Exploring differential gene expression and biomarker potential in systemic lupus erythematosus: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Bioinformatics, Genomics, Immunology, Rheumatology, Statistics
- Keywords
- Systemic lupus erythematosus, Bioinformatics analysis, Disease biomarker, FCER1A, RGS1
- Copyright
- © 2025 Xiao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Exploring differential gene expression and biomarker potential in systemic lupus erythematosus: a retrospective study. PeerJ 13:e19891 https://doi.org/10.7717/peerj.19891
Abstract
Background
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by inflammation and immune-mediated multi-organ system damage, accompanied by clinical manifestations such as fever, hair loss, skin rash, oral ulcers, and joint pain and swelling. SLE has been reported to affect more than 3.4 million people worldwide, of which approximately 90% are women.
Purpose
This study aims to identify and characterize key hub genes implicated in SLE through comprehensive bioinformatics analyses, providing a theoretical foundation for the development of more effective therapeutic strategies.
Methods
Two datasets were procured from the Gene Expression Omnibus (GEO) database: GSE13887 and GSE10325. Differentially expressed genes (DEGs) were identified and subjected to functional enrichment analysis, protein-protein interaction (PPI) network construction, and receiver operating characteristic (ROC) curve analysis to evaluate potential hub genes. The top 20 significantly upregulated and downregulated DEGs, alongside the top 15 enriched Gene Ontology (GO) terms and five Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways, were screened from both datasets. Quantitative real-time PCR (RT-q PCR) was utilized to validate hub gene expression in CD3 + T cells from peripheral blood samples of SLE patients. Concurrently, flow cytometry was employed to quantify inflammatory cytokines in peripheral blood samples.
Results
Bioinformatics analyses identified 1,912 DEGs in GSE13887 and 52 DEGs in GSE10325, with eight DEGs common to both datasets. Functional enrichment analysis underscored critical biological processes, notably cell-mediated cytotoxicity and cell killing. PPI network and enrichment analyses highlighted seven hub genes, among which FCER1A and RGS1 demonstrated consistent expression trends across datasets and clinical samples—FCER1A was significantly downregulated, while RGS1 was upregulated in SLE patients. ROC curve analysis confirmed their strong diagnostic potential (AUC > 0.7). Principal component analysis (PCA) further highlighted distinct gene expression profiles differentiating SLE patients from healthy controls. Clinical validation via RT-q PCR and flow cytometry corroborated these findings, demonstrating decreased FCER1A expression and increased RGS1 expression in CD3 + T cells from SLE patients. Moreover, elevated plasma levels of IL-6 and TNF-α, coupled with diminished IL-10 levels, were observed in SLE patients. These findings suggest that FCER1A and RGS1 are promising biomarkers for SLE diagnosis.
Conclusions
FCER1A and RGS1 are significantly associated with SLE and serve as potential biomarkers for distinguishing SLE patients from healthy individuals. Their involvement in SLE pathogenesis underscores their potential as targets for future diagnostic and therapeutic interventions.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disorder characterized by multisystemic involvement, affecting various organs such as the skin, kidneys, joints, and central nervous system (Hoi et al., 2024). It impacts approximately 3.4 million individuals globally (Tian et al., 2023) and predominantly affects women of childbearing age, with a female-to-male ratio of approximately 10:1 (Fanouriakis et al., 2021). In recent decades, the incidence of SLE has shown a continuous upward trend (Barber et al., 2021; Gergianaki et al., 2017). The global prevalence of SLE varies across regions, ranging from 20 to 150 cases per 100,000 people. The disease burden is notably higher among certain racial and ethnic groups, including Black, Asian, and Hispanic populations (Barber et al., 2021; Siegel & Sammaritano, 2024).
The pathogenesis of SLE is intricate, involving a multifactorial interplay of genetic, epigenetic, environmental, and hormonal factors (Tsokos, 2024; Tsokos, 2011). Its pathogenesis is involved in the alteration of whole immune system. Dysregulated T cell responses are pivotal in the immunopathology of SLE, with CD4+ T helper (Th) cells, particularly Th1, Th2, Th17, and regulatory T cells (Tregs), playing crucial roles in disease progression (Sharabi & Tsokos, 2020). An imbalance in Th1/Th2 cytokine profiles and the heightened presence of proinflammatory cytokines, such as interleukin 6 (IL-6), interleukin 17 (IL-17), and interferon-α (IFN-α), are strongly associated with disease activity and organ damage (Li et al., 2022). Moreover, impaired Treg function exacerbates immune dysregulation and fosters the generation of pathogenic autoantibodies (Brusko, Putnam & Bluestone, 2008). B cell hyperactivity and the subsequent overproduction of autoantibodies, such as anti-dsDNA and anti-Smith (Sm) antibodies, are defining features of SLE (Pisetsky, Bossuyt & Meroni, 2019; Karrar & Cunninghame Graham, 2018). These autoantibodies form immune complexes that are deposited in various tissues, instigating complement activation, inflammation, and tissue damage (Tsokos et al., 2016). Furthermore, neutrophil extracellular traps (NETs) have been implicated in the pathogenesis of SLE by exacerbating self-antigen exposure and perpetuating type I interferon expression (Papayannopoulos, 2018).
Despite significant advances in the understanding of disease mechanisms, the early diagnosis and management of SLE remain challenging due to its heterogeneous clinical manifestations and unpredictable progression (Dörner & Furie, 2019; Durcan, O’Dwyer & Petri, 2019). Current therapeutic approaches primarily target immunosuppression and symptom alleviation; however, these strategies are frequently accompanied by substantial side effects and variable therapeutic efficacy (Fanouriakis et al., 2019). The diagnosis of SLE is predominantly based on clinical presentation and laboratory testing. The American College of Rheumatology (ACR) and the Systemic Lupus International Collaborating Clinics (SLICC) have established classification criteria, which incorporate both clinical manifestations (e.g., cutaneous rashes, joint involvement, renal dysfunction) and serological markers, including the presence of antinuclear antibodies (ANA), anti-dsDNA antibodies, Sm antibodies, and reduced complement levels (Styrkarsdottir et al., 2021). Additional diagnostic modalities include urinalysis to assess renal involvement and skin biopsies in cases of cutaneous lupus.
However, these diagnostic methods are not without their limitations. First, the clinical manifestations of SLE are highly heterogeneous and may overlap with those of other autoimmune diseases, rendering early diagnosis challenging (Dörner & Furie, 2019). For instance, symptoms such as fatigue, arthralgia, and rashes are not exclusive to SLE and may occur in a variety of other conditions, leading to delayed identification and intervention. Secondly, the presence of autoantibodies such as ANA lacks specificity for SLE, as these antibodies may also be found in other autoimmune disorders or even in healthy individuals (Durcan, O’Dwyer & Petri, 2019). Furthermore, some patients with SLE may test negative for certain autoantibodies, complicating the diagnostic process. Lastly, these diagnostic methods often fail to adequately capture disease activity or predict organ damage, thus limiting their effectiveness in monitoring disease progression and guiding therapeutic strategies (Fanouriakis et al., 2019). Traditional diagnostic approaches are time-intensive and cumbersome, hindering their utility in the timely diagnosis, treatment, and prognosis of patients.
Genetic testing has increasingly become a pivotal tool in the diagnosis and treatment of SLE, significantly enhancing both the accuracy and efficiency of diagnosis and therapeutic strategies. By identifying genetic biomarkers linked to SLE, these tests facilitate earlier and more precise diagnoses, thereby enabling timely interventions and optimizing patient management. Consequently, genetic screening is now an essential component of clinical practice, not only refining diagnostic precision but also paving the way for more personalized treatment approaches. Through the identification of disease-associated genes via genetic testing, coupled with the validation of corresponding biomarkers through techniques such as flow cytometry, SLE can be diagnosed and managed more swiftly and effectively, allowing for a more targeted approach to treatment. This, in turn, improves both short-term clinical outcomes and long-term disease control (Ghodke-Puranik, Olferiev & Crow, 2024; Vasquez-Canizares, Wahezi & Putterman, 2017; Lee et al., 2022; Horisberger et al., 2022; Zhu et al., 2023).
The purpose of this study is to explore genes, biomarkers and molecular processes related to SLE in combination with comprehensive bioinformatics analysis, and verify them through clinical data to better diagnose SLE.
Materials and Methods
Research participants and sample collection
This study is a retrospective study. A total of 45 SLE patients and 40 healthy controls (HCs) were collected from Nanfang Hospital of Southern Medical University from January 2024 to December 2024. All SLE patients met the 2012 ACR SLE disease classification criteria (Petri et al., 2012; Tan et al., 1982). All the SLE patients with concurrent infection, acute concurrent illness, and use of probiotics or antibiotics within 1 month before admission were excluded. The collected HCs also needed to have no history of known autoimmune diseases and be of comparable age to SLE patients. All the participants were collected two mL EDTA-K2 blood sample.
Ethics approval was granted by the Ethics Committee of Nanfang Hospital, under the surveillance of ethical number: NFEC-2025-026. All the methods used were in accordance with the approved guidelines. Written informed consent was required from all patients and healthy volunteers in the study.
The SLE patients and HCs in this study were comparable in age and gender (Table 1). Average age of the SLE and HC groups was 38.09 ± 11.97 and 42.20 ± 10.34, respectively (p = 0.099).
| General information | SLE (n = 45) | HC (n = 40) | p value | |
|---|---|---|---|---|
| gender | Female | 42 (93.33%) | 39 (97.5%) | 0.368 |
| Male | 3 (6.67%) | 1 (2.5%) | ||
| Age, years | 38.09 (15–74) | 42.20 (24–67) | 0.099 | |
| WBC, 109/L | 6.72 (1.96–21.97) | 6.00 (3.65–9.54) | 0.695 | |
| LYM,109/L | 1.74 (0.07–3.92) | 2.07 (1.38–3.04) | 0.0082 | |
| NEU, 109L | 4.36 (1.12–17.84) | 4.63 (1.72–52.80) | 0.269 | |
| RBC, 1012/L | 4.29 (2.46–5.74) | 4.62 (3.64–5.18) | 0.0032 | |
| HGB, g/L | 124.71 (78–163) | 134.80 (99–165) | 0.0032 | |
| HCT, L/L | 0.38 (0.25–0.48) | 0.41 (0.33–0.49) | 0.0022 | |
| PLT, 109/L | 225.09 (9–377) | 260.78 (144–387) | 0.051 | |
| SLEDAI | 9.44 (1–24) | —— | —— | |
Datasets
Two raw datasets, GSE13887 and GSE10325, were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). GSE13887 was an analysis of CD3-positive T cells isolated from 10 SLE patients and nine HCs. GSE10325 was an analysis of freshly isolated lymphocytes (CD4+ T cells and CD19+ B cells) and CD33+ myeloid subsets from the blood of 28 HCs and 39 SLE patients.
Identification of differentially expressed genes
Differentially expressed genes (DEGs) between SLE and HCs were identified using the limma package (version 3.64.1) in R (version 4.2.1). The GSE13887 and GSE10325 datasets were first converted into expression matrices, appropriately grouped, and normalized. Normalization and differential expression analysis were performed using the limma package. Genes with an adjusted p-value (false discovery rate, FDR) < 0.05 and an absolute log2 fold change (|log2FC|) ≥ 0.5 were considered statistically significant. DEGs were classified as upregulated or downregulated based on whether their log2FC values were above 0.5 or below −0.5, respectively. An online Venn diagram tool was employed to identify overlapping DEGs between SLE and HC. The results were visualized using ggplot2 (version 3.4.4) for volcano plots (with thresholds set at p ≤ 0.05 and |log2FC| ≥ 1) and box plots to assess normalization across datasets. Heatmaps were generated using the ComplexHeatmap package (version 2.13.1), while unique and shared DEGs were further illustrated using the VennDiagram package (version 1.7.3) (Gu, Eils & Schlesner, 2016).
Gene Ontology term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis
The R language packages clusterProfiler (version 4.4.4) and ggplot2 were used to perform Gene Ontology (GO) term and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis on common DEGs (co-DEGs) and visualize the results, with a threshold of p value < 0.05, and bar charts, bubble charts, and chord plots were drawn (Yu et al. , 2012).
Construction of protein-protein interaction network and identification of hub genes
The PPI network of DEGs that may play an important role in the progression of SLE was constructed using the STRING online database (https://www.string-db.org/). The protein–protein interaction (PPI) network was performed using Cytoscape (version 3.10.2) (http://www.cytoscape.org/) to better visualize the interaction information. Hub genes with connectivity ≥10 were selected using the CytoHubba (version 0.1).
Hub gene validation
Receiver operating characteristic (ROC) analysis was performed on the data using the R language package pROC (version 1.18.0). The results were visualized using ggplot2 to plot the ROC curve of the hub gene. The area under the curve (AUC) of the ROC curve corresponding to the hub gene was used to evaluate the ability to distinguish between SLE and HC. The expression profiles of the hub genes in the two data sets were used as variables for principal component analysis (PCA) to obtain PC1 and PC2, and ggplot2 was used to visualize the results.
CD3+ T cell isolation from SLE and HC periphal blood samples
Human primary PBMC were isolated from periphral blood with EDTA-K2 by density gradient centrifugation using Ficoll. 1 X 106 CD3+ T cells were enriched by positive selection with an CD3+ T cells isolation kit (130-097-043, Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of T cell were analyzed by Flow cytometry (CantoII, BD Bioscience, Franklin Lakes, NJ, USA) staining with anti-CD3 -PE antibody (562310, clone: HIT3A, BD Bioscience, Franklin Lakes, NJ, USA).
Real-time quantitative polymerase chain reaction
The sorted CD3+ T cells were lyzed with TRIzol reagent (Cat. #G3013 Servicebio, Wuhan, China). After chloroform substitute (Cat. #G3014 Servicebio, Wuhan, China) isolation, RNA were percipitated by isopropanol by centrifugation at 4 °C, 15,000 g, 15 min. After that, RNA were rinsed with 2 times 75% ethanol. The cDNA were synthesized with SweScript RT I First Strand cDNA Synthesis Kit (Cat. # G331-1, Servicebio, Wuhan, China) by Random Hexamer Primer (Cat. #G331-4, Servicebio, Wuhan, China). The RNA template were added one µg.
The qPCR were conducted by using Servicebio® 2 ×SYBR Green qPCR Master Mix (Cat. #G3326-1, Servicebio, Wuhan, China). The reation volume were used 20 µL in each wells, cDNA templated were diluted in 10 times with DEPC water and 2 µL, forward and reverse primers were added 0.4 µL. The qPCR were performed with Roche Cobas 4800 PCR machine (Roche, Basel, Switzerland). This experiment used a two-step reaction procedure: pre-denaturation: 95 °C, 2–5 min; cyclic amplification (35–45 cycles): 95 °C denaturation 5–15 s; 60–68 °C annealing/extension 20–60 s (simultaneous fluorescence collection, SYBR Green collection at the end point, TaqMan collection in real time). Melting curve (SYBR Green): gradually increase the temperature from 65 °C to 95 °C and monitor the fluorescence. Relative gene expression was calculated using the 2−ΔΔCt method, and the results were normalized using GAPDH. Primer sequences are shown in Table S1.
Flow cytometry
The FCER1A and RGS1 protein expression levels of CD3+ T cells in plasma collected from HCs and SLE patients and the levels of inflammatory factors in plasma were detected by flow cytometry. Samples were analyzed using a BD FACS Fortassa flow cytometer (BD Biosciences, USA). Data acquisition was performed using BD FACSDiva software, and at least 10,000 events were recorded for each sample. Polyclonal rabbit anti-human RGS1 antibody (Cat# LS-C162570-400; LSBio, Seattle, WA, USA) and FITC-conjugated goat-anti-rabbit secondary antibodies were used for RGS1 analysis (Jiang et al., 2024). Polyclonal rabbit anti-human RGS1 antibody (Cat# LS-C162570-400; LSBio, Seattle, WA, USA) and FITC-conjugated goat-anti-rabbit secondary antibodies were used for RGS1 analysis. A rabbit polyclonal FCER1 alpha Monoclonal Antibody (Cat# 16-5899-82, Functional Grade, eBioscience, San Diego, CA, USA) was used for FCER1A analysis (Greer et al., 2014).
Statistics
All data processing and analysis were performed in GraphPad Prism 9.0. To compare two groups of variables, the Mann–Whitney U test was applied. Fisher’s exact test was performed to analyze the statistical significance between two variable data sets. P < 0.05 was considered statistically significant. Statistical significance is reported as follows: p < 0.05(*), p < 0.01(**), p < 0.001(***), p < 0.0001(****), ns(not significant).
Results
Identification of DEGs
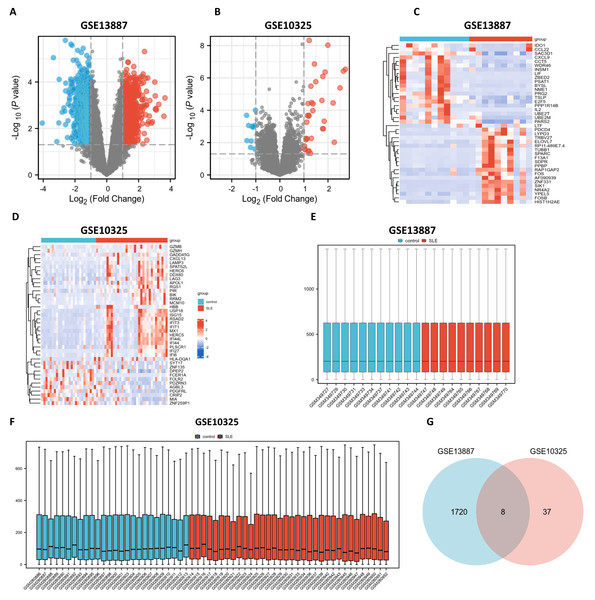
Utilizing bioinformatics analysis through R software (version 4.2.1), a total of 1,912 DEGs were identified in the GSE13887 dataset, while 52 DEGs were detected in the GSE10325 dataset. Subsequent hierarchical clustering analysis was performed on these DEGs, prioritizing the top 20 upregulated and downregulated genes from GSE13887 (Table S3) and GSE10325 (Table S4), respectively. Volcano plots and heatmaps were generated to visualize the clustering results for GSE13887 (Figs. 1A, 1C) and GSE10325 (Figs. 1B, 1D). The heatmaps distinctly illustrated a high degree of consistency in sample clustering.
Figure 1: Volcano plots, heat maps, box plots, and a Venn diagram of GSE13887 and GSE10325 datasets.
(A) Volcano plot of GSE13887 dataset. The x-axis represents log2 (Fold Change), and the y-axis represents −log10 (P value). Red dots represent upregulated genes, and blue dots represent downregulated genes. (B) Volcano plot of GSE10325 dataset. (C) Heat map of the top 20 upregulated and downregulated EP-DEGs in GSE13887 dataset. Each row represents a gene, and each column represents a sample. Red represents high expression levels, and blue represents low expression levels. (D) Heat map of GSE10325 dataset. (E) Box plot of GSE13887 dataset, with no significant difference in median and upper and lower quartiles. (F) Box plot of GSE10325 dataset. (G) Venn diagram of common DEGs in GSE13887 and GSE10325 datasets.Following data normalization and comparative evaluation across datasets, box plots were constructed to confirm the normalization quality for GSE13887 (Fig. 1E) and GSE10325 (Fig. 1F). The results demonstrated that the data distribution across both datasets conformed to established quality standards, affirming the robustness and cross-comparability of the microarray data. A Venn diagram (Fig. 1G) was subsequently generated to illustrate the overlap of DEGs between the two datasets, identifying eight shared DEGs.
Enrichment analysis of DEGs
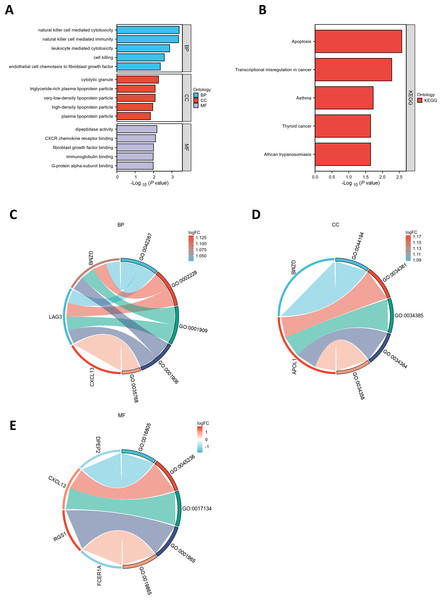
GO and KEGG pathway enrichment analyses were conducted to elucidate the functional roles of the eight common differentially expressed genes (co-DEGs). GO terms were categorized into biological process (BP), cellular component (CC), and molecular function (MF), with the top five most significantly enriched terms in each category selected based on the lowest p-values (Table S5). These results were visualized via bar charts (Fig. 2A) and bubble plots (Fig. S1A). The co-DEGs were predominantly associated with processes such as cell killing, leukocyte-mediated cytotoxicity, natural killer cell-mediated immunity, and natural killer cell-mediated cytotoxicity.
Figure 2: Functional enrichment analysis of DEGs.
(A) Bar plot of GO enrichment analysis results for DEGs. The x-axis represents GO terms, while the y-axis displays −log10 (p-value) for each term. (B) Bar plot of KEGG pathway enrichment analysis results. (C-E) Chord diagrams depicting EP-DEGs enriched in the top five GO categories of biological processes (BP), cellular components (CC), and molecular functions (MF). Red indicates upregulation, blue denotes downregulation, and color intensity corresponds to the magnitude of expression changes.Subsequently, the five most significantly enriched KEGG pathways were identified (Table S6) and represented through bar plots (Fig. 2B) and bubble charts (Fig. S1B). The co-DEGs were chiefly involved in pathways related to transcriptional dysregulation in cancer, apoptosis, thyroid cancer, African trypanosomiasis, and asthma.
PPI network and hub genes
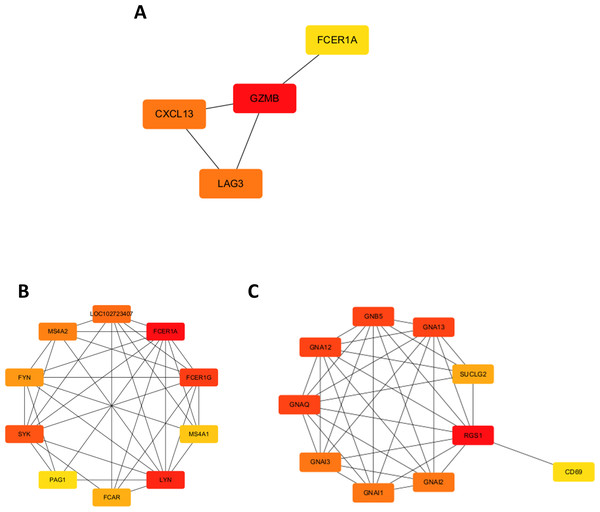
A chord diagram was generated to illustrate the enrichment of genes within the top five GO categories across BP, CC, and MF, identifying seven key genes: GZMB, LAG3, APOL1, CXCL13, RGS1, FCER1A, and DPEP2. Notably, RGS1, CXCL13, and GZMB were enriched in at least two GO categories (Figs. 2C–2E). The DEGs were uploaded to the STRING database to construct the PPI network (Fig. S2). Using the MCODE plugin, a tightly interconnected protein cluster consisting of CXCL13, GZMB, LAG3, and FCER1A was identified (Fig. 3A).
Figure 3: Identification of hub genes in the PPI network and functional enrichment analysis of FCER1A and RGS1.
(A) Identification of four hub genes (CXCL13, GZMB, LAG3, FCER1A) using CytoHubba in Cytoscape. (B) Top ten hub genes identified in the FCER1A PPI network using CytoHubba in Cytoscape. (C) Top ten hub genes identified in the RGS1 PPI network using CytoHubba in Cytoscape.Overall, seven hub genes—GZMB, LAG3, APOL1, CXCL13, RGS1, FCER1A, and DPEP2—were selected based on both the PPI network and chord diagram analyses. Volcano plot assessments of their expression levels in the GSE13887 and GSE10325 datasets revealed that only FCER1A and RGS1 exhibited consistent expression trends across both datasets, with FCER1A being downregulated and RGS1 upregulated (Fig. S3). These two genes were further analyzed using the STRING database to construct an additional PPI network (Fig. S4), visualized in Cytoscape. MCODE analysis identified a highly interconnected protein cluster comprising ten genes (Figs. 3B, 3C).
Prognostic value of hub genes
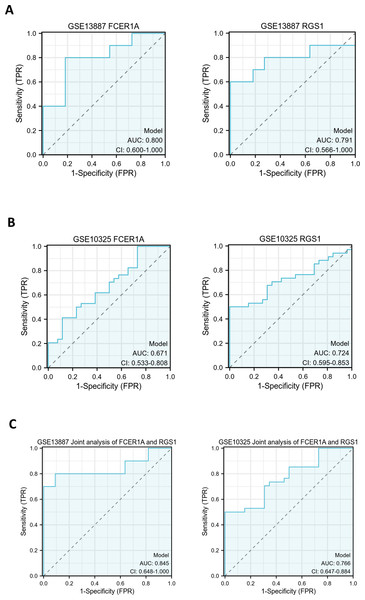
ROC curves were generated to evaluate the diagnostic efficacy of FCER1A and RGS1, both individually and in combination, using their expression levels in the GSE13887 and GSE10325 datasets (Fig. 4). Both genes demonstrated strong diagnostic potential, with area under the curve (AUC) values exceeding 0.7. The combined analysis of these two genes exhibited superior diagnostic performance.
Figure 4: Hub gene ROC curve.
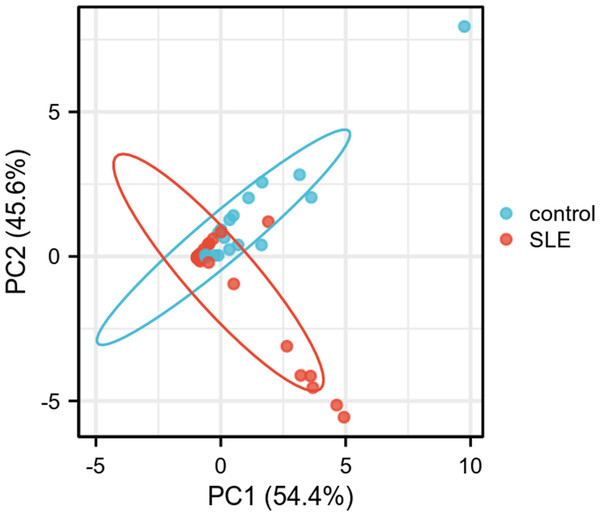
(A) ROC curve analysis of FCER1A and RGS1 hub genes in the GSE13887 dataset. (B) ROC curve analysis of FCER1A and RGS1 hub genes in the GSE10325 dataset. (C) Joint ROC curve analysis of FCER1A and RGS1 genes in the GSE13887 and GSE10325 datasets.PCA was performed to further validate their discriminatory power. The PCA plot, derived from dimensionality reduction of gene expression data in both HCs and SLE patients, demonstrated clear separation between the two groups based on the expression profiles of FCER1A and RGS1 (Fig. 5). The first two principal components (PC1 and PC2) accounted for 100% of the explained variance. SLE samples clustered predominantly on the negative axis of PC1, while HCs were localized on the positive axis, indicating significant differences in gene expression patterns between the groups and highlighting the robust discriminatory capability of these two genes.
Figure 5: PCA plot of two genes.
PC1 and PC2 are the first and second principal components, respectively, explaining the different expressions of potential variants. The plots represent different samples, red for SLE group and blue for control group.Verification by other datasets and clinical samples
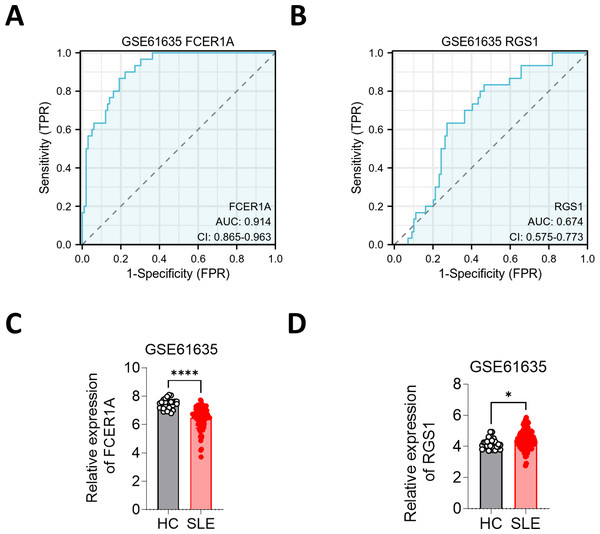
To further substantiate these findings, the expression of FCER1A and RGS1 was validated using the GSE61635 dataset, which corroborated their diagnostic efficacy. The expression trends observed in GSE61635 were consistent with those from GSE13887, GSE10325, and clinical samples, with FCER1A downregulated and RGS1 upregulated in SLE patients relative to HCs (Fig. 6).
Figure 6: Validation of FCER1A and RGS1 in other datasets.
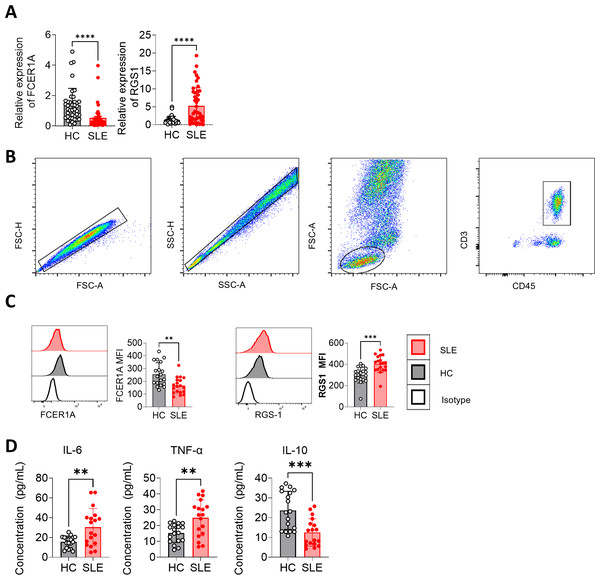
(A, B) ROC curve analysis of FCER1A and RGS1 in GSE61635 dataset (C,D) Comparison of the expression of FCER1A and RGS1 in SLE patients and HCs Comparison between the two groups was performed using Mann–Whitney U test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****), ns (not significant).Peripheral blood samples were collected, and CD3+ T cells were isolated using magnetic bead separation. RNA extraction followed by RT-qPCR revealed significant downregulation of FCER1A (p < 0.0001) and upregulation of RGS1 (p < 0.0001) in SLE patient samples compared to HCs (Fig. 7A). These findings were corroborated by flow cytometry, which demonstrated a marked reduction in FCER1A protein levels (p < 0.01) and a significant increase in RGS1 protein expression (p < 0.001) in the plasma of SLE patients (Figs. 7B, 7C).
Figure 7: Validation of FCER1A and RGS1 expression in CD3 + T cells and assessment of inflammatory cytokines in plasma.
(A) RT- q PCR analysis of FCER1A and RGS1 mRNA expression in CD3 + T cells from healthy controls and SLE patients. (B) Flow cytometry gating strategy. (C) Protein expression levels of FCER1A and RGS1 in CD3 + T cells from SLE patients and healthy controls, measured by flow cytometry. (D) Plasma levels of IL-6, TNF-α, and IL-10 in SLE patients and healthy controls. Statistical comparisons were performed using the Mann–Whitney U test: p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).Additionally, flow cytometric analysis of inflammatory cytokines revealed elevated plasma levels of IL-6 and TNF-α (p < 0.01), alongside a significant reduction in IL-10 (p < 0.001) in SLE patients compared to HCs (Fig. 7D). These results further support the potential of FCER1A and RGS1 as robust biomarkers for the diagnosis and pathophysiological understanding of SLE.
Discussion
SLE is a multifactorial autoimmune disorder characterized by widespread inflammation and immune-mediated damage across multiple organ systems, with a notably higher prevalence in women (Kaul et al., 2016). The pathogenic mechanisms underlying SLE are intricate and interdependent, encompassing T cell dysregulation, B cell hyperactivity, and an elevation in proinflammatory cytokines (Parodis, Gatto & Sjöwall, 2022; Wangriatisak et al., 2022; Yap & Lai, 2013). SLE manifests in a diverse array of clinical presentations and follows an unpredictable trajectory, complicating early diagnosis and therapeutic intervention (Dörner & Furie, 2019; Durcan, O’Dwyer & Petri, 2019). Hence, the identification of SLE-related biomarkers, genes, or signaling pathways is imperative for advancing diagnostic accuracy, therapeutic strategies, and prognostic evaluations in SLE.
Bioinformatics has emerged as a robust tool for predicting potential therapeutic targets and biomarkers in autoimmune diseases such as SLE. For instance, Zhao et al. (2021) demonstrated through a comprehensive bioinformatics analysis that targeting IFI27 holds promising therapeutic potential for SLE. Shen et al. (2022) identified a set of interferon-stimulated genes as potential diagnostic biomarkers for SLE. Leveraging transcriptomic data, Wu et al. (2024) established that the pathogenesis of COVID-19 shares certain similarities with that of SLE and screened small-molecule compounds, thereby suggesting potential molecular targets for the treatment of both COVID-19 and SLE in combination. Li et al. (2023) conducted an in-depth exploration of the molecular characteristics of NET-associated genes (NRGs) in SLE, identifying three prospective biomarkers (HMGB1, ITGB2, and CREB5), and categorizing three distinct clusters based on these key biomarkers.
In this study, we conducted an analysis of the GSE13887 and GSE10325 datasets associated with SLE using bioinformatics tools, and identified eight co-DEGs. This was followed by functional enrichment analysis. Among these, seven hub genes—FCER1A, RGS1, CXCL13, DPEP2, LAG3, APOL1, and GZMB—were extracted by constructing a PPI network, and subsequently validated in peripheral blood samples of SLE patients through RT-qPCR and flow cytometry. Upon evaluating the expression levels of genes across the two datasets, we identified two genes, FCER1A and RGS1, whose expression trends were consistent in both the datasets and clinical samples. FCER1A, a high-affinity IgE receptor, is significantly downregulated in SLE patients, suggesting potential impairment in antigen presentation and immune regulation (Andiappan et al., 2021; Leffler et al., 2019; He et al., 2020). Conversely, RGS1, a G protein signaling regulator, is notably upregulated, and its expression is associated with T cell migration and immune response modulation (Jiang et al., 2024; Bai et al., 2021). Functional enrichment analysis revealed that DEGs were significantly associated with biological processes such as “leukocyte-mediated cytotoxicity”, “natural killer cell-mediated immunity”, and “apoptosis”. KEGG pathway analysis highlighted the enrichment of pathways related to “cancer transcriptional dysregulation”, “apoptosis”, and the “thyroid cancer pathway”, underscoring the involvement of immune dysregulation and apoptosis in the pathogenesis of SLE. Previous studies have established that inflammation, the over activation of immune pathways, and compromised apoptotic mechanisms are central to the pathogenesis of SLE (Crow, 2023; Sutanto & Yuliasih, 2023; Mistry & Kaplan, 2017; Mahajan, Herrmann & Munoz , 2016). Our findings align with these reports, emphasizing the pivotal role of the hub genes identified within these pathological processes. Among the identified hub genes, FCER1A plays a critical role in immune regulation, participating in IgE-mediated signaling, while RGS1 modulates G protein-coupled receptor signaling pathways, influencing immune cell migration and function. Likewise, CXCL13 is known to regulate B cell trafficking and follicular helper T cell responses, both of which contribute to the pathogenesis of SLE (Hui et al., 2024; Schiffer, Worthmann & Haller, 2015). The serine protease GZMB has been implicated in cytotoxic T cell-mediated apoptosis, a crucial mechanism in immune homeostasis (Thompson & Cao, 2024). Additionally, DPEP2, LAG3, and APOL1 exhibit well-established immunoregulatory effects, though their precise contributions to SLE progression remain to be fully characterized. Simultaneously, we assessed the levels of inflammatory mediators IL-6, TNF-α, and IL-10 in the collected plasma samples. It was observed that IL-6 and TNF-α levels were elevated, while IL-10 levels were diminished. IL-6, a proinflammatory cytokine, plays a pivotal role in B-cell differentiation, T-cell activation, and the production of acute-phase proteins. The increased levels of IL-6 in SLE patients indicate its involvement in driving systemic inflammation, enhancing autoantibody production, and contributing to disease activity (Karampetsou et al., 2019; Talaat et al., 2015). TNF-α is a potent proinflammatory cytokine that plays a significant role in inflammation, tissue damage, and apoptosis in SLE. Elevated TNF-α levels are strongly associated with disease severity and organ damage (Richter et al., 2023; Ghorbaninezhad et al., 2022). IL-10, recognized for its anti-inflammatory properties, modulates excessive immune responses and ensures immune tolerance. Reduced IL-10 levels in SLE patients impair its protective function in suppressing inflammation and preventing immune dysregulation. A deficiency in IL-10 may foster the persistence of auto reactive B and T cells, thereby exacerbating autoimmunity (Moore et al., 2001; Biswas, Bieber & Manz, 2022).
Previous studies have made significant strides in identifying biomarkers for autoimmune diseases (ADs) in general. For instance, a review by Gibson et al. (2010) highlighted the challenges in diagnosing and predicting outcomes in autoimmune disorders, emphasizing the need for proteomic strategies to discover early biomarkers. The authors discussed the potential of proteomic platforms to reflect the complexity of autoimmune disease processes, suggesting that these approaches could lead to more accurate and timely diagnoses. Another study by Kruta et al. (2024) explored the application of machine learning for precision diagnostics of autoimmune diseases. The authors developed an integration pipeline to preprocess and integrate various types of health data, including clinical, laboratory, and multi-omics data, to improve the accuracy of machine learning models in classifying autoimmune diseases. Their results demonstrated that integrating multiple data types significantly enhanced the prediction accuracy, highlighting the potential of machine learning in personalized medicine for autoimmune conditions. A comprehensive review by Vivas, Boumediene & Tobón (2024) provided an overview of the latest advancements in predicting autoimmune diseases, including both traditional biomarkers and innovations in artificial intelligence. The authors discussed the potential of AI tools in predicting SLE, emphasizing the importance of early diagnosis and intervention. They also highlighted the need for further research to develop robust predictive models that can be applied in clinical settings. However, these studies summarized the advancements in biomarkers for ADs and outlined directions for future perspectives; in particular, these methods still require further investigation.
Despite these insights, our study still has some limitations. Although clinical samples provided preliminary validation, the functional role of the identified hub genes in the pathogenesis of SLE still needs to be further explored through in vitro experiments and animal models, and we are currently trying to establish a multicenter study for further validation.
In summary, our bioinformatics analysis identified key hub genes, including FCER1A and RGS1, which may serve as potential biomarkers and therapeutic targets for SLE. As research continues to unravel the complexities of SLE, the integration of genomic data with clinical findings will pave the way for improved diagnostic tools, personalized treatments, and better outcomes for SLE patients in the future.
Conclusion
In this study, we employed integrative bioinformatics analysis and clinical validation to identify and verify FCER1A and RGS1 as potential biomarkers for SLE. These genes exhibited consistent differential expression patterns across multiple independent datasets and patient-derived clinical specimens, with FCER1A markedly downregulated and RGS1 significantly upregulated in individuals with SLE. Functional enrichment and PPI analyses implicated these genes in critical immunological processes, including immune regulation, cell-mediated cytotoxicity, and apoptosis—hallmarks of SLE pathogenesis. Moreover, dysregulated levels of pro- and anti-inflammatory cytokines in patient plasma further substantiate the immunopathological relevance of these findings. Collectively, our results provide a foundation for the potential application of FCER1A and RGS1 in SLE diagnosis and suggest their promise as targets for future therapeutic strategies. Further mechanistic studies are warranted to elucidate their functional roles in disease progression.
Supplemental Information
Raw data obtained from qPCR experiments analyzing the expression levels of key genes such as FCER1A and RGS1 in patients with systemic lupus erythematosus (SLE) and healthy controls
The data include the Ct value for each sample, the Ct value for reference genes such as GAPDH, and the relative expression levels calculated using the ΔΔCt method. These data were used to validate the RNA sequencing results and further evaluate the potential of these genes as biomarkers for SLE.
Raw data from qPCR experiments analyzing the expression levels of key genes (e.g., FCER1A and RGS1) in patients with systemic lupus erythematosus (SLE) and healthy controls
These data are new and were recently supplemented with samples. The data include the Ct value for each sample, the Ct value for reference genes (e.g., GAPDH), and the relative expression levels calculated using the ΔΔCt method. These data were used to validate the RNA sequencing results and further evaluate the potential of these genes as biomarkers for SLE.
Raw data of cytokine IL-6, IL-10, and TNF-α expression levels
The expression of cytokine in SLE patients and healthy controls.
MIQE Checklist
A set of internationally recognized standardized guidelines that aims to standardize the design, execution and reporting of real-time quantitative PCR (qPCR) experiments and ensure the reliability, transparency and reproducibility of experimental data.
Supplemental tables
Supplemental Table 1: Primer Sequences. Lists the primer sequences used for gene expression analysis, including GAPDH, FCER1A, and RGS1.
Supplemental Table 2: Top 20 up-regulated and down-regulated genes in GSE13887 dataset. Presents the top 20 genes with the most significant up-regulation and down-regulation in the GSE13887 dataset, including their log2 fold change and p-values.
Supplemental Table 3: Top 20 up-regulated and down-regulated genes in GSE10325 dataset. Lists the top 20 genes with the most significant up-regulation and down-regulation in the GSE10325 dataset, including their log2 fold change and p-values.
Supplemental Table 4: Top 5 GO terms of each category for DEGs between Control and SLE. Summarizes the top 5 Gene Ontology (GO) terms for Biological Processes (BP), Cellular Components (CC), and Molecular Functions (MF) enriched in differentially expressed genes (DEGs) between control and SLE samples.
Supplemental Table 5: Top 5 KEGG pathways for DEGs between Control and SLE. Highlights the top 5 KEGG pathways significantly enriched in DEGs between control and SLE samples, including the associated genes and p-values.
Supplemental figures
Supplemental Figure 1: Bubble plots of GO and KEGG pathway enrichment analysis results. (A) The bubble plot shows the top 5 processes enriched in EP-DEGs in Biological Processes (BPs), Cellular Components (CCs), and Molecular Functions (MFs). The size of the bubbles represents the number of genes, and the color corresponds to different p-values. (B) The bubble plot visualizes the top 5 significantly enriched KEGG pathways in DEGs (co-DEGs). The size of the bubbles represents the number of genes, and the color corresponds to different p-values.
Supplement Figure 2: Construction of a Protein-protein Interaction (PPI) network. The PPI network highlights key genes such as CXCL13, RGS1, LAG3, GZMB, GADD45G, APOL1, DPEP2, and FCER1A, showing their interactions in the context of SLE.
Supplemental Figure 3: Gene validation in GSE10325 and GSE13887 datasets. (A-G) Volcano plots of hub genes expressed in GSE10325 and GSE13887 datasets, showing differential expression patterns. (H) Volcano plot of FCER1A and RGS1, genes with consistent expression levels in GSE10325 and GSE13887 datasets.
Supplemental Figure 4: Construction of the FCER1A and RGS1 PPI network. (A-B) The PPI network focuses on FCER1A and RGS1, illustrating their interactions and potential roles in SLE.
Bubble plots of GO and KEGG pathway enrichment analysis results
(A) The bubble plot shows the top 5 processes enriched in EP-DEGs in BPs, CCs, and MFs. (B) The bubble plot visualizes the top 5 significantly enriched KEGG pathways in DEGs (co-DEGs).
Construction of a hub gene Protein-protein interaction (PPI) network
Fepicts a construction of a hub gene protein-protein interaction (PPI) network.
Gene validation in GSE10325 and GSE13887 datasets
(A–D) Volcano plots of hub genes expressed in GSE10325 and GSE13887 datasets.
Gene validation in GSE10325 and GSE13887 datasets
(E–G) Volcano plots of hub genes expressed in GSE10325 and GSE13887 datasets; (H) Volcano plot of FCER1A and RGS1, genes with consistent expression levels in GSE10325 and GSE13887.
Construction of the FCER1A and RGS1 Protein-protein interaction (PPI) network
Displays a construction of the FCER1A and RGS1 protein-protein interaction (PPI) network.