Effects of drought stress and Morchella inoculation on the physicochemical properties, enzymatic activities, and bacterial community of Poa pratensis L. rhizosphere soil
- Published
- Accepted
- Received
- Academic Editor
- Imren Kutlu
- Subject Areas
- Microbiology, Molecular Biology, Plant Science, Soil Science
- Keywords
- Drought-stress, Enzyme activity, Morchella inoculation, Poa pratensis L., Rhizosphere bacterial community, Soil physicochemical properties
- Copyright
- © 2025 Yin et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Effects of drought stress and Morchella inoculation on the physicochemical properties, enzymatic activities, and bacterial community of Poa pratensis L. rhizosphere soil. PeerJ 13:e18793 https://doi.org/10.7717/peerj.18793
Abstract
Background
Soil microorganisms are crucial for plant growth, and both plants and their associated rhizosphere microbes are impacted by changes in soil moisture. Inoculation with beneficial fungi can improve bacterial community structure and soil parameters.
Aim
Under drought stress conditions, the effects of inoculation with Morchella on the physicochemical properties, enzyme activity, and bacterial community structure of the rhizosphere soil of Poa pratensis were studied.
Methods
High-throughput sequencing was employed to study rhizosphere soil bacterial communities in both Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil subjected to moderate (50% soil moisture) and severe (30% soil moisture) drought stress, as well as under normal water conditions (70% soil moisture).
Results
Morchella inoculation significantly increased the alkaline nitrogen (AN) and available phosphorus (AP) contents, protease activity (PA), and alkaline phosphatase activity (APA) of Poa pratensis rhizosphere soil. Both Morchella inoculation and drought stress significantly altered the abundance and diversity of the P. pratensis rhizosphere community. The Chao1, Shannon, and Pielou diversity indices decreased with increasing drought stress. The effect of Morchella inoculation was improved under moderate drought stress and unstressed conditions. In addition, Morchella inoculation may help to stabilize the rhizosphere bacterial community under various levels of soil moisture.
Introduction
Plant growth and developmental processes not only shape plant health but are also essential to the survival of soil microorganisms (De Vries et al., 2020). These soil-level physical, biological, and chemical processes make up the “plant-soil-microbe” trinity (Das et al., 2022). Furthermore, rhizospheric microorganisms strongly influence agricultural production, plant growth, plant drought resistance, and plant disease resistance (Wang et al., 2019a; Ahmad et al., 2022; Wang et al., 2022; Lau & Lennon, 2012; Niu et al., 2017; Zhang et al., 2021).
Morel mushrooms (Morchella spp.) have culinary, therapeutic, nutritional, scientific, and commercial significance (Du & Yang, 2021; Sambyal & Singh, 2021). Humans began collecting and consuming wild morel mushrooms in the 19th century and later transitioned to cultivating them artificially (Du & Yang, 2021; Liu et al., 2022a). In China, large-scale Morchella farming began around the turn of the century, and there are more than 8,000 acres of Morchella farms today (Du & Yang, 2021; He et al., 2018).
Morchella cultivation is primarily soil-based and has raised many questions regarding how Morchella cultivation affects soil chemistry, physical structure, and ecology, as well as nearby plant communities. At present, there are relatively few reports on the impact of morel inoculation on plants. In 2021, a study found that intercropping Morchella with peach trees significantly enhanced the physical structure, fertility, and enzymatic activity of the soil, and increased peach yields (Song et al., 2021). The bulk of the soil microbiome associated with Morchella production is reported to be composed of Pedobacter, Pseudomonas, Stenotrophomonas, Flavobacterium (Benucci et al., 2019). Under continuous cropping regimes, an increased richness and diversity of soil microbiome was observed, but the abundance of Lactobacillus and Bacillus exhibited a notable decrease. The reduction in Morchella fruit body yield has been attributed to the enrichment of pathogenic fungi and acidified soil, which has aggravated over the years (Yue et al., 2024).
Kentucky bluegrass (Poa pratensis L.) is a widespread perennial grass that exhibits superior drought tolerance, hardiness, and regeneration qualities, and is commonly used as a turf grass and for animal feed (Kim et al., 2022; Kumar et al., 2022). However, little is known regarding the effects of Morchella inoculation and drought stress on the P. pratensis rhizosphere soil’s physicochemical characteristics, enzymatic activity, and bacterial community structure. There have been no reports on the interaction and symbiosis between Morchella and Poa pratensis L. However, during our investigation and research, we found that when collecting Morchella in the wild, Poa pratensis L. often appears near it. Therefore, in response to this discovery, we conducted the following research to verify whether Morchella inoculation has an impact on the growth of Poa pratensis L. Here, we conducted an indoor pot experiment to study how Morchella inoculation treatment and drought stress affects the P. pratensis rhizosphere. We utilized DNA amplicon sequencing of the 16S rRNA gene to study the rhizosphere soil bacterial community. The results presented here will be of great significance for both Morchella and P. pratensis farmers to improve the health of the crops, maximize yields, and ensure operational sustainability.
Materials and Methods
Experimental design
The soil for this experiment was provided by the Academy of Agriculture and Forestry Sciences experimental base at Qinghai University, which is located at N36°43.2034′ and E101°45.1752′ in the North District of Xining, Qinghai Province, China. Specifically, the soil was collected from the top 0–20 cm in a greenhouse used previously for Brassica chinensis L. cultivation. The soil had a calcareous chestnut texture, with a pH of 8.2, and contained 1.98 g/kg total nitrogen, 1.79 g/kg total phosphorus, and 17.99 g/kg total potassium. The indoor pot experiments employed three replications and a two-factor randomized block design. The experiment consisted of two Morchella inoculation treatments (inoculated (X) and uninoculated (K)) and three drought stress treatments (30%, 50%, and 70% soil moisture content).
Pot experiment
Each 12 × 15 cm diameter plastic pot was filled with 300 g of pre-prepared soil. Morchella-inoculated pots were established by adding 10 g of Morchella inoculant to each pot and using sterile wooden sticks to mix the soil. The Morchella sextelata variety QJ-2 was utilized for inoculation purposes. To prepare the inoculum, the stock culture maintained on potato dextrose agar (PDA) medium was transferred to sterile substrate for mycelium propagation. The substrate was composed of the following ingredients: 72.80% wood chips, 19.40% bran, 4.80% humus soil, 1% sucrose, 1% calcium superphosphate, 1% gypsum, and a water content ranging from 60% to 65%. The inoculated substrate was maintained at 22 °C for 30 days until the mycelium fully filled the cultivation bag. Uninoculated pots were established by adding 10 g of sterile substrate to each pot and using sterile wooden sticks to mix the soil. Thirty surface-sterilized P. pratensis seeds were distributed evenly across the soil surface in each pot. The pots were transferred to a light incubator maintained at 23 °C, 80% humidity, 3,000 Lx light intensity, and 16 h of light per day (Su, Qi & Yin, 2024). The humidity was adjusted to 60%, and the temperature was kept at 18 °C at night. Pots were watered every 2 days with distilled water using the constant weight method according to their assigned drought stress treatment.
Determination of plant growth performance
Following emergence, the seedlings in the pots were randomly thinned, with only 10 plants ultimately being retained. To evaluate the growth performance of P. pratensis, the plant height and leaf number per plant were measured 100 days after sowing.
Determination of soil chemical properties
Rhizosphere samples were collected 100 days after sowing. To ensure that each sample contained at least 30 g of soil, all rhizosphere soil from each of the three pots used for each treatment was removed. Soil samples were frozen in liquid nitrogen for cryopreservation. In all, three lyophilization tubes were collected for each of the six treatments, for a total of eighteen sample tubes. Soil alkaline nitrogen (AN) content was determined according to the method of Prasad (1965). The determination was carried out using a Kjeldahl nitrogen analyzer, following a brief procedure as follows: 10 mL of 4 M NaOH and 0.1 g of FeSO4 were added to 1 g of air-dried soil. After distillation, the ammonium is absorbed by boric acid solution, and the AN content was calculated after titrating it with 0.005 M sulfuric acid. Soil available phosphorus (AP) content was determined according to the method of Olsen et al. (1954). A total of 50 mL of 0.5 M NaHCO3 (pH = 8.5) was added to 2.5 g of air-dried soil, and the suspension was shaken for 30 min in an oscillator for extraction. 10 mL of filtrate was mixed with 5 mL of molybdenum-antimony solution and incubated at room temperature for 30 min. The AP content was quantified with a spectrophotometer. Soil available potassium (AK) content was determined according to the method of Leaf (1958). 50 mL of 1 M NH4OAc (pH = 7) was added to 5 g of air-dried soil. After shaking for 30 min, the filtrate was collected and subjected to measurement of excitation intensity using a flame photometer. The AK content was then calculated.
Determination of soil enzymatic activity
Soil catalase (CAT) activity was determined according to the method of Minczewski & Marczenko (1973). Briefly, 40 mL of distilled water and 5 mL of 3% hydrogen peroxide solution were added to 2 g of fresh soil. The mixture was shaken for 30 min and subsequently filtered. A volume of 25 mL of the filtrate was titrated with 0.1 M potassium permanganate until the solution turned pink. Soil alkaline phosphatase (APA) activity was determined according to the method of Tabatabai & Bremner (1969). Briefly, 2 g fresh soil was gently mixed with 5 mL CaCl2 and 1 mL p-nitrophenyl phosphate (pNPP). After incubation at 37 °C for 1 h, the reaction was terminated with 0.5 M NaOH. The mixture was then filtered, and pNP content was determined with a spectrophotometer. Soil protease (PA) activity was determined according to the method of Speir & Ross (1975). A total of 5 g of fresh soil was thoroughly mixed with 10 mL of 2% casein. After incubation at 50 °C for 2 h, the reaction was terminated by adding 17.5% trichloroacetic acid. The protein content in the filtrate was determined spectrophotometrically, and the PA activity was subsequently calculated.
Soil DNA extraction and PCR amplification
Total microbial DNA was recovered from soil samples using the Soil Microbial DNA Quick Extraction Kit (Mo Bio Laboratories Inc., Carlsbad, CA, USA). Following PCR amplification of the complete soil microbial DNA sample, a sequencing library was created. The V3-V4 variable region, specific to the bacterial 16S rRNA, was amplified using the upstream primer 341F 5-CCTACGGGNGGCWGCAG-3 and the downstream primer 806R 5-GGACTACHVGGGTATCTAAT-3 (Klindworth et al., 2013). The following thermal conditions were applied for amplification: 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s for a total of 35 cycles; and a final extension step at 72 °C for 10 min. After passing quality control, the PCR amplification products were cut, recovered, and quantified using a Quanti Fluor fluorometer. The sequencing junctions were ligated and the sequencing libraries were created. Finally, an Illumina sequencer PE250 was used to sequence a mixture of equal amounts of the purified amplification products.
Bioinformatics analysis
Sequencing was followed by data filtering, quality control, and single-base precision amplicon sequence variant (ASV) clustering. ASV clustering is equivalent to clustering operational taxonomic units (OTUs) with 100% similarity (Bokulich et al., 2013; Callahan et al., 2016). Chimera filtering was used to splice the bipartite data using overlap and quality control to obtain high-quality, clean data. Following the acquisition of the ASVs/OTUs (DeSantis et al., 2006; Pruesse et al., 2007; Wang et al., 2007), α-diversity analysis (Caporaso et al., 2010), β-diversity analysis, and community function prediction were carried out. The species annotation method was applied sequentially following the analytical process (Asshauer et al., 2015; Louca, Parfrey & Doebeli, 2016).
Statistical analysis
Microsoft Excel was used to calculate percentages and SPSS 23.0 was used for statistical analysis. Statistically significant differences among groups were determined using one-way ANOVA. Histograms were created with Origin 2021. The R packages psych, igraph, labdsv, Vegan, ggplot2, and pheatmap were used to calculate inter-species correlation coefficients, create inter-species correlation network and ternary diagrams, perform redundancy and correlation analyses, and graph the outputs.
Results
Plant growth performance
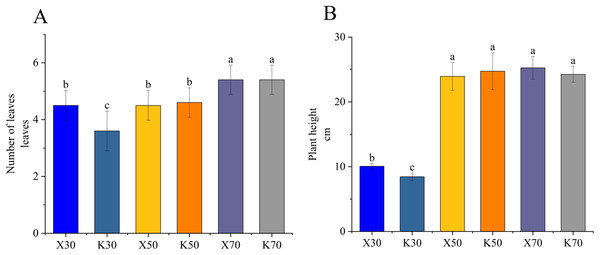
As illustrated in Fig. 1, the X30 treatment group exhibited significantly greater plant height and leaf number per plant compared to the K30 treatment group, showing enhanced growth performance of Morchella-inoculated P. pratensis plants under severe drought conditions. However, the growth-promoting effect was not readily apparent under 50% and 70% moisture conditions. These findings indicate that the growth promotion resulting from Morchella inoculation is contingent upon the moisture content of the soil.
Figure 1: Growth performance of Morchella-inoculated and uninoculated Poa pratensis.
X: Morchella-inoculated. K: uninoculated. (A) Number of leaves per plant. (B) Plant height. In each graph, different letters among bars indicate statistically significant differences (P < 0.05).Rhizosphere soil physicochemical properties and enzymatic activities
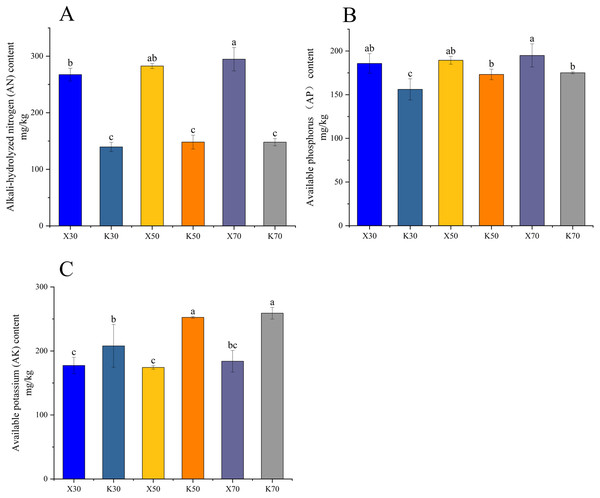
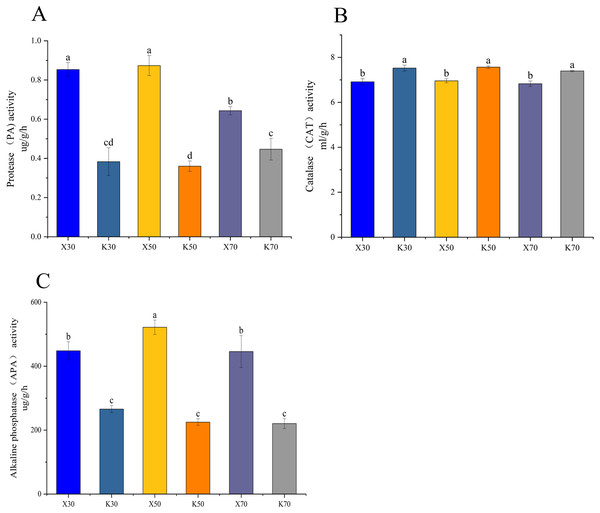
Morchella inoculation considerably decreased the AK content, increased the AN and AP content, enhanced the PA and APA activity, and diminished the CAT activity, in Poa pratensis rhizosphere soil (Figs. 2, 3). In Morchella-inoculated samples, soil AN increased with soil water content, and was significantly higher in the 70% treatment than in the 30% treatment (Fig. 2A). In uninoculated samples, soil AP increased with soil water content, and was significantly higher in the 50% and 70% treatments than in the 30% treatment (Fig. 2B). Furthermore, soil APA activity was notably higher in the 50% treatment as compared to the 30% and 70% treatments, whereas the PA activity was considerably lower in the 70% treatment than in both the 30% and 50% treatments (Figs. 3A, 3C).
Figure 2: Physicochemical properties of Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil.
X: Morchella-inoculated. K: uninoculated. (A) Protease (PA) activity. (B) Catalase (CAT) activity. (C) Available potassium (AK) content. In each graph, different letters among bars indicate statistically significant differences (P < 0.05).Figure 3: Enzymatic activities of Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil.
X: Morchella-inoculated. K: uninoculated. (A) Alkaline nitrogen (AN) content. (B) Available phosphorus (AP) content. (C) Alkaline phosphatase (APA) activity. In each graph, different letters among bars indicate statistically significant differences (P < 0.05).High-throughput sequencing results
After sequence quality control, denoising, splicing, and chimera removal, a total of 944,833 reads were recovered from Morchella-inoculated samples, with an average of 104,981 reads per sample. In addition, a total of 44,533 ASVs, with an average of 4,948 ASVs per sample, were identified in Morchella-inoculated samples. A total of 914,435 reads were recovered from uninoculated samples, with an average of 101,603 reads per sample. A total of 43,018 ASVs, with an average of 4,779 ASVs per sample, were identified in uninoculated samples (Table S1).
Microbiological community analysis
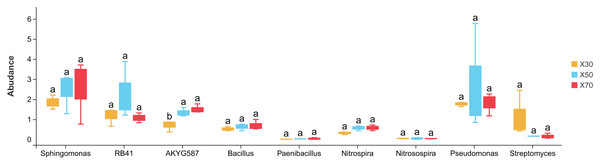
After excluding the “unclassified” and “other genera” categories, the top-ten bacterial genera across all samples were Sphingomonas, RB41, AKYG587, Bacillus, Flavobacterium, Pseudomonas, Pir4_lineage, Pirellula, JGI_0001001-H03, and Stenotrophobacter (Fig. 4). In comparison to the uninoculated treatments (K30, K50, and K70), the abundance of Bacillus decreased by 0.99%, 1.68%, and 1.38% in X30, X50, and X70, respectively. In contrast, the abundance of Pseudomonas exhibited an increase of 1.46%, 2.53%, and 2.18% in X30, X50, and X70, respectively. For JGI_0001001-H03, its abundance increased by 1.28%, 1.49%, and 0.76% in X30, X50, and X70, respectively. Specifically concerning RB41 and AKYG587, their respective abundance levels decreased by 1.64% and 0.85% in X30. Sphingomonas abundance decreased with increasing water stress, falling by 0.73% in X30 and 1.01% in K30 compared to X70 and K70, respectively. Overall, Morchella inoculation appears to significantly alter the bacterial community structure of P. pratensis rhizosphere soil under various levels of drought stress.
Figure 4: Relative abundances of Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil bacteria under various levels of drought stress.
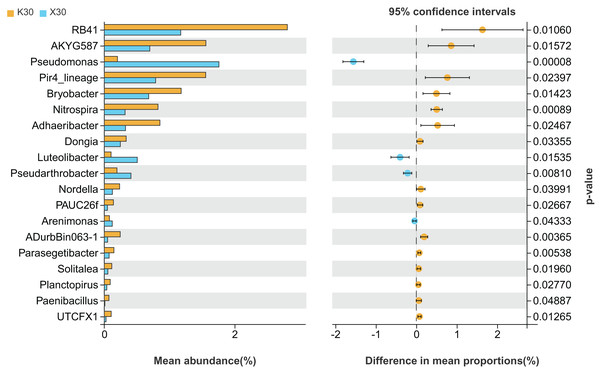
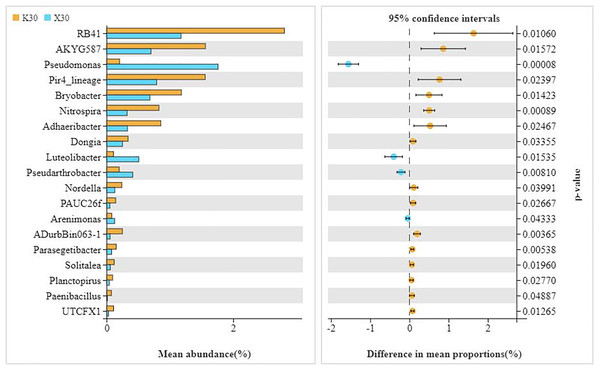
X: Morchella-inoculated. K: uninoculated.We observed substantial variation in the abundances of 19 different bacterial genera between X30 and K30 (Fig. 5), with the abundances of 15 bacterial genera being higher in K30 than in X30. In contrast, the abundances of Pseudomonas, Luteolibacter, Pseudarthrobacter, and Arenimonas were significantly higher in X30 compared to K30. These results suggest that Morchella inoculation under severe drought stress may lead to decreased rhizosphere community diversity and abundance.
Figure 5: Comparison of indicator genera between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under severe drought stress (30% soil moisture).
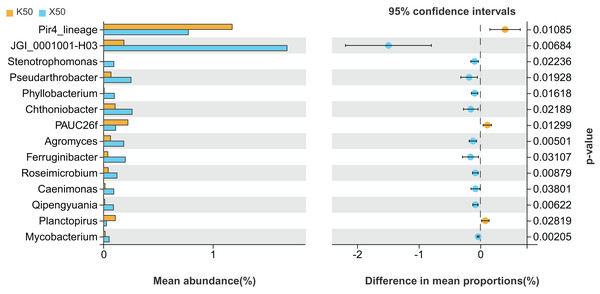
X: Morchella-inoculated. K: uninoculated. Note: Only shown are bacterial genera with significantly different (P < 0.05) relative abundances between treatments.There were significant differences (P < 0.05) in the abundances of 14 different bacterial genera between X50 and K50 (Fig. 6), including JGI 0001001-H03, Stenotrophomonas, Pseudarthrobacter, and Phyllobacterium, among others. Among these, the abundances of Pir4 lineage, PAUC26f, and Planctopirus were significantly lower in X50 than in K50, while the remaining 11 genera were significantly higher in X50. These results suggest that Morchella inoculation under moderate drought stress may lead to increased rhizosphere community diversity and abundance.
Figure 6: Comparison of indicator genera between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under moderate drought stress (50% soil moisture).
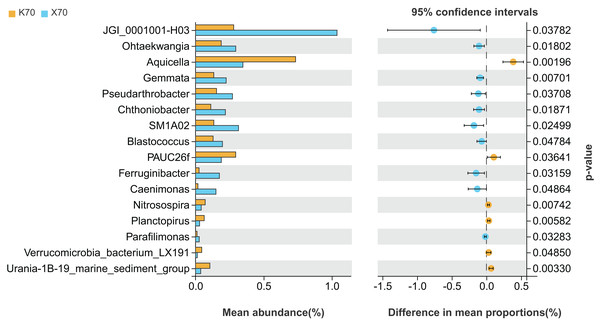
X: Morchella-inoculated. K: uninoculated. Note: Only shown are bacterial genera with significantly different (P < 0.05) relative abundances between treatments.There were significant differences (P < 0.05) in the abundances of 16 different bacterial genera between X70 and K70 (Fig. 7). The abundances of 10 genera, including JGI_0001001-H03, Ohtaekwangia, Gemmata, and Pseudarthrobacter, were significantly higher in X70 than in K70. The abundances of Aquicella, PAUC26f, Nitrosospira, Planctopirus, Verrucomicrobia_bacterium_LX191, and Urania-1B-19_marine_sediment_group were significantly lower in X70 than in K70. The findings suggest that under adequately watered conditions, the introduction of Morchella results in an increase in the diversity of the rhizosphere bacterial community.
Figure 7: Comparison of indicator genera between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under unstressed conditions (70% soil moisture).
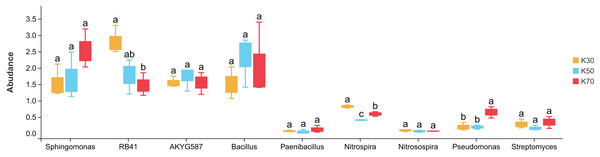
X: Morchella-inoculated. K: uninoculated. Note: Only shown are bacterial genera with significantly different (P < 0.05) relative abundances between treatments.There were notable differences among bacterial genera in uninoculated P. pratensis rhizosphere soil under different water conditions (Fig. 8), including for RB41, Nitrospira, and Pseudomonas. The abundance of RB41 was significantly higher in K30 than in K70, and the abundance of Nitrospira was significantly higher in K30 than in K50 and K70. The abundance of Pseudomonas was significantly higher in K70 than in K30 and K50. Fewer differences were observed in Morchella-inoculated P. pratensis rhizosphere soil under different water conditions (Fig. 9). Specifically, the only notable difference was that of AKYG587 in X30, the abundance of which was significantly lower in X30 than in X50 and X70. Taken together, these results suggest that Morchella inoculation may help to stabilize the rhizosphere bacterial community under various levels of soil moisture.
Figure 8: Comparison of indicator genera in uninoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
Different letters among bars indicate statistically significant differences (P < 0.05). Note: Only shown are bacterial genera with significantly different (P < 0.05) relative abundances between treatments.Figure 9: Comparison of indicator genera in Morchella-inoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
Different letters among bars indicate statistically significant differences (P < 0.05). Note: Only shown are bacterial genera with significantly different (P < 0.05) relative abundances between treatments.Alpha diversity
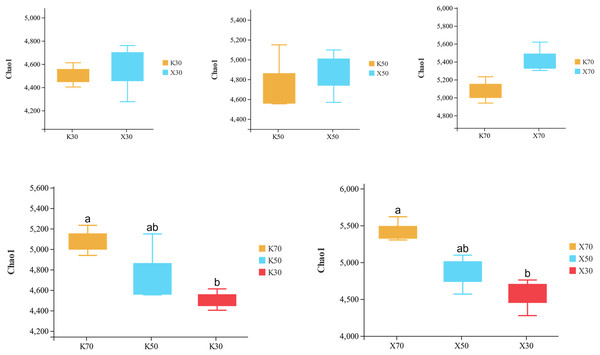
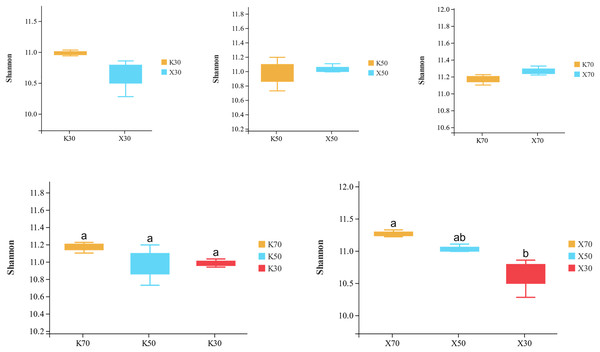
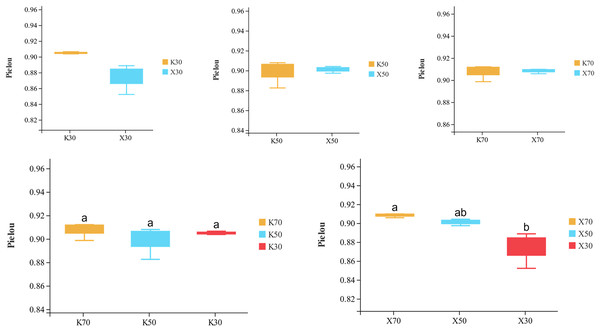
Alpha diversity is primarily utilized to evaluate bacterial diversity among different ecological zones. Considerable differences in species richness (Chao1 index) were observed between moisture conditions, with 70% > 50% > 30% across both Morchella-inoculated and uninoculated soils (Fig. 10). In addition, both the Shannon and Pielou indices were significantly higher under 70% moisture than under 30% moisture (Figs. 11 and 12). In this experiment, we observed that soil moisture had a greater impact on alpha diversity than did inoculation.
Figure 10: Comparison of Chao1 diversity index between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
X: Morchella-inoculated. K: uninoculated. Different letters among bars indicate statistically significant differences (P < 0.05).Figure 11: Comparison of Shannon diversity index between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
X: Morchella-inoculated. K: uninoculated. Different letters among bars indicate statistically significant differences (P < 0.05).Figure 12: Comparison of Pielou diversity index between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
X: Morchella-inoculated. K: uninoculated. Different letters among bars indicate statistically significant differences (P < 0.05).Beta diversity
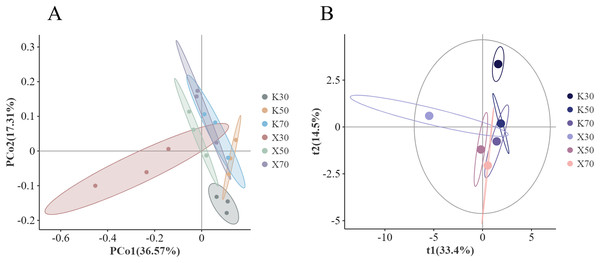
The genus-level principal co-ordinates analysis (PCoA) analysis based on Bray distances showed considerable separation between certain groups (Fig. 13A), particularly the 30% soil moisture treatments. Differences in bacterial community structure were explained by PCo1 in 36.57% of cases and PCo2 in 17.31%. In general, uninoculated soil samples were concentrated between −0.1–0.2 along the PCo1 axis, but more scattered across the PCo2 axis. The Morchella-inoculated soil samples tended to be distributed more randomly along both the PCo1 and PCo2 axes. The PCo2 axis was found to be extended by the overall tendency of progressive decline with decreasing water content.
Figure 13: Comparison of β-diversity between Morchella-inoculated and uninoculated Poa pratensis rhizosphere soil under various water levels (30%, 50%, and 70% soil moisture).
X: Morchella-inoculated. K: uninoculated. (A) PCoA analysis. (B) PLS-DA analysis.PLS-DA analysis revealed that t1 explained 33.4% of the variation in bacterial community structure between treatments, while t2 explained 14.5% of the variation (Fig. 13B). The Morchella-inoculated soil samples were primarily found near the 0 attachment and the negative end of the t1 axis, while the absolute value of the t2 axis was within 2.5. The uninoculated samples were more dispersed along the t2 axis, primarily between the t1 axis 0 and 2.5. The t2 axis was found to be extended by the propensity to progressively rise with diminishing water.
Correlation between soil factors and soil community structure
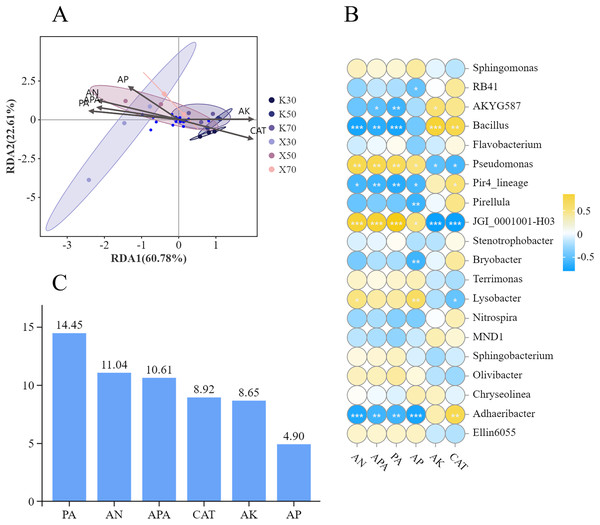
To evaluate any correlations between soil physicochemical properties and enzymatic activities and rhizosphere bacterial community structure, we performed a redundancy analysis (RDA) (Fig. 14A). Overall, RDA1 and RDA2 contributed 60.78% and 22.61% of the explanatory factors, respectively, while the two axes combined contributed 83.39%. The redundancy analysis further indicated that Morchella-inoculated and uninoculated P. pratensis rhizosphere soil had different bacterial community structures. Four soil variables (AN, AP, APA, and PA) were strongly associated with the Morchella inoculation treatment group. Conversely, AK and CAT were more strongly associated with the uninoculated treatment group.
Figure 14: Correlations between soil physicochemical properties and rhizosphere bacterial community structure.
(A) RDA analysis. (B) Correlations between soil properties and relative abundances of bacterial genera. (C) Explanatory value of soil properties. Asterisks (*, **, and ***) signify the level of correlation significance at p < 0.05, p < 0.01, and p < 0.001, respectively.Bacterial community structure was significantly influenced by soil factors (Fig. 14B). The relative abundance of Bacillus showed a significant positive correlation with both AK content and CAT activity, whereas it exhibited a significant negative correlation with the levels of AN, APA, and PA activity. The relationship between the relative abundance of Pseudomonas and soil factors was exactly the opposite of that of Bacillus. Specifically, AN and AP content, as well as APA and PA activity, were positively correlated with the relative abundance of Pseudomonas; whereas AK content and CAT activity were negatively correlated. The correlation between the relative abundance of JGI_0001001-H03 and soil factors displayed a similar pattern to that observed in Pseudomonas as a whole, despite differences in correlation and significance. The relative abundance of Adhaeribacter also demonstrated significant correlations with multiple soil factors. Notably, a negative correlation was observed with the content of AN, AP, APA, and PA activity, whereas a positive correlation was found with CAT activity.
The distribution of soil bacteria was most significantly influenced by PA activity (Fig. 14C), which contributed 14.45%, followed by AN content. The distribution of soil bacteria was least significantly influenced by AP content, which contributed 4.9%.
Discussion
Previous studies have shown that inoculating plants with fungi could yield significant growth-promoting effects. For instance, it is found that inoculation of arbuscular mycorrhiza not only significantly increases the plant height, ear length, ear weight, straw yield, and grain yield of barley, but also improves the plant’s ability to absorb nitrogen, phosphorus, and potassium elements (Masrahi et al., 2023). A soil fungus, JF27 (Aspergillus terreus JF27), isolated from the rhizosphere of chili peppers, can significantly increase tomato yield and quality, while also enhancing the tomato’s resistance to pathogens (Yoo et al., 2018). The isolation of endophytic fungi in Bromus tectorum revealed that Morchella species could infect plant roots in a non-mycorrhizal manner, demonstrating a mutualistic relationship with this fire-tolerant plant (Baynes et al., 2012). A Morchella crassipes strain collected from post-fire Populus simonii stands exhibited root colonization, significantly enhanced drought tolerance, increased biomass, and improved resistance to Fusarium in sweet corn (Yu et al., 2016).
In the current study, the introduction of Morchella resulted in changes in soil nutrients and enzyme activities. Specifically, it led to an increase in AN and AP content, as well as PA and APA activity. Additionally, a decrease in AK content and CAT activity was also observed. This is in agreement with a previous study that found intercropping Morchella with peach trees significantly enhanced soil fertility and structure, including increasing total N, total P, and effective P contents (Song et al., 2021). Changes in soil texture resulting from Morchella inoculation may be the primary driver of these improved soil physicochemical properties. Fungal amendments have been shown to promote soil aggregation and induce changes in soil hydrological properties. Formation of aggregates was found to be positively correlated with fungal biomass, through which soil particles were linked by hyphae (Angulo et al., 2024). The tight relationship among soil texture, nutrient availability, and root exudates may serve as the underlying mechanism responsible for the alterations observed in soil chemical properties and the rhizosphere microbiome after Morchella inoculation of P. pratensis plants (Adeniji et al., 2024).
Species richness (Chao1 index) was higher in the Morchella inoculation treatments than in the uninoculated treatments, although the result was not statistically significant. In addition, Chao1 was lower in severely water stressed (30% soil moisture) treatments compared to moderate water stressed (50%) treatments and unstressed (70%). Similarly, in Morchella-inoculated treatments, both the Shannon and Pielou indices decreased as soil moisture decreased. The PCoA analysis of β-diversity showed that Morchella-inoculated P. pratensis rhizosphere soil samples were more evenly distributed along the PCo1 and PCo2 axes, while the uninoculated soil samples were concentrated along PCo1. Overall, the impact of Morchella-inoculation on the α-diversity of rhizosphere bacteria is not significant. This phenomenon is also observed in other studies, with some bacteria increasing in abundance and others decreasing, while the overall change (α-diversity) remains minimal (Duan et al., 2021; Xiong et al., 2014). Drought can have a significant impact on rhizosphere microorganisms because changes in soil moisture alter nutritional characteristics, enzyme activity, and bacterial community composition (Tian et al., 2018; Brockett, Prescott & Grayston, 2012). The significant impact of drought stress on α-diversity was confirmed in both the Morchella-inoculated and Morchella-uninoculated treatment groups in our study.
Across all treatments, the ten most abundant bacterial genera were Sphingomonas, RB41, AKYG587, Bacillus, Flavobacterium, Pseudomonas, Pir4 lineage, Pirellula, JGI 0001001-H03, and Stenotrophobacter. Morchella inoculation tended to decrease the abundance of Bacillus while increasing the abundance of Pseudomonas and JGI 0001001-H03. In addition, Morchella inoculation may have helped to stabilize the rhizosphere bacterial community under various levels of soil moisture. Even under severe drought stress (30% soil moisture), the abundances of Pseudomonas, Luteolibacter, Pseudarthrobacter, and Arenimonas were substantially higher in X30 than in K30. Under moderate drought (50% soil moisture), Morchella inoculation increased the relative abundances of JGI 0001001-H03, Stenotrophomonas, Pseudarthrobacter, and Phyllobacterium. Under unstressed conditions, Morchella inoculation increased the relative abundances of JGI 0001001-H03, Ohtaekwangia, Gemmata, and Pseudarthrobacter. Under both mild drought stress and normal moisture conditions, JGI 0001001-H03 tends to be more prevalent in Morchella-inoculated soils than in uninoculated soils. The introduction of fungi, particularly growth-promoting species, notably influences the structure and composition of the bacterial community within the rhizosphere. For instance, inoculation of Trichoderma harzianum T-63 significantly increased the relative abundance of Pseudomonas, Flavobacterium, and Arthrobacter in the rhizosphere soil of alfafa plants (Zhang et al., 2019). Moreover, Trichoderma-bacteria co-inoculations (usually with Pseudomonas and Bacillus) often exhibits synergistic effects on plant growth promotion and stress resistance (Poveda & Eugui, 2022). It remains to be further verified whether the changes in the rhizosphere bacterial community caused by the introduction of Morchella are associated with the enhanced drought resistance of P. pratensis.
The use of bacterial biofertilizers and inoculants has been shown to significantly decrease the abundance of soil pathogens and increase the abundance of beneficial microbes such as Flavobacterium, Pseudomonas, and Agrobacterium (Fuentes-Ramirez & Caballero-Mellado, 2006; Qin et al., 2017; Tao et al., 2020; Wang et al., 2019b). In particular, Pseudomonas has been found to produce growth-promoting hormones, solubilize P, increase soil inorganic nutrients, promote plant growth, and manage plant diseases (Schillaci et al., 2022; Garcia-Salamanca et al., 2013; Preston, 2004; Hu et al., 2016). Furthermore, Pseudomonas induces systemic resistance (ISR) in plants by secreting certain organic compounds and participating in the breakdown of soil macromolecular organophosphorus into small fractions of phosphate esters (Zhu et al., 2021; Olanrewaju, Glick & Babalola, 2017; Iavicoli et al., 2003; Schuhegger et al., 2006). In another study, it was found that the mycorrhizal fungus Suillus luteus tended to increase the abundance of Pseudomonas, resulting in improved soil physicochemical properties, including soil organic matter and total N (Zhou et al., 2022). Fungi can alleviate both abiotic and biotic stresses in plants, such as enhancing drought resistance, enhancing tolerance to high and low temperatures, enhancing salt and acid tolerance, alleviating the toxicity of heavy metals to plants, and improving plant disease and insect resistance (Zhang, Gu & Duan, 2018). Fungal inoculation can promote the transformation of organic matter and accelerate the degradation of nitrogen and phosphorus compounds in soil, providing sufficient nitrogen and phosphorus for plant growth and development (Wei et al., 2021).
Notable differences in the mean abundances of RB41, Nitrospira, and Pseudomonas were observed between soil moisture levels in uninoculated samples. Conversely, in Morchella-inoculated samples, the only notable difference was that of AKYG587 in X30, the abundance of which was significantly lower in X30 than in X50 and X70. Recently, it has been reported that RB41 significantly affects how effectively soil organisms like Acidophilus utilize nutrients. In addition, the addition of fermented food waste to the soil improved RB41 colonization, and RB41 favorably enhanced the nutritional level of the soil (Meng et al., 2022; Liu et al., 2022b). Nitrospira participates in the nitrogen cycle by converting nitrite into plant-available nitrate. The abundance of Nitrospira in the rhizosphere can be significantly increased, especially under intercropping practices (Chen et al., 2018; Tang et al., 2020). The presence of Nitrospira may indirectly enhance plant drought resistance by increasing nitrogen transformation. Recently, a survey of oil palm soil with a low incidence of soil disease showed that it contained higher relative abundances of several beneficial bacterial taxa, including AKYG587 and Calditrichaeota (Goh et al., 2020). In other studies, the relative abundance of AKYG587 increased dramatically after Pseudomonas or Bacillus biocontrol agents were introduced to the soil, and soil health and plant biomass was also significantly improved (He et al., 2019; Zhao et al., 2019).
Correlation analysis indicated that four environmental variables (AN, AP, APA, and PA) were strongly associated with Morchella inoculation. It was shown that the activities of soil catalase, sucrase, cellulase, and urease are significantly improved by interplanting Morchella and peach trees (Song et al., 2021). Fungal secretions can significantly improve soil microbial structure, enzymatic activity, and N accumulation (Wang et al., 2014; Vázquez et al., 2000; Zhang et al., 2024). The relative abundance of Bacillus was significantly positively correlated with AK content and CAT activity. A significant positive correlation was found between the relative abundance of Pseudomonas and the contents of AN and AP as well as the activities of APA and PA. As one of the earliest utilized PGPR, Bacillus has been manifested to be effective in promoting the release of soil N and P, enhancing root activity and plant growth, and to accumulate nutrients in the aboveground tissues (Radhakrishnan, Hashem & Abd_Allah, 2017; Liu et al., 2017a). Pseudomonas and other beneficial bacterial species are relatively abundant in the Morchella rhizosphere soil (Benucci et al., 2019; Pion et al., 2013; Liu et al., 2017b).
Conclusions
In this study, we conducted an indoor pot experiment to study the effects of Morchella inoculation and drought stress on the P. pratensis rhizosphere community. Morchella inoculation significantly increased the contents of AN and AP and the activities of PA and APA, and decreased the content of AK and the activity of CAT. Both Morchella inoculation and drought stress significantly altered the abundance and diversity of the P. pratensis rhizosphere community. Sphingomonas, RB41, AKYG587, Bacillus, Flavobacterium, Pseudomonas, Pir4, and 10 other genera were the dominant bacterial genera across different treatments. The Chao1, Shannon, and Pielou diversity indices decreased with increasing drought stress. According to PCoA and PLS-DA analyses, Morchella inoculation and water stress both affected bacterial community structure. The effect of Morchella inoculation was improved under moderate drought stress and unstressed conditions. In Morchella-inoculated samples, the abundances of only a few soil bacteria were significantly influenced by moisture levels.