First three new species of free-living marine nematodes of the Molgolaimus (Nematoda: Desmodoridae) from the continental shelf of the Brazilian coast (Atlantic Ocean)
- Published
- Accepted
- Received
- Academic Editor
- Federica Semprucci
- Subject Areas
- Aquaculture, Fisheries and Fish Science, Taxonomy, Zoology, Biological Oceanography
- Keywords
- South Atlantic, Taxonomy, Nematode diversity
- Copyright
- © 2025 Manoel et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. First three new species of free-living marine nematodes of the Molgolaimus (Nematoda: Desmodoridae) from the continental shelf of the Brazilian coast (Atlantic Ocean) PeerJ 13:e19156 https://doi.org/10.7717/peerj.19156
Abstract
Three new species of the Molgolaimus (Nematoda: Desmodoridae) are described from sample sediments collected in the South Atlantic, along the continental shelf break of Northeastern Brazil. This is the first time that new species of Molgolaimus have been described from sample sediments collected in the Brazilian coast. Molgolaimus sigmoides sp. nov. is characterized by four small cephalic sensillae, a buccal cavity with three small teeth, S-shaped spicules and gubernaculum with dorsal-caudal apophysis. Molgolaimus paralongispiculum sp. nov. possesses four setiform cephalic sensillae, a buccal cavity with three teeth, thin and elongated spicules and gubernaculum with anteriorly oriented apophysis. Molgolaimus brevispiculum sp. nov. is characterized by its possession of four setiform cephalic sensillae, an unarmed buccal cavity, short spicules and absent gubernaculum. We propose to amend the diagnosis of the genus, redistribute Molgolaimus species into subgroups 1b1 and 1b2, and to rearrange the order of presentation of subgroups 4a and 4b.
Introduction
The genus Molgolaimus Ditlevsen, 1921 was erected based on two specimens obtained from sample sediments collected in the Auckland Islands, New Zealand, by Dr. Th. Mortensen during his expedition to the Pacific between 1914 and 1916 (Ditlevsen, 1921). Molgolaimus was originally classified within Microlaimidae (Micoletzky, 1922; Gerlach & Riemann, 1973; Gerlach & Riemann, 1974) presumably based on similarities to the genus Microlaimus De Man, 1880 in head and amphidial fovea shape, arrangement of head sensilla, and buccal cavity structure (Leduc, Fu & Zhao, 2019). Since then, the taxonomic position of the genus has been investigated and modified over time, both based on morphology and phylogenetic analyses of the small subunit (SSU) sequences.
Jensen (1978) reviewed Microlaimidae and, based on a series of morphological characteristics, including the morphology of the gonads of males and females, erected the family Molgolaimidae Jensen, 1978 to accommodate two subfamilies: Aponematinae Jensen, 1978, with a single genus Aponema Jensen, 1978 and Molgolaiminae Jensen, 1978, for Molgolaimus and Prodesmodora Micoletzky, 1923 (Jensen, 1978; Muthumbi & Vincx, 1996). The other genera existing until then (Bolbolaimus Cobb, 1920; Calomicrolaimus Lorenzen, 1976; Ixonema Lorenzen, 1971a and Microlaimus) remained within Microlaimidae.
In his phylogenetic analyses, Lorenzen (1981), based on the morphology of the gonads of males and females, disagreed with Jensen’s (1978) establishment of the family Molgolaimidae and downgraded the family as a subfamily Molgolaiminae in Desmodoridae Filipjev, 1922 (Muthumbi & Vincx, 1996; Shi & Xu, 2016). The same author reintegrated Aponema into Microlaimidae and moved Prodesmodora to the subfamily Prodesmodorinae (Lorenzen, 1981). In the same study, Lorenzen placed Molgolaimus in its own single-genus subfamily within the Desmodoridae and established the monophyly of the superfamily Desmodoroidea (Filipjev, 1922) (Order Desmodorida De Coninck, 1965) based on the presence of only one anterior testis in males (Leduc, Fu & Zhao, 2019).
Leduc, Verdon & Zhao (2018), based on SSU sequences phylogenetic analyses which placed Molgolaimus demani Jensen, 1978 in a monophyletic clade with Microlaimidae sequences, proposed that Molgolaimus be removed from Desmodoroidea and placed within Microlaimoidea Micoletzky, 1922. The same authors also proposed that the family Molgolaimidae be reinstated and moved to accommodate Molgolaimus within Microlaimoidea. Additionally, they erected the order Microlaimida Leduc, Verdon & Zhao, 2018 to accommodate the superfamily Microlaimoidea. The order Microlaimida, based on the results obtained by Leduc, Verdon & Zhao, 2018, began to include the families Microlaimidae, Molgolaimidae, Monoposthiidae Filipjev, 1934 and Aponchiidae Gerlach, 1963.
However, Leduc, Fu & Zhao (2019) explained that the molecular analysis provided by Leduc, Verdon & Zhao (2018) ignored the fact that Molgolaimus demani was synonymized with Microlaimus tenuispiculum De Man, 1922 by Lorenzen (1981), Lorenzen (1994) based on the structure of the reproductive system. Leduc, Fu & Zhao (2019), using SSU sequences from Molgolaimus kaikouraensis Leduc, Fu & Zhao, 2019 and another species, referred to as Molgolaimus sp., performed new molecular analyses and found no support for placing Molgolaimus with either the Desmodorida or Microlaimida. The results of SSU phylogenetic analysis obtained by Leduc, Fu & Zhao (2019) suggest that Molgolaimus should be classified with Chromadorida Chitwood, 1933. Recently, Sun & Huang (2024) carried out molecular analyses using SSU sequences from Molgolaimus longicaudatus Sun & Huang, 2024. The results obtained by these authors showed that Molgolaimus longicaudatus clustered within Desmodoridae clade, supporting the opinion of Lorenzen.
In order to facilitate the identification of the different species of the genus, Fonseca, Vanreusel & Decraemer (2006) distinguished four groups of Molgolaimus species based on a frequency distribution for spicules length (Group 1: species with spicules <35 µm; Group 2: spicules ranging between 35 and 53 µm; Group 3: spicules ranging between 53 and 80 µm; Group 4: spicules longer than 80 µm). Groups 1 and 4 were divided into subgroups (1a, 1b1, 1b2 and 1c; 4a and 4b, respectively) based on relative spicules length, as well as body length and ratios of body dimensions.
Molgolaimus species are present in all oceans, ranging from shallow water regions to the deep sea (Fonseca, Vanreusel & Decraemer, 2006), including records in estuarine areas (Warwick, 1970; Zhou et al., 2020; Sun & Huang, 2024). Along the Brazilian coast, at a generic level, this taxon was previously recorded in articles published in indexed journals and dissertations/thesis for different habitats: continental shelf adjacent to the Santos Estuarine System, São Paulo (Yaginuma, 2011); Slope (Moura, 2013) and Canyons and adjacent areas (Silva, 2012) in the Campos Basin, Rio de Janeiro and in the Capibaribe River estuary, Pernambuco (Cavalcante, 2023). At a specific level, recorded the presence of Molgolaimus abyssorum Muthumbi & Vincx, 1996 and Molgolaimus lazonus Vitiello, 1970; Jensen, 1978 in a deep-sea region of the Campos Basin, Rio de Janeiro (Lira, 2005; Venekey, 2017).
In the present study, representatives of the genus Molgolaimus were found from samples collected in the South Atlantic, along the break of the Continental Shelf in Northeast Brazil. Here we describe three new species of Molgolaimus. This is the first time that new species of this taxon have been described from sample sediments collected on the Brazilian coast. We also propose to amend the diagnosis of the genus, redistribute Molgolaimus species into subgroups 1b1 and 1b2, and to rearrange the order of presentation of subgroups 4a and 4b.
Material and methods
Study area and sampling. This information were previously described in Manoel, Neres & Esteves (2024). Table 1 presents details of the collection stations relevant to this study. For sediment collection, a box-corer was used, while meiofauna samples were collected with a corer with dimensions of 10 cm × 10 cm. The sediment samples were taken in triplicate. The material collected was placed in plastic pots and fixed with 4% formaldehyde.
| Station | Latitude | Longitude | Depth |
|---|---|---|---|
| 2 | S05°42′54.42″ | W34°59′31.92″ | 60 m |
| 4 | S06°27′06.06″ | W34°45′53.64″ | 56 m |
| 9 | S08°38′20.46″ | W34°45′45.12″ | 47 m |
| 10 | S08°56′36.78″ | W34°50″16.02″ | 54 m |
| 11 | S09°15′30.54″ | W34°57′13.14″ | 87 m |
| 16 | S10°44′59.28″ | W36°25′32.88″ | 58 m |
| 17 | S11°00′00.54″ | W36°49′58.98″ | 54 m |
| 23 | S13°04′10.32″ | W38°25′46.98″ | 65 m |
Sample processing. In laboratory, sediment samples were washed with filtered water through sieves of 0.5 mm and 0.045 mm mesh. The remaining samples in the smaller mesh sieve were extracted with SICOL-40 colloidal silica solution (specific gravity 1.18) (Somerfield, Warwick & Moens, 2005).
Under a stereomicroscope, Nematodes was sorted out and deposited in a small glass container containing Solution 1 described by De Grisse (1969). The specimens were then transferred to glycerin and diaphanized using the methodology described by De Grisse (1969), and subsequently mounted permanently on glass slides (Cobb, 1920). At the generic level, Nematodes were identified using keys provided by Warwick, Platt & Somerfield (1998) and Decraemer & Smol (2006). At a specific level, the identification was performed by comparing the characteristics of the species found with those mentioned in the original descriptions. Drawings and photographs were produced utilizing an Olympus CX 31 optical microscope equipped with a drawing tube. Body measurements were obtained with the assistance of a mechanical map meter.
For scanning electron microscopy (SEM), individuals were obtained by disassembling glycerin-paraffin slides. These specimens were rehydrated with distilled water according to the method described by Abolafia (2015). The individuals were then placed in a meiofauna processing manufactured container described by Abolafia (2015), gradually dehydrated in a graded ethanol series (10% during one day and 20, 30, 40, 50, 60, 70, 80, 90, 92, 95, first 100 and second 100% along the second day, changing from one concentration to the following every 2 h) and dried in a critical-point dryer. The specimens were removed from the container, deposited on an aluminum stub covered with conductive tape, coated with gold and examined under a TM4000 SEM at 10 kV with BSE detector.
The holotype and one female paratype for each species are deposited in the Nematoda Collection at the Museum of Oceanography Prof. Petronio Alves Coelho (MOUFPE) in Brazil. Additional paratypes are deposited in the Meiofauna Laboratory within the Zoology Department at the Federal University of Pernambuco (NM LMZOO-UFPE).
The electronic edition of this article, formatted in Portable Document Format (PDF), constitutes a published study in accordance with the guidelines set forth by the International Commission on Zoological Nomenclature (ICZN). Consequently, the new names presented in this electronic version are considered effectively published under the Code based solely on the electronic edition. This research, along with its nomenclatural actions, has been duly registered in ZooBank, the ICZN’s online registration system. The ZooBank LSIDs (Life Science Identifiers) can be accessed and the related information can be viewed using any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:01681568-8A01-47FC-96E5-DE43C7529F14. The online version of this research is preserved and can be accessed through the following digital repositories: PeerJ, PubMed Central, and CLOCKSS.
Results
Systematics
| Taxonomic classification, after De Ley, Decraemer & Eyualem (2006) |
| Class Chromadorea Inglis, 1983 |
| Subclass Chromadoria Pearse, 1942 |
| Order Desmodorida De Coninck, 1965 |
| Suborder Desmodorina De Coninck, 1965 |
| Superfamily Desmodoroidea Filipjev, 1922 |
| Family Desmodoridae Filipjev, 1922 |
| Subfamily Molgolaiminae Jensen, 1978 |
| GenusMolgolaimusDitlevsen, 1921 |
Diagnosis. (After Leduc, Fu & Zhao, 2019) Cuticle finely striated, but may appear smooth under light microscopy (prominently annulated in M. pecticauda). Inner and outer labial papillae are small and often challenging to differentiate. Cephalic setae positioned slightly anterior or posterior to the head constriction. Amphidial fovea round and situated posterior to the head constriction. Buccal cavity small and weakly cuticularized, featuring small teeth that may sometimes be indistinct. Pharynx cylindrical, narrow and characterized by a prominent posterior bulb, typically spherical. Pharyngeal lumen weakly cuticularized, except within the pharyngeal bulb, where it may be heavily cuticularized. Secretory-excretory pore located anterior to the nerve ring and rarely found posterior to it. Female didelphic-amphidelphic, with reflexed ovaries (position of the genital branches varibles). Male monorchic with single anterior testis (variable position of the genital branch: on the right, on the left or positioned ventrally in relation to the intestine). Spicules of variable length and shape. Gubernaculum present or absent, when present with or without apophysis. Precloacal supplements often observed. Tail of varying shape and length (short and conical to elongated and conico-cylindrical).
Type species: Molgolaimus tenuispiculum Ditlevsen, 1921
Valid species (Table 2)
The valid species were divided into groups and, when relevant, into subgroups following the classification by Fonseca, Vanreusel & Decraemer (2006) with modifications in subgroups 1b1, 1b2, 4a and 4b (Table 2). The list of valid species is in accordance with Leduc, Fu & Zhao (2019), but with the addition of species described later and species described by Bussau (1993). The species described by Bussau (1993) are currently considered valid (Holovachov, 2020). Valid species for which synonyms occur are listed in Appendix S1.
| Group | Subgroup | Valid species |
|---|---|---|
| Group 1 (spicule length < 35 µm) | Subgroup 1a (spicules = 1 cloacal body diameters long) | Molgolaimus brevispiculum sp. nov. |
| M. citrusGerlach, 1959 | ||
| M. cuanensis (Platt, 1973) Jensen, 1978 | ||
| M. euryformisZhou et al. 2020 | ||
| M. haakonmosbiensisPortnova, 2009 | ||
| M. lazonus (Vitiello, 1970) Jensen, 1978 | ||
| M. parallgeni (Vitiello, 1973) Jensen, 1978 | ||
| M. turgofrons (Lorenzen, 1971b) Jensen, 1978 | ||
| Subgroup 1b1 (spicules > 1 and <3 cloacal body diameters long; amph ant/hd ≥ 2) | M. amphimacrusBussau, 1993 | |
| M. drakusFonseca, Vanreusel & Decraemer, 2006 | ||
| M. gaziiMuthumbi & Vincx, 1996 | ||
| M. mareprofundusFonseca, Vanreusel & Decraemer, 2006 | ||
| M. porosusBussau, 1993 | ||
| M. spirifer (Warwick, 1970) Shi & Xu, 2016 | ||
| Subgroup 1b2 (spicules >1 and <3 cloacal body diameters long; amph ant/hd <2) | M. abyssorumMuthumbi & Vincx, 1996 | |
| M. carpediemFonseca, Vanreusel & Decraemer, 2006 | ||
| M. exceptionregulumFonseca, Vanreusel & Decraemer, 2006 | ||
| M. falliturvisusFonseca, Vanreusel & Decraemer, 2006 | ||
| M. gallucciiFonseca, Vanreusel & Decraemer, 2006 | ||
| M. kiwayuiMuthumbi & Vincx, 1996 | ||
| M. longicaudatusSun & Huang, 2024 | ||
| M. minutusJensen, 1988 | ||
| M. pecticauda (Murphy, 1966) Shi & Xu, 2016 | ||
| M. sapiensFonseca, Vanreusel & Decraemer, 2006 | ||
| M. sigmoides sp. nov. | ||
| Subgroup 1c (spicules > 3 cloacal body diameters long) | M. typicusFurstenberg & Vincx, 1992 | |
| M. tyroiMuthumbi & Vincx, 1996 | ||
| Group 2 (spicule length 35–53 µm) | - | M. allgeni (Gerlach, 1950) Jensen, 1978 |
| M. australisFonseca, Vanreusel & Decraemer, 2006 | ||
| M. macilentiFonseca, Vanreusel & Decraemer, 2006 | ||
| M. nettoensisFonseca, Vanreusel & Decraemer, 2006 | ||
| M. sabakiiMuthumbi & Vincx, 1996 | ||
| M. xuxunaraensisFonseca, Vanreusel & Decraemer, 2006 | ||
| Group 3 (spicule length 53–80 µm) | - | M. liberalisFonseca, Vanreusel & Decraemer, 2006 |
| M. unicusFonseca, Vanreusel & Decraemer, 2006 | ||
| M. walbethiFonseca, Vanreusel & Decraemer, 2006 | ||
| Group 4 (spicule length >80 µm) | Subgroup 4a (b = 8–11; spicules = 4–6 cloacal body diameters long) | M. gigaslongincusFonseca, Vanreusel & Decraemer, 2006 |
| M. kaikouraensisLeduc, Fu & Zhao, 2019 | ||
| M. pacificusFonseca, Vanreusel & Decraemer, 2006 | ||
| M. tenuispiculumDitlevsen, 1921 | ||
| Subgroup 4b (species not included in subgroup 4a) | M. gigasproximusFonseca, Vanreusel & Decraemer, 2006 | |
| M. longispiculum (Timm, 1961) Jensen, 1978 | ||
| M. paralongispiculum sp. nov. | ||
| M. tanaiMuthumbi & Vincx, 1996 |
Invalid species
The species listed below are considered taxon inquirendum for being poorly described (Jensen, 1978).
| Molgolaimus labradorensis (Allgén, 1957) Jensen, 1978 |
| Molgolaimus tenuicaudatus (Allgén, 1959) Jensen, 1978 |
| Molgolaimus tenuilaimus (Allgén, 1932) Jensen, 1978 |
Description of new species
Type material. Four males and two females found. Holotype male (MOUFPE 0026), paratype female 1 (MOUFPE 0027), 3 male paratype (493–495 NM LMZOO-UFPE) and paratype female 2 (496–497 NM LMZOO-UFPE).
| Molgolaimus sigmoidessp. nov. | Holotype | Male paratypes (n = 3) | Paratype female 1 | Paratype female 2 |
|---|---|---|---|---|
| Body length | 376.5 | 357–368 | 397 | 390 |
| Cephalic setae length | <2 | <2 | <2 | <2 |
| Head diameter | 6 | 4.5–5.5 | 5 | 5 |
| Distance from anterior end to cephalic setae | 3 | 2.5–3 | 3 | 3 |
| Distance from anterior end to amphidial fovea | 10 | 7–9 | 10 | 8.5 |
| Distance from anterior end to amphidial fovea in relation to head diameter | 1.7 | 1.5–1.7 | 2 | 1.7 |
| Amphidial fovea diameter (maximum width) | 4.5 | 4.5–4.5 | 3.5 | 4 |
| Body diameter at level of the amphidial fovea | 8.5 | 8–9 | 9 | 9 |
| % of the amphidial fovea diameter in relation to corresponding body diameter | 53% | 50–58% | 39% | 44% |
| Pharynx length | 73 | 65.5–68.5 | 68.5 | 70 |
| Position of nerve ring from anterior end | 43 | 39–42 | 40 | 42 |
| Nerve ring position in relation to pharynx length (%) | 59% | 57–63% | 58% | 60% |
| Pharyngeal bulb diameter | 13 | 12–13 | 11 | 14 |
| Body diameter at level of the pharyngeal bulb | 16 | 15–16 | 13 | 17 |
| % of basal bulb diameter in relation to corresponding body diameter | 81% | 80–85% | 85% | 82% |
| Maximum body diameter | 18 | 16 | 15 | 20.5 |
| Anal or cloacal body diameter | 15 | 14–14.5 | 10 | 11.5 |
| Tail length | 56 | 51–54 | 65 | 54 |
| Length of spicules along arc | 30 | 29–31.5 | * | * |
| Length of spicules along cord | 17.5 | 16–18 | * | * |
| Length of gubernaculum | 5 | 4.5–6 | * | * |
| Width of gubernaculum | 4 | 4 | * | * |
| Length of apophysis | 5 | 4.5–7 | * | * |
| Length of spicules along arc in relation to cloacal body diameter | 2 | 2–2.2 | * | * |
| Distance from anterior end to vulva | * | * | 170 | 175.5 |
| Position of vulva from anterior end (%) | * | * | 43% | 45% |
| Body diameter in vulva region | * | * | 15 | 19.5 |
| Anterior ovary length | * | * | 105 | 69 |
| Posterior ovary length | * | * | 107 | 70 |
| Reproductive system length | 200 | 181–211.5 | 118.5 | 139 |
| % of reproductive system in relation to body length | 53% | 50–59% | 30% | 36% |
| a | 21 | 23 | 26.5 | 19 |
| b | 5 | 5–5.5 | 6 | 6 |
| c | 7 | 7 | 6 | 7 |
| c’ | 4 | 4 | 6.5 | 5 |
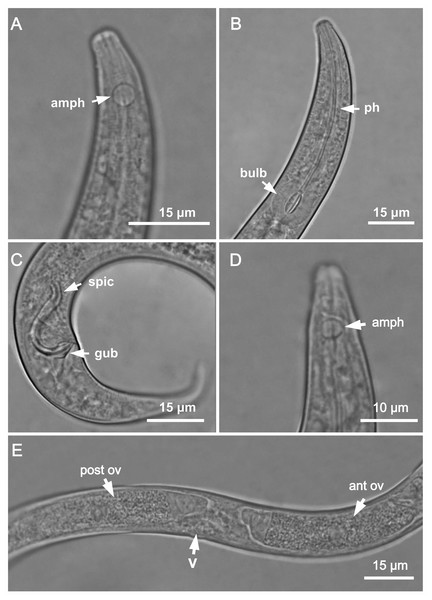
Figure 1: Molgolaimus sigmoides sp. nov. Holotype male, paratype female 1 and paratype female 2.
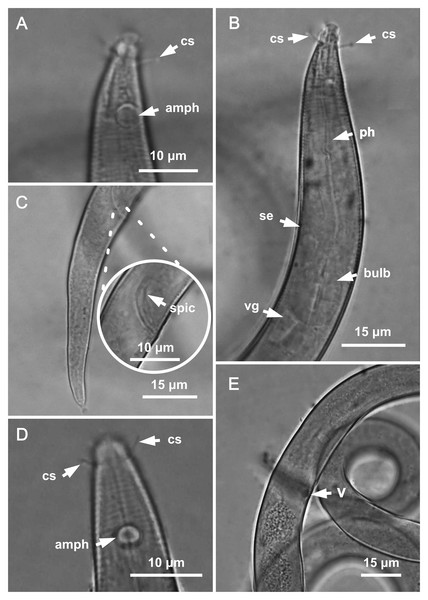
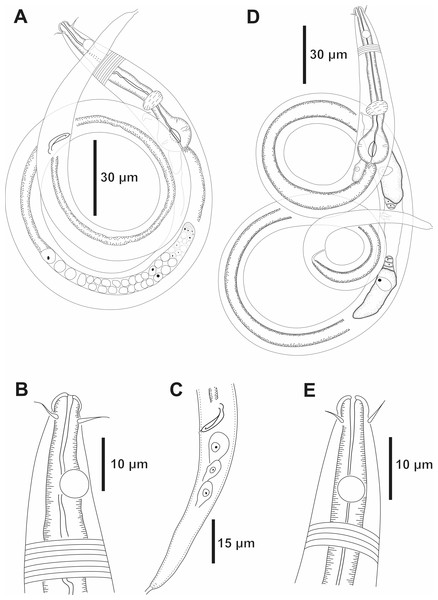
Holotype male: (A) anterior end (arrow indicating amphidial fovea = amph), (B) anterior region (arrows indicating pharynx = ph and basal bulb = bulb), (C) posterior end (arrows indicating spicule = spic and gubernaculum = gub). Paratype female 2: (D) anterior region (arrow indicating amphidial fovea = amph). Paratype female 1: (E) reproductive system (arrows indicating vulva = V; anterior ovary = ant ov and posterior ovary = post ov).Figure 2: Molgolaimus sigmoides sp. nov. Holotype male and paratype female 1.
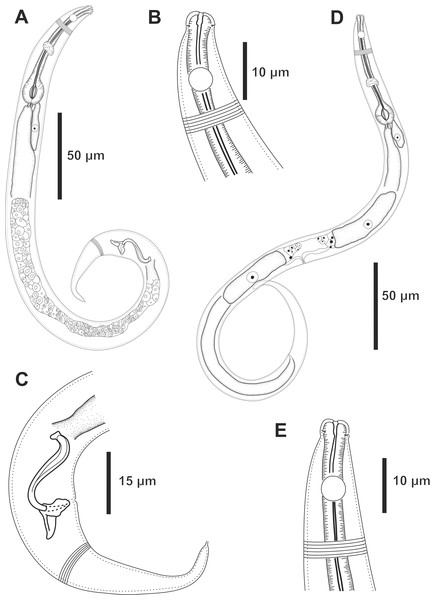
Holotype male: (A) overview, (B) anterior end, (C) posterior region. Paratype female 1: (D) overview, (E) anterior end.Type locality. South Atlantic Ocean, Continental shelf of the State of Bahia, Brazil, station 23 (S13°04′10.32″ W38°25′46.98″), December 11, 2019, 65 m.
Locality of paratypes. Paratype female 1: South Atlantic Ocean, Continental shelf of the State of Rio Grande do Norte, Brazil, station 2 (S05°42′54.42″ W34°59′31.92″), November 28, 2019, 60 m. Paratype males (1–3): South Atlantic Ocean, Continental shelf of the State of Sergipe, Brazil, station 17 (S11°00′00.54″ W36°49′58.98″), December 12, 2019, 54 m. Paratype female 2: South Atlantic Ocean, Continental shelf of the State of Sergipe, Brazil, station 16 (S10°44′59.28″ W36°25′32.88″), December 09, 2019, 58 m.
Etymology. The spicules of Molgolaimus sigmoides sp. nov. has a S-shaped structure. In Greek, sigma (ς) corresponds to the letter S in the Latin alphabet.
Holotype male. Body cylindrical 376.5 µm long. Maximum body diameter corresponding to three times the head diameter. Cuticle faintly striated. Somatic setae not observed. Head slightly set off from the rest of the body by a slight constriction. Inner and outer labial sensilla indistinct. Four short cephalic setae (<two µm long) located at the level of buccal cavity and slightly anterior to head constriction (three µm from anterior end). Amphidial fovea circular, located 10 µm from anterior end (1.7 times the head diameter) and occupying 53% of corresponding body diameter. Buccal cavity small, narrow, with lightly slightly cuticularized walls. Three small teeth, difficult to see (a slightly larger dorsal tooth and two smaller ventrosublateral). Pharynx muscular (73 µm long), surrounding buccal cavity, consisting of narrow, cylindrical anterior portion and with conspicuous spherical posterior bulb (81% of corresponding body diameter). Pharyngeal lumen slightly cuticularized, except in the pharyngeal bulb where the valves are more cuticularized. Nerve ring situated at 59% of the pharynx length from anterior end. Secretory-excretory pore not observed. Ventral gland located posterior to the pharyngo-intestinal junction. Cardia partially surrounded by intestine. Reproductive system with single anterior outstretched testis to the left of the intestine. Spicules S-shaped, arched ventrally at the anterior end and dorsally at the posterior end (2 times the cloacal body diameter). Gubernaculum surrounding the spicules at the distal end and with dorsal-caudal apophyses. Precloacal supplements absent. Caudal glands not observed. Tail conico-cylindrical, about four times the cloacal body diameter. The cylindrical portion of the tail represents about 18% of its total length. Spinneret short.
Paratype female. Similar to male. Body measuring 397 µm in length, with a maximum diameter of 15 µm (three times the head diameter). Amphidial fovea, occupying 39% of corresponding body width and located 10 µm from anterior end. Basal bulb occupying 85% of the corresponding body diameter. Nerve ring situated at 58% of the pharynx length, from anterior end. Vulva located 170 µm from anterior end, at 43% of body length. Reproductive system didelphic, with reflexed ovaries. Anterior gonad situated to the right side of the intestine, posterior gonad to the left side of the intestine. Tail conico-cylindrical, about 6.5 times the anal body diameter. The cylindrical portion of the tail represents about 24% of its total length.
Diagnosis. Molgolaimus sigmoides sp. nov. characterized by its body length (357–397 µm). Cuticle finely annulated. Head slightly set off. Four short cephalic setae (<two µm long) located at the level of buccal cavity and slightly anterior to head constriction. Amphidial fovea occupying 50–58% of the corresponding body diameter in the males and 39–44% in the female, located at about 1.5–2 times the head diameter. Buccal cavity with three small teeth, one dorsal and two ventrosublateral, the dorsal is slightly larger. Muscular pharynx with conspicuous spherical posterior bulb (80–85% of the corresponding body diameter). Pharyngeal lumen slightly cuticularized, except in the pharyngeal bulb where the valves are more cuticularized. Spicules S-shaped (2–2.2 times the cloacal body diameter). Gubernaculum surrounding the spicules at the distal end and with dorsal-caudal apophysis. Tail conico-cylindrical which corresponds to 4–6.5 times the cloacal or anal body diameter.
Differential diagnosis. Molgolaimus sigmoides sp. nov. resembles Molgolaimus sapiens Fonseca, Vanreusel & Decraemer, 2006 and Molgolaimus xuxunaraensis Fonseca, Vanreusel & Decraemer, 2006 mainly due to the peculiar shape of the spicules (S-shaped), precloacal supplements absence and by present short cephalic setae (two µm long in M. sapiens; <two µm long in M. sigmoides sp. nov. and not see in M. xuxunaraensis). However, M. sigmoides sp. nov. it is the only species of Molgolaimus that has a gubernaculum with a dorsal-caudal apophysis. This characteristic differentiates the new species both from those morphologically closest to it (M. sapiens and M. xuxunaraensis) and from the other valid species of the genus.
Type material. Five males and two females found. Holotype male (MOUFPE 0028), paratype female 1 (MOUFPE 0029), 4 male paratype (498–501 NM LMZOO-UFPE) and paratype female 2 (502–503 NM LMZOO-UFPE).
| Molgolaimus paralongispiculum sp. nov. | Holotype | Male paratypes (n = 4) | Paratype female 1 | Paratype female 2 |
|---|---|---|---|---|
| Body length | 811.5 | 693–778.5 | 790.5 | 732 |
| Cephalic setae length | 3 | 3–3.5 | 3.5 | 2.5 |
| Head diameter | 7 | 5–7 | 7 | 7 |
| Distance from anterior end to cephalic setae | 6 | 5–6 | 6 | 6 |
| Cephalic setae in relation to head diameter (%) | 43% | 43%–50% | 50% | 36% |
| Distance from anterior end to amphidial fovea | 9 | 6.5–11 | 9 | 8 |
| Distance from anterior end to amphidial fovea in relation to head diameter | 1.3 | 0.9–1.8 | 1.3 | 1.1 |
| Amphidial fovea diameter (maximum width) | 5.5 | 5–6 | 6 | 6 |
| Body diameter at level of the amphidial fovea | 10 | 8–10 | 11 | 10 |
| % of the amphidial fovea diameter in relation to corresponding body diameter | 55% | 60% | 55% | 60% |
| Pharynx length | 107.5 | 101.5–109 | 110.5 | 97 |
| Position of nerve ring from anterior end | 70 | 62–68 | 70.5 | 61 |
| Nerve ring position in relation to pharynx length (%) | 65% | 60–62% | 64% | 63% |
| Pharyngeal bulb diameter | 21 | 19–21.5 | 21.5 | 19 |
| Body diameter at level of the pharyngeal bulb | 27 | 23.5–28 | 27 | 25 |
| % of basal bulb diameter in relation to corresponding body diameter | 78% | 76–83% | 80% | 76% |
| Maximum body diameter | 33 | 28–35 | 36 | 31 |
| Anal or cloacal body diameter | 20.5 | 20.5–22 | 18 | 20 |
| Tail length | 96 | 74.5–98.5 | 80.5 | 82 |
| Length of spicules along arc | 140.5 | 134–145 | * | * |
| Length of spicules along cord | 94 | 109–125 | * | * |
| Length of spicules along arc in relation to cloacal body diameter | 6.9 | 6.1–7.1 | * | * |
| Length of gubernaculum | 19 | 19–20.5 | * | * |
| Length of gubernaculum in relation to length of spicules along arc (%) | 14% | 13–14% | * | * |
| Distance from anterior end to vulva | * | * | 367.5 | 334.5 |
| Position of vulva from anterior end (%) | * | * | 46% | 46% |
| Body diameter in vulva region | * | * | 36 | 31 |
| Anterior ovary length | * | * | 124.5 | 156 |
| Posterior ovary length | * | * | 174 | 136.5 |
| Reproductive system length | 480 | 391.5–439.5 | 237 | 214 |
| % of reproductive system in relation to body length | 59% | 53–57% | 32% | 29% |
| a | 25 | 22–27 | 22 | 24 |
| b | 8 | 6.5–8 | 7 | 8 |
| c | 8 | 8–9 | 10 | 9 |
| c’ | 5 | 4–4.5 | 4 | 4 |
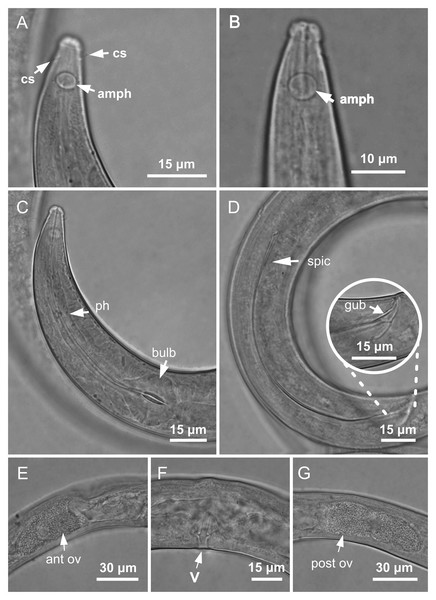
Figure 3: Molgolaimus paralongispiculum sp. nov. Holotype male and paratype female 1.
Holotype male: (A) anterior end (arrows indicating amphidial fovea = amph and cephalic setae = cs), (C) anterior region (arrows indicating pharynx = ph and basal bulb = bulb), (D) posterior end (arrow indicating spicule = spic and gubernaculum = gub). Paratype female: (B) anterior end (arrow indicating amphidial fove a= amph), (E) reproductive system (arrow indicating anterior ovary = ant ov), (F) reproductive system (arrow indicating vulva = V), (G) reproductive system (arrow indicating posterior ovary = post ov).Figure 4: Molgolaimus paralongispiculum sp. nov. Holotype male and paratype female 1.
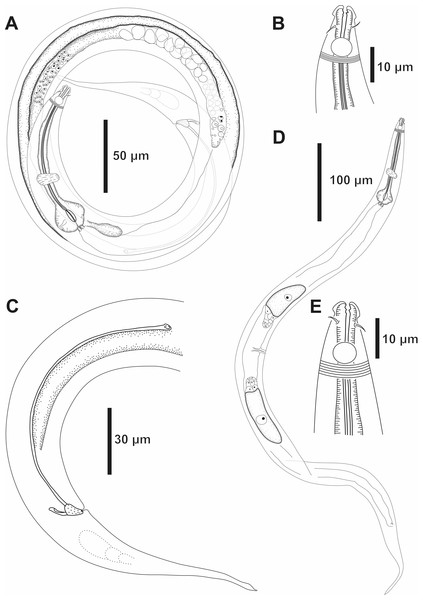
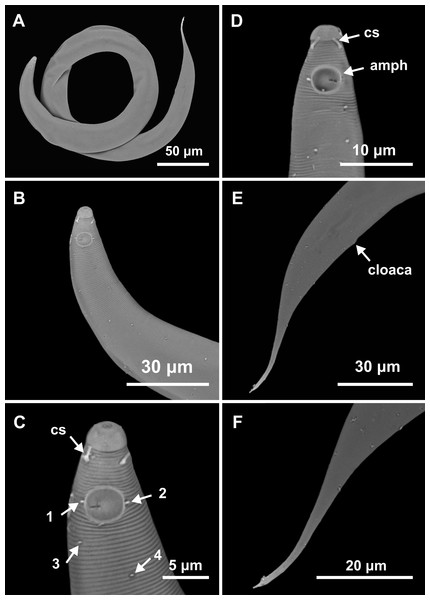
Holotype male: (A) overview, (B) anterior end, (C) posterior region. Paratype female 1: (D) overview, (E) anterior end.Figure 5: Molgolaimus paralongispiculum sp. nov. Male paratypes 3 and 4, SEM photographs.
Paratype 3: (A) overview, (B) anterior region, (C) anterior end (arrows indicating cephalic setae = cs and papillae positioned on both lateral edges of the amphidial fovea = 1 and 2; arrows indicating papillae found in the cervical region = 3 and 4); Paratype 4: (D) anterior end (arrows indicating amphidial fovea = amph and cephalic setae = cs); Paratype 3: (E) posterior region; (F) cylindrical portion of the tail.Type locality. South Atlantic Ocean, Continental shelf of the State of Pernambuco, Brazil, station 9 (S08°38′20.46″ W34°45′45.12″), November 26, 2019, 47 m.
Locality of other paratypes. South Atlantic Ocean, Continental shelf of the State of Alagoas, Brazil, station 11 (S09°15′30.54″ W34°57′13.14″), November 26, 2019, 87 m.
Etymology. The species name refers to similarity with species Molgolaimus longispiculum (Timm, 1961) Jensen, 1978.
Holotype male. Body cylindrical 811.5 µm long. Maximum body diameter corresponding about 4 times the head diameter. Cuticle faintly striated. Small papillae present throughout the body without a distribution pattern (observed in Paratypes 3 and 4, SEM photographs). Head off from the rest of the body by a constriction. Inner and outer labial sensilla indistinct. Four setiform cephalic setae (about 0.4 times the head diameter) located behind the head constriction (about six µm from anterior end). Amphidial fovea circular, located nine µm from anterior end (about 1.3 times the head diameter) and occupying 43% of corresponding body diameter. A small papilla associated with a cuticular pore present on both lateral edges of each amphidial fovea (distinctly visible in Paratypes 3 and 4, SEM photographs). Buccal cavity small, narrow, with slightly cuticularized walls. Three small teeth, a slightly larger dorsal tooth and two smaller ventrosublateral. Pharynx muscular (107.5 µm long), surrounding buccal cavity, consisting of narrow, cylindrical anterior portion and with conspicuous pyriform posterior bulb (78% of corresponding body diameter). Pharyngeal lumen slightly cuticularized, except in the pharyngeal bulb where the valves are more cuticularized. Nerve ring situated at 65% of the pharynx length from anterior end. Secretory-excretory pore not observed. Ventral gland located posterior of the pharyngo-intestinal junction. Cardia partially surrounded by intestine. Reproductive system with single anterior outstretched testis located ventrally to the intestine (germinal zone slightly to the right of the intestine). Spicules thin and elongated (6.9 times the cloacal body diameter). Gubernaculum funnel-shaped surrounding the spicules at the distal end and with anteriorly oriented apophysis. Precloacal supplements absent. Three caudal glands. Tail conico-cylindrical, about five times the cloacal body diameter. The cylindrical portion of the tail represents about one third of its total length. Spinneret short.
Paratype female. Similar to male. Body measuring 790.5 µm in length, with a maximum diameter of 36 µm (about five times the head diameter). Cephalic setae corresponding 0.5 times the head diameter. Amphidial fovea occupying 55% of corresponding body width and located nine µm from anterior end. Basal bulb occupying 80% of the corresponding body diameter. Nerve ring situated at 64% of the pharynx length, from anterior end. Secretory-excretory pore and ventral gland not observed. Vulva located 367.5 µm from anterior end, at 46% of body length. Reproductive system didelphic, with reflexed ovaries. Anterior gonad situated to the left side of intestine, posterior gonad to the right side of intestine. Tail conical with cylindrical terminal portion, about four times the anal body diameter. The cylindrical portion of the tail represents about 38% of its total length. Spinneret short.
Diagnosis. Molgolaimus paralongispiculum sp. nov. characterized by its body length (693–811.5 µm). Cuticle finely annulated. Head set off. Four setiform cephalic setae (about 0.4–0.5 times the head diameter) located behind the head constriction. Amphidial fovea occupying 55–60% of the corresponding body diameter, located at about 0.9–1.8 times the head diameter. Buccal cavity with three small teeth, one dorsal and two ventrosublateral, the dorsal is slightly larger. Muscular pharynx with conspicuous pyriform posterior bulb (76–83% of the corresponding body diameter). Pharyngeal lumen slightly cuticularized, except in the pharyngeal bulb where the valves are more cuticularized. Spicules thin and elongated (6.1–7.1 times the cloacal body diameter). Gubernaculum surrounding the spicules at the distal end and with anteriorly oriented apophysis. Tail conico-cylindrical (4–5 times the cloacal or anal body diameter).
Differential diagnosis. Molgolaimus paralongispiculum sp. nov. and four other species (Molgolaimus longispiculum; Molgolaimus tenuispiculum Ditlevisen, 1921; Molgolaimus gigasproximus Fonseca, Vanreusel & Decraemer, 2006 and Molgolaimus gigaslongincus Fonseca, Vanreusel & Decraemer, 2006) are the only representatives of the genus that have spicules >100 µm in length. Of these, M. longispiculum and M. paralongispiculum sp. nov. do not possess precloacal supplements, which helps to distinguish them from the other species mentioned above.
Considering only male specimens (female paratype described for M. longispiculum is immature), M. paralongispiculum sp. nov. shares the following features with M. longispiculum: de Man’s ratios b (7.4 in M. longispiculum and 6.5–8 in the new species), c (8.2 in M. longispiculum and 8–9 in M. paralongispiculum sp. nov.) and c’ (4.7 in M. longispiculum and 4–5 in M. paralongispiculum sp. nov.); total body length (737 µm long in M. longispiculum and 693–811.5 µm long in M. paralongispiculum sp. nov.) and the length of the cephalic setae (3 µm long in M. longispiculum and 3–3.5 µm long in M. paralongispiculum sp. nov.). However, the new species differs from M. longispiculum in relation to: position of the cephalic setae (cephalic setae positioned after the head constriction and below the level of the buccal cavity in M. paralongispiculum sp. nov. vs positioned close to the head constriction and at the same level of the buccal cavity in M. longispiculum); tail shape (conico-cylindrical in the new species vs conical with slightly swollen tip in M. longispiculum); de Man’s ratio a (22–27 in M. paralongispiculum sp. nov. vs 35.5 in M. longispiculum) and the shape of the gubernaculum (surrounding the spicules at the distal end and with anteriorly oriented apophysis in the new species vs lamellar in M. longispiculum).
Type material. One male and one female found. Holotype male (MOUFPE 0030) and paratype female (MOUFPE 0031).
Type locality. South Atlantic Ocean, Continental shelf of the State of Bahia, Brazil, station 23 (S13°04′10.32″ W38°25′46.98″), November 12, 2019, 65 m.
Locality of paratype female. South Atlantic Ocean, Continental shelf of the State of Pernambuco, Brazil, station 10 (S08°56′36.78″W34°50″16.02″), November 26, 2019, 54 m.
Etymology. The specific epithet of the species name is due to its relatively short spicules length. Latin brevis: short in length.
| Molgolaimus brevispiculum sp. nov. | Holotype | Female paratype |
|---|---|---|
| Body length | 465 | 633 |
| Cephalic setae length | 5.5 | 4.5 |
| Distance from anterior end to cephalic setae | 3 | 2.5 |
| Head diameter | 4 | 5 |
| Cephalic setae in relation to head diameter (%) | 131% | 90% |
| Distance from anterior end to amphidial fovea | 10 | 12 |
| Distance from anterior end to amphidial fovea in relation to head diameter | 2.4 | 2.4 |
| Amphidial fovea diameter (maximum width) | 5.5 | 5 |
| Body diameter at level of the amphidial fovea | 10 | 10 |
| % of the amphidial fovea diameter in relation to corresponding body diameter | 55% | 50% |
| Pharynx length | 70.5 | 79.5 |
| Position of nerve ring from anterior end | 46.5 | 51.5 |
| Nerve ring position in relation to pharynx length (%) | 66% | 65% |
| Distance from anterior end to secretory-excretory pore | 50.5 | 54 |
| Pharyngeal bulb diameter | 13 | 16 |
| Body diameter at level of the pharyngeal bulb | 16 | 19 |
| % of basal bulb diameter in relation to corresponding body diameter | 81% | 84% |
| Maximum body diameter | 16 | 17 |
| Anal or cloacal body diameter | 13 | 11 |
| Tail length | 60 | 70 |
| Length of spicules along arc | 13 | * |
| Length of spicules along cord | 10.5 | * |
| Length of spicules along arc in relation to cloacal body diameter | 1.0 | * |
| Distance from anterior end to vulva | * | 298 |
| Position of vulva from anterior end (%) | * | 47% |
| Body diameter in vulva region | * | 17 |
| Anterior ovary length | * | 80.5 |
| Posterior ovary length | * | 69 |
| Reproductive system length | 306 | 106 |
| % of reproductive system in relation to body length | 66% | 17% |
| a | 29 | 37 |
| b | 7 | 8 |
| c | 8 | 9 |
| c’ | 5 | 6 |
Figure 6: Molgolaimus brevispiculum sp. nov. Holotype male and paratype female.
Holotype male: (A) anterior end (arrows indicating cephalic setae = cs and amphidial fovea = amph), (B) anterior region (arrows indicating cephalic setae = cs; pharynx = ph; secretory-excretory pore = se; basal bulb = bulb and ventral gland = vg), (C) posterior end (arrow indicating spicule = spic). Paratype female: (D) anterior end (arrows indicating cephalic setae = cs and amphidial fovea = amph), (E) reproductive system (arrow indicating vulva = V).Figure 7: Molgolaimus brevispiculum sp. nov. Holotype male and paratype female.
Holotype male: (A) overview, (B) anterior end, (C) posterior region. Paratype female: (D) overview, (E) anterior end.Holotype male. Body cylindrical 465 µm long. Maximum body diameter corresponding to about 4 times the head diameter. Cuticle faintly striated. Somatic setae not observed. Head slightly set off from the rest of the body by a slight constriction. Inner and outer labial sensilla indistinct. Four cephalic setae (about 1.3 times the head diameter) located behind the head constriction (three µm from anterior end). Amphidial fovea circular, located 10 µm from anterior end (about 2.4 times the head diameter) and occupying 55% of corresponding body diameter. Buccal cavity tubular, narrow, with slightly cuticularized walls. Teeth not observed. Pharynx muscular (70.5 µm long), surrounding buccal cavity, consisting of narrow, cylindrical anterior portion and with conspicuous spherical posterior bulb (81% of corresponding body diameter). Pharyngeal lumen slightly cuticularized. Nerve ring situated at 66% of the pharynx length from anterior end. Secretory-excretory system short. Secretory-excretory pore located just below the nerve ring (about 72% of the pharynx length). Ventral gland located posterior to the pharyngo-intestinal junction. Cardia not observed. Reproductive system with single anterior outstretched testis to the left of the intestine. Short spicule, with slightly cephalized anterior end (1 time the cloacal body diameter). Gubernaculum and precloacal supplements absent. Tail conical, about 5 times the cloacal body diameter.
Paratype female. Similar to male. Body measuring 633 µm in length, with a maximum diameter of 17 µm (3.4 times the head diameter). Amphidial fovea, occupying 50% of corresponding body width and located 12 µm from anterior end. Basal bulb occupying 84% of the corresponding body diameter. Nerve ring situated at 65% of the pharynx length, from anterior end. Secretory-excretory pore located just below the nerve ring (about 68% of the pharynx length). Ventral gland located posterior to the pharyngo-intestinal junction. Vulva located 298 µm from anterior end, at 47% of body length. Reproductive system didelphic, with reflexed ovaries. Anterior ovary situated to the right side of the intestine, posterior ovary to the left side of the intestine. Tail conical, about six times the anal body diameter.
Diagnosis. Molgolaimus brevispiculum sp. nov. characterized by its body length (465–633 µm). Cuticle finely annulated. Head slightly set off. Four cephalic setae (0.9–1.3 times the head diameter) located behind the head constriction. Amphidial fovea occupying 50–55% of the corresponding body diameter, located at about 2.4 times the head diameter to the anterior end. Buccal cavity unarmed. Muscular pharynx with cuticularized lumen and conspicuous spherical posterior bulb (81–84% of the corresponding body diameter). Secretory-excretory system short. Secretory-excretory pore located just below the nerve ring. Short spicule, with slightly cephalized anterior end (one time the cloacal body diameter). Gubernaculum and precloacal supplements absent. Tail conical (five to six times the cloacal or anal body diameter).
Differential diagnosis (Table 6). Only males of Molgolaimus typicus (Furstenberg & Vincx, 1992) and Molgolaimus drakus (Fonseca, Vanreusel & Decraemer, 2006) have been described, thus the comparison between those species and Molgolaimus brevispiculum sp. nov. is only based on males. The absence of gubernaculum is a common characteristic between M. typicus, M. drakus and M. brevispiculum sp. nov., which are thus different from the other species of the genus Molgolaimus. Nonetheless, other features as the absence of precloacal supplements, the spicules length, the cephalic setae length, the size of the amphidial fovea in relation to the body diameter and the de Man’s ratio help in differentiating those three species (Table 6). In addition, M. brevispiculum sp. nov. differs from M. typicus with regard to the position of the secretory-excretory pore, with this structure located at the same level as the amphidial fovea in M. typicus and just below the nerve ring in the new species.
| M. typicus | M. drakus | M. brevispiculumsp. nov. | |
|---|---|---|---|
| Body length | 456 µm | 495–575 µm | 465 µm |
| a | 35 | 35.5–42.7 | 29 |
| b | 5.6 | 6.1–7 | 7 |
| c | 6.1 | 7–7.9 | 8 |
| c’ | 6.8 | 5.3–6.2 | 5 |
| Cephalic setae length | 2 µm | 2 µm | 5.5 µm |
| amph% | 40% | 38–44% | 55% |
| Precloacal supplements | 2 small papillae | 1 small papillae | – |
| Spicules length | 30 µm | 19–20 µm | 13 µm |
| Gubernaculum | – | – | – |
Discussion
Even with the use of phylogenetic analyses based on SSU sequences to investigate the taxonomic position of Molgolaimus, this question remains unresolved. The two most recent phylogenetic studies involving species of this genus presented divergent results. The phylogenetic analyses performed by Leduc, Fu & Zhao (2019) suggest that Molgolaimus should be classified with the Chromadorida and not the Desmodorida or Microlaimida, while those performed by Sun & Huang (2024) indicate that the genus belongs to Desmodoridae (Desmodorida). These studies used different parameters to perform their respective analyses (e.g., number of Orders and genus); Sun & Huang (2024) did not use SSU sequences of taxa belonging to Chromadorida, making it impossible to compare with the findings of Leduc, Fu & Zhao (2019), thus making it implausible to confirm or refute their observations regarding the phylogenetic position of the genus. Similar to Leduc, Verdon & Zhao (2018), previous studies investigated the phylogenetic position of Molgolaimus using the same tool and based on SSU sequences of Molgolaimus demani (Meldal, 2004; Meldal et al., 2007; Cavalcante, 2010; Leduc & Zhao, 2016), neglecting the fact that this species was transferred to the genus Microlaimus by Lorenzen (1981), and is therefore considered a synonym of Microlaimus tenuispiculum. Considering this conflict and while the taxonomic position of Molgolaimus remains under investigation, here we adopt the classification provided by De Ley, Decraemer & Eyualem (2006), which was based on both molecular evidence and morphological characteristics.
The Molgolaimus species grouping proposed by Fonseca, Vanreusel & Decraemer (2006), based on the absolute length of the spicules is clear and helps in the separation/identification of species. However, the division of subgroups is not as simple, especially the division between subgroups 1b1 and 1b2. Leduc, Fu & Zhao (2019) listed the valid species of the genus following the division proposed by Fonseca, Vanreusel & Decraemer (2006). When listing the species belonging to subgroup 1b1, they mentioned that this subgroup includes species whose spicules length measure between one and three times the cloacal body diameters. When listing the species of subgroup 1b2, they repeated the same information (species whose spicules lengths are equivalent to between one and three cloacal body diameters). The difference between subgroups 1b1 and 1b2 in the aforementioned list cannot be clearly identified. Furthermore, the order of presentation of group 4 (species with spicules lengths >80 µm), composed of subgroups 4a and 4b, does not follow a logical sequence. Subgroup 4a lists species that are not included in the grouping parameters of subgroup 4b (de Man’s ratio b = 8–11, spicules = 4–6 cloacal body diameters long). However, by logical sequence, the species whose adopted criteria allow for the division of the subgroup, should be presented first and, subsequently, those that are not included in the adopted criteria. In order to make the separation and identification of Molgolaimus species more practical, we propose a modification of the division of subgroups 1b1 and 1b2 and the rearrangement of the order of presentation of subgroups 4a and 4b.
To modify the division of subgroups 1b1 and 1b2, we adopted the relative position of the amphidial fovea as a parameter, i.e., the ratio of the distance between the anterior edge of the amphidial fovea in relation to the anterior end of the body divided by the head diameter (amph ant/hd) (Table 7). Although other characteristics can be used to designate the subgroups in question, this character is mentioned in most of the descriptions of Molgolaimus species. Furthermore, when absent in the description, it can be checked using the available images. The adopted proportion is used in other genera, such as Microlaimus, as part of a set of taxonomic tools to express similarity relationships or to identify differences between species (Kovalyev & Tchesunov, 2005; Gagarin & Tu, 2014; Revkova, 2020; Lima, Neres & Esteves, 2022; Manoel, Neres & Esteves, 2024). As in Fonseca, Vanreusel & Decraemer (2006), the subgroups proposed here are only based on male individuals. As a criterion for separating subgroups, we used the ratio amph ant/hd described/measured from the holotype of each species. The variations found in the paratypes, when available, were indicated in parentheses in Table 7.
| Species | Spicules length (µm) | Group | spic/cbd | amph ant/hd | Subgroup |
|---|---|---|---|---|---|
| M. amphimacrus | 34 | 1 | 2.4 | 3.5 | 1b1 |
| M. drakus | 20 (19) | 1 | 1.5 | 2.4 | 1b1 |
| M. gazii | 29 | 1 | 2 | 2.6 (3) | 1b1 |
| M. mareprofundus | 32 (29) | 1 | 1.9 (1.6) | 2 (1.7) | 1b1 |
| M. porosus | 25 | 1 | 1.9 | 2.8 | 1b1 |
| M. spirifer | 25 (22–23) | 1 | 1.5 (1.2–1.3) | 3 (2.7–3) | 1b1 |
| M. abyssorum | 20 (18–23) | 1 | 2 | <1 | 1b2 |
| M. carpediem | 26 (23) | 1 | 1.6 (1.4) | 1.2 (1.25–1.5) | 1b2 |
| M. exceptionregulum | 28 (29–31) | 1 | 1.6 (1.7–1.8) | 1.8 (2 –2.2) | 1b2 |
| M. falliturvisus | 26 | 1 | 1.7 | 1.4 | 1b2 |
| M. gallucci | 22 (22–27) | 1 | 1.7 (1.6–2.1) | 1.75 (1.8) | 1b2 |
| M. kiwayui | 20 (22) | 1 | 1.7 | 1.5 (1) | 1b2 |
| M. longicaudatus | 33 (32–35) | 1 | 1.8 (1.9) | 1 (1.2) | 1b2 |
| M. minutus | 23 (25) | 1 | 2.1–1.9 | 1.4 | 1b2 |
| M. pecticauda | 31.5 | 1 | 1.7 | 1.8 | 1b2 |
| M. sapiens | 35 | 1 | 1.8 | 1.6 | 1b2 |
| M. sigmoides sp. nov. | 30 (29–31.5) | 1 | 2 (2.1–2.2) | 1.7 (1.5–1.7) | 1b2 |
Molgolaimus species belonging to Group 1 that concomitantly have spicules lengths larger than one (>1) and smaller than three (<3) cloacal body diameters; ratio amph ant/hd greater than or equal to two (≥2) are included in subgroup 1b1 (Table 7). Species that concomitantly have spicules lengths greater than one (>1) and less than three (<3) cloacal body diameters; ratio amph ant/hd less than two (<2) are included in subgroup 1b2 (Table 7). The species M. exceptionregulum (Fonseca, Vanreusel & Decraemer, 2006), M. longicaudatus, M. pecticauda (Murphy, 1966; Shi & Xu, 2016) and M. sapiens which were previously part of subgroup 1b1, now appear in subgroup 1b2. The species M. amphimacrus (Bussau, 1993) and M. porosus (Bussau, 1993), which are now considered valid (Holovachov, 2020), were placed in subgroup 1b1. The other species remained in their original subgroups. In relation to group 4, following a logical sequence, subgroup 4b is now named subgroup 4a, which now includes species that simultaneously present: de Man’s ratio b = 8–11, spicules = 4–6 cloacal body diameters long (see Table 2). Subgroup 4b is then intended for those species that are not included in the criteria mentioned above (see Table 2).
The new species M. sigmoides sp. nov. belongs to subgroup 1b2 (spicules length >1 and <3 cloacal body diameter; ratio amph ant/hd <2). Molgolaimus sigmoides sp. nov. possess a gubernaculum with a dorsal-caudal apophysis, a characteristic never before reported for the genus. Mainly due to this, the new species can be confused and classified as belonging to the genus Aponema Jensen, 1978. However, it is important to note that females of all Aponema species possess a reproductive system with two opposite and outstretched ovaries. In Molgolaimus, the female reproductive system has two reflected ovaries. In appearance, the molgolaimids may be very similar to Microlaimidae but differ sharply with antidromously reflected ovaries (Tchesunov, 2014). Furthermore, representatives of the family Molgolaimidae have a slightly cuticularized pharynx associated with a pronounced pharyngeal bulb with sclerotized valves. This set of characteristics was not observed in Aponema species or in any representative of the family Microlaimidae Micoletzky, 1922.
Molgolaimus paralongispiculum sp. nov. belongs to subgroup 4b. Similarly to what was observed in Molgolaimus tanai Muthumbi & Vincx, 1996, M. paralongispiculum sp. nov. possess testis ventrally positioned in relation to the intestine. In most species of the genus the male genital branch is positioned to the right or left of the intestine. Although it is not always possible to clearly visualize this structure, it is important to record the variation that exists within the genus. The variation in the position of the germinal branch in relation to the intestine was also added to the diagnosis of the genus.
Molgolaimus brevispiculum sp. nov. belongs to subgroup 1a (spicules = 1 cloacal body diameter long). In absolute numbers, M. brevispiculum sp. nov. is the species with the smallest spicules among the representatives of the genus. Additionally, the gubernaculum is absent in this species. The absence of the gubernaculum is a rare characteristic in the genus, having only been detected in two other species (M. typicus and M. drakus). The possibility of the presence or absence of the gubernaculum in species of Molgolaimus was added to the diagnosis of the genus.
Although the occurrence of the genus Molgolaimus has already been recorded for the Brazilian coast, none of these studies include the description of new species of the genus. This study presents three new species of Molgolaimus for the first time, originally described from sediments collected on the Continental Shelf break of Northeastern Brazil. This finding contributes significantly to knowledge about the richness of species of Molgolaimus found in the South Atlantic Ocean. Furthermore, we provide critical elements that lead to the reorganization of the division of Molgolaimus species within previously existing subgroups, thus facilitating the identification/differentiation of species within the genus. Considering that the taxonomic position of Molgolaimus remains uncertain, additional efforts should be made to resolve this issue.