Effect of floods on the δ13C values in plant leaves: a study of willows in Northeastern Siberia
- Published
- Accepted
- Received
- Academic Editor
- Miquel Gonzalez-Meler
- Subject Areas
- Climate Change Biology, Biogeochemistry, Ecohydrology
- Keywords
- Siberia, Stable carbon isotope, Willows, River lowland, Photosynthesis, Waterlogging, Flooding, Stomatal regulation
- Copyright
- © 2018 Fan et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Effect of floods on the δ13C values in plant leaves: a study of willows in Northeastern Siberia. PeerJ 6:e5374 https://doi.org/10.7717/peerj.5374
Abstract
Although stable carbon isotopic composition (δ13C) of plants has been widely used to indicate different water regimes in terrestrial ecosystems over the past four decades, the changes in the plant δ13C value under waterlogging have not been sufficiently clarified. With the enhanced global warming in recent years, the increasing frequency and severity of river floods in Arctic regions lead to more waterlogging on willows that are widely distributed in river lowland. To investigate the δ13C changes in plants under different water conditions (including waterlogging), we measured the δ13C values in the leaves of willows with three species, Salix boganidensis, S. glauca, and S. pulchra, and also monitored changes in plant physiology, under several major flooding conditions in Northeastern Siberia. The foliar δ13C values of willows varied, ranging from −31.6 to −25.7‰ under the different hydrological status, which can be explained by: (i) under normal conditions, the foliar δ13C values decrease from dry (far from a river) to wet (along a river bank) areas; (ii) the δ13C values increase in frequently waterlogged areas owing to stomatal closure; and (iii) after prolonged flooding periods, the δ13C values again decrease, probably owing to the effects of not only the closure of stomata but also the reduction of foliar photosynthetic ability under long period of waterlogging. Based on these results, we predict that plant δ13C values are strongly influenced by plant physiological responses to diverse hydrological conditions, particularly the long periods of flooding, as occurs in Arctic regions.
Introduction
Over the past four decades, stable carbon isotopic composition (δ13C, ‰ relative to Vienna Pee Dee Belemnite, VPDB) of plants has been widely employed as a conventional tool to estimate changes in carbon flux as plant physiology responds to environmental changes. The magnitude of isotopic fractionation is indeed highly dependent on physiological conditions (Farquhar, Ehleringer & Hubick, 1989; Robinson, 2001). For instance, it is well known that carbon isotopic fractionation (Δ13C) in plants is a function of the ratio of leaf intercellular-(ci) to atmospheric (ca) CO2 concentrations (ci/ca) (Farquhar & Sharkey, 1982; Farquhar, Ehleringer & Hubick, 1989), as given in Eq. (1): (1) where, δ13Ca and δ13Cp are the δ13C values of atmospheric CO2 and photosynthate, respectively; while a and b are the carbon isotopic fractionations associated with CO2 diffusion and enzymatic carboxylation (carbon fixation) in plant leaves, respectively.
The ci/ca ratio is usually determined from the balance between the CO2 supply controlled by stomatal conductance and CO2 consumption via the carboxylation related to photosynthetic activity. When the stomata closes (e.g., in response to a large water deficit and high evaporation rates due to high ambient temperature, (Meidner & Mansfield, 1968; Willmer & Fricker, 1996)), low CO2 supply reduces ci, leading to a decrease in the Δ13C values and, ultimately, an increase in the δ13Cp values. On the other hand, when CO2 consumption decreases as a consequence of reducing photosynthetic activity (e.g., due to the limitations of light and nutrients (Hall & Krishna, 1999)), a large ci increases the Δ13C values and, ultimately, decreases the δ13Cp values. These effects are expressed by Eq. (2), (2) where A is the photosynthetic rate, gc is the stomatal conductance of CO2, and gs is stomatal conductance which equals 1.6 times gc.
Combining Eqs. (1) and (2), the Δ13C and δ13Cp values are given by the standard Eq. (3): (3)
Thus, the plant δ13C values are primarily controlled by both stomatal conductance (gs) for CO2 and photosynthetic activity (A) (Farquhar & Richards, 1984). For example, under constant A, the δ13Cp values are controlled mainly by the gs. Drought-induced low gs decreases the Δ13C values and increases the δ13Cp values. In contrast, moisture-induced high gs increases the Δ13C values and decreases the δ13Cp values (Farquhar & Richards, 1984; Knight, Livingston & Van Kessel, 1994; Korol et al., 1999; Barbour & Farquhar, 2000; Warren, McGrath & Adams, 2001; Huang et al., 2008; Peri et al., 2012). Under constant gs, however, the δ13Cp values are primarily controlled by A, which is strongly correlated with light intensity (Yakir & Israeli, 1995) and nutrient availability (Ripullone et al., 2004; Duursma & Marshall, 2006; Kranabetter et al., 2010). Enhanced A decreases the Δ13C values and, ultimately, increases the δ13Cp values (O’Leary, 1988; Farquhar, Ehleringer & Hubick, 1989).
With respect to the river flooding, there is physiological evidence that stomata can also be closed in response to waterlogging conditions (Gomes & Kozlowski, 1980; Olivella et al., 2000; Copolovici & Niinemets, 2010); though, such evidence does not include isotope data, such as the Δ13C or δ13Cp values. If the CO2 gs term in Eq. (3) decreases due to the low stomatal conductance during waterlogging, low foliar Δ13C (high foliar δ13Cp) values will appear very similar to the values observed under drought conditions. Indeed, previous studies have reported changes in the δ13Cp value under both, natural and simulated waterlogging conditions. Anderson et al. (2005) found that tree-ring δ13C values for the pond cypress Taxodium ascendens in their natural environments are positively correlated with the total annual precipitation; similarly, Li & Sugimoto (2017) reported an increase in needle δ13Cp values for the larch Larix gmelinii in waterlogging pot experiments. The latter study attributed the increase in larch needle δ13Cp values to low gs caused by waterlogging.
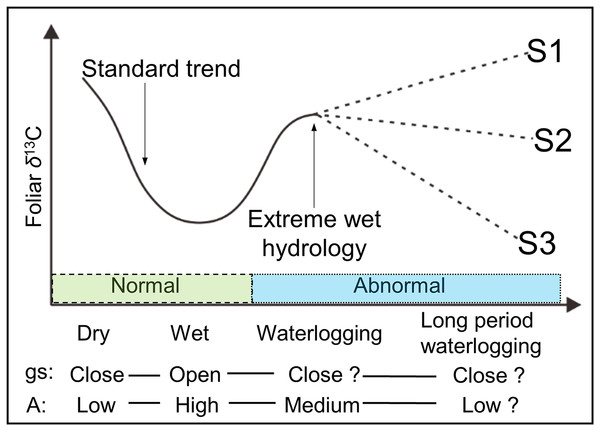
Although Anderson et al. (2005) reported a decreased gs with an increased A under very wet conditions, most physiological experiments have demonstrated that not only gs, but also A is apparently reduced under waterlogging (Gomes & Kozlowski, 1980; Copolovici & Niinemets, 2010; Li & Sugimoto, 2017). Based on these findings, we hypothesize that the Δ13C (and δ13Cp) values in plant leaves are not exclusively under the controlling of stomata (gs), because the photosynthetic rate (A) is also not constant in waterlogging. Moreover, net A and chlorophyll contents were observed decreasing without any change in either gs or ci/ca, in a continuous waterlogging experiment with okra Abelmoschus esculentus (Ashraf & Arfan, 2005), a waterlogging-tolerant plant. Thus, the possible changes in the foliar δ13C value under long period (continuous) waterlogging, which are assumed more dependent on changes of photosynthetic rate, are shown in Fig. 1 under predicted scenarios 1, 2, and 3 (S1, S2, and S3). Moreover, it is possible, as S3, that under low gs, a reduction of A can lead to lower δ13Cp values. Thus, Δ13C (and δ13Cp) values will be potentially changed in terms of frequency, magnitude, and duration of waterlogging.
Figure 1: Schematic view of the possible foliar δ13C values under various hydrological conditions.
gs and A are stomatal conductance and photosynthetic activity, respectively. Dry and wet are without waterlogging, and waterlogging and long period waterlogging represent continual and continuous waterlogging, respectively. Possible changes in the foliar δ13C value are shown for assumed scenarios (S1, S2, and S3).The Arctic region is highly sensitive and responsive to climatic changes (Giorgi, 2006). Thus, increases in atmospheric temperature significantly affect the hydrology in this region, including prevailing spring floods (Shahgedanova, 2002; Shiklomanov et al., 2007; Tan, Adam & Lettenmaier, 2011). For example, with rising temperature, the annual average discharge rate from the 19 largest rivers in the Arctic increased by approximately 10% from 1977 to 2007 (Overeem & Syvitski, 2010). Since the topography of the Arctic river lowlands is relatively flat, spring flooding strongly influences riparian plant communities. Shrubs which can stand high moisture levels, predominate over low moisture-preferring trees like larch and pine in areas along rivers under recurrent spring floods (Troeva et al., 2010). For instance, in the wide Indigirka River lowland near Chokurdakh village Russia in Northeastern Siberia, one sixth of a 10 × 10 km2 area is covered by dwarf shrub willow (Salix) (T. Morozumi, 2015, personal communication) and particularly being abundant on river banks. Thus, because willows in this area are exposed to an increase frequency of river floods and have high chances to be submerged, they are a good candidate species to study the effects of flooding on the δ13Cp values of leaves in relation to A and gs.
The objective of this study was to determine the effects of flooding on the δ13C values in willow leaves under four major hydrological conditions: dry, wet, and short and long period waterlogging (Fig. 1). We measured the δ13C values of bulk leaves from willows growing under these flooding regimes in the Indigirka River lowland of Northeastern Siberia.
Materials and Methods
Study area
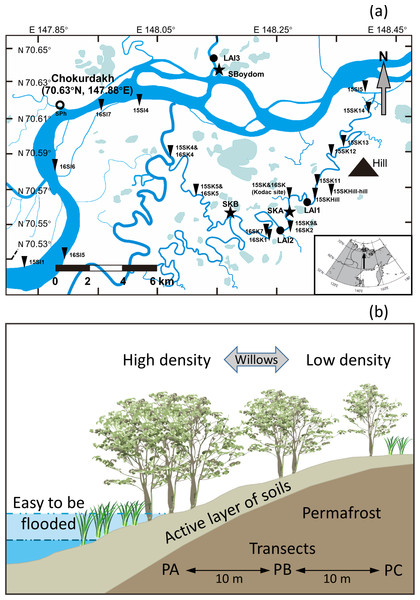
The study site is located in the Indigirka River lowland near Chokurdakh (70°38′N, 147°53′E), Sakha Republic (Yakutia), Russian Federation (Fig. 2). Mean annual air temperature in the region between 1950 and 2016 was −13.7 °C, ranging from −33.9 °C in January (the coldest month) to 10.1 °C in July (the warmest month). Mean annual precipitation between 1950 and 2008 was 209 mm year−1 (Yabuki et al., 2011). The Indigirka River lowland, including rivers, lakes, wetlands, hills, and floodplains, is frequently flooded during spring and summer. Soils in the region are loamy or silty-loamy alluvial soils with black- to grayish-olive color along the riverbanks (Troeva et al., 2010). The average depth of the active layer in soils is approximately 30 cm on land and one m near the river in the summer. The local vegetation consists of aquatics, sphagnums mosses, graminoids, shrubs (mainly the willow Salix sp. and the dwarf birch Betula nana), alders, larches, and pines. Between 1970 and 2016, the average intra-annual water level cycle of the Indigirka River was 70 ± 83 mm for April and May (late winter, pre-flooding), increasing to 600 ± 93 mm for June–August (spring and summer, flooding season); then, gradually receding to 343 ± 146 mm for September and October (autumn and early winter, post-flooding), and declining further to 56 ± 26 mm in winter (after October). Field experiments were approved by Hokkaido University, and Institute for Biological Problems of Cryolithozone, Siberian Branch of Russian Academy of Science, and North-Eastern Federal University.
Figure 2: Sampling sites and schematic illustration of a transect.
(A) Sampling sites near Chokurdakh village in the study region, northeastern Siberia. Thick and thin blue lines represent the Indigirka River and its tributaries, respectively. Areas filled with light blue represent lakes. Triangles (18), stars (3), filled black circles (3) and empty circle (1) indicate the sampling sites, three transects (SKA, SKB, and SBoydom), three sites for production measurement (LAI1∼3) and one site for photosynthesis monitoring (SPh). More sampling sites see Table A2. (B) A schematic illustration of a transect.Willows in the Indigirka River lowland
The common willow species observed in 2015–2017 were Salix boganidensis, S. pulchra, S. glauca, S. richardsonii, S. viminalis, S. alaxensis, S. fuscescens, and S. hastata. Most species were ≤1 m tall, except for a few species such as S. boganidensis, S. alaxensis, and S. fuscescens, which were two to three m in height. Diameter at breast height generally ranged between one and six cm. Maximum root depth was approximately one m at the riverbank, but was highly variable and depended on various factors such as the thickness of the active soil layers and moisture levels where the willows grew. Willows were distributed more densely along the riverbanks than on dry lands.
Observations conducted with a GardenWatchCam time-lapse camera (Brinno, Inc., Taipei City, Taiwan) showed that the buds of willow leaves opened around the first few weeks of June, when the snow had melted and the daily average air temperature had increased to >0 °C. The leaves and stems grew rapidly, within 10 days after bud opening, and were fully developed by mid-July. Willow leaf biomass peaked by the end of July, and this observation was consistent with a normalized difference vegetation index (NDVI) study in Alaska (Boelman et al., 2011). Aboveground net primary production (ANPP, newly formed stems and leaves in each year) and the leaf area index (LAI) of the willows in 2016 were measured using the direct harvesting method (Jonckheere et al., 2004) in three blocks which were predominated by willows. ANPP was 63, 119, and 117 g m−2·a in each of the three blocks, and the LAI was 0.59, 0.71, and 1.59 in each of the three blocks (Table A1).
Samples
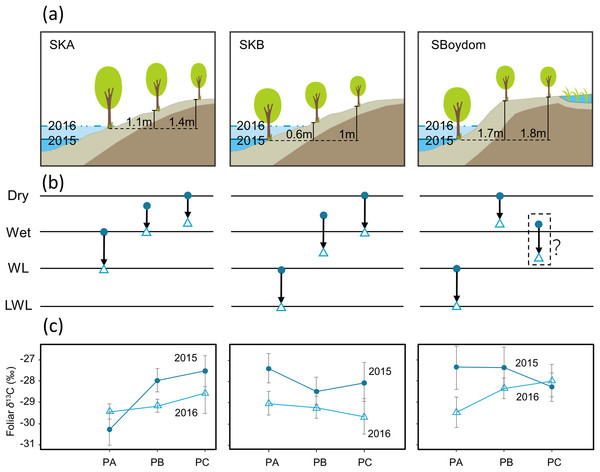
In the summer of 2015 and 2016, we collected leaves from the locally dominant willows Salix boganidensis, S. glauca, and S. richardsonii on three sets of 20 m transects (SBoydom, SKA, and SKB) from the river. SBoydom is located between the mainstream Indigirka and the wetland; while, SKA and SKB are situated next to a secondary tributary, Kryvaya (Fig. 2; Table A2). Three points, named PA, PB, and PC, were marked on each transect based on their distance to the river. The maximum thaw depth was always found at PA. This layout was designed based on the differences in intra- and inter-annual flooding conditions (Figs. 2 and 3). PAs at SKB and SBoydom were continually waterlogged throughout the growing season in 2015 and continuously waterlogged until July in 2016 (Fig. 3). PB at SKB and PA at SKA were flooded only in 2016 (Fig. 3).
Figure 3: Schematic view of each transect, possible changes in the hydrological conditions, and foliar δ13C values (‰).
(A) Schematic view of each transect with the highest water levels observed in each case in 2015 (blue) and 2016 (light blue) and the height of PB and PC, compared to PA (black line). (B) Possible changes (black arrows) in the hydrological conditions from 2015 (filled circles) to 2016 (open triangles) in each point on transects. Dry and wet are without waterlogging, and WL and LWL represent waterlogging (continual) and long period waterlogging (continuous), respectively. (C) The foliar δ13C values (‰) found in willows were reported as mean ± SD.Four current-year top shoots were collected at each point at the end of the growing season (the end of July) in both 2015 and 2016. Current-year shoots were also randomly sampled from willows in local scale on the Indigirka River lowland during the same period. A total of 31 sites with different locations were used in 2015 and 2016 (Fig. 2; Table A2). At least four current-year shoots were collected at each location to obtain representative data for each site.
The details of sampling sites, locations, species, and sampling numbers are shown in Fig. 2 and Table A2. All samples were immediately dried at 60 °C for 48 h after collecting.
Stable carbon isotope analysis
Dried leaves were milled into fine powder with liquid N2 and dried again at 60 °C for 48 h; each sample was then wrapped in a tin capsule and injected into an elemental analyzer (Flash EA 1112; Thermo Fisher Scientific, Bremen, Germany), connected to an isotope ratio mass spectrometry (IRMS, Delta V; Thermo Fisher Scientific, Bremen, Germany) through a continuous-flow carrier-gas system (Conflo III; Thermo Fisher Scientific, Bremen, Germany). The stable carbon isotopic composition was reported in the standard δ notation relative to VPDB. A laboratory standard was injected after every ten samples to verify that the analytical accuracy was better than 0.1‰. To reduce the effect of sampling heterogeneity in δ13C within a single site, four samples were measured and the average isotopic composition was reported for each site, with the standard deviation ranging from 0.0 to 1.4‰ (average, 0.7‰).
Photosynthetic rate and stomatal conductance analyses
Supporting data on the foliar δ13C values, the photosynthetic rate and stomatal conductance of willow leaves were monitored in the field in 2017 using a portable porometer (LCpro+; ADC BioScientific Ltd, Hoddesdon, Herts, UK) equipped with a conifer chamber and a lighting system. The photosynthetic rate (A) of S. boganidensis, S. richardsonii, and S. glauca under different light levels (10–955 μmol m−2 s−1) was measured to obtain light response curves and thus, to identify the saturation light intensity.
Site SPh near Chokurdakh village, was set up in the summer of 2017 to monitor the conditions in former transects SKA, SKB, and SBoydom, since the extremely high flooding caused all these three sites totally submerged for the entire summer of 2017. Under gradient flooding conditions on site SPh (-PA: submerged till July 20; -PB: submerged till July 15; -PC: without submergence during the observation period), temporary changes were measured in the photosynthetic rate (A) and stomatal conductance (gs) of S. richardsonii, S. glauca, and S. boganidensis in response to a single saturated light exposure at 600 μmol m−2 s−1 around noon, the rest of chamber conditions were set to match ambient conditions. For each measurement at the points (PA, PB, or PC), a total of 12 leaves from four trees were marked for leaf ADC data recording for more than six times on any leaf of them. Average of all records was calculated for each measurement. Measurements were taken five times every 2–3 days between July 13, 2017 and July 27, 2017. The leaves were also collected after whole monitoring period to check the foliar δ13C values.
Statistical analysis
Linear Mixed Models (LMMs) were used to clarify differences in the foliar δ13C value among willows growing in three transects in 2015 and 2016. Foliar δ13C value was set as the response variable, with flooding condition was set as the fixed effect, and species (i.e., S. boganidensis, S. richardsonii, and S. glauca) was set as a random effect. Similar analyses by LMMs were also used to figure out any differences in the foliar δ13C value among the willows randomly collected on the Indigirka River lowland in 2015 and 2016. Foliar δ13C value was set as the response variable, the flooding condition was assigned as the fixed effect, and the location (along the mainstream or the tributary), and species (Table A2), were set as random effects. Tukey’s test was used as a post hoc analysis for multiple comparisons. The lme4 package (Bates, Maechler & Walker, 2015) of R (R Core Team, 2015) was used to build the LMMs.
Results
Foliar δ13C in the transects
Foliar δ13C values differed among SKA, SKB, and SBoydom, and between years (Fig. 3). Along transect SKA in 2015, the mean foliar δ13C values were −30.3 ± 0.8‰ for PA (close to the river but without submergence), which was much lower than those for PB (−28.0 ± 0.6‰) and PC (−27.5 ± 0.7‰). A similar trend was also observed in 2016, when the water level was high; in this case, the foliar δ13C values for PA (−29.4 ± 0.4‰) was lower than those for PB (−29.2 ± 0.3‰) and PC (−28.6 ± 0.9‰); although, the difference was small. For the inter-annual changes from 2015 to 2016 on transect SKA, an increase in foliar δ13C value was observed for PA (+0.8 ± 0.6‰); whereas, a decrease was recorded for both, PB (−1.2 ± 0.5‰) and PC (−1.0 ± 0.8‰).
In 2015, the sampling point at PAs along both, the SKB and SBoydom transects, were sometimes waterlogged (“WL”; Fig. 3); whereas, all points PBs and PCs were not. A similar trend for the foliar δ13C values was found in both, SKB and SBoydom transects, as the foliar δ13C values for PAs (−27.4 ± 1.1‰ and −27.3 ± 0.7‰, respectively) were higher than those for PBs (−28.5 ± 0.6‰ and −27.4 ± 1.0‰, respectively), and PCs (−28.1 ± 0.9‰ and −28.3 ± 0.7‰, respectively) (Fig. 3). In 2016, although PAs were also but always waterlogged (“LWL”; Fig. 3), PBs and PCs were not, trends in the foliar δ13C value were apparently different between SKB and SBoydom. In SKB, the foliar δ13C values for PA (−29.0 ± 0.6‰) were slightly higher than those for PB (−29.2 ± 0.5‰) and PC (−29.7 ± 0.9‰); whereas, in SBoydom, values for PA (−29.5 ± 0.7‰) were slightly lower than those for PB (−28.3 ± 0.5‰) and PC (−28.0 ± 0.8‰) (Fig. 3). For the inter-annual changes in SKB and SBoydom, decreases in the foliar δ13C value were observed at all points in all transects, except for PC in SBoydom. The differences in the foliar δ13C value between the PB and PC within each transect ranging from −0.5 to +0.9‰; however, those for the PA significantly deviated (−2.5 to +0.9‰) from the mean value of the PB and PC.
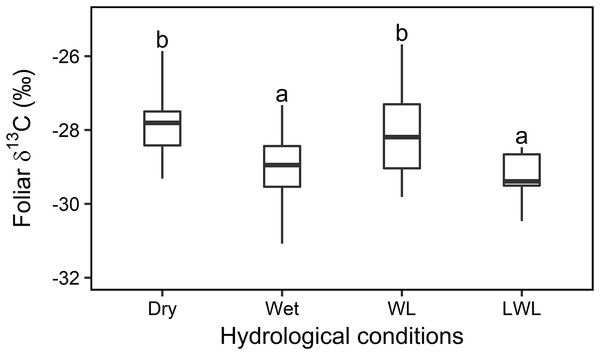
Overall, statistical analysis of the foliar δ13C values from transects (in Fig. 3) showed a significant difference (F3,42 = 42.276, P < 0.01, Fig. 4) among the four major hydrological conditions (i.e., “Dry,” “Wet,” “WL,” and “LWL,” in Figs. 3 and 4). The foliar δ13C values were high in dry and waterlogged continually conditions. Conversely, values under wet and continuous long period waterlogging were consistently low.
Figure 4: Statistical analysis for the foliar δ13C values (‰) under four different hydrological conditions in transects in 2015 and 2016.
Box-and-whisker plot of the statistical analysis for the foliar δ13C values (‰) under four different hydrological conditions in sampling transects established in year 2015 and 2016. Different letters over the numbers indicate statistically significant differences according to Turkey’s post hoc test and Linear Mixed Model. Dry and wet are without waterlogging, and WL and LWL represent waterlogging (continual) and long period waterlogging (continuous), respectively.Spatial distribution in the foliar δ13C of willows
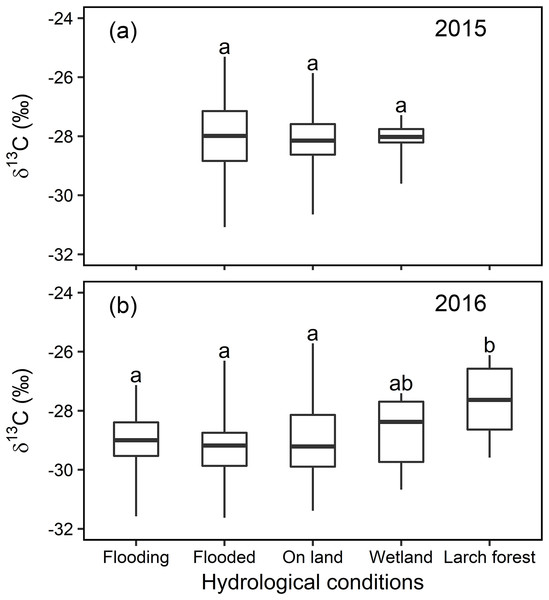
Neither significant nor large differences were detected in the foliar δ13C value in any of the willows growing in the 31 randomly selected sampling sites in local scale (with different locations) in Indigirka River lowland during the same sampling periods at the end of the growing seasons of 2015 and 2016 (Fig. 5). The willow foliar δ13C values ranged from −31.1 to −25.3‰ in 2015, and from −31.6 to −25.7‰ in 2016. Statistical analysis shows that there were no significant differences between the four environmental conditions (flooding, flooded, on land, and wetland) in 2015 or 2016. Moreover, the δ13C values measured for willow leaves collected in a larch forest in 2016 (−27.6 ± 1.2‰) were significantly higher than those sampled anywhere else in the same year (F4,168 = 2.58, P = 0.039).
Figure 5: Statistical analysis for the foliar δ13C values (‰) under different hydrological conditions at local scale in 2015 and 2016.
Box-and-whisker plot of the statistical analysis for the foliar δ13C values (‰) under different environments with distinct hydrological conditions at local scale in year 2015 (A) and 2016 (B), different letters over the numbers indicate statistically significant differences according to Tukey’s post hoc test and the Linear Mixed Model.Photosynthetic rate and stomatal conductance
Photosynthetic rate (A) gradually increased asymptotically and reached to about six μmol m−2 s−1 for S. richardsonii, about six to eight μmol m−2 s−1 for S. glauca, and about 8–12 μmol m−2 s−1 for S. boganidensis, under 400–600 μmol m−2 s−1 (irradiation scanning covered the range from 10 to 955 μmol m−2 s−1) (Fig. A1). Therefore, the saturating light intensity for willow leaves in the Indigirka River lowland was found in the range from 400 to 600 μmol m−2 s−1. Maximum photosynthetic rate A recorded in leaves of S. boganidensis was the highest among all three species (Fig. A1).
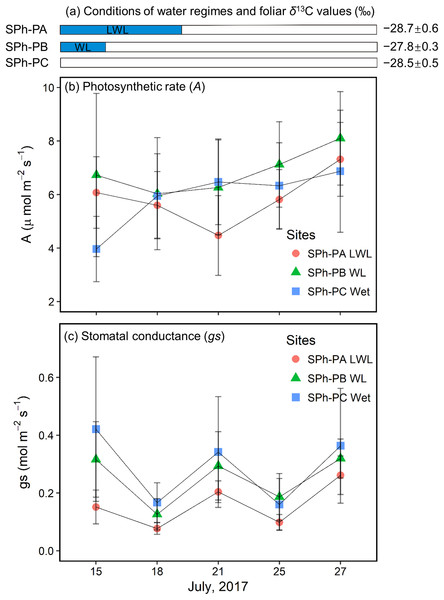
In the summer of 2017, willows at the SPh-PA were continuously submerged until July 20; willows at the SPh-PB had just come out of the water when monitoring began on July 15; while, willows at the SPh-PC were not submerged during the monitoring period (Fig. 6A), although all three points at transect SPh were within 20 m from the river. The largest decrease (−1.6 ± 1.4 μmol m−2 s−1) in A at SPh-PA during the monitoring period, was registered on July 21 when the waterlogging just finished; whereas, after flooding A was observed to follow a slow recovery over the last few days (Fig. 6B). A similar decrease-increase trend in A was also detected in the willow leaves at SPh-PB, and also, in this case, the lowest A was recorded soon after waterlogging finished on July 18 (−0.7 ± 2.6 μmol m−2 s−1 lower than first measurement) (Fig. 6B). However, at SPh-PC, A continuously increased, compared to the initial measurement. These values corresponded with the waterlogging gradients in SPh, that A was reduced under waterlogging and could recover if waterlogging was over. On the other hand, among points, compared to SPh-PC, the lowest gs values were found at SPh-PA; while, intermediate values were recorded in SPh-PB (Fig. 6C). Thus, gs, apparently correlated to the degree of waterlogging among SPh-PA, -PB, and -PC.
Figure 6: Hydrological conditions in SPh, and results of physiology monitoring.
(A) Hydrological conditions in SPh-PA (circles), -PB (triangles), and -PC (squares), with the period of submergence shown in blue shaded bars and relative foliar δ13C values (‰), and (B) photosynthetic rate (A, μmol m−2s−1) and (C) stomatal conductance (gs, mol m−2s−1) with fixed radiation (600 μmol m−2s−1), during July 13th–27th. Mean ± SD.Discussion
River water level and leaf formation
As mentioned before, the willow leaves began opening after the first week in June and finished growing by the end of July. This suggests that the foliar δ13C values of willow leaves recorded hydrological conditions experienced from early mid-June to the date of collection, which is a longer period than that of the in situ observation and monitoring period for hydrological conditions. However, it is known that the river water level is gradually decreased but by within approximately one m during the term of leaf growing (Fig. A2). Moreover, only small differences were found in the foliar δ13C values between top and bottom of a single current-year shoot, approximately 0.5 ± 0.1‰ (Fig. A3), which may have experienced leaf formation over different periods. Thus, in present field observation study, the hydrological conditions observed during July are assumed to be almost the same or very similar to those for the early part of the growing season.
Foliar δ13C values under different hydrological conditions
Foliar δ13C values in normal dry-wet conditions
In the normal dry-wet SKA transect, in the absence of waterlogging during 2015, S. boganidensis grew at PA with more available water than those at PB or PC. The willow leaves at PA were largely depleted in 13C, with the difference of δ13C value by about 2‰, relative to those at PB or PC (Fig. 3). These results are consistent with the well-known fact that, under dry conditions, low gs results in high foliar δ13C values; whereas, under wet conditions, high gs leads to low foliar δ13C values (Eq. (3)); all of which are consistent with the common findings with respect to gs in numerous studies (Chen, Bai & Han, 2002; Peri et al., 2012; Schifman et al., 2012). The decrease in the foliar δ13C value between 2015 and 2016 at PBs and PCs along transects SKA and SKB, and at PB on the transect SBoydom are thus, also attributable to stomatal regulation of gas exchange between dry and wet conditions.
Foliar δ13C values under sporadic waterlogging
Willow leaves at the PAs of SKB and SBoydom in 2015 have the δ13C values similar to or higher than those in their respective PB, even though PAs and PBs were situated under wet and dry conditions, respectively. The high δ13C values in PAs can be explained by stomatal closure under waterlogging conditions. Decreasing gs was demonstrated under extremely wet conditions (Gomes & Kozlowski, 1980; Olivella et al., 2000; Copolovici & Niinemets, 2010; Li & Sugimoto, 2017). We suppose, as has been reported, that the flooding reduces root hydraulic conductance and thereby, leaf water potential (Olivella et al., 2000; Islam & Macdonald, 2004), which in turn leads to decreased gs. Else et al. (2001) also suggested that stomatal closure upon waterlogging is caused by the production of abscisic acid, which may be related to the decrease of root hydraulic conductance and leaf water potential. Moreover, in the present study, we observed a rapid recovery in A but a slow recovery in gs after waterlogging ended (Figs. 6B and 6C). Thus, when the waterlogging was short and continual, low gs can contribute more to the foliar δ13C values than A, resulting in the high foliar δ13C values at SPh-PB, although the lack of statistical significance (Fig. 6A). The co-occurrence of low gs with high foliar δ13C values was also observed under waterlogging in field trials (Ewe & Sternberg, 2003; Anderson et al., 2005) as well as in pot experiments (Li & Sugimoto, 2017). Therefore, we suggest that gs contributes more than A to the foliar δ13C values under sporadic waterlogging caused by medium flooding (“WL”; Fig. 3). The combined results of this and previous research indicates that increasing of the foliar δ13C values (+0.8 ± 0.6‰) at SKA-PA between 2015 and 2016 were caused by stomatal closure in response to the waterlogging in 2016 (“WL”; Fig. 3). Very similar increases in the foliar δ13C value were also observed in the SBoydom-PC, even though it was relatively distant from the river. The wetlands near SBoydom-PC may cause waterlogging similar to that experienced in the riverside sites (Fig. 3).
Foliar δ13C values under continuous long period waterlogging
Sporadic waterlogging (“WL”; Fig. 3) related to medium flooding increased the willow foliar δ13C values. In 2016, however, the foliar δ13C values at SKB-PA (waterlogging) were only slightly higher (approximately 0.4‰) than those at SKB-PB and SKB-PC. The foliar δ13C values at SBoydom-PA (waterlogging) were even lower (approximately 1‰) than those at SBoydom-PB. Moreover, between 2015 and 2016 the foliar δ13C values at PAs on SKB and SBoydom decreased by 1.7 ± 0.6‰ and 2.1 ± 0.9‰, respectively, despite waterlogging occurring in both years. To date, very few studies have investigated the reasons for the lack of changes or negative shifts in foliar δ13C value under waterlogging conditions. We propose that, under the long period waterlogging (“LWL”; Fig. 3) as caused by large flooding observed at PAs on SKB and SBoydom in 2016, the changes in the A are also important factors controlling the foliar δ13C values, besides gs. In the present study, low A was observed during submergence, before July 20 and more flooded SPh-PA (Fig. 6C). It has been suggested that waterlogging induces low carboxylation rate by reducing the amount or activity of Rubisco enzyme (Vu & Yelenosky, 1992; Islam & Macdonald, 2004). According to Eq. (3), the foliar δ13C values should be dependent on both A and gs; thus, low A may have caused the negative shifts in the foliar δ13C value in LWL compared to WL, as the foliar δ13C values at SPh-PA were slightly lighter than at SPh-PB, albeit insignificantly so (Fig. 6A). Therefore, the low foliar δ13C values observed in the willows at the PAs on SKB and SBoydom in 2016, can be explained by this continuously low photosynthetic activity under long period waterlogging caused by large flooding. The low δ13C values, A, and gs under large flooding were previously reported for a pot experiment involving the invasive wetland grass Phalaris arundinacea (Waring & Maricle, 2012). The aforementioned findings together suggest the hypothesis that long period waterlogging (or large flooding) significantly reduces the foliar δ13C values compared to sporadic waterlogging (or small flooding), and that the contribution of A and gs is highly dependent on the frequency and magnitude of waterlogging events.
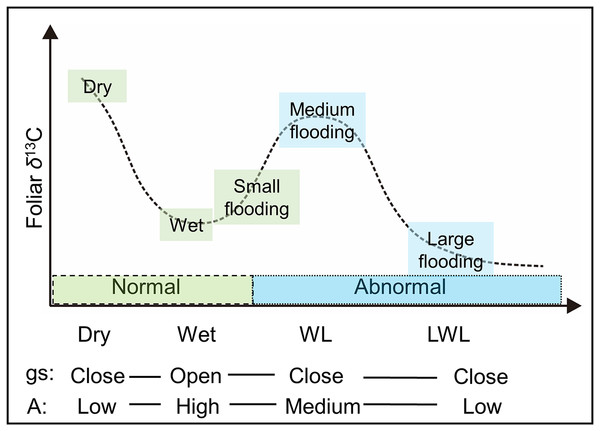
Thus, the large differences (Fig. 4) in hydrological conditions (i.e., “Dry,” “Wet,” “WL,” and “LWL”; Fig. 3) found in transects suggest that the possible foliar δ13C values can correspond to scenario 3 in Fig. 1 (i.e., reduced foliar δ13C values in long period waterlogging), which can be well interpreted by that both A and gs are affected by hydrological gradients (Fig. 7).
Figure 7: The possible foliar δ13C values with respect to physiological responses to various hydrological conditions.
gs: stomatal conductance, A: photosynthesis activity. Dry and wet are without waterlogging, and WL and LWL represent waterlogging (continual) and long period waterlogging (continuous), respectively.However, we note that scenario 3 in this study is a very simplified, schematic hypothesis, without quantitative meaning. There are other several potential factors for controlling the foliar δ13C values, for example mesophyll conductance (gm) (Evans et al., 1986). As gs and gm were found tightly coupled (Vrábl et al., 2009), and both controlled the limitation of CO2 diffusion. Therefore, in our field study of the determining factors of foliar δ13C values, we mainly focused on gs which can be directly monitored. Detailed changes in the δ13C value particularly between and within the four hydrological conditions will be illustrated in further studies with determination of these potential factors, including but not limited to gm changes under different water regimes, the quantitative meaning of gs and gm on foliar δ13C values, and the features of gs and gm in species with different water tolerance.
Spatial difference in the willow foliar δ13C value
Linear Mixed Models analysis of the local scale random sampling indicated that only the willows in the dry larch forest were slightly but statistically enriched in 13C (F4,168 = 2.58, P = 0.039), compared to the other conditions, in 2016. It is accepted that the foliar δ13C values are higher in dry than in wet conditions, due to stomatal regulation. In contrast, there was no statistical difference in the foliar δ13C value for either year or among the hydrological conditions tested here (flooding, flooded, on land, and wetland) (Fig. 5). The first three conditions were situated near the river and at different levels of flooding, whereas the fourth was never affected by flooding, although it was still abundant in water. These results are likely consistent with the lack of difference in the foliar δ13C value among various hydrological conditions in mesic regions or periods, as reported previously (Garten & Taylor, 1992; Alstad et al., 1999). Nevertheless, relatively minor variations in the δ13C value in willows growing under these hydrological conditions cannot be explained by the common dry-wet stomatal regulation theory mentioned above. On the other hand, our transect data in this study can explain why there was a slight variation in the foliar δ13C value among random sampling sites in local scale in response to hydrological gradients as follows.
In 2015, the water level in the river was low. Consequently, the hydrological status of the willows growing nearest the river in the “Flooded” zone, ranged from slight flooding (similar to “Wet” in Figs. 3 and 4; i.e., “small flooding” in Fig. 7) to continual flooding (results in continual waterlogging, e.g., “WL” in Figs. 3 and 4; i.e., “medium flooding” in Fig. 7). Slight flooding near the wet condition zone caused the stomata to open and low foliar δ13C values. In contrast, medium flooding resulted in stomatal closure and high foliar δ13C values. Therefore, under the “Flooded” condition, the δ13C values varied between low and high. This behavior resembles the positive-negative shift in the foliar δ13C value for the “On land” zone under dry-wet conditions. The same interpretation applies to the foliar δ13C values measured in the “Wetland” (Fig. 5).
In contrast, in 2016, the water level in the river was high. Therefore, the hydrological status of the willows growing nearest the river in the “Flooding” zone varied between continual flooding (results in continual waterlogging, e.g., “WL” in Figs. 3 and 4; i.e., “medium flooding” in Fig. 7) and continuous flooding (leads to long period waterlogging, e.g., “LWL” in Figs. 3 and 4; i.e., “large flooding” in Fig. 7). Large flooding reduced both, A and foliar δ13C values. As was the case for 2015, the conditions in the “Flooded” and “Wetland” zones of 2016 ranged between slight and continual waterlogging (similar to “Wet” and “WL,” respectively in Figs. 3 and 4), although only waterlogging in “Flooded” zones was caused by floods (i.e., “small flooding” and “medium flooding”; Fig. 7). Therefore, the foliar δ13C values ranged between low and high in the “Flooding,” “Flooded,” and “Wetland” areas. These responses resemble the positive-negative shift in the foliar δ13C value observed for “On land” under dry-wet conditions.
In previous studies (Garten & Taylor, 1992; Alstad et al., 1999), very small differences in the foliar δ13C value of plants growing near rivers were detected among the diverse hydrological conditions, where waterlogging frequently occurred. These minor differences can also be explained by the physiological responses of willows related to the different hydrological conditions (Fig. 7). If the δ13C values in other organs correlate with those determined by the leaves, then historical records of the wide swings in hydrological conditions could be reconstructed using the δ13C records, such as those obtained from tree ring cellulose.
Conclusions
To illustrate the effects of hydrological conditions on the δ13C values in leaves, we measured the foliar δ13C values of willows at three different points, along three transects near the Indigirka River, under several major hydrological conditions (Fig. 7). Under normal hydrological conditions, the foliar δ13C values were lower under wet conditions (along rivers and/or during a wet year) than under dry conditions (far from the river and/or during a dry year), because the former conditions allowed for stomatal opening. On the other hand, under abnormal hydrological conditions, such as waterlogging, high foliar δ13C values were found, because medium flooding induced stomatal closure. Moreover, long period waterlogging decreased foliar δ13C value by reducing photosynthetic activity. Thus, there was a small variation in the foliar δ13C value (−31.6 to −25.7‰) in the Indigirka River lowland, despite large diversity in the hydrological conditions (Fig. 5). These results demonstrate that the foliar δ13C values reflect hydrological conditions even in mesic environments (Fig. 7). If the foliar δ13C values correlate with those in other organs and tissues (such as tree-ring cellulose), they can be used to reconstruct the hydrological and vegetation changes that have occurred in mesic regions. We suggest that further clarifying of the effects of waterlogging on the foliar or tree ring δ13C values in conducting laboratory experiments under controlled conditions and in field can be highly useful for better interpretation in the δ13C values of plant products.
Supplemental Information
Site No., location, distance to river, dominant species, production, ANPP, and LAI for three 2.5 m × 2.5 m plots in summer of 2016.
Details of sampling site with site No., sampling data, river name, hydrological information, willow species (sampling number) and location of this study.
I and K river represent Indigirka and Kryvaya river.
Photosynthesis light response curve of willows under the light levels from 10 to 955 μmol m−2 s−1.
Photosynthesis light response curve of the willows S. boganidensis (red cycles), S. glauca (green triangles), and S. richardsonii (blue squares) under the light levels from 10 to 955 μmol m−2 s−1. The values were reported as means ± SD.
The river water level (cm) from 1st June to 31st July, for the average in 2010–2014, 2015 and 2016.
The average river water level during 2010-2014 was shown in circles, the water level in 2015 and 2016 was shown in triangles and diamonds, respectively. The arrow lines indicate the observed growing season, with fast growing period in wide arrow line and followed by slow growing period in narrow arrow line.
The foliar δ13C values (‰) of the leaves according to the leaf-open order.
The foliar δ13C values (‰) of the leaves according to the leaf-open order, upon single shoot at each point of PA (empty symbols) and PC (filled symbols) in SBoydom (circles) and SKA (triangles). The different points are shown in different symbols.
Results of foliar δ13C values (‰).
Including samples collected in 2015, 2016 and leaves for ADC measurements.
Data for ADC monitoring at SPh-PA, PB and PC under different hydrological conditions.
ADC monitoring includes especially photosynthetic activity and stomatal conductance.
Data for photosynthesis light response curve of the willows S. boganidensis (red cycles), S. glauca (green triangles), and S. richardsonii (blue squares).
River water level data from 2010 to 2016.
Raw data for Figure A2.
Results of foliar δ13C values (‰) on single shoot from bottom to top.
Raw data for Figure A3.