A new tuskless walrus from the Miocene of Orange County, California, with comments on the diversity and taxonomy of odobenids
- Published
- Accepted
- Received
- Academic Editor
- Nicholas Pyenson
- Subject Areas
- Evolutionary Studies, Paleontology, Zoology
- Keywords
- Odobenidae, Walrus, Phylogeny, Tusks, Pacific, Diversity, Neodobenia, Capistrano formation, Oso member, Miocene
- Copyright
- © 2018 Magallanes et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. A new tuskless walrus from the Miocene of Orange County, California, with comments on the diversity and taxonomy of odobenids. PeerJ 6:e5708 https://doi.org/10.7717/peerj.5708
Abstract
We describe Titanotaria orangensis (gen. et. sp. nov.), a new species of walrus (odobenid) from the upper Miocene Oso Member of the Capistrano Formation of Orange County, California. This species is important because: (1) It is one of the best-known and latest-surviving tuskless walruses; (2) It raises the number of reported odobenid taxa from the Oso Member to four species making it one of the richest walrus assemblages known (along with the basal Purisima of Northern California); (3) It is just the second record of a tuskless walrus from the same unit as a tusked taxon. Our phylogenetic analysis places T. orangensis as sister to a clade that includes Imagotaria downsi, Pontolis magnus, Dusignathus spp., Gomphotaria pugnax, and Odobeninae. We propose new branch-based phylogenetic definitions for Odobenidae, Odobeninae, and a new node-based name (Neodobenia) for the clade that includes Dusignathus spp., G. pugnax, and Odobeninae. A richness analysis at the 0.1 Ma level that incorporates stratigraphic uncertainty and ghost lineages demonstrates maximum peaks of richness (up to eight or nine coeval lineages) near the base of Odobenidae, Neodobenia, and Odobenini. A more conservative minimum curve demonstrates that standing richness may have been much lower than the maximum lineage richness estimates that are biased by stratigraphic uncertainty. Overall the odobenid fossil record is uneven, with large time slices of the record missing on either side of the Pacific Ocean at some times and biases from the preserved depositional environments at other times. We recognize a provisional timescale for the transition of East Pacific odobenid assemblages that include “basal odobenids” (stem neodobenians) from the Empire and older formations (>7 Ma), to a mixture of basal odobenids and neodobenians from the Capistrano and basal Purisima (7–5 Ma), and then just neodobenians from all younger units (<5 Ma). The large amount of undescribed material will add new taxa and range extensions for existing taxa, which will likely change some of the patterns we describe.
Introduction
The modern walrus, Odobenus rosmarus (Linnaeus, 1758), is an iconic Arctic species and the last surviving member of a lineage that first appeared in the middle Miocene (∼16 Ma). For much of their evolutionary history, odobenids lacked the characteristic tusks, molluscivory, and Arctic distribution of O. rosmarus, but instead were much more diverse, widespread, and ecologically varied (Fig. 1). Most of the odobenid fossil record (until the Pliocene) is from the North Pacific, with 21 accepted species (Appendix 1) assigned to 18 genera. The high number of monotypic genera reflects a highly imbalanced, pectinate phylogeny. The majority of odobenid taxa lack diagnostic apomorphies, but rather fall along a continuum between generalized, ancestral pinnipedimorph taxa with many plesiomorphic characters (e.g., complex-crowned double-rooted teeth, no tusks, relatively small body size) and more O. rosmarus-like taxa (e.g., peg-like single-rooted teeth, tusks, relatively large body size; Boessenecker & Churchill, 2013; Tanaka & Kohno, 2015). But the history of odobenids is not as simple as that of an anagenetic lineage culminating in the modern species. For example, the most morphologically derived taxon is extinct (Valenictus Mitchell, 1961, lacks all teeth except its tusks). Also, multiple species of odobenid can be found in the same formation (Barnes, 1988; Deméré, 1994a; Tanaka & Kohno, 2015; Velez-Juarbe, in press) including even the coexistence of tusked and tuskless forms (Boessenecker, Perry & Schmitt, 2014: table 1).
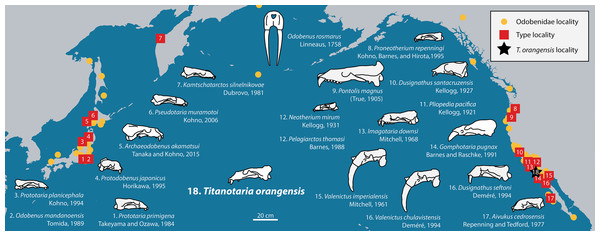
Figure 1: Distribution of fossil odobenid localities in the Pacific.
Circles represent published localities. Numbered squares represent type localities. A star represents the locality of Titanotaria orangensis (OCPC 11141). Figured skulls are known from complete or partial cranial material and are drawn to scale referencing line drawings by Robert W. Boessenecker. The specimens facing right are found in the Western Pacific, while specimens facing left are found in the Eastern Pacific. Occurrence data and basemap were obtained from the Paleobiology Database using the following parameters: family = Odobenidae. Major contributors to this data set were John Alroy and Mark Uhen.The evolution of odobenid assemblages has received some recent attention (Boessenecker, 2017; Velez-Juarbe, 2017; Boessenecker & Churchill, 2018), and whereas some patterns are beginning to emerge, those studies note the existence of many undescribed specimens that could change our understanding once studied. In this study, we describe a new specimen of tuskless odobenid (OCPC 11141) from the upper Miocene Oso Member of the Capistrano Formation from Lake Forest, Orange County, California (Fig. 2). Although it was discovered in 1992 and is one of the more complete odobenid specimens known, OCPC 11141 has never been figured or described. The only mention of this specimen in the peer-reviewed literature is as part of a faunal list for the Oso Member (Barboza et al., 2017). This specimen is significant because it represents one of the best-known and latest-surviving tuskless odobenids, and therefore provides important data for the phylogeny of odobenids as well as the evolution of pinniped assemblages in the East Pacific.
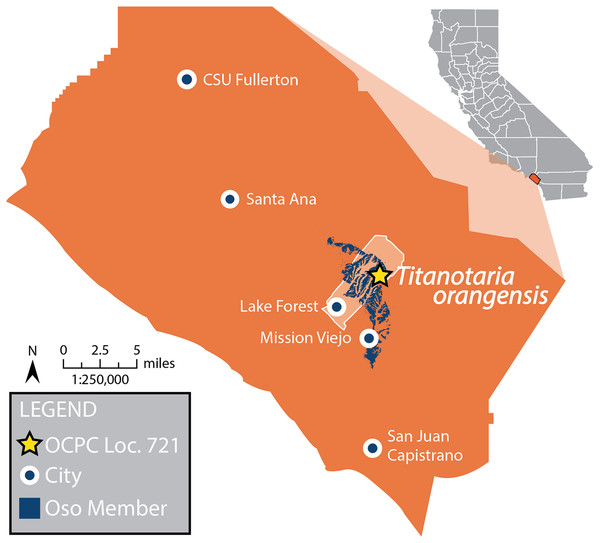
Figure 2: Map of Orange County, CA, and type locality for Titanotaria orangensis.
Map of Orange County, California, showing where OCPC 11141 was discovered in the Oso Member (extent shown in blue) of the Capistrano Formation, Lake Forest (outline in white), Orange County (in orange), California. A star represents the locality (OCPC Locality 721) of Titanotaria orangensis (OCPC 11141). Map modified from Barboza et al. (2017).In addition to describing OCPC 11141 and naming a new genus and species, we perform a phylogenetic analysis that we use as the basis for assessing odobenid phylogenetic taxonomy and lineage richness through time. For the richness analysis, we incorporate stratigraphic uncertainty and phylogenetic relationships (ghost lineages) across 0.1 Ma intervals (n = 173). We use the results of these analyses to present an overview of the odobenid fossil record, review the timing and recently proposed causes for richness changes, and highlight biases that should affect interpretations.
Materials and Methods
Material examined
Aivukus cedrosensis Repenning & Tedford, 1977 (LACM 154671, cast of type); Dusignathus santacruzensis Kellogg, 1927 (LACM 1527, cast of type; LACM 3011, 4342); Dusignathus seftoni Deméré, 1994a (LACM 155310, cast of type; LACM 135545, cast of SDNHM 20801); Gomphotaria pugnax Barnes & Raschke, 1991 (LACM 105151, 121508; LC 7750); Imagotaria downsi Mitchell, 1968 (LACM 144453, cast of type; USNM 23858, 184060); Neotherium mirum Kellogg, 1931 (LACM 4319, 52172, 98147, 123000, 123002, 127697; UCMP 81665); Odobenidae Allen, 1880 gen. et sp. indet. (LACM 135920); Odobenus rosmarus (LACM 31336, 52376, 52423, 72561); Odobenus mandanoensis (NMNS 18911); Ontocetus Leidy 1859 sp. (LACM 150001, cast of SFMCV-0001); Pelagiarctos Barnes, 1988 sp. (SDNHM 131041); Pelagiarctos thomasi Barnes, 1988 (LACM 121501); Pliopedia pacifica Kellogg, 1921 (LACM 57055, cast of type); Pontolis magnus True, 1905 (LACM 101151, cast of type); Proneotherium Barnes in Kohno, Barnes & Hirota 1995 sp. (LACM 128412); Protodobenus japonicus Horikawa, 1995 (LACM 140726, cast of type); Prototaria planicephala Kohno, 1994 (LACM 134826, cast of type); Prototaria primigena Takeyama & Ozawa, 1984 (LACM 130432, cast of type); Valenictus imperialensis Mitchell, 1961 (LACM 3926).
Phylogenetic methods
We added Titanotaria orangensis to the 23 taxon, 90 character, data matrix of Boessenecker & Churchill (2013) with the addition of Archaeodobenus akamatsui Tanaka & Kohno, 2015 and character 91 from Tanaka & Kohno (2015). We analyzed the data set with all unordered characters as well as with some characters ordered. For the analysis with ordered characters, we identified 21 characters that have more than two-character states, form a plausible transformation series, and are phylogenetically informative for the Odobenidae. The ordered characters are 5, 9, 11, 33, 37, 51–53, 55, 56, 61, 62, 70, 71, 74, 76, 77, 80, 82, 83, and 86. For three characters (52, 71, 86) we changed the number applied to character states so that they could be easily analyzed as ordered (switched 0s and 1s). For character 5 we combine states 1 and 2 into state 1 “1 = foramina coalesced, 2 = trilobed pits” (in this case state 3 became state 2). The coding for character 4 matches that of the (Boessenecker & Churchill, 2013) matrix, which is different from that described by their character descriptions (in which the descriptions for states for 2 and 1 are reversed). Also, following their character guide text we change the coding of I. downsi from 0 to ? for this character 4. Character 17 included a state 3 for Erignathus and Monachus, but there is no state 3 described; we change this character to state 1 for these taxa. For I. downsi, we updated character 48 (from 0 to 1) based on Repenning & Tedford (1977; USNM 23858). For N. mirum we updated character 59 (from 0 to 1) based on Velez-Juarbe (in press). Character 61 was updated for G. pugnax (from 1 to 0) based on the type. Character 79 was coded as state 0 for Callorhinus ursinus (Linnaeus, 1758) and Allodesmus kernensis Kellogg, 1922 by Boessenecker & Churchill (2013), but is corrected here to 1. We include the character codings for P. magnus that were included in Boessenecker & Churchill (2013) and Tanaka & Kohno (2015) while noting that these character states have not yet been described.
We analyzed the data matrix (Data S1) in PAUP* 4.0a build 159 (Swofford, 2002) using a heuristic search algorithm with 1,000 stepwise additions. We assessed support for each node with a bootstrap analysis of 1,000 replicates with 100 addition sequence replicates and Bremer decay indices.
Taxonomy
The electronic version of this article in portable document format will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: LSIDurn:lsid:zoobank.org:pub:7826C97C-3994-4519-89F0-603E6135F3DB The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central, and CLOCKSS.
Richness curve methods
A time constrained richness curve was constructed to show the minimum, maximum count of known and inferred odobenid lineages (richness) present throughout their history, taking into account chronostratigraphic uncertainty and ghost lineages. For each taxon, we estimated a minimum and maximum possible age of occurrence by performing a literature review of chronostratigraphic data to build on estimates presented by recent studies (Boessenecker & Churchill, 2015; Barboza et al., 2017; Kloess & Parham, 2017; Velez-Juarbe, 2017; Boessenecker & Churchill, 2018). We exclude the recently described Nanodobenus arandai Velez-Juarbe & Salinas-Márquez, 2018, because it appeared late in the review process for this paper and is the least chronostratigraphically constrained odobenid taxon (15.7 and 9.2 Ma, 6.5 Ma range is more than double that of any other taxon known from just one site). Detailed explanations for the age ranges of all taxa are included in Appendix 1. We used these data and the phylogenetic trees from our preferred hypothesis to generate a stratigraphically-constrained cladogram which was used to count the minimum and maximum number of lineages at 0.1 Ma time slices (0.0–17.3 Ma, 173 time slices).
The maximum lineage count was determined by assuming that each taxon spanned from the oldest to the youngest possible age of the rock units that include referred specimens. Six taxa are known from multiple formations (I. downsi, D. santacruzensis, Ontocetus emmonsi Leidy, 1859, Pelagiarctos [incl. P. thomasi], P. pacifica, V. imperialensis) and so the maximum age range of both units was used for their broadest possible age range. The broadest possible age range of four presumably valid taxa that are not included in the cladistic analysis (Odobenus mandanoensis Tomida, 1989, P. pacifica, P. planicephala, V. imperialensis) were included in the maximum count. We also extended the maximum age range of the Valenictus lineage based on Boessenecker (2017).
The minimum lineage count was generated by assuming originations and extinctions within the possible range that gave the lowest possible number of lineages at any given time. For the most part this involved counting ghost lineages since, at any time within the actual estimate range, a taxon can be presumed to be extinct or unoriginated. However, two taxa are known from more than one formation that do not overlap in time (I. downsi, O. emmonsi) providing a “known age range” for those taxa that was always included in the minimum count.
Maximum and minimum lineage counts from the nine trees from the ordered character analysis that varied among the ingroup were compared, and the maximum and minimum age estimates for each 0.1 Ma bin among the trees was included in the curves. We did not include undescribed or insufficiently characterized specimens that have been mentioned in recent papers (Appendix 2). By excluding these specimens we bias our richness estimates downward and so comparisons with other studies must be evaluated in this context. Nevertheless, this exercise is useful as an exploration of other factors that influence estimates of odobenid richness, as well as an estimate from the more established part of the record that can be used as a baseline for future studies.
In addition to the maximum and minimum global curves, we also generated a curve based on maximum lineage counts from an analysis where all taxa from outside the east Pacific were pruned from the trees. The purpose of this curve is to demonstrate the relative contribution of the East Pacific sites to the fossil record of odobenids.
Systematic Paleontology
MAMMALIA Linnaeus, 1758
CARNIVORA Bowdich, 1821
ODOBENIDAE Allen, 1880
TITANOTARIA gen. nov.
Type and included species—Titanotaria orangensis sp. nov.
Etymology—Titan- for the nickname of California State University, Fullerton, the Titans, to recognize the partnership with the County of Orange (Orange County Parks) that formed the John D. Cooper Archaeology and Paleontology Center; -otaria for Otaria Péron, 1816 the generotypus for the Otariidae, from which other fossil odobenid names have been derived, for example, Imagotaria Mitchell, 1968, Gomphotaria Barnes & Raschke, 1991, Prototaria Takeyama & Ozawa, 1984, and Pseudotaria Kohno, 2006.
Diagnosis—as for species.
TITANOTARIA ORANGENSIS sp. nov.
“Odobenidae sp. A” Barboza et al. (2017: 2, 15)
Holotype—OCPC 11141, a male individual, including a skull, mandible, and postcrania including a nearly complete appendicular and axial skeleton. The missing elements include parts of the right scapula, humerus, and radius, as well as some ribs, some of the autopodia, and most of the pelvis.
Etymology—orange- for the County of Orange, California, USA, where the holotype was found; -ensis for the Latin word meaning “originating in’ (i.e., from).
Locality—The type specimen was found at OCPC Locality 721, in Lake Forest, Orange County, California, during paleontological mitigation for the construction of the Foothill Transportation Corridor (California State Route 241). Collected by David Alexander, July 9, 1993.
Horizon—The type specimen comes from the Oso Member of the upper Miocene Capistrano Formation. The Oso Member is a nearshore facies with fossils of marine and terrestrial vertebrates. A preliminary list of the Oso Member assemblage can be found in Barboza et al. (2017) and includes at least three other putative taxa of walruses, as well as sea cows, whales, desmostylians, sea turtles, crocodylians, sharks, fish, birds, elephants, rhinos, and horses. Using biostratigraphic correlations, Barboza et al. (2017) constrained the age of the Oso Member to 6.6–5.8 Ma.
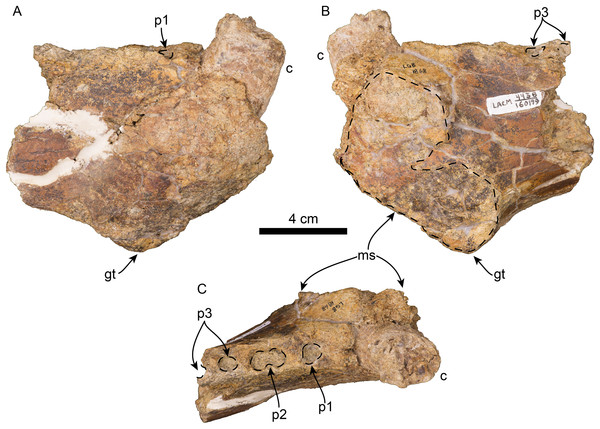
Referred specimen and locality—LACM 160199, anterior end of right mandible, missing all teeth except the base of the canine. LACM Loc. 4438, Lake Mission Viejo, Mission Viejo, Orange Co., CA; collected by L.G. Barnes, August 4, 1978.
Diagnosis—Titanotaria orangensis can be diagnosed from neodobenians (see Phylogenetic Taxonomy) by having a short mandibular symphysis, C1/c1 that are proportional in size, lingual cingulum well-developed on P1 and P2, double-rooted p2–4 and double-rooted m1. Titanotaria orangensis can be distinguished from neodobenians and P. magnus by having postcanine crowns with enamel, a posterior crista on c1, paraconid cusps on the lower post-canines, and a double-rooted P4. Titanotaria orangensis can be distinguished from I. downsi, P. magnus, and neodobenians by having a double-rooted P3. It can be distinguished from I. downsi and Pelagiarctos sp. by having a mandibular symphysis that is less than 50% the length of the horizontal ramus. Titanotaria orangensis can be further distinguished from I. downsi by having a ventral tuberosity of the zygomatic root, from Pelagiarctos sp. by having a sinuous ventral margin of the mandible, and from P. thomasi by having an unfused mandibular symphysis. Titanotaria orangensis can be distinguished from all other known odobenids (excluding I. downsi, P. magnus, neodobenians) by having a non-converging posterior nasal suture, short glenoid fossa, and a large mastoid process.
Description
The type specimen (OCPC 11141) consists of a cranium, mandibles, and associated postcrania including a nearly complete appendicular and axial skeleton. The missing elements include parts of the right scapula, humerus, and radius, as well as some ribs, some of the autopodia, and most of the pelvis. Well-preserved tightly fused cranial sutures and an associated baculum indicate that the holotype of Titanotaria orangensis is an adult male (suture age 35; Sivertsen, 1954), with an estimated body length of 3.3 m (based on cranial, mandibular and dental measurements; Churchill, Clementz & Kohno, 2015). This body length estimate for T. orangensis is similar to that of some undescribed odobenids from the Monterey Formation, G. pugnax from the Capistrano Formation, and O. rosmarus (Velez-Juarbe, 2017; Lydersen, 2018).
For this report, we describe the cranium, mandibles, and phylogenetically relevant postcranial elements (humerus, radius, scapholunar, astragalus, calcaneum, entocuneiform, metacarpal 1, and baculum) of OCPC 11141. We list the phylogenetic characters (see Phylogenetic methods) in our description when that area is being described following a modified convention of Domning (1994) and Parham & Pyenson (2010). We use the format (c. n1[n2]) where n1 corresponds to the character and n2 corresponds to the character state.
Cranium
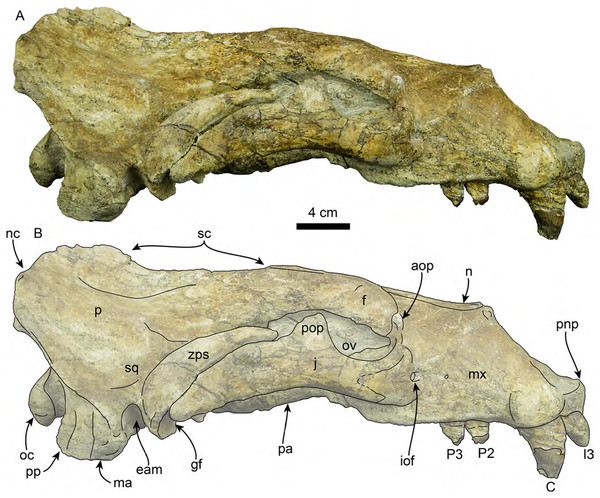
The cranium (Figs. 3–7) is nearly complete and preserves diagnostic characters of the rostrum, frontal, parietal, zygomatic arches, palate, basicranium, and dentition. The skull also exhibits asymmetry, with the right side being more robust. This asymmetry is obvious when comparing the outline of the orbits and the relative dorsoventral thickness of the zygomatic arches. We attribute this asymmetry to a pathology on the left side of the skull, likely from a healed injury; a similar case was described in a modern pinniped by Howell (1925). Unless stated otherwise, the description of the cranium is based on the right side of the skull.
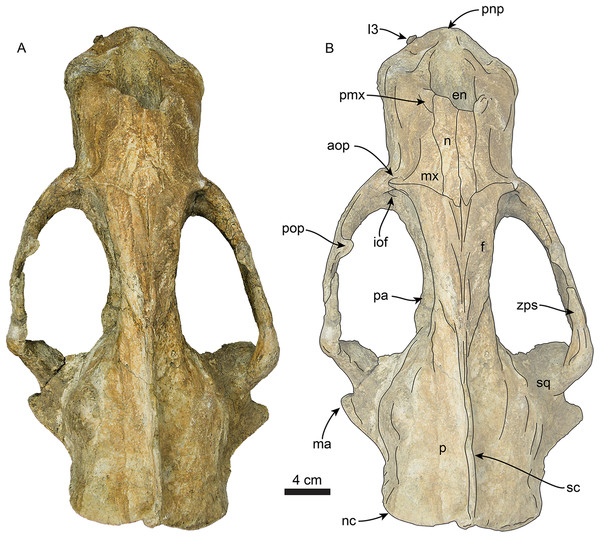
Figure 3: Skull of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Skull in dorsal view, without labels (A) and labelled (B). Abbreviations: aop, antorbital process; en, external nares; f, frontal; I3, upper third incisor; iof, infraorbital foramen; ma, mastoid process; mx, maxilla; n, nasal; nc, nuchal crest; p, parietal; pa, palatine; pmx, premaxilla; pnp, prenarial process; pop, postorbital process; sc, sagittal crest; sq, squamosal; zps, zygomatic process of squamosal.Figure 4: Skull of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
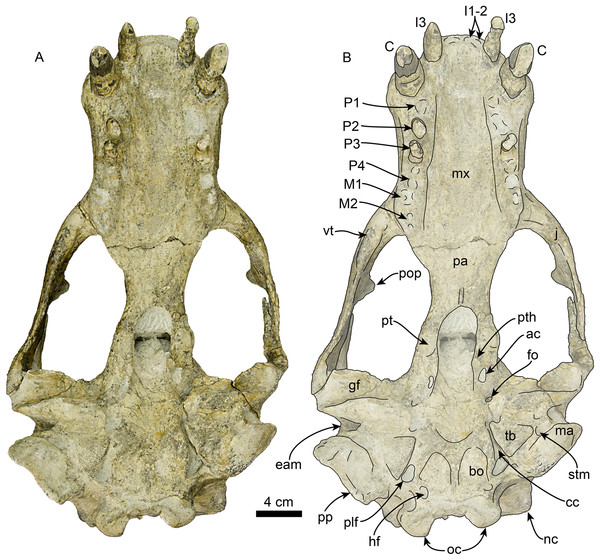
Skull in ventral view, without labels (A) and labelled (B). Abbreviations: ac, alisphenoid canal; bo, basioccipital; C, upper canine; cc, carotid canal; eam, external auditory meatus; fo, foramen ovale; gf, glenoid fossa; hf, hypoglossal foramen; j, jugal; I1–3, upper incisors 1–3; M1–2, upper molars 1–2; ma, mastoid; mx, maxilla; nc, nuchal crest; oc, occipital condyles; P1–4, upper premolars 1–4; pa, palatine; plf, posterior lacerate foramen; pop, postorbital process; pp, paroccipital process; pt, pterygoid; pth, pterygoid hamulus; stm, stylomastoid foramen; tb, tympanic bulla; vt, ventral tuberosity.Figure 5: Skull of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
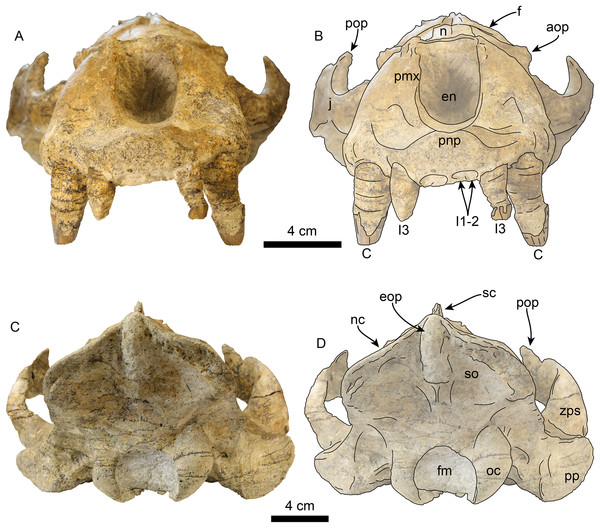
Skull in right lateral view, without labels (A) and labelled (B). Abbreviations: aop, antorbital process; C, upper canine; eam external auditory meatus; f, frontal; gf, glenoid fossa; I3, third upper incisor; iof, infraorbital foramen; j, jugal; ma, mastoid; mx, maxilla; n, nasal; nc, nuchal crest; oc, occipital condyle; ov, orbital vacuity; P2–3, upper premolars 2–3; p, parietal; pa, palatine; pnp, prenarial process; pop, postorbital process; pp, paroccipital process; sc, sagittal crest; sq, squamosal; zps, zygomatic process of squamosal.Figure 6: Skull of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Skull in anterior (A–B), and posterior (C–D), views. Abbreviations: aop, antorbital process; C, upper canine; en, external nares; eop, external occipital protuberance; f, frontal; fm, foramen magnum; I1–3, upper incisors 1–3; j, jugal; n, nasal; nc, nuchal crest; oc, occipital condyle; pmx, premaxilla; pnp, prenarial process; pop, postorbital process; pp, paroccipital process; sc, sagittal crest; so, supraoccipital; zps, zygomatic process of squamosal.Figure 7: Skull of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
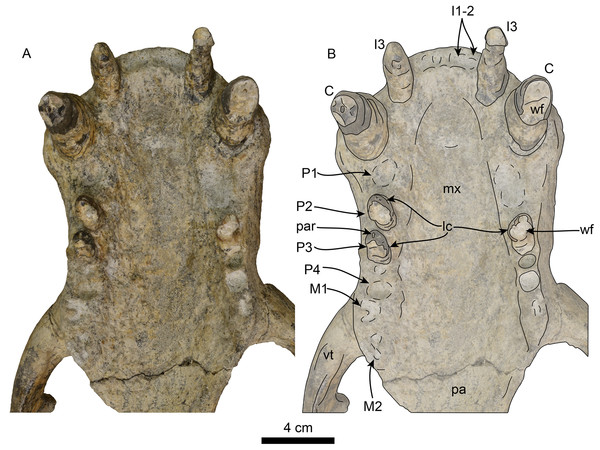
Detail of palatal region, without labels (A) and labelled (B). Abbreviations: C, upper canine; I1–3, upper incisors 1–3; lc, lingual cingulum; M1–2, upper molars 1–2; mx, maxilla; P1–4, upper premolars 1–4; pa, palatine; par, paracone; vt, ventral tuberosity; wf, wear facet.Rostrum—The rostrum (Figs. 3–7; Table 1) is anteroposteriorly elongate and expanded anterolaterally at about the level of the C1 roots. In lateral view, the premaxilla has a triangular outline, with its anterior end recurved and elevated slightly above the maxillary alveolar row. In dorsal view, the ascending processes of the premaxilla extend posteriorly creating short overlap between the nasal and maxilla (c. 3[1]). A prominent, knob-like, prenarial process is present on the anterodorsal tip of the premaxilla (c. 1[1]). The surface posterolateral to this process forms a shallow, oval concavity, dorsal to the space between the canine and incisors, and is interpreted as the area of origin for the m. lateralis nasi (Kastelein, Gerrits & Dubbeldam, 1991). The anterior narial opening is transversely compressed and dorsoventrally elliptical with thick lateral and ventral margins (c. 2[1]) (Fig. 3). Dorsally, the nasals are long (∼60% of the rostrum length), and their lateral edges are roughly parallel to each other with a posterior termination that forms a broad V-shaped frontal/nasal suture (c. 4[1]).
| Total length, premaxilla to intercondylar notch | 403 |
| Facial length | 136.42 |
| Orbit length | 43, 57 |
| Temporal fossa length | 29, 42 |
| C1–M2 toothrow length | L. (to shrunken M1): 127: R: 149 |
| P1–M2 toothrow length | L. (to shrunken M1): 89 R: 113 |
| External nares height | 39 |
| Transverse width of external nares | 33 |
| Length of nasals | 94 |
| Maximum transverse width of nasals | 36 |
| Transverse width of rostrum at C1 | 128 |
| Narrowest width of rostrum | 106 |
| Transverse width of palate at M2 | 85 |
| Bizygomatic width | 238 |
| Transverse width at mastoid | 220 |
| Transverse width across tympanic bullae | 142 (Note: L side does not reach edge of skull) |
| Transverse width at paroccipital process | 143 |
| 18. Transverse width of condyles | 87 |
| 19. Height of foramen magnum | 38 |
| 20. Width of foramen magnum | 44 |
| Anteroposterior length, mastoid, and paroccipital process | 43, 55 |
| Width of choanae between pterygoid hamuli | 30 |
| Infraorbital foramen, height/transverse width | L: 18/20; R: 11/14 |
| Least interorbital width | 45 |
| Interorbital width at supraorbital process | No supraorbital process |
| Transverse width of braincase | 142 |
| Anteroposterior length of sagittal crest | 140 or 199 depending on where the anterior edge of the crest is |
| Greatest depth of zygomatic arch | L: 45; R: 51 |
| Height of occipital shield | 93 |
| C1 crown height/mesiodistal length/buccolingual width | L: 25*/20/20, R: 24*/23/20 |
| P1 crown height/mesiodistal length/buccolingual width | L: Over prepared, can’t see R: a 15/12 |
| P2 crown height/mesiodistal length/buccolingual width | L: Over prepared, can’t see R: 7/19/12 |
| P3 crown height/mesiodistal length/buccolingual width | L: 6*/15/14 R: 13/16/12 |
| P4 crown height/mesiodistal length/buccolingual width | L: a 24/12 R: a 20/15 |
| M1 crown height/mesiodistal length/buccolingual width | L: a 15/6 R: a 17/12 |
| M2 crown height/mesiodistal length/buccolingual width | L: Not present R: a 17/7 |
Notes:
Modified from Boessenecker & Churchill (2018).
a, alveoli; L, left; R, right.
The maxilla is well preserved in dorsal and lateral aspect. In lateral view, the maxillary alveolar row is sinuous and swollen near the roots of the canines. The maxilla forms the anterior portion of the orbital wall and lacks a fossa muscularis (c. 14[1]); together with the frontal, the maxilla forms the antorbital process (c. 15[1]). In ventral view, the palatal margins of the maxilla are nearly parallel anteriorly, becoming more sinuous at the level of m1–2 (c. 10[0]). The palate is widest near the canines and transversely arched (c. 9[1]). The palatal width index (70) is greater than that of Proneotherium repenningi Barnes in Kohno, Barnes & Hirota 1995 (Deméré & Berta, 2001: 52–60), owing to its more parallel toothrows. The transverse palatal arch index of T. orangensis is greater than that of P. repenningi and N. mirum, becoming higher between P3–4 and again smaller at the level of M1–2 (Table 2; Deméré & Berta, 2001). The incisive foramina are largely obscured by sediment so their morphology is not discernible, but are located at about the level of the posterior half of the canines (c. 5[?]). The maxillo-palatine suture is fused; the palatines are elongated, fused along the midline, and posterolaterally expanded into the orbital region (c. 11[1]); their posterior margin is rounded.
| C1 palate width | M1 palate width | Palate width index | |
|---|---|---|---|
| 61 | 87 | 70 | |
| Tooth position | Transverse palatal depth | Transverse palatal width | Transverse palatal arch index |
| C1 | 12 | 61 | 19 |
| P1 | 12 | 53 | 22 |
| P2 | 14 | 60 | 23 |
| P3 | 24 | 67 | 35 |
| P4 | 26 | 72 | 36 |
| M1 | 26 | 87 | 29 |
| M2 | 22 | 84 | 26 |
Note:
Based on Deméré & Berta (2001).
Zygomatic Arch—The zygomatic arch is dorsoventrally broad, (Figs. 3–6) with a low, oval ventral tuberosity on the maxillary root (c. 7[1]) (Fig. 4). The dorsal and ventral struts of the maxillary root give the infraorbital foramen an elliptical outline. The infraorbital foramen is relatively small (c. 6[0]) with a maximum diameter of ∼14 mm, which is about the same diameter as in the much smaller skulls referred to I. downsi (USNM 23858 and USNM 184060; Repenning & Tedford, 1977). The jugal is dorsoventrally thick and has a relatively small triangular postorbital process (c. 18[0]), similar to that of D. seftoni and unlike the much larger process of Valenictus chulavistensis Deméré, 1994a. Anteriorly, the jugal-maxilla articulation is largely fused with the maxilla with an anterodorsal and anteroventral process, the latter not approaching the toothrow (c. 8[0]). Posteriorly, the zygomatic process of the jugal extends to the level just anterior to the preglenoid process on the right side, while is much shorter on the left (100 vs. 59 mm long). The zygomatic process of the squamosal is dorsally curved, relatively short and dorsoventrally thin relative to the jugal (c. 19[1]). The preglenoid process is relatively long, and the glenoid fossa is mediolaterally broad, deep, and is oriented anteroventrally (c. 26[0]). The squamosal fossa on the dorsal surface of the zygomatic root is relatively broad relative to the proportionately narrow fossa of I. downsi (e.g., USNM 184060), slopes anteroventrally (c. 25 [0]), and there is no indication of the presence of a transverse ridge (c. 27[0]).
Frontal—In dorsal view (Fig. 3), the interorbital bar is transversely narrow with parallel lateral margins that diverge anteriorly making the frontal widest anteriorly (c. 17[1]). Anteriorly, the frontals have a transversely concavo-convex contact with the maxilla, that continues along the lateral surface of the skull toward the antorbital region. The frontal lacks a supraorbital process (c. 16[1]). The temporal crests arise just posterior to the contact with the nasals, these then merge posteriorly form the anterior portion of the sagittal crest. In the orbito-temporal region the ventral margin of the frontal is dorsally convex and contacts the maxilla anteriorly and parietal posteriorly.
Orbit—The orbit (Figs. 3, 5 and 6) has an anterodorsal orbital wall that preserves a frontal-maxillary suture that bisects the antorbital process (c. 15[1]). A low ridge extends posteriorly from the antorbital process, forming the ventral edge of the orbit. A sediment filled orbital vacuity is located anteriorly in the orbital wall (c. 20[1]). The posterior margin of the orbital wall is formed by the orbitosphenoid and frontal. The orbitosphenoid is plate-like and extends anteriorly forming part of the optic foramen (c. 21[0]). The ventral half of the orbito-temporal region is dorsally convex and continues posteriorly to the pterygoid strut. The pterygoid strut is dorsoventrally thickened and transversely broad (c. 13[2]; Fig. 4). The hamular process of the pterygoid is small, transversely compressed, anteroposteriorly elongated, and projects posteroventrally (c. 12[0]).
Parietal—The parietal (Figs. 3 and 5) is fused with the frontals anteriorly and the squamosals laterally. The temporal surface is smoothly convex, with no visible pseudosylvian sulcus (c, 28[1]). In dorsal view, the parietals form most of the broad, boxlike braincase, which is roughly as wide transversely as long anteroposteriorly. The sagittal crest is prominent and elongate, extending from the frontal anteriorly and connecting with the nuchal crest posteriorly. The sagittal crest has a sinuous profile in lateral view, unlike the low posterodorsally sloping crest seen in other odobenids (e.g., I. downsi, N. mirum; Repenning & Tedford, 1977; Kohno, Barnes & Hirota, 1995). The nuchal crest is transversely wide and projects posterodorsally obscuring the occipital condyles (c. 32[0]). From the dorsal midline, the nuchal crest curves posterolaterally, then anteroventrally, reaching the mastoid process.
Basicranium—The basioccipital (Fig. 4) is broad and pentagonal in ventral view with anteriorly converging lateral margins and a prominent medial margin (c. 30[1]). The hypoglossal foramen is rounded and located anterior to the occipital condyle. The posterior lacerate foramen is rounded and located anterolateral to the hypoglossal foramen (c. 31[0]); anteromedially it merges with the carotid canal. The carotid canal is partially underlapped anteromedially by the tympanic bulla; both anterior and posterior openings are of similar diameter (∼9 mm) (c. 24[0]). The hyoid fossa is shallow (<10 mm) and relatively small, facing mainly posteroventrally; it is separated from the smaller (∼6 mm) stylomastoid foramen by a low ridge. The mastoid processes are large and broad (c. 33[1]), in contrast to the more delicate processes of N. mirum and early pinnipeds, while the paroccipital process is plate-like, posteriorly directed, and laterally continuous with the mastoid process (c. 34[3]). The tympanic bullae are well preserved, their ventral surface has a triangular outline and their ventral surface is shallowly convex, without any ornamentation. The ecto and entotympanic portions are indistinguishable from each other. There is no distinct posterior bullar process, as in the type of I. downsi (Mitchell, 1968). The stylomastoid foramen opens anterolaterally. The foramen ovale and alisphenoid canal are relatively small (∼14 mm diameter).
Occipital region—Posteriorly, the occipital surface is broadly concave, with a pentagonal outline. A thick external occipital protuberance extends ventrally from the convergence of the sagittal and nuchal crests to about 45 mm dorsal to the foramen magnum. The occipital condyles are large, robust, and form the margins of the circular foramen magnum. As with most of the skull, the occipital condyles are asymmetric, with the left condyle being noticeably smaller, and less prominent than the right one.
Mandible and dentition
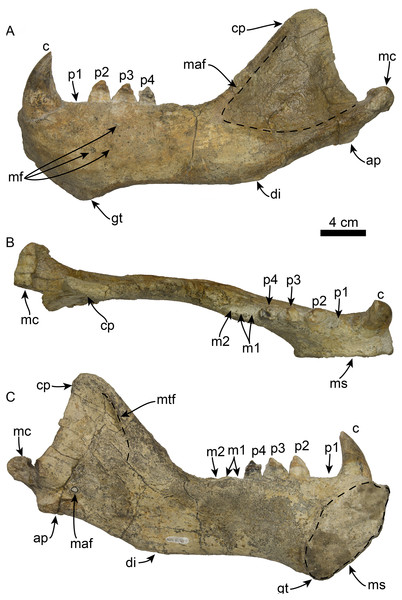
OCPC 11141 preserves both left and right mandibles while LACM 160199 is the anterior part of a right mandible (Figs. 8 and 9). In dorsal view, the articulated mandibles form a nearly parallel arch (c. 44[0]) and lack a mandibular furrow and edentulous mandibular terminus (c. 40[0], 41[0]). The right mandible lacks preserved dentition with the exception of a fragment of the canine. The left mandible preserves the canine as well as p2–4. The horizontal ramus has a straight dorsal and a moderately sinuous ventral margins (c. 39[0], 45[1]). The genial tuberosity is located at the posteroventral margin of the symphysis at a level between p1–2, and extends well below the ventral margin of the horizontal ramus forming the deepest part of the horizontal ramus (c. 37[2], 42[0], 43[1]). The mandibular symphysis is unfused with a rugose symphyseal surface, and occupies less than 50% of the length of the horizontal ramus (c. 35[0], 36[0]). The anterior surface of the symphysis is smooth, and curves anterodorsally from the genial tuberosity (c. 38[0], 40[0]). Several small, anteriorly oriented, mental foramina are present between p1–3 and the ventral border of the ramus. The anterior margin of the mandibular foramen is directed anteroventrally (c. 48[0]).
Figure 8: Mandible of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Left mandible in lateral (A), occlusal (B), and medial (C), views. Abbreviations: ap, angular process; c, lower canine; cp, coronoid process; di, digastric insertion; gt, genial tuberosity; m1–2, lower molars 1–2; maf, masseteric fossa; mc, mandibular condyle; maf, mandibular foramen; mf, mental foramina; ms, mandibular symphysis; mtf, mandibular temporal fossa; p1–4, lower premolars 1–4.Figure 9: Referred partial mandible of Titanotaria orangensis n. gen. et sp. (LACM 160199).
Left mandible in lateral (A), medial (B), and occlusal (C), views. Abbreviations: c, lower canine; gt, genial tuberosity; p1–3, lower premolars 1–3; ms, mandibular symphysis.The coronoid process has a triangular outline and is anteroposteriorly narrow relative to the length of the mandible (c. 50[0]), its anterior edge ascends posterodorsally at about 52° from the horizontal ramus. The masseteric fossa is shallow, as in I. downsi (USNM 23858), and extends to a point near the anterior end of the coronoid process. Medially, the coronoid process has a shallow mandibular temporal fossa. The mandibular condyle is elevated only slightly above the level of the tooth row (c. 47[0]). The digastric insertion is moderately enlarged but not to the degree observed in P. magnus (c. 46[0]; Boessenecker & Churchill, 2013). The angular process is reduced, forming a small medial shelf (c. 49[1]).
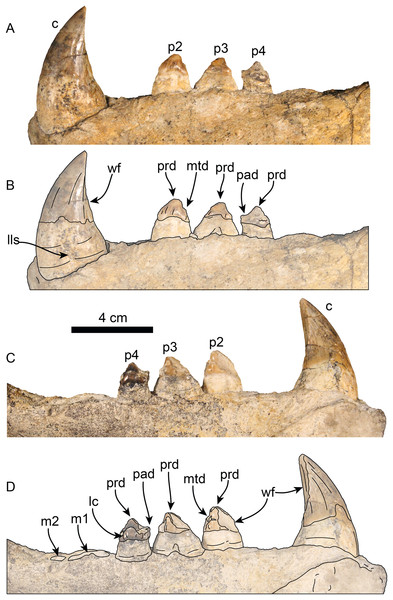
Upper dentition—The preserved upper dentition (Fig. 7) and alveoli indicate a dental formula of I1–3, C1, P1–4, M1–2. The teeth preserved include left and right I3, C1 and P3, and right P2; while the rest of the teeth are represented by their respective alveoli. The alveolar morphology of I1–2 indicate anteroventrally directed single-rooted teeth. Both left and right I3 are present, but heavily worn, they are single-rooted, long and slender (c. 51[0], 52[0]). In occlusal view, I3 are laterally compressed and elliptical in cross section. The left I3 retains a small portion of the crown on its mesial side that begins 25 mm below the alveolar margin and has a distal wear facet; the crown of the right I3 is completely worn.
The canines are conical, ventrally directed, robust, and proportionate in size with respect to c1 (c. 55[0]). The base of the crown is oval in cross section. The enamel is smooth, and thick; the distal wear facet is large and elliptical on the left canine, and relatively smaller on the right.
Only the right P2 and left and right P3 are preserved. The alveolar morphology of P1 indicates a single-rooted tooth. P2 has a single, bi-lobed root, (c. 72[0]) and the crown is bulbous, and heavily worn on its distal surface. The paracone is partially preserved but extremely worn and broken at the apex; the occlusal wear has obliterated all other cusps. P2 also preserves a reduced crenulated lingual cingulum and lacks a labial cingulum (c. 71[0]).
P3 is double-rooted with cylindrical anterior and posterior root lobes that are of equal width as the crowns (c. 64[0], 74[0]). The crowns on both left and right P3 are poorly preserved, however, the right P3 preserves relatively more enamel with visible cusps. As in P2, the crown bulbous and the paracone forms the apex of the crown. Tooth wear on the distal surface has obliterated all other cusps. The lingual cingulum is partially worn, but it still preserves some of the crenations; there is no labial cingulum.
Although P4 and M1 are missing, the alveolar morphology indicates they were double-rooted teeth with cylindrical anterior and posterior root lobes (c. 76[1], 77[1]). M2 was apparently much smaller than M1; the preserved alveoli indicate it was a double-rooted tooth (c. 80[0]).
Lower dentition—The left and right mandibles of OCPC 11141 (Fig. 10; Table 3) preserve c1 and p2–4, and c1, respectively; alveoli for p1, m1–2, for a tooth count of i?, c, p1–4, m1–2 (c. 53[?], 61[0]). The tooth row is relatively short making up less than 40% of the mandible length (c. 65[1]). The crowns have well-developed enamel (c. 62[0]), with wear surfaces on the apex and distal and mesial surfaces (c. 82[1]). The incisors are missing in both mandibles, and their alveolar morphology is obscured by sediment.
Figure 10: Lower dentition of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Left lower dentition in labial (A–B) and lingual (C–D), views. Abbreviations: c, lower canine; lc, lingual cingulum; lls, lingual longitudinal sulcus; m1–2, lower molars 1–2; mtd, metaconid; p2–4, lower premolars 2–4; pad, paraconid; prd, protoconid; wf, wear facet.| Total length | 231, 247 |
| Length of toothrow, c1–m2 | 140, 146 |
| Height at genial tuberosity | 86, 82 |
| Greatest length of symphysis | 62 |
| Greatest height at coronoid process | 121, 115 |
| Transverse width of mandibular condyle | 38*, 58 |
| Length of masseteric fossa | ∼106, ∼109 (Not distinct) |
| Length of coronoid | 118, 118 |
| c1 crown height/mesiodistal length/buccolingual width | 33*/24/19, n/a |
| p1 crown height/mesiodistal length/buccolingual width | L: Alveolus poorly preserved R: 15/12 |
| p2 crown height/mesiodistal length/buccolingual width | L: 9/15/12 R: Alveolus poorly preserved |
| p3 crown height/mesiodistal length/buccolingual width | L: 9/13/1 R: Alveolus poorly preserved |
| p4 crown height/mesiodistal length/buccolingual width | L: 11*/14/11 R: Alveolus poorly preserved |
| m1 crown height/mesiodistal length/buccolingual width | Alveoli poorly preserved |
| m2 crown height/mesiodistal length/buccolingual width | Alveoli poorly preserved |
Notes:
Modified from Boessenecker & Churchill (2018).
a, alveoli; L, left; R, right.
The lower canines are nearly as large as the upper ones, vertically oriented, and posteriorly curved (c. 56[0], 60[0]). The crown is conical, transversely compressed, with an oval cross section; the enamel is smooth and thick with a posterior crista (c. 57[0], 58[0]), and posterior wear facet formed by occlusion with the upper canines. The lingual and labial surfaces of the canine roots exhibits longitudinal sulci that terminate below the base of the crown, giving it a bilobed cross section (c. 59[1]) as in I. downsi, P. thomasi, and N. mirum (Mitchell, 1968; Barnes, 1988; Velez-Juarbe, in press).
The first premolar (p1) is missing on the type and referred mandibles, however, the alveoli indicate a single, cylindrical root. The second through fourth premolars (p2–4) are double-rooted or bilobed. Their crowns are bulbous with oval outline (c. 63[1]) and are worn on their mesial and distal surfaces. Premolar 1 has a prominent protoconid cusp; mesiolingual wear preclude us from determining if there were other cusps (c. 68[?]); distally, the crown is worn but there are remnants of a small metaconid (c. 70[0]). Lingually, a crenulated cingulum is present (c. 67[1]), while distolabially there is an incipient cingulum.
The left p3 is double-rooted with divergent and bulbous anterior and posterior root lobes that result in a root as wide as the crown (Character 64[0], 73[0]). The crown is bulbous and as in p2, the crown has a prominent protoconid cusp, the wear facets have nearly obliterated other cusps. The lingual cingulum is worn mesially and distally, but it was prominent with small crenulations.
The left p4 resembles p3 and is double-rooted with cylindrical anterior and posterior root lobes, but which are not as wide as the crown. Although the crown is damaged on its labial side, the lingual surface is well preserved; the crown is bulbous with small mesial and distal wear facets; with a prominent, crenulated lingual cingulum that borders a small talonid basin (c. 69[1]), prominent protoconid and paraconid cusps [c. 66 [0]).
The molars are not preserved in any of the mandibles. But, the alveolar morphology suggests m1 has double-rooted cylindrical roots while m2 had a single, oval root (c. 78[0], 79[0], 81[0]).
Postcrania
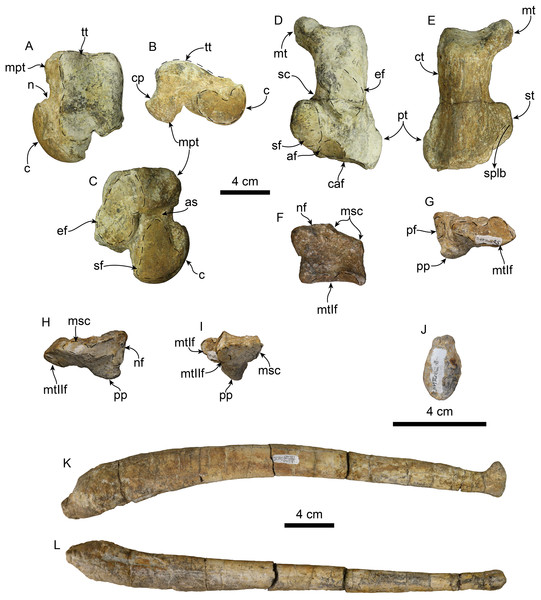
The holotype includes an associated, mostly complete postcranial skeleton. However, here we describe the baculum, atlas, and phylogenetically informative postcranial elements (Figs. 10–12; Tables 4–7), which include the atlas, axis, humerus, radius, scapholunar, metacarpal 1, astragalus, calcaneum, and entocuneiform.
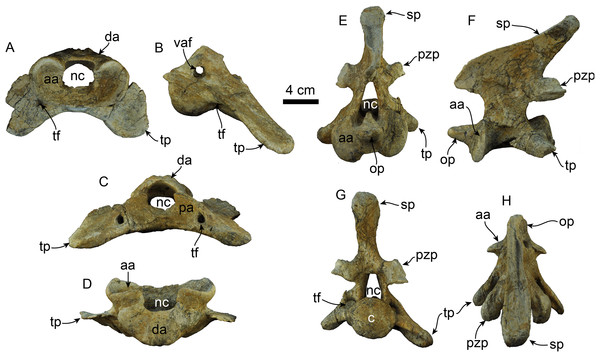
Figure 11: Atlas and axis of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
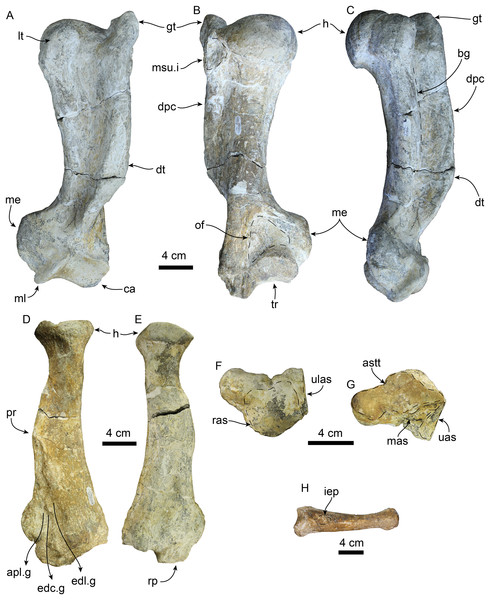
Atlas in anterior (A), left lateral (B), posterior (C), and dorsal (D) views; axis in anterior (E), left lateral (F), posterior (G), and dorsal (H) views. Abbreviations: aa, anterior articular surface; c, centrum; da, dorsal arch; nc, neural canal; op, odontoid process; pa, posterior articular surface; pzp, postzygapophysis; sp, spinous process; tf, transverse foramen; tp, transverse process; vaf, vertebrarterial foramen.Figure 12: Forelimb elements of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Left humerus in anterior (A), posterior (B), and medial (C) views; left radius in lateral (D), and medial (E), views; left scapholunar in proximal (F), and dorsodistal (G), views; left metacarpal I in dorsal (H) view. Abbreviations: apl.g, groove for tendon of M. abductor policis; astt, articular surface for trapezium and trapezoid; bg, bicipital groove; ca, capitulum; dpc, deltopectoral crest; dt, deltoid tubercle; edc.g, groove for tendon of M. extensor digitorum communis; edl.g, groove for tendon of M. extensor digitorum lateralis; gt, greater tubercle; h, head; iep, insertion for M. extensor pollicis; lt, lesser tubercle; mas, magnum articular surface; me, medial epicodyle; ml, medial lip of trochlea; msu.i, insertion of M. supraspinatus; of, olecranon fossa; pr, pronator ridge; ras, radial articular surface; rp, radial process; tr, trochlea; uas, unciform articular surface; ulas, ulnar articular surface.| Atlas | |
| Width across anterior articular surface | 111 |
| Maximum height along midline | 78 |
| Maximum width across transverse processes | 191+ |
| Height/width of neural canal anteriorly | 36/36 |
| Diameter of transverse foramen | 10 |
| Width across posterior articular surface | 79 |
| Axis | |
| Width across anterior articular surfaces | 75 |
| Maximum height along midline | 152 |
| Height of neural spine | 84 |
| Height/width of neural canal anteriorly | 32/18 |
| Maximum width across postzygapophyses | 79 |
| Maximum width across transverse processes | 117 |
| Maximum diameter of transverse foramina | 17 |
| Maximum length | 112 |
| Maximum length of neural spine | 140 |
| Height/width of centrum posteriorly | 43.54 |
| Scapholunar | |
| Maximum length | 80 |
| Maximum width | 64 |
| Metacarpal I | |
| Maximum length | 160 |
| Maximum width/height proximally | 44/31 |
| Maximum width/height distally1 | 33/26 |
| Astragalus | |
| Maximum length | 95 |
| Maximum width | 76 |
| Transverse width of capitulum | 58 |
| Maximum height of capitulum | 34 |
| Width of neck | 45 |
| Calcaneus | |
| Maximum length | 106 |
| Maximum distal width | 75 |
| Maximum proximal width | 59 |
| Entocuneiform | |
| Width/height of metatarsal I articular surface | 47/23 |
| Maximum width/length dorsally | 54/45 |
| Width/height of navicular facet | 30/25 |
| Width/height of mesocuneiform facet | 21/9 |
| Baculum | |
| Maximum length | 342 |
| Proximal diameter | 34 |
| Maximum height of distal end | 28 |
| Mid-shaft diameter | 24 |
Atlas—The atlas (Figs. 11A–11D) is virtually complete, missing only a fragment of the right transverse process. The anterior articular surface is shallowly concave transversely and dorsoventrally, forming a nearly continuous surface ventrally. The dorsal arch has a low midline crest dorsally. The neural canal is round in outline. The vertebrarterial foramina are round (diam. ∼10 mm) and open laterally. The transverse processes are arcuate, flange-like and oriented posteroventrally. The transverse foramina open anteriorly within a shallow fossa at the base of the transverse processes; posteriorly they open anteromedially on the transverse process. The posterior articular surfaces are shallowly concave dorsoventrally and are smaller than their anterior counterparts. The articular surface for the odontoid process is shallowly convex and faces dorsally.
Axis—The axis (Figs. 11E–11H) is nearly complete, missing only the distal end of the left transverse process. The odontoid process is cylindrical and forms a continuous surface with the anterior articular surfaces, which are shallowly convex. Proximally, the dorsal surface of the odontoid forms a low keel, which continues posteriorly into the floor of the neural canal. The neural canal is oval to subtriangular in outline. The transverse processes are oriented posteroventrally and are pierced proximally by the transverse foramina. The postzygapophyses are oriented posteriorly with relatively large mammilary processes. The neural spine is tall and slopes posterodorsally with a blade-like outline in lateral view (c. 91[1]), similar to the condition of A. akamatsui (Tanaka & Kohno, 2015). The dorsal surface of the neural spine is flat and transversely expanded. The posterior surface of the centrum is shallowly convex with a rounded outline.
Baculum—The baculum (Figs. 13J–13L) is nearly cylindrical, dorsally arched, and elliptical in cross-section. The ventral surface is nearly flat, while dorsally it is rounded. The proximal end is bulbous with a rough surface, while distally its diameter becomes smaller toward the distal end. The distal end is anteriorly convex and dorsoventrally expanded in the form of dorsal and ventral knobs. The baculum of T. orangensis is 10% of the estimated body length (34.2 cm/est body length 331 cm). This is considerably smaller than that of O. rosmarus, which has the relatively largest baculum among pinnipeds, with its length being equivalent to around 18% of the body length (Fay, 1982; Miller, 2018).
Figure 13: Hindlimb elements and baculum of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
Left astragualus in tibial (A), medial (B), and ventral (C) views; left calcaneum in dorsal (D), and plantar (E) views; left entocuneiform in dorsal (F), distal (G), proximal (H), and lateral (I), views; baculum in proximal (J), right lateral (I), and ventral (L) views. Abbreviations: af, accessory facet; astragalar sulcus; c, capitulum; caf, cuboid articular facet; cp, calcaneal process; ct, calcaneal tuber; ef, ectal facet; mpt, medial plantar tuberosity; msc, mesocuneiform facet; mt, medial tuberosity; mtIf, metatarsal I facet; n, neck; nf, navicular facet; pf, pisiform facet; pp, plantar process; pt, peroneal tubercle; sc, sulcus calcanei; sf, sustentacular facet; splb, sulcus for peroneus longus and peroneus brevis tendon; st, sustentaculum tali; tt, tibial trochlea.Humerus—The left humerus is laterally bowed (Figs. 12A–12C; Tables 5 and 6), and relatively robust, with length/least diameter and proximal width/greatest length ratios between those of Valenictus spp. (more robust/stocky) and those of the more slender D. seftoni and I. downsi (Repenning & Tedford, 1977; Deméré, 1994a; Table 6). The greater tuberosity is prominent extending proximally beyond the level of the head as in I. downsi, G. pugnax, and D. seftoni (Mitchell, 1968; Repenning & Tedford, 1977; Barnes & Raschke, 1991; Deméré, 1994a). The humeral head is large and rounded. The lesser tuberosity is knob-like and does not extend proximally beyond the level of the head, thus differing from the condition of I. downsi and being more similar to D. seftoni (Mitchell, 1968; Repenning & Tedford, 1977; Deméré, 1994a). In lateral or medial view the deltopectoral crest is moderately prominent, resembling the condition observed in P. pacifica and Valenictus spp., and differing from the more prominent crest of I. downsi (Mitchell, 1961; Repenning & Tedford, 1977; Deméré, 1994a). The deltopectoral crest gradually tapers distally approaching the radial fossa, and curves toward the medial lip of the trochlea. The deltoid tubercle is on the pectoral crest (c. 83[0]). The distal articular surface has a saddle-like outline in posterior or anterior views (Figs. 12A and 12B). The medial lip of the trochlea is prominent with a diameter greater than that of the capitulum (c. 84[1]). The coronoid and radial fossae are present, with the former being deeper and wider. The medial epicondyle is prominent, with an oval outline as in I. downsi and D. seftoni, and unlike the more prominent medial epicondyle that distinguishes Valenictus spp. (Mitchell, 1961, 1968; Repenning & Tedford, 1977; Deméré, 1994a).
| Greatest length, tuberosity to radial capitulum | 345 |
| Length, head to radial capitulum | 331 |
| Length, lesser tuberosity to radial capitulum | 324 |
| Transverse width across tuberosities | 116 |
| Greatest transverse width of head | 96 |
| Transverse width at narrowest part of shaft | 54 |
| Anteroposterior width at midshaft | 74 |
| Greatest width across epicondyles | 125 |
| Greatest anteroposterior diameter of medial edge of trochlea | 70 |
| Greatest anteroposterior diameter of radial capitulum | 43 |
| Greatest width of distal articulation | 83 |
| Transverse width of entepicondyle | 36 |
Note:
Modified from Deméré (1994a).
| GL | LD | TW | GL/LD | TW/GL | ||
|---|---|---|---|---|---|---|
| Titanotaria orangensis | OCPC 11141 | 345 | 54 | 116 | 6.38 | 0.33 |
| Valenictus chulavistensis | SDSNH 367861 | 326 | 56 | 120 | 5.82 | 0.37 |
| Valenictus chulavistensis | SDSNH 383121 | 315 | 55 | 120 | 5.72 | 0.38 |
| Valenictus chulavistensis | SDSNH 383151 | 306 | 56 | 119 | 5.46 | 0.39 |
| Valenictus chulavistensis | SDSNH 353751 | 263 | 61 | 109 | 4.31 | 0.41 |
| Valenictus chulavistensis | SDSNH 383001 | 300 | 63 | 112 | 4.76 | 0.37 |
| Valenictus imperialensis | LACM 39261 | 253 | 51 | 102 | 4.96 | 0.40 |
| Dusignathus seftoni | SDSNH 438731 | 346 | 62 | 96 | 5.68 | 0.26 |
| Imagotaria downsi | USNM 238702 | 265 | 42 | 85 | 6.30 | 0.22 |
| Imagotaria downsi | USNM 238652 | 226 | 37 | 65 | 6.10 | 0.29 |
Notes:
GL, greatest length; LD, least diameter of shaft; TW, transverse width at tuberosities.
Radius—The radius (Figs. 12D and 12E) is stout with its distal end anteroposteriorly expanded with a large radial process (c. 85[2]); it is similar in size to one of the referred radii of I. downsi (Repenning & Tedford, 1977: table 9). Proximally, the head of the radius is rounded and with a shallowly concave articular surface. The proximal end of the diaphysis is transversely narrow relative to the distal end, and oval in cross section. The pronator ridge is prominent and located about midlength on the shaft. Distally, the radial crest is rounded in lateral view, and together with the radial process, it forms the widest part of the radius (Table 7). The radial process is not as displaced medially as that of Imagotaria. Distally, the anterolateral surface is marked by relatively deep grooves for the M. extensor metacarpi pollicis and M. extensor digitorum.
| Length | 277 |
| Greatest width, proximal articulation | 73 |
| Anteroposterior length, distal termination | 95 |
| Anteroposterior length, proximal articulation | 54 |
| Depth of shaft at pronator teres origin | 58 |
| Width of shaft at pronator teres origin | 35 |
Note:
Modified from Repenning & Tedford (1977).
Scapholunar—Proximally (Fig. 11E), the radial articular surface is anteroposteriorly convex with a subrectangular outline. Distally (Fig. 11F), the articular surface for the trapezium and trapezoid is oval and deeply concave transversely. The unciform articular surface is elongated and shallowly concave and is continuous posteriorly with the cuneiform articular surface. The magnum articular surface is deeply concave, forming a distinct pocket (c. 87[1]) as in other odobenids (Repenning & Tedford, 1977; Deméré, 1994b).
Metacarpal 1—The first metacarpal (Fig. 11) is elongated, curved laterally, and slender, resembling that of I. downsi (Repenning & Tedford, 1977). The proximal surface of the bone is smoothly concave transversely and dorsoventrally convex. Proximally, the dorsal surface has a shallow oval depression for the insertion of M. extensor pollicis (c. 86[1]). The distal articular trochlea has a rounded outline.
Astragalus—The head of the astragalus (Fig. 12) is prominent and dorsoventrally flattened; the neck is well defined and the articular surface is dorsoventrally and transversely convex. Ventrally, the navicular facet is continuous with a broad, oval (longer than wide) sustentacular facet, markedly differing from the relatively narrow navicular facet of Valenictus (Deméré, 1994a; Boessenecker, 2017). The sustentacular facet is separated from the ectal facet by a narrow astragalar sulcus. The ectal facet is concave and lunate, and separated from the oval, fibular facet by a low ridge. Dorsally, the navicular facet bears a median notch, and is separated from the trochlea by a groove. The trochlea is rectangular in outline with a short, shallow intertrochlear pit along its distal edge; the articular surface is convex and divided by a shallow anteroposteriorly-oriented groove. The lateral trochlear ridge is longer than the medial trochlear ridge. The calcaneal process is short and rounded (c. 88[1]). The medial plantar tuberosity is rounded and more robust and prominent than the nearly inconspicuous lateral plantar tuberosity.
Calcaneum—The calcaneal tuber is elongated with a prominent medial process (= internal tuberosity of Repenning & Tedford (1977)) (c. 89[1]; Figs. 13D and 13E), as in most odobenids and Allodesmus spp. (Mitchell, 1966; Deméré, 1994b; Tonomori et al., 2018). The posterior articular surface is convex and broad. The sustentaculum tali is robust, extending as far medially as the medial process of the calcaneal tuber. The ectal face is anteroposteriorly convex, with an oval outline, and oriented obliquely to the long axis of the bone. The sustentacular facet is flat posteriorly, becoming concave distally; a secondary shelf in the sustentaculum is missing, as in I. downsi and V. chulavistensis (Repenning & Tedford, 1977; Deméré, 1994a). The groove for the peroneus longus tendon extends along the distoventral surface of the sustentaculum tali. Distally, the calcaneum is mediolaterally broader than proximally, similar to the condition in I. downsi, but unlike the proportionately broader distal end of the calcanei of V. chulavistensis (Repenning & Tedford, 1977; Deméré, 1994a). On the distal surface, the cuboid articular surface is broad, subrounded, and makes up most of the distal surface of the calcaneum.
Entocuneiform—The entocuneiform (Figs. 13F–13H) has an irregularly pentagonal outline, and is mediolaterally wide and dorsoventrally flattened (Table 4). In proximal (posterior) view the entocuneiform is divided into two facets. The medial facet for the navicular is shallowly convex, L-shaped and oriented proximally, while the mesocuneiform facet is oriented proximolaterally, both forming a broad angle (c. 90[1]). Proximally, the medial surface has a suboval pisiform facet. The anterolateral surface of the bone has a ventrolaterally oriented oval facet for metatarsal II. Ventrally, there is a rounded, prominent, plantar process on the proximomedial corner. Anteriorly, the articular surface for metatarsal I is irregularly oval, with a dorsomedial depression; the surface is transversely concave and dorsoventrally convex.
Results
Phylogenetic results
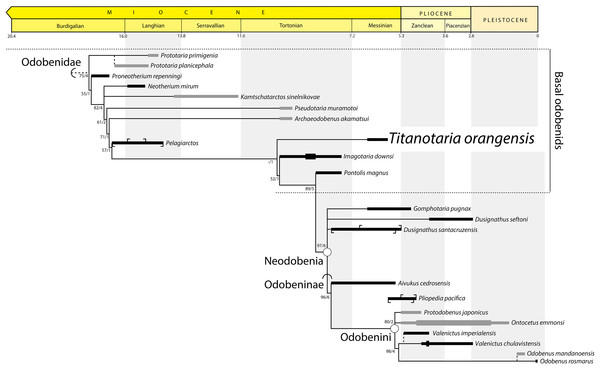
Our phylogenetic analysis without ordered characters recovered 27 equally parsimonious trees of 265 steps. An analysis with ordered characters, recovered 27 equally parsimonious trees of 274 steps (Fig. 13). Both analyses recover nine trees that vary within the ingroup (Odobenidae). In the strict consensus trees for both analyses, the topology for the ingroup is almost entirely pectinate except for three polytomies that can be resolved to form exclusive sister taxa: (1) A polytomy that includes N. mirum, Kamtschatarctos sinelnikovae Dubrovo, 1981, and odobenids more closely related to O. rosmarus. The shortest trees include N. mirum and K. sinelnikovae as sister taxa or paraphyletic with either taxon as more basal; (2) A polytomy that includes D. seftoni, D. santacruzensis, and odobenids more closely related to O. rosmarus. The shortest trees include a monophyletic Dusignathus Kellogg, 1927 and a paraphyletic Dusignathus, with D. santacruzensis as sister to the Odobeninae. In the unordered analysis, G. pugnax is always sister to the Dusignathus polytomy, but the shortest trees from the ordered analysis also include G. pugnax as more closely related to O. rosmarus than a monophyletic Dusignathus. (3) A polytomy that includes P. japonicus, O. emmonsi, and odobenids more closely related to O. rosmarus. The shortest trees include P. japonicus as either the sister taxon of O. emmonsi or the sister taxon of a clade that includes O. emmonsi and odobenids more closely related to O. rosmarus. Titanotaria orangensis is found to be sister to a clade that includes I. downsi and all other more crownward odobenids, albeit with low support. Titanotaria orangensis, Pelagiarctos sp., and I. downsi represent a grade of phenetically similar taxa at the base of the clade that includes P. magnus with Dusignathus, G. pugnax, and Odobeninae.
Phylogenetic taxonomy
The first study to provide phylogenetic definitions for Odobenidae and its subclades was Deméré (1994b), which demonstrated the paraphyly of the “Imagotariinae” Mitchell, 1968, and provided phylogenetic definitions for Dusignathinae Mitchell, 1968, Odobenidae Allen, 1880, Odobeninae Mitchell, 1968, and the new taxon Odobenini Deméré, 1994b. Since that time, all workers have accepted the non-monophyly of the “Imagotariinae,” although this term is used as an informal grade by some authors (Boessenecker & Churchill, 2013). Given that I. dowsni is more closely related to P. magnus and other late Miocene odobenids, the continued referral of the most basal middle Miocene forms (with more plesiomorphic characters) as imagotariines is misleading. Some studies have chosen to refer to more basal forms as “archaic odobenids,” although the boundary of this informal grouping can differ even within a single paper (see Tanaka & Kohno, 2015: fig. 11 vs. text).
The definition of Dusignathinae Mitchell, 1968, proposed by Deméré (1994b) is more exclusive than that used by Repenning & Tedford (1977) who included all non-odobenines in this subfamily. Deméré’s (1994b: 103) definition was based on “the most recent ancestor of Pontolis and Dusignathus,” a grouping that has been found to be paraphyletic by all later analyses (Kohno, 2006; Boessenecker & Churchill, 2013; Tanaka & Kohno, 2015; this study). Kohno (2006) proposed an amended phylogenetic definition for the Dusignathinae as “a clade containing the most recent common ancestor of Dusignathus and Gomphotaria and all of its descendants.” Since that time the concept of the Dusignathinae has been restricted to those two genera, but even the amended definition becomes problematic in light of recent analyses that show that it is paraphyletic and even question the monophyly of the genus Dusignathus (Boessenecker & Churchill, 2013; Tanaka & Kohno, 2015; this study). In light of this history, we do not propose a phylogenetic definition for Dusignathinae and question the further use of this name as the only way to ensure its monphyly is to restrict it to D. santacruzensis.
Setting aside “imagotariines” and “dusignathines,” there are three groupings (Odobenidae, Odobeninae, and Odobenini) that have crystallized in their usage over the past 14–50 years and are consistent with the phylogenetic definitions proposed by Deméré (1994b). We propose slightly amended definitions for two of these names by making them branch-based instead of node-based. We also propose a new name for an often-discussed and well-supported clade of odobenids. The phylogenetic names used in this study are listed below.
Neodobenia, new clade name
Definition—The least inclusive clade that includes Odobenus rosmarus (Linnaeus, 1758), Dusignathus seftoni Deméré, 1994a, and Gomphotaria pugnax Barnes & Raschke, 1991.
Reference phylogeny—Figure 14 of this study.
Figure 14: Preferred phylogenetic tree showing position of Titanotaria orangensis n. gen. et sp. (OCPC 11141).
A stratigraphically-constrained strict consensus of 27 trees from the analysis with ordered characters. Outgroups (non-odobenids) not shown. Node- (circle) and branch-based (semicircle) phylogenetic names and bootstrap/decay values indicated. The length of ranges indicates age uncertainty for the formation, not taxon duration, with tick marks indicating the limits of specific units. In cases where formation ages do not overlap we show a known range (thick line). Taxa that do not occur in East Pacific formations are dark grey. Non-analyzed congeners indicated by dashed lines, with Pliopedia pacifica placed near the Odobenini. The lineage of Valenictus is extended to accommodate a specimen from the Purisima Formation. Protodobenus japonicus is sister to Odobenini in some trees.Etymology—Neo- for “new;” -odobenia for Odobenidae.
Comments—One of the best supported nodes of our analysis unites the clade Odobeninae (see below) with the “dusignathines” (in the recent usage sense of a grade or clade that includes G. pugnax and Dusignathus spp., but not P. magnus). We do not use D. santacruzensis to define Neodobenia because it is poorly known, and in any case is always either sister to D. seftoni or else more closely related to O. rosmarus than D. seftoni in all analyses. Because this clade unites two important groupings of odobenids, and is evidently worth talking about (see mentions in Deméré, 1994b; Kohno, 2006; Boessenecker & Churchill, 2013), we feel it is useful to name. Neodobenia is diagnosed by single-rooted, simplified teeth. Most neodobenians are thought to have tusks except for P. japonicus and possibly A. cedrosensis (broken canines). We refer to all taxa on the stem of Neodobenia, informally, as “basal odobenids.”
Odobenidae Allen, 1880, converted clade name
Definition—All taxa more closely related to Odobenus rosmarus (Linnaeus, 1758) than to Phoca vitulina Linnaeus, 1758 or Otaria byronia (De Blainville, 1820).
Reference phylogeny—Figure 14 of this study.
Comments—The first phylogenetic definition for Odobenidae was provided by Deméré (1994b: 102): “The Odobenidae are defined as the monophyletic group containing the most recent common ancestor of Neotherium and Odobenus and all of its descendants.” Later, Berta, Sumich & Kovacs (2006: 496) provided an amended definition for the Odobenidae: “Monophyletic group containing the common ancestor of stem odobenids Imagotaria, Kamtschatarctos, Neotherium, Proneotherium, Pseudotaria, Prototaria, Pelagiarctos, and crown odobenids and all of their descendants including Aivukus, Dusignathus, Gomphotaria, Ontocetus, Pliopedia, Pontolis, Protodobenus, and Valenictus and Odobenus.” This definition is problematic because it misapplies the crown clade concept (meant to be defined by most recent common ancestor of extant species) to apply to a clade name that is defined by extinct taxa. Both the Deméré (1994b) and Berta, Sumich & Kovacs (2006) definitions have the same content as a total clade name (Cantino & De Queiroz, 2014) for the extant Odobenus rosmarus. Therefore, we propose a branch-based Odobenidae, which is consistent with recent usage and complements the construction of its sister taxon, the branch-based Pan-Otariidae Gill, 1866 (Velez-Juarbe, 2017).
Odobeninae Mitchell, 1968, converted clade name
Definition—All taxa more closely related to Odobenus rosmarus (Linnaeus, 1758) than to Dusignathus santacruzensis Kellogg, 1927, Dusignathus seftoni Deméré, 1994a, or Gomphotaria pugnax Barnes & Raschke, 1991.
Reference phylogeny—Figure 14 of this study.
Comments—The first phylogenetic definition for Odobeninae was provided by Deméré (1994b: 103): “The Odobeninae are here defined as the monophyletic group containing the most recent common ancestor of Aivukus and Odobenus and all of its descendants.” This definition includes all those species more closely related to O. rosmarus than to “dusignathines” and so we propose a branch-based Odobeninae.
Odobenini Deméré, 1994b
Definition—”The monophyletic group containing the most recent common ancestor of Alachtherium [Ontocetus emmonsi] and Odobenus” (Deméré, 1994b: 104). The type species of Alachtherium is Alachtherium cretsii Du Bus, 1867, which was later referred to Ontocetus emmonsi Leidy, 1859, by Kohno & Ray (2008).
Reference phylogeny—Deméré (1994b: fig. 1).
Comments—Deméré (1994b) included all odobenines except Aivukus cedrosensis Repenning & Tedford, 1977 in his node-based definition of Odobenini. We do not propose a branch-based or otherwise emended phylogenetic definition for this name because it is less clear that this would capture the original intent (at the time it was coined, Odobenini was diagnosed by 14 unequivocal synapomorphies) and usage. Shortly after Deméré (1994b) was published, Protodobenus japonicus Horikawa, 1995, was described from the early Pliocene of Japan (published in parallel, neither paper cited each other). Protodobenus japonicus has been placed in the Odobenini by some later authors (Deméré, Berta & Adam, 2003) and not others (Boessenecker & Churchill, 2013). Our phylogenetic analysis alternatively resolves this taxon as the sister to O. emmonsi within Odobenini or as the sister taxon to Odobenini.
Richness curve results
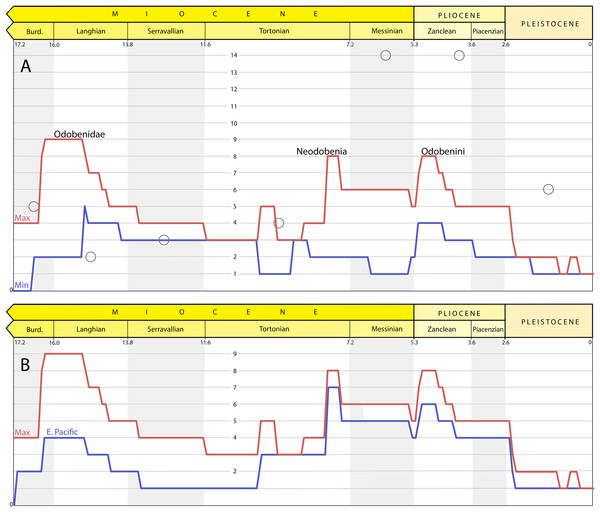
The results of the maximum and minimum lineage count are shown in Fig. 15 (top). The maximum lineage count shows peaks near the base of Odobenidae, Neodobenia, and Odobenini. The lineage count is as high as nine in the Langhian and eight in the Messinian and as low as three and four in the intervening stages (Serravallian and Tortonian); the period of low lineage counts is between ∼15 and 8 Ma. After the Miocene, the maximum linage count decreases with a precipitous drop at the end of the Pliocene.
Figure 15: Richness estimates for Odobenidae.
(A) Maximum (red) and minimum (blue) lineage counts accounting for ghost lineages and stratigraphic uncertainty. Peaks on the maximum richness curve labelled with corresponding clade originations. Open circles are from stage-binned analysis that includes undescribed specimens (includes both “small- and large-bodied” forms; Boessenecker & Churchill, 2018). (B) Maximum overall (red) and maximum of trees with East Pacific taxa only (blue) lineage counts.The minimum lineage count reaches a high of four briefly in the Langhian and throughout the Zanclean. The difference between the maximum and minimum curves is almost entirely determined by stratigraphic uncertainty because maximum lineage counts among the 27 trees were identical for 107 of the 173 time slices (61%) and within one for the other 67 (39%).
The results of the maximum lineages count when all taxa from outside the East Pacific were pruned from the trees is shown in Fig. 15 (bottom). When compared to the maximum global curve, the difference between the two curves is the relative input of lineages from outside the East Pacific. After 10 Ma, the richness curve is dominated by East Pacific lineages as shown by the close tracking of the two curves.
Discussion
Our assessment of odobenid richness takes into account stratigraphic uncertainty and ghost lineages at 0.1 Ma intervals, giving insights into the timing of diversification events from a phylogenetic perspective. The overall pattern from our maximum richness curve (Fig. 15) generally conforms to the pattern shown by the stage-binned analysis of Boessenecker & Churchill (2018) with a few exceptions. Because that previous analysis incorporated undescribed material and used larger bins, we only highlight instances where our analysis estimates greater richness or the possibility of a different timescale for richness changes.
Our maximum diversity curve shows a peak near the origin of Odobenidae ∼16–15 Ma (∼early Langhian) that is higher than that of Boessenecker & Churchill (2018). In our analysis, this peak is determined, in part, by the fact that this is a time when multiple taxa from both the East and West Pacific are known. Another factor is the antiquity and phylogenetic position of Pelagiarctos sp., which necessitates ghost lineages for more basal, but younger, taxa (Fig. 14). P. thomasi is known from a single jaw (Sharktooth Hill Bonebed, Round Mountain Silt), and so was excluded from the first two cladistic analyses of odobenids (Deméré, 1994b; Kohno, 2006). Boessenecker & Churchill (2013) describe and refer additional jaw material from the “Topanga” Formation to Pelagiarctos sp. and include it in an updated matrix. They found it to be a sister taxon to I. downsi based on character states that at the time were unequivocal synapomorphies, but were later found in T. orangensis (c. 59 [1], 67[1]) and A. akamatsui (c. 67[1]). A Pelagiarctos-based OTU was excluded from the latest phylogenetic analysis of odobenids (Tanaka & Kohno, 2015). Those authors include a section on the “Enigmatic odobenid Pelagiarctos,” where they argue that Pelagiarctos is still too poorly known to be included in phylogenetic analyses. The lack of additional material referable to Pelagiarctos is vexing considering it is known from two formations (including at least one bonebed). A recently described specimen from the Sharktooth Hill Bonebed (Velez-Juarbe, in press) suggests that, despite the antiquity of the site (15.9-15.2 Ma) it may include more crownward odobenids. A partial jaw (LACM 135920) was hypothesized to be a new taxon more closely related to P. magnus than to other basal odobenids, i.e., even more crownward than Pelagiarctos. Unfortunately, the incomplete nature of LACM 135920 precludes inclusion in a cladistic analysis. It remains to be seen whether the phylogenetic position of Pelagiarctos and LACM 135920 should be refined or if, as requisite ghost lineages suggest, early Langhian odobenids were even more diverse than currently thought.
After about 15 Ma, the fossil record of odobenids becomes much more incomplete. For example, there is just one described taxon from the Serravallian (13.8–11.6 Ma), K. sinelkovae, which is poorly constrained stratigraphically (Appendix 1) and in need of redescription (Boessenecker & Churchill, 2013: 12). The recently described N. arandai (Velez-Juarbe & Salinas-Márquez, 2018) may also be Serravallian, but its age is poorly constrained (15.7–9.2 Ma). The low number of odobenids is in contrast to the non-odobenid pinniped Allodesmus, which may have three to four described taxa in the Serravallian (Boessenecker & Churchill, 2018; Tonomori et al., 2018). Boessenecker & Churchill (2018) suggest the possibility of competitive displacement of odobenids and Allodesmus, which seems plausible given their undeniable similarity in size, morphology, and presumably aspects of their ecology. Debey & Pyenson (2013) suggest that Allodesmus is a deep-diving taxon, which may also be a factor that may favor their preservation in certain formations over odobenids. The number of observed odobenid lineages remains low until the late Tortonian or early Messinian (8–7 Ma), coincident with both the extinction of Allodesmus (Boessenecker & Churchill, 2018) and the diversification of neodobenians.
The exact timing of the neodobenian diversification event is unclear, the potentially oldest members (A. cedrosensis and D. santacruzensis from the Almejas Formation) have poor temporal constraints (8.0–5.5 Ma, Appendix 1). As such, our maximum richness curve shows a potential peak before the Messinian (7.2–5.3 Ma), earlier than where Boessenecker & Churchill (2018) show their major increase in richness. An undescribed specimen referred to Gomphotaria from the Towsley Formation was reported from the Tortonian by Boessenecker & Churchill (2018) would seemingly confirm a pre-Messinian origin for neodobenians. However, parts of the Towsley Formation are considered to be latest Miocene or even Pliocene in age by other authors (Squires, 1991; Yeats, Huftile & Stitt, 1994; 6.5–5.0 Ma), which means there are no definitive pre-Messinian neodobenians. Therefore, with the available data, we can recognize a provisional timescale for the transition from odobenid assemblages that include just basal odobenids from Empire and older formations (>7 Ma), to a mixture of basal odobenids and neodobenians from the Capistrano and basal Purisima Formations (∼7–5 Ma), and then just neodobenians from all younger units (<5 Ma). In this regard, T. orangensis (6.6–5.8 Ma) is an important addition to our understanding of odobenid assemblages through time because it is one of the best-known and latest-surviving basal odobenids.
The Messinian peak of odobenid richness coincides with the evolution of more specialized feeding systems of neodobenians, including the evolution of tusks and molluscivory, which displaced them from basal odobenids ecologically. Basal odobenids go extinct before the Pliocene, and odobenid richness by that time is dominated by molluscivorous odobenines (Odobenini). Odobenids show a decrease in known richness in the Piacenzian by and ultimately a precipitous drop in the Pleistocene along with other taxa. Recent studies have attributed this extinction event to the reduction of the neritic zone, for example, shallow marine foraging areas, utilized by the molluscivorous Odobenini (Boessenecker, 2013a, 2017; Pimiento et al., 2017).
In contrast to the maximum lineage count for odobenids, the minimum lineage counts remain more stable (Fig. 15). Small peaks near the base of clade origins (Odobenidae, Neodobenia, Odobenini) mirror that of the maximum lineage richness curve. Aside from another peak in the Zanclean, which coincides with the diversification of Odobenini, this analysis alone cannot refute that the standing richness of odobenids was more than three species for most of their history (but see below). Two factors can account for this low estimate relative to the stage-binned analysis of Boessenecker & Churchill (2018). First, we have excluded abundant undescribed material (Appendix 2). Second, since each bin was calculated independently, it means that a lineage with an uncertain range can be considered to not have diverged at one 0.1 Ma interval and then be considered extinct in the next, whichever resulted in the lowest possible lineage count. In this way our curve shows the minimum at any given time, but no single time-constrained tree matches the entire minimum curve. With this in mind, a key comparison for our minimum estimate of odobenid richness is formations that include more than one species of odobenid. Most formations have just one or two species known, but starting in the late Tortonian we begin to see formations with up to four species, although some of these have not been formally described (Appendix 2). For example, with the addition of T. orangensis and unpublished specimens (Barboza et al., 2017; Velez-Juarbe, 2017; Appendix 2), the Oso Member might have four species of odobenids. The same is potentially true for the contemporaneous basal Purisima Formation and slightly older Empire Formation (Boessenecker, 2017; Appendix 2). Although these counts are based on undescribed material, they complement the increased richness suggested by the maximum curve of our conservative analysis and previous studies.
The largest discrepancy between the maximum and minimum estimates is during the Messinian diversification of neodobenians (nine vs. one lineage) and can be attributed to aforementioned stratigraphic uncertainty surround the origination of Neodobenia. For example, if P. magnus and T. orangensis went extinct before the deposition of the Almejas Formation, then the intervening time interval would just include a single estimated lineage, the ghost lineage of Neodobenia. This example demonstrates the large impact of stratigraphic uncertainty as a bias to increase and potentially overestimate lineage counts.
The most important bias to consider when interpreting odobenid richness is the temporal and geographic distribution of odobenid-bearing deposits. Our analysis of maximum lineage counts from the East Pacific closely matches that of the corresponding global curve, indicating that after 9.0 Ma the overall shape of the curve is largely driven by East Pacific taxa. Therefore, the pattern of increased odobenid richness seen here could be influenced by regional depositional patterns in the East Pacific. Kloess & Parham (2017) show that in California, where most of the formations in this study are from, the fossil record between 15 and 10 Ma is characterized by just a few formations that were deposited at upper to mid bathyal depths, whereas after this time many more deposits from shallow water are known. The deeper water deposits correspond to a time of low odobenid richness, which may reflect a reduced preservation potential. It remains to be tested how much the timescale for the transition from Allodesmus, to basal odobenids, to neodobenian assemblages reflects the timing of these depositional changes among regions.
A corollary of the large impact of East Pacific taxa on late Miocene richness estimates is that the West Pacific record is relatively poor (Fig. 1); there are no definitive records of West Pacific odobenids recorded between 9.5 and 5.3 Ma, including the time of highest estimated richness for odobenids in the East Pacific. The faunal list of Miyazaki et al. (1995) suggests that this record may be improved, but even then the record is sparse when compared with the amount of undescribed material from the East Pacific (Appendix 2). Given this unevenness, it is not possible to know whether the diversification events and assemblage transitions seen in the East Pacific happened in a similar way in the West Pacific. In light of the unevenness of the odobenid fossil record and stratigraphic uncertainty, estimates of odobenid richness and their correlations should be treated with caution. The amount of undescribed material mentioned in the literature (Appendix 2) shows great potential for future study. In addition to describing these specimens and placing them in a phylogenetic context, new discoveries and additional chronostratigraphic work on odobenid-bearing sites will help test, refine, and refute the patterns presented here.
Conclusions
We describe Titanotaria orangensis (gen. et. sp. nov.), a new species of walrus (odobenid) from the upper Miocene Oso Member of the Capistrano Formation of Orange County, California. This species is important because: (1) It is one of the best-known and latest-surviving basal odobenids; (2) It raises the number of reported odobenid taxa from the Oso Member to four species making it one of the most rich walrus assemblages known (along with the basal Purisima of Northern California); (3) It is just the second record of a tuskless walrus from the same unit as a tusked taxon. Our phylogenetic analysis places T. orangensis as sister to a clade that includes I. downsi, P. magnus, Dusignathus spp., G. pugnax, and Odobeninae. We propose new branch-based phylogenetic definitions for Odobenidae, Odobeninae, and a new node-based name (Neodobenia) for the clade that includes Dusignathus spp., G. pugnax, and Odobeninae. A richness analysis at the 0.1 Ma level that incorporates stratigraphic uncertainty and ghost lineages demonstrates maximum peaks of richness (up to eight or nine coeval lineages) near the base of Odobenidae, Neodobenia, and Odobenini. A more conservative minimum curve demonstrates that standing richness may have been much lower than the maximum richness estimates that are biased by stratigraphic uncertainty. Overall the odobenid fossil record is uneven, with large time slices of the record missing on either side of the Pacific Ocean at some times and biases from the preserved depositional environments at other times. We recognize a provisional timescale for the transition of an East Pacific odobenid assemblages that includes “basal odobenids” (stem neodobenians) from the Empire and older formations (>7 Ma), to a mixture of basal odobenids and neodobenians from the Capistrano and basal Purisima (7–5 Ma), and then just neodobenians from all younger units (<5 Ma). The large amount of undescribed material will add new taxa and range extensions for existing taxa, which will likely change some of the patterns we describe.
Appendix 1. List of Valid Taxa with Chronostratigraphic Assessments
Aivukus cedrosensis Repenning & Tedford, 1977
Age range 8.0–5.5 Ma.
Occurrence: East Pacific, lower part of the Almejas Formation, Cedros Island, Mexico.
Maximum age: Boessenecker & Churchill (2015, suppl. info.) report a maximum age of the Almejas Formation of ∼8 Ma based on Barnes (1998). Barnes (1998) tentatively reports the maximum age as ∼9 Ma and notes correlations with other upper Miocene formations. Barnes (2008) later refines the maximum age to ∼8 Ma, which we use here but note that more detailed faunal correlations can provide a more refined age.
Minimum age: Boessenecker & Churchill (2015, suppl. info.) estimate the minimum age of the Almejas Formation to be 5.7 Ma based on isotopic analyses of volcanic beds above the unit (Sawlan & Smith, 1984). Because the age of the volcanic bed is 5.7 ± 0.2 Ma, we report the minimum possible age 5.5 Ma.
Archaeodobenus akamatsui Tanaka & Kohno, 2015
Age range: 10.0–9.5 Ma.
Occurrence: West Pacific, Ichibangawa Formation, Hokkaido, Japan.
Maximum age: Fission track dating derived from a tuff layer within the underlying Subestsu Formation are dated at approximately 13 ± 1 Ma (Tsuji et al., 1992). However, we follow Tanaka & Kohno (2015) who accept a maximum age of 10.0 Ma based on the stratigraphic study of Takano et al. (1996).
Minimum age: We follow Tanaka & Kohno (2015) who accept a minimum age of 9.5 Ma based on the stratigraphic study of Takano et al. (1996).
Dusignathus santacruzensis Kellogg, 1927
Age range: 8.0–5.3 Ma.
Occurrence: East Pacific, Purisima Formation, California, USA and lower part of the Almejas Formation, Cedros Island, Mexico.
Maximum age: As for A. cedrosensis (see above).
Minimum age: The minimum age is based on the basal Purisima Formation (6.9–5.3 following Boessenecker, 2017).
Dusignathus seftoni Deméré, 1994a
Age range: 3.6–2.5 Ma.
Occurrence: East Pacific, “lower member” (sensu Deméré, 1983), San Diego Formation, California, USA.
Maximum age: The maximum age is 4.2 Ma based on Vendrasco et al. (2012).
Minimum Age: The minimum age is based on the occurrence of the planktonic foraminifers that indicate deposition during the California margin planktonic foraminiferal zone 6 (Kennett, Rozo-Vera & Machain Castillo, 2000) which has an age range from 3.25 to 2.5 Ma (Kucera & Kennett, 2000).
Gomphotaria pugnax Barnes & Raschke, 1991
Age range: 6.6–4.9 Ma.
Occurrence: East Pacific, unnamed siltstone member, Capistrano Formation, California, USA.
Maximum age: The maximum age is based on the maximum age of the Oso Member, 6.6 Ma (Barboza et al., 2017), which grades laterally into the basal part of the unnamed siltstone member.
Minimum age: The minimum age is based on diatom biostratigraphy from the vertebrate bearing layers (Deméré & Berta, 2005).
Imagotaria downsi Mitchell, 1968
Age range: 10.0–7.6 Ma.
Occurrence: East Pacific, Monterey Formation localities include the Great Lakes Carbon Co. Mine and the Celite Company No. 38 Quarry near Lompoc, California, USA. Imagotaria downsi is also known from the upper sandstones of the Santa Margarita Formation of California. Other specimens of I. downsi are reported from the Towsley and Monterey Formations of California, however, this material is largely fragmentary so they are not considered when constraining the age range of this taxon following Boessenecker & Churchill (2015, suppl. info.).
Maximum age: Our maximum age determination for I. downsi follows that of Boessenecker & Churchill (2015). The maximum age is based on the age of the upper sandstones of the Santa Margarita Formation. The Santa Margarita Formation is summarized by Repenning & Tedford (1977) to have an age range from 12 to 9 Ma based on fossil invertebrates and equid teeth. Referred specimens of I. downsi were recovered from the lower limit of the upper sandstones. Repenning & Tedford (1977) correlated that particular layer to those found above and below the Moraga Formation in the Berkeley Hills of California based on the presence of Hipparion De Christol, 1832. This Moraga Formation is dated at 10 Ma (Repenning & Tedford, 1977).
Minimum age: The minimum age is based on biostratigraphic data from the Monterey Formation, Celite Company No. 38 Quarry near Lompoc. Diatoms yielded from this locality are indicative of Schrader’s Diatom Zone XI (Repenning & Tedford, 1977). The limits of Schrader’s Diatom Zone XI is equivalent to the Thalassiosira antiqua zone (Barron, 1986), which is based on the FO of T. antiqua and the LCO of Thalassionema schraderi (8.6–7.6 Ma fide Gradstein et al., 2004).
Minimum range: Because I. downsi is known from multiple formations with non-overlapping ages, the minimum possible age range is from 9.0 to 8.6 Ma, with 9.0 Ma being the estimated age youngest age of the Santa Margarita Formation and 8.6 Ma being the maximum estimated age of the Monterey Formation at Celite Company No. 38 Quarry near Lompoc.
Kamtschatarctos sinelnikovae Dubrovo, 1981
Age range: 14.1–11.6 Ma.
Occurrence: West Pacific, Etolon Formation, Kamchatka, Russia.
Maximum age: The maximum age is based on the underlying Kakert Formation, which is shown to include at least part of chron C5Acn (Shilo & Minyuk, 2006; Gladenkov, 2013), the base of which is 14.07 Ma (Hilgen, Lourens & Van Dam, 2012).
Minimum age: The minimum age is based on the minimum age of the middle Miocene, i.e., the top of the Serravallian (Hilgen, Lourens & Van Dam, 2012).
Neotherium mirum Kellogg, 1931
Age range: 15.9–15.2 Ma.
Occurrence: East Pacific, Sharktooth Hill Bonebed, Round Mountain Silt Member, Temblor Formation, California, USA.
Maximum and minimum age: The maximum and minimum ages are based on Pyenson et al. (2009) who showed that the bonebed was deposited between 15.9 and 15.2 Ma using paleomagnetism (Prothero, Francisco & Denke, 2008) and biostratigraphy (Kohno et al., 1995).
Odobenus mandanoensis Tomida, 1989
Age range: 0.8–0.5 Ma.
Occurrence: Western Pacific, Mandano Formation, Kazusa Group, Boso Peninsula, Japan.
Maximum age: The maximum age is based on the Byakubi-E tephra within the underlying Kokumoto Formation, which is dated at 0.773 Ma (Kazaoka et al., 2015).
Minimum age: The minimum age is based on the youngest limit of the Kazusa Group (Ito & Katsura, 1992; Kazaoka et al., 2015).
Odobenus rosmarus (Linnaeus, 1758)
Age range: 0.1–0.0 Ma.
Occurrence: The modern walrus (O. rosmarus) is restricted to Arctic regions, but the fossil record extends as far south as the San Francisco Bay of California in the Pleistocene (Harington, 1984; Dyke et al., 1999).
Maximum age: The oldest fossil record of Odobenus rosmarus comes from a glaciomarine clay found in British Columbia, Canada dated to 70,000 years ago (Harington & Beard, 1992). Older fossils assigned to Odobenus from Japan and Russia are reviewed by Boessenecker & Churchill (2015, suppl. info.). The oldest fossil record of Odobenus is reported by Kohno et al. (1995) which is dated at 2.7–2.0 Ma based on associated diatoms of the Neodenticula koizumii zone. Given that the sister taxon, Valenictus, is thought to be as old as 4.2 Ma (minimum possible age of V. imperialensis), these fossils are effectively accommodated by counting ghost lineages.
Minimum age: It is extant.
Ontocetus emmonsi Leidy, 1859
Age range: 5.3–1.1 Ma.
Occurrence: Fossil occurrences of O. emmonsi range from the eastern United States, Europe, and Morocco (Kohno & Ray, 2008; Vélez-Juarbe, Wood & Pimiento, 2016; Boessenecker, Boessenecker & Geisler, 2018). Fossils from Japan have been referred to the genus Ontocetus (Kohno, Narita & Koike, 1998).
Maximum age: The maximum age is based on oldest age of the Palmetto Fauna of the upper Bone Valley Formation (Morgan, 1994; Webb et al., 2008). The maximum age for this unit has been given as 5.8 Ma (Boessenecker, Boessenecker & Geisler, 2018) because that is the maximum age of the latest Hemphillian (Hh4, 5.8–4.9 Ma, Hilgen, Lourens & Van Dam, 2012), but Morgan (1994) demonstrates an absence of Messinian marine mammals in the Palmetto Fauna, and placed the lower limit at the base of the Zanclean (also the base of the Pliocene).
Minimum age: The minimum age is based on a recently described specimen from the Austin Sand Pit of South Carolina, dated to 1.8–1.1 Ma (Boessenecker, Boessenecker & Geisler, 2018).
Minimum range: The minimum range of O. emmonsi is from 4.9 to 1.8 Ma, which is the youngest age of the Upper Bone Valley Formation (Palmetto Fauna; 5.3–4.9 Ma; Morgan, 1994; Hilgen, Lourens & Van Dam, 2012) and the oldest age of the possible age from the Austin Sand Pit (1.8–1.1 Ma; Boessenecker, Boessenecker & Geisler, 2018).
Pelagiarctos Barnes, 1988
Age range: 16.5–14.5 Ma.
Occurrence: East Pacific, Sharktooth Hill Bonebed, Round Mountain Silt Member, Temblor Formation, California, USA (Barnes, 1988) and “Topanga” Formation, California, USA (Boessenecker & Churchill, 2013).
Maximum age: The maximum age is based on benthic foraminifera found in the “Topanga” Formation (Raschke, 1984) that correspond with the early Relizian California stage, which begins at 16.5 Ma (Gradstein et al., 2004).
Minimum age: The minimum age is based on benthic foraminifera found in the “Topanga” Formation (Raschke, 1984) that correspond with the early Luisian California stage, which ends at 14.5 Ma (Gradstein et al., 2004).
Comments: Here, we provisionally combine P. thomasi with “Pelagiarctos sp.” of Boessenecker & Churchill (2013) since they can’t be definitely distinguished. Pelagiarctos thomasi is from the Sharktooth Hill Bonebed (15.8–15.2 Ma, see N. mirum above).
Pliopedia pacifica (Kellogg, 1921)
Age range: 5.8–4.7 Ma.
Occurrence: East Pacific, Paso Robles and Etchegoin Formations, California, USA.
Maximum age: For the Etchegoin Formation, Loomis (1990) gives a basal age of 5.5 Ma based on diatoms, but Loomis (1992) bases the maximum age on a tuff from within a lower formation (∼7.0 Ma). The presence of cervid deer from the Etchegoin (Merriam, 1915) can help constrain its age since cervids are not known in North America before Hh4 (Tedford et al., 2004), the base of which is 5.8 Ma (Hilgen, Lourens & Van Dam, 2012). The base of the Paso Robles Formation is not well constrained, but is thought to be Pliocene (Repenning & Tedford, 1977), which gives a maximum age of 5.333 Ma (Cohen, Harper & Gibbard, 2017) for the Paso Robles Formation.
Minimum Age: The age of the P. pacifica from the Paso Robles Formation is usually given as 6–5 Ma (Deméré, 1994b; Boessenecker, 2013b), ultimately based on an ad hoc assessment by Repenning & Tedford (1977) which considered the level of morphological differentiation of P. pacifica. Later studies show that the Huichica Tuff (4.76 ± 0.02) occurs in the Paso Robles Formation, the type specimen was collected at the base of the Paso Robles so we can constrain its minimum possible age to 4.74 Ma. This is slightly younger than the youngest possible age of the specimens from the Etchegoin Formation. Repenning & Tedford (1977) report that the Etchegoin specimens were collected below a tuff that is later identified as the Lawlor Tuff by Loomis (1992). The Lawlor Tuff has a youngest possible age of 4.823 Ma (4.834 ± 0.011 Ma; Sarna-Wojcicki et al., 2011).
Pontolis magnus (True, 1905)
Age range: 8.6–7.6 Ma.
Occurrence: East Pacific, Empire Formation, Oregon, USA.
Maximum and minimum age: The maximum and minimum ages are based on diatom biostratigraphy. The study of Barron (1986) showed that the Empire Formation is in the Thalassiosira antiqua zone, which is based on the FO of T. antiqua and the LCO of Thalassionema schraderi (8.6–7.6 Ma fide Gradstein et al., 2004). Prothero, Lau & Armentrout (2001) suggested that the Empire Formation was deposited between 8.7 and 6.5 Ma based on paleomagnetism and biostratigraphy, but did not include the addendum of Barron (1981, fig. 7) which restricted the type section to Schrader’s Diatom Zone XI. This zone is equivalent to the Thalassiosira antiqua zone (Barron, 1986), which is based on the FO of T. antiqua and the LCO of Thalassionema schraderi (8.6–7.6 Ma fide Gradstein et al., 2004).
Proneotherium repenningi Barnes in Kohno, Barnes & Hirota 1995
Age range: 17.3–16.6 Ma.
Occurrence: East Pacific, “Iron Mountain Bed” of the Astoria Formation, Oregon, USA.
Maximum and minimum age: The maximum and minimum age is based on Prothero et al. (2001) who showed that the “Iron Mountain Bed” was deposited between 17.3 and 16.6 Ma based on paleomagnetism and biostratigraphy.
Protodobenus japonicus Horikawa, 1995
Age range: 5.3–4.5 Ma.
Occurrence: West Pacific, lower member of the Tamugigawa Formation, Niigata, Japan.
Maximum and minimum age: The maximum and minimum ages are based on estimates from Takano (1990).
Prototaria planicephala Kohno, 1994
Age range: 16.4–15.1 Ma.
Occurrence: West Pacific, Moniwa Formation, Miyagi, Japan.
Maximum and minimum age: The maximum and minimum ages are based on planktonic foraminiferal zone N8 (Kohno, 1994; Gradstein et al., 2012).
Prototaria primigena Takeyama & Ozawa, 1984
Age range: 15.1–14.7 Ma.
Occurrence: West Pacific, muddy fine-grained sandstone unit of the Shimo Formation, Fukui, Japan.
Maximum and minimum age: The maximum and minimum ages are based on planktonic foraminifera that correspond to the lower part of Zone N9 Kohno et al. (1995), which ranges from 15.10 to 14.66 Ma (Gradstein et al., 2012).
Pseudotaria muramotoi Kohno, 2006
Age range: 10.0–9.5 Ma.
Occurrence: West Pacific, Ichibangawa Formation, Hokkaido, Japan.
Maximum age: As for A. akamatsui.
Minimum age: As for A. akamatsui.
Titanotaria orangensis gen. et. sp. nov.
Age range: 6.6–5.8 Ma.
Occurrence: East Pacific, Oso Member, Capistrano Formation, California, USA.
Maximum and minimum age: The maximum and minimum ages are based on Barboza et al. (2017).
Valenictus chulavistensis Deméré, 1994a
Age range: 4.5–2.5 Ma.
Occurrence: East Pacific, “lower member” (sensu Deméré, 1983) of the San Diego Formation and San Joaquin Formation of California, USA.
Maximum age: The maximum age (4.5 Ma) is based on the San Joaquin specimen, which is about 4.5–4.3 Ma, constrained by the basal age of the San Joaquin Formation (Hosford Scheirer & Magoon, 2007) and a tuff bed in the San Joaquin Formation that was dated to 4.3 Ma (Repenning & Tedford, 1977).
Minimum age: The minimum age (2.5 Ma) is based on the San Diego Formation. The minimum age of that formation is determined by the occurrence of the planktonic foraminifers assigned to California margin planktonic foraminiferal zone 6 (Kennett, Rozo-Vera & Machain Castillo, 2000) which has an age range from 3.25 to 2.5 Ma (Kucera & Kennett, 2000). The maximum age of the San Diego Formation is 4.2 Ma (Vendrasco et al., 2012).
Note: We assume that V. imperialensis is a valid sister taxon to V. chulavistensis thereby necessitating a ghost lineage between the two species. We further extend the maximum age of the Valenictus lineage to 5.3 Ma following the report of a specimen from the Purisima Formation Boessenecker (2017) estimated to be between 5.3 and 4.9 Ma.
Valenictus imperialensis Mitchell, 1961
Age range: 5.2–4.2 Ma.
Occurrence: East Pacific, the Deguynos Formation, California, USA.
Maximum and minimum age: The maximum and minimum ages are based on its occurrence in the Deguynos Formation, which is well dated by Dorsey et al. (2011).
Appendix 2. List of Undescribed or Insufficiently Characterized Specimens that Have Been Mentioned in Recent Papers Since 2017, but Are Not Included in Our Richness Estimate. By Its Focus on Recent Papers Looking at Pinniped Diversity, This Is Not Meant to Be an Exhaustive List of Unpublished Odobenid Material. Taxa Are Listed Alphabetical Order By Formation (where Known)
Capistrano Formation, California, USA
In addition to G. pugnax and T. orangensis, at least two undescribed taxa of odobenids have been mentioned from the Capistrano Formation (Barboza et al., 2017; Velez-Juarbe, 2017; Boessenecker & Churchill, 2018). Barboza et al. (2017) and Velez-Juarbe (2017) both list LACM 118967, and the latter lists LACM 150922, as representative specimens of these undescribed taxa. Boessenecker & Churchill (2018: suppl. data) indicate that Deméré, Berta & Adam (2003) list P. magnus from the Capistrano Formation, but those data are not given in that paper.
Deguynos Formation, California, USA
Boessenecker & Churchill (2018: suppl. data) cite an abstract (Atterholt, Jefferson & Schachner, 2007) for the presence of a potential new species of Valenictus in the Deguynos Formation, the type strata of V. imperialensis.
Empire Formation, Oregon, USA
Besides P. magnus, the Empire Formation is known to have additional taxa, although they are currently undescribed. Boessenecker & Churchill (2018: suppl. data) cite Deméré (1994b), who lists USNM 335594 and 335599 as “Imagotaria sp. cf. I. downsi,” an abstract (Sha[h]bazi et al., 2016), and personal observations to account for two undescribed taxa.
Monterey Formation, California, USA
In addition to I. downsi, Velez-Juarbe (2017) lists two taxa from the Monterey Formation and provides two specimen numbers (LACM 123282 and 122444) as their representatives. Velez-Juarbe (2017) also mentions two specimens (LACM 4324, 17588) from the Valmonte Diatomite Member that he hypothesizes could be a new taxon. LACM 4324 was described by Lyon (1941) and referred to P. magnus.
Purisima Formation, California, USA
Boessenecker, Perry & Schmitt (2014) and Boessenecker (2017) list four species of odobenids from the basal Purisima Formation. Currently Dusignathus is the only one that is described (Kellogg, 1927; Boessenecker, 2013b, 2017).
San Diego Formation, California, USA
In addition to D. seftoni and V. chulavistensis, Boessenecker & Churchill (2018: suppl. data) cite Boessenecker (2013b) which provides a specimen (LACM 28432) and a preliminary diagnosis for a new taxon.
Santa Margarita Sandstone, California, USA
In addition to I. downsi, Boessenecker & Churchill (2018: suppl. data) list two taxa “Imagotaria n. sp. 1 and 2.” One of these new species is based on “Desmatophocine A” of Barnes (1972), UCMP 85197. This taxon is incorrectly listed as coming from the Round Mountain Silt by Boessenecker & Churchill (2018: table 9). The other undescribed taxon is based on material described by Repenning & Tedford (1977) and is most likely USNM 23858.
“Topanga” Formation, California, USA
In addition to Pelagiarctos sp., Velez-Juarbe (2017) mentions “Neotherium sp.” from the “Topanga” Formation of Orange County. The specimen number for this material is LACM 123713. Boessenecker & Churchill (2018: suppl. data) lists cf. Imagotaria citing personal observations.
Towsley Formation, California, USA
Boessenecker & Churchill (2018: suppl. data) cite an abstract (Berkoff & Barnes, 1988) that diagnoses a new species of Gomphotaria. Although Berkoff & Barnes (1988) report the age of this specimen as being 10–9 Ma, which was later expanded upon by Whitmore & Barnes (2008), parts of the Towsley are considered Late Miocene or even Pliocene by other authors (Squires, 1991; Yeats, Huftile & Stitt, 1994; 6.5–5.0 Ma).
Other Japanese Formations
Miyazaki et al. (1995, fig. 2) lists two odobenids in the late Messinian: “Imagotaria cf. downsi” and “Imagotariinae,” but only cites a reference for one of them (Imagotariinae, Nakagawa, 1994) which is missing from the literature cited section of that manuscript. We were given the full citation for the Nakagawa (1994) paper, as well as another (Aizu Fossil Research Group, 1985), during review, but were unable to evaluate them before resubmission.
Supplemental Information
Data matrix used for phylogenetic analysis.
25 taxon, 91 character, data matrix of based on Boessenecker and Churchill (2013) with the addition of Titanotaria orangensis and Archaeodobenus akamatsui and character 91 from Tanaka & Kohno (2015). Coding changes noted in Phylogenetic Methods.