Comprehensive evaluation of physiological response and cold tolerance of domesticated Cinnamomum camphora (L.) Presl under low temperature stress
- Published
- Accepted
- Received
- Academic Editor
- Imren Kutlu
- Subject Areas
- Agricultural Science, Plant Science
- Keywords
- Cinnamomum camphora (L.) Presl, Cold tolerance, Domestication cultivation, Low temperature stress, Physiological index
- Copyright
- © 2024 Ding et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Comprehensive evaluation of physiological response and cold tolerance of domesticated Cinnamomum camphora (L.) Presl under low temperature stress. PeerJ 12:e18590 https://doi.org/10.7717/peerj.18590
Abstract
Improving cold tolerance is one of the key techniques for introducing southern tree species to northern China. To provide a theoretical basis for the introduction and cold-tolerance cultivation of Cinnamomum camphora (L.) Presl, the physiological response and cold tolerance of acclimated Cinnamomum camphora (L.) Presl trees were studied. In this experiment, the cold tolerance physiological indexes of Cinnamomum camphora (L.) Presl were measured in an indoor artificial simulation of low temperature stress. The results showed that under low temperature stress, the Cinnamomum camphora (L.) Presl are cultivated for 1 year, 5 years, 10 years and 15 years, respectively, at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C. The relative electrical conductivity change ranges were 26.26%–13.98%, 30.71%–19.24%, 37.36%–27.18%, 44.16%–32.24%, 63.21%–52.05%, and 86.43%–76.24%; the MDA content ranges were 12.10 mmol/g–3.25 mmol/g, 15.90 mmol/g–4.08 mmol/g, 10.53 mmol/g–2.05 mmol/g, 23.20 mmol/g–5.35 mmol/g, 31.30 mmol/g–5.89 mmol/g, and 36.47 mmol/g–8.13 mmol/g, respectively. The relative water content change ranges were 95.35%–65.92%, 71.36%–49.67%, 54.67%–34.89%, 43.12%–23.12%, 26.03%–11.21%, and 23.03%–8.15%, respectively. At the same stress temperature, these indices decreased with increasing cultivation time, and the degree of membrane damage was reduced accordingly. The osmoprotectants were soluble protein, soluble sugar, and free proline which were increased gradually with cultivation time. at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C, the soluble protein content change ranges were 3.51–6.18 mg/g, 6.24–9.95 mg/g, 9.44–19.59 mg/g, 14.23–28.36 mg/g, 17.34–33.19 mg/g, and 25.15–32.23 mg/g. the soluble sugar content change ranges were 9.64-26.97 mg/g, 15.37–39.86 mg/g, 26.63–53.97 mg/g, 45.49–76.75 mg/g, 52.74–81.24 mg/g, and 55.61–85.34 mg/g. the free proline content change ranges were 55.83–85.23 µg/g, 68.95-89.87 µg/g, 95.38-214.38 µg/g, 219.19-389.89 µg/g, 321.28-453.65 µg/g, and 381.23-478.96 µg/g, respectively. The osmoprotectants increased the cell stability and enhanced the cold tolerance of Cinnamomum camphora (L.) Presl. Protective enzyme activity were catalase, superoxide dismutase, peroxidase which were increased gradually with cultivation time, catalase reached a maximum at about −15 °C SOD reached a maximum at about −5 °C and POD reached a maximum at about −10 °C. These results indicated that the leaves of Cinnamomum camphora (L.) Presl could reduce stress damage by increasing the activity of antioxidant enzymes to clear the active oxygen in the body. A principal component analysis showed that the relative water content, soluble protein content, soluble sugar content, and superoxide dismutase activity could be used as important indexes for cold hardiness in Cinnamomum camphora (L.) Presl. The comprehensive evaluation showed that with the increase of domestication cultivation years, Cinnamomum camphora (L.) Presl. cold tolerance gradually improved.
Introduction
Cinnamomum camphora (L.) Presl is a broad-leaved evergreen tree of the Lauraceae family, which is mainly distributed in various places south of the Huai River Basin. It is an excellent landscape tree species. It is also an important economic tree species in China, used for its timber and in the production of aromatic oils (Rong & Jun, 2012; Hua et al., 2000). With the rapid development of urban construction and the additional requirements of landscaping, the demand for evergreen and aromatic tree species in the north of Huai River is growing. In order to enrich the garden tree species in the north and improve the landscape of winter garden plants, landscape workers in Shandong, Henan, Jiangsu and other regions north of the Huai Rive are attempting to introduce Cinnamomum camphora (L.) Presl, but the problem of wintering frost damage in this species has not yet been solved (Su et al., 2015). Plant cold tolerance is a genetic characteristic formed by long-term adaptation of plants to low temperature environment. Plant cold tolerance gene expression is closely related to external environmental conditions and endogenous rhythm and physiological conditions of plant development, and domestication cultivation is of great significance to improve plant cold tolerance. Ren, Yue & Yi (2021) found domesticated four varieties of Crataegus pinnatifida Bunge with 5 years of low temperature acclimation, after domestication, the cold tolerance of four varieties of Crataegus pinnatifida Bunge was significantly improved. Low winter temperatures limit the introduction and popularization of Cinnamomum camphora (L.) Presl north of the Huai River. Therefore, studying the influence of northern transplanting time on the cold tolerance of Cinnamomum camphora (L.) Presl and comprehensively evaluating its cold tolerance can provide a theoretical basis for the domestication and cold tolerance cultivation of Cinnamomum camphora (L.) Presl in the north.
Under low temperature stress, the cell membrane permeability of plants increases (Wen, Fei & Xue, 2007), the relative water content of plant tissues decreases (Rong et al., 2021), the membrane lipid phase transition and the malondialdehyde (MDA) content of membrane lipid peroxidation products both increase (Gui et al., 2018), osmoprotectants (soluble sugar, soluble protein and free proline) accumulate (Sheng et al., 2021), and protective enzyme activities, such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) increase (Yi et al., 2021; Qi & Xiao, 2013; Ye et al., 2014). The comprehensive evaluation of several physiological and biochemical indicators related to cold tolerance is part of the principal methods for the study of cold tolerance in plants. Li et al. (2015) established a comprehensive evaluation method of Vitis vinifera L. cold tolerance by using the subordination function to analyze the correlation between Vitis vinifera L. physiological indicators and cold tolerance. Ni et al. (2020) found that the relative conductivity, relative water content, soluble sugar content, and catalase activity were related to the cold tolerance of Citrus reticulata Blanco, by using the subordination function and a principal component analysis. Low temperature is an important environmental stimulus signal. The temperature of cold tolerance exercise in different plants is different, and the influence of the duration of freezing tolerance exercise on the full expression of cold tolerance heredity also differs (Cheng, 2012). Timmis, Flewelling & Talbert (1994) established a mathematical model for temperature in a cold tolerance exercise of Pseudotsuga menziesii (Mirb.) Franco. However, there are few reports on the impact of domestication time on cold tolerance in landscape plants in the north. Studying low temperature tolerance of Cinnamomum camphora (L.) Presl in a natural environment is both time-consuming and has low controllability. Comparing the physiological and biochemical indexes of Cinnamomum camphora (L.) Presl in an indoor artificial simulation of low temperature stress can effectively replace lengthy field experiments. Therefore, this study analyzed the physiological response of Cinnamomum camphora (L.) Presl to low temperature stress by simulating low temperature stress, and established a comprehensive evaluation method of cold tolerance, in order to provide theoretical research for the introduction, domestication, and cold tolerance cultivation of Cinnamomum camphora (L.) Presl.
Materials & Methods
Material selection and low temperature treatment of Cinnamomum camphora (L.) Presl
Cinnamomum camphora (L.) Presl trees were chosen from a garden green space in Shangqiu city, Henan Province, China. Shagnqiu city is located between 114°49′-116°39′ N, 33°43′-34°52′E, at an altitude of 50 m. The cultivation years (y.) of the chosen plants were 1 y, 5 y, 10 y, and 15 y. The best period of the experiment needs is April-May or August-October. Ten plants of each cultivation period with the same growth potential were planted in the same direction and position of the crown, and annual branches with a diameter of 0.20−0.25 cm were chosen, with 10 leaves in the same position of each branch, and three repetitions in each treatment. All sampling was done while being cautious to avoid hurting branches in the process of picking the leaves. The collected Cinnamomum camphora (L.) Presl leaves are cleaned, dried, and put into self-sealing bags for later use. Each repeated leaf was divided into six parts on average, one part was stored in a refrigerator at 4 °C as the control, and the other five parts were treated at 0 °C, −5 °C, −10 °C, −15 °C, and −20 °C, respectively, and then dropped to the target temperature at a rate of 4 °C/h for 24 h. They were then gradually heated to 4 °C in the same way and placed for 12 h, and then the cold tolerance related indexes were measured.
Physiological and biochemical parameters
The determination method is based on the principles and techniques outlined in the textbook “Physiological and Biochemical Experimental Principles and Technology”, authored by He (2000).
Determination of cell membrane damage
The relative electrical conductivity (REC) of the blade was measured using a conductometer (Rui et al., 2017). The content of malondialdehyde (MDA) was determined by the phenobarbitone acid method (Sheng & Zhu, 2019). The relative water content (RWC) was determined according to Pradhan’s method: the relative water content (%) =[(fresh weight-dry weight)/(saturated fresh weight-dry weight)] ×100 (Pradhan et al., 2019).
Determination of osmoprotectants
The content of soluble sugar was determined by colorimetry. The content of free proline (Pro) was stained by acid ninhydrin. Soluble protein content was established by the coomassie brilliant blue method (Cheng et al., 2021).
Determination of protective enzyme systems
Superoxide dismutase (SOD) activity was determined by the nitrogen blue tetrazole method; Catalase (CAT) activity was established by the ultraviolet absorption method; And peroxidase (POD) activity was established using the guaiacol method (Cheng et al., 2021; He, 2000).
Comprehensive evaluation method of cold tolerance
The cold tolerance of Cinnamomum camphora (L.) Presl was comprehensively evaluated by principal component analysis and the weighted membership function method (Ying et al., 2023), and the calculation methods were as follows:
(1) (2)
In Eq. (1): U (Xi) is the membership function value of index i; Xi is the measured value of the index i; Xmax and Xmin are the minimum and maximum values of the index i, respectively.
(3) (4)
In Eqs. (3) and (4), Pi represents the contribution rate of the i comprehensive index in the principal component analysis, Wi represents the weight, and D represents the evaluation value of the comprehensive index for different cultivation years.
Data processing and analysis
Data processing and graphing were performed using Microsoft Office Excel 2010, while variance analysis (ANOVA), correlation analysis, and principal component analysis (PCA) to assess Cinnamomum camphora (L.) Presl cold tolerance were conducted using SPSS 26.0 software (IBM, Armonk, NY, USA).
Results
Effects of low temperature stress on cell membrane damage of Cinnamomum camphora (L.) Presl leaves with different cultivation lengths
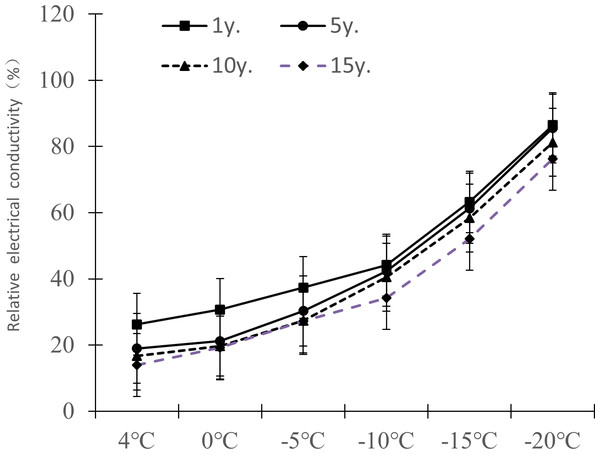
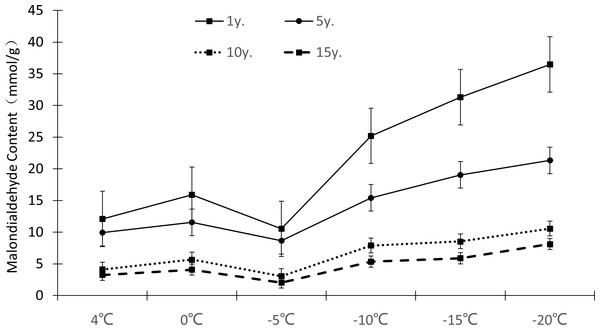
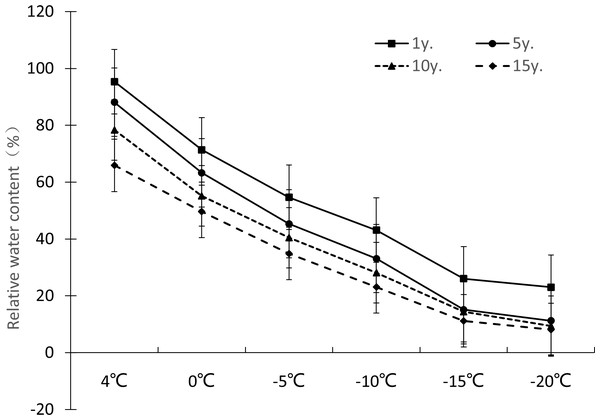
The relative electrical conductivity, relative water content, and MDA content of plant leaves under low temperature stress reflects the damage degree of the plant cell membrane, and the lower the value, the less affected it is by stress. Figures 1–3 show that with the decrease in temperature, the change trend of relative electrical conductivity (Fig. 1) and MDA content (Fig. 2) of Cinnamomum camphora (L.) Presl leaves increased gradually as the temperature decreased. The relative water content (Fig. 3) decreased as the temperature decreased, but the change ranges differed.
Figure 1: Impacts of low temperature stress on relative electronic conductivity of Cinnamomum camphora (L.) Presl leaves.
Figure 2: Impacts of low temperature stress on malondialdehyde content of Cinnamomum camphora (L.) Presl leaves.
Figure 3: Impacts of low temperature stress on relative water content of Cinnamomum camphora (L.) Presl leaves.
The relative electrical conductivity change ranges of Cinnamomum camphora (L.) Presl leaves at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 26.26%–13.98% , 30.71%–19.24%, 37.36%–27.18%, 44.16%–32.24%, 63.21%–52.05%, and 86.43%–76.24%. respectively (Fig. 1). The results of a matched sample T-test showed that the relative electrical conductivity of Cinnamomum camphora (L.) Presl leaves varied significantly (P <0.05; Table 1).
| Cold resistance index | Correlation analysis and matched sample T-test | ||||
|---|---|---|---|---|---|
| 1y. | 5y. | 10y. | 15y. | ||
| REC | 1y. | 1 | 0.997** | 0.996** | 0.999** |
| 5y. | 0.997** | 1 | 1.000** | 0.997** | |
| 10y. | 0.996** | 1.000** | 1 | 0.996** | |
| 15y. | 0.999** | 0.997** | 0.996** | 1 | |
| CAT | 1y. | 1 | 0.997** | 0.996** | 0.994** |
| 5y. | 0.997** | 1 | 0.996** | 0.996** | |
| 10y. | 0.996** | 0.996** | 1 | 1.000** | |
| 15y. | 0.994** | 0.996** | 1.000** | 1 | |
| MDA | 1y. | 1 | 0.998** | 0.985** | 0.969** |
| 5y. | 0.998** | 1 | 0.982** | 0.970** | |
| 10y. | 0.985** | 0.982** | 1 | 0.988** | |
| 15y. | 0.969** | 0.970** | 0.988** | 1 | |
| SOD | 1y. | 1 | 0.700 | 0.838* | 0.817* |
| 5y. | 0.700 | 1 | 0.943** | 0.945** | |
| 10y. | 0.838* | 0.943** | 1 | 0.997** | |
| 15y. | 0.817* | 0.945** | 0.997** | 1 | |
| POD | 1y. | 1 | 0.902* | 0.863* | 0.868* |
| 5y. | 0.902* | 1 | 0.974** | 0.954** | |
| 10y. | 0.863* | 0.974** | 1 | 0.995** | |
| 15y. | 0.868* | 0.954** | 0.995** | 1 | |
| Soluble protein | 1y. | 1 | 0.982** | 0.915* | 0.913* |
| 5y. | 0.982** | 1 | 0.973** | 0.967** | |
| 10y. | 0.915* | 0.973** | 1 | 0.996** | |
| 15y. | 0.913* | 0.967** | 0.996** | 1 | |
| Sugar | 1y. | 1 | 0.988** | 1.000** | 0.994** |
| 5y. | 0.988** | 1 | 0.987** | 0.991** | |
| 10y. | 1.000** | 0.987** | 1 | 0.994** | |
| 15y. | 0.994** | 0.991** | 0.994** | 1 | |
| Pro | 1y. | 1 | 0.997** | 0.966** | 0.962** |
| 5y. | 0.997** | 1 | 0.966** | 0.964** | |
| 10y. | 0.966** | 0.966** | 1 | 0.999** | |
| 15y. | 0.962** | 0.964** | 0.999** | 1 | |
| RWC | 1y. | 1 | 1.000** | 0.999** | 0.998** |
| 5y. | 1.000** | 1 | 0.999** | 0.999** | |
| 10y. | 0.999** | 0.999** | 1 | 0.998** | |
| 15y. | 0.998** | 0.999** | 0.998** | 1 | |
Notes:
In the table, the correlation between physiological index of Cinnamomum camphora (L.) Presl at different cultivation times is indicated, ** indicating a significant difference in paired T-test (P < 0.01), and * indicating a significant difference (P < 0.05).
The MDA content change ranges of Cinnamomum camphora (L.) Presl leaves at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 12.10 mmol/g−3.25 mmol/g, 15.90 mmol/g−4.08 mmol/g, 10.53 mmol/g−2.05 mmol/g, 23.20 mmol/g−5.35 mmol/g, 31.30 mmol/g−5.89 mmol/g, and 36.47 mmol/g−8.13 mmol/g, respectively (Fig. 2). The single factor analysis of variance showed that the MDA content of Cinnamomum camphora (L.) Presl leaves varied significantly between different lengths of cultivation (P <0.05; Table 1)
The relative water content change ranges of Cinnamomum camphora (L.) Presl leaves at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 95.35%-65.92%, 71.36%-49.67%, 54.67%-34.89%, 43.12%-23.12%, 26.03%-11.21%, and 23.03%–8.15%. respectively. The single factor analysis of variance showed that the relative water content of Cinnamomum camphora (L.) Presl leaves varied significantly between different lengths of cultivation (P <0.05; Table 1). The relative comprehensive analysis of Cinnamomum camphora (L.) Presl leaves from trees with different lengths of cultivation showed that as cultivation time increased, the adaptability of Cinnamomum camphora (L.) Presl was enhanced, and low temperature damage in the cell membrane of Cinnamomum camphora (L.) Presl leaves was weakened (Fig. 3).
Effects of low temperature stress on osmoprotectants in Cinnamomum camphora (L.) Presl leaves with different cultivation lengths
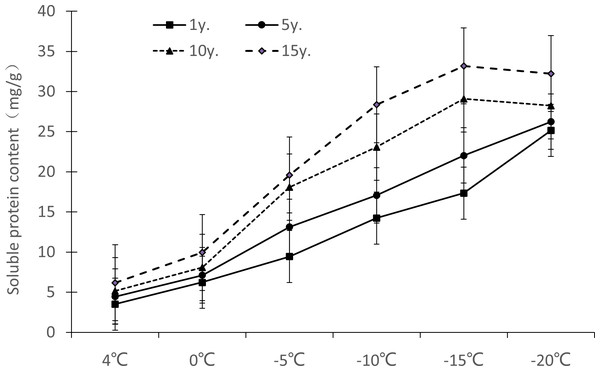
Osmoprotectants provide a regulation mode under low temperature stress. The osmotic potential of cell fluid is reduced by actively accumulating lytic substances in cells. In order to prevent excessive cell water loss under low temperature stress, the change trend content of soluble protein (Fig. 4) in Cinnamomum camphora (L.) Presl gradually increased, did not differ by length of cultivation. The content of soluble sugar (Fig. 5) and free proline (Fig. 6) increased in an S-shaped curve by cultivation year.
Figure 4: Impacts of low temperature stress on soluble protein of Cinnamomum camphora (L.) Presl leaves.
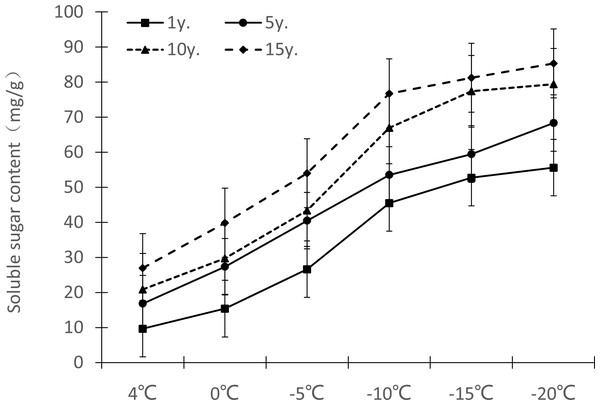
Figure 5: Impacts of low temperature stress on soluble sugar content of Cinnamomum camphora (L.) Presl leaves.
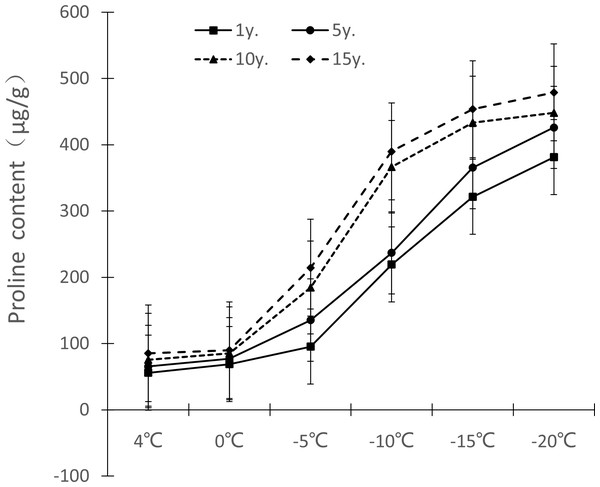
Figure 6: Impacts of low temperature stress on proline content of Cinnamomum camphora (L.) Presl leaves.
It can be seen from Fig. 4 that the soluble protein content change ranges of Cinnamomum camphora (L.) Presl leaves at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 3.51−6.18 mg/g, 6.24−9.95 mg/g, 9.44–19.59 mg/g, 14.23–28.36 mg/g, 17.34–33.19 mg/g, and 25.15–32.23 mg/g respectively. Therefore, the soluble protein content of Cinnamomum camphora (L.) Presl leaves increased gradually with the increase of cultivation years, and the difference was significant (P <0.05; Table 1). The maximum value of soluble protein appeared at −15 °C in trees cultivated for 10 y or 15 y and at −20 °C in trees cultivated for 1 y or 5 y.
Changes in soluble sugar content in Cinnamomum camphora (L.) Presl leaves under low temperature stress are shown in Fig. 5. As the temperature decreased, the soluble sugar content gradually increased. From 1 y to 15 y, the soluble sugar content change ranges at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 9.64–26.97 mg/g, 15.37–39.86 mg/g, 26.63–53.97 mg/g, 45.49–76.75 mg/g, 52.74–81.24 mg/g, and 55.61–85.34 mg/g respectively. As cultivation time increased, the soluble sugar under low temperature stress also increased, and the difference was significant (P <0.05; Table 1).
The changes of free proline content in Cinnamomum camphora (L.) Presl leaves under low temperature stress are shown in Fig. 6. The free proline content gradually increased as the temperature decreased. From 1 y. to 15 y, the free proline content change ranged at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 55.83–85.23 µg/g, 68.95–89.87 µg/g, 95.38–214.38 µg/g, 219.19–389.89 µg/g, 321.28–453.65 µg/g, and 381.23–478.96 µg/g, respectively. As years of cultivation increased, the free proline under low temperature stress also increased and the difference was significant (P <0.05; Table 1); for free proline, the change range was the largest at −10 °C, with 170.10 µg/g cultivated for 1 to 15 years, which increased by 77.88% compared with 1 y.
Effects of low temperature stress on protective enzymes of different cultivation lengths Cinnamomum camphora (L.) Presl leaves
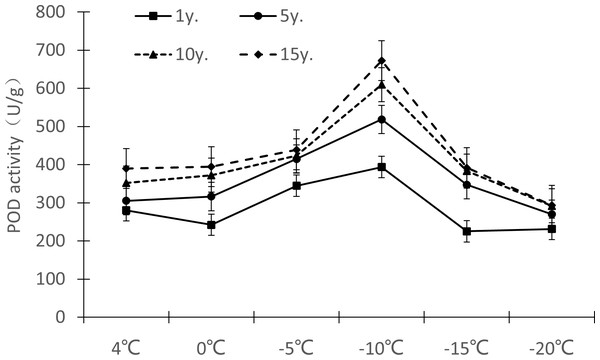
Protective enzymes help regulate a plant under low temperature stress, as endogenous active oxygen species scavengers, CAT, SOD and POD can remove excess active oxygen species in the body to a certain extent, maintain the metabolic balance of active oxygen species, and protect the membrane structure, thus reducing the damage of active oxygen species in plants, so that Cinnamomum camphora (L.) Presl has the ability to tolerate low temperature stress. It can be seen from Figures 7–9 that the change trends of CAT activity (Fig. 7), SOD activity (Fig. 8), and POD activity (Fig. 9) of Cinnamomum camphora (L.) Presl leaves under different cultivation years under low temperature stress are basically consistent.
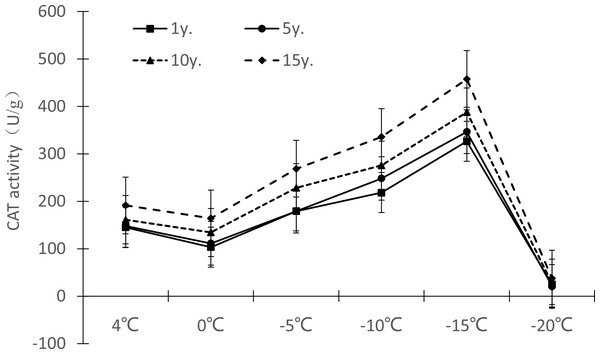
Under low temperature stress, the change trend of CAT activity in Cinnamomum camphora (L.) Presl leaves did not differ by length of cultivation (Fig. 7), with CAT activity first decreasing, then increasing, and then decreasing again as the temperature decreased. The CAT activity of Cinnamomum camphora (L.) Presl decreased from 4 °C to 0 °C, and increased significantly from 0 °C to −15 °C. When the temperature was lower than −15 °C, the CAT activity decreased sharply, indicating that the CAT activity reached a maximum at about −15 °C. Cultivation for 15 years is better than 1 year, the increments of CAT activity at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 31.72%, 58.99%, 49.87%, 53.88%, 40.21% and 52.27%, respectively.
Figure 7: Impacts of low temperature stress on CAT activity of Cinnamomum camphora (L.) Presl leaves.
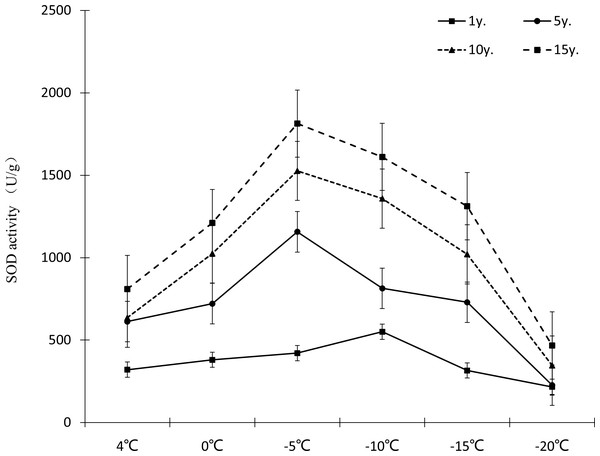
Figure 8: Impacts of low temperature stress on SOD activity of Cinnamomum camphora (L.) Presl leaves.
Figure 9: Impacts of low temperature stress on POD activity of Cinnamomum camphora (L.) Presl leaves under low temperature stress.
Under low temperature stress, SOD activity of Cinnamomum camphora (L.) Presl leaves first increased and then decreased as the temperature decreased (Fig. 8), with SOD activity reaching its highest point at −5 °C. Cultivation for 15 years is better than 1 year, the increments of SOD activity at 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C were 152.60%, 218.33%, 331.07%, 192.81% and 316.11% respectively. The analysis of variance showed that when the temperature was above −15 °C, as the temperature decreased, the stimulating effect of cultivation years on SOD activity of Cinnamomum camphora (L.) Presl was gradually enhanced.
The response trend of low temperature stress to POD activity in Cinnamomum camphora (L.) Presl was consistent by length of cultivation (Fig. 9). When the temperature decreased from 4 °C to 0 °C, the POD activity of Cinnamomum camphora (L.) Presl leaves decreased, When the temperature decreased from 0 °C to −10 °C, the POD activity of Cinnamomum camphora (L.) Presl leaves increased significantly. When the temperature was lower than −10 °C, the POD activity of Cinnamomum camphora (L.) Presl leaves decreased sharply after reaching a maximum around −10 °C.
Under the temperature treatment of 4 °C, 0 °C, −5 °C, −10 °C, −15 °C and −20 °C, the POD activity of Cinnamomum camphora (L.) Presl leaves cultivated for 1 to 15 years changed by 38.98%, 62.74%, 27.45%, 70.93%, 73.88%, and 26.70%, respectively, indicating that the POD activity of Cinnamomum camphora (L.) Presl leaves gradually increased as the temperature decreased until −15 °C.
Correlation analysis and matched sample T-test of different cultivation years on cold tolerance index of Cinnamomum camphora (L.) Presl
Table 1 shows that longer cultivation time of Cinnamomum camphora (L.) Presl is positively correlated with the physiological index of Cinnamomum camphora (L.) Presl, and the difference is significant, except for SOD. The difference in SOD changes between 1 y and 5 y of cultivation is not significant, but reaches significance after 5 y of cultivation.
Comprehensive cold tolerance and principal component analysis of cold tolerance e of the Cinnamomum camphora (L.) Presl in different cultivation years
The principal component analysis of nine physiological indexes of Cinnamomum camphora (L.) Presl leaves related to cold tolerance showed that the contribution rate of the first two principal components were 52.01% and 32.89%, and the cumulative variance contribution rate was 84.90% (Table 2).
| Principal component Indicators of cold resistance |
Prin. 1 | Prin. 2 |
|---|---|---|
| Relative conductivity | 0.84 | −0.53 |
| MDA | 0.28 | −0.75 |
| Relative water content | −0.97 | −0.05 |
| CAT | 0.24 | 0.66 |
| SOD | 0.07 | 0.94 |
| POD | 0.14 | 0.86 |
| Soluble protein | 0.98 | 0.11 |
| Soluble sugar | 0.97 | 0.18 |
| Free proline | 0.99 | −0.03 |
| Eigenvalue | 4.68 | 2.96 |
| Contribution ratio (%) | 52.01% | 32.89% |
| Weight coefficient (W) | 0.61 | 0.39 |
The first principal component corresponds to the larger eigenvectors of soluble protein content, soluble sugar content, and free proline content, which reflect the change of osmoprotectants content, and the relative water content reflects the membrane permeability. The second principal component corresponds to the larger eigenvector of SOD enzyme activity, which reflects the change of the protective enzyme system.
Using Eq. (3), the weights of the two comprehensive indexes were 0.61 and 0.39, respectively. The weighted membership function value D was then calculated under each treatment using Eq. (4), and sorted according to the D value (Table 3). Table 3 shows that more cultivation years improves the cold tolerance of Cinnamomum camphora (L.) Presl.
| Measurement indicators | Cultivation years of C. camphora moving | |||
|---|---|---|---|---|
| 1y. | 5y. | 10y. | 15y. | |
| Relative conductivity | 0.37 | 0.37 | 0.38 | 0.38 |
| MDA | 0.44 | 0.45 | 0.48 | 0.45 |
| Relative water content | 0.40 | 0.41 | 0.41 | 0.42 |
| CAT | 0.47 | 0.48 | 0.49 | 0.49 |
| SOD | 0.45 | 0.52 | 0.54 | 0.55 |
| POD | 0.36 | 0.37 | 0.36 | 0.36 |
| Soluble protein | 0.42 | 0.42 | 0.42 | 0.44 |
| Soluble sugar | 0.44 | 0.44 | 0.49 | 0.49 |
| Free proline | 0.41 | 0.42 | 0.51 | 0.51 |
| Comprehensive evaluation (D) | 0.80 | 0.81 | 0.85 | 0.86 |
| Sorting of cold resistance | 4 | 3 | 2 | 1 |
Discussion
Relative water content is an important index to measure plant water content. Under stress conditions, the relative water content in plants decreases as the degree of stress increases. The results of this study showed that the relative water content of Cinnamomum camphora (L.) Presl leaves decreased gradually under low temperature stress, but the lower the cultivation time, the more serious the low temperature stress and the poorer the water retention capacity.
When plants suffer from adversity, the integrity of the cell membrane is destroyed, resulting in the leakage of intracellular electrolytes (Thakur & Nayyar, 2013). Previous studies have shown that under low temperature stress, the weaker the cold tolerance, the greater the membrane permeability and the higher the relative electrical conductivity of the plants (Li et al., 2015; Ni et al., 2020). In this study, the relative electrical conductivity of Cinnamomum camphora (L.) Presl increased, indicating that the cell membrane structure of Cinnamomum camphora (L.) Presl leaves was damaged to varying degrees by low temperature stress.The shortest cultivation times had the largest increase in relative electrical conductivity, the smallest change in relative electrical conductivity of cultivation of 15 y. Cinnamomum camphora (L.) Presl leaves.
MDA is the result of membrane lipid peroxidation in plants in response to external changes, which are toxic to cells. These findings are consistent with the physiological responses observed by Fan et al. (2015) of different Olea europaea L. and Ni et al. (2020) of different Citrus reticulata Blanco under temperature stress. The level of cold tolerance of plants is negatively correlated with MDA content, meaning the weaker the cold tolerance, the higher the MDA content. In this study, the MDA content of Cinnamomum camphora (L.) Presl leaves increased gradually, indicating that low temperature stress caused damage to Cinnamomum camphora (L.) Presl leaves, with the shortest cultivation time showing the greatest change range, indicating the maximum degree of peroxidation, and the weakest cold tolerance.
Plants can adjust the concentration of cell fluid in vivo to adapt to changes in the external environment through osmoprotectants. Soluble sugar and proline are important osmoprotectants in plants that can reduce cell water potential and increase cell stability, which is closely linked to stress tolerance (Farhangi-Abriz & Torabian, 2017). In this study, the soluble sugar content and proline content of Cinnamomum camphora (L.) Presl leaves in different cultivation years increased in different degrees under low temperature stress, which showed that the Cinnamomum camphora (L.) Presl that had been cultivated longer were able to actively produce regulatory substances to adapt to environmental changes in order to resist low temperature stress. Consistent with the research results of Ying et al. (2019), proline content produced by plants with strong cold tolerance is higher than that of plants with weak cold tolerance. In this study, The longer the cultivation of Cinnamomum camphora (L.) Presl leaves, the proline content changed the most and increased the most, which indicated that Cinnamomum camphora (L.) Presl had strong cold tolerance. The soluble protein of Cinnamomum camphora (L.) Presl gradually increased with the increase of cultivation years under low temperature stress, being highest for 15 year old while lowest for 1 year old, which was consistent with the study of Chen et al. (2024). The increase of soluble protein induced higher content of bound water in cells, thereby reducing the possibility of death of plants due to intracellular freezing. therefore, its content could reflect the degree of frost damage of plants.
When plants are under external stress, they can maintain a balance of reactive oxygen species in vivo by protecting the enzyme system, and can directly decompose H2O2 into H2O and O2, thus reducing the damage of reactive oxygen species (Hui et al., 2019) and ensuring growth and development (Theocharis, Clément & Barka, 2012). In this study, the activities of catalase, superoxide dismutase, and peroxidase were all changed under low temperature stress, which indicated that under low temperature stress, Cinnamomum camphora (L.) Presl leaves cleared reactive oxygen species in vivo by increasing the activity of antioxidant enzymes, and improved the damage caused by stress. However, the stress level increased too high, the plant could no longer react and the damage of cells is gradually irreversible, and the enzyme activity will gradually decline until it is lost.
Conclusion
In this experiment, the membership function method was used to comprehensively evaluate the cold tolerance of the Cinnamomum camphora (L.) Presl in four separate cultivation years. The cold tolerance of trees that had been cultivated for 15 y was the strongest, and was the weakest in those that had only been cultivated for 1 y, which indicates that the cold tolerance of the Cinnamomum camphora (L.) Presl can be improved by lengthening cultivation time. For planting these trees in the north, domestication cultivation and moving cultivated trees from the south to the north area may provide better outcomes. In the initial transplanting stage of Cinnamomum camphora (L.) Presl, anti-freezing protection measures should be taken to avoid huge economic losses. A principal component analysis showed that relative electrical conductivity, soluble protein, soluble sugar content, and catalase activity could be used as important indexes for cold tolerance analysis of Cinnamomum camphora (L.) Presl. and the cumulative variance contribution rate was 84.90%. Among these factors, relative electrical conductivity was the most representative. These results could provide some reference for seed selection or northward migration of Cinnamomum camphora (L.) Presl.
The important physiological parameters changed sharply at minus 10–15 degrees. Thus we extrapolated that the critical temperature of the cold damage of Cinnamomum camphora (L.) Presl was in the range of minus 10–15. Which is consistent with the research results Jin et al. (2023).
The results of this study not only provide theoretical support for screening cold tolerance varieties of Cinnamomum camphora (L.) Presl, but also provide a basis for further study of the physiological mechanism of the Cinnamomum camphora (L.) Presl under low temperature stress. However, molecular differences of cold-tolerance varieties of Cinnamomum camphora (L.) Presl with different cultivation years need to be further explored.