Identifying a cis-element in PtoCP1 promoter for efficiently controlling constitutive gene expression in Populus tomentosa
- Published
- Accepted
- Received
- Academic Editor
- Mohammad Irfan
- Subject Areas
- Agricultural Science, Molecular Biology, Plant Science
- Keywords
- Populus tomentosa, PtoCP1 promoter, 5′ end deletion, MYB-TGACG cis-element, Gene expression
- Copyright
- © 2024 Peng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Identifying a cis-element in PtoCP1 promoter for efficiently controlling constitutive gene expression in Populus tomentosa. PeerJ 12:e18292 https://doi.org/10.7717/peerj.18292
Abstract
Gene expression is regulated by transcription factors binding to cis-elements in promoters. However, efficient cis-elements for genetic engineering are rarely reported. In this study, we identified an 11 bp cis-element in the PtoCP1 promoter that drives strong constitutive gene expression in Populus tomentosa. A 2,270 bp promoter region upstream of the PtoCP1 gene’s translation start site was cloned and named ProPtoCP1. This promoter controls GUS reporter gene expression in the roots, leaves, and stems of Arabidopsis seedlings. Based on the location and density of cis-elements, the PtoCP1 promoter was divided into four fragments by 5′-end deletions. GUS staining and RT-qPCR revealed a key cis-element at −466 to −441 bp essential for gene expression. Further analysis showed that the MYB-TGACG cis-element is a positive regulator, whereas neither MYB nor TGACG alone drove gene expression. This study enhances our understanding of gene expression regulation by cis-elements and provides a valuable tool for genetic engineering.
Introduction
The Chinese white poplar (P. tomentosa Carr.), a commercially valuable tree species used for timber production, belongs to the section Populus (Leuce). It is a diploid (2n = 2× = 38) and outcrossed perennial species (Tian, Xie & Qu, 2013; Xun et al., 2021). Proteases play a crucial role in the growth and development of Chinese white poplar. The main cysteine proteases in plants are categorized into the Papain and Legumain types, with other members of the family falling under the Caspase and Calpain types (Yan, Yang & Han, 2005). Among these, the Papain cysteine proteases (PLCPs) are the most prevalent and extensively studied (Liu et al., 2018). Cysteine proteases are expressed in various tissues and are involved in the degradation of related proteins during different biological processes in plants.
The cysteine protease CEP1 in Arabidopsis is responsible for mediating tapetal programmed cell death (PCD) and secondary wall thickening. The expression level of CEP1 is closely associated with the level of PCD in the tapetum layer and pollen fertility (Zhang et al., 2014). The Arabidopsis cysteine protease γVPE indirectly influences PCD in xylem fiber cells by activating the maturation of the CEP1 protease (Cheng et al., 2019). HvPAP14, identified in barley, is involved in the normal turnover of chloroplast proteins and potentially degrades macromolecular proteins during leaf senescence (Frank et al., 2019). The cysteine protease gene PtCP5 impacts seed germination by mobilizing Populus storage proteins during this process (Liu et al., 2021).
Furthermore, the expression of cysteine proteases in many plant species is influenced by biotic and abiotic stressors. For instance, the transcription levels of four C1A cysteine protease genes increase in response to drought stress in barley plants. Knockout lines of proteases encoded by the genes HvPap-1 and HvPap-19 in these plants result in changes in cuticle thickness and stomatal area in the leaves, which aids in preventing fungal infections and mite predation (Gomez-Sanchez et al., 2019). In tomato plants, SA-induced PCD is associated with rapid upregulation of specific cysteine proteases in root tissues and increased proteolytic activity. This is attributed to the downregulation of protease inhibitors (Kovács et al., 2016). Ultimately, the tissue-specific expression of cysteine proteases and their involvement in the stress response are vital for plants to adapt better to their environment.
The function of cysteine proteases is directly influenced by their expression. In sweet potatoes, the SPCP2 gene expression significantly increases in naturally aged leaves, but is nearly absent in mature green leaves, stems, and roots (Chen et al., 2010). Similarly, the expression level of the CP gene increases during leaf senescence in Picrorhiza kurrooa Royle ex Benth (Jai, Sanjeeta & Shruti, 2015). The SAG12 protein, known as a reference gene for leaf senescence, also shows significant increase in expression during this process (James et al., 2018). However, there is limited research on the cis-elements and transcription factors responsible for regulating cysteine protease expression. Some studies suggest that WRKY75 acts as a positive regulator of leaf senescence by directly binding to the SAG12 and SAG29 promoters, thus activating their expression. Expression of age-related genes (SAG) is inhibited in wrky75 mutants and increased in WRKY75 overexpressing plants (Du et al., 2012).
To investigate the specificity of PtoCP1 gene expression, we obtained the upstream promoter sequence of the gene and performed GUS staining in the leaves, roots, and stems of Arabidopsis thaliana. Our findings indicate that a key MYB-TGACG cis-element plays a regulatory role in gene expression, as demonstrated through 5′end deletion and site-specific mutation experiments. This study identifies important elements involved in cysteine protease gene expression and provides valuable information for genetic engineering.
Materials and Methods
Growth of plant materials
Wild-type tobacco (Nicotiana benthamiana) seeds were vernalized in the dark at 4 °C for 3 days and then planted in nutrient soil (substrate: vermiculite = 3:1) using toothpicks. The pots were covered with plastic wrap and placed in a laboratory light incubator set at 25 °C, 60% humidity, and a 16-h light/8-h darkness cycle. The plastic wrap was removed when the plants reached two weeks of age. The plants were cultivated until they were 5 weeks old for transient transformation and GUS staining of tobacco leaves.
Col-0 wild-type Arabidopsis seeds were also vernalized in the dark at 4 °C for 3 days and then planted in nutrient soil (substrate: vermiculite = 3:1) using toothpicks. The pots were covered with plastic wrap and placed in a laboratory light incubator set at 25 °C, 60% humidity, with a consecutive alternating culture of 16 h light at 23 °C, 60% humidity, and 8 h dark. The plastic wrap was removed when the plants reached the stage of having six true leaves and were cultivated until they reached the flowering stage.
Wild-type Populus alba × Populus glandulosa were grown on ½ MS solid medium in a greenhouse at a constant temperature of 25 °C, with a 14 h light/10 h dark photoperiod. ½ MS solid medium included 1.5% (w/v) sucrose, 2.18 g l −1 ½ Murashige and Skoog salts, 100 mg l −1 inositol, 0.1 mg l −1 1−naphthaleneacetic acid (NAA) and 6 g l −1 agar, pH 6.15.
Transformation of plant materials
Transient transformation of tobacco leaves: Prior to the immediate infection of tobacco leaves, it is necessary to ensure that they have absorbed an ample amount of water. During the infection process, remove the needle from a 1 mL syringe and carefully inject an appropriate quantity of the corresponding bacterial suspension into the lower epidermis of tobacco leaves that are 4–5 weeks old. Gently and evenly push the syringe until the desired infection area is reached, and immediately cease injection. Once the injection process is complete, use filter paper to wipe away any remaining bacterial liquid on the leaves. Proceed by marking the leaves and covering the plants with plastic film to prevent evaporation of the bacterial liquid and maintain moisture. Incubate the plants in darkness at room temperature for 36 h, allowing the bacterial solution to fully penetrate the leaves. Finally, transfer the plants to normal cultivation under 12 h of light for GUS histochemical staining.
Stable transformation of Arabidopsis: The Agrobacterium-mediated transformation method using the flower-dip technique was employed. To promote the growth of lateral branches, carefully cut the main stem of Arabidopsis thaliana with scissors, and then infect the inflorescence of wild Arabidopsis thaliana that has been cultivated for a specific duration. Before the initial infection, remove any mature fruit pods and immerse the entirety of the Arabidopsis inflorescence in water for approximately 30 s. Once infected, cover the entire plant with plastic wrap to maintain moisture. Allow the plant to undergo dark cultivation for 24 h, and then transfer it to a well-lit environment for growth. Sterilize and select transformant seeds on half-strength MS agar medium supplemented with 1.5% (w/v) sucrose and 50 mg/L kanamycin sulfate. To further verify positive seedlings, perform PCR identification using a rapid DNA extraction kit on plants that have successfully survived on the antibiotic-containing culture medium. In the PCR process, upstream primers should target sequences on the promoter, whereas downstream primers should target sequences on GUS (F: 5′-TAAAAGCCTCCACCTCTCGC-3′, R: 5′-AACTGTTCGCCCTTCACTGC-3′). Ultimately, utilize T3 generation homozygous positive plants for subsequent experiments.
Stable transformation of Populus alba × Populus glandulosa: Take small sections of Populus alba × Populus glandulosa leaves and place them on solid medium WPM supplemented with 0.1 mg/l NAA. Pre-culture the leaves in darkness at a temperature of 25 °C for 2–3 days. Subsequently, infect the leaves with A. tumefaciens GV3101 at an optical density of 0.6 for a duration of 10–15 min at a wavelength of 600 nm. Use carbenicillin sodium (250 mg/l) and kanamycin (20 mg/l) for selection purposes. Replace the selective medium every 2 weeks until adventitious buds begin to develop.
Cloning of the PtoCP1 promoter
The genomic database for JGI P. trichocarpa (https://phytozome.jgi.doe.gov/pz/portal.html) was utilized to locate the corresponding PtCP1 genomic sequence. Our method involved extracting the PtoCP1 genome DNA from a 100 mg portion of fresh P. tomentosa leaves, following the manufacturer’s instructions and using the extracted genomic DNA as the template. Specific primers (F: 5′-CTAAATGTGCAATTTGTAACCATCA-3′, R: 5′-CGAGAATATTGCAAACCTTAACTTC-3′) were designed with reference to the sequence of the PtCP1 genome to clone the 2,270 bp PtoCP1 gene promoter. The cloned promoter was then connected to the pMD18-T vector and sent to a biological company for sequencing. For promoter expression analysis, promoter fragments with correct sequencing were constructed into the pBI121 expression vector. We would like to highlight that the synthesis of primers and the sequencing services were provided by Beijing Tsingke Biotech Co., Ltd.
Bioinformatics analysis of PtoCP1 promoter sequence cis-element
The online sequence analysis of the PtoCP1 promoter of the P. tomentosa cysteine protease gene was performed using the PlantCARE (http://bioinformatics.psb.Ugent.be/webtools/plantcare/html/) and PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace) databases to predict the cis-element on the promoter.
Vector construction
In the vector construction experiment for the promoter fragments, the following upstream primers were employed to amplify the full-length promoter and 5′ deletion: P0 (F: 5′-CTATGACCATGATTACGCCAAGCTTTGTGCAATTTGTAACCATCA-3′), P1 (F: 5′-GACCATGATTACGCCAAGCTTCCTGGCTTGAAAGGTTGGACT-3′), P2 (F: 5′-GACCATGATTACGCCAAGCTTAACCGTTAGGCGTCACCAT-3′), P3 (F: 5′-GACCATGATTACGCCAAGCTTCACAGTAAGCGATAACGGAACTT-3′), and P4 (F: 5′-GACCATGATTACGCCAAGCTTCATGTACTTTGCTCCTTGACCAG-3′). The downstream universal primer used was PR (R: 5′-GACTGACCACCCGGGGATCCGATTTACTTGTTTTTGTGGGTGGTC-3′). The upstream primers contained a segment of the homologous arm sequence and a Hind III restriction site upstream of the vector, whereas the downstream primer contained a segment of the homologous arm sequence and a BamH I restriction site downstream of the vector. The PCR reaction involved a 50 µL mixture comprising 25 µL of 2× Phanta Max Buffer, 1 µL of the dNTP Mix, 2 µL of the 10 µM upstream primer, 2 µL of the 10 µM downstream primer, 1 µL of template, and ddH2O to complete the volume. The PCR reaction procedure included incubation at 95 °C for 3 min, followed by cycles at 95 °C for 15 s, 60 °C for 15 s, 72 °C for 15 s, and a final extension step at 72 °C for 5 min, with a subsequent holding step at 4 °C. We employed 35 cycles for steps 2 to 4. The amplified target fragments were homologously recombined into the pBI121 expression vectors, with the 35S promoters removed using Hind III and BamH I, following the instructions provided in the seamless cloning kit. The resulting conjugate product was then transformed into competent E. coli DH5α, and the positive single colony was screened in LB medium containing Kan. The correctness of the recombinant product was verified using PCR, double enzyme digestion, and sequencing. To construct the vector for cis-elements connecting 35Smini composite fragments, the upstream primers for the target fragment were as follows: ABC (F: 5′-GACCATGATTACGCCAAGCTTCCGTTACGTCACATGTGCCTTCGCAAGACCCTTC-3′), AAA (F: 5′-GACCATGATTACGCCAAGCTTCCGTTACCGTTACCGTTACCTTCGCAAGACCCTTC-3′), BBB (F: 5′-GACCATGATTACGCCAAGCTTCGTCACGTCACGTCACCTTCGCAAGACCCTTC-3′), and CCC (F: 5′-GACCATGATTACGCCAAGCTTCATGTGCATGTGCATGTGCCTTCGCAAGACCCTTC-3′). The downstream universal primer used was ABC (R: 5′-GACTGACCACCCGGGGATCCCGTGTTCTCTCCAAATGAAC-3′).
For the vector construction of site-mutated cis-elements connecting 35Smini composite fragments, the upstream primers for the target fragment were (A)BC (F: 5′-GACCATGATTACGCCAAGCTTCTAGGACGTCACATGTGCCTTCGCAAGACCCTTC-′), A(B)C (F: 5′-GACCATGATTACGCCAAGCTTCCGTTACAGCACATGTGCCTTCGCAAGACCCTTC-3′), and AB(C) (F: 5′-GACCATGATTACGCCAAGCTTCCGTTACGTCACCGAGGCCTTCGCAAGACCCTTC-3′). The downstream universal primer used was ABC (R: 5′-GACTGACCACCCGGGGATCCCGTGTTCTCTCCAAATGAAC-3′).
GUS histochemical staining
Five 7-day-old stable transgenic Arabidopsis seedlings were randomly selected and placed in 2 mL centrifuge tubes. The leaves from transient tobacco infections were placed in a suitably sized petri dish. Pre-cooled 90% acetone (−20 °C) was added to the tubes and petri dish to completely cover the material. The tissue was treated at room temperature for 20–30 min to fix it and partially remove the chlorophyll. The plant material was then rinsed with distilled water, and a GUS staining buffer was added to cover it completely. The buffer contained 100 mM sodium phosphate (pH 7.2), 10 mM EDTA, 1% (v/v) Triton X-100, and 0.5 mg/L X-Gluc, as modified by Berger et al. (2011). The tubes and petri dish were attached to a vacuum pump for 40 min, kept away from light at 37 °C, and incubated overnight. The material was then eluted through an ethanol gradient (50%, 70%, and 95%), gently shaking for 40 min each time. The 95% ethanol was used until the green color of the leaves was completely removed. GUS staining was observed by the naked eye or with an anatomic microscope.
Expression analysis by real-time qPCR
To analyze differences in GUS expression levels driven by different promoter fragments, RNA was extracted from 7-day-old transgenic Arabidopsis leaves. RT-qPCR was performed using SYBR Green qPCR Mix (TIANGEN, Beijing, China) on an iQ5 Multicolor Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA, USA). The primer sequence for detecting the reporter gene GUS was (F: 5′-TAACCACAAACCGTTCTACTT-3′, R: 5′-ACTGATACTCTTCACTCCACA-3′), and the primer sequence for the internal reference gene Actin was (F: 5′-CGTATGAGCAAGGAGATCAC-3′, R: 5′-CACATCTGTTGGAAGGTGCT-3′). The PCR conditions were 95 °C for 15 min, 95 °C for 10 s, 55 °C for 20 s, 72 °C for 30 s, 39 cycles were employed from steps 2 to 4. Data were analyzed using iQ5 (Bio-Rad) software, and differences in gene expression were calculated using the 2−∆∆Ct method. β-actin was used as an internal control to quantify the relative expression levels of genes in the samples.
Results
ProPtoCP1 cloning and bioinformatics analysis of the cis-elements
To determine the expression of the PtoCP1 gene, we designed primers (Forward: 5′-CTAAATGTGCAATTTGTAACCATCA-3′, Reverse: 5′-CGAGAATATTGCAAACCTTAACTTC-3′) based on the PtCP1 sequence (Genbank Accession Number: XM_006381559.1) available at the JGI (https://phytozome-next.jgi.doe.gov/blast-search). We successfully cloned a 2,270 bp PtoCP1 promoter sequence from P. tomentosa Carr., which we named ProPtoCP1. The fragment size was measured from the translation initiation site (ATG). The CDS sequence similarity between PtoCP1 (Populus tomentosa) and PtCP1 (Populus trichocarpa) was found to be 98.50%. We analyzed the PtoCP1 promoter sequence using the Plant CARE (http://bioinformatics.psb.Ugent.be/webtools/plantcare/html/) and PLACE (https://www.dna.affrc.go.jp/PLACE/?action=newplace) promoter analysis software to predict the presence of cis-elements (Fig. 1 and Table 1). The PtoCP1 promoter region was found to contain numerous photoresponsive elements (T-box, BOX4, and GT1-motif), as well as plant hormone and abiotic stress response elements such as methyl jasmonic acid (MeJA) response element (TGACG-motif), SA response element (W BOX), gibberellic acid (GA) response element (CARE), abscisic acid (ABA), and drought response elements (CNGTTR, YAACKG, WAACCA, and CANNTG). These findings suggest that the expression of the PtoCP1 gene may be regulated by multiple factors, including abiotic stress and hormones.
Figure 1: The ProPtoCP1 cis-acting element analysis.
| Cis-element | Sequence (5′–3′) | Start position | Function |
|---|---|---|---|
| CARE | CAACTC | −1,799, −1,652, −1,405 | GA response element |
| ATCT-motif | AATATAATCC | −1,142 | Light response element |
| T-box | ACTTTG | (−1,794), (−1,740), −107 | Light response element |
| BOX 4 | ATTAAT | −2,079, −1,549, −1,351, −728, −652, −615 | Light response element |
| GT1-motif | GRWAAW GGTTAA |
−1,828, −1,748, (−1,661), (−1,636), (−1,458), (−1,435), (−1,166), (−1,016), (−1,008), (−969), −958, (−920), (−876), (−794), (−736), (−330), (−307), (−248), (−239), (−45), | Light response element |
| TGACG-motif | TGACG | (−454), (−444) | TGA binding site, MeJA, SA response element |
| W BOX | TTGACY | −987, −899, −97 | WRKY binding site, SA, pathogen, damage response element |
| MYB | CNGTTR YAACKG WAACCA |
−2,257, (−2,053), (−1,907), −1,744, (−464), −429 | MYB binding site, ABA, drought etc., response element |
| MYC | CANNTG | −1,972, −1,838, −1,754, −1,459, −1,471, −1,361, −450, −134 | MYC binding site, ABA, drought etc., response element |
Note:
() indicates promoter sequence antisense chain; R: A/G; W: A/T; Y: C/T; N: A/T/C/G; K:G/T.
Analysis of key fragments of PtoCP1 promoter expressed by driver gene
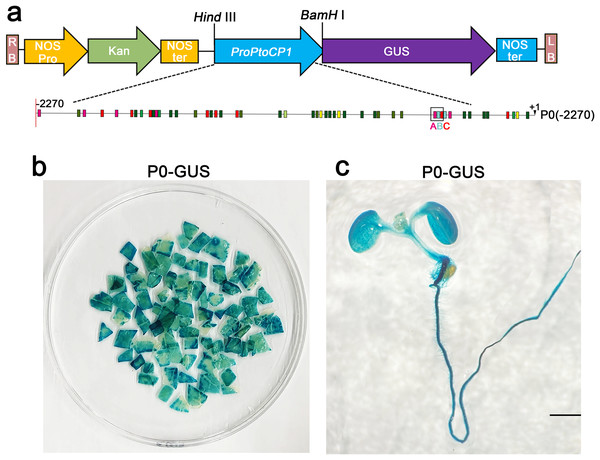
To assess the activity of the PtoCP1 promoter fragment, we fused the full-length PtoCP1 promoter fragment P0 (−2,270 bp) with the GUS reporter gene (Fig. 2A). This recombinant expression vector was then transiently transformed into tobacco leaves using the Agrobacterium-mediated method. Additionally, we transformed it into A. thaliana to generate stably transformed plants. The GUS staining results revealed that the PtoCP1 promoter fragment drives GUS expression in tobacco leaves (Fig. 2B). GUS staining was also detected in the leaves, roots, and stems of 7-day-old transgenic Arabidopsis seedlings (Fig. 2C).
Figure 2: GUS staining of PtoCP1 full-length promoters in tobacco leaf and 7-day-old transgenic Arabidopsis thaliana seedlings.
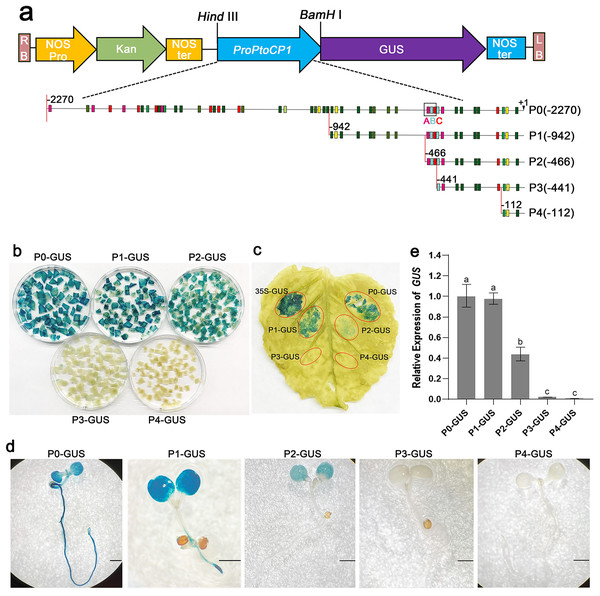
(A) Schematic representation of the PtoCP1 full-length promoter fragments fusion with GUS; (B) GUS staining of different tobacco leaves during the same period; (C) GUS staining of the 7-day-old transgenic Arabidopsis seedlings. Bar = 1 mm.To determine the key fragments of the PtoCP1 promoter that regulate protease expression via MYB elements, we designed primers based on the MYB element locations in the promoter. We then truncated the full-length promoter by deleting the 5′-end, resulting in four promoter deletion fragments named P1 (−942 bp), P2 (−466 bp), P3 (−441 bp), and P4 (−112 bp). Each of these promoter deletion fragments was fused with GUS reporter genes (Fig. 3A). Subsequently, the recombinant vectors containing the full-length promoter and the deletion promoter fragments were introduced into tobacco leaves. The GUS staining results showed that compared to P0, the GUS staining of P1 exhibited no significant change, whereas the GUS staining intensity of P2 decreased. Furthermore, the GUS staining in P3 (−441 bp) and P4 (−112 bp) almost completely disappeared (Figs. 3B and 3C). To validate these findings, we stably transformed recombinant vectors containing the PtoCP1 promoter and the 5′-end deletion series into Arabidopsis. The GUS staining and RT-qPCR results indicated (Figs. 3D and 3E) that there was no significant difference in GUS expression in transgenic Arabidopsis leaves driven by P1 and P0. However, GUS expression driven by P2 decreased by approximately one fold, whereas P3 and P4 showed negligible GUS gene transcription. These results suggest that an essential gene expression element is located in the region between P2 and P3 (−466 to −441 bp).
Figure 3: GUS activities analysis of PtoCP1 promoters full-length and deletion series in transgenic Arabidopsis thaliana seedlings and tobacco leaf.
(A) Schematic representation of diferent promoter fragments fusion with GUS; (B) GUS staining in leaves of tobacco; (C) GUS staining; (D) GUS staining of the transgenic Arabidopsis seedlings, Bar = 1 mm; (E) Expression levels of GUS under the control of a PtoCP1 promoter full-length and deletion fragments in transgenic Arabidopsis thaliana seedlings. The relative difference of GUS gene expression was detected at the P value 0.05 level, and different letters represented significant difference.An important MYB-TGACG cis-element of PtoCP1 driven gene expression
Upon comparing the fragments P2 (−466 bp) and P3 (−441 bp), we observed that three cis-elements, namely MYB, TGACG, and MYC (referred to as A, B, and C, respectively), were found in close proximity between the P2 and P3 regions. It is possible that these cis-elements play a crucial role in determining gene expression.
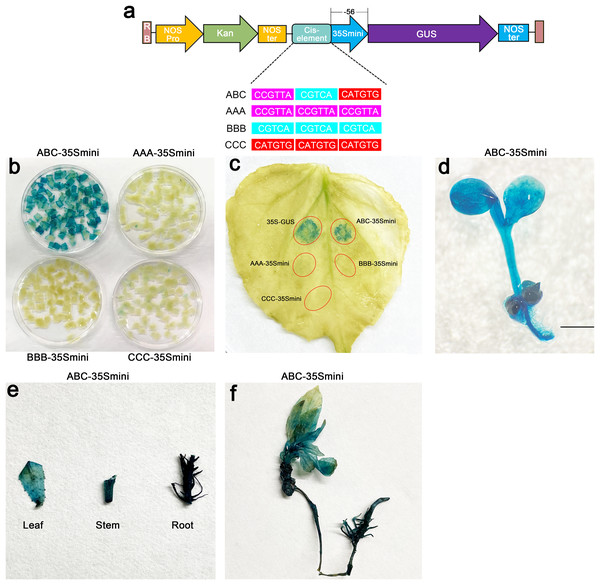
To further explore the role of these three elements, we studied the three elements together (ABC) or each element individually in three groups (AAA, BBB, and CCC). These elements were then combined with the −56 bp CaMV 35S minimum promoter (35Smini) to create four fused fragments (ABC-35Smini, AAA-35Smini, BBB-35Smini, and CCC-35Smini). Each fused fragment was then coupled with GUS reporter genes (Fig. 4A). The recombinant expression vector was transiently transformed into tobacco leaves, and GUS staining revealed that ABC-35Smini effectively drives GUS transcription, whereas AAA-35Smini, BBB-35Smini, and CCC-35Smini do not (Figs. 4B and 4C). These results indicate that A, B, or C elements alone cannot determine gene expression.
Figure 4: GUS activities analysis under the control in fusion of the PtoCP1 promoter candidate cis-element with the 35Smini.
(A) Schematic representation that the combination of cis-element and 35Smini fragment is fused with GUS. Purple background: MYB, blue background: TGACG-motif, red background: MYC, white capital base: element motif. (B) GUS staining in different leaves of the same tobacco plant; (C) GUS staining on the one tobacco leaf; (D) GUS staining in transgenic Arabidopsis thaliana seedlings. Bar = 1 mm; (E and F) GUS staining in transgenic Populus alba × Populus glandulosa seedlings.Furthermore, we introduced the ABC-35Smini recombinant vector into Arabidopsis and Populus alba × Populus glandulosa to investigate whether A, B, and C elements are sufficient to drive gene expression. GUS staining was observed in the leaves, roots, and stems of transgenic Populus alba × Populus glandulosa seedlings, as well as in transgenic 7-day-old Arabidopsis seedlings (Figs. 4D–4F). This result demonstrates that the ABC cis-element is a positive active element for gene expression.
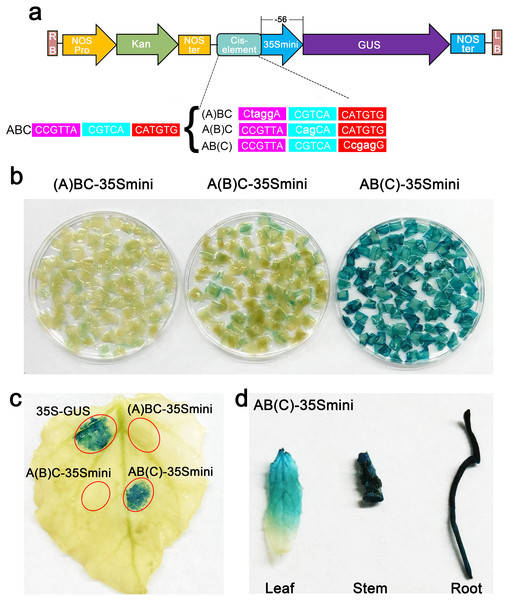
To further explore the core structure of the ABC cis-elements, we subjected the three candidate elements A, B, and C to site-specific mutation and connected them to the 35Smini promoters, resulting in (A)BC-35Smini, A(B)C-35Smini, and AB(C)-35Smini, respectively (Fig. 5A). GUS staining results indicated that a mutation in element A or B completely abolished GUS activity, whereas a mutation in element C did not affect GUS activity (Figs. 5B and 5C). To confirm these findings, we stably transformed the AB(C)-35Smini recombinant vector into Populus alba × Populus glandulosa. GUS staining was observed in transgenic Populus alba × Populus glandulosa seedlings (Fig. 5D). These results reveal that the MYB-TGACG cis-elements, but not MYB-TGACG-MYC, are the key elements determining gene expression.
Figure 5: GUS activities analysis under the control in fusion of the site-mutation cis-element with the 35Smini.
(A) Schematic representation that the combination of site- mutation cis-element and 35Smini fragment is fused with GUS. Purple background: MYB, blue background: TGACG-motif, red background: MYC, white capital base: element motif, ( ): mutant elements, white bold lowercase base: mutation site; (B) GUS staining in different leaves of the same tobacco plant; (C) GUS staining on the one tobacco leaf; (D) GUS staining in transgenic Populus alba × Populus glandulosa seedings.Discussion
Understanding the properties of promoters is crucial for studying the function of genes in specific tissues and organs (Tian, Xie & Qu, 2013). However, the mechanism of gene activity in plants remains largely unexplored (Xun et al., 2021). Previous studies have identified positive regulatory regions in the SlDREBA4 promoter (−1,095 to −730 bp and −162 to −38 bp) responsible for the promoter heat response (Yaschenko et al., 2022). Similarly, three positive regulatory regions in response to infestation by Nilaparvata lugens Stal (BPH) were identified in the rice OsAOS promoter through truncation experiments (Deng et al., 2020). The GmPRP2 promoter, cloned from soybean, exhibits root preference in soybean and transgenic A. thalianaand its core fragment for root preference expression is likely between −369 bp and +1 bp (Li et al., 2022).
The G-box (CACGTG) cis-element on the SmCP promoter of the Solanum melongena cysteine protease gene interacts specifically with a nuclear protein in leaves. Its strong binding activity to the G-box is consistent with the high expression of SmCP (Roy et al., 2012). The S2 site (ATACA), which was identified in the nuclear RPL21 gene promoter encoding a plastid ribosomal protein, functions as a leaf-specific cis-element. The activation of the S2 site in leaves is achieved through its interaction with the leaf transcription factor S2F (Xu et al., 2003). In this study, we conducted the first characterization of the PtoCP1 promoter of P. tomentosa and discovered a gene expression element, the MYB-TGACG-motif cis-element, within the region from −466 to −441 bp.
Transcription factor binding is regulated by various interactions, primarily involving cis-element binding (Lagrange et al., 1997). The gene expression regulation network mediated by transcription factors plays a vital role in plant growth, development, and stress response pathways. MYB transcription factors constitute one of the most widely distributed families of transcription factors in plants and are involved in development and stress response by binding to the MYB cis-element in target gene promoters (Mehrotra et al., 2013). Previous studies have demonstrated that the TGACG-motif serves as a recognition binding element for the TGA family (Wang, Niu & Zheng, 2021; Idrovo Espín et al., 2012). TGA transcription factors, belonging to the basic region leucine zipper (bZIP) family, serve as crucial regulatory factors in various cellular processes. TGA proteins bind to their target DNA sequences as dimers through a conserved bZIP domain, and their activity is influenced by different hormonal pathways, interacting proteins, and regulatory elements (Qi et al., 2022). Fourteen genes encoding TGA transcription factors have been identified in Hevea brasiliensis, with HbTGA1 showing the highest expression in latex (Tomaž, Gruden & Coll, 2022). Wang et al. (2019) have reported strong expression of TGA1 and TGA4 in mature organs of A. thaliana, including the leaf base, petioles, pedicel axils, and the base of floral organs (Guo et al., 2022). Our findings indicate a close connection between MYB and TGACG elements, jointly determining gene expression. Neither the individual MYB nor TGACG elements alone can drive gene expression, suggesting potential interaction between MYB and TGA transcription factors. The MYB family can be categorized into 1R-, R2R3-, 3R-, and 4R-MYB proteins based on the number of MYB domains (Wang et al., 2019), whereas TGA transcription factors can be divided into multiple subfamilies. Therefore, further verification is required to determine the specific types of TGA and MYB proteins that bind to the MYB (CCGTTA) and TGACG cis-elements.
Conclusion
In this study, we identified the PtoCP1 promoter for the first time and identified two core cis-elements (MYB-TGACG) that are associated with gene expression between −466 and −441 bp. Further analysis showed that MYB-TGACG was a positive regulatory element of gene expression. It is of great significance to further understand the gene expression of cysteine protease, and provides a valuable gene expression element for genetic engineering.