In vitro performance in cotton plants with different genetic backgrounds: the case of Gossypium hirsutum in Mexico, and its implications for germplasm conservation
- Published
- Accepted
- Received
- Academic Editor
- Axel Tiessen
- Subject Areas
- Biodiversity, Biotechnology, Conservation Biology, Plant Science
- Keywords
- Crop wild relatives, GMOs, In vitro germplasm conservation, 13 Aichi Biodiversity Targets, Ex situ conservation, Primary genetic pool
- Copyright
- © 2019 Hernández-Terán et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. In vitro performance in cotton plants with different genetic backgrounds: the case of Gossypium hirsutum in Mexico, and its implications for germplasm conservation. PeerJ 7:e7017 https://doi.org/10.7717/peerj.7017
Abstract
One of the best ex situ conservation strategies for wild germplasm is in vitro conservation of genetic banks. The success of in vitro conservation relies heavily on the micropropagation or performance of the species of interest. In the context of global change, crop production challenges and climate change, we face a reality of intensified crop production strategies, including genetic engineering, which can negatively impact biodiversity conservation. However, the possible consequences of transgene presence for the in vitro performance of populations and its implications for biodiversity conservation are poorly documented. In this study we analyzed experimental evidence of the potential effects of transgene presence on the in vitro performance of Gossypium hirsutum L. populations, representing the Mexican genetic diversity of the species, and reflect on the implications of such presence for ex situ genetic conservation of the natural variation of the species. We followed an experimental in vitro performance approach, in which we included individuals from different wild cotton populations as well as individuals from domesticated populations, in order to differentiate the effects of domestication traits dragged into the wild germplasm pool via gene flow from the effects of transgene presence. We evaluated the in vitro performance of five traits related to plant establishment (N = 300): propagation rate, leaf production rate, height increase rate, microbial growth and root development. Then we conducted statistical tests (PERMANOVA, Wilcoxon post-hoc tests, and NMDS multivariate analyses) to evaluate the differences in the in vitro performance of the studied populations. Although direct causality of the transgenes to observed phenotypes requires strict control of genotypes, the overall results suggest detrimental consequences for the in vitro culture performance of wild cotton populations in the presence of transgenes. This provides experimental, statistically sound evidence to support the implementation of transgene screening of plants to reduce time and economic costs in in vitro establishment, thus contributing to the overarching goal of germplasm conservation for future adaptation.
Introduction
Interest in plant germplasm conservation addresses the need to preserve a diverse genetic pool, thus providing options for future decision-making (Rockstrom et al., 2014). Such options must include genetic and phenotypic diversity to face current and future challenges in crop production. The conservation of these variants can help in developing or finding solutions to disease, changing environments, and low yields, among others, and is necessary for safeguarding biodiversity and cultural identity (Hawkes, 1977; Plucknett et al., 1983; Hajjar & Hodgkin, 2007). In most crops, the largest genetic variation exists in the Crop Wild Relatives (CWR) found in the centers of origin of the species (Hawkes, 1977). Accordingly, research groups in food security identified CWR as a target group for conservation (Harlan, 1965; Hunter & Heywood, 2011; Castañeda Álvarez et al., 2016). Due to the importance of CWR for conservation, international agreements have made the in situ and ex situ preservation of their genetic diversity one of their goals (Aichi Target 13) (Leadley et al., 2014).
Plant tissue culture methods represent a robust approach for many purposes, from being a tool designed to pursue a variety of basic research questions to helping ex situ preservation of genetic diversity (Engelmann, 1991; Gosal & Kang, 2012). The most successful example of tissue culture in commercial and conservation applications is micropropagation: the propagation of plants from small parts under in vitro conditions. The success of micropropagation in tissue culture is due to ease of having multiple genetic clones from different geographic locations, thus lowering the risk of loss of such genotypes (Kumar & Reddy, 2011; Rajasekharan & Sahijram, 2015).
Despite the great advantages of micropropagation for in vitro conservation of plant species, different factors can compromise its success, such as culture medium composition, environmental conditions and genotype, among others (Li et al., 2002; Tyagi et al., 2004; Kumar & Reddy, 2010). In fact, it has been shown that different cultivars of the same species can have different in vitro performance or success (Gubis et al., 2003; Pathi & Tuteja, 2013). This reveals the sensitivity of in vitro culture to even small genetic variation. In the past few decades, the use of Genetically Modified Organisms (GMO) has become extensive (ISAAA, 2017) and a source of new genetic variation even in countries that are considered centers of origin of important crops (Lu, 2008). In some cases, the release of GMOs in areas with CWR or with other crops and weeds, has caused gene flow events across populations of different economically important cultivars, such as maize, cotton, papaya, bent grass, alfalfa and canola (Quist & Chapela, 2001; Warwick et al., 2008; Piñeyro Nelson et al., 2009; Wegier et al., 2011; Greene et al., 2015; Manshardt et al., 2016). Thus, given the extensive use of GMO technologies in economically important cultivars, it becomes relevant to analyze all evidence related to the effects of this introduced variation on the in vitro culture germplasm conservation efforts.
Mexico is the center of origin for cotton (Gossypium hirsutum L.) (Ulloa et al., 2006; Burgeff et al., 2014), and its metapopulations have been found on the coasts of the country, while extensive cultivars can be observed in the northern states, and backyard/home garden plants and native varieties have been reported in the southeastern states (Velázquez-López et al., 2018). Previous studies have described the genetic diversity of the Mexican metapopulations, including the presence of transgenes in some of them (Wegier et al., 2011), suggesting gene flow associated with specific transformation events (i.e., transgene introduction) from extensive cultivars to metapopulations. Ellstrand (2018) has posed the hypothesis that the majority of Mexican cotton metapopulations do not correspond with wild relatives of cotton (truly wild), but are instead a mix of escaped cultivar individuals that have evolved in wild conditions (weedy-wild). Nonetheless, even if the cotton metapopulations are weedy-wild relatives, they are part of the primary genetic pool (Heywood et al., 2007) and are thus of conservation interest due to their genetic diversity (Ellstrand, 2018). Phenotypic consequences of genetic flow (including transgene flow) into wild and domesticated lines in other species have been suggested in previous studies (Hernández-Terán et al., 2017); therefore, in vitro culture performance could also be affected, in principle, by genetic modification. This could have important consequences for the success of germplasm conservation strategies.
In the present study, and given the genetic diversity of G. hirsutum in Mexico, we analyzed experimental evidence of the effects of transgene presence on the in vitro performance of representative population clones of G. hirsutum diversity, and reflect on the implications of such effects for ex situ genetic conservation. For these reasons, and given that transgene presence in cotton metapopulations (hereafter wild germplasm) can be directly attributed to gene flow from domesticated populations, we included individuals from domesticated populations in our comparisons in order to differentiate the effect of domestication traits dragged into the wild germplasm pool via gene flow from the mere presence of transgenes. Thus, we hypothesize that (i) given that transgenes are directed to specific traits (e.g., defense and herbicide tolerance), which are not related to in vitro performance, we will not find differences in such performance between populations with and without transgenes, and (ii) the only differences to be found in the in vitro performance will be those associated with the domestication process, between wild and domesticated populations.
Material and Methods
Experimental design
To evaluate the potential effects of transgene presence on the in vitro culture performance of wild cotton plants and its consequences for germplasm conservation efforts, we conducted a systematic analysis of the performance of specific traits of an in vitro germplasm collection of cotton plants. We included a representative sample of the genetic diversity of wild population plants with (WT) and without (W) transgenes (i.e., no isogenic lines), to test the effect of transgenes on the in vitro performance of metapopulation variants. Given the interest of this study for conservation strategies, we intentionally look for diversity of the genetic background of the analyzed clones, in other words population level diversity. In addition, and as a preliminary attempt to distinguish the effects attributable to transgenes from the effects attributable to flow from domesticated or cultivar populations into wild populations, we included domesticated plants with (DT) and without (D) transgenes in the experiment and analyses.
Germplasm collection
In order to have a germplasm collection that is representative of the genetic diversity of wild cotton populations in Mexico, we collected seeds from individual plants in populations spanning its natural distribution or in metapopulations (Wegier et al., 2011). Ten seeds of each individual plant in the collection were germinated in prepared substrate (Peat Moss, agrolite, vermiculite (3:1:1)) and 50 g of slow-release Osmocote fertilizer (14N-14P-14K, [Scott’s, Marysville, Ohio]), in a greenhouse under controlled conditions (25 ± 5 °C). Once the seedlings emerged, the apex and the first three axillary buds were explanted to start the in vitro culture. We also included germplasm of domesticated plant individuals from farmer’s markets in Mexico City as representatives of the domesticated cotton populations.
In vitro culture establishment and propagation
Establishment
All the experimental procedures were done under the license of the Servicio Nacional de Inocuidad y Calidad Agroalimentaria (SENASICA) trough the Aviso de Utilización Confinada de OGM (folio: 007_2016). Once disinfected (Appendix S1), the axillary buds were transferred to culture tubes containing 6 ml of MS basal medium (Murashige & Skoog, 1962). Each tube was sealed with Parafilm M (Bemis, USA) to prevent contamination. The culture tubes were incubated in a growth room at 24 °C for a 12-hours photoperiod.
Propagation
Propagation was initiated with individual explants that reached 8 cm height or the height of the culture tube, or when the initial culture exhausted the culture medium. Propagation consisted in removing the explants from the culture tubes, cutting each of the new axillary buds and planting them in a culture tube with fresh medium. The process was performed under sterile conditions in a laminar-flow hood (ThermoFisher, Massachusetts, USA). For more information, see Appendix S1.
Detection of transgenes in the germplasm collection
To characterize the populations under evaluation (i.e., Wild (W) and Domesticated (D)), we looked for two constructions of lepidopteran resistance and one of herbicide tolerance (Cry1Ab/Ac, Cry2Ab and CP4EPSPS) in all individuals of the collection. This allowed us to detect 23 of the 33 transgenic cotton events released in Mexico (ISAAA, 2018). For the wild cotton populations (W and WT) we carried out two independent tests to verify the presence of the genetic events: enzyme-linked immunoabsorbent assay (ELISA), and sequencing of Polymerase Chain Reaction (PCR) products. For the domesticated cotton populations (D and DT), transgenic events were verified only with a PCR-sequencing assay. The ELISA tests were performed in duplicate using the following kits: Bt-Cry1Ab/1Ac ELISA Kit, Bt-Cry2A ELISA Kit, and Roundup Ready ELISA Kit (Agdia, Elkhart, Indiana, USA). The results were read in a MultiskanFC Microplate Photometer (ThermoFisher Scientific, Massachusetts, USA). We considered a sample to be positive only when its absorbance was equal to or above three standard deviations from the average intensity of all negative controls and blank samples. In all ELISA plates a blank sample (extraction buffer), a negative, and a positive control provided in each detection kit were included. ELISA results are available as supplementary material (ELISA_results.xslx).
For the PCR assays, DNA extraction was performed in duplicate for each individual, following the DNA Miniprep CTAB method reported in Wegier et al. (2011). The quality and concentration of the DNA were analyzed in a NanoDrop 2000 (ThermoFisher Scientific, Massachusetts, USA). The PCR assay was performed with the primers Cry1Ab/Ac (F 5′ACCGGTTACACTCCCATCGA 3′, R 5′CAGCACCTGGCACGAACT 3′), Cry2Ab (F 5′CAGCGGCGCCAACTCTACG 3′, R 5′TGAACGGCGATGCACCAATGTC 3′), and CP4EPSPS (F 5′GCATGCTTCACGGTGCAA 3′, R 5′TGAAGGACCGGTGGGAGAT 3′) from Eurofins Scientific (Brussels, Belgium). The assay was carried out according to the references provided in Appendix S3. Subsequently, the amplicons result of the PCR assay were verified by Sanger sequencing. The sequencing was done in the Laboratorio de Secuenciación Genómica de la Biodiversidad y de la Salud in the Instituto de Biología, UNAM. Raw sequences are available in GenBank platform (accession number MK089921 to MK089930; Appendix S5).
Data collection
To evaluate the potential consequences of transgene presence for the in vitro performance of cotton populations, we measured different traits of individuals in our germplasm collection. All data analyzed is included as supplementary material (Supplementary_dataset.xlsx).
During the establishment of the in vitro germplasm collection we documented differences in propagation success in a period of two years that included a total of 4,377 axillary buds (corresponding to 74 individual original plants, 27 with transgenes and 47 without transgenes). From this original sample, we randomly selected 20 individuals (five wild with transgenes (WT), five wild without transgenes (W), five domesticated without transgenes (D) and five domesticated with transgenes (DT)), and 15 replicates per individual (N = 300) to evaluate in vitro performance of four phenotypic traits (leaf rate, height rate, microbial growth, and root development). This collection was intended to be a fair representation of the wild cotton metapopulations genetic backgrounds diversity, since it includes five out of the eight populations reported in Mexico (Wegier et al., 2011) plus ten individuals from the domesticated genetic background. Data for these traits were collected weekly during a four-month period. As mentioned above, all the experiments were conducted in a growth room at 24 °C under a 12-hours photoperiod. A detailed scheme of the experimental design is available in the Appendix S2.
Specifics for the evaluated traits are:
-
propagation rate, calculated as the number of buds derived from a single individual every two weeks during two years of continual propagation, or the slope of the linear regression model using the lm function in R (no. buds Vs. time) (WT N = 1800, W N = 2577, D N = 490, DT N = 633);
-
leaf rate, or leaf production rate, calculated as the number of leaves derived from a single individual every week during four months of in vitro culture, or the slope of the linear regression model using the lm function in R (no. leaves Vs. time) (WT N = 75, W N = 75, D N = 75, DT = 75);
-
height rate, or height increase rate, calculated as the height (cm) of a single individual measured every week during four months of in vitro culture or the slope of the linear regression model using the lm function in R (cm Vs. time) (WT N = 75, W N = 75, D N = 75, DT = 75);
-
microbial growth, measured as observable growth of either bacterial or fungal organisms associated to plant tissue, potentially attributable to possible endophyte overgrowth (WT N = 75, W N = 75, D N = 75, DT = 75). In accordance with Quambusch & Winkelmann (2018), we only considered as possible endophytes those microorganisms growing directly on the explant, not in the culture media;
-
root development, determined by the average number of days that it took for the roots to develop after in vitro establishment, multiplied by the number of individuals that developed roots, and divided by the total number of analyzed individuals (WT N = 75, W N = 75, D N = 75, DT = 75).
No transformation of the dataset was carried out for further analysis.
Data analysis
To determine if there are statistical differences among experimental groups for all the traits simultaneously, we carry out a Permutational Multivariate Analysis of Variance (PERMANOVA) (Legendre & Anderson, 1999) based on 1,000 permutations using adonis function in vegan R package (Oksanen et al., 2018). As a post-hoc test we carry out a Wilcoxon rank sums test in order to distinguish differences in the individual traits. This statistical approach was performed based on Rebollar et al. (2017). In addition, to evaluate the potential differences between the analyzed populations, we conducted a Non-Metric Multidimensional Scaling (NMDS) with Manhattan distance in the vegan R package (Oksanen et al., 2018). We selected the NMDS due to its flexibility, which allowed us to use different types of variables and make few assumptions about the nature of the data (Legendre & Legendre, 2012). Given that the number of entries for “propagation rate” was higher than for the rest of the evaluated traits, we subsampled these entries with the “sample” function in R. To evaluate if the subsample dataset was representative of the full dataset, we conducted a paired sample T-test. All the analyses were conducted using R software (version 2.4-6) (R Core Team, 2013).
Results
In vitro culture performance is significantly different between W and WT populations
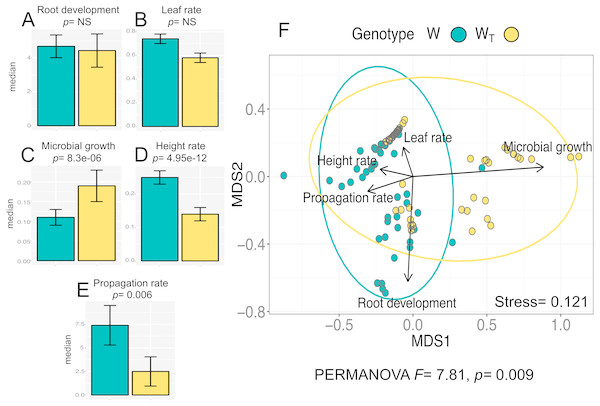
The analysis for the in vitro culture performance traits in wild populations with (WT) and without (W) transgenes shows statistically significant differences between populations for all traits (PERMANOVA, F = 7.81, p = 0.0009) and for three out of the five individual traits according to the Wilcoxon test (“height rate” p = 4.95e−12 ; “microbial growth” p = 8.3e−06; “propagation rate”; p = 0.006). Figure 1A–1E shows the values for all analyzed traits per population. In particular, we want to emphasize that “height rate” as an in vitro performance trait had the largest difference between W and WT populations.
Figure 1: In vitro culture performance traits of W and WT populations.
W, wild populations without transgenes, and WT, wild populations with transgenes. (A–E) median and standard error of all analyzed traits in both populations. P values were obtained through Wilcoxon test. (F) Non-Metric Multidimensional Scaling (NMDS) that include all the analyzed traits in the two populations. The ellipses represent 95% confidence interval around the centroids.Multivariate analysis of in vitro performance traits (NMDS) in W and WT populations (Fig. 1F) shows different phenotypic variations attributable to each population (W and WT). “Propagation rate” is a trait positively related to W population; in contrast, “microbial growth” is positively related to WT population.
In vitro culture performance differs between W and D populations
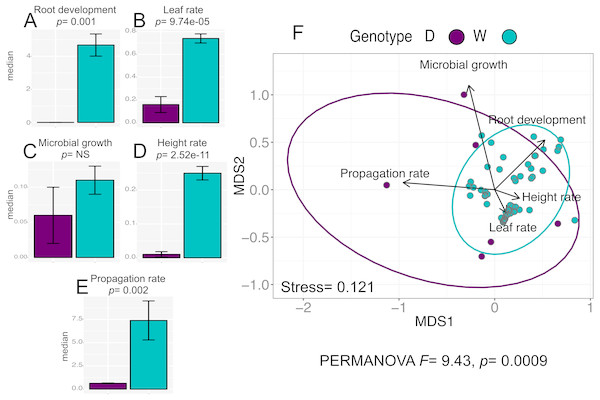
The analysis for the in vitro culture performance traits in wild (W) and domesticated (D) populations does show statistically significant differences between populations for all traits (PERMANOVA, F = 9.43, p = 0.0009), and for four out of the five individual traits: “leaf rate” (Wilcoxon, p = 9.74e−05), “propagation rate” (Wilcoxon, p =0.002), “root development” (Wilcoxon p = 0.001), and “height rate” (Wilcoxon p = 2.52e−11) (Figs. 2A–2E).
Figure 2: In vitro culture performance traits of W and D populations.
D, domesticated populations without transgenes, and W, wild populations without transgenes. (A–E) median and standard error of all analyzed traits in both populations. P values were obtained through Wilcoxon test. (F) Non-Metric Multidimensional Scaling that include all the analyzed traits in the two populations. The ellipses represent 95% confidence interval around the centroids.Moreover, although the graphic representation of multivariate analysis of in vitro performance traits (NMDS) in W and D populations shows overlapping of the ellipses representing each population, the statistical analysis of multivariate differences (PERMANOVA) in both populations is statistically significant (Fig. 2F).
In vitro culture performance differs between WT and DT populations and between D and DT populations
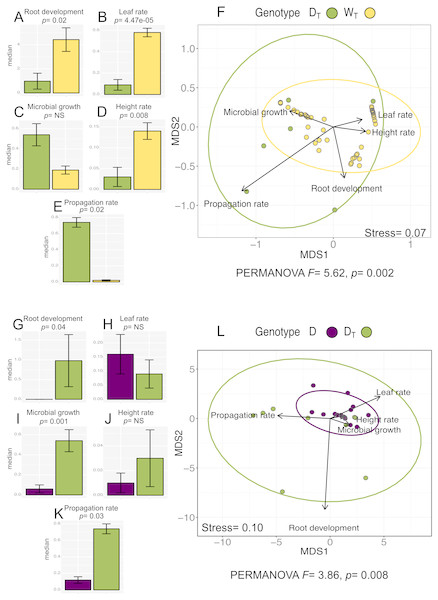
The analysis for in vitro culture performance traits in wild (WT) and domesticated (DT) populations with transgenes shows statistically significant differences between populations in four out of the five in vitro performance traits (Wilcoxon “height rate” p = 0.008; “leaf rate” p = 4.47e−05; “propagation rate” p = 0.02; “root development” p = 0.02) (PERMANOVA, F = 5.62, p = 0.002). Figures 3A–3E shows the values for all the analyzed traits per population. In particular, we want to emphasize that although WT has higher values for “root development”, “leaf rate” and “height rate” traits, DT population has higher “propagation rate”.
Figure 3: In vitro culture performance traits of DT and WT and between D and DT populations.
In genotype, DT, domesticated populations with transgenes, WT, wild populations with transgenes, D: domesticated populations without transgenes. (A–E) (G–K) median and standard error of all analyzed traits in both populations. P values were obtained through Wilcoxon test. (F) (L) Non-Metric Multidimensional Scaling that include all the analyzed traits in the analyzed populations. The ellipses represent 95% confidence interval around the centroids.In the case of the analysis of domesticated populations with (DT) and without (D) transgenes, we also found statistically significant differences in three out of the five analyzed traits (Wilcoxon “microbial growth” p =0.001; “propagation rate” p = 0.03; “root development” p = 0.04) (PERMANOVA, F = 3.86, p = 0.0008) (Figs. 3G–3K), showing that DTin general has a better in vitro performance.
The multivariate analysis of in vitro performance traits (NMDS) in WT and DT populations (Fig. 3F) shows different phenotypic variations attributable to each population (WT and DT), which coincides with the same analysis for W and D populations (with no transgenes identified; Fig. 2F). Moreover, “propagation rate” is positively related to DT population, while the rest of the traits seems positively related to WT population.
In the analysis of D and DTpopulations, the NMDS (Fig. 3L) shows overlapping of the ellipses representing the data set distribution of both populations, despite the significance of the statistical analysis (PERMANOVA).
To look at the full data set (W, WT, D, DT), and beyond the pairwise hypotheses, we carried out the NMDS and PERMANOVA analyses. The results of the full data set analyses are coherent with the pairwise comparisons, which supports the above mentioned observations (Fig. S5).
Discussion
Conservation of the genetic and phenotypic diversity in the CWR has been acknowledged as key in the preservation of diverse gene pools to secure genetic alternatives for future decisions and interventions regarding crop production. Of the different conservation strategies that exist, in vitro gene banks represent a robust approach to preserve genetic diversity without introducing unintended variations into the genetic pool (Engelmann, 1991; Gosal & Kang, 2012). In recent decades, the use of GMOs has become extensive (ISAAA, 2017), and a source of new genetic variation, even in centers of origin of important crops (Lu, 2008). This makes it important to evaluate evidence of the effects, if there are any, of this introduced variation on in vitro culture germplasm conservation efforts.
In order to analyze evidence of potential effects of transgene presence in cotton metapopulations, we compared in vitro performance traits in metapopulations with (WT) and without (W) transgenes. We found significant differences in in vitro culture performance between W and WT populations for three out of the five analyzed traits (Figs. 1A–1E). Previous studies with different crop populations have shown that even small genotypic changes can have major impact in phenotypes and fitness traits both in field experimental settings (Hernández-Terán et al., 2017) and in in vitro culture (Gandonou et al., 2005; Landi & Mezzetti, 2006; Kumar & Reddy, 2011). Thus, it can be argued that the observed differences in in vitro culture performance could be the result of natural genetic variation within and among populations, however when we look at potential differences among W metapopulations, we found no statistically significant differences (Appendix S4). Nonetheless, the observed differences between WT and W populations could be attributed, as in other studies, to pleiotropic effects, where certain phenotypic traits may be linked to and affected by the genetic modification of another trait (Filipecki & Malepszy, 2006). In this sense, some studies have shown that genetic modification can alter metabolic pathways due to position effects of transgenes or somaclonal variation during tissue culture (Agapito-Tenfen et al., 2013; Mesnage et al., 2016). In the specific case of cotton (Wang et al., 2015), some authors have found overexpression of metabolites related to energy metabolism pathways that could indicate an increased demand for energy, and concomitant changes in resource allocation and development. Pleiotropic effects, have also been attributed to bottlenecks, selective sweeps, phenotypic plasticity or gene x environment (GxE) interactions (Remington et al., 2001; Pozzi et al., 2004; Gunasekera et al., 2006; Doust et al., 2014). In addition, ecological costs in different plant species have been associated with the expression of transgenes (Chen et al., 2006). Specifically, some studies show that the physiological production of transgene toxins (e.g., Cry proteins) is extremely costly, limiting the energy destined for growth and reproduction. This trade-off caused by the genetic modification has been found in Brassica (Snow, Andersen & Jorgensen, 1999) and Arabidopsis (Bergelson et al., 1996). Although in our study we cannot attribute these performance differences only to the presence of transgenes (i.e., no strict control of genotypes), we can say that all individuals identified as WT were positive for the expression of transgene proteins in the ELISA approach, which is suggestive of a potential cost that gets reflected in the in vitro performance of WT populations. In general, in vitro performance differences between W and WT could be explained, at least with the information here collected, by ecological costs associated to the expression of transgenes and by potential pleiotropic and GxE interactions associated with small genetic differences.
In order to isolate the effect of domestication from the effect of transgenes on the in vitro performance of cotton populations, we compared the in vitro performance of both wild metapopulations and domesticated populations with and without transgenes. In the case of comparisons of populations without transgenes (W-D) we found significant differences in four out of the five analyzed traits (Figs. 2A–2E). Such phenotypic differentiation, regardless of substantial evolutionary divergence and genetic differentiation (Fang et al., 2017), is somehow unexpected, since differentiation between these populations is the result of a selective process focused on traits that are not related to in vitro performance, such as length, size and color of the fiber, loss of seed dispersal and germination speed (Lubbers & Chee, 2009; Gross & Strasburg, 2010; Velázquez-López et al., 2018). Nonetheless, strong selective forces associated with domestication and divergence times between populations are together of sufficient strength to show phenotypic differentiation even in an environment to which both W and D populations were naïve to (in vitro conditions).
Regarding the comparison of wild (WT) and domesticated (DT) populations with transgenes, we found significant differences in four out of five analyzed traits, with WT populations being better performers than DT populations in the in vitro culture in general (Fig. 3), with the important exception of one trait, “propagation rate”. This better performance of DT populations for “propagation rate” could be the result of a history of selection in in vitro culture, which is part of the conventional process of genetic engineering, through which transgenes are introduced in the domesticated plants’ genetic backgrounds (Hooykaas & Schilperoort, 1992). This suggests that since GMOs have previously gone through an in vitro process, it could be possible that GM plants that have been selected for culture are better adapted to these conditions. In contrast, for the rest of traits, WT populations perform better (higher “height rate”, “leaf rate”, and “root development”), to which a potential mechanistic explanations or hypotheses are hard to articulate. One possibility is that, given the reduced genetic variation of D populations in comparison with W populations, the general performance of D populations might be expected to be worse in environmentally astringent conditions (Flint-garcia, 2013; Lu, 2013), such as in vitro culture.
Regardless of the trait performance direction of populations (WT and DT), we can argue that given the existent differences between D and W genotypes without transgenes (see above and Velázquez-López et al., 2018), it is expected that additional genetic changes (due to gene insertion) could contribute to increased phenotypic differentiation. Nonetheless, given that we did not determine the exact location of the inserted transgenes in the genome, it is not possible to give a mechanistic explanation to the specific trait differences. Overall, we can conclude that our results suggest that the presence of transgenes, originally associated with domesticated populations, has a significant impact on the in vitro performance of the genotypes, regardless of their wild or domesticated origin.
Implications of transgene presence for In vitro wild germplasm conservation
One of the best ex situ conservation strategies for wild germplasm are in vitro banks (Gosal & Kang, 2012). In vitro conservation success depends on efficient and reliable micropropagation or in vitro performance of the species of interest (Mycock, Blakeway & Watt, 2004). Despite the reality of crop intensification, including genetic engineering, the possible consequences of the presence of transgenes for the in vitro performance of populations are poorly documented. In this study, we present results that suggest detrimental consequences for the in vitro culture performance of wild cotton populations in the presence of transgenes, which calls for monitoring transgenes in the plants to be micropropagated for conservation or future genetic improvement, as has been suggested by other authors, as conservation strategies and protocols (Bhatia, 2015). Moreover, it is worth noting that in the present study, with a minimal investment of three primer sets for transgene detection, we were able to identify 23 out of 33 transformation events reported for cotton populations in Mexico (ISAAA, 2018). As it stands, our results provide experimental evidence to support the implementation of transgene screening of plants to reduce time and economic costs in in vitro establishment, helping the overarching goal of germplasm conservation for future adaptation.
In current scenarios of global change, uncertain future conditions pose the major challenge of securing resources for future adaptations (Wise et al., 2014). In this sense, it is of utmost importance to preserve options for future decisions and to guarantee the right to biodiversity and cultural identity for future generations, which includes genetic and phenotypic options (Rockstrom et al., 2014). Crop biodiversity preservation is, in other words, part of our life insurance for future adaptation in a changing planet. In this sense, future work on conservation strategies and policies should put effort in expanding the knowledge about the consequences of transgene presence (Lu, 2013) beyond the immediate gene pool of wild populations. This means extending the efforts breadth towards other interfertile species; in other words, to the genetic primary pool.
Conclusions
The results presented show how transgene presence in CWR cotton populations has negative consequences for their in vitro culture performance. In particular, reviewing our hypotheses, we found that (1) in vitro culture performance is significantly different between W and WT populations, and (2) in vitro culture performance is different between wild and domesticated populations regardless of transgene presence. Overall, our results suggest that the presence of transgenes in wild populations imposes a cost (e.g., metabolic cost of maintaining the expression of toxins) that is reflected in their in vitro performance and that could endanger the success of germplasm conservation efforts. Further studies controlling for specific genotypes and specific transgene constructions would help to better disentangle the costs associated with specific genomic contexts and genetic modifications to improve genetic screenings for in vitro banks.
Supplemental Information
Supplementary material
Appendix S1.Specifics of the in vitro culture technique used in the establishment and propagation of the germplasm collection. Appendix S2. Scheme of experimental design. Appendix S3. Transgene detection of in vitro germplasm collectio. Appendix S4.In vitro performance at metapopulation level. Appendix S5. Raw sequences from Sanger verification of PCR amplicons.
ELISA assays results
Absorbance values per analyzed sample. Tab names refer to the results table for the different analyzed proteins (i.e. Cry2A; Cry1Ab; CP4epsps). Positive values are highlighted in red.