The effectiveness of arbuscular mycorrhizal fungal species (Funneliformis mosseae, Rhizophagus intraradices, and Claroideoglomus etunicatum) in the biocontrol of root and crown rot pathogens, Fusarium solani and Fusarium mixture in pepper
- Published
- Accepted
- Received
- Academic Editor
- Nasim Yasin
- Subject Areas
- Microbiology, Mycology, Plant Science
- Keywords
- Pepper, AMF, Disease severity, Fusarium solani, Fusarium mix, Root colonization, Plant nutrients, Biological control, Root and crown rot
- Copyright
- © 2025 Bilgili
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. The effectiveness of arbuscular mycorrhizal fungal species (Funneliformis mosseae, Rhizophagus intraradices, and Claroideoglomus etunicatum) in the biocontrol of root and crown rot pathogens, Fusarium solani and Fusarium mixture in pepper. PeerJ 13:e18438 https://doi.org/10.7717/peerj.18438
Abstract
This study evaluated the effectiveness of arbuscular mycorrhizal fungi (AMF) species, including Funneliformis mosseae (FM), Rhizophagus intraradices (RI), Claroideoglomus etunicatum (CE), and a Mycorrhizal mix (MM) comprising these three species, on pepper plants (Capsicum annuum L.) inoculated with two isolates of Fusarium solani (48-F. solani and 18-F. solani) and two isolates of Fusarium mix (50-F. mixture and 147-F. mixture). Analysis of variance (ANOVA)-Tukey statistics revealed that the effects of AMF inoculations on morphological parameters, disease severity, root colonization, and total spore numbers in pathogen-infected plants varied significantly depending on the AMF species and pathogen group. AMF colonization significantly reduced disease severity, with disease inhibition (DI) reaching up to 58%, depending on the specific pathogen. However, there were a few instances where the application of AMF did not lead to a reduction in disease severity. Single AMF species were more effective in enhancing the growth of pathogen-treated host plants and suppressing disease compared to the mixed AMF. The mixed AMF was only more effective in balancing pathogen-induced decreases in plant nutrients (Copper (Cu), Magnesium (Mg), Zinc (Zn), and Phosphorus (P)). Among the compared mycorrhizae, C. etunicatum (CE) was the most effective in disease suppression due to its relatively more positive effects on plant root structure, increasing root fresh weight by up to 49% in the CE+pathogen plant group compared to the control group. Root colonization rates were generally higher in plants treated with both mycorrhiza and pathogens compared to plants treated with mycorrhiza alone. Overall, the curative effects of AMFs on plants following pathogen application varied concurrently with disease severity rates caused primarily by pathogens. AMFs demonstrated greater efficacy in combating 18-F. solani, which causes less severe plant disease. However, the effectiveness of AMFs was comparatively lower against 48-F. solani and 147-F. mix., which cause more severe plant disease. This indicates that the efficacy of AMFs varies depending on the specific strain of Fusarium solani, with better results observed against strains that cause less severe plant disease.
Introduction
The pepper (Capsicum annuum L.) plant is a globally significant and widely used crop, consumed both fresh and dried. It is cultivated over an extensive area of 2.05 million hectares, with a global annual production of approximately 35.6 million tons (Faostat, 2021). Turkey contributes around 6–9% of this total production. Pepper cultivation is often impacted by two prevalent fungal diseases: root rot and wilt. These diseases pose a significant threat to the health and productivity of pepper plants, leading to adverse effects on overall crop yield (El-kazzaz et al., 2022). Fungal diseases cause annual yield losses of approximately 14% in pepper crops (Coskun, Alptekin & Demir, 2023). Root and crown rot are common pathogens that occur during the growth period of pepper plants in the field. Over time, affected plants start to exhibit symptoms such as wilting, yellowing, and drying. The pathogen responsible for these diseases can persist in the soil for an extended duration, often in the form of chlamydospores. This soil-borne pathogen has the ability to survive in plant residues, leading to the occurrence of wilting in newly planted crops during subsequent favorable conditions for the disease (Zhang, Yu & Wang, 2021; Devi et al., 2022). The impact of wilt is not limited to individual plants but can spread to other nearby plants depending upon climate and soil conditions (i.e., soil moisture) and irrigation practices, resulting in substantial economic losses across large areas (Panth, Hassler & Baysal-Gurel, 2020; Zhang, Yu & Wang, 2021). Phytophthora capsici, various Fusarium spp., Pythium spp., Rhizoctoniasolani, and Macrophomina phaseolina are well-known soilborne pathogens that frequently contribute to yield losses in vegetable cultivation (Panth, Hassler & Baysal-Gurel, 2020; El-kazzaz et al., 2022). Among these common pathogens, Fusarium wilt is a rapidly spreading disease that severely impacts the quality and productivity of pepper plants, similar to its effects on crops such as cotton, cucumber, chickpea, banana (Pu et al., 2022), necessitating effective management.
Cultural measures (such as crop rotation, drip irrigation, and soil fumigation) and chemical methods are utilized to mitigate economic losses caused by soil-borne diseases (Devi et al., 2022). However, chemical methods are now prohibited due to their detrimental effects on the environment and human health. While cultural measures effectively control disease spread, there can be challenges in implementing them. Soil fumigation is limited due to cost, applicability in large areas, and negative impact on beneficial soil microflora. Factors like ozone layer depletion, climate change, drought, and the development of resistance in diseases and pests to chemicals have prompted producers to explore alternative solutions (Li et al., 2019; Ramírez-Gil & Morales-Osorio, 2020; Devi et al., 2022).
The use of resistant varieties or biological control and protective agents is the most secure in the fight against the disease (Yücel & Özarslandan, 2014; Panth, Hassler & Baysal-Gurel, 2020).
In recent times, the utilization of arbuscular mycorrhizal fungus has gained prominence as a biological control strategy in combating bacterial, viral, fungal, and nematode infections across various plant species (Dey & Ghosh, 2022; Weng et al., 2022).
Arbuscular mycorrhizal fungi (AMF) can have a symbiotic relationship with around 71–90% of plants (Song et al., 2015; Dowarah, Gill & Agarwala, 2022; Dey & Ghosh, 2022; Weng et al., 2022). In sustainable agriculture, AMF is recognized as a biofertilizer and biocontrol agent (Dowarah, Gill & Agarwala, 2022). AMF enhances the nutrient uptake of plants (Dowarah, Gill & Agarwala, 2022). Plants also benefit from AMF in their fight against biotic and abiotic stressors (Wu et al., 2021).
AMFs have been tested against both above-ground and soil-borne diseases. The application of Glomus versiforme mycorrhizae to Salvia miltiorrhiza plants was found to enhance the plant’s resistance to the soil-borne pathogen Fusarium oxysporum. The increased protection of mycorrhizal-preinoculated plants against the pathogen was associated with elevated levels of defense enzymes in the roots, such as phenylalanine ammonia-lyase (PAL), chitinase, and β-1,3-glucanase, following pathogen attack (Pu et al., 2022). Song et al. (2015) investigated the effect of Funneliformis mosseae AMF on early blight disease in tomatoes, caused by the pathogen Alternaria solani. They found that AMF application significantly reduced the severity of the disease. Similarly, for this leaf disease, AMF-treated plants exhibited increased levels of defense enzymes—including β-1,3-glucanase, chitinase, PAL, and lipoxygenase (LOX)—in the leaves following pathogen inoculation. This increase in enzyme levels was associated with the successful reduction of the disease. Several studies have also reported how the change in the quantity and quality of plant root secretions following the application of AMF led to a decrease in the number of pathogens in the root zone (Dowarah, Gill & Agarwala, 2022). The quality and quantity of root secretions might vary based on the type of AMF, the plant, and the root colonization (Dowarah, Gill & Agarwala, 2022). More recent reviews provide a more comprehensive summary of studies on the use of several AMF species against various fungal diseases in diverse plants (Dowarah, Gill & Agarwala, 2022; Dey & Ghosh, 2022; Weng et al., 2022).
The interaction between plants, diseases, and AMF in enhancing plant protection through AMF colonization is complex. The effectiveness of various mycorrhizal fungi can differ, and not all AMF will have the same impact on plant defense. Recent research indicates that the benefits of AMF are influenced by factors such as the type of fungal species, the host plant, as well as soil and environmental conditions (Wu et al., 2021; Boutaj et al., 2022). Aside from these, rhizosphere chemistry, environmental circumstances, and interactions with other microorganisms in the mycorrhizal zone are among the factors influencing AMF’s ability to protect plants (Dowarah, Gill & Agarwala, 2022). The application of mycorrhizae alone or in combination with other mycorrhizae or other biological control microorganisms influences their efficiency (Dowarah, Gill & Agarwala, 2022). Other factors include the amount and timing of AMF inoculation, as well as interactions between AMF and host plants (Weng et al., 2022).
Numerous studies have explored the effects of different AMF species on soil-borne diseases caused by various pathogens, investigating the interactions between mycorrhiza, plants, and pathogens through various parameters (Song et al., 2015; Aljawasim, Khaeim & Manshood, 2020; Wu et al., 2021). However, there is limited research on the impact of AMF inoculation on the defense mechanisms of pepper plants against soil-borne pathogens, particularly in relation to Fusarium spp. (Rodriguez-Heredia et al., 2020; Coskun, Alptekin & Demir, 2023). The Güneydoğu Anadolu Projesi (GAP) region, where the study was performed, faces specific challenges due to monoculture and traditional irrigation methods that promote soil-borne pathogen spread (Bilgili, 2017; Bilgili et al., 2018). Despite the known benefits of AMF in enhancing plant resilience and disease control (Bilgili & Güldür, 2018), comprehensive studies addressing their effectiveness in this region remain scarce. Previous research has highlighted the need for detailed investigations into the mechanisms of AMF interactions and signaling processes with plants, but significant gaps and limited applications persist (Pu et al., 2022).
To address these gaps, this study seeks to test the following hypotheses: (1) AMF inoculation significantly enhances the defense mechanisms of pepper plants against soil-borne pathogens, particularly Fusarium spp., compared to non-inoculated plants; (2) The effectiveness of AMF in disease management varies depending on the specific AMF species and their interactions with the soil environment and pepper plants.
The overall goals of this study were to investigate the effects of three different AMFs—Funneliformis mosseae (FM), Rhizophagus intraradices (RI), and Claroideoglomus etunicatum (CE)—as well as a mycorrhizal mix (MM: FM+CE+RI), used as a biological control agent, on two isolates of soil-borne pathogens (Fusarium solani and Fusarium mix) in pepper plants. We examined these effects both independently and in combination to identify the most effective AMF species for disease suppression. Additionally, the study aimed to assess how these AMFs influence plant growth, nutrient uptake, and the rhizosphere of infected plants. By achieving these objectives, the research seeks to enhance our understanding of AMF efficacy in managing soil-borne pathogens and improving pepper plant health.
Materials and Methods
Potting mixture
Imported white sphagnum sterile peat, with an EC value of 35 mS/m (+/− 25%), a pH range of 5.5−6.5, and the fertilizer content amounting to 1.0 kg/m3 with an NPK ratio of 14:10:18, was used as the plant growth medium in pots (TS 1; Klasmann-Deilmann GmbH, Geeste, Germany). Furthermore, before being employed in the study, the peat was re-sterilized in an autoclave at 134 °C for 20 min. To enhance its moisture holding capacity, perlite, which has a high moisture retention ability, was added to the peat in a 1:1 ratio. (Portions of this text were previously published as part of a preprint; https://doi.org/10.21203/rs.3.rs-3260167/v1.)
Plant material & AMFs
The pepper variety INAN-3363 F1 (Capsicum annuum L.), registered by the GAP Agricultural Research Institute (GAPTAEM), was used as the plant material in this study. AMF used in the study; Claroideoglomus etunicatum (syn. Glomus etunicatum; Sensoy et al., 2007), Rhizophagus intraradices (syn. Glomus intraradices; Demir & Onoğur, 1999), and Funneliformis mosseae (syn. Glomus mosseae; Demir et al., 2015), were obtained from Dr. Semra Demir (Van-Yüzüncüyil University, Van, Turkey). The three species were combined in autoclaved sterile peat to create a mycorrhizal mix. The inoculum of the mycorrhizal mix was prepared in a ratio of CE/FM/RI (1:1:1) using sterile peat. The mycorrhizal fungus inoculum was applied at a rate of 1,000 spores/10 g of peat soil (Menge & Timmer, 1982).
Pathogen isolates
The fungal isolates Fusarium solani and Fusarium mix (consisting of F. solani, F. oxysporum, and F. oxysporum f.sp. vasinfectum) were obtained from a survey conducted in the pepper production areas of the GAP region as part of the projects TAGEM-BS-13/09-03/02-06 and HÜBAK-13168 (Bilgili, 2017). These isolates were used in the study after assessing their virulence. Four specific fungal isolates were included in the study: Fusarium mix-50 (Diyarbakır-Cermik), Fusarium mix-147 (Batman-Hasankeyf), Fusarium solani-18 (Sanliurfa-Kisas), and Fusarium solani-48 (Diyarbakir-Yenisehir).
Molecular diagnosis of isolates of fungal pathogens
The morphologically diagnosed fungal isolates were identified at the species level with molecular studies and analyses. DNA isolation was performed using the Plant Genomic DNA Purification Protocol of the Thermo Scientific GeneJET Plant DNA Purification Mini Kit (K0791, K0792; Thermo Fisher Scientific, Waltham, MA, USA). The amplification was performed at 658 bp and 58 °C (Tables 1 and 2). The ITS-Fuf-r primer (Abd-Elsalam et al., 2003) was used for Fusarium spp., and TEF1-α gene primers (Arif et al., 2012; TEF-Fs4f: ATCGGCCACGTCGACTCT and TEF-Fs4r: GGCGTCTGTTGATTGTTAGC) were used specifically for F. solani.
| Pathogen | Target region | Primer (forward and reverse) | Band length—annealing temperature | Reference |
|---|---|---|---|---|
| Fusarium spp. | ITS region | ITS-Fu-f (CAACTCCCAAACCCCTGTGA) | 398 bp | Abd-Elsalam et al. (2003) |
| ITS-Fu-r (GCGACGATTACCAGTAACGA) | 54 °C | |||
| Fusarium oxysporum | Calmodulin gene | CLOX1 (CAGCAAAGCATCAGACCACTATAACTC) | 534 bp | Mule et al. (2004) |
| CLOX2 (CTTGTCAGTAACTGGACGTTGGTACT) | 60 °C | |||
| ITS region | Fov1-Egf (CCACTGTGAGTACTCTCCTCG) | 438 bp | Abd-Elsalam et al. (2006) | |
| Fov1-Egr (CCCAGGCGTACTTGAAGGAAC) | 53 °C | |||
| Fusarium solani | TEF1-α gene | TEF-Fs4f (ATCGGCCACGTCGACTCT) | 658 bp | Arif et al. (2012) |
| TEF-Fs4r (GGCGTCTGTTGATTGTTAGC) | 58 °C | |||
| ITS region | ITS-Fu2f (CCAGAGGACCCCCTAACTCT) | 595 bp | Arif et al. (2012) | |
| ITS-Fu2r (CTCTCCAGTTGCGAGGTGTT) | 63.5 °C | |||
| ITS2/28S rDNA | ITS-Fs5f (CGTCCCCCAAATACAGTGG) | 485 bp | Arif et al. (2012) | |
| ITS-Fs5r (TCCTCCGCTTATTGATATGCT) | 61 °C |
| DNA sample number | Survey isolate number |
Fusarium spp. Fuf |
F. solani |
F.oysporum f.sp. vasinfectum Fov1egr |
F. oxysporum Clox |
|
|---|---|---|---|---|---|---|
| Fs5 | Fu2 | |||||
| 2 | 18 | + | + | + | ||
| 18 | 147 | + | + | + | + | |
| 28 | 50 | + | + | + | + | |
| 26 | 48 | + | + | + | ||
Experimental design
Pepper seedlings were grown in a medium composed of a 1:1 ratio of peat and perlite, with vermiculite as a covering material, placed in plastic viols (45 cm diameter, 5 cm mesh diameter, 6 cm mesh depth) (Aslanpay & Demir, 2015). Mycorrhizal inoculation was applied by adding 2.5 g of inoculum to the seed bed, while control viols remained uninoculated. Pepper seeds were pre-treated by soaking overnight, washing three times with distilled water, soaking in 2% NaClO for 5 min, and rinsing twice with sterile distilled water before sowing 1 day before the scheduled date. Seedlings were then transplanted into 16 × 18 cm plastic pots with 2–2.5 kg of growing medium. The experiment, conducted in the GAPTAEM Department of Plant Health’s laboratory and climate room, used a randomized plot design with six treatments (FM, CE, RI, MM, Control P, Control N), four replications, and 16 plants per treatment, totaling 96 plants per pathogen. Conditions included 12 h of light per day, 25 °C temperature, and 50–60% relative humidity. Watering was done with distilled water every 2 days until germination and daily thereafter (Vosatka & Gryngler, 1999). After 8 weeks, the mycorrhizal fungi activity was evaluated by measuring root colonization rates and disease severity (Fig. 1).
Figure 1: (A) Root structure of the treatments for 18-F. solani pathogen at the end of the trial (from left to right; Control N; Control P; FM+P; RI+P; CE+P; MM+P). (B) Roots of Control N. (C) Roots of Control P.
Pathogen inoculation into plants
Fungal isolates of Fusarium solani and mixed infections, referred to as Fusarium mix, were collected during a 2014 survey in the pepper production areas of the GAP region. These isolates, which exhibited high virulence, were transferred to PDA nutrient medium in a sterile cabinet. The pathogenic fungal isolates were then placed in 5 mm diameter erlenmayer flasks and incubated for 10 days in a 26 ± 1 °C incubator, following the preparation and autoclaving of an artificial oat culture medium. The spores of the pathogens were adjusted to a density of 1 × 106 CFU/g using the Thoma Lamel before being applied to the plants. Subsequently, the developed medium was inoculated around the roots of the plants by contaminating the potting soil during the transfer of plants from vials to pots. The growth of the pathogens and the formation of lesions were monitored daily in a climate room with 12 h of illumination, at a temperature of 25 ± 1 °C, and a humidity range of 50–60% (Ahmed, Sanchez & Candela, 2000; Alejo-Iturvide et al., 2008; Varma et al., 2009).
Determination of morphological growth parameters of seedlings
The morphological growth parameters of the seedlings were assessed. This included measuring the shoot and root lengths (in cm), the root collar diameter (in mm), the shoot and root fresh weights after harvest (in grams), and the number of leaves per pepper seedling during the harvest. The wet weights of the root and green parts of the plants were measured and then placed in paper bags. These samples were dried in ovens at 70 °C for 48 h to obtain the shoot and root dry weights (Kacar & Inal, 2008).
Determination of micro and macro nutrient contents
The micro and macro nutrient contents were determined separately in both the root zone and shoot of the plants. After harvesting the pepper plants, they were dried and ground. From the ground plant samples, 1 g was taken and subjected to the combustion process (Jones et al., 1991). The filtrates obtained after combustion, using a mixture of nitric and perchloric acid, were used to determine the contents (in mg/kg) of Calcium (Ca), Magnesium (Mg), Phosphorus (P), Potassium (K), Iron (Fe), Manganese (Mn), Zinc (Zn), Sodium (Na), Boron (B), and Copper (Cu) using an ICP-OES device (Jones et al., 1991). For total nitrogen analysis, plant samples underwent three stages: combustion, distillation, and titration, following the procedure described by Kacar & Inal (2008).
Determination of EC and pH in the growing medium
In order to determine the electrical conductivity (EC) and pH values of the growing medium in the different applications, 100 g samples of the potting mixture were weighed and used to prepare saturation paste with distilled water. The pH and EC values of the saturation paste were measured using a pH meter and an EC meter, respectively.
Evaluation of disease severity
Disease symptoms that appeared in plants after the fourth week of pathogen inoculation were evaluated weekly to determine the disease severity. A scale ranging from 0 to 4 was used to assess the disease severity caused by Fusarium solani and Fusarium mix at the end of the 4th, 5th, 6th, and 7th weeks following pathogen inoculation. The scale was defined as follows: 0 indicated no symptoms, 1 represented minor color change in the leaf, slight wilting, or deformation, 2 indicated severe yellowing of the leaf, wilting, and stunting, 3 indicated blackening of the stem, and 4 indicated a completely dry or dead plant. The results of these evaluations were calculated using the formula below to determine the disease severity (%) (Wheeler, 1969);
The disease inhibition rate was calculated to assess the effectiveness of each treatment in suppressing the disease using the formula proposed by De Corato et al. (2020);
Determination of AMF root colonization
Fixation and staining
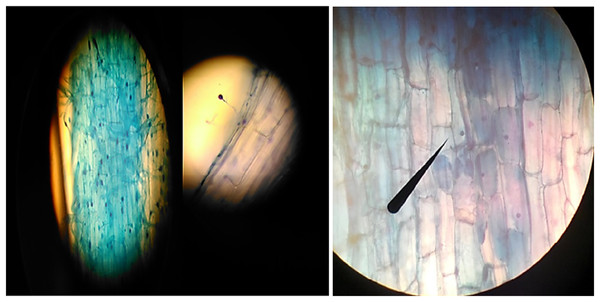
The above-ground parts of the pepper plant were cut, and the root and root collar were carefully removed from the soil. The roots, which were separated from the soil, were thoroughly washed under tap water to clean any soil particles adhering to them. Next, 0.5–1 g pieces of the roots were taken and placed in AFA Fixation liquid, which consists of 90 ml of 70% Alcohol, 5 ml of Formaldehyde, and 5 ml of Acetic acid. The roots were kept in this liquid at +4 °C until the dyeing process. The presence of mycorrhizal fungus and the percentage of colonization were determined by staining the roots in AFA liquid using trypan blue. For the dye solution, a mixture of lactic acid (40 ml), glycerin (80 ml), and distilled water (40 ml) was used, containing 0.4% Trypan blue. This dye solution was adapted from the method of Phillips & Hayman (1970) and modified based on Read, Kouckeki & Hodgsen (1976). The GridLine Intersect Method, as described by Giovannetti & Mosse (1980), was utilized to determine the percentage of colonization of AMF fungi in the dyed roots. Additionally, the number of mycorrhizal spores was determined by staining the roots with Trypan Blue and examining them under a microscope, following the procedure outlined by Read, Kouckeki & Hodgsen (1976) (Fig. 2).
Figure 2: The various appearances of AMF fungi in the roots of pepper seedlings under a light microscope.
Evaluation of the efficacy of AMF Mycorrhizae (Root colonization)
By staining the roots with Trypan Blue, we examined the percentage colonization and the number of mycorrhizal spores in the roots of the pots treated with mycorrhiza under a microscope (Read, Kouckeki & Hodgsen, 1976, Fig. 2). To assess the number of mycorrhizal roots colonized and the efficacy of AMFs, the roots were removed from the pots and washed with tap water after harvest. Subsequently, all roots were placed in capped tubes containing 50% ethyl alcohol, which were kept in the dark in a refrigerator. The roots were then taken out, washed with distilled water, and cut to approximately 5 cm in length from the bottom of the root collar. These sections were wrapped in gauze and sealed with a stapler. The samples were returned to the test tubes, and a 10% KOH solution was added, followed by incubation at 25 °C for 4 days. After the 4th day, the cloth packages were opened, and the roots were washed with distilled water. They were then rewrapped with the cloth packages and submerged in a 1% HCl solution for 3 min. Next, the samples were transferred to a 1% Trypan Blue solution and placed on a magnetic stirrer for 3 h at a mild heat. Finally, the samples were removed from the cloth packages, thoroughly washed with distilled water, and placed on a slide for further examination. The samples were then observed using a light microscope at a magnification of 40x (Koske & Gemma, 1989; Yildirim, 2014).
The percentage of root infection was determined using the calculation method described by Giovannetti & Mosse (1980);
Mycorrhiza total spore count (number/10)
In determining the total number of spores in the soil; samples were taken from the peat perlite mixture in the pots, totaling 10 g. Distilled water (100 ml) was added to the samples, which were then passed through sieves with a mesh size of 53–125 µm. The resulting mixture was centrifuged at 3,000 rpm for 20 min. From the supernatant, 10 ml was extracted and brought up to a total volume of 50 ml by adding 50% sucrose. This mixture was subjected to centrifugation once again at 3,000 rpm for 20 min. Subsequently, 5 ml of the centrifuged samples were placed in a petri dish, and the spore density was examined under a microscope at a magnification of 40x (Gerdeman & Nicolson, 1963; Akay & Karaarslan, 2015).
Statistical analyzes
Statistical analyses were conducted using one-way analysis of variance (ANOVA) to assess differences between groups, followed by Tukey’s Multiple Comparison Test to determine which groups differed significantly. These analyses were carried out using the JMP software.
Results
Plant morphological parameters
All morphological parameters were negatively affected by the application of pathogens to plants in four different pathogen groups. The values of morphological parameters in the control positive groups, where pathogen application was made, were lower than those in the control negative groups across all pathogen groups (Tables 3–6). Depending on the pathogen species, biomass reductions ranged from 10% to 54% in the root parts and from 13% to 56% in the aboveground parts (Table 7). Changes in the fresh and dry weights of shoots and roots were found to be statistically significant in all four groups (p < 0.05), while the significance of other parameters varied depending on the pathogen group and mycorrhiza application.
| 18-F. solani | F | p | Control N | Control P | CE | CE+P | RI | RI+P | FM | FM+P | MM | MM+P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | SL | 1.5 | ns | 35.24 ± 1.40a | 33.67 ± 3.42a | 31.74 ± 1.88a | 29.88 ± 0.96a | 35.37 ± 2.47a | 37.17 ± 3.50a | 33.14 ± 1.25a | 38.00 ± 6.28a | 39.88 ± 1.45a | 26.85 ± 2.39a |
| Morp | RL | 1.6 | ns | 28.99 ± 1.58a | 24.38 ± 3.56a | 30.75 ± 1.62a | 29.39 ± 4.02a | 30.67 ± 2.98a | 26.30 ± 4.34a | 32.26 ± 0.74a | 22.26 ± 1.19a | 33.45 ± 3.61a | 25.95 ± 2.87a |
| Param | RCD | 1.9 | ns | 3.98 ± 0.16a | 3.95 ± 0.27a | 3.67 ± 0.06a | 3.77 ± 0.03a | 3.79 ± 0.24a | 4.11 ± 0.07a | 33.9 ± 0.11a | 3.82 ± 0.21a | 3.81 ± 0.37a | 3.24 ± 0.11a |
| LN | 0.9 | ns | 25.29 ± 1.50a | 21.37 ± 2.40a | 26.00 ± 3.10a | 22.33 ± 0.68a | 26.75 ± 4.39a | 29.75 ± 0.94a | 28.56 ± 1.83a | 264.1 ± 3.92a | 25.85 ± 1.16a | 24.66 ± 3.75a | |
| SFW | 2.2 | ns | 47.28 ± 1.93ab | 34.79 ± 6.44b | 47.60 ± 4.52ab | 47.01 ± 8.03ab | 42.10 ± 8.07ab | 60.97 ± 3.46a | 49.50 ± 2.11ab | 48.50 ± 4.82ab | 35.91 ± 4.34b | 33.90 ± 6.48b | |
| SDW | 2.3 | * | 4.25 ± 0.28ab | 2.90 ± 0.61ab | 4.98 ± 0.70a | 4.51 ± 0.34ab | 4.15 ± 0.82ab | 4.77 ± 0.27ab | 4.4 ± 50.20ab | 4.94 ± 0.45a | 3.27 ± 0.37ab | 2.55 ± 0.78b | |
| RFW | 3.7 | ** | 6.26 ± 0.74ab | 4.08 ± 1.01b | 11.54 ± 0.97a | 9.36 ± 1.48ab | 7.95 ± 2.41ab | 6.46 ± 1.14ab | 5.85 ± 0.43ab | 7.71 ± 0.26ab | 8.34 ± 0.18ab | 3.85 ± 0.98b | |
| RDW | 3.5 | ** | 0.87 ± 0.14ab | 0.53 ± 0.13ab | 1.15 ± 0.10ab | 1.03 ± 0.14ab | 1.29 ± 0.18a | 0.74 ± 0.21ab | 0.75 ± 0.06ab | 0.90 ± 0.02ab | 0.76 ± 0.05ab | 0.37 ± 0.16b | |
| Plant | B | 4.6 | ** | 65.70 ± 3.51a | 54.42 ± 2.67ab | 63.10 ± 1.04ab | 53.38 ± 1.47ab | 67.36 ± 2.06a | 55.50 ± 2.08ab | 62.00 ± 0.85ab | 51.01 ± 0.77b | 68.064 ± 4.71a | 58.16 ± 2.09ab |
| Nutr. | Ca | 0.7 | ns | 13,156 ± 1,465a | 13,286 ± 478a | 13,650 ± 338a | 12,447 ± 738a | 13,244 ± 424a | 15,000 ± 620a | 14,586 ± 319a | 13,134 ± 573a | 13,078 ± 1,828a | 15,616 ± 484a |
| Cu | 5.2 | ** | 18.55 ± 2.08ab | 10.06 ± 1.23b | 17.29 ± 0.97ab | 8.61 ± 1.04b | 22.49 ± 1.75ab | 12.82 ± 1.96b | 21.02 ± 4.07ab | 9.20 ± 0.45b | 30.46 ± 6.4a | 10.12 ± 0.90b | |
| Fe | 4.8 | ** | 94 ± 1.95b | 128 ± 9.40a | 122 ± 3.73a | 119 ± 5.35ab | 127 ± 11.69a | 130 ± 6.37a | 137 ± 7.41a | 112 ± 2.14ab | 120 ± 9.75ab | 143 ± 8.51a | |
| K | 3.8 | ** | 60,396 ± 2,513b | 69,560 ± 5,208ab | 56,501 ± 2,311b | 62,957 ± 4,801ab | 65,057 ± 2,153ab | 71,184 ± 1,331ab | 67,640 ± 2,841ab | 58,722 ± 824b | 77,046 ± 5,124a | 72,183 ± 7,472ab | |
| Mg | 2.9 | ** | 10,245 ± 982ab | 8,172 ± 751ab | 9,241 ± 444ab | 8,406 ± 1,340ab | 8,122 ± 1,408ab | 12,520 ± 408a | 10,433 ± 315ab | 11,362 ± 545ab | 6,586 ± 1,379b | 10,789 ± 531ab | |
| Mn | 14.1 | ** | 32 ± 2.84d | 47 ± 9.40bcd | 57 ± 2.44bc | 51 ± 4.87bcd | 67 ± 3.55b | 91 ± 6.75a | 51 ± 1.01bc | 42 ± 0.98cd | 52 ± 7.41bc | 48 ± 0.94bcd | |
| Na | 9.1 | ** | 361 ± 29.4d | 885 ± 60abc | 481 ± 57cd | 907 ± 83abc | 440 ± 68cd | 986 ± 40.26ab | 566 ± 17.204bcd | 847 ± 30.47abc | 615 ± 133bcd | 1,366 ± 526a | |
| P | 4.4 | ** | 8,102 ± 416abc | 8,068 ± 417abc | 7,203 ± 166abc | 7,356 ± 538abc | 6,425 ± 124c | 9,131 ± 532a | 8,108 ± 289abc | 8,591 ± 322ab | 6,771 ± 838bc | 9,336 ± 468a | |
| Zn | 5.9 | ** | 44 ± 4.06b | 63 ± 11.7abc | 57 ± 5.47abc | 60 ± 5.78abc | 81 ± 4.83a | 71 ± 4.39ac | 66 ± 4.53abc | 42 ± 1.88bc | 83 ± 9.23a | 75 ± 5.84abc | |
| Growth | pH | 1.7 | ns | 6.27 ± 0.02a | 6.06 ± 0.14a | 6.09 ± 0.01a | 6.46 ± 0.09a | 5.97 ± 0.29a | 6.03 ± 0.03a | 6.23 ± 0.09a | 6.32 ± 0.25a | 6.54 ± 0.15a | 6.14 ± 0.12a |
| Medium | EC | 1.2 | ns | 0.32 ± 0.03a | 0.26 ± 0.01a | 0.27 ± 0.07a | 0.26 ± 0.03a | 0.49 ± 0.14a | 0.35 ± 0.02a | 0.44 ± 0.15a | 0.27 ± 0.01a | 0.27 ± 0.01a | 0.22 ± 0.01a |
| TN | 4.6 | ** | 3.61 ± 0.20abc | 3.98 ± 0.32abc | 2.85 ± 0.32c | 3.89 ± 0.79abc | 3.38 ± 0.55bc | 5.03 ± 0.24abc | 5.96 ± 1.23a | 3.69 ± 0.54abc | 2.56 ± 0.13c | 5.54 ± 0.38ab | |
| P | 5.9 | *** | 69.60 ± 1.67ab | 50.81 ± 0.76bc | 34.52 ± 1.32c | 62.95 ± 19.7ab | 65.17 ± 6.84ab | 67.04 ± 1.17ab | 64.20 ± 5.14ab | 64.93 ± 2.49ab | 64.38 ± 3.08ab | 76.66 ± 2.96a | |
| K | 33.9 | *** | 75.70 ± 5.25c | 89.00 ± 17.5c | 46.20 ± 7.31c | 81.50 ± 8.64c | 73.70 ± 1.63c | 84.30 ± 3.46c | 70.87 ± 4.38c | 77.60 ± 1.66c | 187.30 ± 4.17b | 247.70 ± 28.03a | |
| Cu | 1.9 | ns | 0.63 ± 0.07a | 0.47 ± 0.03a | 0.42 ± 0.02a | 0.56 ± 0.10a | 0.61 ± 0.07a | 0.56 ± 0.06a | 0.44 ± 0.03a | 0.43 ± 0.01a | 0.63 ± 0.05a | 0.64 ± 0.02a | |
| Fe | 0.6 | ns | 14.19 ± 0.43a | 14.64 ± 1.24a | 16.36 ± 0.14a | 15.71 ± 4.72a | 18.99 ± 2.52a | 16.73 ± 3.42a | 14.83 ± 0.98a | 11.76 ± 1.0a | 16.45 ± 5.24a | 17.30 ± 3.37a | |
| Mn | 2.1 | ns | 1.61 ± 0.12ab | 1.62 ± 0.11ab | 1.60 ± 0.08ab | 2.05 ± 0.43ab | 2.02 ± 0.34ab | 1.94 ± 0.41ab | 1.38 ± 0.26ab | 1.04 ± 0.11b | 2.19 ± 0.67ab | 2.71 ± 0.36a | |
| Zn | 0.9 | ns | 1.19 ± 0.06a | 1.26 ± 0.09a | 1.34 ± 0.07a | 1.61 ± 0.23a | 1.68 ± 0.08a | 1.61 ± 0.14a | 2.32 ± 0.94a | 1.14 ± 0.06a | 1.79 ± 0.33a | 1.83 ± 0.18a |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g). *Significant at p < 0.05; ** Significant at p < 0.01; ns: not significant. Treatment groups with different letters (a, b, c, d or their combinations) are statistically different from each other.
| 48-F. solani |
F | p | Control N | Control P | CE | CE+P | RI | RI+P | FM | FM+P | MM | MM+P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | SL | 3.8 | ** | 35.24 ± 1.40a | 30.24 ± 2.6ab | 31.74 ± 1.88ab | 27.50 ± 4.35ab | 35.37 ± 2.47a | 31.30 ± 2.13ab | 33.14 ± 1.25a | 28.86 ± 2.13ab | 39.88 ± 1.45a | 20.90 ± 2.85b |
| Morp | RL | 4.3 | ** | 28.99 ± 1.58a | 24.40 ± 4.1ab | 30.75 ± 1.62a | 24.01 ± 0.83ab | 30.67 ± 2.98a | 25.54 ± 1.64ab | 32.26 ± 0.74a | 26.01 ± 4.56ab | 33.45 ± 3.61a | 13.43 ± 2.34b |
| Param | RCD | 1.8 | ns | 3.98 ± 0.16a | 3.24 ± 0.34a | 3.67 ± 0.07a | 3.52 ± 0.26a | 3.79 ± 2.24a | 3.61 ± 0.14a | 3.39 ± 0.11a | 3.55 ± 0.26a | 3.81 ± 0.37a | 3.07 ± 0.07a |
| LN | 1.1 | ns | 25.29 ± 1.50a | 16.88 ± 4.72 | 26.06 ± 3.10a | 21.66 ± 3.30a | 26.75 ± 4.39a | 21.41 ± 3.96a | 28.56 ± 1.83a | 25.05 ± 6.15a | 25.83 ± 1.16a | 19.38 ± 2.48a | |
| SW | 2.2 | * | 47.28 ± 1.93a | 23.92 ± 12.1ab | 47.60 ± 4.52a | 33.34 ± 8.55ab | 42.10 ± 8.07ab | 33.76 ± 8.57ab | 49.50 ± 2.11a | 31.96 ± 12.62ab | 35.91 ± 4.34ab | 17.65 ± 5.44b | |
| SDW | 3.1 | * | 4.25 ± 0.28ab | 1.87 ± 0.87b | 4.98 ± 0.7a | 2.95 ± 0.74ab | 4.15 ± 0.82ab | 2.93 ± 0.77ab | 4.45 ± 0.20ab | 2.87 ± 1.12ab | 3.27 ± 0.37ab | 1.37 ± 0.40b | |
| RFW | 4.7 | ** | 6.26 ± 0.74ab | 4.06 ± 1.3b | 11.54 ± 0.97a | 5.95 ± 0.46ab | 7.95 ± 2.41ab | 2.93 ± 0.98b | 5.85 ± 0.43ab | 4.41 ± 2.18b | 8.34 ± 0.18ab | 1.59 ± 0.37b | |
| RDW | 7.2 | ** | 0.87 ± 0.14abc | 0.40 ± 0.1cd | 1.15 ± 0.10ab | 0.53 ± 0.07bcd | 1.29 ± 0.18a | 0.45 ± 0.09cd | 0.75 ± 0.06abcd | 0.44 ± 0.21cd | 0.76 ± 0.05abcd | 0.12 ± 0.03d | |
| Plant | B | 1.9 | ns | 65 ± 3.51a | 50 ± 1.9a | 63 ± 1.04a | 59 ± 2.7a | 67 ± 2.06a | 53 ± 1.83a | 62 ± 0.85a | 57 ± 0.61a | 68 ± 4.71a | 55 ± 0.91a |
| Nutr. | Ca | 0.4 | ns | 13,156 ± 1,465a | 15,110 ± 7a | 13,650 ± 338a | 14,167 ± 2,046a | 13,244 ± 424a | 14,370 ± 30a | 14,586 ± 319a | 16,350 ± 1,513a | 13,078 ± 1,828a | 14,615 ± 1,195a |
| Cu | 2.1 | * | 18.55 ± 2.0ab | 14.7 ± 0.4ab | 17.29 ± 0.97ab | 15.45 ± 4.92ab | 22.49 ± 1.75ab | 12.82 ± 0.13ab | 21.02 ± 4.07ab | 10.08 ± 1.08b | 30.46 ± 6.40a | 12.08 ± 2.29ab | |
| Fe | 3.8 | ** | 94 ± 1.95b | 131 ± 4.2ab | 122 ± 3.73a | 130 ± 8.10a | 127 ± 11.69a | 124 ± 5.75ab | 137 ± 7.41a | 125 ± 5.91ab | 120 ± 9.75ab | 115 ± 3.35ab | |
| K | 4.5 | ** | 60,396 ± 25.13bc | 68,520 ± 2,340abc | 56,507 ± 2,311c | 73,942 ± 5,352ab | 65,057 ± 2,153abc | 72,875 ± 9,675abc | 67,640 ± 2,841abc | 77,030 ± 5,903ab | 77,046 ± 5,124a | 82,155 ± 1,175a | |
| Mg | 1.6 | ns | 10,245 ± 982a | 10,110 ± 89a | 9,241 ± 444a | 7,371 ± 1,330a | 8,122 ± 1,408a | 8,734 ± 973a | 10,433 ± 315a | 11,299 ± 763a | 6,586 ± 1,379a | 10,188 ± 832a | |
| Mn | 12.5 | ** | 32 ± 2.84e | 74 ± 2.3abcde | 57 ± 2.44bcd | 79 ± 6.57abc | 67 ± 3.55bcd | 106 ± 12.64a | 51 ± 1.01de | 86 ± 22.07ab | 52 ± 7.41cde | 83 ± 11.76abcd | |
| Na | 10.9 | ** | 361 ± 29.4d | 985 ± 16abcd | 481 ± 57cd | 1,166 ± 271a | 440 ± 68.12d | 1,007 ± 13.15abc | 566 ± 17.20cd | 1,061 ± 66.61ab | 615 ± 133bcd | 1,297 ± 91a | |
| P | 2.4 | ns | 8,102 ± 416a | 7,596 ± 62a | 7,203 ± 166a | 7,530 ± 711a | 6,425 ± 124a | 7,453 ± 107a | 8,108 ± 289a | 8,452 ± 548a | 6,771 ± 838a | 8,925 ± 149a | |
| Zn | 5.2 | ** | 44.58 ± 4.06b | 80.7 ± 1.7ab | 57.20 ± 5.47ab | 71.32 ± 7.69ab | 81.73 ± 4.83a | 72.8 ± 4.21ab | 66.4 ± 4.53ab | 59.5 ± 8.11ab | 83 ± 9.23a | 78.5 ± 9.64ab | |
| Growth | pH | 1.7 | ns | 6.26 ± 0.02a | 6.41 ± 0.15a | 6.08 ± 0.01a | 5.91 ± 0.06a | 5.97 ± 0.51a | 6.13 ± 0.39a | 6.22 ± 0.09a | 6.58 ± 0.14a | 6.54 ± 0.15a | 6.51 ± 0.24a |
| Med | EC | 0.9 | ns | 0.31 ± 0.03a | 0.34 ± 0.01a | 0.27 ± 0.07a | 0.26 ± 0.02a | 0.49 ± 0.14a | 0.26 ± 0.02a | 0.44 ± 0.15a | 0.28 ± 0.003a | 0.26 ± 0.02a | 0.45 ± 0.18a |
| TN | 2.2 | ns | 3.61 ± 0.20a | 4.00 ± a | 2.85 ± 0.17a | 4.41 ± 1.58a | 3.38 ± 0.55a | 3.82 ± 0.86a | 5.96 ± 1.23a | 4.16 ± 1.0a | 2.56 ± 0.13a | 5.62 ± 0a | |
| P | 2.7 | * | 69.59 ± 1.67a | 63.44 ± 14.8ab | 34.52 ± 1.32b | 63.88 ± 5.11ab | 65.17 ± 6.84ab | 62.86 ± 5.84ab | 64.19 ± 5.14ab | 63.85 ± 9.59ab | 64.37 ± 3.08ab | 80.22 ± 9.97a | |
| K | 11.9 | ** | 75.70 ± 5.25cd | 138.70 ± 26.6ab | 46.20 ± 7.31d | 83.70 ± 1.58bcd | 73.70 ± 1.63cd | 118.90 ± 13.4bc | 70.87 ± 4.38cd | 115.70 ± 19.68bc | 187.30 ± 4.17a | 106.90 ± 15.8bc | |
| Cu | 1.9 | ns | 0.62 ± 0.07a | 0.63 ± 0.04a | 0.42 ± 0.02a | 0.60 ± 0.04a | 06.0 ± 0.08a | 0.62 ± 0.02a | 0.43 ± 0.03a | 0.51 ± 0.07a | 0.63 ± 0.05a | 0.45 ± 0.07a | |
| Fe | 0.8 | ns | 14.19 ± 0.43a | 16.56 ± 4.2a | 16.36 ± 0.14a | 206.3 ± 1.92a | 18.99 ± 2.52a | 15.22 ± 3.65a | 14.82 ± 0.98a | 15.02 ± 4.22a | 16.44 ± 5.24a | 12.00 ± 1.82a | |
| Mn | 1.7 | ns | 1.61 ± 0.12a | 2.66 ± 0.3a | 1.60 ± 0.07a | 2.36 ± 0.21a | 2.02 ± 0.34a | 2.09 ± 0.41a | 1.38 ± 0.27a | 1.59 ± 0.65a | 2.19 ± 0.67a | 1.33 ± 0.10a | |
| Zn | 0.8 | ns | 1.18 ± 0.06a | 1.55 ± 0.2a | 1.34 ± 0.07a | 1.81 ± 0.14a | 1.67 ± 0.08a | 1.64 ± 0.08a | 2.31 ± 0.94a | 1.40 ± 0.29a | 1.70 ± 0.33a | 1.43 ± 0.11a |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g). *Significant at p < 0.05; ** Significant at p < 0.01; ns: not significant. Treatment groups with different letters (a, b, c, d or their combinations) are statistically different from each other.
| 50-F. mix |
F | p | Control N | Control P | CE | CE+P | RI | RI+P | FM | FM+P | MM | MM+P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | SL | 4.1 | ** | 35.24 ± 1.40a | 32.21 ± 3.19a | 31 ± 1.88ab | 28.4 ± 1.45ab | 35.37 ± 2.47a | 32.4 ± 0.77ab | 33.14 ± 1.25a | 32.14 ± 2.25ab | 39.8 ± 1.45a | 21.66 ± 2.18b |
| Morp | RL | 1.6 | ns | 28.99 ± 1.58a | 24.28 ± 4.30a | 30.7 ± 3.25a | 30.03 ± 3.0a | 30.67 ± 2.98a | 30.30 ± 3.52a | 32.26 ± 0.74a | 24.81 ± 1.90a | 33.4 ± 3.61a | 23.60 ± 1.60a |
| Param | RCD | 2.3 | * | 3.98 ± 0.16a | 3.39 ± 0.32ab | 3.6 ± 0.07ab | 3.79 ± 0.16ab | 3.79 ± 0.24ab | 3.69 ± 0.10ab | 3.39 ± 0.11ab | 3.78 ± 0.38ab | 3.8 ± 0.37ab | 2.82 ± 0.12b |
| LN | 1.0 | ns | 25.29 ± 1.50a | 23.0 ± 3.62a | 26.0 ± 3.10a | 23.5 ± 1.88a | 26.75 ± 4.39a | 30.0 ± 2.98a | 28.56 ± 1.83a | 24.0 ± 3.46a | 25.8 ± 1.16a | 18.33 ± 3.92a | |
| SFW | 4.2 | ** | 47.28 ± 1.93a | 33.99 ± 7.97ab | 47.6 ± 4.52a | 38.8 ± 7.21ab | 42.10 ± 8.07a | 54.49 ± 8.60a | 49.50 ± 2.11a | 42.24 ± 7.61a | 35 ± 4.34ab | 10.21 ± 4.72b | |
| SDW | 3.4 | ** | 4.25 ± 0.28a | 3.11 ± 0.72ab | 4.98 ± 0.70a | 3.53 ± 0.48ab | 4.15 ± 0.82a | 4.53 ± 0.92a | 4.45 ± 0.20a | 3.80 ± 0.77ab | 3.2 ± 0.37ab | 0.80 ± 0.31b | |
| RFW | 4.3 | ** | 6.26 ± 0.74ab | 5.46 ± 1.32b | 11.5 ± 0.97a | 7.80 ± 1.03ab | 7.95 ± 2.41ab | 3.30 ± 1.47b | 5.85 ± 0.43ab | 7.13 ± 1.51ab | 8.3 ± 0.18ab | 1.70 ± 0.32b | |
| RDW | 4.2 | ** | 0.87 ± 0.14a | 0.78 ± 1.15ab | 1.15 ± 0.10a | 0.71 ± 0.16ab | 1.29 ± 0.18a | 0.67 ± 0.16ab | 0.75 ± 0.06ab | 0.68 ± 0.16ab | 0.7 ± 0.05ab | 0.15 ± 0.03b | |
| Plant | B | 0.7 | ns | 65.7 ± 3.51a | 66.5 ± 3.71a | 63.2 ± 0.90a | 63.6 ± 2.36a | 67.3 ± 2.06a | 61.8 ± 1.80a | 62.0 ± 0.85a | 65.1 ± 2.92a | 68.0 ± 4.71a | 68.4 ± 1.8a |
| Nutr. | Ca | 2.8 | ** | 13,156 ± 1,465b | 12,291 ± 1,062b | 13,707 ± 301b | 14,190 ± 858ab | 13,244 ± 424a | 15,533 ± 238ab | 14,586 ± 319ab | 13,670 ± 739b | 13,078 ± 1828b | 20,310 ± 179a |
| Cu | 1.5 | ns | 18.5 ± 2.08a | 16.3 ± 1.01a | 17.1 ± 0.68a | 28.8 ± 10.43a | 22.4 ± 1.75a | 24.8 ± 2.95a | 21.0 ± 4.07a | 27.2 ± 7.29a | 30.4 ± 6.4a | 23.3 ± 0.6a | |
| Fe | 8.9 | ** | 94 ± 1.95c | 101 ± 2.84bc | 124 ± 3.0ab | 129 ± 4.42ab | 127 ± 11.69ab | 122 ± 0.77ab | 137 ± 7.41a | 99 ± 2.70bc | 120 ± 9.75abc | 153 ± 2.1a | |
| K | 12.6 | ** | 60,396 ± 2,513cd | 59,466 ± 1,987cd | 58,177 ± 1,871d | 70,678 ± 1,891bc | 65,057 ± 2,153bcd | 74,811 ± 1,702b | 67,640 ± 2,841bcd | 68,216 ± 2,895bcd | 77,046 ± 5,124b | 96,950 ± 1,291a | |
| Mg | 1.4 | ns | 10,245 ± 982a | 8,017 ± 1,173a | 9,314 ± 404a | 28,074 ± 1,976a | 8,122 ± 1,408a | 11,170 ± 225a | 10,433 ± 315a | 9,465 ± 572a | 6,586 ± 1,379a | 12,070 ± 145a | |
| Mn | 22.7 | ** | 32.3 ± 2.84e | 55.0 ± 4.22cd | 57.6 ± 1.73cd | 70.3 ± 6.49bc | 67.6 ± 3.55bc | 92.0 ± 4.57a | 51.2 ± 1.01d | 54.5 ± 2.30cd | 52.5 ± 7.41cd | 81.9 ± 1.2ab | |
| Na | 11.4 | ** | 361 ± 29.42c | 310 ± 18.83c | 528 ± 44.78bc | 497 ± 49.65bc | 440 ± 68.12bc | 455b ± 22.07c | 566 ± 17.2b | 562 ± 45.41bc | 615 ± 133b | 1147 ± 28a | |
| P | 1.4 | ns | 8,102 ± 416a | 6,698 ± 556a | 7,310 ± 137a | 7,822 ± 220a | 6,425 ± 124a | 7,702 ± 200a | 8,108 ± 289a | 19,505 ± 1,266a | 6,771 ± 838a | 10,100 ± 88a | |
| Zn | 7.8 | ** | 44.5 ± 4.06d | 54.0 ± 4.21bcd | 55.5 ± 3.78cd | 76.3 ± 12.03abc | 81.7 ± 4.83a | 87.1 ± 7.57a | 66.4 ± 4.53abcd | 58.9 ± 4.31abcd | 83.7 ± 9.23ab | 90.3 ± 1.7a | |
| Grow | pH | 3.4 | ** | 6.26 ± 0.02ab | 6.13 ± 0.24ab | 6.08 ± 0.01ab | 6.04 ± 0.01ab | 5.97 ± 0.29ab | 5.61 ± 0.03b | 6.22 ± 0.09ab | 6.27 ± 0.12ab | 6.54 ± 0.15a | 6.61 ± 0.29a |
| Med | EC | 2.2 | ns | 0.31 ± 0.03a | 0.39 ± 0.10a | 0.27 ± 0.07a | 0.28 ± 0.03a | 0.49 ± 0.14a | 0.70 ± 0.09a | 0.44 ± 0.15a | 0.44 ± 0.05a | 0.26 ± 0.02a | 0.29 ± 0.06a |
| TN | 4.2 | ** | 3.61 ± 0.20ab | 2.92 ± 0.32b | 2.85 ± 0.32b | 3.99 ± 0.53ab | 3.38 ± 0.55ab | 5.01 ± 0.60ab | 5.96 ± 1.23a | 4.93 ± 0.93ab | 2.56 ± 0.13b | 5.48 ± ab | |
| P | 4.9 | ** | 69.59 ± 1.67a | 63.77 ± 11.99a | 34.52 ± 1.32b | 49.07 ± 6.09ab | 65.17 ± 6.84a | 74.23 ± 1.20a | 64.19 ± 5.14a | 61.79 ± 6.21a | 64.37 ± 3.08a | 71.30 ± 2.95a | |
| K | 18.3 | ** | 73.70 ± 5.25cde | 108.80 ± 17.90cd | 46.20 ± 7.31e | 54.00 ± 9.08de | 73.70 ± 1.63cde | 124.60 ± 16.4bc | 70.87 ± 4.38cde | 75.80 ± 1.31cde | 187.30 ± 4.17a | 176.90 ± 27.03ab | |
| Cu | 2.7 | ns | 0.62 ± 0.07a | 0.55 ± 0.04a | 0.42 ± 0.02a | 0.38 ± 0.04a | 0.60 ± 0.08a | 0.63 ± 0.03a | 0.43 ± 0.03a | 0.46 ± 0.03a | 0.63 ± 0.05a | 0.54 ± 0.04a | |
| Fe | 1.8 | ns | 14.19 ± 0.43a | 18.33 ± 2.05a | 16.36 ± 0.14a | 15.40 ± 0.89a | 18.99 ± 2.52a | 21.52 ± 1.12a | 14.82 ± 0.98a | 13.42 ± 2.14a | 16.44 ± 5.24a | 18.96 ± 1.48a | |
| Mn | 3.4 | ** | 1.61 ± 0.12b | 2.55 ± 0.35ab | 1.60 ± 0.07ab | 1.48 ± 0.12ab | 2.02 ± 0.35ab | 2.96 ± 0.11a | 1.38 ± 0.26b | 1.25 ± 0.08b | 2.19 ± 0.67ab | 2.00 ± 0.39ab | |
| Zn | 0.9 | ns | 1.18 ± 0.06a | 1.53 ± 0.08a | 1.34 ± 0.06a | 1.38 ± 0.18a | 1.67 ± 0.08a | 1.97 ± 0.02a | 2.31 ± 0.94a | 1.29 ± 0.11a | 1.70 ± 0.33a | 1.66 ± 0.14a |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g). *Significant at p < 0.05; ** Significant at p < 0.01; ns: not significant. Treatment groups with different letters (a, b, c, d, e or their combinations) are statistically different from each other.
| 147-F .mix |
F | p | Control N | Control P | CE | CE+P | RI | RI+P | FM | FM+P | MM | MM+P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant | SL | 1.7 | ns | 35.24 ± 1.40a | 30.77 ± 2.01a | 31.74 ± 1.88a | 26.27 ± 1.72a | 35.37 ± 2.47a | 27.69 ± 1.35a | 33.14 ± 1.25a | 31.94 ± 9.96a | 39.88 ± 2.05a | 23.53 ± 1.88a |
| Mor | RL | 2.2 | ns | 28.99 ± 1.58a | 24.81 ± 2.61a | 30.75 ± 1.62a | 29.20 ± 2.51a | 30.67 ± 2.98a | 22.60 ± 2.84a | 32.26 ± 0.74a | 21.55 ± 7.75a | 33.45 ± 3.61a | 20.70 ± 1.97a |
| Par. | RCD | 3.9 | ** | 3.98 ± 0.16a | 3.88 ± 0.22ab | 3.67 ± 0.07ab | 3.11 ± 0.25ab | 3.79 ± 0.25ab | 3.33 ± 0.03ab | 3.39 ± 0.12ab | 4.36 ± 0.81a | 3.81 ± 0.37ab | 2.65 ± 0.24a |
| LN | 1.1 | ns | 25.29 ± 1.50a | 24.83 ± 3.75a | 26.00 ± 3.10a | 14.00 ± 0.80a | 26.75 ± 4.39a | 16.91 ± 1.08a | 28.56 ± 1.83a | 30.16 ± 13.74a | 25.83 ± 1.16a | 19.77 ± 5.30a | |
| SFW | 2.9 | * | 47.28 ± 1.93ab | 40.94 ± 7.19ab | 47.60 ± 4.52ab | 18.72 ± 1.11b | 42.10 ± 8.07ab | 24.17 ± 3.93ab | 49.50 ± 2.11a | 36.23 ± 17.57ab | 35.91 ± 4.34ab | 19.73 ± 8.51b | |
| SDW | 2.9 | * | 4.25 ± 0.28ab | 3.21 ± 0.56ab | 4.98 ± 0.70a | 2.21 ± 0.42ab | 4.15 ± 0.82ab | 2.00 ± 0.23b | 4.45 ± 0.20ab | 3.26 ± 1.55ab | 3.27 ± 0.37ab | 1.56 ± 0.69b | |
| RFW | 4.3 | ** | 6.26 ± 0.74ab | 4.33 ± 1.03b | 11.54 ± 0.97a | 8.11 ± 0.09ab | 7.95 ± 2.41ab | 2.11 ± 0.77b | 5.85 ± 0.43ab | 3.82 ± 1.91b | 8.34 ± 0.18ab | 4.43 ± 1.76ab | |
| RDW | 5.4 | ** | 0.87 ± 0.14abc | 0.61 ± 0.13bc | 1.15 ± 0.10ab | 0.69 ± 0.07abc | 1.29 ± 0.18a | 0.30 ± 0.08c | 0.75 ± 0.06abc | 0.77 ± 0.07abc | 0.76 ± 0.06abc | 0.35 ± 0.12c | |
| Plant | B | 0.9 | ns | 65.7 ± 11.64a | 65.2 ± 3.67a | 63.1 ± 1.04a | 67.2 ± 4.78a | 67.3 ± 2.06a | 73.8 ± 5.19a | 62.0 ± 0.85a | 64.7 ± 1.06a | 68.0 ± 4.71a | 65.4 ± 0.93a |
| Nutr. | Ca | 1.3 | ns | 13,156 ± 1,465a | 15,611 ± 597a | 13,650 ± 338a | 15,960 ± 1,748a | 13,244 ± 424a | 13,812 ± 418a | 14,586 ± 319a | 15,875 ± 523a | 13,078 ± 1,828a | 16,876 ± 433a |
| Cu | 2.6 | * | 18.5 ± 2.08ab | 16.97 ± 1.23ab | 17.29 ± 0.97ab | 15.66 ± 1.21ab | 22.49 ± 1.75ab | 14.7 ± 0.68ab | 21.02 ± 4.07ab | 10.80 ± 2.26b | 30.46 ± 6.40a | 21.19 ± 2.55ab | |
| Fe | 4.4 | ** | 94 ± 1.95b | 112 ± 6.37ab | 122 ± 3.73a | 117 ± 10.0ab | 127 ± 11.69a | 131 ± 9.84a | 137 ± 7.41a | 102 ± 1.59ab | 120 ± 9.75ab | 130 ± 7.92ab | |
| K | 6 | ** | 60,396 ± 2,513bcd | 74,268 ± 3,812abc | 56,501 ± 2,311cd | 78,400 ± 6,270abc | 65,057 ± 2,153bcd | 80,785 ± 4,681ab | 67,640 ± 2,841abcd | 46,149 ± 15,656d | 77,046 ± 5,124abc | 92,920 ± 8,262a | |
| Mg | 3.5 | ** | 10,245 ± 982ab | 10,197 ± 929ab | 9,241 ± 444ab | 5,397 ± 615b | 8,122 ± 1,408ab | 7,599 ± 952ab | 10,433 ± 315ab | 12,610 ± 585a | 6,586 ± 1,379b | 11,333 ± 134ab | |
| Mn | 21 | ** | 32.3 ± 2.85d | 80.2 ± 7.09b | 57.5 ± 2.44c | 117 ± 14.23a | 67.6 ± 3.55bc | 89.1 ± 6.72ab | 51.2 ± 1.01c | 61.8 ± 4.37bc | 52.5 ± 7.41cd | 84.5 ± 2.56b | |
| Na | 4.6 | ** | 361 ± 29.42b | 674 ± 58.26a | 481 ± 57.9ab | 705 ± 82.20a | 440 ± 68.12ab | 710 ± 75.00a | 566 ± 17.20ab | 716 ± 39.06a | 615 ± 133ab | 724 ± 132a | |
| P | 3.0 | ** | 8,102 ± 416a | 7,526 ± 554a | 7,203 ± 166a | 6,625 ± 1,042a | 6,425 ± 124a | 6,496 ± 121a | 8,108 ± 289a | 8,578 ± 286a | 6,771 ± 838a | 8,586 ± 195a | |
| Zn | 7.2 | ** | 44.5 ± 4.07bc | 61.5 ± 7.94abc | 57.2 ± 5.47abc | 68.3 ± 10.25abc | 81.7 ± 4.83a | 78.5 ± 8.45a | 66.4 ± 4.53ab | 31.2 ± 0.80c | 83.7 ± 9.23a | 83.9 ± 6.07a | |
| Grow | pH | 1.4 | ns | 6.26 ± 0.02a | 5.78 ± 0.19a | 60.8 ± 0.01a | 6.26 ± 0.12a | 5.97 ± 0.29a | 6.06 ± 0.41a | 6.22 ± 0.09a | 6.23 ± 0.13a | 6.54 ± 0.15a | 6.08 ± 0.07a |
| Med | EC | 1.3 | ns | 0.31 ± 0.03a | 0.26 ± 0.02a | 0.27 ± 0.07a | 0.37 ± 0.07a | 0.49 ± 0.15a | 0.61 ± 0.13a | 0.44 ± 0.16a | 0.29 ± 0.10a | 0.26 ± 0.02a | 0.37 ± 0.12a |
| TN | 4.5 | ** | 3.61 ± 0.20b | 4.46 ± 0.45ab | 2.85 ± 0.33b | 2.14 ± 0.30b | 3.38 ± 0.55b | 4.57 ± 0.45ab | 5.96 ± 1.23a | 3.73 ± 0.46ab | 2.56 ± 0.13b | 4.18 ± ab | |
| P | 7.6 | ** | 69.59 ± 1.67a | 71.08 ± 9.32a | 34.52 ± 1.32b | 37.70 ± 3.80b | 65.17 ± 6.84a | 66.17 ± 5.63a | 64.19 ± 5.14a | 77.71 ± 7.36a | 64.37 ± 3.08a | 69.62 ± 4.33a | |
| K | 3.4 | ** | 75.70 ± 5.25b | 114.80 ± 4.75ab | 46.20 ± 7.31b | 94.20 ± 18.01ab | 73.70 ± 1.63ab | 134.70 ± 28.10ab | 70.87 ± 4.38b | 165.10 ± 75.00ab | 187.30 ± 4.17a | 101.5 ± 20.3ab | |
| Cu | 2.2 | ns | 0.62 ± 0.07a | 0.68 ± 0.01a | 0.42 ± 0.01a | 0.59 ± 0.08a | 0.60 ± 0.08a | 0.67 ± 0.03a | 0.43 ± 0.03a | 0.44 ± 0.02a | 0.63 ± 0.05a | 0.36 ± 0.18a | |
| Fe | 1.3 | ns | 14.19 ± 0.43a | 21.04 ± 4.71a | 16.36 ± 0.14a | 12.17 ± 2.20a | 18.99 ± 2.52a | 21.25 ± 3.31a | 14.82 ± 0.98a | 13.01 ± 0.51a | 16.44 ± 5.24a | 12.10 ± 6.09a | |
| Mn | 1.9 | ns | 1.61 ± 0.12a | 2.85 ± 0.47a | 1.60 ± 0.08a | 1.69 ± 0.13a | 2.02 ± 0.35a | 2.88 ± 0.72a | 1.38 ± 0.26a | 1.43 ± 0.20a | 2.19 ± 0.67a | 1.33 ± 0.72a | |
| Zn | 0.9 | ns | 1.18 ± 0.06a | 1.66 ± 0.12a | 1.34 ± 0.07a | 1.60 ± 0.09a | 1.67 ± 0.08a | 2.00 ± 0.15a | 2.31 ± 0.94a | 1.25 ± 0.08a | 1.70 ± 0.33a | 1.69 ± 0.12a |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g). *Significant at p < 0.05; ** Significant at p < 0.01; ns: not significant. Treatment groups with different letters (a, b, c, d or their combinations) are statistically different from each other.
| 18 F.solani | 48 F.solani | 50 F.mix | 147 F.mix | ||
|---|---|---|---|---|---|
| Plant Morp | SL | −4.46 | −14.19 | −8.60 | −12.68 |
| Parameters | RL | −15.90 | −15.83 | −16.25 | −14.42 |
| RRM | −0.75 | −18.59 | −14.82 | −2.51 | |
| LN | −15.90 | −33.25 | −9.05 | −1.82 | |
| SFW | −26.42 | −49.41 | −28.11 | −13.41 | |
| SDW | −31.76 | −56.00 | −26.82 | −24.47 | |
| RFW | −34.82 | −35.14 | −12.78 | −30.83 | |
| RDW | −39.08 | −54.02 | −10.34 | −29.89 | |
| Plant Nutr | B | −17.17 | −23.08 | 1.22 | −0.76 |
| Ca | 0.99 | 14.85 | −6.57 | 18.66 | |
| Cu | −45.77 | −20.32 | −11.89 | −8.52 | |
| Fe | 36.17 | 39.36 | 7.45 | 19.15 | |
| K | 15.17 | 13.45 | −1.54 | 22.97 | |
| Mg | −20.23 | −1.32 | −21.75 | −0.47 | |
| Mn | 46.88 | 131.25 | 70.28 | 148.30 | |
| N | 145.15 | 172.85 | −14.13 | 86.70 | |
| P | −0.42 | −6.25 | −17.33 | −7.11 | |
| Zn | 43.18 | 81.02 | 21.35 | 38.20 | |
| Growth med | pH | −3.35 | 2.40 | −2.08 | −7.67 |
| EC | −18.75 | 9.68 | 25.81 | −16.13 | |
| TN | 10.25 | 10.80 | −19.11 | 23.55 | |
| P | −27.00 | −8.84 | −8.36 | 2.14 | |
| K | 17.57 | 83.22 | 47.63 | 51.65 | |
| Cu | −25.40 | 1.61 | −11.29 | 9.68 | |
| Fe | 3.17 | 16.70 | 29.18 | 48.27 | |
| Mn | 0.62 | 65.22 | 58.39 | 77.02 | |
| Zn | 5.88 | 31.36 | 29.66 | 40.68 |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g).
The negative effects of pathogen application on plant growth parameters were mostly counteracted by mycorrhizal application in the 18-F. solani pathogen group and partially in the 50-F.mix and 147-F.mix pathogen groups. Except for a few instances, the application of CE, RI, and FM in the 18-F. solani group was largely effective in reversing the pathogen’s effects on all morphological parameters, whereas the MM was largely ineffective in countering these effects (Table 8). The CE+P group had 49% more root dry weight compared to the pathogen-free control group (Table 8). In the 48-F. solani group, only the FM application (FM+P) was able to eliminate the negative effect of pathogen application on the number of leaves. In the 50-F. mix pathogen group, The CE application (CE+P) effectively countered the negative effects of the pathogen on root length and root fresh weight, showing 3.6% and 24% more root length and root fresh weight compared to the pathogen-free control group (Table 8). The RI application (RI+P) countered the pathogen’s negative effects on root length, as well as shoot fresh and dry weight. The FM application (FM+P) successfully mitigated the pathogen-induced reduction in root fresh weight.
| 18-F.solani | 48-F. solani | 50-F.mix | 147-F.mix | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE+P | RI+P | FM+P | MM+P | CE+P | RI+P | FM+P | MM+P | CE+P | RI+P | FM+P | MM+P | CE+P | RI+P | FM+P | MM+P | ||
| Plant morp | SL | −15.2 | 5.5 | 7.8 | −23.8 | −22.0 | −11.2 | −18.1 | −40.7 | −19.4 | −8.0 | −8.8 | −38.5 | −25.5 | −21.4 | −9.4 | −33.2 |
| Parameters | RL | 1.4 | −9.3 | −23.2 | −10.5 | −17.2 | −11.9 | −10.3 | −53.7 | 3.6 | 4.5 | −14.4 | −18.6 | 0.7 | −22.0 | −25.7 | −28.6 |
| RRM | −5.3 | 3.3 | −4.0 | −18.6 | −11.6 | −9.3 | −10.8 | −22.9 | −4.8 | −7.3 | −5.0 | −29.1 | −21.9 | −16.3 | 9.5 | −33.4 | |
| LN | −11.7 | 17.6 | 4.4 | −2.5 | −14.4 | −15.3 | −0.9 | −23.4 | −7.1 | 18.6 | −5.1 | −27.5 | −44.6 | −33.1 | 19.3 | −21.8 | |
| SFW | −0.6 | 29.0 | 2.6 | −28.3 | −29.5 | −28.6 | −32.4 | −62.7 | −17.8 | 15.2 | −10.7 | −78.4 | −60.4 | −48.9 | −23.4 | −58.3 | |
| SDW | 6.1 | 12.2 | 16.2 | −40.0 | −30.6 | −31.1 | −32.5 | −67.8 | −16.9 | 6.6 | −10.6 | −81.2 | −48.0 | −52.9 | −23.3 | −63.3 | |
| RFW | 49.5 | 3.2 | 23.2 | −38.5 | −5.0 | −53.2 | −29.6 | −74.6 | 24.6 | −47.3 | 13.9 | −72.8 | 29.6 | −66.3 | −39.0 | −29.2 | |
| RDW | 18.4 | −14.9 | 3.4 | −57.5 | −39.1 | −48.3 | −49.4 | −86.2 | −18.4 | −23.0 | −21.8 | −82.8 | −20.7 | −65.5 | −11.5 | −59.8 | |
| Plant Nutr | B | −18.8 | −15.5 | −22.4 | −11.5 | −9.2 | −18.5 | −12.3 | −15.4 | −3.2 | −5.9 | −0.9 | 4.1 | 2.3 | 12.3 | −1.5 | −0.5 |
| Ca | −5.4 | 14.0 | −0.2 | 18.7 | 7.7 | 9.2 | 24.3 | 11.1 | 7.9 | 18.1 | 3.9 | 54.4 | 21.3 | 5.0 | 20.7 | 28.3 | |
| Cu | −53.6 | −30.9 | −50.4 | −45.4 | −16.7 | −30.9 | −45.7 | −34.9 | 55.7 | 34.1 | 47.0 | 25.9 | −15.6 | −20.8 | −41.8 | 14.2 | |
| Fe | 26.6 | 38.3 | 19.1 | 52.1 | 38.3 | 31.9 | 33.0 | 22.3 | 37.2 | 29.8 | 5.3 | 62.8 | 24.5 | 39.4 | 8.5 | 38.3 | |
| K | 4.2 | 17.9 | −2.8 | 19.5 | 22.4 | 20.7 | 27.5 | 36.0 | 17.0 | 23.9 | 12.9 | 60.5 | 29.8 | 33.8 | −23.6 | 53.9 | |
| Mg | −18.0 | 22.2 | 10.9 | 5.3 | −28.1 | −14.7 | 10.3 | −0.6 | 174.0 | 9.0 | −7.6 | 17.8 | −47.3 | −25.8 | 23.1 | 10.6 | |
| Mn | 59.4 | 184.4 | 31.3 | 50.0 | 146.9 | 231.3 | 168.8 | 159.4 | 117.6 | 184.8 | 68.7 | 153.6 | 262.2 | 175.9 | 91.3 | 161.6 | |
| N | 151.2 | 173.1 | 134.6 | 278.4 | 223.0 | 178.9 | 193.9 | 259.3 | 37.7 | 26.0 | 55.7 | 217.7 | 95.3 | 96.7 | 98.3 | 100.6 | |
| P | −9.2 | 12.7 | 6.0 | 15.2 | −7.1 | −8.0 | 4.3 | 10.2 | −3.5 | −4.9 | 140.7 | 24.7 | −18.2 | −19.8 | 5.9 | 6.0 | |
| Zn | 36.4 | 61.4 | −4.5 | 70.5 | 60.0 | 63.3 | 33.5 | 76.1 | 71.5 | 95.7 | 32.4 | 102.9 | 53.5 | 76.4 | −29.9 | 88.5 | |
| Growth med | pH | 3.0 | −3.8 | 0.8 | −2.1 | −5.6 | −2.1 | 5.1 | 4.0 | −3.5 | −10.4 | 0.2 | 5.6 | 0.0 | −3.2 | −0.5 | −2.9 |
| EC | −18.8 | 9.4 | −15.6 | −31.3 | −16.1 | −16.1 | −9.7 | 45.2 | −9.7 | 125.8 | 41.9 | −6.5 | 19.4 | 96.8 | −6.5 | 19.4 | |
| TN | 7.8 | 39.3 | 2.2 | 53.5 | 22.2 | 5.8 | 15.2 | 55.7 | 10.5 | 38.8 | 36.6 | 51.8 | −40.7 | 26.6 | 3.3 | 15.8 | |
| P | −9.6 | −3.7 | −6.7 | 10.1 | −8.2 | −9.7 | −8.2 | 15.3 | −29.5 | 6.7 | −11.2 | 2.5 | −45.8 | −4.9 | 11.7 | 0.0 | |
| K | 7.7 | 11.4 | 2.5 | 227.2 | 10.6 | 57.1 | 52.8 | 41.2 | −26.7 | 69.1 | 2.8 | 140.0 | 24.4 | 77.9 | 118.1 | 34.1 | |
| Cu | −11.1 | −11.1 | −31.7 | 1.6 | −3.2 | 0.0 | −17.7 | −27.4 | −38.7 | 1.6 | −25.8 | −12.9 | −4.8 | 8.1 | −29.0 | −41.9 | |
| Fe | 10.7 | 17.9 | −17.1 | 21.9 | 45.4 | 7.3 | 5.8 | −15.4 | 8.5 | 51.7 | −5.4 | 33.6 | −14.2 | 49.8 | −8.3 | −14.7 | |
| Mn | 27.3 | 20.5 | −35.4 | 68.3 | 46.6 | 29.8 | −1.2 | −17.4 | −8.1 | 83.9 | −22.4 | 24.2 | 5.0 | 78.9 | −11.2 | −17.4 | |
| Zn | 35.3 | 35.3 | −4.2 | 53.8 | 53.4 | 39.0 | 18.6 | 21.2 | 16.9 | 66.9 | 9.3 | 40.7 | 35.6 | 69.5 | 5.9 | 43.2 | |
Note:
SL, Shoot Length (cm); RL, Root Length (cm); RCD, Root CCC Diameter (mm); LN, Number of Leaves; SFW, Shoot Fresh Weight (g); SDW, Shoot Dry Weight (g); RFW, Root Fresh Weight (g); RDW, Root Dry Weight (g).
Plant nutritions
The Tables 3–6 shows changes in plant nutrient contents following pathogen and mycorrhiza treatments in four different pathogen groups. Ca, Fe, K, Mn, Na, and Zn ratios in plants increased in all groups following pathogen application (p < 0.05). Comparing the control positive groups with pathogen applications to the control negative groups, the levels of these nutrients are higher in the control positive groups. In these nutrients, increases of up to 18%, 39%, 22%, 148%, and 171% were observed after pathogen application compared to the pathogen-free negative control group, respectively (Table 7).
After applying the pathogen, decreases in the contents of the plants were seen in the remaining other nutrients, B, Cu, Mg, and P. In 18-F. solani and 147-F. mix pathogen groups, the reduction in Mg and P contents caused by pathogen application was statistically significant (p < 0.05), but in 48-F. solani and 50-F. mix pathogen groups, it was found to be statistically insignificant (p > 0.05). The decrease in Cu was statistically insignificant (p < 0.05) only in the 50-F.mix pathogen group, but significant (p > 0.05) in the other three.
In all pathogen groups, the decreases in these nutrients following pathogen application were effectively balanced, particularly with MM (MM+P) application. Plants treated with MM application and inoculated with 50-F. mix pathogen showed increases of 54%, 62%, 60%, 17%, 153%, 217%, and 102% in these nutrients, respectively, compared to the negative control group (Table 8). The reductions in these three nutrients in the 18-F. solani group were countered with RI (RI+P), FM (FM+P), and MM (MM+P) applications. The decreases in Mg and P elements in the 48-F. solani group were balanced with FM (FM+P) and MM (MM+P) applications. In the 50-F. mix group, the decreases in Cu and Mg were balanced with CE (CE+P), RI (RI+P), FM (FM+P), and MM (MM+P) applications, while the decrease in P was balanced with FM (FM+P) and MM (MM+P) applications. For the 147-F. mix group, the pathogen-induced decreases in Mg and P were balanced with FM (FM+P) and MM (MM+P) applications, while the decrease in Cu was only balanced with MM (MM+P) application.
Growth medium
In terms of growth medium EC and pH values, there were no statistically significant differences between treatments in different pathogen groups (Tables 3–5) (p > 0.05). However, when comparing the N, P, and K contents, significant differences were found between applications in different pathogen groups (p < 0.05). Following pathogen application, the contents of N and K increased by up to 23% and 83%, respectively, while the content of P decreased by up to 27%, depending on the species of the pathogen (Table 7). For Fe, Cu, Zn, and Mn elements, there were no statistically significant differences between treatments in any of the pathogen groups.
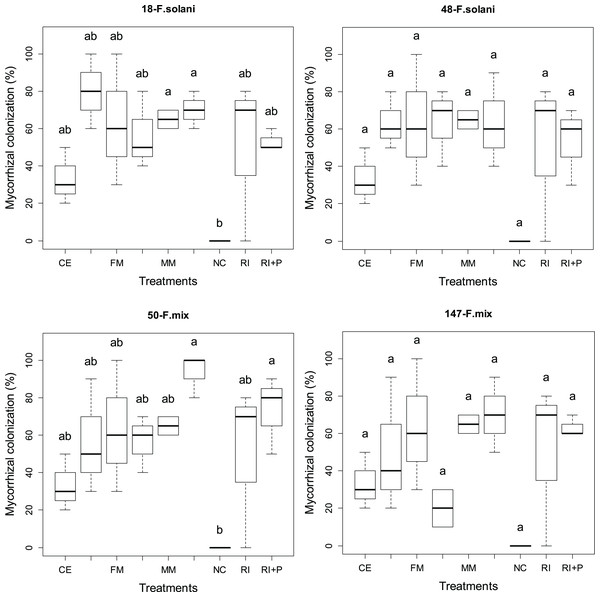
Root colonization
Figure 3 presents the percentage of root colonization achieved through four different AMF applications in various pathogen groups. Statistically significant differences (p < 0.05) in root colonization were observed among different AMF applications in all pathogen groups. The rates of root colonization varied across different pathogen and mycorrhiza application groups, ranging from 33% to 65% in plants treated solely with mycorrhiza and from 53% to 93% in plants where mycorrhiza and pathogen were applied together. The highest average root colonization (93%) was obtained from the MM+P application in the 50-F. mix group (Fig. 3).
Figure 3: The statistical comparison of total spore counts of arbuscular mycorrhizal fungi (AMF) among the four pathogens studied.
In all pathogen groups, for the three mycorrhizal species, plants treated with mycorrhiza and pathogen together (CE+P, RI+P, MM+P) exhibited higher root colonization compared to plant groups treated only with mycorrhiza (CE, RI, MM). Conversely, for the FM species, the plant groups treated solely with mycorrhiza showed higher rates of root colonization than the groups treated with both pathogen and mycorrhiza together, except in the 48-F. solani pathogen group. In the 48-F. solani pathogen group, the FM and FM+P groups displayed an equal level of colonization (Fig. 3).
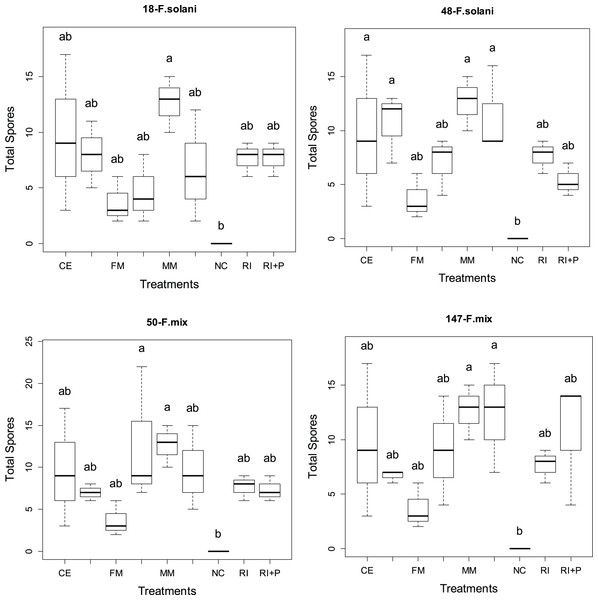
Total spore counts
Figure 4 display the total number of spores obtained from four different AMF applications in various pathogen groups. Significant statistical differences (p < 0.05) in the total spore count were observed among AMF applications in all pathogen groups. The total spore rates varied across pathogen and mycorrhiza application groups, ranging from 3.63 to 12.66 in plants treated solely with mycorrhiza and from 4.66 to 12.66 in plants treated with both mycorrhiza and pathogen. Among all pathogen groups, the MM application resulted in the highest overall spore count (12.66). Additionally, the FM+P application in the 50-F. mix pathogen group also yielded the highest overall spore count, along with MM (Fig. 4).
Figure 4: The statistical comparison of the percentage of mycorrhizal colonization by arbuscular mycorrhizal fungi (AMF) among the four different pathogens used in the study.
Disease severity
Table 9 provides a comparison of disease severity and disease suppression rates for different pathogen and mycorrhiza treatment groups. With the exception of the 147-F. mix group (p < 0.05), there were no statistically significant differences (p > 0.05) in the severity and suppressiveness of the disease between the applications. However, the severity of the disease decreased in plant groups with mycorrhizae at varying rates compared to plants inoculated with pathogens alone. Despite exhibiting the most severe disease, the 147-F. mix group was followed by the 48-F. solani, 50-F. mix, and 18-F. solani groups, in that order. When examining the disease severity data for the 18-F. solani pathogen, it was found that the pathogen’s disease severity was 20.83%. Among the mycorrhizal applications, CE showed the lowest disease severity rate (13.89%), making it the most effective mycorrhizal treatment. It was followed by RI (16.16%) and FM and MM (19.44%). In terms of disease suppression rate, CE exhibited the highest effectiveness (58.32%). When comparing the groups based on disease suppression rate, CE was followed by RI, FM, and MM. When analyzing the percent disease severity of the 48-F. solani pathogen, the Control (+) group exhibited the highest disease severity after 147-F. mix (58.33%). Among the AMF application groups, the disease severity rates for this pathogen were ranked as follows: FM, MM, RI, and CE, respectively. Among the AMF treatments, CE application (48.14%) was the most effective in suppressing this pathogen, followed by RI (40.74%), MM (29.63%), and FM (29.62%).
| 18-F. solani | 48-F. solani | 50-F. mix | 147-F. mix | |||||
|---|---|---|---|---|---|---|---|---|
| Treatments | DS (%) | DI (%) | DS (%) | DI (%) | DS (%) | DI (%) | DS (%) | DI (%) |
| Control N | 0 | 0 | 0 | |||||
| Control P (Pathogen) | 20.83a | – | 56.25a | – | 35.41a | – | 58.33ab | – |
| FM+Pathogen | 19.44a | 41.66a | 52.78a | 29.62a | 38.88a | 22.23a | 77.77a | −16.67b |
| RI+Pathogen | 16.16a | 49.99a | 44.44a | 40.74a | 36.11a | 27.78a | 52.77ab | 20.82ab |
| CE+Pathogen | 13.89a | 58.32a | 38.89a | 48.14a | 41.66a | 16.68a | 33.33b | 50.00a |
| MM+Pathogen | 19.44a | 41.66a | 52.77a | 29.63a | 66.66a | −33.32a | 41.66ab | 37.50ab |
Note:
FM, Funneliformis mosseae; RI, Rhizophagus intraradices; CE, Claroideoglomus etunicatum; MM, Combination of all three mycorrhiza species; DS, Disease severity; DI, Disease inhibition. Treatment groups with different letters (a, b or their combinations) are statistically different from each other.
When examining disease severity for the 147-F. mix pathogen, CE was ranked first, followed by MM, RI, and FM. Similarly, when considering the suppression rate of this 147-F. mix pathogen by AMF, the ranking matched the disease severity percentages (DS%). However, there was a negative suppression rate observed in the FM application (−16.67%). Similarly, a negative disease inhibition rate (−) was obtained in the MM application (−33.32) for the 50-F. mix pathogen.
When examining the disease severity rates for the 50-F. mix pathogen, it was observed that the AMF application groups, in contrast to the other three pathogens, increased the severity of the disease rather than decreasing it. The disease severity in the Control (+) group for the 50-F. mix pathogen was 35.41%. In comparison, MM exhibited a severity of 66.66%, CE had 41.66%, FM had 38.88%, and RI had 36.11%. Similarly, the disease suppression rates for MM, CE, FM, and RI were −33.32%, 16.68%, 22.23%, and 27.78%, respectively.
Discussion
After pathogen application, all plant growth parameters, across all pathogen groups, were generally negatively impacted. On the other hand, mycorrhiza application counteracted this pathogen-caused negativity in plants in the 18-F. solani pathogen group, but it was unable to do so in the 48-F. solani pathogen group, where the disease severity is greater. Only a few instances of mycorrhiza application were able to completely reverse the negative effects brought on by the pathogen in the 50-F. mix and 147-F. mix pathogen groups, where the disease severity is comparatively higher. This finding demonstrates that mycorrhizae are ineffective after a certain level of disease severity. While CE, FM, and RI were successful in counteracting the pathogen’s negative effects on morphological parameters, MM composed of their mixture had no effect. Various studies have also found that AMFs have a positive effect on plant growth parameters in soil-borne pathogen-treated plants (Vigo, Norman & Hooker, 2000; Ozgonen & Erkilic, 2007; Hafez et al., 2013; Aljawasim, Khaeim & Manshood, 2020; Wu et al., 2021). In their study, Aljawasim, Khaeim & Manshood (2020) found a significant increase in both shoot dry weight and root dry weight in plants treated with mycorrhiza compared to plants not applied. Demir et al. (2023) found that the application of AMF, specifically FM and Gigaspora margarita (Gm), had significant effects on the morphological parameters of strawberry plants infected with various pathogens. They noted that different AMF treatments resulted in varying increases in plant fresh weight, dry weight, and length, depending on the specific pathogen involved.
In contrast to plant growth parameters, some plant nutrients (Ca, Fe, K, Mn, Na and Zn) increased after pathogen application, which can be attributed to increased plant nutrient intakes in response to stress. Pathogen application resulted in decreases in nutrient elements such as Cu, Mg, and Zn, similar to plant growth parameters, but these decreases were mostly balanced by mycorrhiza application. Following pathogen application, MM, FM, RI and CE were respectively the most effective in maintaining the decrease in these plant nutrients in plants. CE application was rarely found to be effective in maintaining plant nutrient content. AMFs are an important tool for increasing plant nutrient absorption and stress tolerance in biotic stress conditions, as well as for biological control of cucumber plants treated with soil-borne pathogen (R. solani) (Aljawasim, Khaeim & Manshood, 2020). In Verticillium dahliae-inoculated pepper plants, all macro and micronutrients, except for N, were found to be lower when compared to uninoculated control plants. The application of AMF led to a slight increase in P, Mg, Cu, Mn, and B levels. However, this increase was not statistically significant (Coskun, Alptekin & Demir, 2023).
With the exception of the FM+P case, the coexistence of both AMF and pathogens exhibited synergistic effects on root colonization. The presence of pathogens had a positive impact on the development of different AMFs in the root zone, as shown in Fig. 3. The presence of pathogens in soils has the potential to influence the colonization of AMF and the development of AMF in the presence of pathogens can vary depending on biotic and abiotic conditions and is influenced by the interactions between AMF, hosts, and pathogens (Spagnoletti et al., 2021; Coskun, Alptekin & Demir, 2023). While previous studies have reported that pathogen infection reduces mycorrhizal colonization in pepper (Coskun, Alptekin & Demir, 2023), the researchers investigating the interaction between AMF (Rhizophagus intraradices) and a soilborne pathogen (Fusarium pseudograminearum) on the root colonization of wheat plants found that the simultaneous inoculation of AMF and the pathogen resulted in higher AMF colonization percentages compared to AMF alone (Spagnoletti et al., 2021).
In general, pathogen severity was lower in plants treated with mycorrhizae, which effectively reduced pathogen impact. Disease recovery rates of up to 58% were achieved depending on the mycorrhizal type and pathogen involved. This significant reduction in pathogen severity can be attributed to the modulation of plant nutrient uptake, changes in root morphology, and competition between mycorrhizal fungi and pathogens for colonization sites, as demonstrated by Spagnoletti et al. (2021). Although differences in disease suppression rates among various mycorrhizal treatments were generally not significant, except for one pathogen group, the CE application was notably more effective, showing the highest suppression in three of the four pathogen groups. This superior performance of CE is attributed to its greater positive impact on root fresh and dry weights in infected plants.
Researchers testing different combinations of mycorrhizae against the root rot disease pathogen Rhizoctonia solani in watermelon found that AMF fungi helped to reduce the severity of the disease caused by soil-borne pathogens (Wu et al., 2021). Researchers obtained mix of AMF species (different species including FM and RI) and also a mix of AMF from different genera. Their findings were the opposite of those of our study. They observed that the blend of AMF from different genera performed exceptionally well in watermelon, showing notable improvements in terms of dry weight, photosynthesis rate, percent root colonization, and mycorrhizal dependence. Furthermore, the researchers reported that mycorrhizae from different genera exhibited greater efficacy compared to combinations of mycorrhizae from the same genus but different species, as well as individual mycorrhizae. Hafez et al. (2013) found that AMF mycorrhizae, including FM and RI, had significant effects on disease severity, disease suppression rate, and plant growth parameters in the bean plant when a mix mixture of AMFs was tested against the soil-borne pathogen R. solani. This study results demonstrated that the use of a single AMF species yielded better outcomes compared to a mixture of AMF species. This could be attributed to several factors, including competition between different AMF species for resources and space within the rhizosphere, which may reduce overall effectiveness. Additionally, specific plant-AMF compatibility likely plays a crucial role, where certain AMF species establish a more efficient symbiotic relationship with the plant. Moreover, a single AMF species may be more efficient in resource allocation and better adapted to the specific environmental conditions of our study. These findings suggest that, contrary to the expected synergistic interactions, a single AMF species might offer a more optimal solution for enhancing plant growth and health. The research by Demir et al. (2023) found that both individual treatments with AMF species FM and Gm, as well as their combined application, significantly reduced the severity of diseases caused by three major pathogens—Rhizoctonia fragariae, Fusarium oxysporum, and Alternaria alternata—in strawberry plants compared to control treatments. However, there was no significant difference in disease reduction between the combined AMF treatment and the individual AMF treatments. These results suggest that while AMF applications are effective in mitigating pathogen impact, combining FM and Gm does not provide additional benefits over using either species alone for controlling these specific soil-borne pathogens.
The performance of mycorrhizae in disease suppression may vary depending on the host variety, mycorrhizal variety and environmental conditions (Dowarah, Gill & Agarwala, 2022; Demir et al., 2023). FM reduced the disease severity of P. capsici by 57.2%, 43%, and 91.7% in field greenhouse and controlled climate room conditions, according to Ozgonen & Erkilic (2007). In the current study, FM AMF significantly reduced the severity of disease in 18-F. solani (41.66%), 50-F. mix (22.23%), and 48-F. solani (29.62%), but significantly increased the severity of disease in 147-F. mix (16.67%). These findings from our study and those of Ozgonen & Erkilic (2007) show that FM is effective against P. capsici and F. solani pathogens, but not against Fusarium mix disease pathogens.
Spagnoletti et al. (2021) demonstrated that inoculation with Rhizophagus intraradices (R. intraradices) significantly reduces disease severity and enhances plant growth parameters. Specifically, this treatment resulted in a 75.7% reduction in Fusarium pseudograminearum pathogen density and a 39% decrease in disease severity. These effects are attributed to increased antioxidant enzyme activity and decreased lipid peroxidation, which indicate improved redox balance and reduced oxidative stress in AMF-inoculated plants. Additionally, R. intraradices alleviates pathogen impact by competing for root colonization sites, thereby limiting pathogen establishment and enhancing plant tolerance.
Demir et al. (2023) observed that the application of AMF, including Funneliformis mosseae and Gigaspora margarita, significantly reduced the severity of soil-borne fungal diseases such as Rhizoctonia fragariae, Fusarium oxysporum, and Alternaria alternata in strawberry plants. Specifically, disease severity for Rhizoctonia fragariae decreased from 81.25% to 20.00%, for Fusarium oxysporum from 50.00% to 25.00%, and for Alternaria alternata from 42.50% to 20.00%, reflecting reductions of approximately 75%, 50%, and 53%, respectively. These reductions can be attributed to AMF’s impact on several underlying mechanisms. AMF improves nutrient uptake and enhances plant physiological functions, which contribute to higher levels of total phenolic content, antioxidant activity, and phosphorus content.
In the study conducted by Liu et al. (2018), it was observed that although AMF positively contributed to plant growth, it did not show any effect against powdery mildew in Standing milkvetch (Astragalus adsurgens), a legume forage plant. In fact, the disease index was higher in AMF-inoculated plants compared to plants that were not inoculated with AMF. Additionally, the researchers reported that there was no association between AMF colonization rate and crop morphological parameters or defense enzyme activities in the crop.
Conclusions
The study revealed that various AMF and a mycorrhizal mix have differing effects on pepper plants. The rates of disease severity suppression varied according to pathogen groups and mycorrhizae. Individual application of mycorrhizae was found to be more effective in suppressing disease severity than mixture application. Because of their higher positive impacts on plant root and vegetative parts, CE and RI have been shown to be more effective in disease suppression. While mycorrhiza mix was important in balancing the decreases in plant nutrient content following the application of the pathogen, it did not contribute to the correction of the decreases in plant growth parameters. Notably, the combination of MM with 50 F. mix pathogen was found to significantly enhance Ca, Fe, and K levels. In general, excluding FM, plants treated with mycorrhiza in conjunction with the pathogen colonized at a higher rate than plants treated with mycorrhiza alone. There is no clear result in terms of total number of spores, with some cases showing a decrease with the application of the disease and others showing an increase. However, plants treated only with mycorrhizal mix had the highest total spore count.