Genome-wide characterization and expression analysis of PP2CA family members in response to ABA and osmotic stress in Gossypium
- Published
- Accepted
- Received
- Academic Editor
- Edward Braun
- Subject Areas
- Agricultural Science, Bioinformatics, Molecular Biology, Plant Science
- Keywords
- Gene family, Gossypium, Protein interaction, Phylogeny, Clade A type 2C protein phosphatases
- Copyright
- © 2019 Lu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Genome-wide characterization and expression analysis of PP2CA family members in response to ABA and osmotic stress in Gossypium. PeerJ 7:e7105 https://doi.org/10.7717/peerj.7105
Abstract
Clade A type 2C protein phosphatases (PP2CAs), as central regulators of abscisic acid (ABA) signaling, negative control growth, development and responses to multiple stresses in plants. PP2CA gene families have been characterized at genome-wide levels in several diploid plants like Arabidopsis and rice. However, the information about genome organization, phylogenesis and putative functions of PP2CAs in Gossypium is lacking. Here, PP2CA family members were comprehensively analyzed in four Gossypium species including the diploid progenitor Gossypium arboreum, G. raimondii and the tetraploid G. hirsutum and G. barbadense, and 14, 13, 27, and 23 PP2CA genes were identified in the genomic sequences of these plants, respectively. Analysis results showed that most Gossypium PP2CAs were highly conserved in chromosomal locations, structures, and phylogeny among the four cotton species. Segmental duplication might play important roles in the formation of the PP2CAs, and most PP2CAs may be under purifying selection in Gossypium during evolution. The majority of the PP2CAs were expressed specifically in diverse tissues, and highly expressed in flowers in G. hirsutum. The GhPP2CAs displayed diverse expression patterns in responding to ABA and osmotic stress. Yeast-two hybrid assays revealed that many GhPP2CAs were capable of interaction with the cotton ABA receptors pyrabactin resistance1/PYR1-like/regulatory components of ABA receptors (PYR1/PYL/RCAR) GhPYL2-2D (Gh_D08G2587), GhPYL6-2A (Gh_A06G1418), and GhPYL9-2A (Gh_A11G0870) in the presence and/or absence of ABA. These results gave a comprehensive view of the Gossypium PP2CAs and are valuable for further studying the functions of PP2CAs in Gossypium.
Introduction
Protein phosphorylation and dephosphorylation, as two central mechanisms of cellular signal transduction, play pivotal roles in many biological processes including growth, development, and adaptations to various environmental stimuli in plants (Schweighofer, Hirt & Meskiene, 2004). They are catalyzed by protein kinases and phosphatases, respectively. Phosphatases are generally categorized into serine/threonine (Ser/Thr) phosphatases and tyrosine phosphatases according to the different amino acid residues they dephosphorylate. Based on biochemical and pharmacological properties, Ser/Thr phosphatases can be further classified into three large families: phosphoprotein phosphatases (PPs), phosphoprotein metallophosphatases, and aspartate-based protein phosphatases (Schweighofer, Hirt & Meskiene, 2004; Kerk, Templeton & Moorhead, 2007; Fuchs et al., 2013; Singh et al., 2015). The PPs includes PP1, PP2A, PP2B, PP4, PP5, PP6, and PP7, the phosphoprotein metallophosphatases consist of Mg2+/Mn2+-dependent type 2C protein phosphatases (PP2Cs) and other Mg2+-dependent phosphatases (Schweighofer, Hirt & Meskiene, 2004; Singh et al., 2010, 2015; Fuchs et al., 2013). PP2Cs, which play key roles in dephosphorylation events in plants, belong to a large subfamily, and can be further divided into 11 clades (A–K) in Arabidopsis and rice (Singh et al., 2010) and 12 clades (A–L) in Brachypodium distachyon (Cao et al., 2016). Among these, Clade A proteins of PP2Cs (PP2CAs) are the ones of well-studied PP2Cs in Arabidopsis, and they have been shown to have important roles in controlling abscisic acid (ABA) signaling, and negatively regulate plant growth, development and response to various biotic and abiotic stresses in plants (Tähtiharju & Palva, 2001; Fuchs et al., 2013; Singh et al., 2015). In Arabidopsis genome, nine PP2CA members have been identified. They are ABI1 (ABA insensitive 1), ABI2, HAB1 (Homology to ABI1), HAB2, AHG1 (ABA hypersensitive germination 1), HAI1 (Highly ABA-induced PP2C1), HAI2, HAI3, and AHG3/AtPP2CA (Fuchs et al., 2013). These genes, particularly ABI1, ABI2, and AHG3/PP2CA alone or cooperatively control ABA-mediated transpiration, stomatal closure, seed germination and root growth, and are involved in the regulation of many abiotic stress responses like drought, high salinity, cold, heat, and potassium deprivation (Schweighofer, Hirt & Meskiene, 2004; Rubio et al., 2009; Singh et al., 2015). Some PP2CAs also play important roles in responses to pathogen attack (Schweighofer, Hirt & Meskiene, 2004; Singh et al., 2015). PP2CAs are functionally redundant, and their expression is upregulated by high concentrations of ABA (Rubio et al., 2009; Singh et al., 2015). Moreover, PP2CAs physically interact with numerous cytosolic and nuclear localized proteins such as AtHB6 (Homeobox protein 6), CIPK8 (Calcineurin B–like protein-interacting protein kinase 8), CIPK24, and SnRK2s (Sucrose nonfermenting one-related protein kinases subfamily two proteins) (Ohta et al., 2003; Fuchs et al., 2013; Singh et al., 2015). SnRK2s exert central and positive roles in ABA signal cascade in plants (Fujii & Zhu, 2009; Fujii et al., 2009).
Recently, ABA receptors pyrabactin resistance1/PYR1-like/regulatory components of ABA receptors (PYR1/PYL/RCAR) (named PYLs for simplicity) have been found (Ma et al., 2009; Park et al., 2009). This is a breathtaking discovery in plants. PP2CAs were identified as coreceptors, specifically interact with PYLs and control ABA signaling. In the presence of ABA, ABA binds to PYLs, further interacts with and inhibits the activities of PP2CAs; thereby releasing and activating SnRK2s. SnRK2s subsequently regulate multiple downstream transcriptional factors and other proteins to trigger ABA responses (Fujii et al., 2009; Geiger et al., 2009; Lee et al., 2009).
PP2C gene families including PP2CAs have been analyzed at genome-wide levels in Arabidopsis, rice, maize and B. distachyon (Xue et al., 2008; Fujita et al., 2009; Wei & Pan, 2014; Cao et al., 2016). The domain structure of PP2CAs was also studied (Schweighofer, Hirt & Meskiene, 2004). Moreover, the expression patterns of PP2CAs have been examined in response to ABA and multiple stresses in Arabidopsis, rice, maize and B. distachyon (Xue et al., 2008; Wei & Pan, 2014; Zhang et al., 2017a). However, genomic information and expression profiles of PP2CAs in cotton is unknown to date.
Cotton is the most important fiber crop which provides the spinnable lint for the textile industry in the world. The yield and quality of cotton are adversely affected by many abiotic stresses such as drought and high salinity, which are governed by ABA signaling (Hauser, Waadt & Schroeder, 2011; Liang et al., 2017; Ullah et al., 2017). Therefore, it is essential for us to uncover the functional mechanisms of PP2CAs in ABA signal transduction pathway in cotton. Here, we carried out a genome-wide identification of PP2CA gene family in diploid Gossypium arboreum (A2) and G. raimondii (D5), and their descendant tetraploid species G. hirsutum (AD1) and G. barbadense (AD2). The evolutionary relationships of these PP2CAs were analyzed. Changes in the transcriptional levels of the PP2CAs were also investigated in diverse tissues and in response to ABA and osmotic stress in G. hirsutum. Furthermore, the interactions between G. hirsutum PP2CAs and several GhPYLs were detected by the yeast-two hybrid method. These results may be valuable for further functional characterization of cotton PP2CAs in ABA signaling in the future.
Materials and Methods
Analysis of the PP2C family in four Gossypium species
To explore all the members of the PP2C family in Gossypium, the protein sequences of 80 AtPP2Cs were initially applied as queries to search against the databases of G. arboreum (BGI-CGB v2.0 assembly genome), G. raimondii (JGI assembly v2.0 data), G. hirsutum (NAU-NBI v1.1 assembly genome) (www.cottongen.org), and G. barbadense (http://database.chgc.sh.cn/cotton/index.html), respectively, using the BLAST program with default setting (E-value < e−10) (Camacho et al., 2009). After removing the redundant sequences from the data set, the putative Gossypium PP2Cs were then characterized using the PP2C model (PF00481) (http://pfam.xfam.org/) by the Hmmer software (http://hmmer.org/), and the proteins without a PP2C catalytic domain were deleted. The molecular weight (MW) and isoelectric point of the Gossypium PP2Cs were predicted by the online tool ExPaSy (http://web.expasy.org/protparam/), which can give various physico-chemical properties of a protein based on its amino acid sequence (the extinction coefficient and the absorbance of a native protein in water at 280 nm were used). The composition and position of exons and introns of the PP2CAs were obtained from CottonGen (https://www.cottongen.org/) and characterized by the Gene Structure Display Server tools (http://gsds.cbi.pku.edu.cn/) (Hu et al., 2015). The conserved domains of PP2CAs were validated in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) using the automatic mode (Marchler-Bauer et al., 2017). The MEME program (meme-suite.org/tools/meme) was applied to determine the motifs of PP2CAs in Gossypium (“any number of repetitions” to be distributed in sequences was set). The locations of Gossypium PP2CAs in chromosomes were assessed using MapInspect software (http://www.mybiosoftware.com/mapinspect-compare-display-linkage-maps.html).
Analysis of synteny and Ka/Ks of PP2CAs
The homologous regions of PP2CAs in Gossypium were identified by MCScanx software (http://chibba.pgml.uga.edu/mcscan2/) and syntenic blocks were determined by the CIRCOS program (http://www.circos.ca/). The syntenic maps of the PP2CAs were obtained using the circos-0.69±3 software with default parameters (http://www.circos.ca/). Some genes located within the same or adjacent intergenic region were regarded as tandem duplications. The nucleotide substitution parameters Ka (nonsynonymous) and Ks (synonymous) were assessed by the PAML program (http://abacus.gene.ucl.ac.uk/software/paml.html). Then, the ratio of Ka/Ks was calculated. Ka/Ks <1 means purifying selection; Ka/Ks = 1 indicates neutral selection, while Ka/Ks >1 represents positive selection (Hurst, 2002).
Phylogenetic analysis of PP2CAs
The PP2CA databases were downloaded for Arabidopsis thaliana (http://www.arabidopsis.org/), Theobroma cacao (http://cocoagendb.cirad.fr), Ricinus communis (http://castorbean.jcvi.org), Populus trichocarpa (http://www.phytozome.net/poplar), Glycine max (http://www.phytozome.net/soybean), B. distachyon (http://plants.ensembl.org/Brachypodium_distachyon/Info/Index), Oryza sativa (http://rapdb.dna.affrc.go.jp), and the four Gossypium species mentioned above. The amino acid sequences of PP2CAs were aligned by the MUSCLE software (Edgar, 2004), and a phylogenetic tree of the PP2CAs was generated using the IQ-TREE server (http://www.iqtree.org/) following the maximum likelihood (ML) method (Nguyen et al., 2015; Trifinopoulos et al., 2016). The best-fitting model was chosen using the ModelFinder (Kalyaanamoorthy et al., 2017) and the support was assessed using the ultrafast bootstrap (Minh, Nguyen & Von Haeseler, 2013). Evolutionary tree was visualized using the FigTree v1.4.4 software (available from https://github.com/rambaut/figtree/releases).
Measurements of GhPP2CAs expression in tissues and in response to ABA or osmotic stress
For measuring the expression of GhPP2CAs in tissues in each experiment, seeds of G. hirsutum L. acc. Texas Marker-1 (TM-1) were sown in pots containing the mixed nutrient soil (rich soil:vermiculite = 2:1, v/v) in a growth chamber. After 21 days, about two gram samples of roots, stems, or leaves were collected from 10 plants. About 20 flowers were collected 1 day post anthesis, and about five gram fibers were obtained from ovules 23 days post anthesis of cotton plants grown in the fields. For monitoring the expression of GhPP2CAs after ABA treatment or under osmotic stress, TM-1 seeds were germinated and planted in liquid 1/2 MS medium (Murashige & Skoog, 1962) in a growth chamber (the medium was aerated. day/night temperature cycle of 28/26 °C, 14 h light/10 h dark, and about 50% relative humidity). Three weeks later, the plants were sprayed with 100 μM ABA or treated with 10% PEG6000 (dissolved in medium) for 0, 3, 6, 12, and 24 h, respectively. Then, about two gram roots were sampled, frozen in liquid nitrogen and stored at −70 °C. Total RNA was extracted from some of the samples and cDNA was generated according to the method described previously (Ma et al., 2012; Zhang et al., 2017b).
Quantitative real-time RT-PCR (qRT-PCR) experiments were constructed in an ABI 7,500 real-time PCR amplifier using the cDNA, SYBR Green Master mix, the specific primers of GhPP2CA genes (Table S1). GhUBQ7 was used as an internal control (Lu et al., 2017). Experiments were independently repeated three times. The interval between two repeated experiments was 7–10 days.
Monitoring protein interaction by yeast-two hybrid method
The CDS sequences of GhPYLs (GhPYL2-2D, GhPYL6-2A, and GhPYL9-2A) and GhPP2CAs were amplified, and cloned into pGADT7 and pGBKT7 vectors, respectively, using gene specific primers (Table S2). After sequencing, the fused vectors were transformed into AH109. The cotransformants were plated on nonselective SD/-Leu/-Trp solid medium and selective SD/-Leu/-Trp/-His/-Ade solid medium as described previously (Lu et al., 2017; Zhang et al., 2017b).
Results
Genome-wide analysis of PP2CAs in four Gossypium species
To identify the putative PP2CA family members in Gossypium, the amino acid sequences of 80 Arabidopsis PP2Cs (Xue et al., 2008) were used to survey the Gossypium databases. Putative PP2Cs were assigned to a total of 114, 116, 239, and 232 genomic sequences that were retrieved from G. arboreum, G. raimondii, G. hirsutum, and G. barbadense, respectively. They were individually denominated as GaPP2Cs, GrPP2Cs, GhPP2Cs, and GbPP2Cs (Table S3). According to the phylogenetic relationships of PP2Cs between Gossypium and Arabidopsis, the Gossypium PP2Cs could be clustered into 12 clades (A–L) (Figs. S1–S4). The PP2CAs possessed 14 GaPP2CAs, 13 GrPP2CAs, 27 GhPP2CAs, and 23 GbPP2CAs, respectively. They were named individually according to their gene identifiers (Table 1). In this report, we focused on the PP2CA family members in the four Gossypium species.
| Gene identifier | Gene name | Size (aa) | Mass (kDa) | pI |
|---|---|---|---|---|
| Cotton_A_00941 | GaPP2CA1 | 420 | 46.9 | 6.61 |
| Cotton_A_01365 | GaPP2CA2 | 419 | 45.9 | 6.39 |
| Cotton_A_02676 | GaPP2CA3 | 494 | 53.4 | 5 |
| Cotton_A_03112 | GaPP2CA4 | 400 | 43.4 | 5.12 |
| Cotton_A_05714 | GaPP2CA5 | 470 | 51.8 | 5.82 |
| Cotton_A_11854 | GaPP2CA6 | 593 | 66 | 5.51 |
| Cotton_A_12895 | GaPP2CA7 | 416 | 45.4 | 6.07 |
| Cotton_A_14028 | GaPP2CA8 | 573 | 62.4 | 4.69 |
| Cotton_A_18842 | GaPP2CA9 | 393 | 43 | 5.25 |
| Cotton_A_25088 | GaPP2CA10 | 413 | 45.1 | 5.15 |
| Cotton_A_25089 | GaPP2CA11 | 413 | 45.1 | 5.15 |
| Cotton_A_27758 | GaPP2CA12 | 390 | 43.3 | 8.34 |
| Cotton_A_31719 | GaPP2CA13 | 413 | 45.4 | 5.55 |
| Cotton_A_34085 | GaPP2CA14 | 242 | 26.6 | 5.73 |
| Gh_A03G0373 | GhPP2CA1 | 416 | 45.9 | 8.09 |
| Gh_A05G0308 | GhPP2CA2 | 558 | 60.9 | 4.69 |
| Gh_A05G0782 | GhPP2CA3 | 416 | 45.4 | 6.07 |
| Gh_A05G1136 | GhPP2CA4 | 494 | 53.5 | 5.09 |
| Gh_A05G3030 | GhPP2CA5 | 413 | 45.4 | 5.52 |
| Gh_A06G0579 | GhPP2CA6 | 416 | 45.9 | 5.64 |
| Gh_A07G0123 | GhPP2CA7 | 471 | 52 | 5.58 |
| Gh_A08G2192 | GhPP2CA8 | 463 | 51.4 | 7.56 |
| Gh_A10G0578 | GhPP2CA9 | 397 | 42.8 | 5.76 |
| Gh_A10G1998 | GhPP2CA10 | 409 | 44.6 | 5.1 |
| Gh_A12G2380 | GhPP2CA11 | 420 | 46 | 6.27 |
| Gh_A13G0184 | GhPP2CA12 | 179 | 20 | 5.71 |
| Gh_A13G1741 | GhPP2CA13 | 400 | 43.3 | 5.12 |
| Gh_D03G1169 | GhPP2CA14 | 258 | 28.1 | 8.6 |
| Gh_D04G0612 | GhPP2CA15 | 413 | 45.5 | 5.61 |
| Gh_D05G0410 | GhPP2CA16 | 558 | 60.8 | 4.69 |
| Gh_D05G1309 | GhPP2CA17 | 494 | 53.5 | 5 |
| Gh_D05G3907 | GhPP2CA18 | 417 | 45.4 | 5.83 |
| Gh_D06G0657 | GhPP2CA19 | 393 | 43 | 5.49 |
| Gh_D07G2383 | GhPP2CA20 | 471 | 51.9 | 5.91 |
| Gh_D08G2557 | GhPP2CA21 | 463 | 51.3 | 6.48 |
| Gh_D10G0622 | GhPP2CA22 | 342 | 38.1 | 8.74 |
| Gh_D10G2305 | GhPP2CA23 | 411 | 45 | 5.05 |
| Gh_D12G2508 | GhPP2CA24 | 418 | 45.8 | 6.42 |
| Gh_D13G0199 | GhPP2CA25 | 416 | 46.2 | 6.53 |
| Gh_D13G2089 | GhPP2CA26 | 400 | 43.4 | 5.17 |
| Gh_Sca051315G01 | GhPP2CA27 | 118 | 12.8 | 4.93 |
| GOBAR_AA03316 | GbPP2CA1 | 413 | 45.4 | 5.52 |
| GOBAR_AA08348 | GbPP2CA2 | 409 | 44.6 | 5.1 |
| GOBAR_AA12929 | GbPP2CA3 | 416 | 45.8 | 8.07 |
| GOBAR_AA17958 | GbPP2CA4 | 393 | 43 | 5.31 |
| GOBAR_AA26179 | GbPP2CA5 | 418 | 45.9 | 6.39 |
| GOBAR_AA27223 | GbPP2CA6 | 456 | 50.6 | 7.16 |
| GOBAR_AA30591 | GbPP2CA7 | 470 | 51.8 | 5.58 |
| GOBAR_AA32894 | GbPP2CA8 | 558 | 60.9 | 4.67 |
| GOBAR_AA34839 | GbPP2CA9 | 416 | 45.4 | 6.07 |
| GOBAR_AA37246 | GbPP2CA10 | 558 | 60.8 | 4.69 |
| GOBAR_DD04210 | GbPP2CA11 | 416 | 46.1 | 6.76 |
| GOBAR_DD07153 | GbPP2CA12 | 371 | 40.7 | 6.07 |
| GOBAR_DD08720 | GbPP2CA13 | 392 | 43.2 | 7.2 |
| GOBAR_DD10426 | GbPP2CA14 | 385 | 42.4 | 6.64 |
| GOBAR_DD12855 | GbPP2CA15 | 302 | 33.4 | 6.46 |
| GOBAR_DD17767 | GbPP2CA16 | 389 | 42 | 4.96 |
| GOBAR_DD22444 | GbPP2CA17 | 400 | 43.3 | 5.11 |
| GOBAR_DD29723 | GbPP2CA18 | 408 | 44.9 | 8.52 |
| GOBAR_DD30544 | GbPP2CA19 | 411 | 45 | 5.05 |
| GOBAR_DD32331 | GbPP2CA20 | 226 | 25.2 | 5.58 |
| GOBAR_DD35228 | GbPP2CA21 | 470 | 51.8 | 5.82 |
| GOBAR_DD37768 | GbPP2CA22 | 470 | 51.8 | 5.82 |
| GOBAR_DD38067 | GbPP2CA23 | 494 | 53.5 | 5 |
| Gorai.001G013500 | GrPP2CA1 | 471 | 51.9 | 5.91 |
| Gorai.003G128900 | GrPP2CA2 | 416 | 45.9 | 8.09 |
| Gorai.004G284400 | GrPP2CA3 | 463 | 51.2 | 6.34 |
| Gorai.008G282600 | GrPP2CA4 | 414 | 45.4 | 6.3 |
| Gorai.009G042600 | GrPP2CA5 | 558 | 60.8 | 4.65 |
| Gorai.009G096200 | GrPP2CA6 | 416 | 45.4 | 5.73 |
| Gorai.009G143300 | GrPP2CA7 | 494 | 53.4 | 5.04 |
| Gorai.010G076700 | GrPP2CA8 | 393 | 43 | 5.48 |
| Gorai.011G071200 | GrPP2CA9 | 347 | 38.8 | 8.43 |
| Gorai.011G268200 | GrPP2CA10 | 411 | 44.9 | 5 |
| Gorai.012G072200 | GrPP2CA11 | 409 | 44.9 | 5.7 |
| Gorai.013G022100 | GrPP2CA12 | 415 | 46.1 | 6.2 |
| Gorai.013G230300 | GrPP2CA13 | 400 | 43.3 | 5 |
It was found that the predicted coded amino acid lengths of Gossypium PP2CAs ranged from 118 to 593, with an average of 420. These PP2CAs had MWs of 12.8 kDa (GhPP2CA27) to 66 kDa (GaPP2CA6). The mean theoretical pIs of PP2CAs were approximately 5.9 with a minimum of 4.65 (GrPP2CA5) and a maximum of 8.74 (GhPP2CA22) (Table 1).
Phylogenetic and structural analysis of PP2CAs in Gossypium
In order to understand the evolutionary relationship among GaPP2CAs, GrPP2CAs, GhPP2CAs, and GbPP2CAs, we conducted a phylogenetic tree using the protein sequences of the Gossypium PP2CAs (Fig. 1A). As expected, most of GaPP2CAs were individually clustered closely with their corresponding orthologs of GhPP2CAs (GhPP2CA1–13) and GbPP2CAs (GbPP2CA1–10) in A genomes, and a majority of GrPP2CAs individually clustered closely with their homologs of GhPP2CAs (GhPP2CA14–27) and GbPP2CAs (GbPP2CA11–23) in D genomes. Noteworthily, GaPP2CA10 clustered together with GaPP2CA11, and a similar case occurred between GbPP2CA21/GbPP2CA22 and GbPP2CA16/GbPP2CA23. Moreover, homologues of 14 GaPP2CAs and of 13 GrPP2CAs were found in the G. hirsutum At and Dt subgenomes, respectively; and homologs of 10 GaPP2CAs (except GaPP2CA1, GaPP2CA3, GaPP2CA4, and GaPP2CA14) and 11 GrPP2CAs (except GrPP2CA6 and GrPP2CA9) were detected in the G. barbadense At′ or Dt′ subgenomes, respectively. Additionally, three pairs of paralogues with high sequence similarity in GbPP2CAs including GbPP2CA8/GbPP2CA10, GbPP2CA16/GbPP2CA23, GbPP2CA21/GbPP2CA22 were clustered together. They were seemingly derived from GaPP2CA8, GrPP2CA7, and GrPP2CA1, respectively.
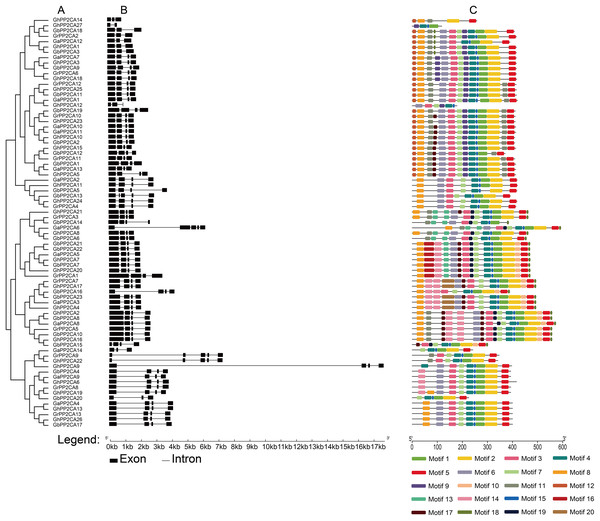
Figure 1: Phylogenetic relationships, gene structures, and conserved motifs of PP2CA genes in Gossypium.
(A) The phylogenetic tree was generated by the maximum likelihood (ML) method, with ultrafast bootstrap. (B) Exon/intron architectures of Gossypium PP2C genes. The black boxes indicate exons, and the black lines represent introns. The sizes of exons and introns were determined by the scale at the bottom. (C) Distributions of conserved motifs. The motifs are displayed by 20 different color boxes.Most PP2CAs had three to four exons except that GaPP2CA6, GrPP2CA9, GhPP2CA22, GbPP2CA13 possessed five exons, and GhPP2CA27 had two exons. Among the PP2CA genes, GaPP2CA6, GrPP2CA9, GhPP2CA9, and GhPP2CA22 individually had a longer intron sequence than other genes did (Fig. 1B). These results indicate that the exon/intron structures of the Gossypium PP2CA genes were highly conserved.
The motif compositions of the PP2CA proteins were analyzed in the four Gossypium species. Twenty putative motifs named motif 1 to motif 20 were identified. Among those, motif 1, 2, 3, 4, 5, 6, and 7 existed in every cluster and the majority of the PP2CA members. Moreover, most orthologous PP2CA proteins in the four Gossypium plants had the same or very similar compositions and distributions of motifs, suggesting that the PP2CA members in the same cluster likely share similar functions (Fig. 1C).
Chromosomal distributions of PP2CAs in Gossypium
To determine the putative evolutionary relationships of the Gossypium PP2CA genes, the positions of the genes on chromosomes were analyzed. We found that the distributions of these PP2CAs were uneven. The 14 GaPP2CAs, 13 GrPP2CAs, 27 GhPP2CAs, and 23 GbPP2CAs were distributed on 8, 9, 16, and 14 chromosomes, respectively. Most of the chromosomes contained one PP2CA gene. By contrast, some chromosomes individually had two PP2CA genes. They were D11 and D13 in G. raimondii, At10, At13, Dt05, Dt10, and Dt13 in G. hirsutum, and Dt′07 and Dt′13 in G. barbadense. Besides, each of D09 and Dt′05 owned three PP2CAs. A13, At05, and At′05 separately possessed four PP2CAs. In contrast, GaPP2CA5, GaPP2CA8, GaPP2CA14, GhPP2CA18, GhPP2CA20, GhPP2CA27, GbPP2CA2, and GbPP2CA12 were located on scaffolds, in which contigs were not spliced into any chromosome in genomic mapping.
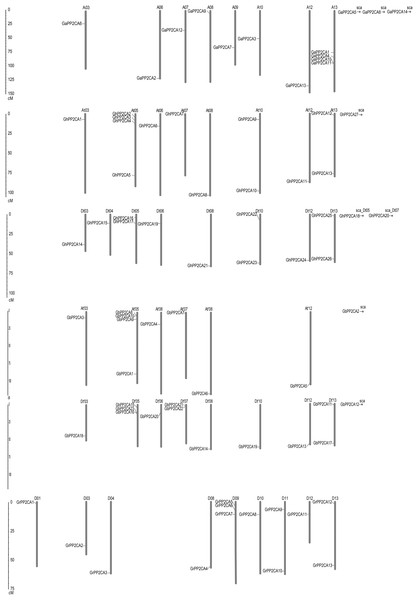
We compared the positions of the orthologs of GaPP2CAs, GrPP2CAs, and GhPP2CAs or GbPP2CAs in chromosomes. As expected, most homologs of GaPP2CAs and GrPP2CAs in G. hirsutum were located in their corresponding At subgenomes and Dt subgenomes. A similar situation also occurred in G. barbadense. However, homologous genes of GaPP2CAs and GrPP2CAs were barely located in their corresponding homoeologous chromosomes and collinear loci in G. hirsutum and G. barbadense (Fig. 2). For example, GaPP2CA7 was found in chromosome A09, however, its ortholog GhPP2CA3 and GbPP2CA9 were present in chromosome At05 and At′05, respectively. These results imply that specific, unique, and complex variation events in PP2CA-contained homoeologous chromosomes may happen within each of the two diploid and tetraploid species during genetic evolution.
Figure 2: Positions of Gossypium PP2CA genes on chromosomes.
GaPP2CAs, GrPP2CAs, GhPP2CAs, and GbPP2CAs were from G. arboreum, G. raimondii, G. hirsutum, and G. barbadense, respectively.Synteny analysis of PP2CA genes
During evolutionary processes, tandem and segmental duplications contribute to expanding gene family in plants (Cannon et al., 2004). We examined the duplication relationship of the PP2CAs among G. arboreum, G. raimondii, and G. hirsutum (the related database for G. barbadense was lacking). It was found that GaPP2CA10 and GaPP2CA11 joined together, and GbPP2CA16 and GbPP2CA23 clustered together in the chromosome. There are less than five genes between each pair of the genes, suggesting that the two pairs of genes are tandemly duplicated.
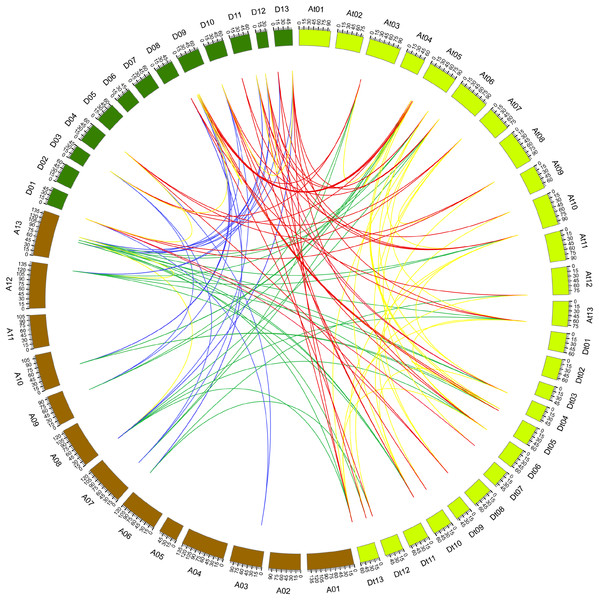
The synteny relationship of gene pairs was also explored among GaPP2CAs, GrPP2CAs, and GhPP2CAs. A total of 136 homologous gene pairs were observed in 133 collinearity blocks. Most of the blocks had one gene pair. Some blocks owned two gene pairs (GrPP2CA9/GhPP2CA22, GrPP2CA10/GhPP2CA23) between chromosomal D11 and Dt10. Another block harbored three gene pairs (GrPP2CA5/GhPP2CA2, GrPP2CA6/GhPP2CA3, GrPP2CA7/GhPP2CA4) between chromosomal D09 and At05 (Fig. 3). These findings imply that segmental duplication plays major roles in generating PP2CAs during evolution in Gossypium.
Figure 3: Genome-wide synteny results of PP2CA genes from G. arboreum, G. raimondii, and G. hirsutum.
Green lines linked gene pairs between G. arboreum and G. hirsutum, red lines connected gene pairs between G. raimondii and G. hirsutum, blue lines bridged gene pairs between G. arboreum and G. raimondii, yellow lines joined gene pairs within individual species in G. arboreum, G. raimondii, and G. hirsutum.Analysis of Ka/Ks values of PP2CAs
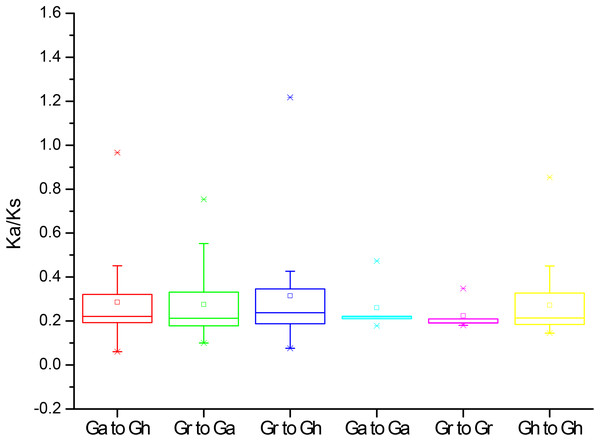
To further understand the evolution processes among Gossypium PP2CAs, the effects of selection on duplication of PP2CA genes were determined. The Ka and Ks substitutions, and Ka/Ks values were calculated for the homologous gene pairs among GaPP2CAs, GrPP2CAs, and GhPP2CAs. The mean values of Ka/Ks for these gene pairs between species Ga/Gh, Gr/Ga, Gr/Gh, Ga/Ga, Gr/Gr, Gh/Gh were 0.22, 0.21, 0.22, 0.21, 0.19, and 0.21, respectively. All of them were less than 1, indicating that the formation of these genes were mainly under purifying selection during evolution. The Ka/Ks ratios for the two gene pairs GrPP2CA11/GhPP2CA15 and GrPP2CA3/GhPP2CA21 were higher than 1, suggesting that the two gene pairs were generated under positive selection and the selection likely has effects to change these genes during evolution (Fig. 4).
Figure 4: The Ka/Ks values of the homologous PP2CA gene pairs in Gossypium.
(Ga), (Gr), and (Gh) represented G. arboreum, G. raimondii, and G. hirsutum, respectively.Phylogenetic analysis of PP2CAs in Gossypium and other plants
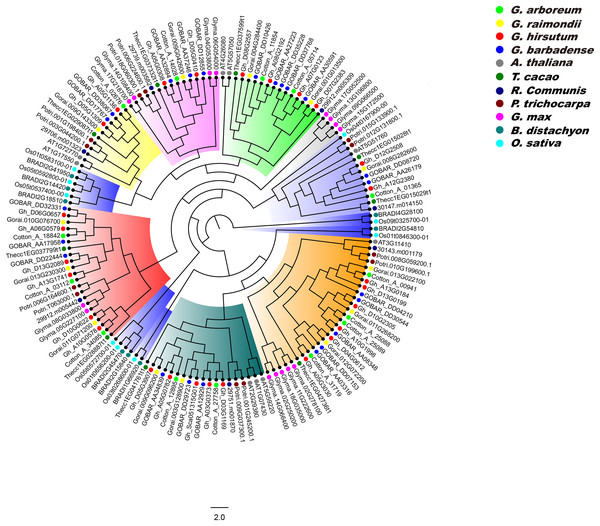
We constructed a phylogenetic tree of PP2CA proteins in G. arboreum, G. raimondii, G. hirsutum, G. barbadense, A. thaliana, T. cacao, R. communis, P. trichocarpa, G. max, B. distachyon, and O. sativa using the ML method, and analyzed the evolutionary relationships of these PP2CAs. It was found that the PP2CAs included both dicotyledonous and monocotyledonous members (Fig. 5). This suggests that these PP2CAs formed before the divergence of eudicots and monocots and are in general highly conserved. Indeed, the PP2CAs from the eudicots Gossypium, cacao, poplar, castor, soybean and Arabidopsis clustered more closely, and those of the monocots rice and distachyon clustered together. Moreover, many PP2CAs from Gossypium clustered more closely with those from cacao than from poplar, castor, soybean and Arabidopsis (Fig. 5), indicating that PP2CAs of Gossypium had closer relationship with those of cacao than those of other plants. As expected, PP2CAs in the four Gossypium species always clustered together, in line with their homologous evolutionary relationships (Fig. 5).
Figure 5: Phylogenetic tree of PP2CAs in Gossypium and other plants.
The multiple alignment was performed by MUSCLE program. IQTREE was used to create the maximum likelihood with Dayhoff model. The phylogenetic relationship of PP2CAs in G. arboreum, G. raimondii, G. hirsutum, G. barbadense, A. thaliana, T. cacao, R. communis, P. trichocarpa, G. max, B. distachyon, and O. sativa were analyzed.Expression patterns of GhPP2CA genes in different tissues
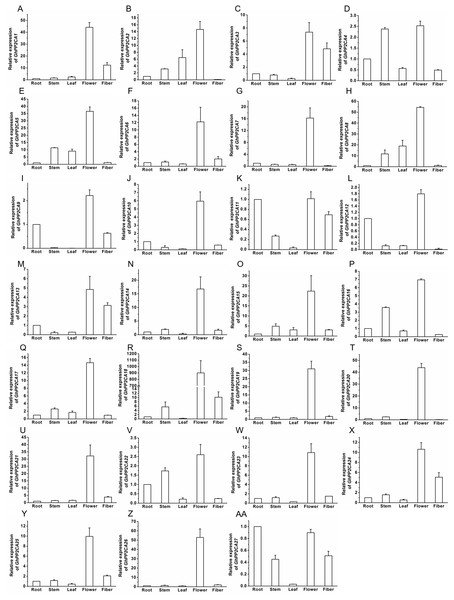
The transcript abundances of 27 GhPP2CAs in various tissues were measured by qRT-PCR to determine the putative functions of the PP2CAs in cotton. The results showed that all of the GhPP2CAs were highly expressed in flowers. GhPP2CA11 and GhPP2CA27 were also preferentially expressed in roots. Moreover, the transcriptional levels of GhPP2CA3, 11, 13, 27 were high in fibers. The transcripts of GhPP2CA4, 16, 22 were abundant in stems. These results imply that most cotton PP2CA members may function in reproductive development, and some PP2CAs also play roles in some specific tissues like roots, fibers, and stems (Fig. 6).
Figure 6: Transcription levels of GhPP2CAs in cotton tissues.
The genes preferentially expressed in flowers (A–Z) and fibers (AA). Gene GhUBQ7 was used as the internal control. The expression level of the gene in roots was set as 1. The data were mean ± SE. Statistical analyses were carried out by student’s t-test to determine the differences in gene abundances between roots and other tissues.Transcriptional changes of GhPP2CAs in responses to ABA and osmotic stress
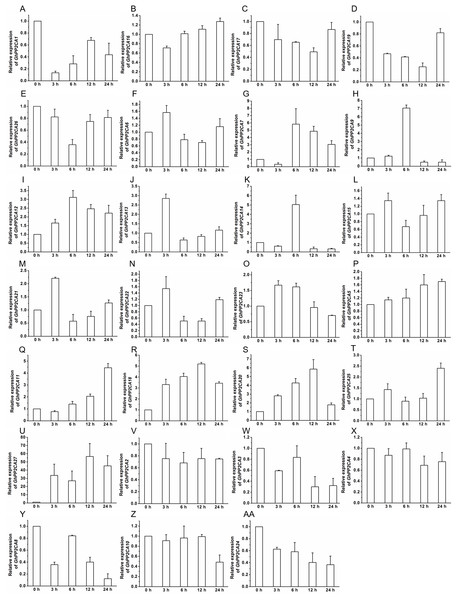
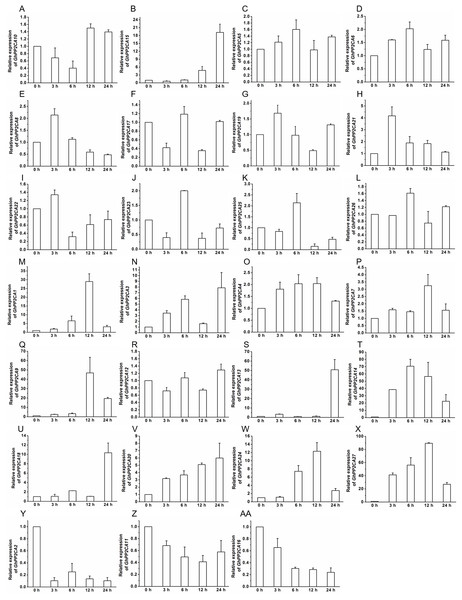
To gain insight into the roles of GhPP2CAs in ABA signaling, transcriptional abundances of GhPP2CAs in roots were detected after treatments with 100 μM ABA or 10% PEG6000 for indicated periods of time. We observed that the transcriptional levels of some GhPP2CA genes such as GhPP2CA5, 11, 18, 20, 25, 27 continually increased with the extension of ABA treatment time. In contrast, the expression levels of some members including GhPP2CA2–4, 8, 10, 24 had decreasing trends. The expression levels of some genes were decreased at 3 or 6 h but increased at 12 or 24 h. These genes included GhPP2CA1, 16, 17, 19, 26. The expression levels of several genes increased at 3 or 6 h but decreased at 12 or 24 h. These genes were GhPP2CA6, 7, 9, 12–15, 21–23 (Fig. 7). Treatment of cotton seedlings with PEG6000 also altered the expression of most GhPP2CA genes (Fig. 8). The majority of GhPP2CAs were upregulated after treatments with PEG for a short time period and downregulated afterward. For example, the transcriptional levels of GhPP2CA8 and GhPP2CA21 were prominently enhanced at 3 h, and then reduced at 6, 12, and 24 h, while those of GhPP2CA5, 6, 17, 23, 25, 26 were pronouncedly increased at 6 h and decreased at 12 and 24 h. By contrast, the expression of some genes was significantly elevated at 12 or 24 h post PEG treatment. These genes included GhPP2CA1, 3, 4, 7, 9, 12–14, 18, 20, 24, 27. The overall trend of expression of GhPP2CA10 and GhPP2CA15 was increased while that of GhPP2CA2, 11, 16 was decreased under osmotic stress (Fig. 8). Together, these data suggest that GhPP2CAs exhibit diverse expression patterns in responses to ABA and osmotic stress.
Figure 7: Expression of GhPP2CA genes in response to ABA.
The relative expression of GhPP2CAs was monitored after treatments with 100 μM ABA for indicated periods of time. The expression levels of the genes were prominently reduced at 3 or 6 h but elevated at 12 or 24 h (A–E), and were increased at 3 or 6 h but decreased at 12 or 24 h (F–O). The transcription abundances of the genes were generally enhanced (P–U), and diminished (V–AA), respectively, with the prolongation of treatment time. Cotton gene GhUBQ7 was applied as the internal control. The gene expression value at 0 h was set as 1. The values were mean ± SE. Statistical analyses were carried out by student’s t-test to determine the differences in gene abundances between 0 h and other treatment times.Figure 8: Expression of GhPP2CA genes in response to osmotic stress.
The relative expression of GhPP2CAs was determined after exposure upon 10% PEG6000 for indicated time. The transcriptional levels of the genes were clearly decreased at 3 or 6 h but increased at 12 or 24 h (A and B), and rose at 3 or 6 h but dropped down at 12 or 24 h (C–L), the expression of the genes in (M–X) was elevated, and that in (Y–AA) was reduced with the extension of treatment time. GhUBQ7 was used as the internal control. The gene expression value at 0 h was set as 1. The values were mean ± SE. Statistical analyses were carried out by student’s t-test to determine the differences in gene abundances between 0 h and other treatment times.Many GhPP2CAs interact with GhPYL2-2D, GhPYL6-2A, and GhPYL9-2A
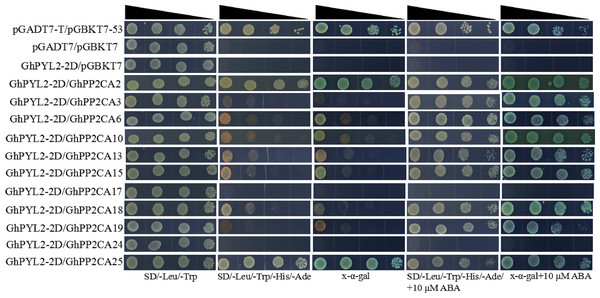
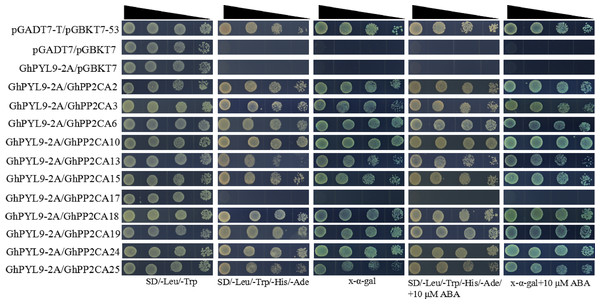
Clade A type 2C protein phosphatases have been documented to interact with ABA receptor PYLs in ABA signal pathway (Ma et al., 2009; Park et al., 2009). Accordingly, we investigated the interactions between GhPP2CAs and GhYPLs in the absence or presence of ABA by yeast-two hybrid method. A total of 11 GhPP2CAs were cloned, and three GhPYLs GhPYL2-2D (Gh_D08G2587), GhPYL6-2A (Gh_A06G1418), and GhPYL9-2A (Gh_A11G0870) were randomly selected and cloned. These genes were fused into yeast vectors, and yeast-two hybrid experiments were performed. In the absence of ABA, GhPP2CA2, and GhPP2CA25, respectively, interacted with GhPYL2-2D while multiple GhPP2CAs like GhPP2CA2, 3, 6, 10, 13, 15, 18, 19, 25 individually interplayed with GhPYL2-2D in the presence of ABA (Fig. 9). In contrast, several GhPP2CAs could, respectively, interact with GhPYL6-2A or GhPYL9-2A either with or without ABA. These GhPP2CAs included GhPP2CA2, 6, 10, 13, 15, 18, 19, 24, 25. Besides, GhPP2CA3 interact with GhPYL9-2A but not with GhPYL6-2A either in the presence or absence of ABA (Figs.10 and 11). These results imply that GhPP2CAs differentially interact with GhPYLs in responding to ABA in cotton.
Figure 9: Analysis of interactions between GhPP2CAs and GhPYL2-2D by yeast-two hybrid method.
Narrowing triangles indicate the reduced cell densities in the dilution series from left to right.Figure 10: Assay of interactions between GhPP2CAs and GhPYL6-2A using yeast-two hybrid technology.
Narrowing triangles show the reduced cell densities in the dilution series from left to right.Figure 11: Determination of interactions between GhPP2CAs and GhPYL9-2A by yeast-two hybrid method.
Narrowing triangles reveal the reduced cell densities in the dilution series from left to right.Discussion
Clade A type 2C protein phosphatases are central components of ABA signal transduction pathway, and negatively control ABA and stress responses in plants (Fuchs et al., 2013; Singh et al., 2015). They have been identified in several plants including Arabidopsis, rice, maize and B. distachyon in recent years (Xue et al., 2008; Wei & Pan, 2014; Cao et al., 2016). However, phylogenesis and putative functions of PP2CAs in Gossypium remain elusive. In the present study, 14, 13, 27, and 23 PP2CA genes were characterized in genomes of G. arboreum, G. raimondii, G. hirsutum, and G. barbadense, respectively (Table 1). Compared to the number of PP2CAs in Arabidopsis (9), rice (10), maize (16), and B. distachyon (8), that in G. hirsutum and G. barbadense was great (Xue et al., 2008; Wei & Pan, 2014; Cao et al., 2016). This suggests that more complex and elaborate ABA signaling mechanisms modulated by PP2CAs may exist in the upland and island cotton species. Conceivably, the high number of PP2CAs of the two species is related to their tetraploid nature. The two plants retain most PP2CA homologs of both G. arboreum and G. raimondii but not a copy of either progenitor during evolution. This may be due to long-term human selection with these two tetraploid cotton species for higher yields, growth in hotter and drier regions, day neutral flowering, and adaptation to agronomic areas far outside their original habitats. These altered characteristics may be associated with more PP2CA proteins and complex ABA signal mechanisms in the cultivated cotton plants than in wild plants and in the greater opportunities to accumulate sequences, amenable to mutation and selection, in a tetraploid genome than in a diploid genome.
We noticed that 27 GhPP2CAs and 23 GbPP2CAs individually had their corresponding orthologs in G. arboreum or G. raimondii (Fig. 1), indicating that those PP2CAs in G. hirsutum and G. barbadense are ancestrally related to those PP2CAs in the two diploid species. Additionally, no orthologous genes of GaPP2CA1, 3, 4, 14, GrPP2CA6, and GrPP2CA9 were observed in G. barbadense (Fig. 1). This hints that these genes are possibly lost, or these genes arose after the tetraploid species appeared and separated from the diploid species during the evolutionary processes.
The structures and the numbers of introns and exons in PP2CAs were similar among the four Gossypium species as well as Arabidopsis, rice, maize and B. distachyon (Xue et al., 2008; Wei & Pan, 2014; Cao et al., 2016), suggesting that the PP2CAs undergo conserved evolutionary processes even after the divergence of monocotyledons and dicotyledons. Colinearity results showed that 136 homologous gene pairs existed among GaPP2CAs, GrPP2CAs, and GhPP2CAs (Fig. 3), indicating that PP2CA genes expand primarily through segmental duplication of DNA. Segmental duplicates may be more often maintained through subsequent gene subfunctionalization compared to tandem duplicates (Lynch & Conery, 2000). Accordingly, these PP2CAs probably had diverse functions in Gossypium. Moreover, in agreement with our results, Arabidopsis phosphatase family genes also showed segmental duplication (Cannon et al., 2004), suggesting the evolutionary mechanism of PP2CAs may be conserved in plants. The mean value of Ka/Ks for a majority of PP2CA homologous gene pairs was about 0.2, significantly less than 1 (Fig. 4). This hints that most mutations occurred in the genomic sequences of PP2CAs in G. arboreum, G. raimondii, and G. hirsutum during evolution were detrimental for plant survival, or the mutations-caused traits were not required for man. Thus, these mutated genes were gradually eliminated, and only those we found were kept during the long-time selection.
Phylogenetic results showed that the PP2CA members from monocotyledonous plants clustered together, and similar results occurred in dicotyledonous PP2CAs (Fig. 5). This suggests that great changes in DNA sequences of the PP2CAs have taken place after isolation of monocotyledons and dicotyledons although these genes shared a common ancestor. PP2CAs in Gossypium always clustered together with those in T. cacao rather than with those in A. thaliana, R. communis, P. trichocarpa, G. max, B. distachyon, and O. sativa (Fig. 5), pointing to the closer evolutionary relationship of Gossypium with T. cacao. That is, most of homologous PP2CA members in Gossypium and cacao were generated before separation of the two genera from the common ancestor. The common PP2CAs across all plants show that they still have core functions essential to basic plant survival and functions. Differences in PP2CAs that follow differentiation of different genera and even species show that they are still diverse and can accommodate functions specific to the survival of species and even in response to selection by man. “Housekeeping” PP2CAs could probably be subtracted from the picture to illuminate the more unique ones to better understand functions of individual PP2CAs and their roles in specific species, traits, or even agronomic performance of specific cultivars.
Transcript abundance analysis indicated that the majority of the GhPP2CAs was predominantly expressed in flowers (Fig. 6), suggesting that GhPP2CA-mediated ABA signaling may be of great importance in flower development of cotton. High expression of GhPP2CAs in flowers was likely due to the importance of timing flowering to environmental conditions of native Gossypium plants. Because evolution of some species is tied to long-term human selection of cotton plants with high yields of fibers and good adaptations to hot and dry growth conditions. Cotton yields are closely associated with flowering in agronomic conditions created by man, which often are much different from the natural habitats of wild or ancestral Gossypium species (e.g., cultivation only in summers of temperate–tropical latitudes instead of perennial growth in tropical latitudes closer to the equator). Drought and hot stresses should limit flower development. PP2CAs are negative regulators of the adverse stresses; and therefore, may facilitate flowering of cotton in these newer environments. The expression of most GhPP2CAs was upregulated after treatment with ABA or PEG6000 (Figs. 7 and 8), in good agreement with the results from AtPP2CAs, OsPP2CAs, and BdPP2CAs (Xue et al., 2008; Cao et al., 2016). These findings imply that PP2CAs are essential for plant response to ABA and osmotic stress.
The interactions between 11 GhPP2CAs and three GhPYLs were examined. The results revealed that most GhPP2CAs can individually interact with the three GhPYLs in the absence or presence of ABA (Figs. 9–11). GhPYLs are homologs of AtPYLs and some GhPYLs have been suggested to be functional ABA receptors (Liang et al., 2017; Zhang et al., 2017b). These data indicate that a large number of GhPP2CAs may play roles via interactions with GhPYLs in ABA-dependent or ABA-independent manner in cotton. The detailed mechanisms of GhPP2CAs in ABA signaling will be further explored in the future.
Conclusions
In total, 14, 13, 27, and 23 PP2CA genes were characterized from G. arboreum, G. raimondii, G. hirsutum, and G. barbadense, respectively. These genes shared high similarity in chromosomal locations, structures, and phylogeny among the species. Most of them might be under purifying selection during evolution. Moreover, PP2CAs displayed specific expression patterns in tissues and diverse expression profiles in response to ABA and osmotic stress in G. hirsutum. Yeast-two hybrid experiments indicated that most GhPP2CAs interacted with GhPYL2-2D, GhPYL6-2A, and GhPYL9-2A with or without ABA. These findings provide essential information for in-depth investigations of the functions of PP2CAs in Gossypium in the future.
Supplemental Information
Gene primers used forquantitative real-time RT-PCR experiments.
Primer Premier 5 software was used to design the primers depending on the CDS of the GhPP2CA genes. The lengths of amplified fragment ranged from 80 bp to 300 bp.
Gene primers applied for yeast two-hybrid experiments.
Primer Premier 5 software was used to design the primers to amplify the CDS fragments of GhPP2CA genes..
PP2C family members in Gossypium.
114, 116, 239 and 232 PP2Cs were identified from G. arboretum, G. raimondii, G. hirsutum and G. barbadense, respectively.
Phylogenetictree of PP2Cs in G. arboretum and Arabidopsis..
The phylogenetic tree of PP2Cs in G. arboretum and A. thaliana were generated by IQTREE server using the maximum likelihood with Dayhoff model. The PP2Cs were clustered into 12 clades (A-L) being indicated by different colors.
Phylogenetic tree of PP2Cs in G.raimondii and Arabidopsis.
The phylogenetic tree of PP2Cs in G. raimondii and A. thaliana were generated by IQTREE server using the maximum likelihood with Dayhoff model. The PP2Cs were clustered into 12 clades (A-L) being indicated by different colors.
Phylogenetictree of PP2Cs in G. hirsutum and Arabidopsis..
The phylogenetic tree of PP2Cs in G. hirsutum and A. thaliana were generated by IQTREE server using the maximum likelihood with VT+F+G4 model. The PP2Cs were clustered into 12 clades (A-L) being indicated by different colors.
Phylogenetic tree of PP2Cs in G. barbadense and Arabidopsis.
The phylogenetic tree of PP2Cs in G. barbadense and A. thaliana were generated by IQTREE server using the maximum likelihood with VT+F+G4 model. The PP2Cs were clustered into 12 clades (A-L) being indicated by different colors.
Alignment files and newick treefiles.
Raw data for phylogenetic trees.