2017. Males were calling on plants close to trunk ridges filled with rainwater. Photographs: Jiří Moravec.Distribution and habitat
Until now, O. vilarsi was recorded in localities inside the interfluve between the Negro and Solimões rivers, as well as at the left bank of the upper Negro River (Figs. 1A and 1B). Such a wide geographic range covering approximately 212,000 km2 indicates that O. vilarsi is widely distributed across the extreme North-western Brazil (state of Amazonas) and the adjacent Southern Venezuela. The western most known locality is ca. 40 km from the Colombian border. Therefore, its occurrence can be expected in Colombian territory.
During field surveys in the vicinity of the municipality of Novo Airão, O. vilarsi was recorded both in relatively undisturbed forests and in heavily altered habitats adjacent to communities and farmlands. It was found both in the semi-open white-sand forest known as campinarana (canopy below 20 m; Fig. 12A) and in high closed forests (canopy above ca. 20 m; Fig. 12B). No individuals were found in the open white-sand vegetation known as campina (canopy below 10 m). After dusk, individuals were found perched on vegetation (mostly on narrow vertical trunks of smaller trees) ca. 50–200 cm above the ground. In two cases, adult individuals were found hidden in open vertical plastic tubes, used for the delimitation of INPA study plots. Other hylid species found in sympatry with O. vilarsi at Novo Airão include Boana boans (Linnaeus, 1758), B. aff. geographica, B. lanciformis (Cope, 1871), Dendropsophus minutus (Peters, 1872), O. taurinus (in low abundance compared to O. vilarsi), Scinax ruber (Laurenti, 1768), Scinax sp., and Trachycephalus cunauaru Gordo, Toledo, Suárez, Kawashita-Ribeiro, Ávila, Morais & Nunes, 2013.
Several non-reproductive specimens of O. vilarsi at Jaú National Park were observed in both flooded and unflooded rainforests (known as igapó and terra firme forests, respectively; canopy above ca. 20 m), campinarana (canopy below 20 m), and campina (comprising shrubs and exposed sand). Specimens were perched on horizontal branches of small trees or on vertical trunks. Other hylid species found in sympatry with O. vilarsi at the Jaú National Park are listed in Neckel-Oliveira & Gordo (2004).
Similarly, individuals recorded during the field survey at the Brazilian foothills of the Pico da Neblina, close to the Venezuelan border, were found perched on horizontal branches of small trees, in an ecotonal white-sand forest (campinarana) with a low canopy and several small temporary puddles. In this region, O. vilarsi occurs in sympatry with the hylids Boana aff. cinerascens, B. lanciformis, Dendropsophus minutus, D. tintinnabulum (Melin, 1941), Osteocephalus aff. taurinus, Scinax ruber and S. cruentommus (Duellman, 1972).
In fact, all individuals of O. vilarsi analysed in this study were recorded in localities covered by white-sand forest, or in close vicinity. Such an association indicates an ecological preference of this species to this particular and threatened type of Amazonian forest. In fact, the estimated distribution of O. vilarsi presented herein mostly coincides with the occurance of this forest type inside the Amazon in North-western Brazil and adjacent Colombia and Venezuela (Fig. 1A).
Discussion
The results of the phylogenetic analysis based on five mitochondrial markers are in good agreement with the previous broader phylogenetic hypotheses proposed for Osteocephalus by Ron et al. (2012) and Jungfer et al. (2013). Monophyly of the five species groups was strongly supported. The main difference between the present study and previous phylogenetic analyses lies in the topology of the species groups. Whereas Ron et al. (2012) and Jungfer et al. (2013) recovered the O. planiceps species group as sister to the O. leprieurii species group, our analyses placed this group in a sister position to the O. alboguttatus species group. Different topologies concerning these three phylogenetic hypotheses may reflect differences in specimen sampling, analysed genes and amount of missing data from each dataset.
Our phylogenetic and morphological analyses revealed O. vilarsi to be a valid species belonging to the O. planiceps species group. It is interesting however, that one individual of O. vilarsi (AMNH 1312546, Venezuelan slopes of the Pico da Neblina mountain range) was already recognised at the specific level in most of the previous phylogenetic hypotheses concerning the genus. Due to the absence of other voucher specimens and lack of comparative morphological data, the individual from the Venezuelan slope of the Pico da Neblina was wrongly associated with other Osteocephalus (e.g. O. leprieurii, O. ‘leprieurii’, or O. planiceps CA1; Wiens et al., 2006; Moravec et al., 2009; Wiens et al., 2010; Ron et al., 2012; Salerno et al., 2012; Jungfer et al., 2013).
The apparent strong ecological association of O. vilarsi to white-sand ecosystems (e.g. primary and secondary white-sand forests, as well as ecotonal zones between white-sand forests, igapó and terra firme forests) represents a rarely reported example of such an association in Amazonian anurans. To the best of our knowledge, a similar ecological association is known only in the case of the casque-headed frog Aparasphenodon venezolanus (Mertens, 1950), whose distribution partially overlaps with that of O. vilarsi at Jaú National Park (De Carvalho et al., 2018). Despite rarely reported in anurans, such a specific association is widely known and studied in Amazonian birds, which present highly endemic white-sand assemblages (Adeney et al., 2016; Borges et al., 2016).
As the white-sand forests cover large but patchily distributed portions of the upper and middle Negro River basin (Adeney et al., 2016), we expect O. vilarsi to present a similar distribution pattern across this area. White sand ecosystems belong to the most sensitive vegetation types in Amazonia (Adeney et al., 2016), and the effects of their exploration and alteration on the dynamics of O. vilarsi populations in different parts of the Amazon are still unknown.
Osteocephalus vilarsi is a remarkable case of a widely distributed Amazonian anuran species, which has been overlooked for decades. Its rediscovery illustrates how an integrative approach to the study of Amazonian amphibians is vital. Our extensive examination of morphological, molecular, geographical, ecological, and bioacoustical data, combined with the revision of museum collections has updated and extended the geographic range of the species (previously known only from its type locality) to an area covering over 200,000 km2 at the Negro-Solimões Interfluve and surrounding Brazilian and adjacent Venezuelan areas (the species is also expected to occur in adjacent Colombia). The scientific knowledge concerning O. vilarsi should rapidly increase, since the data reported herein (including morphological variation in adults, juveniles and tadpoles and advertisement call characteristics) should serve as a basis for further identification of individuals belonging to this species.
Conclusion
Osteocephalus vilarsi (Melin, 1941) has been rediscovered 75 years after its description. Based on morphological and molecular analyses, O. vilarsi is replaced from the O. taurinus species group to the O. planiceps species group. Morphological variation of adult, subadult and juvenile specimens, morphology of tadpoles, advertisement call, and natural history of O. vilarsi are described for the first time. Biogeographic data demonstrate that O. vilarsi, previously known only from its type locality, is widely distributed across the interfluve between the Negro and Solimoês rivers in North-western Brazil and adjacent southern Venezuela. The species displays strong ecological association to white-sand ecosystems (primary and secondary white-sand forests).
Appendix I
Additional specimens examined. Symbols: * = photograph.
Osteocephalus buckleyi: BOLIVIA: Pando: Santa Crucito (NMP-P6V 73945), Pando (CBF 1262, CBF 2150–51).
Osteocephalus cabrerai: PERU: Loreto: 21 km W of Iquitos (NMP-P6V 71144/1–2).
Osteocephalus castaneicola: BOLIVIA: Pando: San Antonio (CBF 6051 (holotype), NMP-P6V 73810/1–3 (paratopotypes), NMP-P6d 28/2009).
Osteocephalus deridens: PERU: Loreto: Anguilla (NMP-P6V 71263), 35 km SW of Iquitos (NMP-P6V 71262/1–5).
Osteocephalus fuscifacies: ECUADOR: Napo: Aliñahuí, 5 km W of Ahuano (ZFMK 68660* (paratype)).
Osteocephalus mimeticus: PERU: Huánuco/Ucayali: El Boquerón del Padre Abad (ZFMK 33352, ZFMK 36319).
Osteocephalus mutabor: ECUADOR: Napo: Río Chaloyacu on Carretera Narupa – Coca (ZFMK 66237* (paratype)).
Osteocephalus oophagus: BRAZIL: Amazonas: Reserva Forestal Adolfo Ducke (ZFMK 57137–38* (paratypes)).
Osteocephalus planiceps: ECUADOR: Napo: Puerto Misahaulli at La Cruz Blanca (MCZ-A 111190, MCZ-A 111188), Limoncocha (MCZ-A 98000, MCZ-A 98019, QCAZA 63543*), Laguna Taracoa (MCZ-A 97755); Orellana: Yasuni National Park (QCAZA 14844*, QCAZA 20797*, QCAZA40987*, QCAZA 51085*, QCAZA 55257*, QCAZA 55297–8*, QCAZA 55364*, QCAZA 55378*, QCAZA 55380*, QCAZA 64119–21*, QCAZA 64125–31*), Napo River (QCAZA 43891*, QCAZA 44420*); Pastaza: Lorocachi (QCAZA 55857*, QCAZA 55874*, QCAZA 55879*, QCAZA 55881*, QCAZA 55908*, QCAZA 55924*, QCAZA 55999*, QCAZA 56011*, QCAZA 56017*, QCAZA 56046*, QCAZA 56061*, QCAZA 56624*), Campo Villano (QCAZA 38702*), Pupalyacu (QCAZA 56624*); Morona Santiago: Comunidad Jempekat (QCAZA 54404*), San José de Morona (QCAZA 73720*), Marantian Wildlife Reserve (QCAZA 75756*); Sucumbíos: Cuyabeno Wildlife Reserve (QCAZA 37786*, QCAZA 52426*), Sacha Lodge (QCAZA 42288*), La Selva Amazon Ecolodge (QCAZA 44060*). PERU: Loreto: Anguilla (NMP-P6V 71264/1–2), Tarapoto surr., 21 km W of Iquitos (NMP-P6V 71204/1–2), Puerto Almendras (NMP-P6V 71174/1–5), Nauta (ANSP 11399, holotype*). Ucayali: Regional Conservation Area Imiría (NMP-P6V 74913).
Osteocephalus subtilis: BRAZIL: Acre: Cruzeiro do Sul (MZUSP 60561* (holotype)).
O. taurinus: BOLIVIA: Pando: Nacebe (NMP-6V 72172/1–2), Pando (CBF 1281, 1300-02, 2147-48, 43333). PERU: Loreto: Puerto Almendras (NMP-P6V 71184); Ucayali: Regional Conservation Area Imiría (NMP-P6V 74441).
O. vilarsi: BRAZIL: Amazonas: Missão Taracuá (INPA-H 40458, INPA-H 40470, INPA-H 40461, GNM 488 (holotype; photographs and data available in Jungfer, 2010)); Rio Negro Sustainable Development Reserve (INPA-H 40468, INPA-H 40456, INPA-H 40459, INPA-H 40465, INPA-H 40460, INPA-H 40462, INPA-H 40454, INPA-H 40452, INPA-H 40471 (tadploes)); Seringalzinho Village, Jaú National Park (INPA-H 40464, INPA-H 40472, INPA-H 40467, INPA-H 40455, INPA-H 40463, INPA-H 40466, INPA-H 40473); 5 km south Seringalzinho Village, Jaú National Park (INPA-H 10940, INPA-H 10942, INPA-H 10939, INPA-H 10944); Miratucu Lake, Jaú National Park (INPA-H 40469, INPA-H 40453, INPA-H 40457); east bank of Ayuanã River (CZPB-AA 239); northern bank of Japurá River (CZPB-AA 1421); Pico da Neblina National Park (INPA-H 37243–4, INPA-H 37246, INPA-H 37245).
Osteocephalus yasuni: ECUADOR: Napo: Yasuní Scientific Research Station (QCAZA 11336* (holotype), QCAZA 10879* (paratopotype), QCAZA 11329* (paratopotype)). PERU: Ucayali: Regional Conservation Area Imiría (NMP-P6V 74442/1–4).
Supplemental Information
Sampling localities of Osteocephalus vilarsi in the Amazonas state, Brazil.
Symbols: * = specimens previously published as Osteocephalus planiceps. References are available in the main text.
Samples of the Osteocephalus and Dryaderces genera used for the phylogenetic analyses.
See text for abbreviations.
Morphometric measurements of specimens of Osteocephalus vilarsi and O. planiceps used in the Principal Component Analyses (PCA).
Abbreviations are described in the main text.