Visualizing and quantifying biomineral preservation in fossil vertebrate dental remains
- Published
- Accepted
- Received
- Academic Editor
- Philip Reno
- Subject Areas
- Paleontology, Biogeochemistry
- Keywords
- Biomineral presevation, Diagenesis, Trace elements, REE, Microcathodoluminescence, Dental fossils
- Copyright
- © 2025 Cowen et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Visualizing and quantifying biomineral preservation in fossil vertebrate dental remains. PeerJ 13:e18763 https://doi.org/10.7717/peerj.18763
Abstract
In this study, we attempt to illustrate fossil vertebrate dental tissue geochemistry and, by inference, its extent of diagenetic alteration, using quantitative, semi-quantitative and optical tools to evaluate bioapatite preservation. We present visual comparisons of elemental compositions in fish and plesiosaur dental remains ranging in age from Silurian to Cretaceous, based on a combination of micro-scale optical cathodoluminescence (CL) observations (optical images and scanning electron microscope) with in-situ minor, trace and rare earth element (REE) compositions (EDS, maps and REE profiles), as a tool for assessing diagenetic processes and biomineral preservation during fossilization of vertebrate dental apatite. Tissue-selective REE values have been obtained using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS), indicating areas of potential REE enrichment, combined with cathodoluminescence (CL) analysis. Energy dispersive X-ray spectroscopy (EDS) mapping was also used to identify major elemental components and identify areas of contamination or diagenetic replacement. We conclude that the relative abilities of different dental tissues to resist alteration and proximity to the exposure surface largely determine the REE composition and, accordingly, the inferred quality of preserved bioapatite.
Introduction
Assessing the preservation quality of fossil hard tissues such as bone, dentine, enamel or enameloid is fundamental to research that utilizes this material as a source of biogeochemical data. Isotopic and elemental proxies derived from fossil bioapatite rely on specimens which have not experienced significant late-diagenetic alteration to accurately reflect palaeobiology or the environmental conditions in the past. The chemical composition of fossil bone tissues, including trace elements and stable light isotope ratios, may provide valuable information on the biology of extinct species, such as thermometabolism (e.g., Amiot et al., 2007; Bernard et al., 2010; Eagle et al., 2011; Rey et al., 2017; Séon et al., 2020; Leuzinger et al., 2022), diet (e.g., Heuser et al., 2011; Owocki et al., 2020; Klock et al., 2022), ecology and environments (e.g., Bryant & Froelich, 1995; Fricke et al., 2008; Amiot et al., 2010; Goedert et al., 2018, 2020; De Rooij et al., 2022; Thibon et al., 2022). The ability to make such inferences depends on the preservation quality of the fossil remains, and at present there exists no definitive methodology for screening out diagenetic alteration.
To better understand the effects of diagenesis and to discriminate the primary (or closest-to-primary) geochemical signal from early diagenetic secondary overprinting, a spatially resolved compositional analysis of the histological sections of fossil bioapatite is required. In this study we combine spectroscopic mapping techniques including cathodoluminescence (CL) and energy dispersive spectroscopy (EDS) analysis with in-situ rare earth element (REE) analysis to visualize compositional changes. We examine plesiosaur teeth and lungfish dental plates from the Lower Cretaceous, as well as Devonian fish scales to compare potential biomineral preservation in enamel, enameloid, and dentinous tissues.
The mineral component of vertebrate hard tissues is composed of biological apatite, commonly present in the form of carbonate hydroxyapatites, which stabilize to fluorapatite [Ca5(PO4)3F] during diagenesis as the carbonate component diminishes and is replaced by fluorine (Trotter & Eggins, 2006; Keenan et al., 2015; Lübke et al., 2017). Depending on the conditions and environment of burial, the processes of fossilization may lead to the modification of preserved biominerals through ionic exchange and rearrangements in the primary structure throughout the incorporation of foreign ions in the crystal lattice. These substitutions may include rare earth elements (REE) for Ca2+ in Ca sites (Burton & Wright, 1995; Bryant & Froelich, 1995; Trueman & Tuross, 2002; Trueman et al., 2006; Kocsis, Trueman & Palmer, 2010; Heuser et al., 2011).
REE composition of fossil vertebrate hard tissues is an established tool for determining the extent of reworking and chemical changes during taphonomy (Trueman, 1999, 2013; Kohn & Cerling, 2002). Rare earth elements are also commonly used in the reconstruction of past environments (Grandjean et al., 1987; Kemp & Trueman, 2003; Lécuyer, Reynard & Grandjean, 2004; Fadel et al., 2015; Žigaitė et al., 2016; Ivanova et al., 2022), principally as a proxy to provenance, taphonomy and diagenesis. The incorporation of REEs and other trace elements into bioapatite predominantly takes place post-mortem (Toyoda & Tokonami, 1990) due to the infiltration from either sediment pore water, or directly from surrounding water bodies.
Apatite, with its very high affinity for REE, frequently contains at least two to three orders of magnitude higher REE concentrations than any other mineral phase present in the fossil bones and teeth (Trueman & Palmer, 1997; Kohn, Schoeninger & Barker, 1999; Trueman, 1999). Concentrations of REE in fossil apatite from marine basins are higher than any other sedimentary mineral and commonly 5–6 orders of magnitude higher than seawater (Kolodny et al., 1996). The REE reside in the two calcium sites in the apatite lattice and are normally present in living bone at the ppb level (Shaw & Wasserburg, 1985), while fossil bones yield much higher REE levels, usually in the 103 ppm range (Kolodny et al., 1996).
The REE composition of apatitic fossils is taxon-independent since the REE do not appear to be physiologically vital trace elements and in vivo bone concentrations are several orders of magnitude lower than diagenetic concentrations (Trueman, 1999). Wright, Schrader & Holser (1987) argued that ichthyoliths (disarticulated dermal and dental fish remains), concentrated at the sediment-water interface, exhibit an enrichment in REEs, with no discernible fractionation of REEs occurring during this process. However, Reynard, Lécuyer & Grandjean (1999) convincingly argue for fractionation between seawater and ichthyoliths. Debate remains (summarized by Ivanova et al. (2022)) as to whether REE uptake occurs only during early diagenesis or whether the process occurs continuously. Two main mechanisms exist for REE trapping in phosphates—adsorption and substitution (Reynard, Lécuyer & Grandjean, 1999; Trueman & Tuross, 2002).
However, the adsorption process is in equilibrium and desorption of REE3+ ions can occur over time as argued by Li et al. (2021). Herwartz et al. (2011, 2013a, 2013b) have disputed the view set out by Reynard, Lécuyer & Grandjean (1999) that adsorption and substitution represent “early” and “late” stages of diagenesis. Further, Chen et al. (2015) have shown that in order to capture the composition of contemporary seawater, REE adsorption must occur close to the sediment-water interface, as even shallow burial can result in fractionation during early diagenesis.
Cathodoluminescence (CL) is achieved through the excitation of the sample mineral with a continuous high-energy electron beam to produce photon emission, generally in the visible spectral range (Barbin, 2013). CL analysis has been used extensively as a tool to assess preservation quality and diagenetic impact in fossil enamel (e.g., Götze et al., 2001; Schoeninger et al., 2003; Ségalen et al., 2008; Owocki et al., 2020; Richard et al., 2022). In assessing biomineral preservation in apatitic fossil hard tissues, CL provides a relatively quick means of identifying areas of diagenetic replacement (Ségalen et al., 2008), without further destruction of the thin section.
Substitution by other elements of Ca sites in the crystal lattice of apatite can be detected through CL, with the elements responsible for the substitution discernible based on the wavelength and hue of the photon emission. For example, substitution by Mn2+ produces a yellow or orange hue (Gaft et al., 1997) of between 565 and 585 nm, while unaltered biogenic apatite emits a dull blue luminescence of approximately 400 nm (Schoeninger et al., 2003). Hättig et al. (2019) have shown that Mn2+ incorporation can cause CL emission in enameloid from recent sharks, and thus CL alone cannot be relied upon as a diagenetic indicator. Areas of REE substitution were associated with distinct bands with sharp emission lines between 300 and 1,000 nm (Gaft et al., 1997; Blanc et al., 2000; Habermann et al., 2000; Ségalen et al., 2008). Notably, Gaft et al. (1997) showed that the luminescence bands are absent where adsorption has occurred and are only present as a result of substitution.
Energy dispersive spectroscopy (EDS) is a widely used scanning electron microscope SEM technique for determining the elemental composition of specimens. EDS has previously been used to study the distribution of elements within dental remains in relation to their structure and functional use (e.g., Enax et al., 2012; Dumont et al., 2009,2011) and to compare the element composition present in the teeth of different groups of organisms (Lübke et al., 2015).
Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS) is an in-situ form of mass spectrometry useful for down-hole compositional depth profiling, which provides reliable quantitative high-resolution REE and major element compositions with only minor destruction of the thin section (see Trotter & Eggins, 2006; Žigaitė et al., 2016).
In this study we use cathodoluminescence-microscopy and spectroscopy (micro-CL) combined with EDS and in-situ LA-ICP-MS on fossil bioapatite, using several types of dental fossils, and the same thin and thick sections to be able cross-verify the results of these three complementary techniques.
Materials and Methods
Samples investigated in this study include dermal scales from jawless and jawed fishes from the Devonian of Svalbard as well as plesiosaur tooth crowns and fossil lungfish (Dipnoi) dental plates from the Cretaceous of southeastern Australia. Portions of this section were previously published as part of our previous articles (Žigaitė et al., 2015, 2016, 2020; Fadel et al., 2015).
The Devonian fish dermal denticles were obtained from the palaeontological collections of the Paris National Natural History Museum (Museum national d’histoire naturelle), France. Original sampling of this material was from the Andrée Land Group of Spitsbergen Island, Svalbard archipelago, Norway. The scales analysed derive from two taxa, the thelodont Talivalia svalbardae and an undescribed putative chondrichthyan, both of which come from the Grey Hoek Formation in the upper part of the Andrée Land Group succession. The thelodonts have been described by Žigaitė et al. (2013), and the putative chondrichthyan is currently being described.
The Early Cretaceous plesiosaur and lungfish fossils were sampled from the palaeontological collection of the Melbourne Museum (Museums Victoria, NMV), Melbourne, Australia. One plesiosaur tooth and one lungfish toothplate (see Fig. 1) were selected from the lower Albian, the Eumeralla Formation and uppermost Barremian to lowermost Aptian, the Wonthaggi Formation of south-eastern Australia (Wagstaff et al., 2020). Previous taxonomic evaluations of these plesiosaur teeth suggested leptocledian affinity (Kear, 2006; Kear & Hamilton-Bruce, 2011; Poropat et al., 2018, 2023; Kear et al., 2018); the lungfish toothplates cannot be confidently identified beyond Ceratodontiformes (see Poropat et al. (2018) for discussion).
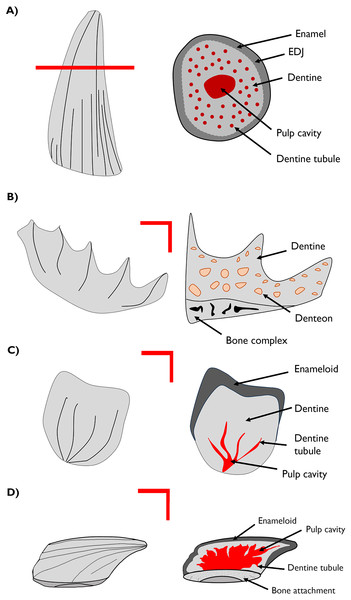
Figure 1: Cross-section drawings of general structures of the materials studied.
(A) Plesiosaur tooth schema and transverse section showing enamel, dentine and dentine tubule. (B) Lungfish plate with longitudinal section showing the dentine composing the structure and the denteon. (C) Chondrichthyan scale with longitudinal section showing the enameloid, dentine and dentine tubule. (D) Thelodont scale with lateral section. EDJ–enamel-dentine junction.All specimen sections are held in The Museum of Evolution Palaeontological Collections (PMU), Uppsala University, Sweden.
Geological settings
Svalbard material
The thelodont and chondrichthyan scales used in this study come from the Andrée Land territory in the northern part of Spitsbergen Island, Svalbard archipelago. Stratigraphically the material originates from the Lower Devonian Old Red Sandstone succession referred to as the Andrée Land Group (Blomeier et al., 2003) and represents deposition in a continental rift basin along the northern margin of the Old Red Sandstone (ORS) landmass. The succession is essentially confined to a major graben with a unique depositional history, involving a shift from coarse clastic red-beds, mainly of alluvial fan and fluvial origin, to a series of more greyish fluvial and possibly deltaic sediments recording a transition from the southern arid zone to the equatorial tropics. The nature of the basin and, more specifically, its palaeoenvironmental conditions are as yet poorly understood, although it plays an important role as a regional niche and separate biogeographical province in the Early Devonian (Žigaitė et al., 2016).
Vertebrate microfossils are quite common in the Andrée Land deposits, and include isolated micromeric elements of the dermal exoskeleton (dentine scales) of acanthodians, chondrichthyans, and thelodonts (Ørvig, 1967; Blom & Goujet, 2002; Žigaitė et al., 2013). The Formation extends from the Lower to Middle Devonian (Blomeier et al., 2003). It is subdivided into three lithological units: the Verdalen, Skamdalen and Tavlefjellet members (Blomeier et al., 2003; Volohonsky et al., 2008). The thelodont scales come both from the Tavlefjellet and Skamdalen, while the undescribed chondrichthyan comes only from the Skamdalen, specifically from the Gråkammen locality (Žigaitė et al., 2013).
Australian material
The Wonthaggi and Eumeralla formations consist of fluvial sandstone and mudstone deposits which formed part of a wider floodplain arising from the rifting of mainland Australia, Tasmania and Antarctica (Mutter et al., 1985). Both formations have previously yielded a diverse array of vertebrate body and ichnofossils (Martin et al., 2012; Poropat et al., 2018; Romilio & Godfrey, 2022).
The informally designated ‘Wonthaggi Formation’ is a unit of the Strzelecki Group assigned to the latest Barremian to earliest Aptian on the basis of palynology (Wagstaff et al., 2020). The Eumeralla Formation from the Otway Group is early Albian in age (Wagstaff et al., 2020). The Wonthaggi Formation’ records evidence of possible freezing in the winter (Wagstaff & Mason, 1989) in contrast with more temperate conditions present in the Eumeralla Formation. Both units are associated with high palaeolatitudes, the position of Australia during the Lower Cretaceous being approximately 60–80° S (Embleton & McElhinny, 1982). An assessment of the floral communities of the Eumeralla Formation by Tossolini et al. (2018) concluded that its warmer climate may have included strong seasonal variations.
Sample preparation
Sectioning and preparation of dental fossils examined, in this study were carried out at the Department of Organismal Biology at Uppsala University, Sweden and at the NordSIM facility, Department of Mineralogy, Swedish Museum of Natural History, Stockholm, Sweden. Sections were taken along the vertical axial plane of each tooth fragment, through both the enamel (or enameloid for scales) and the dentine. The sample sections were selected on the basis of enamel/enameloid thickness to provide a reasonable amount of working material. Thin sections (30 μm) were polished and carbon-coated before CL-spectroscopy analysis by the Biomineralizations and Palaeoenvironment group at the Pierre and Marie Curie University Paris 6, France. The same sections of plesiosaur teeth and the dental plates of lungfish were subsequently analysed through SEM analysis.
Energy dispersive x-ray spectroscopy (EDS)
The chemical composition of the biomineral was investigated using energy-dispersive X-ray spectroscopy (EDS) at the Max Plank Institute for Iron Research, Duesseldorf, Germany in accordance with the method outlined by Dumont et al. (2014). EDS elemental map sections and profiles have been generated for the plesiosaur teeth and the tooth plates of lungfish. SEM imaging was conducted using a Jeol JSM-6500F scanning electron microscope operating at 15 kV with a tungsten filament instrument. The microscope was equipped with an EDAX-TSL EBSD system. The chemical compositions used in mapping were determined using EDAX energy-dispersive X-ray spectrometers (EDS) attached to the electron microscope. The microanalyses were conducted using the EDAX library standard-less procedure with a 20 s dwell time.
Laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS)
All specimens subject to LA-ICP-MS underwent gold sputtering and polishing prior to analysis. The elemental compositions were obtained by LA-ICP-MS at the Imaging and Analysis Centre of the Science Facilities Department, Natural History Museum, London (UK). LA-ICP-MS is a widely used technique to determine in-situ mineral elemental compositions, and offers the necessary high spatial resolution required to analyse the in-situ trace element compositions of separate tissues of micron-sized scales. Analyses were performed using a New Wave Research UP213AI 213 nm aperture imaged laser ablation accessory coupled to a Thermo Elemental PQ3 ICP-MS with an enhanced sensitivity S-option interface. Data were acquired for 120 s at each analysis site on the plesiosaur and lungfish specimens, taking individual points in histologically different regions (dentine or enamel). Background signals were collected for the first ~60 s and the laser was fired at the sample to collect sample signals for the remaining acquisition time. Data were collected using the time resolved method and were processed offline using LAMTRACE software (Simon Jackson, Macquarie University, Sydney). Portions of this text were previously published as part of our previous articles (Fadel et al., 2015; Žigaitė et al., 2015, 2016, 2020).
Elemental concentrations were calculated using the National Institute of Standards and Technology (NIST) standard reference material 612 for calibration and calcium was used for internal standardization. The limit of detection was taken as 1σ of the mean background count, and the data filtered at twice this limit (2σ). Calculated precision was better than 3% RSD (at 1σ error) when using 43Ca as an internal standard. The concentrations of REEs were measured in parts per million and normalized to Post-Archaean Australian Shale (PAAS) concentration values (McLennan, 1989). The obtained in-situ REE compositions are explored below using basic geochemical calculations and quantifications for sedimentary rocks (Reynard, Lécuyer & Grandjean, 1999; Johannesson, Hawkins & Cortés, 2006; Žigaitė et al., 2016 and citations therein). Elemental compositions were measured in parts per million (ppm), and the Al2O3, SiO2, TiO2, MgO, CaO, MnO and FeO oxides, in weight percentages (wt%) (see Tables S1–S7).
The shale-normalized cerium (Ce/Ce*)SN and praseodymium (Pr/Pr*)SN anomalies were calculated using the formula Ce/Ce* = 2CeSN/(LaSN + NdSN) and Pr/Pr* = 2PrSN/(CeSN + SmSN) (Barrat, Bayon & Lalonde, 2023).
Optical CL examination of the samples was performed at the Imaging and Analysis Center (NHMUK) using an OPEA Catodym luminoscope operating at 15 kV and 300-μA. Transmitted light and CL images of the samples were taken using a Nikon D70 digital camera. CL images were subsequently processed in Adobe Photoshop by raising brightness 150%. This was done to enhance the visibility of histological features as well as cracks, in order to visualize any changes in the distribution of secondary elements associated with these features. The luminescence colours and their corresponding wavelengths were then compared to the peak shifts for REE emission spectra (Ségalen et al., 2008).
Results
Optical cathodoluminescence
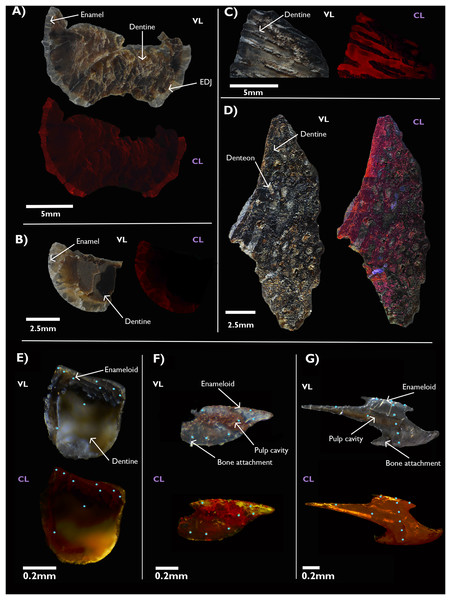
The optical CL images of the specimens from the Eumeralla Formation show a red-orange luminescence present in the biomineralized tissue of all of the samples, most likely attributable to REE substitution in the Ca+ sites of the preserved apatite. Luminescence of this hue is associated with replacement by Eu3+ and Sm2+ ions (Blanc et al., 2000; Ségalen et al., 2008). In the lungfish plate, distinct areas of light blue or violet luminescence can be seen in the matrix infill around the denteons (Fig. 2C). Light blue/violet luminescence is not exclusive to bioapatite, and as it can also be be generated by a number of silicate minerals (Götze, 2012). The EDS maps of this specimen (see Supplemental Figures) show enrichment of silicon and aluminium within this infill. These elements are not present in the original bioapatite, suggesting this luminescence is representative of secondary mineral infilling rather than the preserved dentine.
Figure 2: Visible light photographs (VL) and optical cathodoluminescence (CL) images of fossil vertebrate hard tissues.
Plesiosaur teeth (A, B) and lungfish dental plates (C, D) from the Cretaceous age Eumeralla Formation, Otway basin, Australia. Fish scales from the Devonian of Svalbard, including a chondrichthyan (E) and two thelodont body scales from Gråkammen (F) and Tavlefjellet (G). Teal dots on Svalbard specimens indicate LA-ICP-MS points.In the Devonian fish from Svalbard, a yellow-orange luminescence is observed. Substitution by Dy3+, Sm3+, and Eu3+ ions is associated with these hues (Blanc et al., 2000; Ségalen et al., 2008). Notably, the interior pulp cavity in the thelodont scale from Tavlefjellet (Fig. 2G) appears to luminesce a bright yellow, although it must be noted this luminescence is filtered through the external enameloid. Yellow luminescence can also arise from Mn2+ substitution, which may also contribute to this effect. However, the overall concentration of MnO is lower in the Gråkammen scales in comparison to the Tavlefjellet scale, as measured by in-situ LA-ICP-MS (see below).
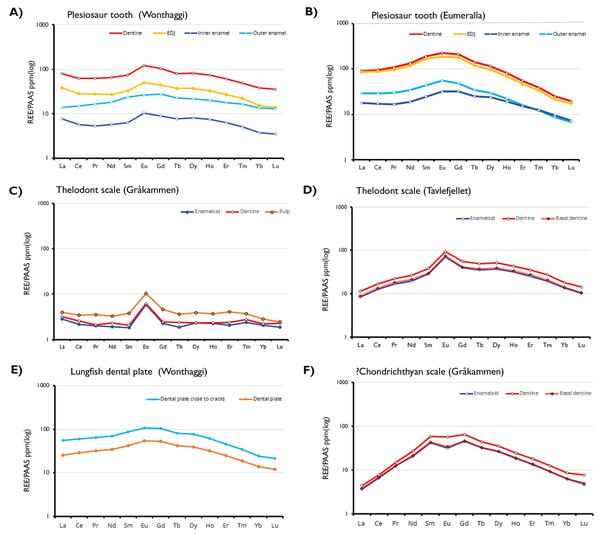
As optical cathodoluminescence imaging is limited to the visible spectrum of light, luminescence in wavelengths outside the visible range is not detected. Thus, despite the abundance of Gd in the specimens being comparable to, or exceeding, that of Sm and Eu (Fig. 3D) the influence of this element on the CL images is not observed as the emission peak of the Gd3+ ion in apatite has a wavelength in the ultraviolet range (Blanc et al., 2000).
Figure 3: Shale-normalized REE profiles of vertebrate hard tissues.
The profiles show in-situ data points for each tissue type and the enameldentine Junction, obtained through LA-ICP-MS. Due to the absence of enamel in the lungfish dental plate, data points have been divided between sites close to cracks and those further away, to determine whether this proximity affects REE uptake.Trace element analysis
The EDS maps (Supplemental Figures) show that secondary elements are concentrated in areas accessible by pore fluids, most significantly in the dentine and internal pores and voids but also at the enamel-matrix interface and in cracks. Differences in secondary mineralization between the two formations appear to be minor and are best explained by the histology of the samples.
The plesiosaur teeth from the Eumeralla Formation exhibit a limited secondary element presence, with high calcium and phosphorous concentrations in both the dentine and enamel. Samples 1122A and 1122B both feature homogenous distribution of Ca and P across the enamel layers (Supplemental Figures). Secondary minerals are largely concentrated in and around cracks. No surficial inclusions are present in these samples.
The Eumeralla Formation lungfish dental plates overall show more widespread secondary mineralization than the plesiosaur teeth, but without the apparent histological differentiation in the distribution of these minerals seen in the plesiosaurs. Sample 1122C exhibits a slight reduction in calcium and phosphorous in areas of cracked dentine. For example, the enamel does not appear to have undergone significant secondary mineralization, both according to the REE concentrations, and the micro-CL and EDS imaging. Sample 1122C exhibits a slight reduction in calcium and phosphorous in areas of cracked enamel and in the vicinity of the enamel-dentine junction. Both specimens 1122C and 1122D exhibit surficial inclusions of Si-, Al-, and Na-rich secondary precipitate minerals. By comparison, the dentine of each of these samples contains a greater number of minerals present in relatively high concentration. For instance, the dentine of sample 1122D has been infiltrated by iron-, aluminium-, and silicon-rich minerals which have crystalized within cavities in the dentine. Outside of these cavities calcium and phosphorous remain abundant, with similar concentrations observed in both enamel and dentine.
REE analysis
REE concentrations are highest in the dentine and lowest in the inner enamel of the plesiosaur teeth. The enamel-dentine junction (EDJ) generally has REE content lower than the dentine but higher than the interior part of the enamel. More REEs are present in the outer part of the enamel than in the inner part. This suggests that the samples experienced approximately the same degree of post-mortem diagenetic alteration, independent of age and burial environments. Contrastingly, in the Svalbard fish scales REE concentrations are substantially higher in the pulp cavity than the outer enameloid layers, with Eu-anomalies present in all samples and tissue types.
Cerium (Ce) and Lanthanum (La) anomalies can be calculated based on the LA-ICP-MS data and represent an important paleoenvironmental indicator, as these anomalies are linked to the oxic state of pore waters (e.g., Reynard, Lécuyer & Grandjean, 1999; Kemp & Trueman, 2003; Patrick et al., 2004). Negative Ce anomalies are associated with oxic conditions, whilst positive anomalies—or the absence of an anomaly—may indicate anoxia (Herwartz et al., 2013b).
Discussion
Most of the enamel present in the samples studied appears to represent the original biomineralized material, with minimal diagenetic alteration. The outermost enamel at the surface of the teeth and dental plates has a higher secondary element content than the inner enamel. The exposure of the outer surface of the hard tissues to the environment may account for this to some extent; it is the area with the most contact with the matrix fluids that are the source of many of the secondary elements. The presence of elevated REE concentrations in the outermost enamel relative to the inner enamel is consistent with the observations of Williams et al. (1997) and Ségalen et al. (2008) that REE integration occurs primarily at the interface between the preserved tissue and the sediment. The density and poor permeability of the outer enamel may shield the inner matrix from significant pore fluid infiltration.
In the Wonthaggi plesiosaur teeth, secondary minerals are more prevalent. In sample 1223A the pulp cavity has undergone extensive infilling, with Al, Si, Fe and Zn present in higher concentrations than the surrounding dentine. The enamel of this sample is less secondarily mineralized, though infilling of cracks by Si- and Al-rich minerals is observed. Sample 1223B also exhibits some secondary mineralization. Whilst there is no infilling of the pulp cavity, the dentine is marked in places by areas exhibiting increased F and C. Secondary inclusions containing Al, Si and C are observed along the internal pulp cavity surface of this tooth, and Fe is also observed to infiltrate the external-most portion of the dentine. The lungfish plates display higher levels of Ca and P than is seen in the dentine of other samples. Secondary mineralization is also present in the lungfish teeth, with extensive infilling of pore spaces and dentine tubules by Si, Al, and Fe. Although infilling is widespread, particularly in sample 1123D (Fig. S11), no large areas of recrystallisation alike those in the Eumeralla Formation specimens are seen.
In both sets of samples Si, Al and Fe are the most abundant elements being primarily present in secondary phases infilling cracks. The probable source of these elements is the matrix in which the specimens were deposited; the formations in which the specimens were found consist of sandstones and mudstones from which high quantities of quartz and clay minerals are to be expected. Fluorine (F) is generally elevated in fossil hard tissues relative to contemporary remains, as in vivo incorporation of F into bioapatite is comparatively low yet fluoride ions readily replace OH- during diagenesis (Ghadimi et al., 2013; Keenan et al., 2015). An exception would be enameloid, which has close chemical composition to geological fluorapatite (Sasagawa et al., 2009; Enax et al., 2012). In these samples, F has accumulated in areas of secondary filling, such as histological cavities and surficial cracks, but is also present within the fossil tissue. The distribution of F within all the analysed tissues is largely homogenous, with no clear distinction between dentine and enamel visible in the EDS maps (see Supplemental Figures).
Secondary elements are marginally more abundant in the lungfish plates than in the plesiosaur teeth. Lungfish do not shed their dental plates (Kemp, 2002), and they are thus only deposited with the death of the animal. During the animal’s lifetime, the outer surface of the plate is susceptible to mechanical wear, which may expose the eroded dentine to secondary elements. Comparison of extant lungfish with fossil populations (Kemp, 2005) has indicated wearing may be exacerbated by environmental stresses such as dietary changes driven by food availability and variations in ambient oxygen concentration. It should also be noted that some lungfish taxa replace eroded enamel with hydroxyapatite enriched petrodentine which is continuously produced (Kemp, 2001; Smith & Krupina, 2001). By contrast, plesiosaurs are known to have experienced continuous tooth shedding and replacement (Kear et al., 2017). Polyphyodonty (tooth shedding) is a trait found in the majority of vertebrate groups and is not indicative of an animal’s metabolism. Kear (2006) noted that the plesiosaur teeth used in this study also exhibited wear to some degree, though not to the extent that inclusions in worn enamel present a significant route for secondary mineral infiltration into the dentine compared to compaction-induced cracks or natural pores.
As with the secondary elements, luminescence is strongly associated with cracks and the outer surfaces of the samples, reflecting the vulnerability of these areas to infiltration by pore waters during diagenesis. The enamel present in the plesiosaur teeth superficially appears to luminesce more strongly than the dentine, contrary to expectations based on the LA-ICP-MS results. We suggest this may result from the translucency of the enamel allowing for more photon transmission than in comparatively more opaque dentine rather than a signal of potentially greater diagenetic alteration. The wavelength of the luminescence, inferred from the hue, is of greater importance to this study than the intensity as it is potentially indicative of whether the REE replacement has occured (Ségalen et al., 2008), though this information is substantially less quantitative in comparison to those derived from methods such as LA-ICP-MS.
Interpretation of the compositional profiles obtained requires histological context, as the histology of fossil tissues determines potential systematic trends in their relative permeability and susceptibility to diagenesis. Enamel and enameloid are more resistant to elemental and mineral replacement and alteration than dentine as they are of a lower porosity and more extensively mineralised, with <2% organic content (Hoppe, Koch & Furutani, 2003) in comparison to approximately 70% in dentine. Dentine is less mineralised in vivo than enamel, and micro-sized tubules increase its porosity and permeability. In lungfish dental plates the dentine is also vascularized (Kemp & Barry, 2006), with voids left by blood vessels providing effective entry-points for groundwater during early diagenesis. These factors increase the potential for infiltration of the dentine by secondary elements, in turn increasing the likelihood of mineral alteration and replacement.
The strong yellow luminescence in the pulp cavity of the Tavlefjellet thelodont scale (Fig. 2G) suggests stronger infiltration of the cavity by REEs relative to the dentine and enameloid. This is supported by our LA-ICP-MS analysis showing REE concentrations in the pulp cavity up to an order of magnitude higher than in other tissues, especially for Eu. Pulp is extensively vascularised and has a greater organic component than dentine, and so it is more susceptible to fluid infiltration. Greater REE enrichment of the pulp cavity tissue in comparison to the other tissues further supports the porosity of hard tissues being a significant factor in diagenetic REE uptake.
The observed REE profiles of the fossils are indicative of limited diagenetic alteration. In the plesiosaur teeth, the degree of preservation in the inner enamel is such that the observed elemental signals can be interpreted as primary. In these fossil specimens REE content varies based on histology and does so in a way that largely mimics the distribution of secondary elements seen in the EDS maps. The dentine of the samples is, with some exceptions, more strongly enriched in trace elements than the enamel. However, the enamel exhibits greater variability in enrichment within the same tissue; while it is generally the case that the outer enamel is more strongly enriched than the inner, both areas possess regions either more strongly or weakly enriched than would be predicted based on histology. Even within the same tooth this is the case, as seen in the Wonthaggi plesiosaur tooth (Fig. 3A). In that specimen, the inner enamel is split between areas of high REE concentration exceeding that of the enamel (approaching 1,000 ppm), and exceptionally low concentration, between 0.1 ppm for LREEs and 1 ppm for HREEs.
All the Australian Cretaceous samples exhibit a slightly “bell shaped” shale-normalized REE profile, with MREE being more abundant than LREE and HREE, though this is most pronounced in the plesiosaur samples. The abundance of MREEs, and in particular Eu, is especially apparent in the cathodoluminescence images. Strong MREE enrichment is often associated with the overprinting of early diagenetic signals by later recrystallization and fractionation (Lécuyer, Reynard & Grandjean, 2004). This pattern supports the interpretation of the specimens as being well preserved, displaying minor REE adsorption from early diagenesis rather than the fractionated incorporation of a significant amount of REEs associated with later overprinting (Fadel et al., 2015; Žigaitė et al., 2015).
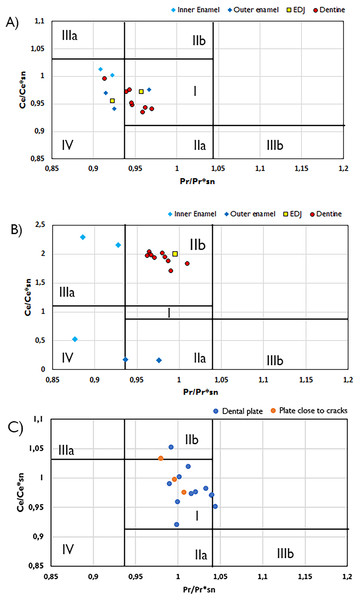
Cerium state varies greatly between tissue types. In Wonthaggi plesiosaur tooth, the Ce anomaly throughout its dentine appears to be influenced by a negative La anomaly, while the enamel is influenced by a positive La anomaly (Fig. 4), while the enamel of both plesiosaur teeth exhibits an overall positive Ce anomaly. The lungfish plate generally displays no Ce anomaly. Positive La anomalies have been linked to riverine conditions (Kulaksız & Bau, 2011), though such anomalies may also arise from pre-diagenetic fractionation (Herwartz et al., 2013a; Ullmann et al., 2021).
Figure 4: Plots of Ce/Ce*SN/vs. Pr/Pr*SN plots with fields indicating potential Ce and La anomalies.
Ce/Ce*SN/vs. Pr/Pr*SN for the Wonthaggi plesiosaur (A), Eumeralla plesiosaur (B) and lungfish dental plate (C). Ce/Ce* = 2CeSN/(LaSN + NdSN) and Pr/Pr* = 2PrSN/(CeSN + SmSN); Field I, no anomaly; IIa, positive La-anomaly causes apparent negative Ce-anomaly; IIb, negative La-anomaly causes apparent positive Ce anomaly; IIIa, real positive Ce anomaly; IIIb, real negative Ce anomaly; IV, positive La-anomaly disguises positive Ce anomaly. Field divisions after Bau & Dulski (1996).The HREE concentrations in our samples are lower than would be expected from ocean waters (Patrick et al., 2004). In the Svalbard fish samples, REE enrichment is more varied (Table 1). The thelodont scales display considerably positive Eu anomalies, which may be attributed to reducing conditions or reworking during diagenesis (see Žigaitė et al., 2016). Positive Eu anomalies are quite usual in sediments, however, in fossil bioapatite Eu presence would suggest locally reducing conditions, likely a result of decomposition of organic matter (Trueman, Benton & Palmer, 2003; Fadel et al., 2015). In a reductive environment, the bivalent Eu2+ is mobile in diagenetic waters, and consequently, the Eu2+ enriched pore water can reprecipitate under low oxygen content, creating a positive Eu anomaly. At the same time, such processes of dissolution and reprecipitation are quite rare, and therefore Eu anomalies alone cannot be used to assess the reductive or oxidizing conditions (Martinez-Ruiz, Ortega-Huertas & Palomo, 1999), but can indicate the difference in diagenetic histories (Žigaitė et al., 2016).
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | ∑REE | (Ce/Ce*)SN | (Pr/Pr*)SN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eumeralla Plesiosaur | 2,608.600 | 5,651.867 | 700.700 | 3,287.333 | 755.846 | 175.873 | 704.833 | 78.924 | 391.260 | 59.140 | 116.013 | 11.774 | 53.973 | 6.542 | 14,602.680 | 0.966 | 0.170468 |
| Wonthaggi Plesiosaur | 1,750.380 | 2,831.316 | 314.556 | 1,270.946 | 234.407 | 74.861 | 275.641 | 35.790 | 219.742 | 42.330 | 101.229 | 11.552 | 62.127 | 8.856 | 7,233.736 | 0.804 | 0.904127 |
| Wonthaggi Lungfish | 1,359.000 | 2,928.533 | 356.013 | 1,469.667 | 298.686 | 73.986 | 308.133 | 40.633 | 232.200 | 39.452 | 86.560 | 9.428 | 47.693 | 6.358 | 7,256.345 | 1.066 | 1.078222 |
|
Gråkammen ?chondricthyan |
149.000 | 556.000 | 116.300 | 771.500 | 259.125 | 41.862 | 235.875 | 27.212 | 132.750 | 19.950 | 41.375 | 4.103 | 19.437 | 2.441 | 2,376.933 | 0.820 | 0.886973 |
| Gråkammen Thelodont | 123.000 | 206.250 | 21.250 | 80.225 | 13.105 | 7.617 | 13.605 | 1.905 | 12.815 | 2.635 | 7.582 | 1.138 | 6.462 | 0.919 | 498.511 | 0.965 | 1.197866 |
|
Tavlefjellet Thelodont |
466.285 | 1,386.000 | 205.285 | 976.714 | 228.857 | 110.842 | 284.714 | 41.242 | 265.714 | 48.642 | 112.942 | 11.997 | 58.585 | 6.937 | 4204.763 | 0.992 | 1.011098 |
Conclusions
The REE distribution patterns in the samples studied herein are indicative of generally minimal diagenetic overprinting, with histological variations that overlap with the secondary element distributions seen in EDS maps (Supplemental Figures). Our data therefore support the conclusion that the primary chemical composition of the fossil bioapatite is largely well preserved in the studied specimens. In particular, the inner enamel of our samples likely consists of mostly unaltered original tissues and should be targeted for palaeobiological proxies, such as stable isotopes compositions and other biogeochemical signatures. We were also able to identify the extent to which secondary elements had infiltrated these samples through diagenetic processes, including their spatial distributions. We conclude that histology is a better indicator of the extent of both preservation quality and secondary replacement than either diagenetic or non-histology-related biological factors.
The distribution of REEs in our samples appears reflective of early-diagenetic uptake having occurred from freshwater surface and/or pore fluids, in agreement with previous paleoenvironmental assessments. Our results themselves provide no further insight into the climate of southeastern Australia in the Early Cretaceous, though the cool environment identified by other studies (Rich, Vickers-Rich & Gangloff, 2002) may have been a factor in the high level of biomineral chemical preservation seen in our samples (Tütken, Vennemann & Pfretzschner, 2008). The elevated quantities of MREEs in the plesiosaur samples may be reflective of the marine conditions (Žigaitė et al., 2016). Given the fluvial interpretation of the Eumeralla Formation (Kear, 2006; Kear et al., 2006; Benson et al., 2013), this further supports the idea of euryhaline behaviour in plesiosaurs (Benson et al., 2013; Bunker et al., 2022, and citations therein).
Mapping of REE and trace element distributions through in-situ spectroscopy and cathodoluminescence allow rapid and accessible visualisation of geochemical composition in fossil bioapatite. In so doing, it allows for areas of significant diagenetic alteration to be identified, providing insight into the specific mechanism(s) of diagenetic change, and potentially, into the temporal sequence of those diagenetic alterations. Additionally, these mapping techniques highlight areas in which primary biomineral composition is likely to be preserved, and thus they provide useful tools to guide palaeobiological, palaeoecological, and palaeoenvironmental analyses. In particular, mapping is likely to benefit the design and spatial targeting while conducting in-situ microanalyses. Consequently, the application of mapping from multiple sources increases confidence in biogeochemistry-based reconstructions of past organisms and environments.
Supplemental Information
Supplementary Tables 1-7.
LA-ICP-MS elelmental composition data supplement.