A novel and enigmatic two-holed shell aperture in a new species of suspension-feeding worm-snail (Vermetidae)
- Published
- Accepted
- Received
- Academic Editor
- Suzanne Williams
- Subject Areas
- Biodiversity, Marine Biology, Taxonomy, Zoology
- Keywords
- Mollusca, Morphology, Barrier reef, Diversity, Anatomy, Western Atlantic, Taxonomy, New taxa, Gastropoda
- Copyright
- © 2019 Bieler et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. A novel and enigmatic two-holed shell aperture in a new species of suspension-feeding worm-snail (Vermetidae) PeerJ 7:e6569 https://doi.org/10.7717/peerj.6569
Abstract
Shell aperture modifications are well known in terrestrial and aquatic gastropods, with apertural lip thickening and tooth development common in species with terminal (determinate) shell growth. In contrast, secondary shell openings are rare in snails and are largely limited to slit shells, keyhole limpets, and abalone of the Vetigastropoda. When such features occur in other groups, they are noteworthy and raise interesting questions concerning the functional/adaptive significance of these shell modifications. Here we report on one such modification in a newly described species of vermetid snail. Members of the worm-snail family Vermetidae are sessile, suspension-feeding caenogastropods found in warm temperate to tropical marine environments worldwide. As juveniles, vermetids permanently cement their shells to hard substrata and subsequently produce irregularly coiled polychaete-like shell tubes with indeterminate growth and typically a simple circular shell aperture. In one previously studied group (genus Cupolaconcha), the aperture can be covered by a shell dome with a central slit that retains its widest opening in the center of the aperture. Vermetid specimens collected in the barrier reefs of Belize and the Florida Keys show an extreme aperture modification previously unknown in Gastropoda, in which the shell opening is covered by an apertural dome that leaves two equal-sized circular holes, each corresponding to the inflow and outflow water exchange currents of the animal’s mantle cavity. The function of this perforated apertural dome is unknown, and it is in some ways antithetical to the suspension feeding habit of these snails. Further field and laboratory-based studies will be needed to clarify the functional significance and trade-offs of this unique morphology. The new taxon, which is not closely related to the previously described dome-building clade Cupolaconcha, is described and named as Vermetus biperforatus Bieler, Collins, Golding & Rawlings n. sp.
Introduction
In most gastropods, both terrestrial and aquatic, the animal interacts with the external world through a single aperture in its shell, which often can be sealed with a horny or calcareous operculum. This opening usually serves for extension of the head-foot for locomotion and feeding, for the exchange of air or water in the mantle cavity that houses the lung or gill(s), and for the removal of waste products and release of gametes. There are some known exceptions to that single-aperture bauplan: Several groups among the marine Vetigastropoda use a secondary outside connection derived from a groove at the periphery of the early shell (selenizone), a character already known from Palaeozoic gastropods (Hickman, 1984). Among the living vetigastropods, the selenizone is variously expressed, for instance as a slit in Pleurotomariidae, a single hole on Fissurellidae, and a series of holes in Haliotidae. However, selenizones do not occur in the other clades of gastropods and secondary shell openings are rare in caenogastropods. One such exception occurs in the slit-worm-snail family Siliquariidae, a cerithioidean caenogastropod group with sessile, mostly irregularly coiling shells that is not closely related to the vermetid worm-snails discussed herein (Bieler & Petit, 2011). Some siliquariids (e.g., genus Tenagodus) live mostly or completely embedded within sponges and have evolved a mutualistic relationship with the surrounding host sponge by sharing water flow through a shell slit or a series of perforations (Bieler, 2004). Modifications of the primary shell aperture by extensions, narrowing, tooth structures etc. are common in both terrestrial and marine gastropods (e.g., Wada & Chiba, 2013; Vermeij, 2015) and have likely evolved independently many times. Vermeij (2007), for instance, has reported that a closed siphonal canal associated with increased protection of soft anteriorly-positioned tissues, has arisen 15 times in caenogastropods with determinate growth. In combination with an often tightly fitting horny or calcareous operculum, apertural modifications complete the protection afforded by the shell and, in land snails, prevent desiccation (Paul, 1991). Operculate land snails have evolved various shell devices to allow gas exchange while the operculum tightly seals the aperture; these structures range from simple bypass slits in the shell aperture to elaborate snorkel structures (Páll-Gergely, Naggs & Asami, 2016). A unique aperture modification has also been reported from a Miocene member of the marine caenogastropod family Melongenidae, in which an adapical septum is formed that closes off the last-formed shell spine at the outer apertural lip (Vermeij & Raven, 2009).
In the marine gastropod family Vermetidae, a suspension-feeding caenogastropod group that is characterized by irregularly coiling shells permanently cemented to hard substrata, apertural modification usually is subtle. No terminal shell growth is evident and the normally circular shell opening is surrounded by regular shell wall without additional thickening or tooth-like structures narrowing or reinforcing the opening. Adult vermetids add shell material to their apertural rims in response to damage, current patterns that influence their suspension feeding, and neighboring obstacles (Schiaparelli & Cattaneo-Vietti, 1999). Some vermetid groups (e.g., members of nominal genera Vermetus, Petaloconchus, Eualetes, Thylacodes, and Thylaeodus; R Bieler, pers. obs., 2018) build comparatively thin-shelled “feeding tubes”, apertural extensions that allow the sessile snail to orient the aperture around obstacles and toward the water current. The terminal portion of feeding tubes at times is slightly narrowed. Such terminal modifications need to be removed when the animal extends the tube, and vermetids are indeed capable of cutting the shell tube by radular action (Schiaparelli & Cattaneo-Vietti, 1999). This ability allows some species (genus Cupolaconcha; see Golding et al., 2014) to build, and when necessary remove, temporary thin-shelled domes that greatly constrict the aperture, only leaving a narrow central elliptical or lens-shaped slit through which the animal accomplishes feeding and gas exchange.
The new species described herein from specimens collected in the Belizean and Florida Keys barrier reefs takes such aperture restrictions to the extreme. Here, the animal builds a temporary apertural shell dome that leaves two small, identical, circular holes as the only connections to the outside world. Comparative morpho-anatomical (and separately developing molecular) investigations show that this species is not a member of the previously described dome-producing clade Cupolaconcha and—partly in preparation for forthcoming genomic and ultrastructural studies involving this newly discovered species—is introduced in the following as Vermetus biperforatus n. sp.
Materials & Methods
Specimens were collected and studied in the context of larger vermetid surveys as outlined by Golding et al. (2014). Field work in Belize in April 2011 was conducted under the auspices of Smithsonian’s Caribbean Coral Reef Ecosystems Program, with specimen exported under permission granted by the Belize Fisheries Department. Work in the protected waters of the Florida Keys is part of the Florida Keys Molluscan Biodiversity Survey (e.g., Bieler & Mikkelsen, 2004) in the Florida Keys National Marine Sanctuary (FKNMS) and was conducted under Research Permit FKNMS-2009-024 to RB. FMNH number designations refer to specimen series lodged as voucher material and accessible in the Field Museum of Natural History (FMNH; database access under “invertebrates” at http://collections-zoology.fieldmuseum.org/). Likewise, USNM numbers refer to voucher specimens lodged in the Smithsonian’s National Museum of Natural History Invertebrate Zoology collection database (USNM; database access at https://collections.nmnh.si.edu/search/iz/). Full author/date references for all mentioned species are recorded in WoRMS (2018) and MolluscaBase (2018).
Morphological Studies: Because only two specimens were found alive and tissues were already removed at the field site for molecular and ultrastructural projects that required specialized preservatives, subsequent opportunities for comprehensive anatomical studies were limited. Specimens attached to or freshly removed from the substratum were photographed using a digital camera in the laboratory or alive in the field to record coloration of the head-foot and mantle. Shell measurements were made using digital calipers to record the maximum aperture diameter, the greatest whorl width, and the length of the shell mass (i.e., greatest length in any direction). One protoconch was obtained from a recently settled juvenile but prior corrosion and partial overgrowth by the postlarval shell hindered obtaining a clean specimen.
Scanning electron microscopy (SEM): The shell material was cleaned of encrustations by immersion in an ultrasonic water bath and examined using SEM to observe sculpture and protoconch shape. The buccal mass was extracted manually from the dissected specimen and the radula cleaned by soaking in 10% NaOH solution for about four hours at 60 °C, then rinsed in distilled water and ethanol. Specimens to be imaged by SEM were mounted on conductive carbon tabs (with the radula and protoconch coated with gold), and examined using a Leo EVO 60 SEM at FMNH.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:6F752A70-A53F-4C82-9F93-9DA64783FEAC. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
| Taxonomic description |
| Subclass Caenogastropoda Cox, 1960 |
| Family Vermetidae Rafinesque, 1815 |
None of the numerous existing nominal genus-group taxa of the family (Bieler & Petit, 2011) clearly encompass the morphological features of this new species. Species of Cupolaconcha, the only other vermetid group with known dome-shaped apertural shell modifications, differ in having large, calcareous, dome-shaped opercula. The overall teleoconch morphology (exclusive of the apertural dome building), with its increasingly knobby longitudinal ribs that are most prominent near the inside “umbilical” part of the adult whorls, is similar to that of some species currently classified in Vermetus that also lack “Petaloconchus-like” internal columellar lamellae (Mediterranean V. triquetrus Bivona-Bernardi, 1832, eastern Atlantic V. bieleri Scuderi, Swinnen & Templado, 2017, and an as-yet unnamed “Vermetus sp.” occurring in Bermuda, R Bieler, pers. obs., 2018). Awaiting an extensive genus-level review of the family, we have therefore opted for placing this new species into Vermetus sensu lato.
Type Locality: Western Atlantic, Meso-American Barrier Reef, Belize, Stann Creek District, about 34 km S of Dangriga; off Little Cat Cay, 16°39.098′N, 088°12.094′W, 21 m depth, on underside of dead coral rock [collecting event BEL2011-004, 17 April 2011; by SCUBA; R Bieler & P Sierwald].
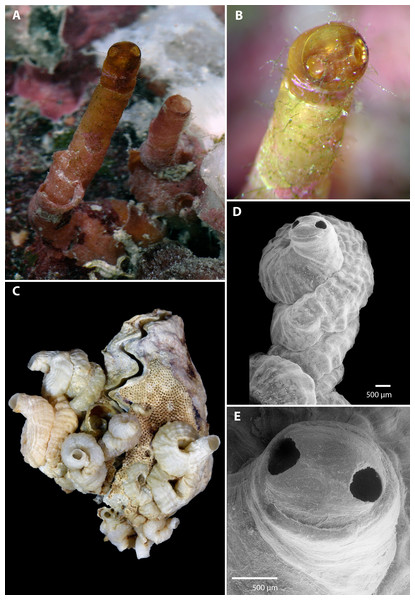
Material examined: Type material: Belize (2 live-collected specimens): Holotype USNM 1480639 ex FMNH 327153 (see Fig. 1A, left) with single attached paratype (Fig. 1A, right). The body of the male holotype was dissected, its posterior body preserved in glutaraldehyde for ultrastructural studies of the gametes, its columellar muscle preserved in RNAlater for molecular studies, and its buccal mass with radula removed for SEM studies. The Belizean material was collected under the auspices of the Smithsonian’s Caribbean Coral Reef Ecosystems Program and the shells of the holotype and attached paratype (with tissue remnants) have been deposited at the National Museum of Natural History in Washington, DC (USNM). The body of the Belizean paratype has been preserved in 95% ethanol and remains cataloged as FMNH 327153.
Florida Keys (empty shells only): 10 paratypes, FMNH 327212. Florida Keys, Monroe County, Missouri Key, 24°40.600′N, 081°14.261′W, clustering and partly overlapping on both valves of a single old shell of the frond oyster Dendostrea frons (Linnaeus, 1758) that was previously attached to holdfasts of Gorgonia sp., ex AS Koto & A Bedell (Koto) collection (undated, 1950–1960s).
Etymology: biperforatus, -a, -um: “two-holed” (used as adjective). The species has been referred to by our research team as the “2-holer” since its discovery.
Description (all live observations and anatomical data are based on the two Belizean type specimens):
Figure 1: Morphology of Vermetus biperforatus (teleoconch shell).
(A) In situ photograph of holotype (left, with apertural dome) and attached paratype (USNM 1480639, Belize); note tapering of smooth erect feeding tubes; diameter of terminal tube of holotype, 1.7 mm. (B) Close-up of shell dome of living holotype; note alternating red-white mantle edge pattern visible through shell dome opening. (C) Cluster of Florida Keys paratypes on partly bryozoan-covered valves of Dendostrea frons (FMNH 327212); specimen with apertural dome in center; greatest dimension of entire cluster in image, 33 mm. (D) Terminal teleoconch whorls showing irregularly but tightly wound whorls with knobby longitudinal sculpture and apertural dome with dual openings; SEM of specimen in center of (C). (E) Close-up of apertural portion of specimen in (D); note narrowed short feeding tube with thinner-shelled dome, and seam between the two apertural holes (SEM). All images by the authors.Teleoconch (Fig. 1): Largest length of attached individual adult shell mass at base 13 mm (11 mm in holotype); length of standing portion (feeding tube) of adult tube above attached shell mass 1–10 mm (10 mm in holotype); feeding tube diameter much narrower (usually about ) than nearest attached whorl; largest diameter of attached shell whorl about 4.0 mm (3 mm in holotype), with sides often drawn out into coarsely wavy flanges; shell coils irregularly piled up or in a pattern resembling a turritellid shell “squashed sideways”, usually with two old feeding tube scars. Shell sculpture of early whorls with irregular axial (transverse) riblets and finer growth marks; later whorls with spiral (longitudinal) ribs becoming increasingly knobby toward aperture, with up to 17 spiral ribs (about 6–10 of which visible on surface of attached shell) that are larger and more prominent toward inner radius of the coil (with one sometimes forming a dorsal shell keel). Feeding tubes smooth except for growth marks. Exposed section of shell somewhat translucent, pale caramel brown (faded greyish white in dead specimens). Apertural shell dome, when present, with two near-circular holes, one on either side of the dome along the equator, each with a maximum diameter of 380–400 µm, with a fine seam connecting their midpoints; dome fragile and readily damaged when still thin-shelled (e.g., it was lost during manipulation of the holotype shell after taking the photographs and was preserved in only one of the ten dead-collected paratypes from the Florida Keys). Shell domes occur at the end of long and thin-walled feeding tubes (holotype, Fig. 1A) and also across thicker-walled apertures that show signs of older tube sections that were broken or cut off (Figs. 1D and 1E). No longitudinal laminar structures on columellar wall.
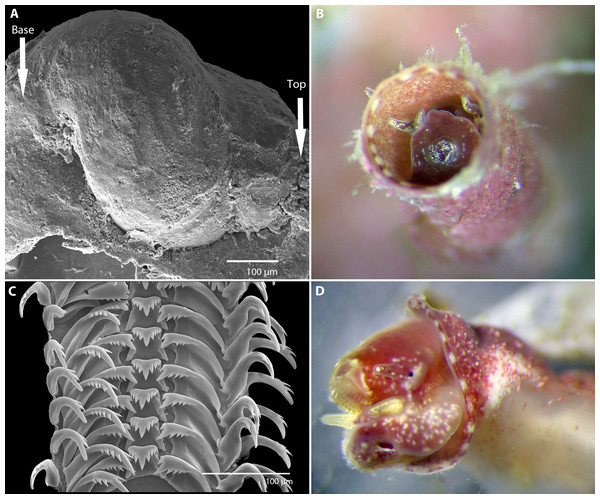
Figure 2: Morphology of Vermetus biperforatus (continued).
(A) Protoconch removed from underside of attached specimen; sculpture obscured by remnants of overlapping teleoconch whorls and the substrate (oyster shell) on which the juvenile settled (SEM, FMNH 327212, Florida Keys). (B) Living paratype specimen from Belize (compare to Fig. 1A, right); note small translucent operculum in center of pedal disk and alternating red-white mantle edge coloration; diameter of aperture, 1.5 mm. (C) Portion of adult radular ribbon; SEM, Belize paratype. (D) Anterior body of paratype after removal from shell (compare to C); oblique view of left head-foot area; note yellowish-white pedal tentacles under the extended radula and small black eye at base of left cephalic tentacle. All images by the authors.Protoconch (Fig. 2A): About 525 µm long and 440 µm greatest width; approximately 2 whorls with the last substantially enlarging; sculpture not ascertained (known only from a single damaged specimen removed from the Florida Keys paratype lot; no protoconch retrievable from Belizean material as early shell whorls were corroded).
Operculum (Fig. 2B): Translucent, with raised spiral lamina, positioned in center of pedal disk; extremely small, its diameter equivalent to about 30% of the width of the maximally extended pedal disk and to about 18% of the inner apertural shell diameter.
Radula (Fig. 2C): Only partially recovered during preparation, the remaining (>50%) of the adult radular ribbon about 500 µm in length with 15 rows. Taenioglossate; rachidian with 3–4 cusps on either side of strong central cusp; lateral teeth strong central and 4–5 outer and 1–2 inner smaller cusps; inner marginal tooth with long and strong main cusp, 4–7 small cusps on outer side, and single longer cusp on inner side; slender outer marginal tooth with 2 (sometimes 3) cusps on inner side.
Soft-body morphology and coloration (Figs. 1B, 2B and 2D): Body (removed from shell) serpentine, about 19 mm in relaxed length; long posterior lobe of digestive gland and gonad about half of its length; columellar muscle a long narrow strip with a deep insertion in the shell (animal capable of deep retraction). Head-foot dusky red with white speckles; on pedal disk, white speckling especially concentrated near and under translucent operculum; cephalic tentacles narrow, tapering towards tip, red with white speckles and a white tip; pedal tentacles pale red to whitish color, relatively short (not extended through aperture in these specimens). Mantle edge with near-rectangular white markings (ca. 18 in the two specimens observed) alternating with red. Alcohol-preserved body fading to overall yellowish-white, without retaining distinctive red or white markings.
Development: Unknown. Holotype male, with sperm morphology currently under investigation.
Habitat and ecology. The live-collected specimens from Belize were attached to the underside of loose, dead coral pieces in an area of hard coral boulders on muddy sand at a depth of 21 m. The empty shell specimens from the Lower Florida Keys were shore-collected at Missouri Key and found attached to both shell valves of a frond oyster Dendostrea frons (Linnaeus, 1758) that in turn was previously attached to a seafan (Gorgonia sp.) holdfast. Such material is frequently cast ashore after storms in that region and corresponding living populations of Gorgonia ventalina Linnaeus, 1758 and G. flabellum Linnaeus, 1758 are common in the nearby near-shore patch reefs and off-shore barrier reef areas, from shallow depths to about 30 m (R Bieler, pers. obs., 2018). Apparently a gregarious form; in both regions, sets of more than one worm-snail specimen were clustering together.
Distribution: Known to date from the barrier reefs of Belize and the Florida Keys, with the two collecting sites more than 1,000 km apart. Several other reef-dwelling vermetid taxa, including Cupolaconcha guana Golding et al., 2014, Dendropoma nebulosum (Dillwyn, 1817), Petaloconchus varians (d’Orbigny, 1839), and Thylacodes decussatus (Gmelin, 1791), are known to occur in both regions (R Bieler, pers. obs., 2018). This species, inconspicuous aside from its 2-holed temporary shell dome, has probably been overlooked in other areas. Consequently, a wide distribution in the Caribbean realm is likely. It does not appear to be common (at least in shallower depths), however, as we have encountered it only twice despite extensive focused collecting efforts in many locales of the Caribbean, the Bahamas, Bermuda, and, especially, Florida. With its shell dome a relatively fragile and temporary structure, more material of this species might be hiding in collections among un- or misidentified material.
Comparison: The overall teleoconch sculpture, with its increasingly knobby longitudinal ribs that are most prominent near the inside “umbilical” radius of the adult whorls, appears most similar to that of species currently included in Vermetus sensu stricto, e.g., as was described for “Vermetus cf. triquetrus” from the Azores by Bieler (1995; now V. bieleri Scuderi, Swinnen & Templado, 2017). The western Atlantic deep-water species Vermetus erectus (Dall, 1888) likewise develops very long and tapering feeding tubes, but has a translucent white shell and a more delicate, somewhat reticulate teleoconch sculpture. Sculpture and growth pattern also are reminiscent of Caribbean Petaloconchus electrinus Mörch, 1862, which, however, has a more brightly colored orange shell, a much more strongly defined sculpture with sharply delineated longitudinal ribs, and internal columellar shell laminae currently deemed characteristic of Petaloconchus.
Discussion
In the majority of vermetid species, protection of the apertural opening is accomplished by a horny (or, rarely, partly calcified) operculum that partially or wholly plugs the shell opening when the animal’s body is withdrawn into the shell tube. In some worm-snail groups (Dendropoma, Novastoa) these opercula can be massive structures deeply anchored into the animal’s foot and forming a protecting lid over a strong shell aperture (Golding et al., 2014; Schiaparelli et al., 2017). Taxa that lack a large operculum (those with small opercula such as Vermetus, or entirely without opercula such as Thylacodes) rely on withdrawing into the shell tube and surrendering to grazing predators the often thin-shelled terminal part of the shell (feeding tube) that is directed toward the food-particle bearing current. Such feeding tubes often are somewhat narrowed near the aperture but normally show no structural enhancement of the apertural shell rim. Members of Cupolaconcha plug the aperture with a dome-shaped and partially calcified operculum and constrict the aperture further by spanning it with a matching apertural shell dome (Golding et al., 2014).
The Cupolaconcha shell dome narrows the aperture to an elliptical or lens-shaped slit that still allows for water exchange to and from the mantle cavity and needs to be removed and rebuilt for accommodating additional shell growth. The function of Cupolaconcha’s slitted dome is not entirely clear, although the aperture restriction would help to reduce sun exposure in specimens that live close to the tidal zone and/or reef crest and even might help prevent desiccation when a specimen falls dry during extreme low tides. The known living specimens of Vermetus biperforatus described herein, however, came from deeper water (>20 m) where a possible function of the dome might be to prevent other organisms from entering the feeding tube that cannot be protected by the extremely small operculum of this species. We did observe the holotype extending its pedal tentacles through the dome holes. Whereas the dual apertural holes allow for water exchange and would accommodate the intake of some plankton and detritus for the animal’s gill-filtering suspension feeding, a biperforatus-type dome hinders the second form of vermetid feeding, one accomplished by extruding, and subsequently eating, mucus webs that trap particles from the current (Morton, 1955).
The apertural dome is a temporary structure as vermetids have no terminal shell growth and continue to extend the shell tube based on individual growth, changes in the environment (e.g., growth of neighboring organisms, change of currents), and repair needs. This necessitates reopening the apertural dome and, apparently, even cutting off parts of the terminal feeding tube with their chitinous rasp-like feeding organ, the radula (Schiaparelli & Cattaneo-Vietti, 1999, and R Bieler, pers. obs., 2018, in other species of Vermetidae). Vermetids have sturdy taenioglossate radulae that are fairly uniform across the family (Bandel, 1984). The animals normally are capable of using their radulae to graze the immediate surroundings reachable by extending their heads from the tube opening (R Bieler, pers. obs., 2018). Members of some groups use their radulae to entrench deep into comparatively hard surfaces such as dead coral and coralline algal layers (e.g., Dendropoma; Golding et al., 2014). As such it is no surprise that vermetid radulae are much sturdier than the suspension-feeding diet of these animals would suggest. A radula capable of effectively excavating calcium-carbonate substratum is well adapted for use in shell modification by cutting off shell tube segments and aperture restrictions when they are no longer needed.
Plasticity of shell form is characteristic of the Vermetidae, and this feature is at least partly responsible for the tremendous challenge that has faced systematists in developing a robust taxonomic and phylogenetic structure for the group (see Keen, 1961; Gould, 1994). Species are highly variable in coiling patterns and shape, with many aspects of their growth reflecting the vagaries of environments in which these snails reside. And while the discovery of the apertural dome in Vermetus biperforatus appears to represent just another feature in the broad behavioral repertoire of this unusual family of snails, its taxonomic relevance begs for a more complete understanding of this trait. What is its functional significance? At what point(s) in a vermetid’s growth is this trait expressed? An initial hypothesis, the dome as a temporary shell modification to facilitate brooding of larvae, was not supported; the living domed specimen from Belize was found to be a male with fully developed sperm (the subject of another study). Do specific environmental cues induce the expression of this trait? Do all individuals develop a dome at some stage? Why is this associated with feeding tubes in some individuals (e.g., Fig. 1A) and not apparently in others (Figs. 1C and 2B)? Are there functional tradeoffs associated with this trait: for instance, if this is a protective structure, can snails continue to feed and reproduce when it is present? And, if it is a defensive covering, against what kind of predator would this be effective? Unfortunately, answers to these questions remain out of reach of the present study.
Collections of vermetids, either live or beach collected, such as those presented in Figs. 1A and 1C, are useful in providing snapshots of vermetid growth and development. Expression patterns, environmental correlates, and functional tradeoffs associated with morphological traits such as Vermetus biperforatus’s apertural dome, however, are best examined through longer-term observations and experiments under lab and field conditions, the latter field option only realistic for shallow-water vermetids. Surprisingly, such studies of vermetid growth have been few and far between. Of these, Schiaparelli & Cattaneo-Vietti (1999) carefully explored the functional morphology of feeding tubes in three vermetid species in response to water flow and underwater obstacles through lab observations and manipulations over the course of eight months. If a reliable source of specimens of Vermetus biperforatus can be obtained, such lab-based experiments could help to address some of the questions outlined above. If this trait is indeed an induced response to an environmental stimulus, such as a predator, it would be interesting to determine the specific source of this phenotypic response. Induced responses to the cues of crab predators can result in better protected apertural margins (thicker shells; apertural teeth) in some intertidal and shallow water caenogastropods (e.g., Appleton & Palmer, 1988; Trussell & Smith, 2000), but such have yet to be reported in vermetids. Given the demonstrated ability of tropical aquarium hobbyists to culture vermetids successfully in aquarium tanks, the potential for such longer-term observation and experimentation is not unrealistic.
Conclusions
The morphology of V. biperforatus stands out as an extreme example of shell modification in Mollusca. Vermetus biperforatus n. sp. is the only gastropod known with the ability of modifying its shell aperture by covering it with a shell dome that has two equal shell openings.