An overview report on the application of heteropoly acids on supporting materials in the photocatalytic degradation of organic pollutants from aqueous solutions
- Published
- Accepted
- Received
- Academic Editor
- Huan-Tsung Chang
- Subject Areas
- Environmental Contamination and Remediation, Green Chemistry
- Keywords
- Heteropolyanions, Phosphotungstic acid, Photocatalytic activity, Polyoxometalate
- Copyright
- © 2018 Nikoonahad et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. An overview report on the application of heteropoly acids on supporting materials in the photocatalytic degradation of organic pollutants from aqueous solutions. PeerJ 6:e5501 https://doi.org/10.7717/peerj.5501
Abstract
Organic pollutants contaminate water resources and the environment when discharged into water streams. Also, the presence of these materials in incompletely treated or untreated wastewater leads to serious environmental hazards. The hydroxyl radicals and holes are regarded as the most oxidant species in the degradation of organic pollutants using the studied composites. The results of this review show that heteropoly acids on supporting materials could be considered as appropriate photocatalysts in the removal of organic pollutant from aqueous solutions.
Introduction
Organic pollutants contaminate water resources and the environment when discharged into water streams. Also, the presence of these materials in incompletely treated or untreated wastewater leads to serious environmental hazards. Therefore, developing some technologies that can destruct these pollutants is of great importance. Several common processes such as condensation, ultrafiltration, membrane separation, and adsorption are applied for removing the organic pollutants. However, these methods cannot completely degrade the pollutants into nontoxic substances; rather, they often are just responsible for transferring these substances to other phases (Yang et al., 2012). In this regard, photocatalytic degradation can be regarded as one of the promising technologies to solve this problem (Lu et al., 2013; Norzaee et al., 2017). During the last decade, heterogeneous photocatalysis has received much attention as an unconventional technology in environmental remediation because of its advantages such as applying mild experimental conditions; i.e., atmospheric pressure and room temperature (Marcì, García-López & Palmisano, 2014). This technique can degrade most of the organic pollutants and mineralize them to final products such as carbon dioxide, water, and other small inorganic molecules (Feng, Shang & Liu, 2014).
In recent years, much research has been conducted into improving the photocatalytic process by preventing/delaying the recombination of the hole-electron pair at the surface of the photocatalyst, enhancing the electron transfer rate (Li, Vorontsov & Jing, 2015; Taghavi et al., 2018a), increasing the specific surface area and porosity of the photocatalyst by introducing a structure directing agent (Feng, Shang & Liu, 2014), enhancing the amount of adsorbed photons by photocatalyst at UVA and visible region, and reducing energy consumption through using LEDs or sunlight as the light source. In addition, some attempts have been made to use sensitizers on the surface of photocatalyst or couple with other semiconductors in order to modify the physiochemical and electronic properties of photocatalyst (Marcì, García-López & Palmisano, 2014). Heteropoly acids (HPA), as green and eco-friendly catalysts, have been proposed as potential candidates to be used as surface modifiers of TiO2 intended for photocatalytic degradation (Hakimi et al., 2014; Li, Vorontsov & Jing, 2015). The compound catalyst could eliminate the difficulties for recycling heteropoly acids and prevent the recombination of hole-electron pairs for both catalysts when heteropoly acids were carried on semiconductors such as TiO2. Thus, an increase in the specific area of heteropoly acid and synergy resulted in enhancing the photocatalytic degradation (Wang, Huang & Yang, 2010). Among heteropoly acids, Keggin-type heteropoly acids have been widely implemented for supporting materials such as semiconductors, resins, and clays and for environmental remediation (Guo & Hu, 2007; Leal Marchena et al., 2015; Lei et al., 2005; Shi et al., 2012; Wei et al., 2012; Zhang et al., 2015). The chemical formula of these acids is [XM12O40]n−, where X indicates the heteroatom such as P5+ or Si4+ and M displays the addenda atom, mostly W or Mo in high oxidation state (Tabatabaee et al., 2015).
The present study was conducted to review the application of Keggin-type heteropoly acids on supporting materials in the photocatalytic degradation of organic pollutants in aqueous solutions.
Survey Methodology
Available reports on the photocatalytic application of Keggin-type heteropoly acid-based composites were searched. For this purpose, important international and national databases were considered for retrieving all related studies; hence, Google Scholar, Science direct, Scopus and PubMed databases were considered to survey peer-reviewed journal articles. The search terms were selected as “Keggin-type heteropoly acid”, “ α–Keggin anions”, “Keggin-type polyoxometalate”, “phosphotagustic acid”, “phosphomolybdic acid”, together with “photocatalytic activity,” “photocatalytic degradation,” “degradation” and “removal”. We strictly searched for publications focusing on the applications of Keggin-type heteropoly acid–based composites in photocatalytic degradation. Research articles that were published before March 2017 were collected based on our search criteria.
Results
Photocatalytic activity
The photocatalytic degradation activity of synthesized nanophotocatalysts was compared with that of other photocatalysts reported in the literature for photocatalytic degradation of various pollutants (Table 1). Based on the obtained results, the synthesized nanophotocatalysts could represent a reasonable photocatalytic degradation activity of organic pollutants, compared to other photocatalysts.
| Catalyst | Pollutant | Light source | V(mL) | pH | Dose | Initial concentration | Time (min) | R(%) | Specific condition | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| H3PW12O40/TiO2 | Congo Red | A 400 W Xe lamp (λ >420 nm) | 200 mL | – | 250 mg | 50 mg/L | 120 | 92 | – | Yang et al. (2005) |
| Methyl Orang | 240 | 72.4 | ||||||||
| Ponceau G | 180 | 94.8 | ||||||||
| Orange II | 240 | 67.2 | ||||||||
| Eriochrome Blue Black B | 180 | 75.8 | ||||||||
| Alizarin S | 240 | 72.8 | ||||||||
| Methylene Blue | 60 | 96 | ||||||||
| Neutral Red | 60 | 98.2 | ||||||||
| Rhodamine B | 60 | 98 | ||||||||
| Fuchsin Acid | 240 | 75 | ||||||||
| H3PMo12O40/TiO2 | Methylene Blue | UV-A (λmax = 365 nm) | – | – | – | – | 30 | 90 | – | Nivea et al. (2014) |
| H3PW12O40/TiO2 | 62 | |||||||||
| Ce- H3PMo12O40/TiO2 | Methylene Blue | A 125 W high-pressure Hg lamp | 50 mL | – | 200 mg | 40 mg/L | 100 | 98 | – | Shi et al. (2012) |
| La- H3PW12O40/TiO2 | 96 | |||||||||
| H3PW12O40/TiO2 | Parathion-methyl | Visible light | 120 mL | – | 200 mg | 50 mg/L | 40 | >95 | – | Guo & Hu (2007) |
| PW11–SiO2 film | Rhodamine B | A 125 W high-pressure Hg Lamp (λmax = 313.2 nm) | 150 mL | – | 1. 25 ×12 × 45 mm | 1 mM/L | 240 | 70 | – | Yang et al. (2003b) |
| Erythrosine BS | 90 | 98.6 | ||||||||
| Methyl Orange | 240 | 42.8 | ||||||||
| Congo Red | 240 | 59.4 | ||||||||
| PW11–TiO2 film | Rhodamine B | 240 | 87 | |||||||
| Erythrosine BS | 90 | 99.4 | ||||||||
| Methyl Orange | 240 | 55.6 | ||||||||
| Congo Red | 240 | 73.2 | ||||||||
| HPW-yttrium-TiO2 | Methyl Orange | A 300 W Xe lamp (λ>365 nm) | 50 mL | 1 | 30 mg | 10 mg/L | 21 | 100 | – | Yajun, Kecheng & Changgen (2011) |
| Immobilized H3PW12O40 (30%) on NH4ZSM5 zeolite | Methyl Orange | A 125 W high-pressure Hg lamp (λmax = 365 nm) | 400 mL | 2.5 | 0.75 g/L | 2.62 mg/L | 240 | 91 | – | Leal Marchena et al. (2015) |
| H3PW12O40/TiO2 | Congo red | A 150 W Xe arc lamp | 10 mL | – | 12 mg | 10 μ M | 30 | 32 | – | Pearson, Bhargava & Bansal (2011a) |
| H3PW12O40/TiO2/Cu | 58 | |||||||||
| H3PW12O40/TiO2/Ag | 71 | |||||||||
| H3PW12O40/TiO2/Pt | 79 | |||||||||
| H3PW12O40/TiO2/Au | 86 | |||||||||
| H3PW12O40/MCM-41 | Imidacloprid | A 300 W Xe light (equipped with 365 nm optical filter, λmax = 365 ±10 nm) | 50 mL | – | 20 mg | 10 mg/L | 300 | 58 | – | Feng, Li & Liu (2012) |
| H3PW12O40/BiVO4 | Methylene blue | A 500 W Xe lamp (with UV cut-off filters, λ>420 nm) | 50 mL | – | 30 mg | 10 mg/L | 360 | 93 | – | Zhang et al. (2013) |
| H3PW12O40/TiO2 | Methyl orange | A 300 W medium-pressure Hg lamp (λmax = 365 nm) | 20 mL | 2 | 10 pieces glass slide (12.7 × 38.1 mm2/piece) | 5 mg/L | 60 | 93.4 | – | Niu & Hao (2011) |
| H3PW12O40/TiO2/Float pearls | Congo red | A 250 W medium-pressure Hg | 100 mL | 7 | 1.5 g/L | 60 mg/L | 70 | 90 | With aeration 2L/min | Yang et al. (2008) |
| H3PW12O40 pillared Mg3Al-LDH | Methyl orange | UV light | 150 mL | – | 60 mg | 0.02 M | 30 | 96.39 | With H2O2 | Zhang et al. (2011) |
| Zeolite-Y/ TiO2/Co2+/H3PMo12O40 | Methyl orange | Two 200 W tungsten filament lamps | 10 mL | – | 75 mg | 5 mg/L | 240 | 51 | In presence of ethanol | Dubey et al. (2006) |
| H3PW12O40/TiO2 film | Rhodamine B | A 300 W Xe lamp (with an IR cut filter, λ = 320–780, 200 mW/cm2) | 120 mL | 4.4 | two pieces of quartz (4.5) mg | 25 mg/L | 240 | >98 | – | Lu et al. (2012) |
| H3PW12O40/TiO2 | p-Nitroaniline | Two 125 W medium-pressure Hg lamps (λmax = 365 nm) | 100 mL | 3 | 0.6 g/L | 10 mg/L | 120 | 95.11 | – | Huang & Liu (2011) |
| H3PW12O40/Polymethylmethacrylate/ Polycaprolactam nanofibrous membrane | Methyl orange | A 300 W high-pressure Hg lamp | 50 mL | 1 | – | 10 mg/L | 30 | 92.7 | – | Li et al. (2016) |
| H3PW12O40/TiO2/SiO2 | Methyl violet | A 500 W Xe lamp (intensity: 1,200 µmol/m2.s) | – | 3 | 2.9 g/L | 10 mg/L | 150 | 95.4 | – | Yang et al. (2016) |
| Methyl orange | 99.9 | |||||||||
| Methyl red | 100 | |||||||||
| Naphthol green B | 93.7 | |||||||||
| Methylene blue | 81 | |||||||||
| H3PW12O40/ZrO2 | 4-nitrophenol | A 50 W high-pressure Hg lamp | 100 mL | – | 100 mg | 0.36 mM/L | 90 | >90 | – | Qiu, Zheng & Haralampides (2007) |
| Methylene blue | 0.065 mM/L | >90 | ||||||||
| H3PMo12O40/MnO2 | Methylene blue | UV light (λmax = 365 nm) | 100 mL | 4 | 50 mg/L | 32 mg/L | 150 | >98 | – | Kannan et al. (2011) |
| H3PW12O40/ZrO2 | Methylene blue | A 400 W high-pressure Hg lamp | 10 mL | 1.16 | 20 mg | 10 mg/L | 15 | 87 | Oxygen flow rate of 5 mL/min | Salavati, Tavakkoli & Hosseinpoor (2012) |
| Congo red | 6.15 | 20 mg/L | 84 | |||||||
| Rhodamin B | 1.27 | 30 mg/L | 87 | |||||||
| Bromothymol Blue | 1.1 | 20 mg/L | 52 | |||||||
| Alizarin | 6.6 | 40 mg/L | 61 | |||||||
| H3PW12O40/Ag-TiO2 | Atrazine | A 300 W Xe lamp (equipped with an IR cut filter, intensity: 200 mW/cm2) | 100 mL | 3.4 | 1 g/L | 5 mg/L | 60 | 98.6 | – | Xu et al. (2013) |
| H3PW12O40/ Activated clay | Methyl orange | a 40 W UV light tube (λmax = 365 nm) | 500 mL | 2 | 1.5 g/L | 10 mg/L | 60 | 78.9 | 0.7 mol/L H2O2 | Wei et al. (2012) |
| H3PW12O40/La-TiO2 | Imidacloprid | A 300 W Xe lamp (λmax ≥ 365 nm) | 50 mL | – | 30 mg | 10 mg/L | 60 | 98.17 | – | Changgen, Gang & Xia (2013) |
| H3PW12O40-TiO2/Bentonite | Methyl orange | Two 15 W UV lamps (λmax = 253.7nm) | – | initial pH of methyl orange solution | 1,000 mg/L | 10 mg/L | 120 | 82.7 | – | Zhang et al. (2015) |
| H3PW12O40/TiO2 film | Bisphenol A | A 300W Xe lamp (equipped with IR cut filter, λ = 320–780 nm) | 100 mL | 8.2 | – | 5 mg/L | 240 | ≈100 | – | Lu et al. (2013) |
| H3PW12O40/TiO2 | Dinitrotoluene | A 300 W Xe lamp (λ = 250–380 nm) | 50 mL | 2 | 0.8 g/L | 40 mg/L | 240 | 95 | – | Feng, Shang & Liu (2014) |
| H3PW12O40/SiO2 | Rhodamin B | A 500 W Xe lamp | – | 2.5 | 0.8 g | 10 mg/L | 120 | 97.7 | – | Yang et al. (2012) |
| H3PW12O40/TiO2 | Nitrobenzene | A 500 W tungsten light (λ = 400–760 nm) | 25 mL | – | 10 mg | 20 mg/L | 390 | 94.1 | – | Wang, Huang & Yang (2010) |
| H3PW12O40/Ag-TiO2 | Sulfamethoxazole | A 500 W Xe lamp (equipped with an IR and 400 nm cut filter, λ = 400–680 nm) | 100 mL | 6.8 | 200 mg | 40 mg/L | 240 | 97.8 | – | (Xu et al., 2012) |
| Ag/AgxH3−xPMO12O40 | Methyl orange | A 300 W Xe lamp (equipped with 420 nm cut-off filter, λ>420 nm) | 20 mL | 1 | 20 mg | 20 mg/L | 60 | 100 | – | Shi et al. (2016) |
| H3PW12O40/TiO2 | Methyl orange | A 300 W Xe lamp (λ ≥ 365 nm) | 50 mL | 2 | 0.6 g/L | 10 mg/L | 18 | 100 | – | Feng & Shang (2012) |
| H3PW12O40/modified cobalt ferrite | Acid Orange 95 | A 9 W (UV-C) | 800 mL | – | 0.01g | 10 mg/L | 30 | 91 | – | Mahmoodi et al. (2016) |
| Acid Red 18 | 99 | |||||||||
| Direct Red 81 | 96 | |||||||||
| PW12O403− immobilized on an anionic exchangeresin | Rhodamine B | A 500 W halogen lamp (equipped with a 450 nm cut-off filter, visible light) | 60 mL | 2.5 | – | 0.02 mM | 240 | >95 | In presence of 2 mM H2O2 | Lei et al. (2005) |
| cucurbit[6]uril- α-Keggin type polysilicontungstate anions | Methyl orange | A 500 W Xe lamp (equipped with a 420 nm cut-off filter) | – | 2.5 | 0.5 g/L | 10 mg/L | 120 | 95.6 | In presence of 1.5 mM H2O2 | Cao et al. (2011) |
| 93.6 | – | |||||||||
| TiO2-NH2-H3PW12O40-Au | Congo red | A 150 W Xe arc lamp | 10 mL | – | 12 mg | 10 μ M | 30 | 77 | – | Pearson et al. (2011b) |
| Ag@AgxH3−xPW12O40 | Methylene Blue | A 300 W Xe arc lamp (λ>400 nm) | 250 | – | – | 12 mg/L | 120 | ≈100 | – | Zhou et al. (2013) |
| HPW-yttrium-TiO2 | Methyl Orange | A 300 W Xe lamp (λ>365 nm) | 50 mL | 1 | 30 mg | 10 mg/L | 21 | 96.6 | – | Yajun, Kecheng & Changgen (2013) |
| PCPs/POM host–guest compound ([Cu(II)2Cu(I)3(OH)4(H2O)2(TPT)4][PW12O40]) | Methyl Orange | A 150 W Xe lamp | 250 mL | 6.3 | 0.15 g | 15 mg/L | 150 | 91 | In presence of 1.5 mM/L H2O2 | Fu et al. (2012) |
| H3PW12O40/In2O3 | Methylene blue | A 400 W high-pressure Hg lamp (λ = 200-400 nm) | 10 mL | 4.3 | 20 mg | 10 mg/L | 15 | 80 | Oxygen flow rate of 5 mL/ min | Salavati & Saedi (2014) |
| Solophenyl red-3BL | 40 mg/L | 49 | ||||||||
| Nylosan black 2-BL-acid | 80 mg/L | 56 | ||||||||
| Methyl orange | 20 mg/L | 26 | ||||||||
| Bromothymol blue | 40 mg/L | 44 | ||||||||
| PW11O39MnII(H2O)5−/ D301R resin | Rhodamine B | A 200 W metal halide lamp (equipped with a 420 nm cut-off filter) | 250 mL | – | 100 mg | 10 μ M/L | 40 | 100 | – | Hua et al. (2014) |
| SiW11/TiO2 | Rhodamine B | A 125 W high-pressure Hg lamp (λmax = 313.2 nm) | 80 mL | – | 0.015 mM | 0.1 mM | 180 | >90 | – | Yang et al. (2003a) |
| GeW11/TiO2 | Rhodamine B | 180 | >90 | |||||||
| PW11/TiO2 | Rhodamine B | 80 | 94.4 | |||||||
| Methyl orange | 180 | >80 | ||||||||
| Erythrosine B. S. | 180 | 90 | ||||||||
| H3PW12O40/TiO2 | Acid brilliant red 3R | – | – | – | – | – | – | 91 | – | Sun et al. (2006) |
| H3PW12O40/TiO2/SiO2 | Rhodamine B | – | – | 1 | 0.2 g | – | >95 | – | Wang & Niu (2007) | |
| H3PW12O40/TiO2 | Methylene blue | Solar light | 200 mL | – | 0.4 g | 50 mg/L | 90 | 95 | – | Lee, Dong & Dong (2010) |
| H3PW12O40/TiO2 | Rhodamine B | A 350 W Xe lamp | 100 mL | – | 100 mg | 25 mg/L | 240 | ≈80 | – | Piao et al. (2013) |
| TiO2/ZnO/H3PMo12O40 | Aniline | 42 LED lamps (3.2 V, λ = 390 nm) | 100 mL | unadjusted | 0.05 g | 50 mg/L | 180 | 38 | In presence of 5 mM/L H2O2 | Taghavi et al. (2018b) |
| TiO2/H3PMo12O40 | 26 | |||||||||
| ZnO/H3PMo12O40 | 45 | |||||||||
| TiO2/ZnO/H3PMo12O40 | Aniline | Two 11 W low-pressure Hg lamps (λmax = 254 nm) | 100 mL | unadjusted | 0.05 g | 50 mg/L | 180 | 71 | – | Taghavi et al. (2018c) |
| 74 | In presence of 5 mM/L H2O2 | |||||||||
| TiO2/H3PMo12O40 | 72 | – | ||||||||
| 77 | In presence of 5 mM/L H2O2 | |||||||||
| ZnO/H3PMo12O40 | 75 | – | ||||||||
| 79 | In presence of 5 mM/L H2O2 |
In some studies, the optical properties of the composite including a photocatalyst in combination with HPA were better than the case of using a photocatalyst and HPA alone (Changgen, Gang & Xia, 2013; Shi et al., 2012; Yang et al., 2004; Zhang et al., 2015). In most cases, the composite had a stronger photon absorption capability compared with its ingredients (Feng, Shang & Liu, 2014; Lu et al., 2012; Zhang et al., 2015). A red shift was observed in the UV-Vis diffuser reflectance spectra (DRS) for various composites, due to the effect of the Keggin unit and other doping materials on the electronic properties of the photocatalyst (Changgen, Gang & Xia, 2013; Lu et al., 2013; Salavati, Tavakkoli & Hosseinpoor, 2012; Shi et al., 2012; Yang et al., 2004). Doping the photocatalyst by HPA has resulted in an increase in the absorption in the visible region along with a high light harvesting efficiency by expanding the light response region of composite (Lu et al., 2013).
HPA can influence the electron transformation and act as the electron shuttle in light illuminating photocatalyst (Shi et al., 2012). Thus, HPA doping compared with supporting materials can lead to the outstanding photocatalytic activity of a composite photocatalyst. The results of the photocatalytic activity of Keggin-type heteropoly acids on supporting materials in the degradation of organic pollutants in aqueous solutions have been presented in Table 1.
Mechanism of degradation
Electron–hole pairs are produced and separated when they receive the energy needed for overcoming their mutual electrostatic attraction. Therefore, electrons move to the surface of the photocatalyst where the hole is transferred to the adsorbed hydroxide to create ∘OH, and accordingly the electron reacts with O2 to form superoxide anion radical (∘O2−) (Ghaneian et al., 2014b; Mahmoodi et al., 2016; Norzaee et al., 2017; Shi et al., 2012).
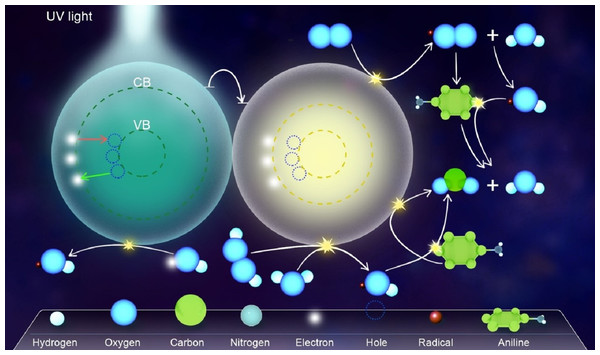
The use of HPA as a doping agent creates a new electronic state in the middle of the photocatalyst band gap, leading to a change in the band gap energy (Shi et al., 2012). Thus, it has some advantages such as a decrease in the chance of recombining the electrons and holes. The electrons excited from the valence band of composite because of absorbing UV/visible photon are transferred to the surface of HPA in the composite. In other words, HPA acts as an electron scavenger that prevents the fast recombination of electron–hole pairs, resulting in an increase in the degradation efficiency of the system (Shi et al., 2012). In other words, the photogenerated electron (e−) at the semiconductor (SEMI) is trapped into an unoccupied W5d state of the Keggin unit by HPA, which leads to a reduction in heteropoly blue (HPA−) (Eqs. (1) and (2)). Given its reducing ability, the HPA− can sensitize the photochemical reduction of O2 to produce supperoxides (∘O2−) (Eq. (3)). Specifically, HPA accelerates electron transfer from the semiconductor to O2 and retards the recombination electron–hole pairs on the semiconductor. Hence, the enhanced quantum efficiency is achieved in composite photocatalyst, compared to the pure semiconductor. Further, the generated ∘O2− can interact with the adsorbed water to produce hydroxyl radical (∘OH) and oxidize the organic target pollutant Eqs. (4) and (5) (Tabatabaee & Abolfazl Mirrahimi, 2011; Zhang et al., 2013). Furthermore, the photogenerated holes on the semiconductor react with the adsorbed water and hydroxyl ions to yield more ∘OH or oxidize the organic pollutant Eqs. (6)–(8) (Ghaneian et al., 2014a; Zhang et al., 2013). As a result, the ∘OH as an active species can degrade the organic pollutant Eq. (9) (Tabatabaee & Abolfazl Mirrahimi, 2011; Zhang et al., 2013). Some of these reactions for the degradation of aniline as an organic pollutant model, using the synthesized nanophotocatlyst, are presented in Fig. 1.
Figure 1: The degradation pathways of aniline as an organic pollutant model, using the composite nanophotocatlyst.
Some of the reactions for the degradation of aniline as an organic pollutant model, using the composite synthesized nanophotocatlyst.(1) (2) (3) (4) (5) (6) (7) (8) (9)
Furthermore, the direct irradiation of HPA on supporting materials also leads to the creation of some reactions. The irradiation of H3PW12O40 results in charging transfer from O2− to W+6 in W − O − W and creating a pair of the hole (O−), along with a trapped electron center (W5+), as shown in the following equation (Antonaraki et al., 2010; Yang et al., 2004): (10)
The strong oxidation ability of HPA∗, charge transfer-excited state, has been confirmed for degrading organic pollutants (Yang et al., 2004). The hole (O−) formed in the charge transfer-excited state of HPA has a strong oxidation ability, which is responsible for oxidizing pollutants (Mahmoodi et al., 2016; Yang et al., 2004). The general reactions involved in degrading organic pollutants in the photocatalytic system containing HPA can be shown by the following equations (Antonaraki et al., 2010; Wei et al., 2012; Zhang et al., 2013):
(11) (12) (13) (14) (15)
In the presence of hydrogen peroxide, the HPA∗ can produce hydroxyl radicals in reaction with H2O2, which is transferred to the reduced state based on the equations as follows (Wei et al., 2012):
(16) (17)
Ultraviolet irradiation of H2O2 is another pathway for generating hydroxyl radical in the presence of H2O2. (18)
Some studies emphasized the role of reactive species and its contribution to each in the presence of scavengers, (Lu et al., 2013; Norzaee et al., 2018). For example, Mahmoodi et al. (2016) evaluated the effect of some anions such as NaCl, NaHCO3, and Na2SO−4 on the photocatalytic decolorization of Acid Orange 95, Acid Red 18, and Direct Red 81 by using H3PW12O40/modified cobalt ferrite composite and observed the retarding effect of anions taken place through their reaction with ∘OH as well as hole based on the Eqs. (19)–(22). They interpret the dominant effect of holes and ∘OH radicals by the fact that owing to the synergistic effect of POM and cobalt ferrite, photoinduced electrons in cobalt ferrite are excited and captured by POM. As a result, a decrease occurs in the production of superoxide anion radical produced via the interaction between photoinduced electrons and oxygen molecules adsorbed on the surface of composite (Mahmoodi et al., 2016).
(19) (20) (21) (22)
Furthermore, an order of SO42−>Cl−>NO3− has been reported for the influential extent of the anion in reducing degradation Rhodamine B (Hua et al., 2014). The hydroxyl radical also has been represented as the main reactive species in photocatalytic degradation of bisphenol A by H3PW12O40/TiO2 film (Lu et al., 2013).
On the contrary, Shi et al. (2016) rejected the significant role of ∘OH species on photocatalytic degradation of methyl orange by Ag/AgxH3−xPMO12O40 under visible light irradiation and introduced ∘O2− and h+ radicals as the main reactive species in the process. Niu & Hao (2011) also considered the most contribution in degradation Methyl orange for holes.
In some studies, the heteropoly acid leachate from composites also investigated. The use of H3PW12O40/In2O3 composite photocatalyst in the photocatalytic degradation of methylene blue during four cycles resulted in a leaching of 0.2–1.4% W. Accordingly, a strong coordination communication was observed between the HPA and the In2O3 facade. The leaching of W was decreased with the increase of application cycle (Salavati & Saedi, 2014). A 2% leaching or loss of POM was reported for H3PMo12O40/MnO2 composite photocatalyst after using the fifth cycle (Kannan et al., 2011). Qu, Guo & Hu (2007) found W concentration within the range of 1.1–3.3 mg/L in the final treated solution by H3PW12O40/ZrO2, which was negligible with respect to its initial concentration. Finally, a strong interaction was reported between the Keggin unit and ZrO2 support with the unchangeable photocatalytic activity of composite for the three-time cycle.
Effect of initial pH of the solution
The pH of the solution plays an important role in the photocatalytic degradation process (Taghi Ghaneian et al., 2016). Most studies in this regard report a good photocatalytic activity of composite photocatalysts including HPA toward organic pollutant in an acidic medium in a pH range of 1–2.5 (Table 1). Hence, we can infer the partial change of the HPW structure from PW12 into PW11 at high pH values (Qiu, Zheng & Haralampides, 2007). The surface of the composite including PW12 in the outermost layer carries a negative charge due to PW12, which results in accelerating the transfer rate of holes and facilitating the separation of electron–hole pairs. As a result, the photogenerated electrons could have enough time to react with the adsorbed O2 on the composite surface to yield reactive oxygen species (∘O2 or H2O2). As previously mentioned, holes can react with the adsorbed water to generate ∘OH. Therefore, decomposition of HPW is responsible for the relatively low photocatalytic activity at neutral and alkaline media (Niu & Hao, 2011).
However, there are some reports with conflicting results in published works, with some of them discussed in detail below.
Hua et al. (2014) observed that the degradation efficiency of rhodamine B increased with an increase in the initial pH ranging 2.5–7 in the homogeneous system (PW11Mn), but the vice versa happened in the heterogeneous system (PW11Mn/D301R resin). Considering a red shift in the maximum visible absorption peak of rhodamine B in presence of PW11Mn, this result is related to the interaction between the catalyst and rhodamine B in the homogeneous system.
Moreover, Changgen, Gang & Xia (2013) reported that the initial pH of the solution within a range of 1–5.88 plays no significant role in degrading imidacloprid by H3PW12O40/La-TiO2 nanocomposite. Further, Wei et al. (2012) confirmed the negligible effect of pH on degradation of methyl orange using activated clay-supported HPW. This effect was observed as a decrease in degradation efficiency with an increase of pH in a range of 1.0–7.0.
Through using H3PW12O40/Ag-TiO2, an increase took place in the degradation of sulfamethoxazole after increasing the pH up to neutral to alkaline conditions (pH = 6.8–8.7), probably due to the changes in sulfamethoxazole acid–base species and an increase in its adsorption on composite photocatalyst (Xu et al., 2012).
HPW composites in combination with H2O2 is found in a wide range of initial pH of the solution in the photocatalytic degradation of organic pollutants (Wei et al., 2012).
The maximum degradation of bisphenol A by H3PW12O40/TiO2 composite catalyst was found at pH 8.2. The increase in photocatalytic activity of the composite catalyst at an alkaline condition (pH = 8.2) compared with acid solution is attributed to an increase in the number of OH reacting with photoinduced holes on the surface of the composite and a consequent increase in hydroxyl radicals generated for the degradation of bisphenol A. On the other hand, the decrease in efficiency at higher pHs (pH = 10.2) is attributed to ionization of bisphenol A and generation of bisphenolate anion (pKa = 9.6–10.2), which leads to the electrostatic repulsion between negatively surface charged composite (pHpzc of TiO2 = 6.25) and bisphenolate anion (Lu et al., 2013). Elsewhere, this composite photocatalyst was used in degrading rhodamine B in which the best results were observed in acidic solutions (pH = 4.4) (Lu et al., 2012).
Effect of photocatalyst dosage
Photocatalyst dosage is regarded as another factor playing a significant role in the photocatalytic process. This dosage should be considered in optimizing the operational conditions. Some studies indicated that an increase in photocatalyst dosage could result in increasing the photocatalytic efficiency (Yang et al., 2012). Therefore, an increase in the photocatalyst dosage does not always lead to the enhancement of photocatalytic activity (Hua et al., 2014; Wang, Huang & Yang, 2010; Yang et al., 2012). An excessive dosage increase, however, may decrease the photocatalytic efficiency. It seems that surplus photocatalyst results in scattering the photons in the solution and decreasing the photons that reach the surface of photocatalyst (Feng & Shang, 2012; Xu et al., 2012; Yang et al., 2012). Some other researchers reported the same results for various composites (Feng & Shang, 2012; Wei et al., 2012; Xu et al., 2012). In contrast, (Changgen, Gang & Xia, 2013) did not report a decrease in photocatalytic activity H3PW12O40/La-TiO2 composite with an increase of photocatalyst dosage within the range of 200–800 mg/L. An explanation for this result is that the maximum applied dosage is still in the optimum range of photocatalyst dosage based on other reports (Wei et al., 2012; Xu et al., 2012).
Effect of pollutant concentration
Several studies have shown that an increase in pollutant concentration leads to a decrease in photocatalytic efficiency through different ways (Feng & Shang, 2012; Lu et al., 2013; Mahmoodi et al., 2016; Niu & Hao, 2011; Norzaee et al., 2017; Xu et al., 2012; Yang et al., 2012).
More pollutant and intermediate products molecules are accumulated on the surface of photocatalyst when an excessive increase takes place in the initial concentration. Accordingly, the generation of reactive oxygen species is reduced because of inhibiting the adsorption of the incident photon for active sites (Lu et al., 2013; Niu & Hao, 2011). As for the dyes, the higher concentration of dye makes the color of solution very deeper and thus results in limiting the penetration of light to the solution depth and reaching the surface of photocatalyst (Feng & Shang, 2012). Besides, a constant intensity of light source and illumination time leads to a constant amount of the generated radicals (Norzaee et al., 2017). Hence, surplus dye molecules cannot be degraded and are represented as a low degradation efficiency (Niu & Hao, 2011).
Nevertheless, the low concentration of target pollutant may lead to a weak interaction between pollutant and composite photocatalyst. Wang, Huang & Yang (2010) reported this point as a reason for low efficiency in degradation of nitrobenzene by H3PW12O40/TiO2 composite photocatalyst in initial concentration 10 mg/L and increasing the efficiency through enhancing the initial concentration of nitrobenzene.
Effect of oxidants
In some studies, oxidants such as H2O2 have been applied to improve photocatalytic activity. Increasing the H2O2 concentration from 0.2 to 0.7 mol/L in a UV/ H3PW12O40/activated clay system could raise the degradation of methyl orange. However, a larger increase in the oxidant dosage results in decreasing the degradation efficiency of methyl orange (Wei et al., 2012).
Cao et al. (2011) reported that the photocatalytic degradation process is accelerated in the presence of H2O2. In addition, it has been evidenced that addition of H2O2 to the photocatalytic system leads to an increase in the hydroxyl radicals produced in the system, which is regarded as a reason for enhancing the degradation rate (Wei et al., 2012).
H2O2 at high concentrations has a negative effect on photocatalytic degradation rate by decreasing the number of hydroxyl radicals in solution because of acting as a hydroxyl radical scavenger at a higher level of concentration. A decrease in hydroxyl radical due to the high concentration of H2O2takes place through the following reactions (Wei et al., 2012):
(23) (24)
Effect of heteropoly acid loading
Various concentrations of heteropoly acid can change the photocatalytic activity of composites (Changgen, Gang & Xia, 2013; Lu et al., 2013). The HPW loading on La-TiO 2nanoparticle (0.3%) within the range of 10–20% resulted in increasing the photocatalytic activity of nanocomposite while the further increase in HPW loading led to a slight decrease (Changgen, Gang & Xia, 2013). Zhang et al. (2015) found same results in preparing H3PW12O40-TiO2/Bentonite and the highest photocatalytic activity achieved at a molar ratio of 0.5:100 for HPW and TiO2, respectively. In addition, the rate constant k for an HPW loading of 20% in H3PW12O40-TiO2 composite was 1.4 times that for an HPW loading of 30%, and approximately 3 times that of an HPW loading of 10% and 40% (Feng & Shang, 2012).
Other studies reported similar results for various composite photocatalysts (Lu et al., 2013; Zhang et al., 2013). The first increase in photocatalytic activity along with an increase in a doping of HPA is attributed to the increase in captured electrons by HPA, as an electron scavenger, a consequent delay in recombination electron–hole pairs, and an increase in the photocatalytic activity (Lee, Dong & Dong, 2010; Zhang et al., 2015). However, a high increase in the doping intensity of HPA is not always favored. A high amount of HPA causes to increase the available electron traps provided by HPA and the distance between electron traps will decrease. Therefore, an excessive amount of HPA in the composite can provide a place for recombining the photogenerated electron–hole pairs, leading to a decrease in photocatalytic activity of composite photocatalyst (Zhang et al., 2013; Zhang et al., 2015). Another reason for this phenomenon might be a reduction in specific areas of composite photocatalyst and accordingly less active sites for light and pollutant introduction (Feng & Shang, 2012; Lu et al., 2013; Zhang et al., 2013).
Photocatalyst recovery
The H3PW12O40/TiO2 composite synthesized by combining sol–gel technology using a nonionic surfactant P123 as a structure directing agent with solvothermal treatment demonstrated a constant photocatalytic activity after three sequent cycles. Accordingly, the prepared composite photocatalyst using this method has a high stability (Feng, Shang & Liu, 2014). Shi et al. (2016) reported the same result for Ag/AgxH3−xPMO12O40 after degrading methyl orange for four cycles. Further, Xu et al. (2012) reported the use of H3PW12O40/Ag-TiO2 composite in degrading Sulfamethoxazole with an insignificant decrease in photocatalytic activity after three cycles. The degradation rate for the first, second, and third cycles were 97.6, 91.7, and 87.7%, respectively. Similar results were reported for H3PW12O40/modified cobalt ferrite composite in the degradation of some dyes (Mahmoodi et al., 2016). This result is consistent with those reported in (Feng, Li & Liu, 2012; Zhang et al., 2013). However, Niu & Hao (2011) obtained different results for five cycles by reusing H3PW12O40/TiO2 in the decomposition of methyl orange. They reported that an increase could take place in decomposition efficiency after an increase in reusing cycles, due to the change in photocatalyst surface property and the bound water produced on the recycled photocatalyst.
Outlook on challenge and perspective
From the catalytic activity point of view, the previous sections showed that application of heteropoly acids on supporting materials in the photocatalytic process for the removal of organic pollutants has been an effective move in this field of study. Increasing photocatalytic activity for composite materials and overcoming problems in regard to the separation of heteropoly acids from the final solution are the most significant achievements of the application of heteropoly acids on supporting materials. Although some researchers also used SiO2, BiVO4, Au, Mg3Al-LDH, ZrO2, MnO2 as supporting materials, most works to date have focused on TiO2 as supporting materials. Future works should focus on materials that have not yet been used such as Fe2O3 to develop composites with desirable separation properties as well as the biotoxicity of treated wastewater, the changes in the environmental toxicity of synthesized composites, and applying facilitating methods for the composite photocatalysts such as HPA in a wider range of pH.
Conclusion
An overview was conducted on the use of Keggin-type HPA on solid supports such as nanophotocatalysts, resins, rare earth elements (REEs), clays, and other materials in the photocatalytic degradation of water and wastewater pollutants. The findings revealed a relatively high photocatalytic activity of composite photocatalysts toward organic pollutants. In addition, the combination of HPA with other semiconductors had some advantages, such as a delay in the recombination of electron-hole pairs, enhanced absorption of UV light for the composite, increased light harvesting of the composite in the visible region, and accelerated electron transfer of semiconductor, especially TiO2 toward O2. For other materials, some advantages such as recycling HPA and preventing secondary pollution because of the solubility of HPA can be mentioned. Moreover, some aspects of the synthesis and application of supported HPA were reviewed in the present study. However, further research is needed for the use of easily recyclable supporting materials, the biotoxicity of treated wastewater, the changes in the environmental toxicity of synthesized composites, and applying facilitating methods for the composite photocatalysts such as HPA in a wider range of pH.