A new species of Gulo from the Early Pliocene Gray Fossil Site (Eastern United States); rethinking the evolution of wolverines
- Published
- Accepted
- Received
- Academic Editor
- Laura Wilson
- Subject Areas
- Evolutionary Studies, Paleontology
- Keywords
- Guloninae, Mustelidae, Carnivora
- Copyright
- © 2018 Samuels et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. A new species of Gulo from the Early Pliocene Gray Fossil Site (Eastern United States); rethinking the evolution of wolverines. PeerJ 6:e4648 https://doi.org/10.7717/peerj.4648
Abstract
The wolverine (Gulo gulo) is the largest living terrestrial member of the Mustelidae; a versatile predator formerly distributed throughout boreal regions of North America and Eurasia. Though commonly recovered from Pleistocene sites across their range, pre-Pleistocene records of the genus are exceedingly rare. Here, we describe a new species of Gulo from the Gray Fossil Site in Tennessee. Based on biostratigraphy, a revised estimate of the age of the Gray Fossil Site is Early Pliocene, near the Hemphillian—Blancan transition, between 4.9 and 4.5 Ma. This represents the earliest known occurrence of a wolverine, more than one million years earlier than any other record. The new species of wolverine described here shares similarities with previously described species of Gulo, and with early fishers (Pekania). As the earliest records of both Gulo and Pekania are known from North America, this suggests the genus may have evolved in North America and dispersed to Eurasia later in the Pliocene. Both fauna and flora at the Gray Fossil Site are characteristic of warm/humid climates, which suggests wolverines may have become ‘cold-adapted’ relatively recently. Finally, detailed comparison indicates Plesiogulo, which has often been suggested to be ancestral to Gulo, is not likely closely related to gulonines, and instead may represent convergence on a similar niche.
Introduction
Wolverines (Gulo gulo) are the largest living terrestrial mustelid, have a circumboreal Holarctic historic distribution, and have commonly been considered a ‘cold-adapted’ species (Bonifay, 1971; Pasitschniak-Arts & Larivière, 1995; Zigouris et al., 2013). Their diet is best characterized as being that of an opportunistic hypercarnivore, either ambushing and/or chasing prey or scavenging carcasses (Pasitschniak-Arts & Larivière, 1995). Though primarily terrestrial, they are capable climbers, swimmers, and diggers (Pasitschniak-Arts & Larivière, 1995; and citations therein). Though wolverines are well-known, their fossil record is relatively sparse and their origin has been controversial (ex. Kurtén, 1970; Harrison, 1981).
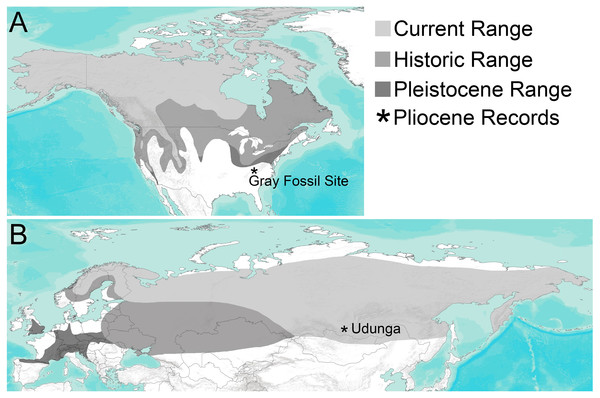
Fossil wolverines have been described from many Pleistocene and Holocene sites in North America and Eurasia, including quite a few outside of the species’ historic distribution (Fig. 1, Table S1 and citations therein). Bryant (1987) compared a large sample of Gulo from the Pleistocene of the Yukon to extant populations and to other Pleistocene records of the genus from North America, which had been described as Gulo gidleyi (Hall, 1936) and later referred to Gulo schlosseri (Kurtén & Anderson, 1980). While Bryant (1987) found some small differences in average size, there was substantial overlap in size ranges and morphology between samples, leading him to conclude that all Quaternary Gulo should be treated as a single species. He did caution that a thorough study of Asian and European specimens was needed (Bryant, 1987), and several subsequent papers, largely focused on European material, have maintained G. schlosseri as a separate species (Pasitschniak-Arts & Larivière, 1995; Kolfschoten, 2001; Nadachowski et al., 2011; Krajcarz, 2012). Until a detailed analysis of specimens from all continents is undertaken, we will follow the taxonomy for Pleistocene wolverines from Eurasia in those studies. Specifically, following Bryant (1987), all Quaternary records of Gulo in North America are considered G. gulo here.
Figure 1: Distribution of Gulo through time in (A) North America and (B) Eurasia.
Current range (light gray) and historic range (medium gray) are based on Pasitschniak-Arts & Larivière (1995), Zigouris et al. (2013); Pleistocene range (dark gray) is based on localities where fossils of Gulo have been found and reported in a wide range of literature sources. The only two known Pliocene occurrences, the Gray Fossil Site in Tennessee and Udunga Fauna of Russia, are highlighted with an asterisk. Terrain base map from ESRI ArcMap 10.5.The origin of wolverines has long been controversial (ex. Zdansky, 1924; Viret, 1939; Kurtén, 1970; Hendey, 1978; Harrison, 1981; Alcalá, Montoya & Morales, 1994; Pasitschniak-Arts & Larivière, 1995; Montoya, Morales & Abella, 2011); mostly due to the sparse pre-Pleistocene records. Only a single record of Gulo prior to the Quaternary has been reported; Gulo minor from the middle Pliocene age Udunga fauna from the Transbaikal region of Russia (Sotnikova, 1982; Sotnikova, 1995; Erbajeva & Alexeeva, 2013). G. minor is morphologically similar to, but substantially smaller than G. gulo. It falls below the range of size variation reported for large extant samples of G. gulo (Bryant, 1987; Anderson, 1998), as well as fossil samples from Europe (Kormos, 1914; Bonifay, 1971; Kurtén, 1973; Döppes, 2001) and North America (Gidley & Gazin, 1938; Kurtén, 1973; Bryant, 1987; Anderson, 1998). Plesiogulo, known from Middle to Late Miocene of Eurasia, Late Miocene and Early Pliocene of North America, and Late Miocene to Early Pliocene of Africa, has often been discussed as a potential close relative of Gulo. While some authors have considered Plesiogulo to be directly ancestral to Gulo (Viret, 1939; Kurtén, 1970; Pasitschniak-Arts & Larivière, 1995), others considered such a line of descent to be unlikely (Zdansky, 1924; Webb, 1969; Hendey, 1978; Harrison, 1981; Xu & Wei, 1987; Alcalá, Montoya & Morales, 1994; Montoya, Morales & Abella, 2011). Alternatively, some authors have suggested independent origins of Gulo and Plesiogulo, with Gulo originating from a fisher or marten-like ancestor in the late Miocene (Samuels & Cavin, 2013) and Plesiogulo from an early Miocene ischyrictine like Iberictis (Ginsburg & Morales, 1992; Alcalá, Montoya & Morales, 1994; Montoya, Morales & Abella, 2011).
Molecular data from a wide range of studies indicate Gulo is part of a clade (Guloninae) that includes fishers (Pekania), martens (Martes), and the tayra (Eira). Among gulonines, wolverines have been consistently found to be most closely related to martens, which form a sister group to the fisher (Koepfli et al., 2008; Wolsan & Sato, 2010; Sato et al., 2012; Li et al., 2014; Malyarchuk, Derenko & Denisova, 2015; Zhu et al., 2016). Koepfli et al. (2008) estimated the Guloninae diverged from other mustelids around 11.0 Ma (95% CI [9.4–12.5 Ma]); whereas Sato et al. (2012) estimated 12.65 Ma (95% CI [10.83–14.72 Ma]). Li et al. (2014) examined the complete mitochondrial genomes of gulonines and estimated the Gulo-Martes clade diverged from Pekania between 8.9 and 7.05 Ma, most likely around 7.6 Ma, while the divergence of Gulo and Martes was between 7.6 and 5.3 Ma, most likely around 6.4–6.3 Ma. Similarly, Malyarchuk, Derenko & Denisova (2015) estimated the divergence of Gulo and Martes to be around 5.6 Ma (95% CI [6.3–4.9] Ma). These estimates coincide with the earliest records of definite gulonine mustelids in the late Miocene, namely Pekania occulta from North America and P. palaeosinensis from Asia (Wang, Tseng & Takeuchi, 2012; Samuels & Cavin, 2013), and Martes ginsburgi and M. wenzensis from Europe (Stach, 1959; Wolsan, 1989a; Anderson, 1994; Sato et al., 2003; Montoya, Morales & Abella, 2011). Each of these molecular estimates for the divergence of Gulo from other closely related gulonines (Martes and Pekania) are long after the earliest records of Plesiogulo, which date to the middle Miocene (MN6) of Turkey and Kazakhstan, between 15.2 and 12.5 Ma (Schmidt-Kittler, 1976; Montoya, Morales & Abella, 2011). Though it seems clear that Plesiogulo falls along a different lineage, new and/or older records could clarify the origin of Gulo.
Here, we describe a new species of Gulo from the Early Pliocene age Gray Fossil Site of Tennessee (Fig. 1). The specimen described here represents the earliest record of Gulo and predates other records of the genus by over one million years. Similarity of this new species to early fishers (Pekania), suggests that wolverines may have originated in North America. In light of this discovery and other fossil records of Gulo, we also discuss the historical biogeography and ecology of wolverines, as well as their commonly accepted ‘cold-adapted’ nature.
Materials and Methods
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: [29DF929D-D054-4912-A2B1-FFEEFD4BDE1B]. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Dental nomenclature follows Ginsburg (1999). Measurements of the teeth, to the nearest 0.01 mm, were made using Mitutoyo Absolute digital calipers. Measurements include anteroposterior length and transverse breadth of the teeth. For the P4, maximum transverse breadth was measured at both the protocone (P4Wpro) and the metastyle (P4Wmet), and for the M1 lengths were measured for both inner (M1Lint) and outer lobes (M1Lext).
Measurements were taken, and comparisons were made, with specimens of extant and extinct mustelids from several collections. Extant samples included Gulo gulo (n = 36), Pekania pennanti (n = 22), Martes americana (n = 11), Martes flavigula (n = 1), Martes foina (n = 1), Martes martes (n = 1), Eira barbara (n = 5); this includes all extant gulonine species other than Martes melampus and Martes zibellina. Fossil samples included Pleistocene and Holocene specimens of Gulo and Pekania from North America, late Miocene specimens of Pekania occulta and Plesiogulo marshalli from North America, as well as casts of Pekania palaeosinensis from Asia and Plesiogulo from North America. All material was also compared to specimens and measurements in a wide range of publications (including Zdansky, 1924; Gidley & Gazin, 1933; Gidley & Gazin, 1938; Chardin & Leroy, 1945; Stach, 1959; Anderson, 1970; Anderson, 1998; Kurtén, 1970; Kurtén, 1973; Hendey, 1978; Harrison, 1981; Bryant, 1987; Wolsan, 1989a; Wolsan, 1989b; Ginsburg & Morales, 1992; Döppes, 2001; Haile-Selassie, Hlusko & Howell, 2004; Montoya, Morales & Abella, 2011; Peigné, 2012; Wang, Tseng & Takeuchi, 2012). Complete measurement data for all mustelids studied are included in Table S2.
Fossil and modern specimens used for comparative illustrations were photographed with the alveolar margin of the upper carnassial (P4) parallel to the photographic plane. This is important to note, as most published fossil mustelids have been photographed with the palate parallel to the photographic plane (Fig. S1A); in that orientation the alveolar margins of the cheek teeth are at a slightly oblique angle, with the lateral portions slightly ventral to the medial. A consequence of this difference in photography and illustration methodology is that some of the lingual portions of the tooth, like the P4 lingual cingulum, are not obscured using our methodology (Fig. S1B).
Specimen Repositories—ETMNH, East Tennessee State University Museum of Natural History—Fossil Collection, Gray, Tennessee; ETVP, East Tennessee State University Museum of Natural History—Comparative Collection, Johnson City, Tennessee; LACM, Natural History Museum of Los Angeles County, Los Angeles, California; MVZ, Museum of Vertebrate Zoology, University of California, Berkeley, California; UCLA, Donald R. Dickey Collection of the University of California, Los Angeles, Los Angeles, California; USNM, United States National Museum of Natural History (Smithsonian Institution), Washington, D.C.
Geological Setting
The Gray Fossil Site in northeastern Tennessee includes deposits that represent an ancient sinkhole containing a small, but deep lake, which gradually filled with sediment (Shunk, Driese & Clark, 2006; Shunk, Driese & Dunbar, 2009). Sediment cores have revealed a series of rhythmites in the upper lacustrine strata, which alternate between fine-grained silty clay layers and coarse-grained, organic rich layers (Shunk, Driese & Clark, 2006; Shunk, Driese & Dunbar, 2009). Shunk, Driese & Dunbar (2009) estimated the sinkhole lake filled with sediment in approximately 4,500 to 11,000 years. Within the sedimentary layers are well-preserved and diverse flora and fauna (ex. Parmalee et al., 2002; Wallace & Wang, 2004; Mead et al., 2012; Zobaa et al., 2011; Ochoa et al., 2012; Ochoa et al., 2016; Worobiec, Liu & Zavada, 2013), which indicate a forested environment was present. Macro- and microfossils indicate the presence of a forest dominated by oak (Quercus), hickory (Carya), and pine (Pinus), as well as a variety of herbaceous taxa (Ochoa et al., 2016; and references therein). Isotopic analyses of carbon and oxygen from teeth of ungulates at the site indicate a relatively dense forest, with some more open grass-dominated habitats nearby, and climate with little seasonal temperature and precipitation variation (DeSantis & Wallace, 2008). Ochoa et al. (2016) interpreted the flora at the Gray Fossil Site as indicative of a woodland or woodland savanna environment characterized by frequent disturbance. The occurrence of bald cypress (Taxodium) and tupelo (Nyssa) leaves and pollen at Gray (Brandon, 2013; Worobiec, Liu & Zavada, 2013) suggest the presence of humid riparian or wetland areas. Fauna includes many taxa indicative of aquatic environments, such as fish, neotenic salamanders, aquatic turtles, Alligator, and beavers (Parmalee et al., 2002; Boardman & Schubert, 2011; Mead et al., 2012; Jasinski, 2013; Bourque & Schubert, 2015).
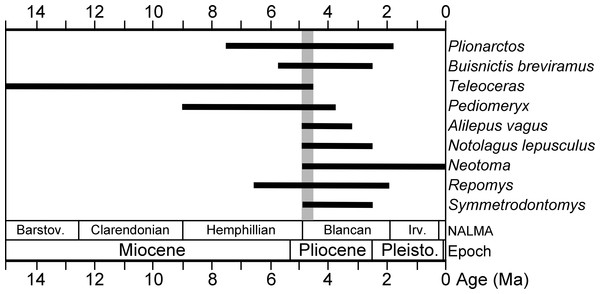
Age of the Gray Fossil Site has been previously reported as constrained between 7 and 4.5 Ma, based on the stratigraphic ranges of the rhino Teleoceras and ursid Plionarctos (Wallace & Wang, 2004). Teleoceras has commonly been considered an index taxon for the Hemphillian NALMA, but it is worth noting that there are several Pliocene records of Teleoceras, including Blancan age specimens from White Bluffs in Washington (Gustafson, 2012), Beck Ranch in Texas (Madden & Dalquest, 1990), and Saw Rock Canyon in Kansas (Prothero & Manning, 1987). It is important to note that the record from Beck Ranch in Texas has been interpreted as a tooth reworked from older sediments (Prothero, 2005) and the report from Kansas does not refer to any cataloged specimens, but the presence of Ogmodontomys sawrockensis indicates Saw Rock Canyon is Blancan in age (Martin & Peláez-Campomanes, 2014; Martin, Peláez-Campomanes & Viriot, 2017). Some of the latest rhino records in North America may be younger than 4.5 Ma and potentially as young as 3.5 Ma (Gustafson, 2012). Gustafson (2012) asserted that such records indicate that the presence of Teleoceras at a site is insufficient justification for assigning a Hemphillian age to the fauna, as has been done for a variety of eastern U.S. localities, specifically the Palmetto fauna (Webb et al., 2008) and Pipe Creek Sinkhole (Farlow et al., 2001; though see (Martin, Goodwin & Farlow, 2002; Martin, 2010) for a revised age of that site).
A number of recently identified taxa from the Gray Fossil Site have good fossil records and limited stratigraphic ranges. Based on first and last appearance data (FAD, LAD) of these taxa derived from the MIOMAP/FAUNMAP Databases (Carrasco et al., 2007; Graham & Lundeliu Jr, 2010; http://www.ucmp.berkeley.edu/neomap/), NOW Database (Fortelius, 2013; http://pantodon.science.helsinki.fi/now/), and recent publications, we can produce a revised estimate of the age of the Gray Fossil Site. The cricetids Neotoma and Symmetrodontomys have their first appearances near the Hemphillian—Blancan transition, approximately 4.9 or 4.8 Ma (Martin, 2000; Martin, Goodwin & Farlow, 2002; Bell et al., 2004; Lindsay, 2008; Martin, Peláez-Campomanes & Viriot, 2017). The leporid Notolagus lepusculus and the mephitid Buisnictis breviramus appeared in, and are restricted to, the early Blancan (FAD 4.9 Ma). Among other taxa at the site are the cricetid Repomys (FAD 6.6 Ma) and the leporid Alilepus vagus (FAD 5.9), which appeared in the Hemphillian and survived into the Blancan. The dromomerycid Pediomeryx appeared earlier in the Miocene, but disappeared near the Hemphillian—Blancan transition (LAD 4.7 Ma) (Voorhies, 1990; Janis & Manning, 1998). However, a recently described record of Pediomeryx from the Lee Creek Mine Local Fauna in North Carolina (Eshelman & Whitmore Jr, 2008) is early Blancan in age, as Marx & Fordyce (2015) bracket the age of the lower Yorktown Formation (including Lee Creek Mine) between 4.9 and 3.9 Ma.
In sum, none of the mammal genera at the Gray Fossil Site are restricted to the late Miocene or Hemphillian NALMA, and some taxa are actually characteristic of early Blancan (Early Pliocene) faunas. For example, the Gray Fossil Site and the early Blancan age Beck Ranch fauna in Texas (Dalquest, 1978) share the following (Teleoceras, Neotoma, Symmetrodontomys, Notolagus lepusculus, Buisnictis breviramus). Other than Neotoma, none of these taxa survived later than the early Blancan (∼2.5 Ma; Bell et al., 2004). Considering the Gray Fossil Site fauna in total, a revised estimate of the age of the Gray Fossil Site is Early Pliocene near the Hemphillian–Blancan transition, likely between 4.9 and 4.5 Ma (Fig. 2).
Figure 2: Stratigraphic ranges of selected mammals from the Gray Fossil Site in Tennessee.
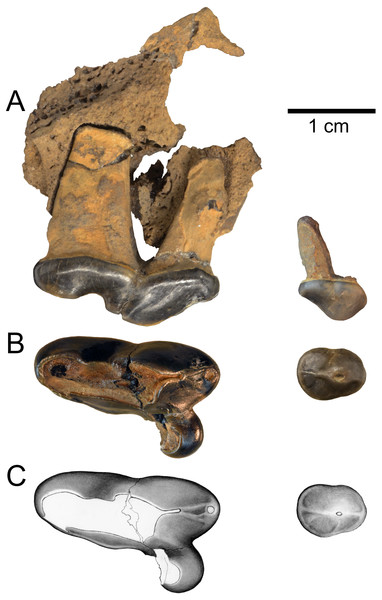
The black bars indicate stratigraphic ranges of genera and species based on first and last appearance dates (data sources are listed in the Geologic Setting section). Overlap in ranges of taxa, between 4.9 and 4.5 Ma, is highlighted with a gray bar.Figure 3: Holotype of Gulo sudorus (ETMNH 3663) from the Gray Fossil Site, Tennessee.
Specimen consists of a right P2 and maxilla fragment with P4. (A) Lateral view. (B) Occlusal view. (C) Original illustration of the specimen by Keila Bredehoeft. Scale bar equals 1 cm. Photographs by Joshua Samuels.Results
Systematic paleontology
| Class MAMMALIA Linnaeus, 1758 |
| Order CARNIVORA Bowdich, 1821 |
| Family MUSTELIDAE Fischer von Waldheim, 1817 |
| Subfamily GULONINAE Gray, 1825 |
| Genus Gulo Pallas, 1780 |
Type Species—Gulo gulo.
Included Species—Gulo minor, Gulo schlosseri, Gulo sudorus (new species).
Distribution—Early Pliocene of Tennessee, middle Pliocene of Russia, Pleistocene to recent of North America and Eurasia (see Table S1 for a listing of fossil sites).
Holotype—ETMNH 3663, partial right maxilla with P2 and P4.
| Species | Specimen No. | Locality | P2L | P2W | P4L | P4Wpro | P4Wmet | M1Lext | M1Lint | M1W | P2L/P4L | P4Wpro/ P4L | P4Wmet/ P4L | P4L/ M1Lint |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gulo sudorus new species | ETMNH 3663 | Gray Fossil Site, TN | 7.93 | 6.05 | 21.23 | 13.19 | 8.09 | 0.374 | 0.621 | 0.381 | ||||
| Gulo gulo (Recent) | Mean (n = 36) Min. Max. |
Various | 6.49 5.40 7.80 |
4.38 3.70 5.29 |
19.79 17.70 23.90 |
11.49 10.30 14.04 |
7.28 6.30 9.30 |
7.10 6.16 8.12 |
8.02 7.29 8.77 |
13.89 12.88 14.92 |
0.328 0.303 0.402 |
0.581 0.518 0.619 |
0.385 0.359 0.409 |
2.646 2.511 2.769 |
| Gulo gulo (Holocene) | Min. (n = 5) Max. Min. (n = 14) Max. |
Middle Butte Cave, ID Moonshiner Cave, ID |
6.9 5.7 5.8 7.0 |
3.9 4.7 3.6 5.0 |
18.0 21.5 18.0 22.2 |
9.6 12.1 9.4 12.9 |
7 8.1 6.6 8.7 |
11.8 13.8 11.9 14.2 |
0.317 0.321 0.315 0.322 |
0.522 0.581 0.533 0.563 |
2.552 2.727 2.571 2.654 |
|||
| Gulo gulo (Pleistocene–Eurasia) | Mean (n = 18) Min. Max. |
Various | 7.17 6.6 7.9 |
4.82 4.3 5.5 |
22.02 19.7 23.2 |
12.96 11.5 14.4 |
0.324 0.301 0.364 |
0.590 0.550 0.619 |
||||||
| Gulo schlosseri (Pleistocene–North America) | USNM V8175 USNM V8176 |
Cumberland Cave, MD | 7.38 | 4.9 | 18.9 21.84 |
11 11.65 |
7.3 8.5 |
6.98 | 9.18 | 14.46 | 0.338 | 0.582 0.533 |
0.386 0.389 |
2.379 |
| Pekania occulta† | JODA 15214 | Rattlesnake Fm., OR | 3.0 | 13.25 | 8.78 | 5.31 | 5.56 | 8.54 | 12.84 | 0.663 | 0.401 | 1.552 | ||
| Pekania palaeosinensis† | IVPP V 18408 Mean (n = 7) Min. Max. |
Baogeda Ula, China Baode, China |
5.8 5.12 4.2 5.7 |
2.19 2.32 1.9 2.5 |
12.38 10.54 9.0 11.5 |
6.29 6.07 4.6 6.7 |
3.95 4 |
5.7 | 6.5 | 10.4 | 0.468 0.486 0.467 0.496 |
0.508 0.576 0.511 0.583 |
0.319 0.364 |
1.692 |
| Pekania diluviana† | USNM V8010 | Cumberland Cave, MD | 10.2 | 6 | 3.9 | 5 | 6 | 9.5 | 0.588 | 0.382 | 1.7 | |||
| Pekania pennanti | Mean (n = 22) Min. Max. |
Various | 5.45 4.66 6.42 |
2.79 2.04 3.18 |
11.54 9.98 13.36 |
6.98 5.83 7.88 |
4.22 3.34 4.91 |
5.78 4.87 6.52 |
6.86 5.47 7.77 |
10.09 8.43 11.51 |
0.472 0.423 0.513 |
0.605 0.575 0.650 |
0.364 0.335 0.403 |
1.695 1.521 1.969 |
| Martes ginsburgi† | VV-11759 | Venta del Moro, Spain | 8.9 | 5.7 | 5.4 | 8.3 | 0.640 | 1.648 | ||||||
| Martes wenzensis† | Holotype | Węże 1, Poland | 5.8 | 3.0 | 12.0 | 5.2 | 6.6 | 10.5 | 0.483 | 0.433 | 1.818 | |||
| Martes americana | Mean (n = 11) Min. Max. |
Various | 4.33 3.21 5.15 |
2.00 1.57 2.23 |
7.80 6.50 9.17 |
4.86 3.90 5.80 |
2.84 1.90 3.51 |
3.79 3.09 4.68 |
5.23 3.78 6.64 |
7.88 6.01 9.36 |
0.557 0.473 0.625 |
0.622 0.582 0.642 |
0.362 0.292 0.396 |
1.536 1.334 1.728 |
| M. martes | LACM 74508 | 5.34 | 2.78 | 9.25 | 6.12 | 3.36 | 4.72 | 7.81 | 9.55 | 0.577 | 0.662 | 0.363 | 1.184 | |
| M. flavigula | LACM 8229 | 5.22 | 2.6 | 10.11 | 6.42 | 3.48 | 4.02 | 5.31 | 9.57 | 0.516 | 0.635 | 0.344 | 1.904 | |
| M. foina | ETVP 5535 | 4.1 | 2.43 | 9.37 | 5.83 | 3.36 | 5.2 | 5.55 | 9.22 | 0.438 | 0.622 | 0.359 | 1.688 | |
| Eira barbara | Mean (n = 5) Min. Max. |
Various | 3.85 3.30 4.31 |
2.84 2.56 3.04 |
10.06 9.23 10.62 |
6.90 6.47 7.49 |
3.66 3.58 3.85 |
3.60 2.65 4.25 |
4.68 3.61 5.57 |
8.73 8.17 9.52 |
0.382 0.347 0.406 |
0.687 0.610 0.743 |
0.365 0.337 0.417 |
2.183 1.905 2.637 |
| Sminthosinis bowleri† | UMMP 52868 UMMP 55214 |
Hagerman, ID | 5.38 4.98 |
2.15 | 9.6 9.65 |
5.35 5 |
4.76 | 7.47 | 0.560 0.516 |
0.557 0.518 |
2.017 | |||
| Ischyrictis zibethoides† | Mean (n = 4) Min. Max. |
Various, Europe | 8.5 | 4.6 | 15.95 14.0 17.8 |
10.1 7.9 12 |
5.6 4.6 6.6 |
7.03 6.65 7.4 |
8.58 6.85 9.9 |
15.25 13.75 16.5 |
0.478 | 0.630 0.564 0.674 |
0.329 0.4 |
1.964 1.798 2.263 |
| Plesiogulo brachygnathus† | Mean (n = 10) Min. Max. |
Shansi, China | 8.15 7.6 8.9 |
5.85 5.0 6.7 |
19.24 17.1 20.5 |
12.87 11.1 14.0 |
9.65 8.4 11.7 |
13.54 12 16.3 |
16.88 13.8 18.4 |
0.420 0.393 0.445 |
0.669 0.649 0.683 |
1.428 1.258 1.583 |
||
| Plesiogulo crassa† | Mean Min. Max. |
Paote, China | 8.15 7.8 8.3 |
5.9 | 19.48 18.3 20.8 |
12.92 12.3 14.2 |
8.4 7.6 9 |
13.22 11.9 14.8 |
16.98 15.8 17.8 |
0.418 | 0.663 | 1.473 | ||
| Plesiogulo minor† | Holotype | K’ingyang, China | 17 | 10.5 | 7.9 | 11.2 | 14.3 | 0.618 | 1.518 | |||||
| Plesiogulo praecocidens† | UPI No. M19 (5) | Paote, China | 17.2 | 10.9 | 7.8 | 12.4 | 13.8 | 0.634 | 1.387 | |||||
| Plesiogulo lindsayi† | F:AM 49384 | Wikieup, Arizona | 9.5 | 6.9 | 23.5 | 17.3 | 9.7 | 14.6 | 20.6 | 0.404 | 0.736 | 1.610 | ||
| Plesiogulo marshalli† | Mean (n = 8) Min. Max. |
Various, North America | 8.01 7.84 8.2 |
5.83 5.7 5.9 |
19.75 18.2 22.04 |
13.25 12.1 15.0 |
8.59 7.81 9.80 |
9.88 8.3 11.87 |
13.95 12.45 15.9 |
17.58 17.0 18.5 |
0.416 | 0.670 0.640 0.696 |
0.434 0.422 0.445 |
1.411 1.238 1.749 |
| Plesiogulo monspessulanus† | L40042 | Langebaanweg, South Africa | 9.7 | 7.2 | 23.2 | 15.6 | 15.4 | 18.6 | 0.418 | 0.672 | 1.506 | |||
| Plesiogulo botori† | KNM-NK 41420 | Narok, Kenya | 24.5 | 16.7 | 10.1 | 15.9 | 21.2 | 0.682 | 1.541 | |||||
| Iberictis azanzae† | Holotype | Artesilla, Spain | 15.5 | ?9.7 | 10.9 | ?16 | 0.626 | 1.422 |
Locality—Gray Fossil Site, Washington County, Tennessee.
Age—Early Pliocene (earliest Blancan).
Diagnosis—P2 broad and robust, and P4 enlarged with a broad paracone and metastyle; these features are typical of Gulo, but not observed in any other gulonines. P4 protocone anteriorly positioned and infraorbital foramen is oval, unlike Eira. P4 lacks an external median rootlet, unlike Pekania. P4 protocone and parastyle larger than in extant Gulo gulo and all other previously described species of Gulo. P4 parastyle includes a small, conical parastylar cusp. P4 metastyle tapers distally, unlike the posteriorly broad and squared metastyle of other Gulo species. P2 not as strongly reduced as in other members of the genus. Infraorbital foramen is located above the anterior root of the P4, rather than anterior to the P4 as is typical of other species of Gulo.
Etymology—“Sudorus” from the Latin for “sweaty”. In reference to the warm, humid climate present in the Early Pliocene of Tennessee relative to the typical boreal habitat that the modern taxon is known for inhabiting.
Description—The holotype specimen (ETMNH 3663) consists of a left maxilla fragment with the P2 and P4 (Fig. 3). There is little of the maxilla preserved, though some anatomical information can be derived from the specimen. A portion of the infraorbital foramen is preserved above the anterior root of the P4 and its lateral margin suggests an oval shape; the foramen is approximately 10.8 mm in dorsoventral diameter. The P2 crown is intact and only a small portion of the posterior surface of the P4 protocone is missing. The P2 shows minor, and P4 moderate, wear; indicating that this was an adult individual.
The P2 is double-rooted, robust, and distinctly larger than in other members of the genus (Table 1). Though it is larger in absolute terms, the size of the P2 relative to the P4 in ETMNH 3663 falls within the range of variation observed in extant Gulo gulo (P2L/P4L, Table 1). The P2 has a single principal cusp and a ridge running along its midline, most distinct posterior to the principal cusp. There is a low, relatively indistinct cingulum on the P2, which is most prominent along its posterior margin.
The P4 is robust and three-rooted, as is typical of the genus. Also typical of Gulo, there is no external median rootlet present on the P4, which is a feature that has been used to characterize Pekania (though see comparisons below). The tooth bears a distinct parastyle and a relatively larger protocone than in other species of Gulo. The parastyle bears a small, round parastylar cusp. A pair of low ridges run along the anterior surface of the paracone; the lateral ridge ends at the posterobuccal edge of the parastylar cusp, the medial ridge forks in two and ends just posterior to the parastyle. Like other gulonines, Gulo sudorus has a relatively deep inflection of the anterior portion of the P4, between the parastyle and protocone. The protocone is particularly large and extends anteromedially from the base of the paracone, projecting nearly as far anteriorly as the parastyle. The metastyle of G. sudorus is mesially broad, tapers distally, and bears a distinct lingual cingulum. There is a small cingulum along the base of the metastyle on the lingual surface of the tooth and a subtle remnant of an anterior cingulum along the anterior margin of the protocone, both of which are common in known gulonines.
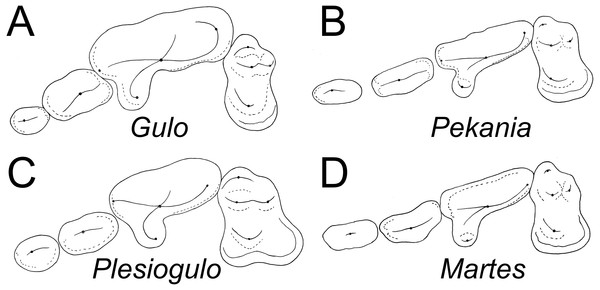
Comparisons—Overall, the morphology of ETMNH 3663 is similar to known fossil and modern specimens of Gulo and other gulonine mustelids (Fig. 4), but has a set of features that distinguish it from other taxa. As in extant and Pleistocene specimens of Gulo, the infraorbital foramen of ETMNH 3663 has an oval shape, but it is located more posteriorly, above the anterior root of the P4, rather than above the P3 or anterior margin of the P4 as in G. gulo (Harrison, 1981). Position and shape of the infraorbital foramen in extant and fossil Pekania and Martes are variable, either oval or round and anterior to the P4 as in Gulo or above the anterior root of the P4. In Eira the infraorbital foramen is round and located above the anterior root or middle of the P4. In Plesiogulo the infraorbital foramen is relatively round in cross section and located above the anterior root or middle of the P4 (Harrison, 1981).
Figure 4: Occlusal morphology of gulonine mustelids and Plesiogulo.
(A) Gulo gulo (ETVP 291). (B) Pekania pennanti (ETVP 601). (C) Plesiogulo marshalli (composite of FAM 23386 and 49230). (D) Martes americana (NAUQSP 2015). Images are scaled to the same P2–M1 length. Original illustrations by Keila Bredehoeft.There is a deep inflection between the parastyle and protocone of the P4 in ETMNH 3663 and G. gulo, and in the latter that inflection is occupied by the posterior margin of the P3 (Fig. 4A). In extant and fossil gulonines, a similar arrangement is also seen in individuals of Martes flavigula, M. martes, and Pekania palaeosinensis, but not in Eira barbara, M. americana, P. occulta, P. diluviana, or P. pennanti.
While a parastylar cusp is occasionally present in the P4 of Gulo gulo, it is never as substantial as in ETMNH 3663. Similarly, the protocone is proportionately larger than in extant Gulo and other fossils of the genus (Table 1). Presence of a large protocone and parastyle is typical of other gulonine mustelids, like Pekania, Martes, and Eira; though the protocone is more posteriorly positioned in Eira than other gulonines. A distally tapered P4 metastyle like that of ETMNH 3663 is seen in Eira, Pekania, and Martes, but not in other species of Gulo. Specifically, in both Gulo gulo and G. schlosseri the P4 metastyle is broad and squared distally, and bears a distinct u-shaped or square extension at the posterior margin of the metastyle blade. That extension runs anteroposteriorly along the buccal margin of the metastyle, then transversely to join the metastyle blade at a nearly right angle (Fig. 4A).
Pekania occulta from the late Miocene of Oregon has a more robust P4 than other Pekania and Martes species, as well as a particularly large P4 protocone and distally tapered metastyle, and an oval-shaped infraorbital foramen located above the anterior root of the P4. Each of those features are similar to ETMH 3663, though P. occulta is smaller and lacks the robust anterior premolars seen in Gulo sudorus. Additionally, as is typical of the genus Pekania, P. occulta bears an external median rootlet on the P4, which is not present in ETMNH 3663.
Due to the fragmentary material known for both taxa, the morphology of Gulo sudorus and G. minor from the middle Pliocene of Asia cannot be directly compared. However, all measurements of the lower dentition of G. minor (Sotnikova, 1982) fall outside the range of variation of extant and fossil samples of Gulo, between 7 and 16% smaller than the smallest extant wolverines studied. In contrast, all dimensions the upper dentition of G. sudorus, other than length and width of the P2, fall within the ranges of G. gulo and G. schlosseri, and all are larger than the means of extant samples (Table 1). As such, it is unlikely that known specimens of G. sudorus and G. minor are from the same taxon.
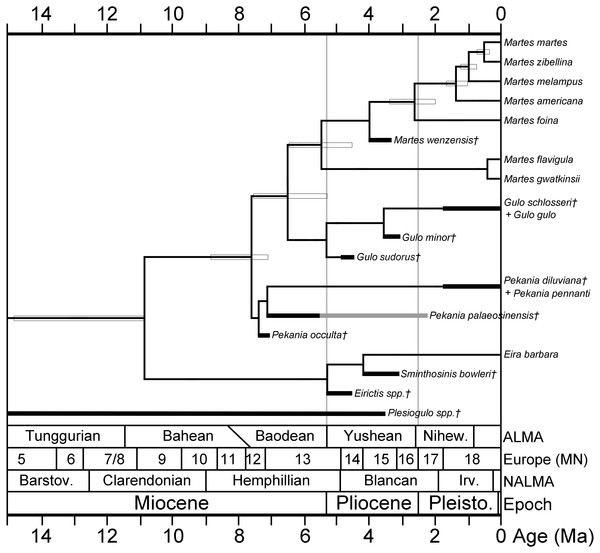
A hypothesis of the evolutionary relationships of gulonine mustelids is presented in Fig. 5. Relationships of extant gulonine taxa are based on molecular phylogenetic studies (Koepfli et al., 2008; Sato et al., 2012; Li et al., 2014); estimated divergence times are based on the protein coding region data set in Li et al. (2014), except the divergence time of Guloninae from other “crown” mustelids (Helictidinae, Ictonychinae, Lutrinae, and Mustelinae) which is based on the multidivtime analysis of Sato et al. (2012). Geologic ages of known gulonine fossils are derived from the MIOMAP/FAUNMAP Databases (Carrasco et al., 2007; Graham & Lundeliu Jr, 2010; http://www.ucmp.berkeley.edu/neomap/), NOW Database (Fortelius, 2013; http://pantodon.science.helsinki.fi/now/), and recent publications; the age of Gulo sudorus is based on the biostratigraphic framework presented in Fig. 2. Note that the placements of extinct taxa in the phylogeny are not based on a cladistic analysis, but rather morphological comparisons made here and in a number of earlier studies. The placement of the extinct Pekania palaeosinensis, Eirictis, and Sminthosinis are based on Wang, Tseng & Takeuchi, 2012, and the placement of M. wenzensis is based on Anderson, 1994; Sato et al., 2003.
Figure 5: Phylogeny of gulonine mustelids with estimated divergence times and geologic ages of known fossils.
Phylogenetic relationships of extant taxa based on cladistics analyses of Koepfli et al. (2008), Sato et al. (2012) and Li et al. (2014). Note that the placements of extinct taxa are not based on a cladistic analysis, rather they are based morphological comparisons made in this manuscript and prior studies. Divergence times (indicated by horizontal boxes) are based on protein coding region data set in Li et al. (2014), except divergence time of Guloninae from other “crown” mustelids (Helictidinae, Ictonychinae, Lutrinae, and Mustelinae) based on multidivtime analysis of Sato et al. (2012). Age ranges of fossil taxa (thick black lines) are based on occurrences and references listed in Table S2 and data sources listed in the Geologic Setting section.The basal placement of Gulo sudorus within the genus is based on the following features: (1) infraorbital foramen is positioned posteriorly in G. sudorus, as in Eira and some species of Pekania and Martes, and in contrast to the anterior position of the foramen in G. schlosseri and G. gulo; (2) P2 larger than in any studied specimens of G. schlosseri and G. gulo, which have reduced anterior premolars; (3) P4 metastyle tapers posteriorly in G. sudorus, as in Ischyrictis zibethoides, Eira, Pekania, and Martes, and in contrast to the broad and posteriorly squared metastyle of G. schlosseri and G. gulo; (4) the P4 protocone and parastyle are particularly large in G. sudorus, as in Ischyrictis zibethoides, Pekania, and Martes, and larger than in G. schlosseri and G. gulo. Each of those features of G. sudorus are present in other gulonine genera and thus may represent primitive features for the Guloninae. Gulo minor is considered derived relative to G. sudorus, as the only differences it shows from G. schlosseri and G. gulo are the following features (from Sotnikova, 1982; Kalmykov, 2015): (1) smaller body size; (2) weaker curvature of the tooth row, with the m1 trigonid offset at an oblique angle to the p3 and p4, whereas the p3, p4, and m1 trigonid are all aligned in G. schlosseri and G. gulo; (3) relatively elongate and narrow p3 and p4 (length/width ratio of premolars higher than in any studied samples of other members of the genus). Given the substantial variability in body size of extant wolverines, with the largest studied samples having dentition 25% larger than the smallest (Table 1), the somewhat smaller size of G. minor is not interpreted as a particularly substantial difference. The difference in the curvature of the toothrow is also not particularly distinctive, as the dental arcade of extant wolverines is highly variable in terms of rotation and alignment of the anterior premolars (Jung et al., 2016). Within the genus Pekania, placement of P. occulta as the most basal member is based on the following features: (1) robust P4, more robust than in other members of the genus, (2) P4 protocone larger than in other members of the genus. Each of these features are similar to Gulo, and thus may represent the primitive state for the Pekania—Gulo/Martes clade.
Discussion
Origin and evolution of Gulo
Origins of the subfamily Guloninae and the genus Gulo have long been uncertain, but recently described fossils have substantially improved our understanding of the group’s evolution. The earliest species that has been referred to any extant gulonine genus is “Martes” laevidens from the early Miocene (MN 3) of Germany (Dehm, 1950). However, Sato et al. (2003) noted the basicranial anatomy of “M.” laevidens indicated it was not a member of the extant genus Martes; the incompletely ossified suprameatal fossa (Wolsan, 1993) is a plesiomorphic trait among mustelids (Hughes, 2012). There are many other described examples of potential early “Martes” species from the early and middle Miocene of Eurasia, North America, and North Africa (Anderson, 1994; Baskin, 1998; Ginsburg, 1999; Hughes, 2012); however; most are not closely related to extant gulonine genera, but instead represent stem groups outside of the crown clade Guloninae (Anderson, 1994; Sato et al., 2003; Wang, Tseng & Takeuchi, 2012; Li et al., 2014). Moreover, some of these “Martes” taxa show similarity to ischyrictines (Ginsburg & Morales, 1992; Montoya, Morales & Abella, 2011) and, in some cases, have been referred to the ischyrictine genera Hoplictis, Plionictis, and Sthenictis (Anderson, 1994; Baskin, 1998; Hughes, 2012).
Similarities of many of these potential early “Martes” species with members of the Guloninae is likely due to the retention of plesiomorphic traits in extant gulonine taxa, or more likely ecomorphological convergence with the genus Martes. Complicating the issue, extant gulonines, including species of Martes and Gulo, are characterized by highly polymorphic dentition (Wolsan, Ruprecht & Buchalczyk, 1985; Wolsan, 1988; Wolsan, 1989b; Döppes, 2001; Jung et al., 2016), and it seems likely that ancestral forms shared the same level of variation. Given the polymorphism observed in extant taxa and incomplete fossil material known for many early and middle Miocene species, early taxa referred to “Martes” are in need of detailed study, which may reveal how they are (or are not) related to gulonines.
Something similar to Ischyrictis zibethoides, which is known from the Early and Middle Miocene (MN 5-8) of Europe (Peigné, 2012), could be ancestral to gulonines. I. zibethoides exhibits a dentition which has some similarity to that of gulonines; the P4 protocone and parastyle are large, and there is a deep inflection in the anterior margin of the tooth. The M1 has a distinctive round lingual margin. Lastly, the age of that taxon is similar to the estimated divergence of gulonines from other mustelids, which molecular estimates placed near 11.0 Ma (Koepfli et al., 2008) or 12.65 Ma (Sato et al., 2012).
The oldest confirmed gulonines are early records of fishers, including Pekania occulta from North America (Samuels & Cavin, 2013) and P. palaeosinensis from Asia (Wang, Tseng & Takeuchi, 2012). Pekania, which was formerly considered a subgenus of Martes, is distinguished by the presence of an external median rootlet on the upper P4 (Anderson, 1994; Samuels & Cavin, 2013). Morphology of P. occulta, particularly the robust P4 with a large protocone, is similar to extant Gulo gulo and G. sudorus, suggesting that species may be similar to the shared ancestor of more recent Pekania and Gulo. The late Miocene ages of P. occulta (Hemphillian NALMA, 7.3–7.05 Ma) and P. palaeosinensis (Baodean ALMA) are consistent with molecular estimates for divergence of Pekania from the Martes/Gulo clade (Koepfli et al., 2008; Sato et al., 2012; Li et al., 2014).
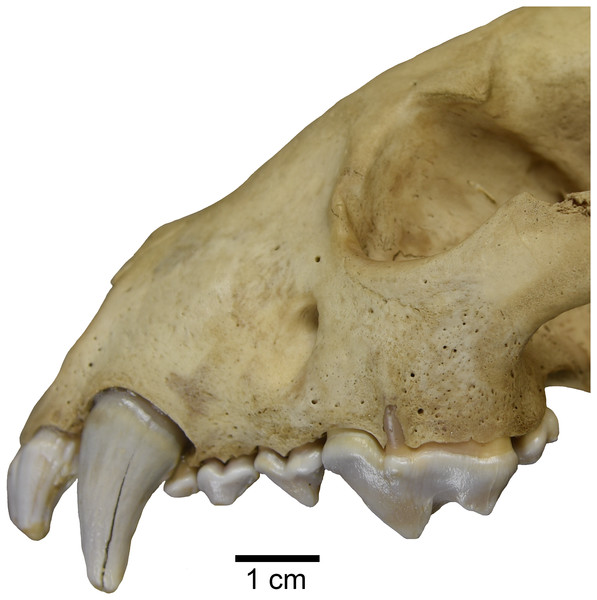
Interestingly, one studied specimen of recent Gulo gulo (ETVP 269) possesses an external median rootlet on both upper carnassials (Fig. 6). Presence of an external median rootlet on the upper P4 is considered diagnostic of the genus Pekania (Anderson, 1970). Though it is only observed in a single specimen, this apparent atavistic trait lends support to both molecular and fossil evidence indicating a close relationship between Pekania and Gulo. If Gulo evolved from a fisher-like ancestor, as we hypothesize, the occurrence of an external median rootlet in ETVP 269 is not as surprising as it would be if the two taxa were not so closely related.
Figure 6: Gulo gulo (ETVP 269) with an atavistic external median rootlet on the upper P4, which is exposed through the lateral surface of the maxilla.
Scale bar equals 1 cm. Photograph by Joshua Samuels.An early representative of the genus Martes may be M. ginsburgi (Montoya, Morales & Abella, 2011) from the late Miocene of Spain (MN 13), which is similar to both M. anderssoni (Schlosser, 1924) from latest Miocene/Early Pliocene and M. zdanskyi (Teilhard de Chardin & Leroy, 1945) from the late Pliocene of China. M. ginsburgi lacks an external median rootlet on the P4 and the m1 morphology is distinct from Pekania (Montoya, Morales & Abella, 2011). M. ginsburgi is also very similar to M. martes, suggesting that it may be similar to the ancestor of extant martens (Montoya, Morales & Abella, 2011). Detailed study of M. ginsburgi, M. anderssoni, and other late Miocene species referred to the genus should help resolve how these taxa are related to extant martens.
Various authors (Anderson, 1994; Sato et al., 2003; Li et al., 2014) have considered Martes wenzensis (Stach, 1959) from the Pliocene (MN 15) of Poland (Wolsan, 1989a) to be the earliest undoubted member of the genus Martes. M. wenzensis is known from multiple skulls and characterized by having longer and more robust carnassials than extant Martes (Stach, 1959). Measurements of M. wenzensis fall within the size range of extant and fossil samples of Pekania (Table 1), but it clearly lacks the external median rootlet of the P4 in that taxon. Ages of both species, about 7.1–5.3 Ma for M. ginsburgi and 4.0–3.3 Ma for M. wenzensis, are consistent with molecular divergence estimates of the Martes/Gulo clade from other gulonines (Koepfli et al., 2008; Sato et al., 2012; Li et al., 2014); comparable in age to G. sudorus and slightly younger than the earliest records of Pekania.
There are a number of distinct evolutionary trends that can be recognized by examination of the teeth of gulonines. Compared to other extant gulonine mustelids, Gulo has strongly reduced anterior premolars (Table 1, Fig. 4). Both Bryant (1987) and Jung et al. (2016) have suggested there is a general evolutionary trend in loss/reduction/rotation of the anterior premolars and enlargement of the carnassials in Gulo, which is a consequence of the hypercarnivorous diet of wolverines. Since the canines and carnassials are the primary teeth used to kill and process prey, and reduction in snout length increases the bite force of those teeth, it is not surprising that the anterior premolars of Gulo are frequently missing or rotated (Jung et al., 2016). The P2 of G. sudorus is larger than all previously described members of the genus (Table 1). In terms of relative size (P2L/P4L), G. sudorus has anterior premolars intermediate in size between the means of G. gulo and related taxa like Pekania and Eira. The premolar morphology of G. sudorus suggests that the evolutionary trend of premolar reduction in the genus Gulo had already begun in the Early Pliocene.
In addition to reduction of the premolars, Gulo has relatively smaller upper molars than other gulonine mustelids (P4L/M1Lint) (Table 1, Fig. 4; Tseng et al., 2009). The P4 length is well over twice as long as the maximum length of the M1 (inner lobe) in all Gulo studied; the only other taxa that approach similar proportions are some specimens of Eira and the tayra-like Sminthosinis bowleri from the Pliocene (early Blancan) of Idaho (Bjork, 1970). Similarly, the ratio of P4 length to the transverse width of the M1 was greater than 1.5 in nearly all Gulo studied, whereas it was less than 1.25 in all other taxa. As in the premolars, the relative size of the M1 and m1 talonid in Gulo likely reflect adaptations for hypercarnivory; reduction of the post-carnassial dentition and shortening the rostrum increase mechanical advantage when biting with both the canines and carnassials.
Plesiogulo and convergent evolution of wolverine-like mustelids
Plesiogulo, known from many mid to late Miocene and Pliocene sites in North America, Eurasia, and Africa, has long been discussed as a potential relative of Gulo (Zdansky, 1924; Viret, 1939; Webb, 1969; Kurtén, 1970; Hendey, 1978; Harrison, 1981; Xu & Wei, 1987; Alcalá, Montoya & Morales, 1994; Pasitschniak-Arts & Larivière, 1995; Montoya, Morales & Abella, 2011). Careful consideration of the morphology of extant gulonine mustelids (Fig. 4) and recently discovered fossil material suggests that similarity between Gulo and Plesiogulo is likely a result of convergence on a similar niche, rather than the result of a close relationship. Eira, Pekania, Martes, and Gulo all have a distinct P4 parastyle (though it is variably well-developed), but only a distinct anterior cingulum or occasionally a weak parastyle are seen in Plesiogulo (contrary to indication that the parastyle is absent; Harrison, 1981). The inner lobe of the M1 is expanded posterolingually in Plesiogulo to form a broad talon, but not in Gulo. Similarly, the m1 talonid is proportionately much longer and wider in Plesiogulo than Gulo, and has a shallow basin Stehlin, 1931; Teilhard de Chardin & Leroy, 1945; Kurtén, 1970). A number of evolutionary reversals (P4 parastyle reappearance, M1 reduction, M1 talon reduction, m1 talonid reduction) would be required for Gulo to have been derived from something like Plesiogulo, as has been previously noted (Zdansky, 1924; Webb, 1969; Harrison, 1981; Xu & Wei, 1987). On the other hand, few changes, other than increased size and robustness of the carnassial and premolars, are necessary for derivation of Gulo from something similar to early species of Pekania (P. occulta, P. palaeosinensis).
Rather than showing similarity to gulonine mustelids, Plesiogulo is most similar to the ischyrictine Iberictis, which is known from the early Miocene (MN4) of Spain and France (Ginsburg & Morales, 1992). Both Plesiogulo and Iberictis display robust anterior premolars with strong cingula, a P4 with a large protocone and relatively broad metastyle, large M1 with a posterolingually expanded inner lobe, and relatively broad and elongate m1 talonid. In contrast, Gulo and Pekania have a relatively narrow P4 metastyle and M1 with inner lobe only slightly longer than the outer lobe, and Gulo also has a distinctly reduced M1 and m1 talonid. In Iberictis and most species of Plesiogulo, the m1 has a distinct metaconid that is approximately equal in height to the paraconid, while the m1 of Gulo has no distinct metaconid. As in Gulo, the m1 metaconid is absent in Plesiogulo praecocidens from the late Miocene of Asia and variably absent in the Pliocene P. monspessulanus (Viret, 1939; Kurtén, 1970; Bonifay, 1971; Hendey, 1978; Alcalá, Montoya & Morales, 1994; Koufos, 2000). Additionally, P. monspessulanus has a single-rooted p2, while the p2 is double-rooted in Gulo, further suggesting the two are not closely related (Hendey, 1978).
Another feature distinguishing Gulo from Plesiogulo are the proportions of the teeth (Table 1, Fig. 4). Plesiogulo and Gulo both have robust premolars, wide relative to their length, but the anterior premolars are smaller in proportion to the carnassial (P2L/P4L) in all samples of Gulo. Similarly, the relative size of the M1 (P4L/M1Lint) in all samples of Gulo is relatively smaller than those of described and examined specimens of Plesiogulo. Additionally, no reported specimens of Plesiogulo display the premolar rotation commonly seen in Gulo (Jung et al., 2016).
Many of the features that are similar between Gulo and Plesiogulo are ones that are functionally important and observed in other carnivorans of similar size and ecology. The extant honey badger Mellivora, extinct Eomellivora (Mellivorinae), and Megalictis (Oligobuninae) all display some strong similarity to Gulo and Plesiogulo, despite having distinct evolutionary histories (Zdansky, 1924; Kurtén, 1970; Hendey, 1978; Harrison, 1981; Valenciano et al., 2016; Valenciano et al., 2017). Superficial similarity of the teeth (robust posterior premolars and carnassials, trenchant talonids, m1 metaconid loss), skull (short rostrum, broad and robust zygomatic arches, prominent sagittal crest), and jaw (deep mandibular corpus) are seen in each of these clades. Additionally, the enamel microstructures of the posterior premolars and molars of Gulo, Plesiogulo, Mellivora, and Eomellivora all display zigzag Hunter-Schreger Bands (Stefen, 2001; Tseng, Wang & Stewart, 2009). Independent evolution of similar craniodental morphology and enamel microstructures in these taxa is likely attributable to relatively large body size and similar lifestyles, specifically a hypercarnivorous or durophagous diet (Van Valkenburgh, 1989; Stefen, 1997; Stefen, 1999).
Further support for the hypothesized convergence of Gulo and Plesiogulo, rather than close relationship, comes from a variety of molecular studies. The earliest occurrences of Plesiogulo are in the middle Miocene (MN 6, about 15.2–12.5 Ma; Schmidt-Kittler, 1976). That age predates some molecular estimates for the divergence of the Guloninae from other mustelids, including the approximately 11.0 Ma estimate of Koepfli et al. (2008), but not the 12.65 Ma estimate of Sato et al. (2012). However, those early occurrences of Plesiogulo precede the molecular estimates of divergence of the Gulo-Martes clade from other gulonines by nearly five million years. In contrast, the ages of recently described fossil specimens of Pekania (Wang, Tseng & Takeuchi, 2012; Samuels & Cavin, 2013), and the new Gulo specimen described here, agree broadly with molecular divergence estimates of those extant genera (Koepfli et al., 2008; Hughes, 2012; Sato et al., 2012; Li et al., 2014; Malyarchuk, Derenko & Denisova, 2015). In addition, the ages of Gulo sudorus and G. minor are similar to the latest records of Plesiogulo in North America and Asia. Given the timing of these finds, it is possible that Plesiogulo was ecologically replaced by Gulo in the Pliocene. It is important to note that the hypothesized convergence described here is supported by morphological and geochronologic evidence, but is not based on analysis of taxa within a cladistic framework, and is thus speculative.
‘Cold-Adaptation’ in Gulo
Presence of Gulo, which is typically considered an inhabitant of boreal habitats (Kvam, Overskaug & Sorensen, 1988; Pasitschniak-Arts & Larivière, 1995), at a site with many vertebrate taxa characteristic of warm and/or humid forested habitats (Alligator, Heloderma, Pristinailurus, and Arctomeles) and a subtropical forest flora is a unique combination among North American biotas. An interesting analog is known from the middle Pliocene age (MN16a) Udunga fauna of the Transbaikal area of Russia (Sotnikova & Kalmykov, 1991; Erbajeva, Alexeeva & Khenzykhenova, 2003; Sotnikova, 2006; Erbajeva & Alexeeva, 2013). The Udunga fauna also has forest-adapted taxa like Parailurus, Parameles, and Arctomeles occurring along with Gulo (Sotnikova, 2006; Ogino et al., 2008; Erbajeva & Alexeeva, 2013). Another site with Gulo outside its current range is the latest Blancan and Irvingtonian (early Pleistocene) age Vallecito Creek Local Fauna of extreme southern California (Cassiliano, 1999). Like Gray, the Vallecito Creek fauna includes Tapirus and the procyonid, Nasua, which has a current and prehistoric range restricted to the southwestern United States, through Mexico and Central America, and into portions of South America. Occurrences and associated faunas of Gray, Udunga, and Vallecito Creek suggest the boreal distribution of extant Gulo gulo may not represent the potential range of habitats occupied by wolverines, at least in the past. Adaptation to cold, boreal habitats within wolverines may have occurred later, in the late Pliocene or Pleistocene, as global climates became substantially colder and those habitats spread across northern Eurasia and North America.
Historical biogeography
These finds also have implications for understanding of historical biogeography and evolution of fishers and wolverines. Koepfli et al. (2008) and Zigouris et al. (2013) both estimate Eurasia as the ancestral area for the origin of these taxa, while Sato et al. (2012) restricted the area of origin to Asia. The oldest known probable gulonine is Ischyrictis zibethoides from the Early/Middle Miocene (MN 5-8) of Europe. Early records of Pekania are known from the late Miocene of North America (early Hemphillian) and Asia (Baodean) (Wang, Tseng & Takeuchi, 2012; Samuels & Cavin, 2013). Similarly, early records of Gulo are known from the earliest Pliocene (early Blancan) of North America and middle Pliocene (Yushean) of Asia (Sotnikova, 1982; Sotnikova, 1995). While there are many species of “Martes” described from the Early and Middle Miocene of Eurasia and North America, the most confidently placed early members of Martes are from the Pliocene (MN 15) of Europe (Stach, 1959; Anderson, 1994; Sato et al., 2003). Earliest members of the tayra clade appear later than the other clades, with Eirictis pachygnatha from the Early Pliocene (Yushean) of Asia (Teilhard de Chardin & Leroy, 1945; Qiu, Deng & Wang, 2004; Wang, Tseng & Takeuchi, 2012) and Sminthosinis bowleri from the middle Pliocene (Blancan) of North America.
Early members of multiple clades (fisher, wolverine-marten, and tayra) show similar distributions in the late Miocene and Pliocene, occurring in both Asia and North America. This suggests connection of these regions via the Bering Land Bridge at the time, which facilitated dispersal of carnivorans between Asia and North America (Qiu, 2003). Consequently, a hypothesis for intercontinental dispersals of the wolverine clade is: (1) origin of gulonines in Eurasia, (2) dispersal of Pekania from Asia to North America in the late Miocene, (3) divergence of Gulo from an ancestor similar to P. occulta in the earliest Pliocene of North America, (4) dispersal of Gulo to Asia in the Pliocene, following the extinction of Plesiogulo, and (5) dispersal of Gulo to Europe in the Pleistocene.
Conclusions
The new material of Gulo described here demonstrates the presence of wolverines in the Early Pliocene of North America, more than 1 million years earlier than other known records of the genus. Differences from the extant species and previously described extinct wolverines indicate the presence of a distinct new species, G. sudorus. Similarity of the new species to both early fishers (Pekania) and later Gulo species suggests that this may represent an intermediate form, which evolved in North America and later dispersed to Eurasia. Presence of Gulo in the Pliocene of Tennessee, at a site with a variety of floral and faunal elements indicative of warm/humid climates, suggests that the ‘cold-adapted’ nature of extant G. gulo may be a relatively recent phenomenon. Comparisons of Gulo and other members of the Guloninae to Plesiogulo suggest that the latter is not closely related to gulonines, but instead likely represents convergence on a similar niche to that occupied by wolverines. The similar timing of the last records of Plesiogulo with the appearance of Gulo in North America and Asia suggest the former may have been ecologically replaced by the latter in the Early Pliocene.
Supplemental Information
Gulo gulo (ETVP 291) photographed in two different orientations
(A) Palate parallel to the photographic plane. (B) Alveolar margin of P4 parallel to the photographic plane. Note that in A the lingual cingulum along the P4 metastyle is obscured, but it is visible in B. Scale bar equals 1 cm. Photographs by Joshua Samuels.
Occurrences of Gulo in the fossil records of North America and Eurasia
Data were derived from a wide range of literature sources, as well as occurrences listed in the MIOMAP/FAUNMAP Databases (Carrasco et al., 2007; Graham & Lundeliu Jr, 2010; http://www.ucmp.berkeley.edu/neomap/) and NOW Database (Fortelius, 2013; http://pantodon.science.helsinki.fi/now/).
Dental measurements (in mm) of gulonines and some other mustelid species used in comparative analyses
Data were derived from measurements of specimens and consultation of cited literature sources.